Effects of Salinity and Dissolved Oxygen Concentration on the Tail-Flip Speed and Physiologic Response of Whiteleg Shrimp, Litopenaeus vannamei
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of Animals
2.2. Experimental Apparatus
2.3. Experimental Design
2.3.1. Salinity Experiment
2.3.2. DO Concentration Experiment
2.3.3. Tail-Flip Speed (Stf)
2.4. Tissue Collection and Biochemical Assessment
2.5. Statistical Analysis
3. Results
3.1. Stf
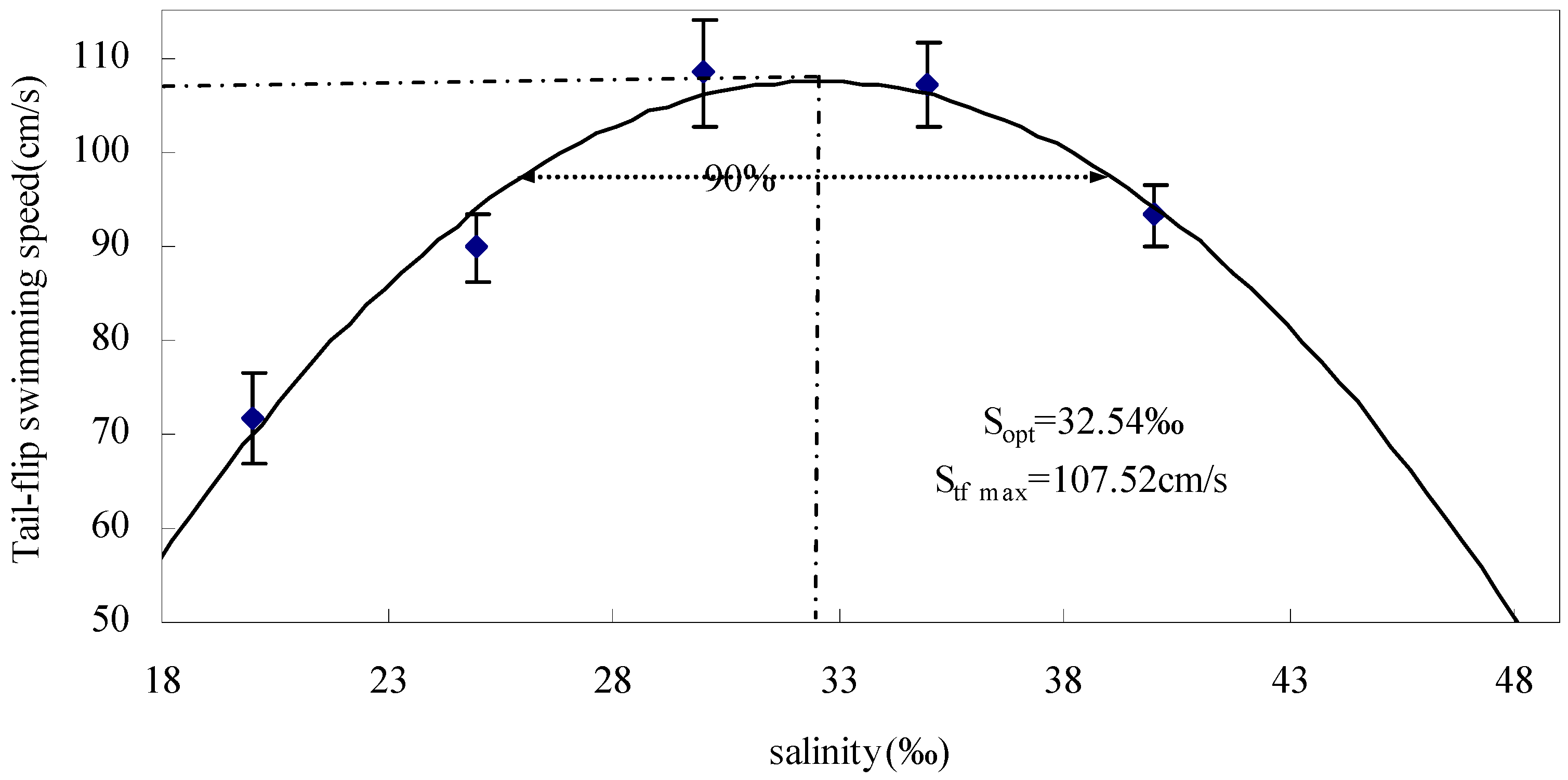
3.1.1. Effect of Salinity
3.1.2. Effect of DO
3.2. Physiologic Response
3.2.1. Effect of Salinity
3.2.2. Effect of DO
4. Discussion
4.1. Effects of Salinity and DO on Stf
4.1.1. Effect of Salinity
4.1.2. Effect of DO
4.2. Physiologic Response
4.2.1. Effect of Salinity
4.2.2. Effect of DO
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sornom, P.; Felten, V.; Médoc, V.; Sroda, S.; Rousselle, P.; Beisel, J.N. Effect of gender on physiological and behavioral responses of Gammarus roeseli (Crustacea Amphipoda) to salinity and temperature. Environ. Pollut. 2010, 158, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Morillo-Velarde, P.S.; Lloret, J.; Marín, A.; Sánchez-Vázquez, F.J. Effects of cadmium on locomotor activity rhythms of the Amphipod Gammarus aequicauda. Arch. Environ. Contam. Toxicol. 2011, 60, 444–451. [Google Scholar] [CrossRef]
- Bernatis, J.L.; Gerstenberger, S.L.; McGaw, I.J. Behavioural responses of the Dungeness crab, Cancer magister, during feeding and digestion in hypoxic conditions. Mar. Biol. 2007, 150, 941–951. [Google Scholar] [CrossRef]
- Claireaux, G.; Webber, D.M.; Kerr, S.R.; Boutilier, R.G. Physiology and behaviour of free-swimming Atlantic cod (Gadus morhua) facing fluctuating salinity and oxygenation conditions. J. Exp. Biol. 1995, 198, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.Z.; Zhang, X.M.; Li, J.; Huang, G.Q. Compensatory growth of Chinese shrimp, Fenneropenaeus chinensis following hypoxic exposure. Aquacult. Int. 2008, 16, 455–470. [Google Scholar] [CrossRef]
- Yu, X.M.; Zhang, X.M.; Duan, Y.; Zhang, P.D.; Miao, Z.Q. Effects of temperature, salinity, body length, and starvation on the critical swimming speed of whiteleg shrimp, Litopenaeus vannamei. Comp. Biochem. Physiol. A 2010, 157, 392–397. [Google Scholar] [CrossRef]
- Zhang, P.D.; Zhang, X.M.; Li, J.; Huang, G.Q. The effects of temperature and salinity on the swimming ability of whiteleg shrimp, Litopenaeus vannamei. Comp. Biochem. Physiol. A 2007, 147, 64–69. [Google Scholar] [CrossRef]
- Saunders, R.L. Respiration of the Atlantic cod. J. Fish. Res. Board Can. 1963, 20, 373–385. [Google Scholar] [CrossRef]
