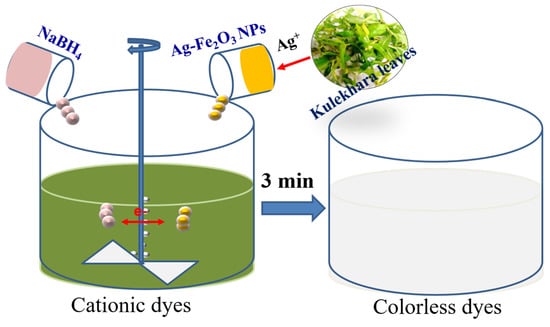
Biogenic Synthesis of Silver-Iron Oxide Nanoparticles Using Kulekhara Leaves Extract for Removing Crystal Violet and Malachite Green Dyes from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticles
2.3. Characterization
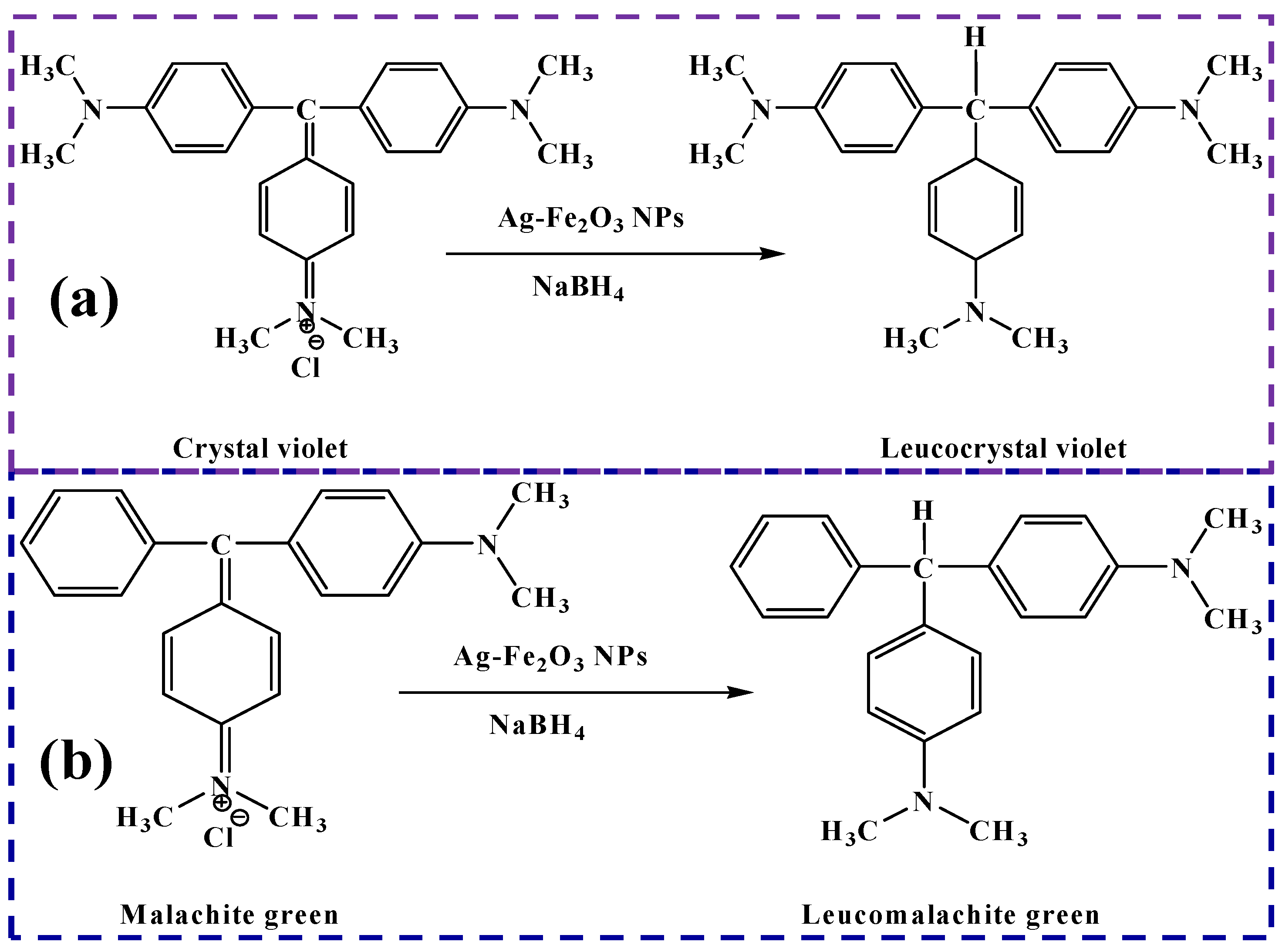
2.4. Removal of Malachite Green and Crystal Violet
3. Results and Discussions
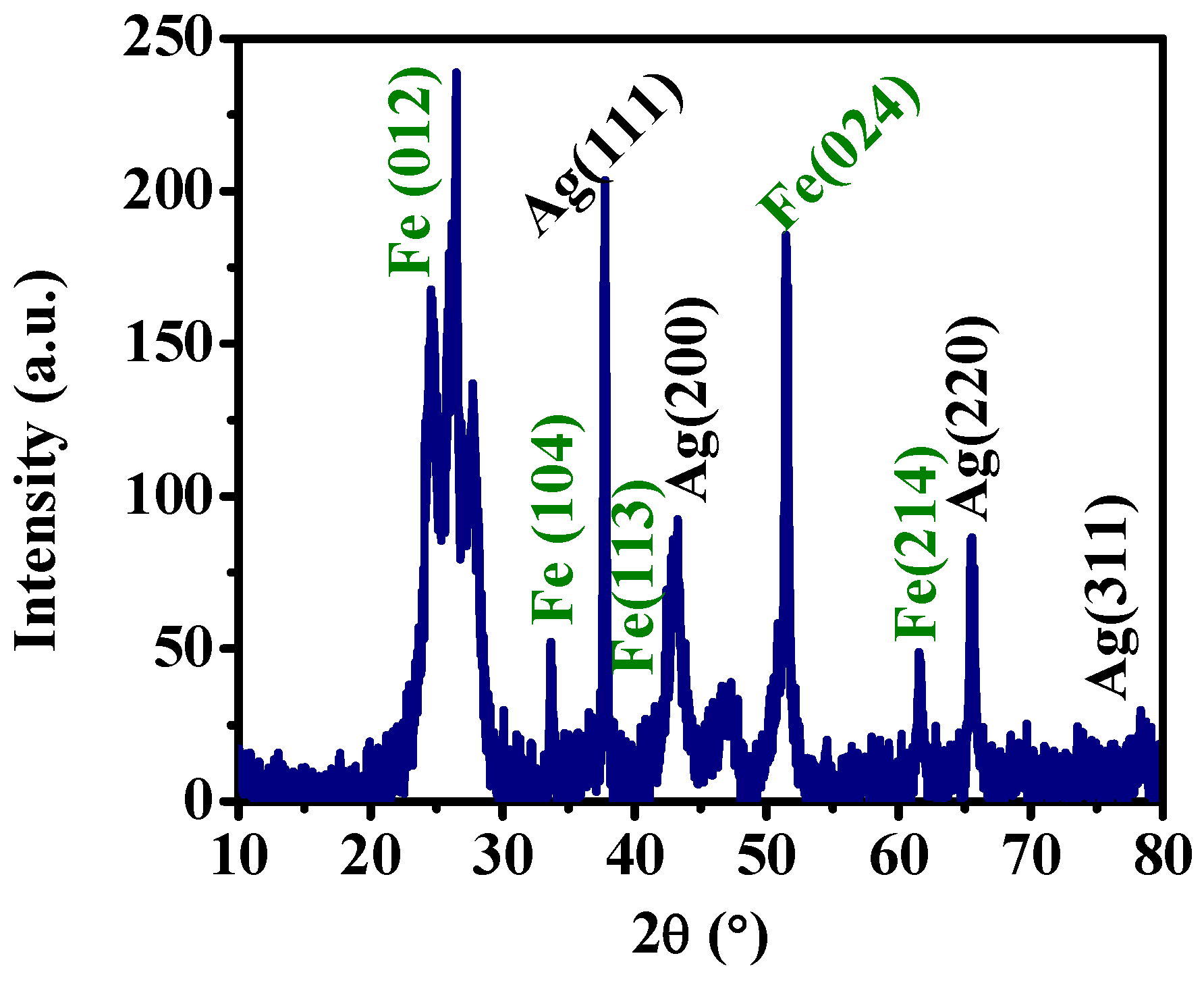
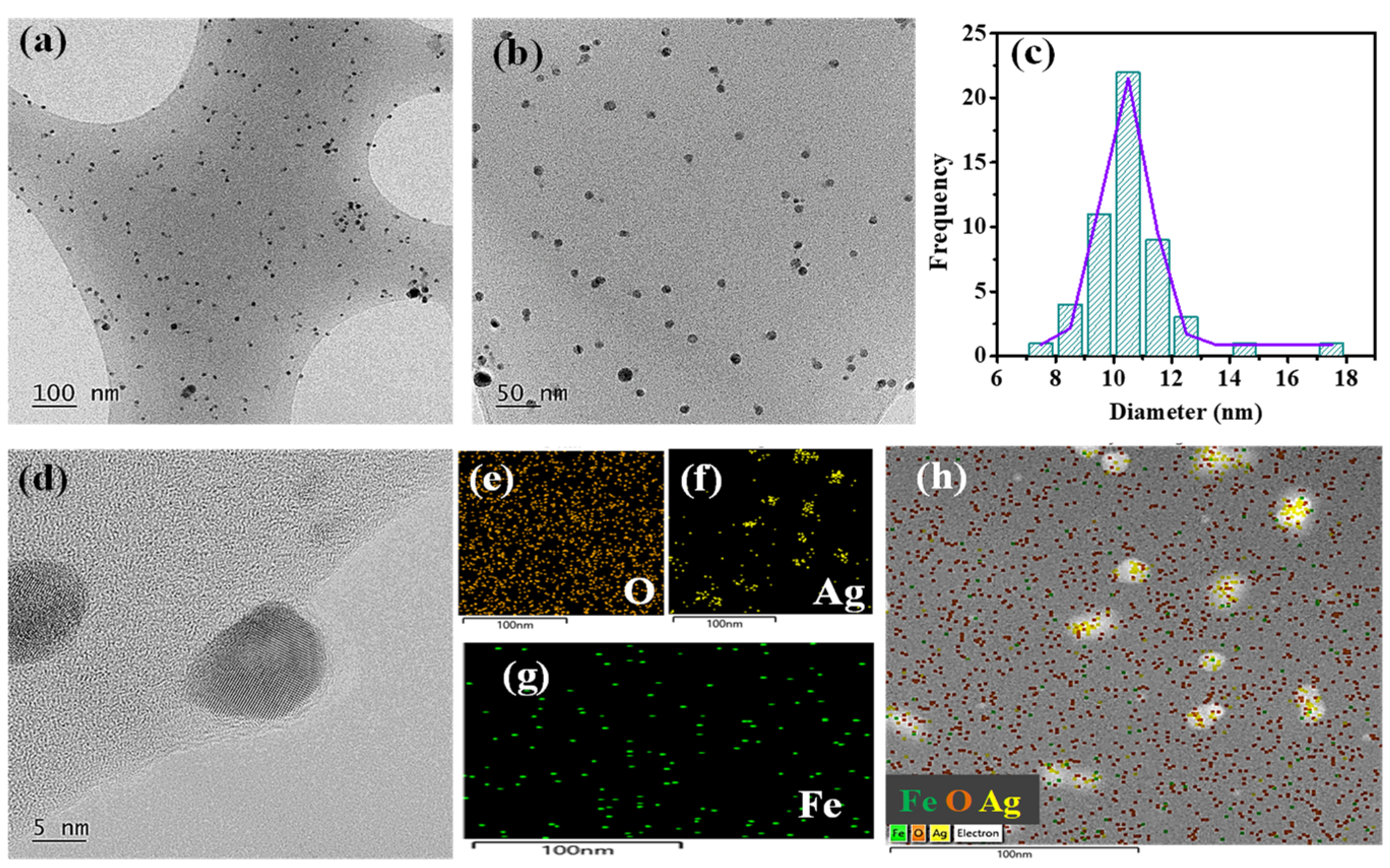
3.1. Synthesis and Characterization of Ag-Fe2O3 Nanoparticles
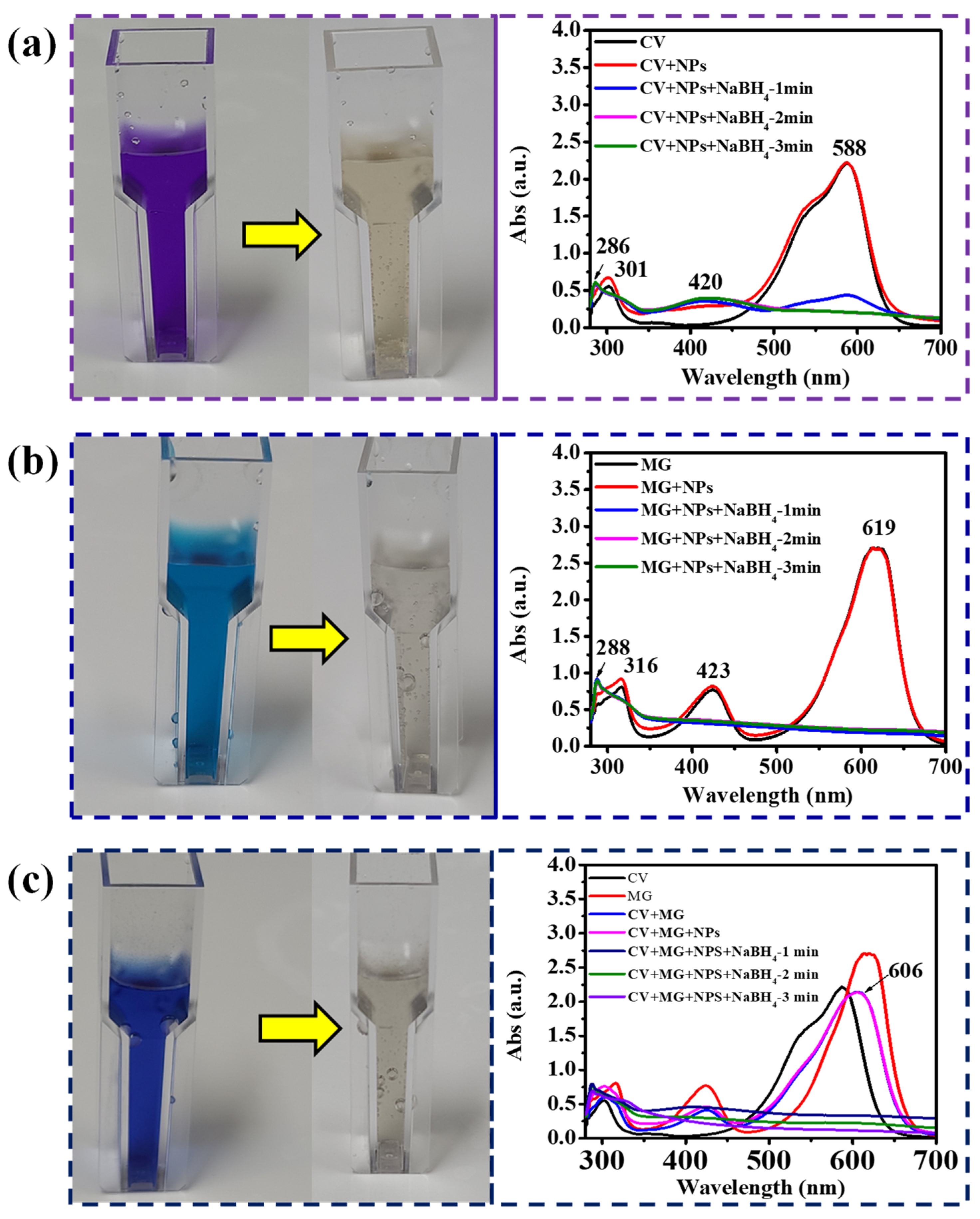
3.2. Catalytic Activity of Ag-Iron Oxide Nanoparticles
3.3. Discussion
3.4. Efficiency Comparison
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oyelude, E.O.; Awudza, J.A.M.; Twumasi, S.K. Removal of malachite green from aqueous solution using pulverized teak leaf litter: Equilibrium, kinetic and thermodynamic studies. Chem. Cent. J. 2018, 12, 81. [Google Scholar] [CrossRef]
- Martins, L.R.; Catone Soares, L.; Alves Gurgel, L.V.; Gil, L.F. Use of a new zwitterionic cellulose derivative for removal of crystal violet and orange II from aqueous solutions. J. Hazard. Mater. 2022, 424, 127401. [Google Scholar] [CrossRef]
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24. [Google Scholar] [CrossRef]
- Ozlem-Caliskan, S.; Ilikci-Sagkan, R.; Karakas, H.; Sever, S.; Yildirim, C.; Balikci, M.; Ertabaklar, H. Efficacy of malachite green mediated photodynamic therapy on treatment of Cutaneous Leishmaniasis: In vitro study. Photodiagnosis Photodyn. Ther. 2022, 40, 103111. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.; Lima, Â.; Sousa, L.G.V.; Rosca, A.S.; Muzny, C.A.; Cerca, N. Crystal Violet Staining Alone Is Not Adequate to Assess Synergism or Antagonism in Multi-Species Biofilms of Bacteria Associated with Bacterial Vaginosis. Front. Cell. Infect. Microbiol. 2022, 11, 1375. [Google Scholar] [CrossRef]
- Krishna Moorthy, A.; Govindarajan Rathi, B.; Shukla, S.P.; Kumar, K.; Shree Bharti, V. Acute toxicity of textile dye Methylene blue on growth and metabolism of selected freshwater microalgae. Environ. Toxicol. Pharmacol. 2021, 82, 103552. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Bamigboye, O.M.; Ogunbiyi, O.D.; Akano, M.T. Toxicity and decontamination strategies of Congo red dye. Groundw. Sustain. Dev. 2022, 100844. [Google Scholar] [CrossRef]
