Effects of Harvesting Intensity on the Growth of Hydrilla verticillata and Water Quality
Abstract
1. Introduction
2. Research Methods
2.1. Experimental Design
2.2. Determination of H. verticillata Growth Indices
2.3. Determination of Water Quality Indicators
2.4. Data Processing
3. Results and Analysis
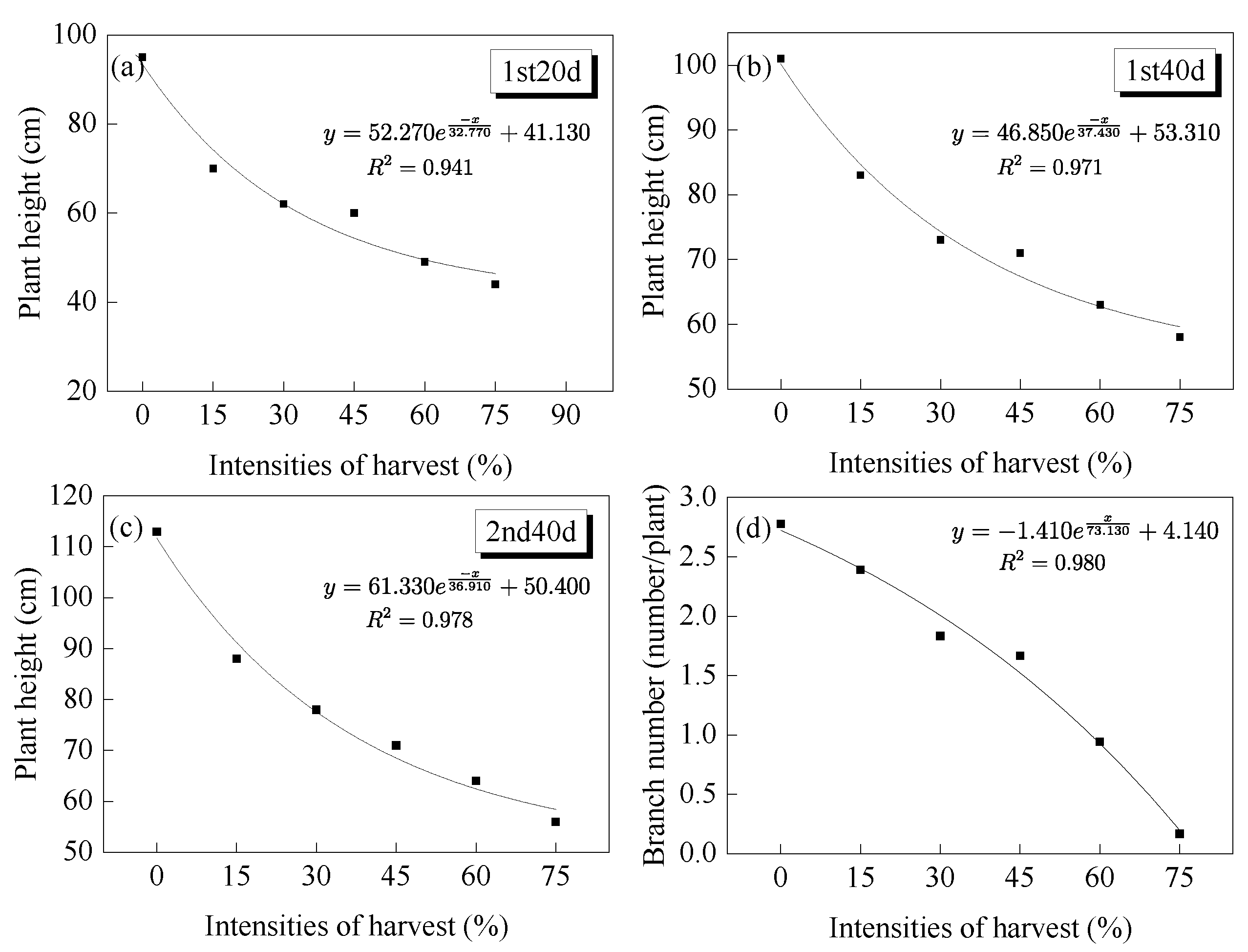
3.1. Changes in Growth Indicators
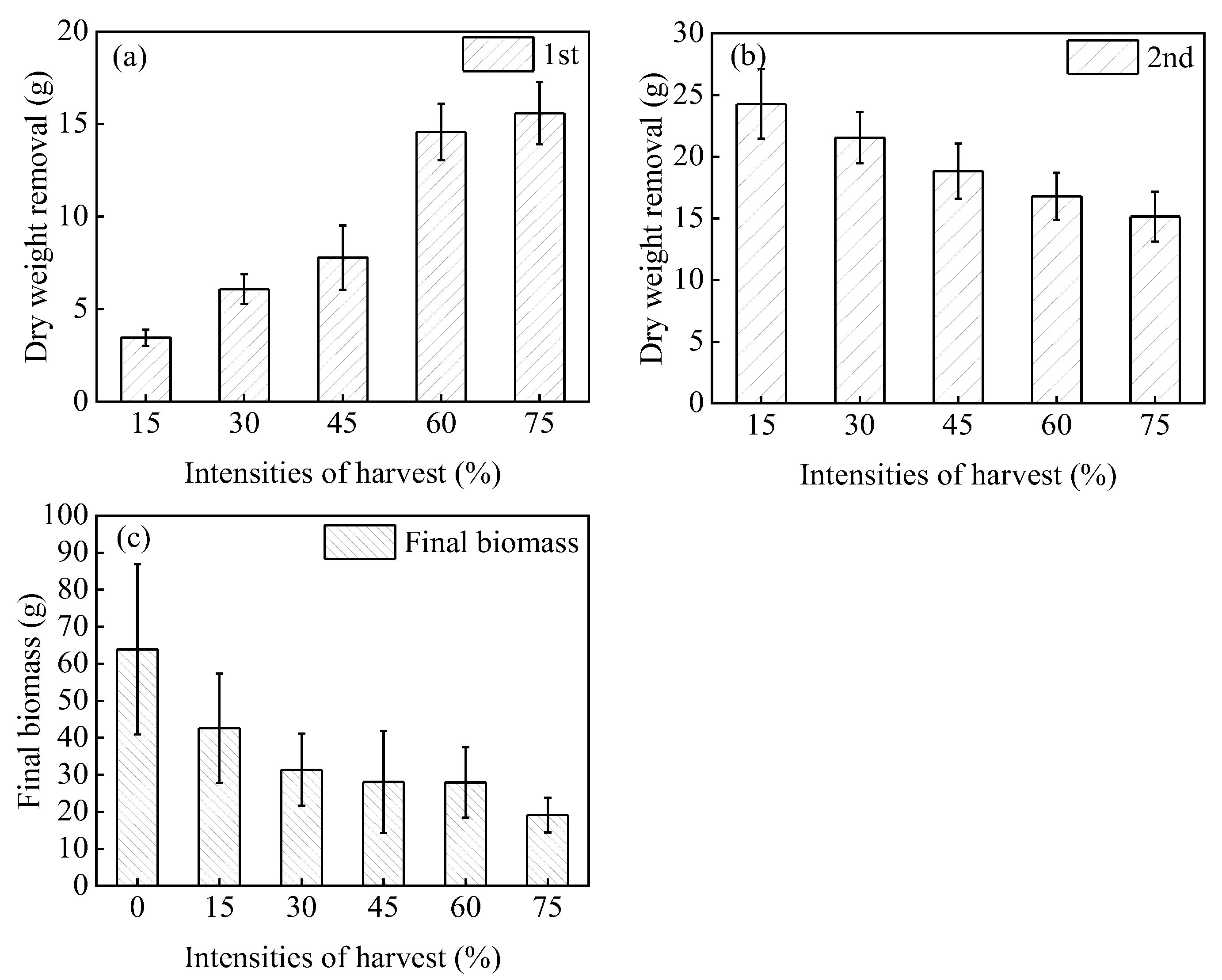
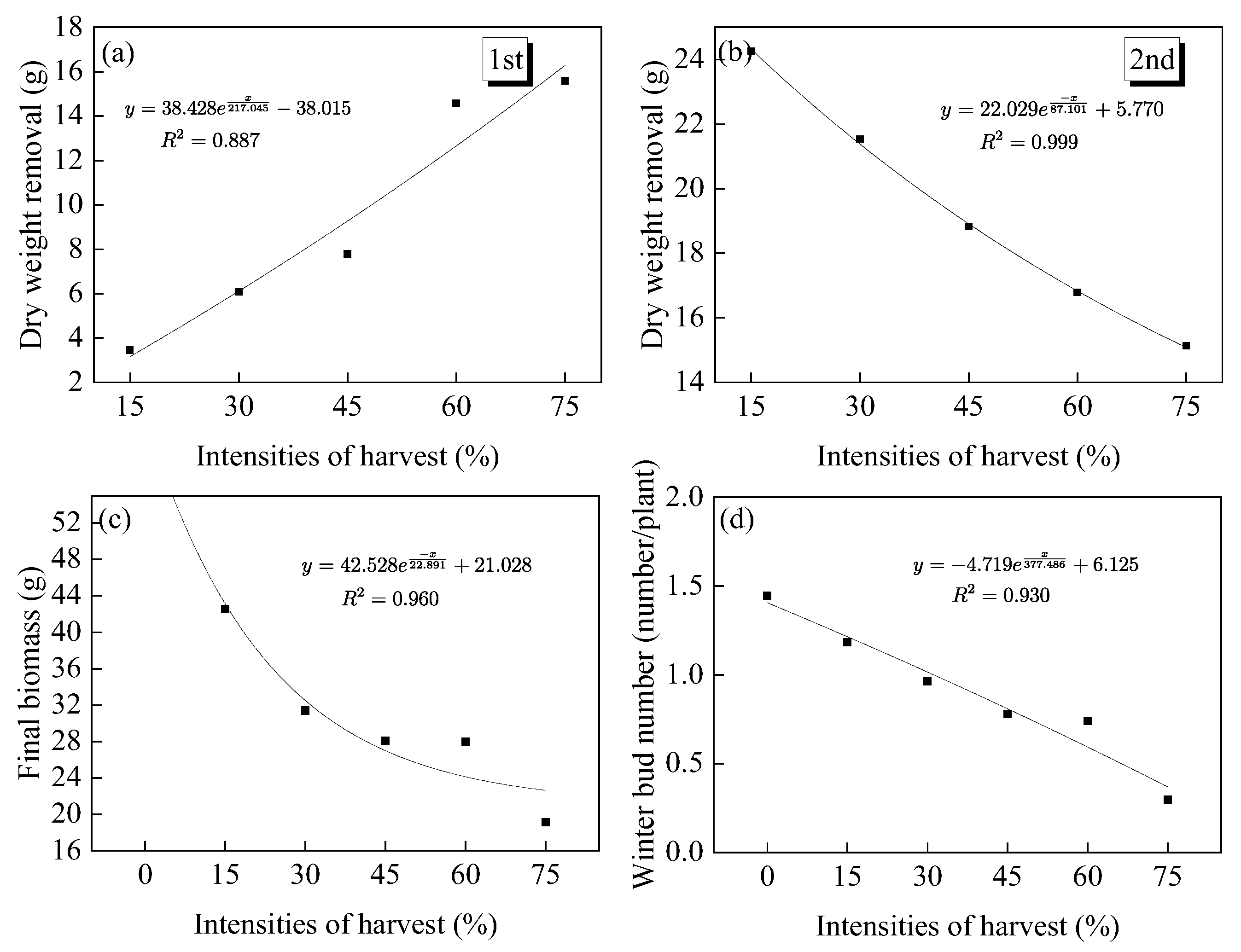
3.1.1. Branch Recovery and Biomass
3.1.2. Number of Branches
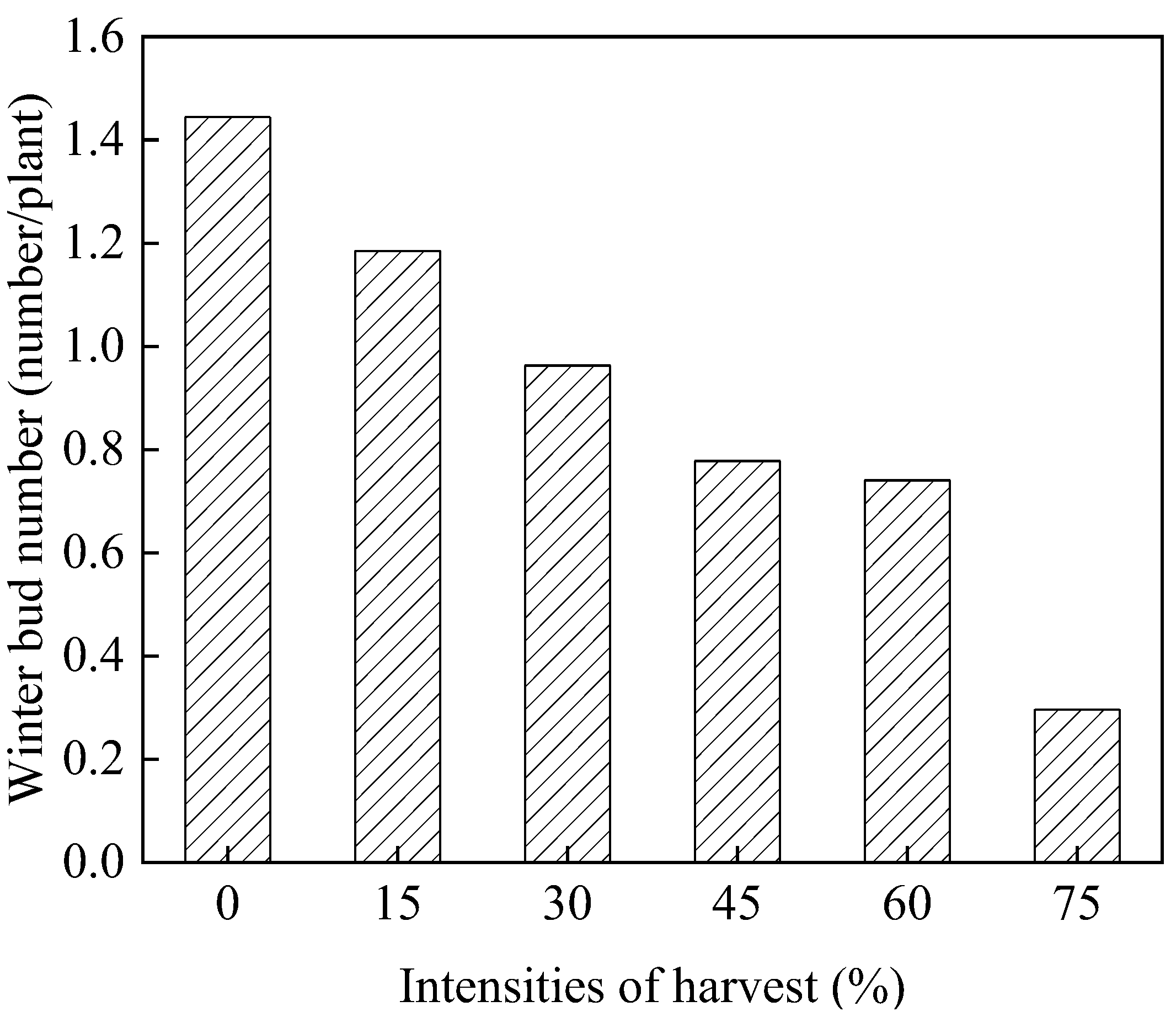
3.1.3. Number of Winter Buds
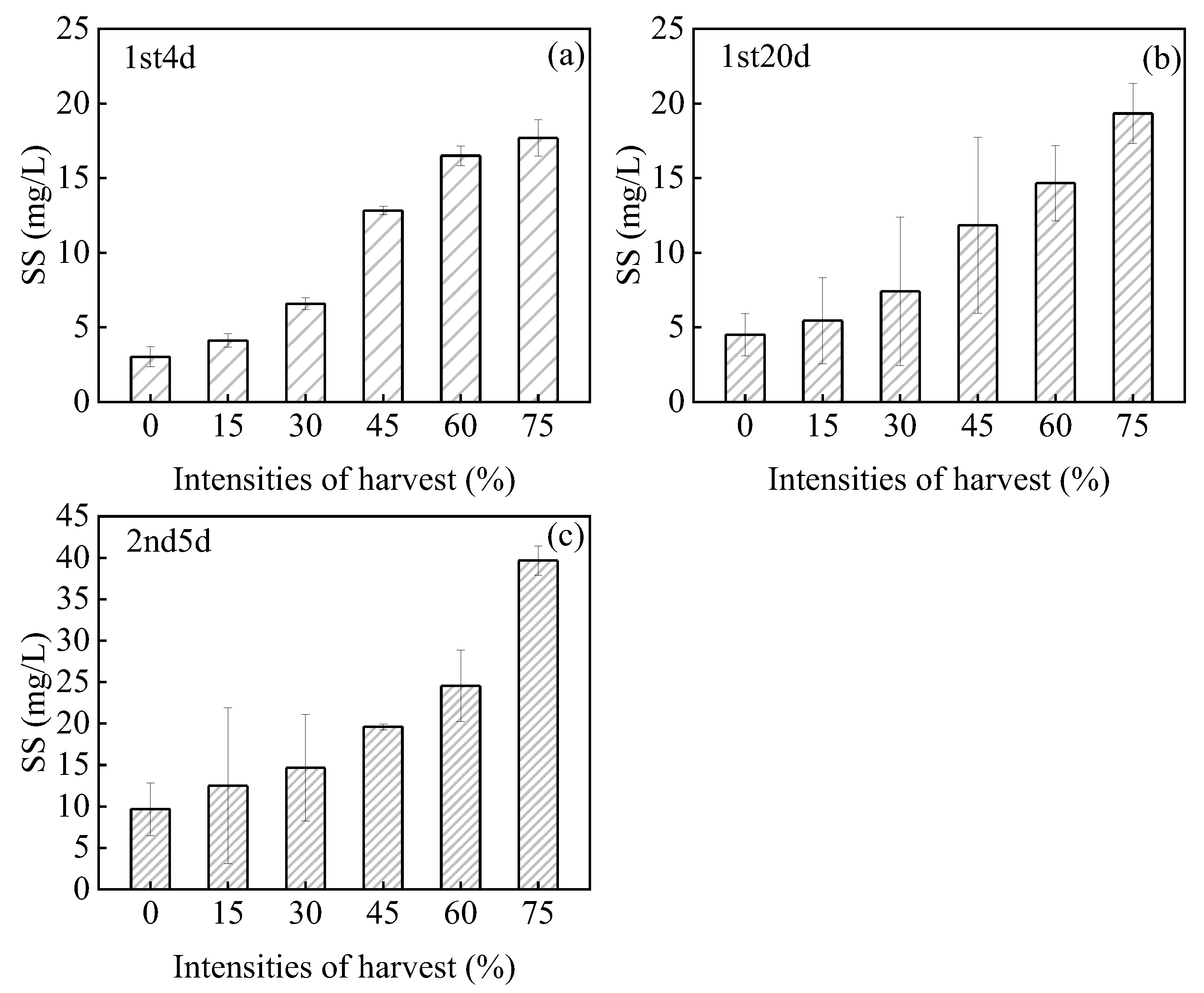
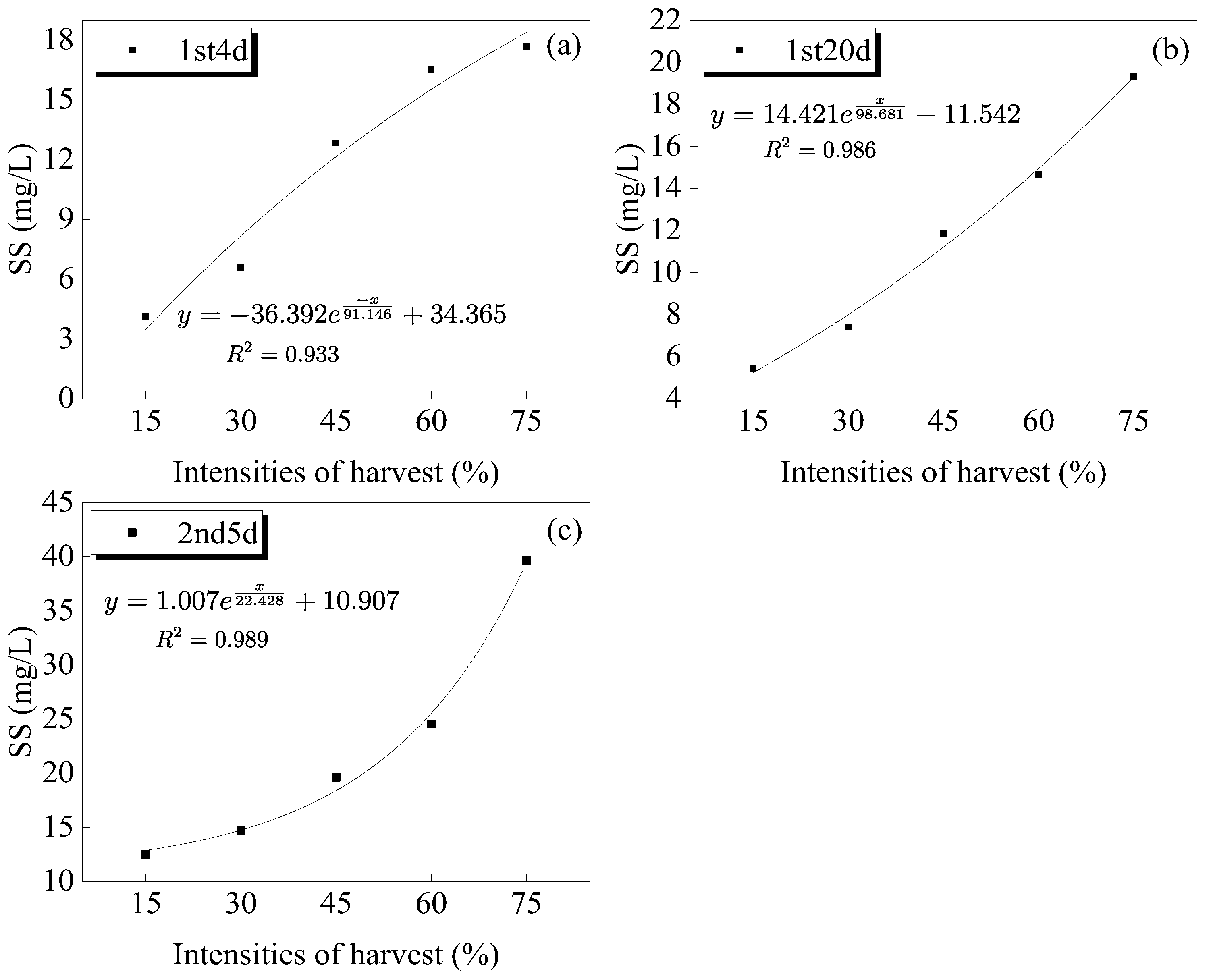