- Nelson, J.A.; Tang, Y.; Boutilier, R.G. The effects of salinity change on the exercise performance of two Atlantic cod (Gadus morhua) populations inhabiting different environments. J. Exp. Biol. 1996, 199, 1295–1309. [Google Scholar] [CrossRef]
- Davis, G.E.; Foster, J.; Warren, C.E.; Doudoroff, P. The influence of oxygen concentration on the swimming performance of juvenile pacific salmon at various temperatures. Trans. Am. Fish. Soc. 1963, 92, 111–124. [Google Scholar] [CrossRef]
- Dahlberg, M.L.; Shumway, D.L.; Doudoroff, O. Influence of dissolved oxygen and carbon dioxide on swimming performance of largemouth bass and coho salmon. J. Fish. Res. Board Can. 1968, 25, 49–70. [Google Scholar] [CrossRef]
- Jones, D.R. The effect of hypoxia and anaemia on the swimming performance of rainbow trout (Salmo gairdneri). J. Exp. Biol. 1971, 55, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Guppy, M.; Fuery, C.J.; Flanigan, J.E. Biochemical principles of metabolic depression. Comp. Biochem. Physiol. B 1994, 109, 175–189. [Google Scholar] [CrossRef]
- Rosas, C.; Sánchez, A.; Díaz-Iglesia, E.; Brito, R.; Martinez, E.; Soto, L.A. Critical dissolved oxygen level to Penaeus setiferus and P. schmitti postlarvae (PL10–18) exposed to salinity changes. Aquaculture 1997, 152, 259–272. [Google Scholar] [CrossRef]
- Rosas, C.; Martinez, E.; Gaxiola, G.; Brito, R.; Díaz-Iglesia, E.; Soto, L.A. The effect of dissolved oxygen and salinity on oxygen consumption, ammonia excretion and osmotic pressure of Penaeus setiferus (Linnaeus) juveniles. J. Exp. Mar. Biol. Ecol. 1999, 234, 41–57. [Google Scholar] [CrossRef]
- Yu, X.M.; Zhang, X.M.; Zhang, P.D.; Yu, C.G. Critical swimming speed, tail-flip speed and physiological response to exercise fatigue in kuruma shrimp, Marsupenaeus japonicus. Com. Biochem. Physiol. A 2009, 153, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Tallmark, B.; Evans, S. Substrate related differences in antipredator behaviour of two gobiid fish species and the brown shrimp and their adaptive value. Mar. Ecol. Prog. Ser. 1986, 29, 217–222. [Google Scholar] [CrossRef]
- Berghahn, R.; Wiese, K.; Lüdemann, K. Physical and physiological aspects of gear efficiency in North Sea brown shrimp fisheries. Helgoland Mar. Res. 1995, 49, 507–518. [Google Scholar] [CrossRef]
- Newland, P.L.; Neil, D.M.; Chapman, C.J. Escape swimming in the Norway lobster. J. Crustac. Biol. 1992, 12, 342–353. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Neila, D.M.; Atkinson, R.J.A.; Bailey, N. An analysis of swimming performance in the Norway lobster, Nephrops norvegicus L. infected by a parasitic dinoflagellate of the genus Hematodinium. J. Exp. Mar. Biol. Ecol. 2000, 247, 169–181. [Google Scholar] [CrossRef]