- Littlefield, N.A.; Blackwell, B.-N.; Hewitt, C.C.; Gaylor, D.W. Chronic Toxicity and Carcinogenicity Studies of Gentian Violet in Mice. Toxicol. Sci. 1985, 5, 902–912. [Google Scholar] [CrossRef]
- Kolya, H.; Tripathy, T. Hydroxyethyl Starch-g-Poly-(N,N-dimethylacrylamide-co-acrylic acid): An efficient dye removing agent. Eur. Polym. J. 2013, 49, 4265–4275. [Google Scholar] [CrossRef]
- Haque, A.N.; Sultana, N.; Sayem, A.S.; Smriti, S.A. Sustainable Adsorbents from Plant-Derived Agricultural Wastes for Anionic Dye Removal: A Review. Sustainability 2022, 14, 11098. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Xu, F.; Sun, B.; Hong, G.; Bao, L. Adsorption of Methylene Blue on Azo Dye Wastewater by Molybdenum Disulfide Nanomaterials. Sustainability 2022, 14, 7585. [Google Scholar] [CrossRef]
- Alam, J.; Shukla, A.K.; Ansari, M.A.; Ali, F.A.; Alhoshan, M. Dye Separation and Antibacterial Activities of Polyaniline Thin Film-Coated Poly(phenyl sulfone) Membranes. Membranes 2021, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Kolya, H.; Sasmal, D.; Tripathy, T. Novel Biodegradable Flocculating Agents Based on Grafted Starch Family for the Industrial Effluent Treatment. J. Polym. Environ. 2017, 25, 408–418. [Google Scholar] [CrossRef]
- Pinheiro, L.R.; Gradíssimo, D.G.; Xavier, L.P.; Santos, A. V Degradation of Azo Dyes: Bacterial Potential for Bioremediation. Sustainability 2022, 14, 1510. [Google Scholar] [CrossRef]
- Kolya, H.; Kuila, T.; Kim, N.H.; Lee, J.H. Bioinspired silver nanoparticles/reduced graphene oxide nanocomposites for catalytic reduction of 4-nitrophenol, organic dyes and act as energy storage electrode material. Compos. Part B Eng. 2019, 173, 106924. [Google Scholar] [CrossRef]
- Kang, C.-W.; Kolya, H. Green Synthesis of Ag-Au Bimetallic Nanocomposites Using Waste Tea Leaves Extract for Degradation Congo Red and 4-Nitrophenol. Sustainability 2021, 13, 3318. [Google Scholar] [CrossRef]
- You, X.; Zhou, R.; Zhu, Y.; Bu, D.; Cheng, D. Adsorption of dyes methyl violet and malachite green from aqueous solution on multi-step modified rice husk powder in single and binary systems: Characterization, adsorption behavior and physical interpretations. J. Hazard. Mater. 2022, 430, 128445. [Google Scholar] [CrossRef]
- Cuong, D.V.; Hou, C.-H. Engineered biochar prepared using a self-template coupled with physicochemical activation for highly efficient adsorption of crystal violet. J. Taiwan Inst. Chem. Eng. 2022, 139, 104533. [Google Scholar] [CrossRef]