3.2. Changes in the Suspended Solids in Water
3.3. Changes in the Water Nutrients
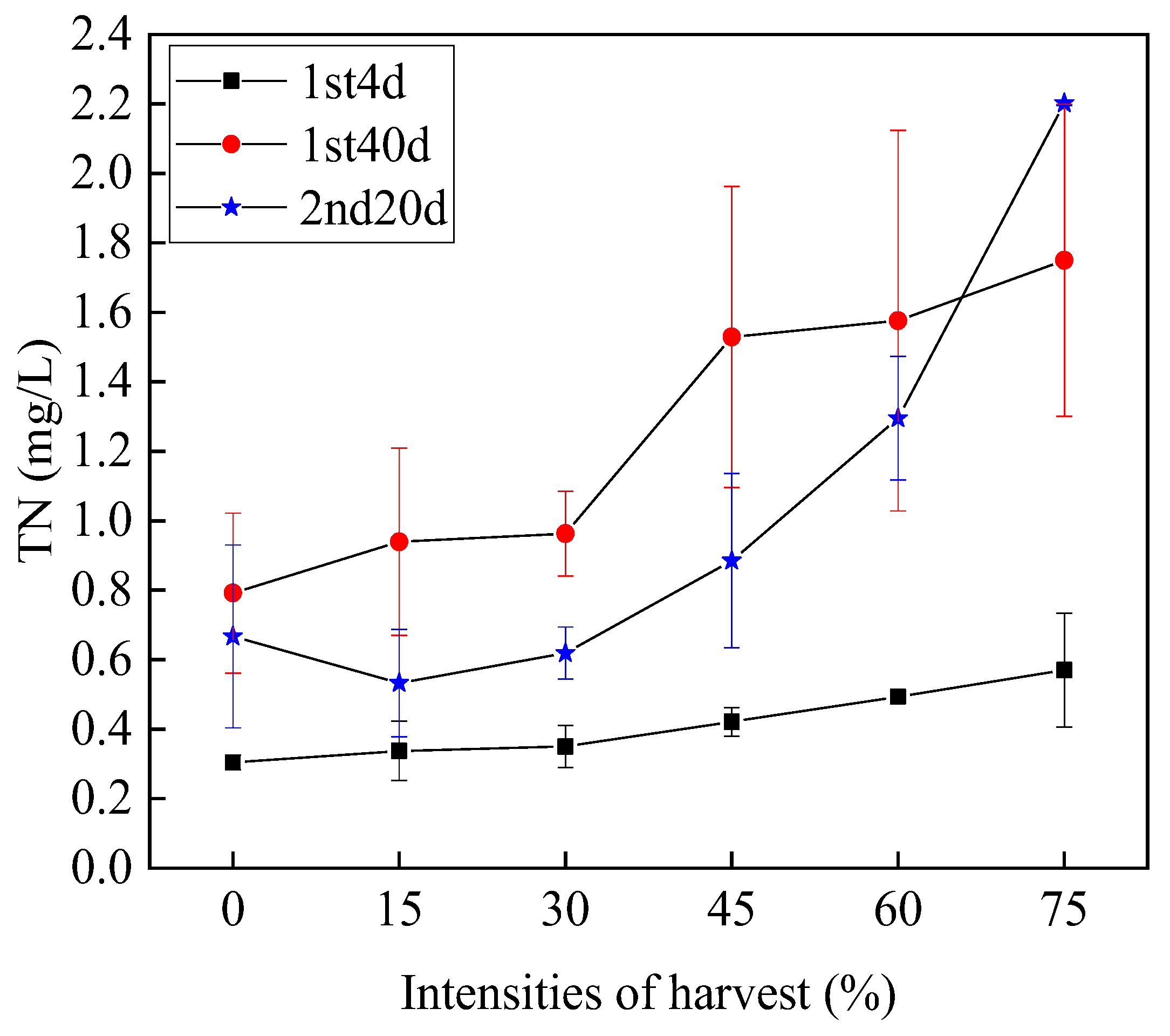
3.3.1. Total Nitrogen
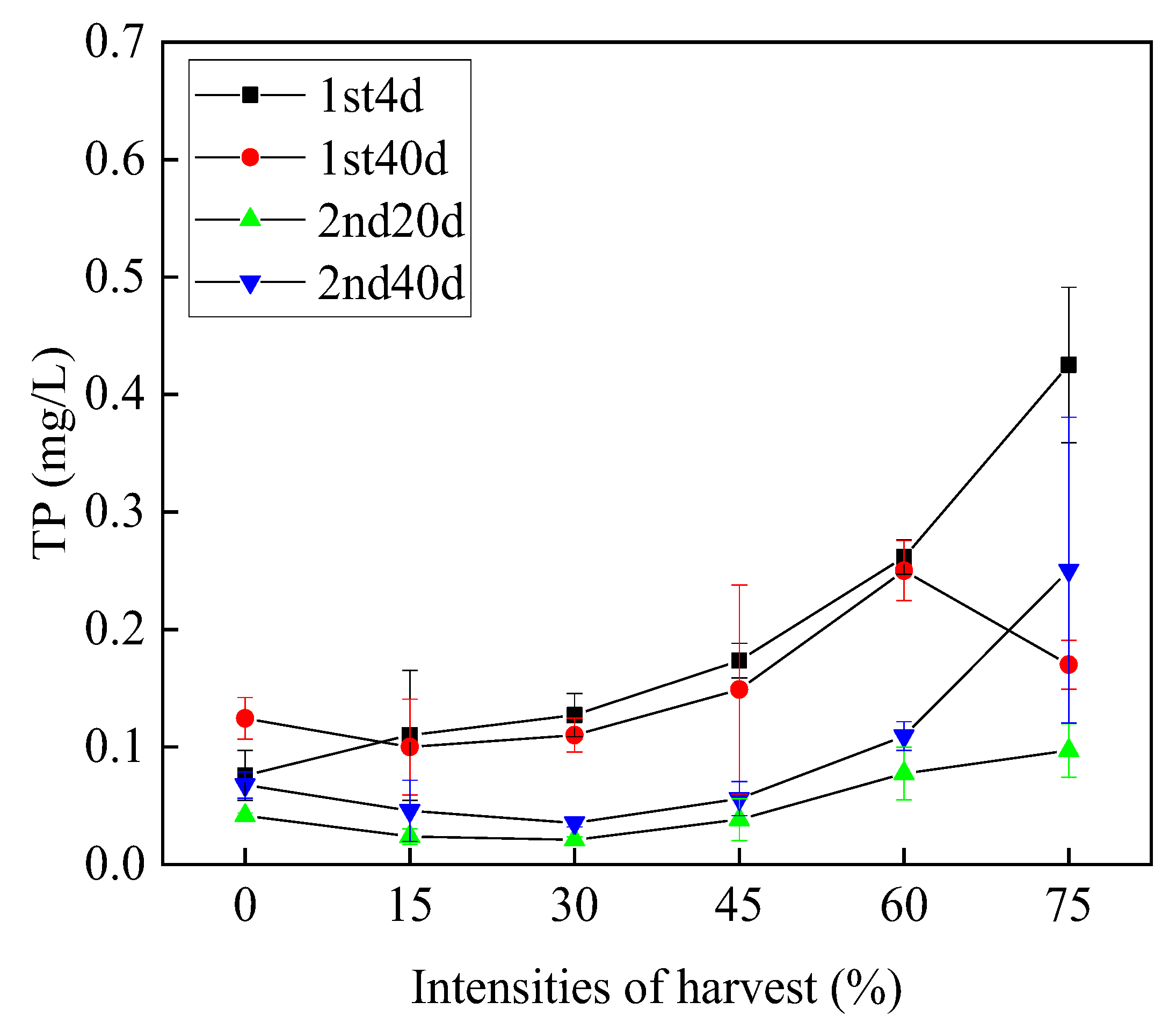
3.3.2. Total Phosphorus
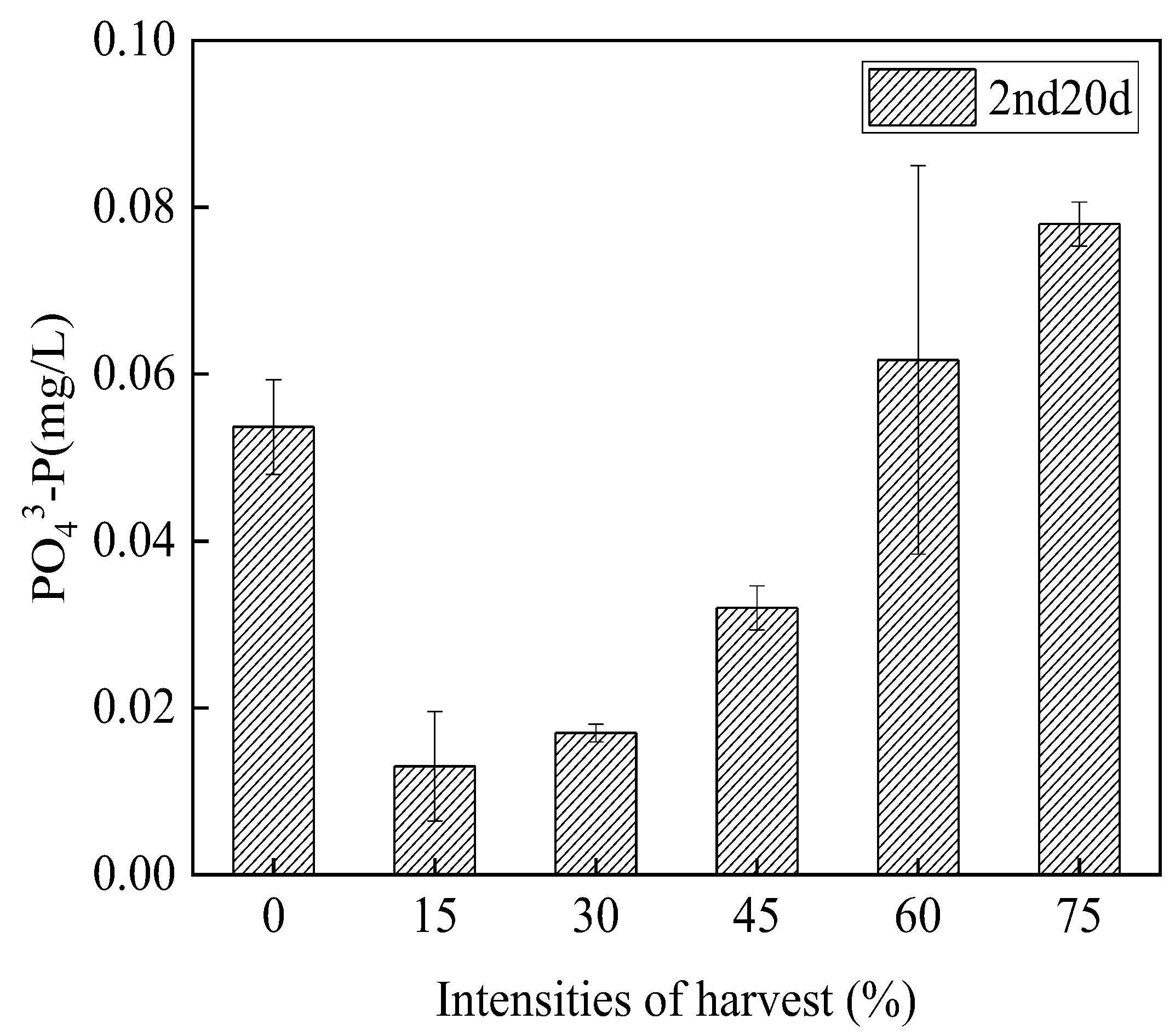
3.3.3. Orthophosphate
3.4. Changes in the Water Organic Matter
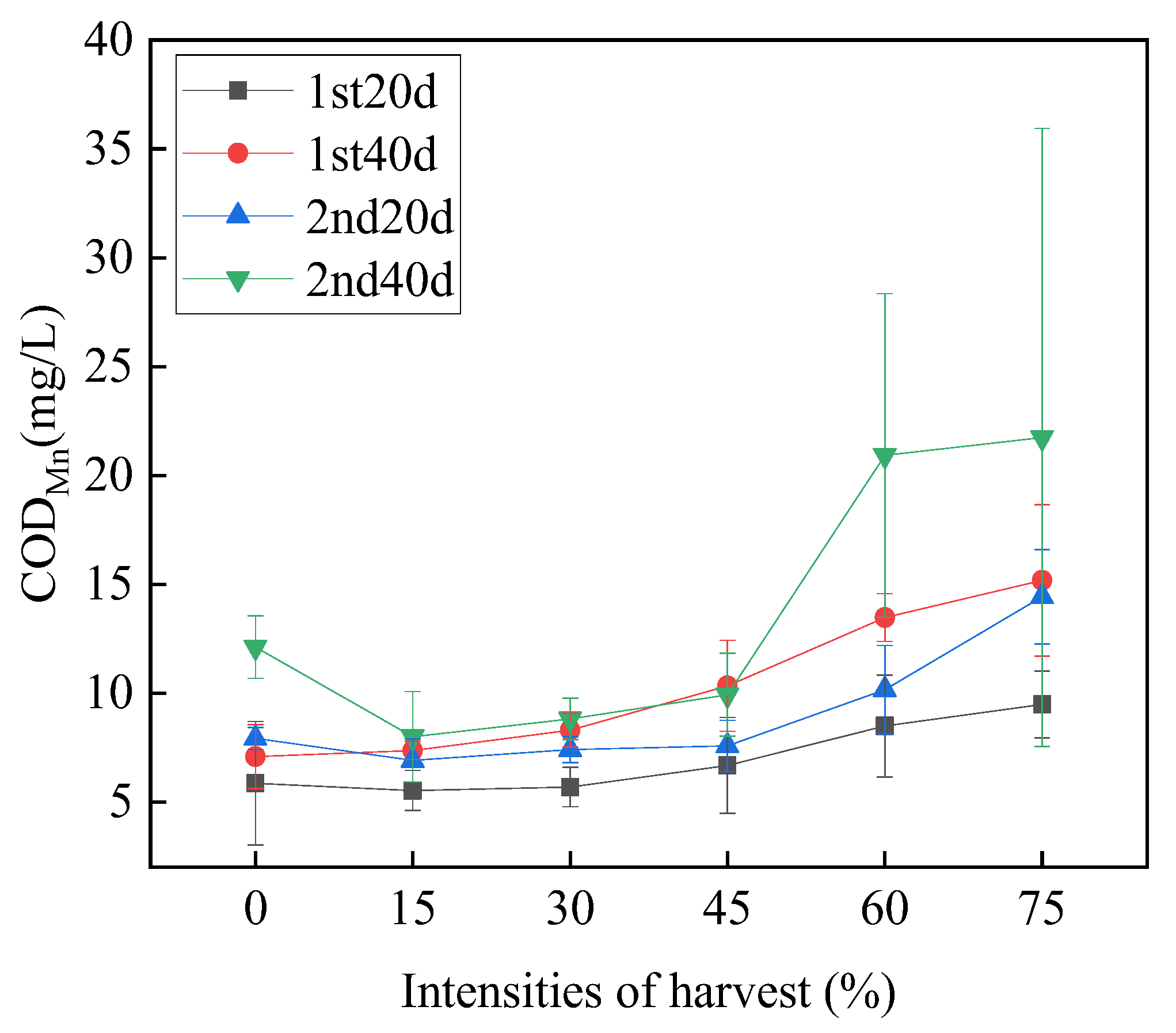
3.4.1. Permanganate Index (CODMn)
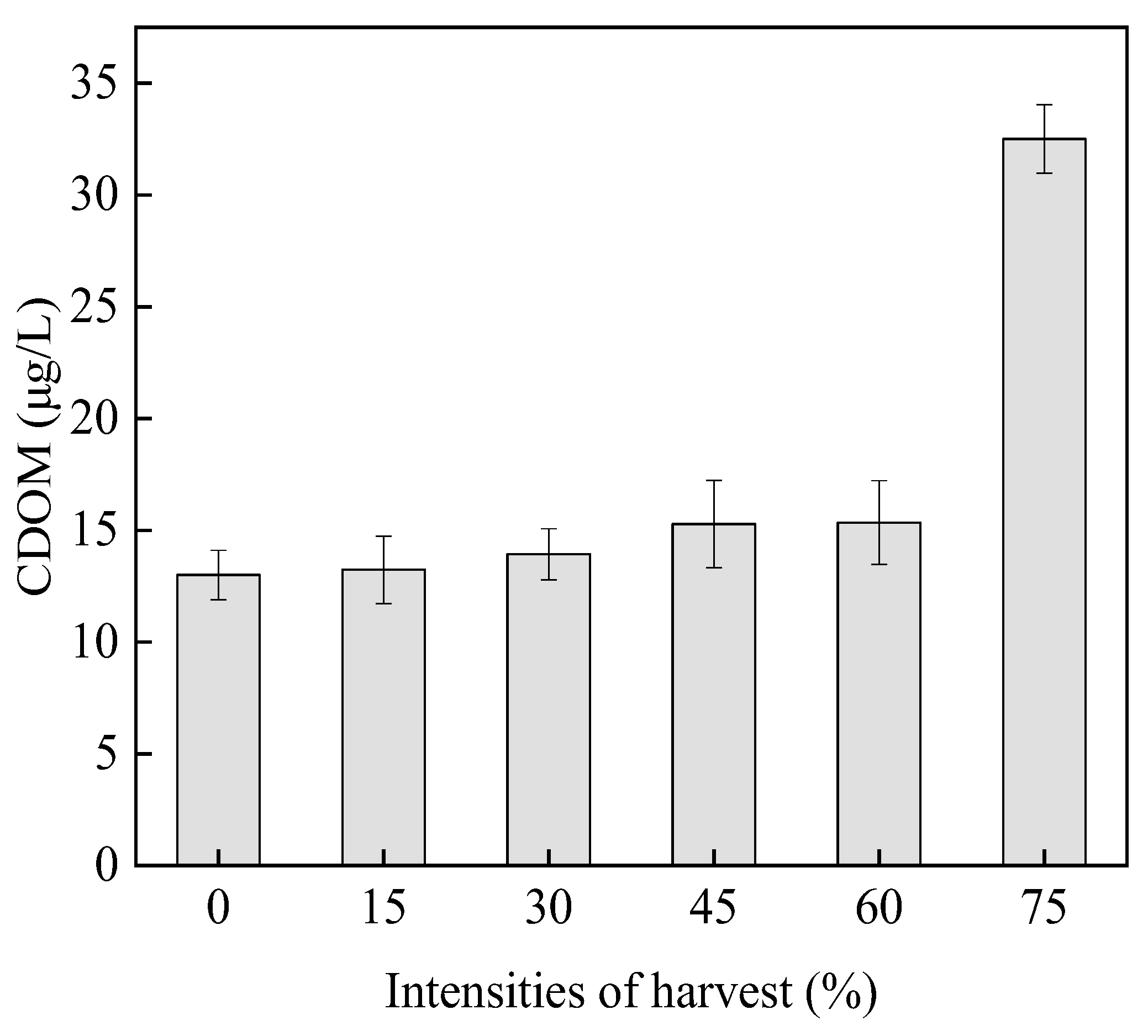
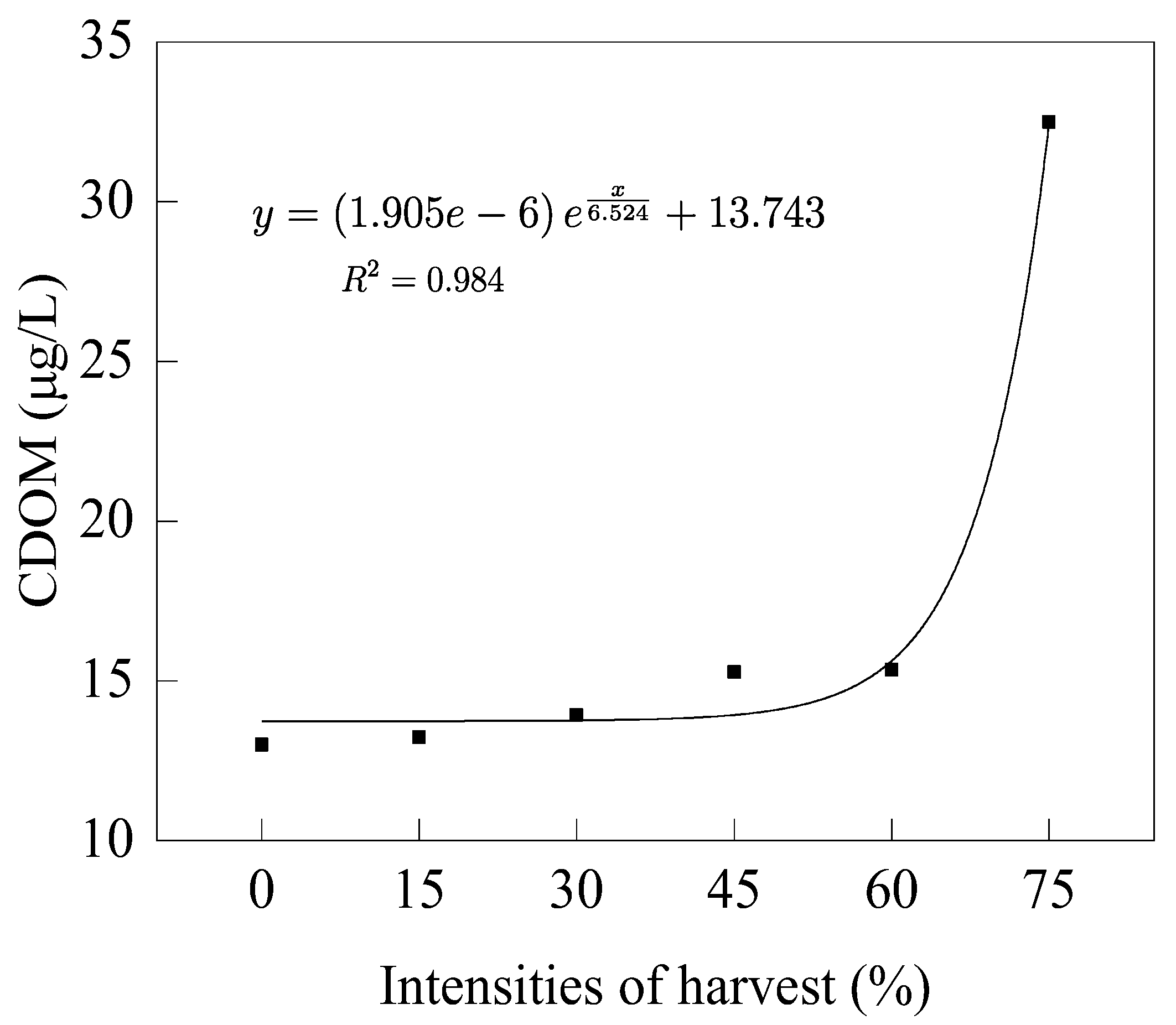
3.4.2. Colored Dissolved Organic Matter
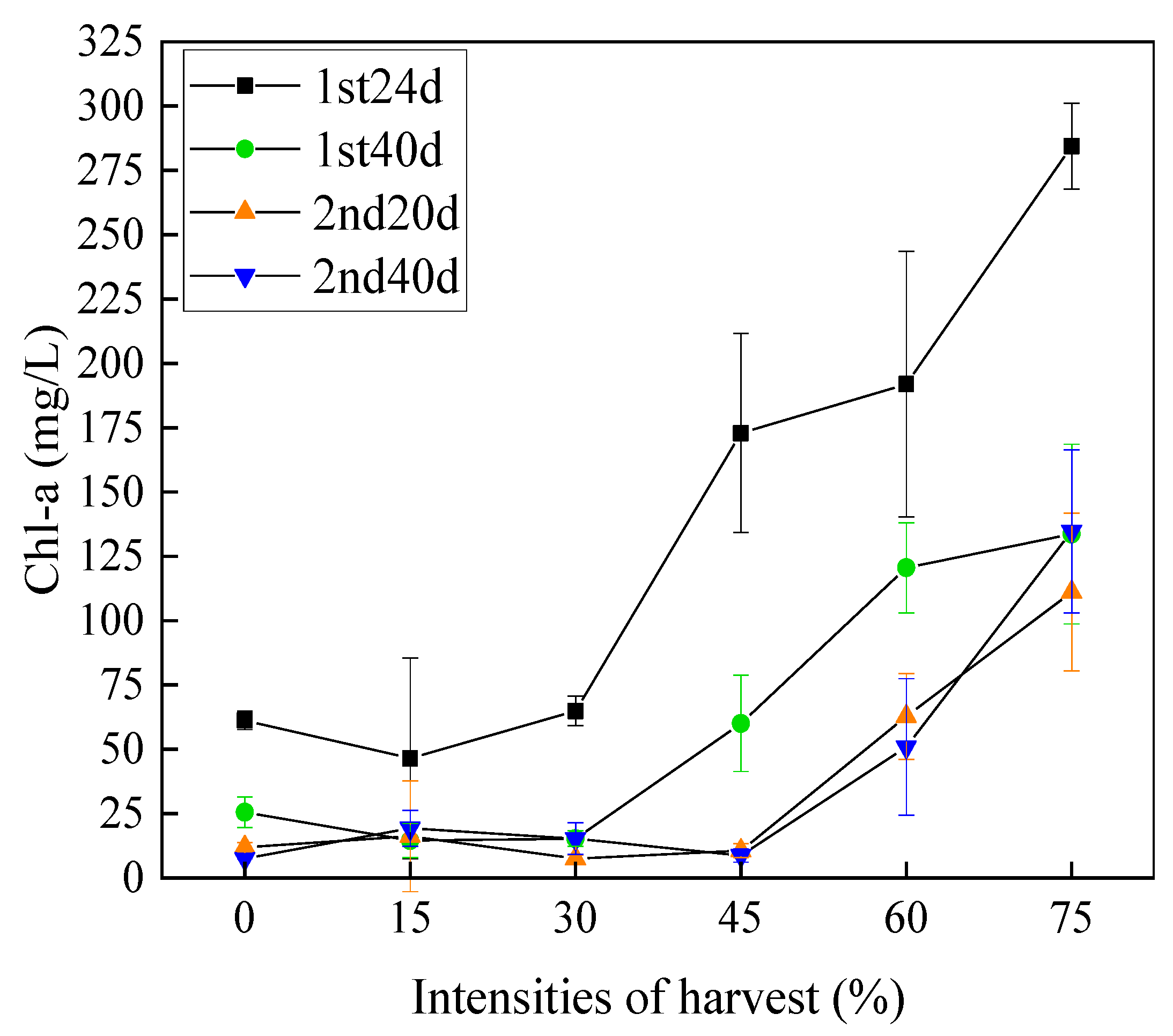
3.5. Chlorophyll a
4. Discussion
4.1. Effects of Harvesting on the Growth and Reproduction of H. verticillata