- Harris, R.R.; Andrews, M.B. Physiological changes in the Norway lobster Nephrops norvegicus (L.) escaping and discarded from commercial trawls on the West Coast of Scotland II. Disturbances in haemolymph respiratory gases, tissue metabolites and swimming performance after capture and during recovery. J. Exp. Mar. Biol. Ecol. 2005, 320, 95–210. [Google Scholar]
- Harris, R.R.; Andrews, M.B. Physiological changes in the Norway lobster Nephrops norvegicus (L.) escaping and discarded from commercial trawls on the West Coast of Scotland 1. Body fluid volumes and hemolymph composition after capture and during recovery. J. Exp. Mar. Biol. Ecol. 2005, 320, 179–193. [Google Scholar] [CrossRef]
- Newland, P.L.; Chapman, C.J.; Nell, D.M. Swimming performance and endurance of the Norway lobster, Nephrops norvegicus. Mar. Biol. 1988, 98, 345–350. [Google Scholar] [CrossRef]
- Daniel, T.L.; Meyhöfer, E. Size limits in escape locomotion of carridean shrimp. J. Exp. Biol. 1989, 143, 245–265. [Google Scholar] [CrossRef]
- Arnott, S.A.; Neil, D.M.; Ansell, A.D. Tail-flip mechanism and size-dependent kinematics of escape swimming in the brown shrimp Crangon Crangon. J. Exp. Biol. 1998, 201, 1771–1784. [Google Scholar] [CrossRef]
- Robertson, L.; Bray, W.; Leung-Trujillo, J.; Lawrence, A. Practical molt staging of Penaeus setiferus and Penaeus stylirostris. J. World Aquacult. Soc. 1987, 18, 180–185. [Google Scholar] [CrossRef]
- Vargas-Albores, F.; Guzmán, M.A.; Ochoa, J.L. An anticoagulant solution for haemolymph collection and prophenoloxidase studies of penaeid shrimp (Penaeus californiensis). Comp. Biochem. Physiol. A 1993, 106, 299–303. [Google Scholar] [CrossRef]
- Amornpiyakrit, T.; Arimoto, T. Muscle physiology in escape response of kuruma shrimp. Am. Fish. Soc. Symp. 2008, 2, 1321–1334. [Google Scholar]
- Robles-Romo, A.; Zenteno-Savín, T.; Racotta, I.S. Bioenergetic status and oxidative stress during escape response until exhaustion in whiteleg shrimp Litopenaeus vannamei. J. Exp. Mar. Biol. Ecol. 2016, 478, 16–23. [Google Scholar] [CrossRef]
- Field, R.H.; Taylor, A.C.; Neil, D.M. Factors affecting swimming ability and its recovery in the Norway lobster (Nephrops norvegicus). J. Mar. Biol. Assn. UK 1991, 71, 707–742. [Google Scholar]
- Li, J.T.; Li, W.T.; Zhang, X.M.; He, P.G. Physiological and behavioral responses of different modes of locomotion in the whiteleg shrimp Litopenaeus vannamei (Boone, 1931) (Caridea: Penaeidae). J. Crustacean Biol. 2018, 38, 79–90. [Google Scholar] [CrossRef]
- Brauner, C.J.; Shrimpton, J.M.; Randall, D.J. Effect of short-duration seawater exposure on plasma ion concentrations and swimming performance in coho salmon (Oncorhynchus kisutch) parr. Can. J. Fish. Aquat. Sci. 1992, 49, 2399–2405. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Cataldi, E.; Owen, S.; Taylor, E.W.; Bronzi, P. Effects of acclimation to brackish water on the growth, respiratory metabolism and exercise performance of Adriatic sturgeon (Acipenser naccarii). Can. J. Fish. Aquat. Sci. 2001, 58, 1104–1112. [Google Scholar] [CrossRef]