- Kumar, S.A.; Jarvin, M.; Inbanathan, S.S.R.; Umar, A.; Lalla, N.P.; Dzade, N.Y.; Algadi, H.; Rahman, Q.I.; Baskoutas, S. Facile green synthesis of magnesium oxide nanoparticles using tea (Camellia sinensis) extract for efficient photocatalytic degradation of methylene blue dye. Environ. Technol. Innov. 2022, 28, 102746. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, H.; Sun, Y.; Ren, J.; Zheng, X.; Sun, Q.; Jiang, S.; Ding, W. Synthesis of novel chitosan-based flocculants with amphiphilic structure and its application in sludge dewatering: Role of hydrophobic groups. J. Clean. Prod. 2020, 249, 119350. [Google Scholar] [CrossRef]
- Ji, Y.; Ma, C.; Li, J.; Zhao, H.; Chen, Q.; Li, M.; Liu, H. A Magnetic Adsorbent for the Removal of Cationic Dyes from Wastewater. Nanomaterials 2018, 8, 710. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar] [CrossRef]
- Gallo-Cordova, A.; Castro, J.J.; Winkler, E.L.; Lima, E.; Zysler, R.D.; del Puerto Morales, M.; Ovejero, J.G.; Streitwieser, D.A. Improving degradation of real wastewaters with self-heating magnetic nanocatalysts. J. Clean. Prod. 2021, 308, 127385. [Google Scholar] [CrossRef]
- Kolya, H.; Mondal, S.; Kang, C.W.; Nah, C. The use of polymer-graphene composites in catalysis. In Polymer Nanocomposites Containing Graphene; Woodhead Publishing: Sawston, UK, 2022; pp. 537–556. [Google Scholar] [CrossRef]
- Kouhbanani, M.A.J.; Beheshtkhoo, N.; Taghizadeh, S.; Amani, A.M.; Alimardani, V. One-step green synthesis and characterization of iron oxide nanoparticles using aqueous leaf extract of Teucrium polium and their catalytic application in dye degradation. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 15007. [Google Scholar] [CrossRef]
- Barozzi, M.; Copelli, S.; Russo, E.; Sgarbossa, P.; Lavagnolo, M.C.; Sandon, A.; Morosini, C.; Sieni, E. Implementation of Magnetic Nanostructured Adsorbents for Heavy Metals Separation from Textile Wastewater. Sustainability 2022, 14, 11785. [Google Scholar] [CrossRef]
- Biswas, S.; Pal, A. Application of biopolymers as a new age sustainable material for surfactant adsorption: A brief review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100145. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, M.; Xu, W.; He, S.; Men, Y. Natural polysaccharides-modified graphene oxide for adsorption of organic dyes from aqueous solutions. J. Colloid Interface Sci. 2017, 486, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.; Shukla, S.K.; Singh, N.B. Water purification by polymer nanocomposites: An overview. Nanocomposites 2017, 3, 47–66. [Google Scholar] [CrossRef]