4.2. Effects of Harvesting H. verticillata on Water Quality
5. Conclusions
- (1)
- Harvesting significantly affected the growth of H. verticillata, limiting the formation of the canopy. H. verticillata could recover quickly after harvesting at medium and low intensities but the recovery rate of H. verticillata was significantly slower after two high-intensity harvests.
- (2)
- Harvesting reduced the accumulation of H. verticillata biomass. Under medium and high-intensity harvesting, H. verticillata plant height and the number of branches decreased significantly, resulting in lower final biomass. This finding indicates that medium and high-intensity harvesting could effectively restrict the accumulation of H. verticillata biomass.
- (3)
- Harvesting had a significant effect on water quality. Low-intensity H. verticillata harvesting improved the water quality, while medium and high-intensity harvesting of H. verticillata significantly deteriorated the water quality.
- (4)
- Phytoplankton increased significantly in the high-intensity harvesting group, and the CDOM concentrations varied with the increase in phytoplankton. Medium and low-intensity harvesting effectively suppressed the growth and reproduction of phytoplankton and the CDOM concentration during the peak H. verticillata growth period.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.S.; He, H.; He, H.Y.; Ma, L.S.; Su, Y.L.; Liu, Z.W. Effect of addition of lanthanum-modified bentonite (Phoslock) in sediments on growth of Hydrilla verticillata under different water nutrient concentration. J. Lake Sci. 2021, 33, 388–396, (In Chinese with English abstract). [Google Scholar]
- Wang, J.J.; Xie, T.; Que, T.Y.; Zhang, S.W.; Qian, X.Q.; Lv, S.P. Dynamics in phosphorus and bacterial community structure as influenced by Vallisneria natans in sediment of Qinhu lake. Chin. J. Ecol. 2022, 10, 413, (In Chinese with English abstract). [Google Scholar]