- McKenzie, D.J.; Cataldi, E.; Taylor, E.W.; Cataudella, S.; Bronzi, P. Effects of acclimation to brackish water on tolerance of salinity challenge by Adriatic sturgeon (Acipenser naccarii). Can. J. Fish. Aquat. Sci. 2001, 58, 1113–1120. [Google Scholar] [CrossRef]
- Chatelier, A.; McKenzie, D.J.; Claireaux, G. Effects of changes in water salinity upon exercise and cardiac performance in the European seabass (Dicentrarchus labrax). Mar. Biol. 2005, 147, 855–862. [Google Scholar] [CrossRef]
- Gracia-López, V.; Rosas-Vázquez, C.; Brito-Pérez, R. Effects of salinity on physiological conditions in juvenile common snook Centropomus undecimalis. Comp. Biochem. Physiol. A 2006, 145, 340–345. [Google Scholar] [CrossRef]
- Lefrancois, C.; Amat, J.N.; Domenici, P.; Kostecki, C.; Ferrari, R. The effect of oxygen and temperature on the energetics of swimming in Mugil cephalus. Comp. Biochem. Physiol. A 2007, 146, S75–S86. [Google Scholar] [CrossRef]
- Rosas, C.; Martinez, E.; Gaxiola, G.; Brito, R.; Díaz-Iglesia, E.; Soto, L.A. Effect of dissolved oxygen on the energy balance and survival of Penaeus setiferus juveniles. Mar. Ecol. Prog. Ser. 1998, 174, 67–75. [Google Scholar] [CrossRef]
- Li, D.; Wei, X.L.; Lin, X.T.; Xu, Z.N.; Mu, X.P. Effects of exercise training on carbohydrate and lipid catabolism in the swimming muscles of Nile tilapia (Oreochromis niloticus). J. Anim. Physiol. Anim. Nutr. 2015, 99, 893–898. [Google Scholar] [CrossRef]
- Schmitt, A.S.C.; Uglow, R. Haemolymph constituent levels and ammonia efflux rates of Nephrops norvegicus during emersion. Mar. Biol. 1997, 127, 403–410. [Google Scholar] [CrossRef]
- Paterson, B.D. The rise in inosine monophosphate and L-lactate concentration in muscle of live penaeid prawn (Penaeus japonicus, Penaeus monodon) stressed by the storage out of water. Comp. Biochem. Physiol. B 1993, 106, 395–400. [Google Scholar] [CrossRef]
- Yoganandhan, K.; Thirupathi, S.; Sahul Hameed, A.S. Biochemical, physiological and hematological changes in white spot syndrome virus-infected shrimp, Penaeus indicus. Aquaculture 2003, 221, 1–11. [Google Scholar] [CrossRef]
- Nordlie, F.G.; Walsh, S.J.; Haney, D.C.; Nordlie, T.F. The influence of ambient salinity on routine metabolism in the teleost Cyprinodon variegatus Lacepède. J. Fish. Biol. 1991, 38, 115–122. [Google Scholar] [CrossRef]
- Swanson, C. Interactive effects of salinity on metabolic rate, activity, growth and osmoregulation in the euryhaline milkfish (Chanos chanos). J. Exp. Biol. 1998, 201, 3355–3366. [Google Scholar] [CrossRef] [PubMed]