- Acar, M.K.; Altun, T.; Gubbuk, I.H. Synthesis and characterization of silver doped magnetic clay nanocomposite for environmental applications through effective RhB degradation. Int. J. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Sengan, M.; Veeramuthu, D.; Veerappan, A. Photosynthesis of silver nanoparticles using Durio zibethinus aqueous extract and its application in catalytic reduction of nitroaromatics, degradation of hazardous dyes and selective colorimetric sensing of mercury ions. Mater. Res. Bull. 2018, 100, 386–393. [Google Scholar] [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient catalytic degradation of dyes using κ-carrageenan stabilized silver nanoparticles nanocatalyst. Carbohydr. Polym. 2020, 230, 115597. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Jing, K.; Lu, L.; Liu, H. Facile synthesis of Ag/AgCl/3D-rGO with rapid catalytic degradation toward Methyl Orange and Rhodamine B. Chem. Eng. Res. Des. 2022, 186, 22–33. [Google Scholar] [CrossRef]
- Naseem, K.; Ali, F.; Tahir, M.H.; Afaq, M.; Yasir, H.M.; Ahmed, K.; muteb Aljuwayid, A.; Habila, M.A. Investigation of catalytic potential of sodium dodecyl sulfate stabilized silver nanoparticles for the degradation of methyl orange dye. J. Mol. Struct. 2022, 1262, 132996. [Google Scholar] [CrossRef]
- Padre, S.M.; Kiruthika, S.; Mundinamani, S.; Ravikirana; Surabhi, S.; Jeong, J.-R.; Eshwarappa, K.M.; Murari, M.S.; Shetty, V.; Ballal, M.; et al. Mono- and Bimetallic Nanoparticles for Catalytic Degradation of Hazardous Organic Dyes and Antibacterial Applications. ACS Omega 2022, 7, 35023–35034. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Chakravarty, A.; Ahmad, I.; Singh, P.; Ud Din Sheikh, M.; Aalam, G.; Sagadevan, S.; Ikram, S. Green synthesis of silver nanoparticles using fruits extracts of Syzygium cumini and their bioactivity. Chem. Phys. Lett. 2022, 795, 139493. [Google Scholar] [CrossRef]
- Kolya, H.; Maiti, P.; Pandey, A.; Tripathy, T. Green synthesis of silver nanoparticles with antimicrobial and azo dye (Congo red) degradation properties using Amaranthus gangeticus Linn leaf extract. J. Anal. Sci. Technol. 2015, 6, 33. [Google Scholar] [CrossRef]
- Basalius, H.; Mani, A.; Michael, A.; Mary, S.M.; Lenin, M.; Chelliah, P.; Siddiqui, M.R.; Wabaidur, S.M.; Islam, M.A. Green synthesis of nano-silver using Syzygium samarangense flower extract for multifaceted applications in biomedical and photocatalytic degradation of methylene blue. Appl. Nanosci. 2022. [Google Scholar] [CrossRef]
- Panyala, N.R.; Peña-Méndez, E.M.; Havel, J. Silver or silver nanoparticles: A hazardous threat to the environment and human health? J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar] [CrossRef]
- Roy, M.; Sarkar, B.C.; Shukla, G.; Vineeta; Debnath, M.K.; Nath, A.J.; Bhat, J.A.; Chakravarty, S. Traditional homegardens and ethnomedicinal plants: Insights from the Indian Sub-Himalayan region. Trees For. People 2022, 8, 100236. [Google Scholar] [CrossRef]
- Konar, A.; Mukherjee, K.; Ghosh, P.; El-Shazly, M. Traditional medicinal plants used in different districts of West Bengal by the tribal communities. J. Pharmacogn. Phytochem. 2022, 11, 104–110. [Google Scholar] [CrossRef]
- Sethiya, N.K.; Ahmed, N.M.; Shekh, R.M.; Kumar, V.; Kumar Singh, P.; Kumar, V. Ethnomedicinal, phytochemical and pharmacological updates on Hygrophila auriculata (Schum.) Hiene: An overview. J. Integr. Med. 2018, 16, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, B.; Rasool, M.G.; Ullah, I.; Rasool, R.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Phytochemicals Mediated Synthesis of AuNPs from Citrullus colocynthis and Their Characterization. Molecules 2022, 27, 1300. [Google Scholar] [CrossRef]
- Yin, Y.; Han, D.; Tai, C.; Tan, Z.; Zhou, X.; Yu, S.; Liu, J.; Jiang, G. Catalytic role of iron in the formation of silver nanoparticles in photo-irradiated Ag+-dissolved organic matter solution. Environ. Pollut. 2017, 225, 66–73. [Google Scholar] [CrossRef]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Maaza, M. Biosynthesis of iron oxide (Fe2O3) nanoparticles via aqueous extracts of Sageretia thea (Osbeck.) and their pharmacognostic properties. Green Chem. Lett. Rev. 2017, 10, 186–201. [Google Scholar] [CrossRef]
- Masek, A.; Latos-Brozio, M.; Chrzescijanska, E.; Podsedek, A. Polyphenolic Profile and Antioxidant Activity of Juglans regia L. Leaves and Husk Extracts. Forests 2019, 10, 988. [Google Scholar] [CrossRef]
- Kolya, H.; Kuila, T.; Kim, N.H.; Lee, J.H. Colorimetric/naked eye detection of arsenic ions in aqueous medium by mango flower extract: A facile and novel approach. Appl. Surf. Sci. 2020, 513, 145760. [Google Scholar] [CrossRef]