- Plaut, I. Resting metabolic rate, critical swimming speed, and routine activity of the euryhaline cyprinodontid, Aphanius dispar, acclimated to a wide range of salinities. Physiol. Biochem. Zool 2000, 73, 590–596. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Cao, Z.D.; Fu, S.J.; Peng, J.L.; Wang, Y.X. The effect of exhaustive chasing training and detraining on swimming performance in juvenile dark barbel catfish (Peltebagrus vachelli). J Comp Physiol B 2009, 179, 847–855. [Google Scholar] [CrossRef]
- Vermeer, G.K. Effects of air exposure on desiccation rate, haemolymph chemistry, and escape behaviour of the spiny lobster, Palinurus argus. Fish. Bull. 1987, 85, 45–51. [Google Scholar]
- Baldwin, J.; Gupta, A.; Iglesias, X. Scaling of anaerobic energy metabolism during tail flipping behaviour in the freshwater crayfish, Cherax destructor. Mar. Freshw. Res. 1999, 50, 183–187. [Google Scholar] [CrossRef]
- Lage, L.P.A.; Plagnes-Juan, E.; Putrino, S.M.; Baron, F.; Weissman, D.; Guyonvarch, A.; Brugger, R.; Nunes, A.J.P.; Panserat, S. Ontogenesis of metabolic gene expression in whiteleg shrimp (Litopenaeus vannamei): New molecular tools for programming in the future. Aquaculture 2017, 479, 142–149. [Google Scholar] [CrossRef]
- Hill, A.D.; Strang, R.H.C.; Taylor, A.C. Radioisotope studies of the energy metabolism of the shore crab Carcinus maenas (L.) during environmental anoxia and recovery. J. Exp. Mar. Biol. Ecol. 1991, 150, 51–62. [Google Scholar] [CrossRef]
- Spicer, J.I.; Hill, A.D.; Taylor, A.C.; Strang, R.H.C. Effect of aerial exposure on concentration of selected metabolites in blood of Norwegian lobster Nephrops norvegicus (Crustacea: Nephropoidea). Mar. Biol. 1990, 105, 129–135. [Google Scholar] [CrossRef]
- Bergmann, M.; Moore, P.G. Survival of decapod crustaceans discarded in the Nephrops fishery of the Clyde Sea area, Scotland. ICES J. Mar. Sci. 2001, 58, 163–171. [Google Scholar] [CrossRef][Green Version]
- Woo, N.Y.S.; Kelly, S.P. Effects of salinity and nutritional status on growth and metabolism of Sparus sarba in a closed seawater system. Aquaculture 1995, 135, 229–238. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, X.M.; Liu, X.X.; Thakur, D.N. Effect of dissolved oxygen on swimming ability and physiological response to swimming fatigue of whiteleg shrimp (Litopenaeus vannamei). J. Ocean. Univ. China 2014, 13, 132–140. [Google Scholar] [CrossRef]
- Heath, A.G. Anaerobic and aerobic energy metabolism in brain and liver tissue from rainbow trout (Salmo gairdneri) and bullhead catfish (Ictalurus nebulosus). J. Exp. Zool. 1988, 248, 140–146. [Google Scholar] [CrossRef]
- Dobson, G.P.; Parkhouse, W.S.; Hochachka, P.W. Regulation of anaerobic ATP-generating pathways in trout fast-twitch skeletal muscle. Am. J. Physiol. 1987, 253, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Hochachka, P.W.; Emmett, B.; Suarez, R.K. Limits and constraints in the scaling of oxidative and glycolytic enzymes in homeotherms. Can. J. Zool. 1988, 66, 1128–1138. [Google Scholar] [CrossRef]
- Somero, G.N.; Childress, J.J. Scaling of ATP-supplying enzymes, myofibrillar proteins and buffering capacity in fish muscle: Relationship to locomotory habit. J. Exp. Biol. 1990, 149, 319–333. [Google Scholar] [CrossRef]
- Goolish, E.M. Aerobic and anaerobic scaling in fish. Biol. Rev. 1991, 66, 33–56. [Google Scholar] [CrossRef]
- Baldwin, J.; Seymour, R.S.; Webb, G.J.W. Scaling of anaerobic metabolism during exercise in the estuarine crocodile (Crocodylus porosus). Comp. Biochem. Physiol. A 1995, 112, 285–293. [Google Scholar] [CrossRef]

| Salinity (‰) | Body Length (cm) | Wet Mass (g) | Average Stf (cm/s) | Average Distance of One Tail-Flip (cm) | Average Total Distance of Tail-Flips (cm) | Average Total Number of Tail-Flips |
|---|---|---|---|---|---|---|
| 20 | 79.79 ± 1.34 a | 5.71 ± 0.25 a | 71.65 ± 4.83 a | 29.27 ± 2.66 a | 463.39 ± 70.56 a | 15.86 ± 2.37 a |
| 25 | 81.53 ± 1.38 a | 6.55 ± 0.37 a | 89.78 ± 3.58 ab | 31.62 ± 2.27 a | 674.10 ± 90.65 ab | 22.85 ± 2.72 ab |
| 30 | 80.33 ± 1.15 a | 6.11 ± 0.27 a | 118.31 ± 10.65 c | 32.33 ± 2.71 a | 865.86 ± 112.23 b | 28.34 ± 2.90 b |
| 35 | 79.10 ± 1.28 a | 5.74 ± 0.28 a | 107.01 ± 4.48 bc | 32.02 ± 2.18 a | 714.06 ± 93.24 ab | 26.27 ± 2.527 b |
| 40 | 81.14 ± 1.26 a | 6.28 ± 0.29 a | 93.29 ± 3.22 b | 31.33 ± 2.09 a | 534.14 ± 51.15 a | 19.34 ± 2.057 a |
| DO (mg/L) | Body Length (cm) | Wet Mass (g) | Average Stf (cm/s) | Average Distance of One Tail-Flip (cm) | Average Total Distance of Tail-Flips (cm) | Average Total Number of Tail-Flips |
|---|---|---|---|---|---|---|
| 1.9 | 79.53 ± 0.98 a | 5.15 ± 0.21 a | 83.42 ± 2.19 a | 25.20 ± 1.47 a | 466.32 ± 31.19 a | 19.89 ± 1.44 a |
| 3.8 | 80.33 ± 1.15 a | 5.82 ± 0.28 a | 93.33 ± 1.82 b | 27.42 ± 1.51 ab | 585.31 ± 30.26 a | 23.86 ± 1.20 ab |
| 6.8 | 78.64 ± 0.81 a | 5.22 ± 0.17 a | 105.02 ± 3.14 c | 31.09 ± 1.48 b | 794.74 ± 64.27 b | 27.26 ± 2.16 b |
| 13.6 | 80.75 ± 1.05 a | 5.83 ± 0.23 a | 120.24 ± 4.47 d | 31.60 ± 2.21 b | 922.73 ± 69.09 b | 33.15 ± 2.265 c |
| Tissue | Salinity (‰) | |||||
|---|---|---|---|---|---|---|
| 20 | 25 | 30 | 35 | 40 | ||
| Plasma | ||||||
| Glucose (mmol L−1) | Control | 1.79 ± 0.13 a | 2.64 ± 0.21 b* | 2.34 ± 0.20 ab* | 3.04 ± 0.40 ab* | 2.12 ± 0.24 ab |
| After exercise fatigue | 1.63 ± 0.19 a | 1.90 ± 0.20 a | 1.55 ± 0.16 a | 1.91 ± 0.22 a | 2.11 ± 0.20 a | |
| Triglyceride (mmol L−1) | Control | 0.07 ± 0.02 a* | 0.18 ± 0.02 b* | 0.16 ± 0.02 b* | 0.15 ± 0.01 b | 0.18 ± 0.03 b |