- Nalbandian, L.; Patrikiadou, E.; Zaspalis, V.; Patrikidou, A.; Hatzidaki, E.; N. Papandreou, C. Magnetic nanoparticles in medical diagnostic applications: Synthesis, characterization and proteins conjugation. Curr. Nanosci. 2016, 12, 455–468. [Google Scholar] [CrossRef]
- Raj, S.; Chand Mali, S.; Trivedi, R. Green synthesis and characterization of silver nanoparticles using Enicostemma axillare (Lam.) leaf extract. Biochem. Biophys. Res. Commun. 2018, 503, 2814–2819. [Google Scholar] [CrossRef]
- Joya, M.R.; Barón-Jaimez, J.; Barba-Ortega, J. Preparation and characterization of Fe2O3nanoparticles. J. Phys. Conf. Ser. 2013, 466, 12004. [Google Scholar] [CrossRef]
- Jang, M.-S.; Lee, Y.-M.; Kim, C.-H.; Lee, J.-H.; Kang, D.-W.; Kim, S.-J.; Lee, Y.-C. Triphenylmethane reductase from Citrobacter sp. strain KCTC 18061P: Purification, characterization, gene cloning, and overexpression of a functional protein in Escherichia coli. Appl. Environ. Microbiol. 2005, 71, 7955–7960. [Google Scholar] [CrossRef]
- Henderson, A.L.; Schmitt, T.C.; Heinze, T.M.; Cerniglia, C.E. Reduction of malachite green to leucomalachite green by intestinal bacteria. Appl. Environ. Microbiol. 1997, 63, 4099–4101. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Nagajyothi, P.C.; Reddy, G.R.; Shim, J.; Yoo, K. Urea assisted ceria nanocubes for efficient removal of malachite green organic dye from aqueous system. Sci. Rep. 2019, 9, 14477. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Kashyap, A.; Kashyap, A.; Vishwakarma, D.; Maholiya, A. Methylene Blue Dye Degradation Using Silver Nanoparticles Synthesized from Andographis Paniculata Leaves Extract. J. Pharm. Res. Int. 2021, 33, 131–139. [Google Scholar]
- Abd El-Aziz, A.R.M.; Gurusamy, A.; Alothman, M.R.; Shehata, S.M.; Hisham, S.M.; Alobathani, A.A. Silver nanoparticles biosynthesis using Saussurea costus root aqueous extract and catalytic degradation efficacy of safranin dye. Saudi J. Biol. Sci. 2021, 28, 1093–1099. [Google Scholar] [CrossRef]