| After exercise fatigue | 0.24 ± 0.04 a | 0.30 ± 0.04 a | 0.31 ± 0.05 a | 0.20 ± 0.03 a | 0.25 ± 0.02 a | |
| Total protein (mg mL−1) | Control | 68.40 ± 7.99 a | 116.91 ± 13.11 a | 85.85 ± 10.43 a | 89.64 ± 17.97 a | 92.08 ± 15.60 a* |
| After exercise fatigue | 80.61 ± 10.58 a | 94.385 ± 13.71 a | 73.23 ± 14.43 a | 74.77 ± 9.45 a | 132.52 ± 7.59 a | |
| Lactate (mmol L−1) | Control | 0.62 ± 0.13 a* | 0.59 ± 0.17 a* | 0.23 ± 0.05 a* | 0.44 ± 0.17 a* | 0.61 ± 0.13 a* |
| After exercise fatigue | 1.32 ± 0.10 a | 1.19 ± 0.24 a | 1.48 ± 0.44 ab | 2.09 ± 0.31 ab | 2.69 ± 0.19 b | |
| Hepatopancreas | ||||||
| Glycogen (mg g−1) | Control | 5.23 ± 0.49 ab* | 6.22 ± 0.85 a | 3.58 ± 0.40 bc | 2.94 ± 0.73 c | 3.85 ± 0.43 bc |
| After exercise fatigue | 3.77 ± 0.19 a | 4.65 ± 0.40 a | 2.56 ± 0.50 a | 3.13 ± 0.48 a | 4.95 ± 1.40 a | |
| Total protein (mg g−1) | Control | 151.28 ± 8.84 a | 131.98 ± 5.35 a | 110.33 ± 22.60 ab | 85.41 ± 7.07 b | 164.82 ± 29.04 ab |
| After exercise fatigue | 133.69 ± 23.79 a | 148.01 ± 16.15 a | 140.10 ± 12.69 a | 98.95 ± 2.39 a | 150.56 ± 14.78 a | |
| Lactate (μmol g−1) | Control | 43.67 ± 5.83 a | 32.01 ± 3.14 a | 30.80 ± 8.23 a | 40.70 ± 3.27 a | 25.25 ± 12.06 a |
| After exercise fatigue | 42.52 ± 4.58 a | 22.00 ± 4.73 a | 38.67 ± 3.71 a | 42.02 ± 3.00 a | 36.39 ± 9.18 a | |
| Abdominal muscle | ||||||
| Glycogen (mg g−1) | Control | 1.78 ± 0.29 a* | 1.88 ± 0.16 a | 2.49 ± 0.40 ab | 1.64 ± 0.29 a | 3.47 ± 0.43 b |
| After exercise fatigue | 3.24 ± 0.30 bc | 2.66 ± 0.35 ab | 1.72 ± 0.32 a | 2.15 ± 0.27 ab | 3.95 ± 0.53 c | |
| Total protein (mg g−1) | Control | 70.84 ± 9.86 a | 100.81 ± 13.19 a | 121.77 ± 14.44 a | 108.05 ± 17.28 a | 71.86 ± 7.30 a* |
| After exercise fatigue | 62.30 ± 12.66 a | 86.43 ± 10.02 a | 88.55 ± 12.59 a | 84.07 ± 11.86 a | 54.11 ± 4.07 a | |
| Lactate (mmol g−1) | Control | 0.44 ± 0.05 a | 0.39 ± 0.04 ab | 0.26 ± 0.03 c | 0.28 ± 0.04 bc | 0.45 ± 0.04 a |
| After exercise fatigue | 0.50 ± 0.07 a | 0.48 ± 0.04 a | 0.33 ± 0.03 b | 0.39 ± 0.05 ab | 0.51 ± 0.02 a | |
| Tissue | DO Concentration (mg L−1) | ||||
|---|---|---|---|---|---|
| 1.9 | 3.8 | 6.8 | 13.6 | ||
| Plasma | |||||
| Glucose (mmol L−1) | Control | 2.03 ± 0.19 ab | 2.04 ± 0.19 ab | 2.30 ± 0.16 a* | 1.52 ± 0.11 b* |
| After exercise fatigue | 2.19 ± 0.24 a | 1.87 ± 0.31 ab | 1.68 ± 0.08 a | 0.93 ± 0.17 b | |
| Triglyceride (mmol L−1) | Control | 0.10 ± 0.02 a | 0.15 ± 0.01 a | 0.12 ± 0.01 a | 0.10 ± 0.02 a |
| After exercise fatigue | 0.06 ± 0.02 a | 0.09 ± 0.04 a | 0.11 ± 0.02 a | 0.10 ± 0.03 a | |
| Total protein (mg mL−1) | Control | 128.88 ± 9.34 b* | 125.79 ± 10.30 b | 92.07 ± 9.85 a | 74.47 ± 10.73 a |
| After exercise fatigue | 163.28 ± 5.29 b | 112.52 ± 10.47 a | 96.17 ± 9.52 a | 110.00 ± 16.98 ab | |