- Ojo, S.A.; Lateef, A.; Azeez, M.A.; Oladejo, S.M.; Akinwale, A.S.; Asafa, T.B.; Yekeen, T.A.; Akinboro, A.; Oladipo, I.C.; Gueguim-Kana, E.B.; et al. Biomedical and Catalytic Applications of Gold and Silver-Gold Alloy Nanoparticles Biosynthesized Using Cell-Free Extract of Bacillus Safensis LAU 13: Antifungal, Dye Degradation, Anti-Coagulant and Thrombolytic Activities. IEEE Trans. Nanobiosci. 2016, 15, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Diana-Carmen, M.; Dumitra, R.; Ana-Maria, G.; Ana-Maria, R.; Andrei, C.V.; Valentin, Z.; Ileana-Denisa, N. Silver Nanoparticles Incorporated on Natural Clay as an Inhibitor against the New ISO SS Bacteria Isolated from Sewage Sludge, Involved in Malachite Green Dye Oxidation. Molecules 2022, 27, 5791. [Google Scholar] [CrossRef] [PubMed]
- Nga, N.T.A.; Raghavendra, V.B.; Sindhu, R.; Alshiekheid, M.; Sabour, A.; Krishnan, R.; Lan Chi, N.T.; Pugazhendhi, A. Green fabrication of silver nanoparticles using Chloroxylon swietenia leaves and their application towards dye degradation and food borne pathogens. Food Chem. Toxicol. 2022, 165, 113192. [Google Scholar] [CrossRef]
- Recio-Sánchez, G.; Tighe-Neira, R.; Alvarado, C.; Inostroza-Blancheteau, C.; Benito, N.; García-Rodríguez, A.; Marcos, R.; Pesenti, H.; Carmona, E.R. Assessing the effectiveness of green synthetized silver nanoparticles with Cryptocarya alba extracts for remotion of the organic pollutant methylene blue dye. Environ. Sci. Pollut. Res. 2019, 26, 15115–15123. [Google Scholar] [CrossRef] [PubMed]
- Bonnia, N.N.; Kamaruddin, M.S.; Nawawi, M.H.; Ratim, S.; Azlina, H.N.; Ali, E.S. Green Biosynthesis of Silver Nanoparticles Using ‘Polygonum Hydropiper’ and Study its Catalytic Degradation of Methylene Blue. Procedia Chem. 2016, 19, 594–602. [Google Scholar] [CrossRef]
- Zhang, N.; Peng, S.; Liu, Z.; Li, Y.; Huang, J.; Li, J.; Wan, H.; Zhou, S.; Gao, Z.; Chen, T. Ag NPs decorated on the magnetic Fe3O4@PDA as efficient catalyst for organic pollutants removal and as effective antimicrobial agent for microbial inhibition. J. Alloys Compd. 2022, 928, 167257. [Google Scholar] [CrossRef]
- Rajasekar, R.; Samuel, M.; Edison, T.N.J.I.; Raman, N. Sustainable synthesis of silver nanoparticles using Alstonia scholaris for enhanced catalytic degradation of methylene blue. J. Mol. Struct. 2021, 1246, 131208. [Google Scholar] [CrossRef]
- Somasundaram, C.K.; Atchudan, R.; Edison, T.N.; Perumal, S.; Vinodh, R.; Sundramoorthy, A.K.; Babu, R.S.; Alagan, M.; Lee, Y.R. Sustainable Synthesis of Silver Nanoparticles Using Marine Algae for Catalytic Degradation of Methylene Blue. Catalysts 2021, 11, 1377. [Google Scholar] [CrossRef]


| Sl No. | Nanoparticles | Dyes | Reaction Conditions | Removal (%) | Time | Ref. |
|---|---|---|---|---|---|---|
| 1. | MMT/iron oxide/Ag | Rhodamine B | Catalyst: 0.448 mg/mL; NaBH4: 27 mM; Dye: 0.08 mM. | 46.0 | 8 min | [30] |
| 2. | Ag NPs | Methylene blue | Catalyst: 0.1 mg/mL; Dye: 10 mg/L. | 84.0 | 64 h | [55] |
| 3. | Ag NPs | Safranin | Catalyst: 0.12 mg/mL; Dye: 10 mg/L. | 84.6 | 72 h | [56] |
| 4. | Au-AgNPs | Malachite green | Catalyst: 0.1 mg/mL; Dye: 40 mg/L. | 90.0 | 48 h | [57] |
| 5. | Bentonite-Ag0 | Malachite green | Catalyst: 0.12 mg/mL; Dye: 5 × 10−5 M. | 86.4 | 5 min | [58] |
| 6. | AgNPs | crystal violet | Catalyst: 10 mg/mL; Dye: NA. | 90.0 | 24 h | [59] |
| 7. | AgNPs | Methylene blue | Catalyst: 0.3 mg/mL; Dye: 3.1 × 10−5 M. NaBH4: 3.0 × 10−5 M. | 100 | 1 min | [60] |
| 8. | Au-Ag NPs | Congo red | Catalyst: 20 μL; Dye: 10−4 M. NaBH4: 20 μL. | 100 | 6 min | [32] |
| 9. | Ag NPs | Methylene blue | Catalyst: 2 mL; Dye: 10−3 M. NaBH4: 0.1 M | 100 | 13 min | [61] |
| 10. | Fe3O4 @PDA-Ag | Methylene blue | Catalyst: 3 mg; Dye: 20 mg/L. NaBH4: 0.1 M | 92.6 | 7 min | [62] |
| 11. | AS-AgNPs | Methylene blue | Catalyst: 0.05 mL; Dye: 100 μM. NaBH4: 100 mM | 97.0 | 27 min | [63] |
| 12. | AgNPs | Methylene blue | Catalyst: 0.05 mL; Dye: 50 μM. NaBH4: 0.005 M | 99.0 | 20 min | [64] |
| 13. | Ag-iron oxide NPs | Crystal violet Malachite green Mixture (1:1 v/v) | Catalyst: 0.5 mL; Dye: 100 mg/L. NaBH4: 0.5 mg/mL | 100 | 3 min | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolya, H.; Kang, C.-W. Biogenic Synthesis of Silver-Iron Oxide Nanoparticles Using Kulekhara Leaves Extract for Removing Crystal Violet and Malachite Green Dyes from Water. Sustainability 2022, 14, 15800. https://doi.org/10.3390/su142315800
Kolya H, Kang C-W. Biogenic Synthesis of Silver-Iron Oxide Nanoparticles Using Kulekhara Leaves Extract for Removing Crystal Violet and Malachite Green Dyes from Water. Sustainability. 2022; 14(23):15800. https://doi.org/10.3390/su142315800
Chicago/Turabian StyleKolya, Haradhan, and Chun-Won Kang. 2022. "Biogenic Synthesis of Silver-Iron Oxide Nanoparticles Using Kulekhara Leaves Extract for Removing Crystal Violet and Malachite Green Dyes from Water" Sustainability 14, no. 23: 15800. https://doi.org/10.3390/su142315800
APA StyleKolya, H., & Kang, C.-W. (2022). Biogenic Synthesis of Silver-Iron Oxide Nanoparticles Using Kulekhara Leaves Extract for Removing Crystal Violet and Malachite Green Dyes from Water. Sustainability, 14(23), 15800. https://doi.org/10.3390/su142315800