| Lactate (mmol L−1) | Control | 1.42 ± 0.32 a* | 0.58 ± 0.12 ab* | 0.55 ± 0.22 ab* | 0.30 ± 0.11 b* |
| After exercise fatigue | 4.60 ± 0.52 c | 2.39 ± 0.12 b | 2.43 ± 0.42 ab | 1.38 ± 0.23 a | |
| Hepatopancreas | |||||
| Glycogen (mg g−1) | Control | 3.44 ± 0.62 a | 4.41 ± 0.31 a | 2.90 ± 0.53 a | 3.09 ± 0.26 a |
| After exercise fatigue | 4.36 ± 0.31 a | 4.27 ± 0.22 a | 3.02 ± 0.28 a | 3.20 ± 0.62 a | |
| Total protein (mg g−1) | Control | 168.16 ± 42.97 a | 151.28 ± 15.69 a | 139.05 ± 23.85 a | 165.15 ± 27.95 a |
| After exercise fatigue | 151.28 ± 24.95 a | 143.82 ± 20.17 a | 149.19 ± 18.56 a | 199.43 ± 41.16 a | |
| Lactate (μmol g−1) | Control | 27.46 ± 12.79 a | 29.17 ± 5.05 a | 23.47 ± 4.00 a | 29.12 ± 9.81 a |
| After exercise fatigue | 35.64 ± 6.21 a | 31.02 ± 12.27 a | 25.74 ± 8.59 a | 22.39 ± 6.14 a | |
| Abdominal muscle | |||||
| Glycogen (mg g−1) | Control | 7.68 ± 1.11 a | 6.87 ± 1.00 a | 3.11 ± 0.62 b | 1.02 ± 0.26 c |
| After exercise fatigue | 6.79 ± 1.21 c | 5.74 ± 1.07 bc | 3.43 ± 0.93 ab | 1.87 ± 0.78 a | |
| Total protein (mg g−1) | Control | 153.17 ± 13.67 a | 155.92 ± 16.30 a | 164.03 ± 18.09 a | 132.27 ± 8.03 a |
| After exercise fatigue | 123.69 ± 14.84 a | 119.32 ± 24.92 a | 155.96 ± 8.10 a | 107.47 ± 14.79 a | |
| Lactate (mmol g−1) | Control | 0.28 ± 0.01 a | 0.34 ± 0.04 a | 0.21 ± 0.02 a | 0.20 ± 0.02 a |
| After exercise fatigue | 0.31 ± 0.02 a | 0.46 ± 0.07 a | 0.26 ± 0.01 a | 0.27 ± 0.03 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Y.; Li, M.; Sun, M.; Wang, A.; Chai, Y.; Dong, J.; Chen, F.; Yu, Z.; Zhang, X. Effects of Salinity and Dissolved Oxygen Concentration on the Tail-Flip Speed and Physiologic Response of Whiteleg Shrimp, Litopenaeus vannamei. Sustainability 2022, 14, 15413. https://doi.org/10.3390/su142215413
Duan Y, Li M, Sun M, Wang A, Chai Y, Dong J, Chen F, Yu Z, Zhang X. Effects of Salinity and Dissolved Oxygen Concentration on the Tail-Flip Speed and Physiologic Response of Whiteleg Shrimp, Litopenaeus vannamei. Sustainability. 2022; 14(22):15413. https://doi.org/10.3390/su142215413
Chicago/Turabian StyleDuan, Yan, Mengyao Li, Ming Sun, Aiyong Wang, Yu Chai, Jing Dong, Fudi Chen, Zhe Yu, and Xiumei Zhang. 2022. "Effects of Salinity and Dissolved Oxygen Concentration on the Tail-Flip Speed and Physiologic Response of Whiteleg Shrimp, Litopenaeus vannamei" Sustainability 14, no. 22: 15413. https://doi.org/10.3390/su142215413
APA StyleDuan, Y., Li, M., Sun, M., Wang, A., Chai, Y., Dong, J., Chen, F., Yu, Z., & Zhang, X. (2022). Effects of Salinity and Dissolved Oxygen Concentration on the Tail-Flip Speed and Physiologic Response of Whiteleg Shrimp, Litopenaeus vannamei. Sustainability, 14(22), 15413. https://doi.org/10.3390/su142215413