- Bai, G.L.; Zhang, Y.; Yan, P.; Yan, W.H.; Kong, L.W.; Wang, L.; Wang, C.; Liu, Z.S.; Liu, B.Y.; Ma, J.M.; et al. Spatial and seasonal variation of water parameters, sediment properties, and submerged macrophytes after ecological restoration in a long-term (6 year) study in Hangzhou west lake in China: Submerged macrophyte distribution influenced by environmental variables. Water Res. 2020, 186, 116379. [Google Scholar] [PubMed]
- Qiu, D.R.; Wu, Z.B.; Liu, B.Y.; Deng, J.Q.; Fu, G.P.; He, F. The restoration of aquatic macrophytes for improving water quality in a hypertrophic shallow lake in Hubei Province, China. Ecol. Eng. 2001, 18, 147–156. [Google Scholar] [CrossRef]
- Gao, Y.M.; Yin, C.Y.; Zhao, Y.; Liu, Z.W.; Liu, P.P.; Zhen, W.; Hu, Y.H.; Yu, J.L.; Wang, Z.X.; Guan, B.H. Effects of Diversity, Coverage and Biomass of Submerged Macrophytes on Nutrient Concentrations, Water Clarity and Phytoplankton Biomass in Two Restored Shallow Lakes. Water 2020, 12, 1425. [Google Scholar] [CrossRef]
- Wang, J.J.; Xie, T.; Que, T.Y.; Zhang, S.W.; Qian, X.Q.; Lv, S.P. Dynamics of phosphorus concentration and bacterial community structure in sediment of Qinhu Lake as influenced by Vallisneria natans. Chin. J. Ecol. 2022, 41, 1787–1795, (In Chinese with English abstract). [Google Scholar]
- Yu, X.H.; Wu, X.D.; Ge, X.G.; Gui, Z.F.; Zhou, M.D.; Bian, L.L.; Liu, L. Effects of harvesting intensity on the growth and water quality of Myriophyllum aquaticum. J. Hydro. 2022, 43, 95–102, (In Chinese with English abstract). [Google Scholar]
- Yang, C.T.; Nan, J.; Li, J.H.; Yi, L.; Yu, J.; Wu, J.B.; Shen, X.B. The role of mechanical harvesting on the recession of aquatic vegetation under an extreme water level increase in a eutrophic shallow lake. Environ. Sci. Pollut. Res. 2021, 28, 61682–61695. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Becares, E. The effect of vegetation harvest on the operation of a surface flow constructed wetland. Water SA 2008, 34, 645–650. [Google Scholar] [CrossRef]
- Xu, W.W.; Hu, W.P.; Deng, J.C.; Zhu, J.M.; Li, Q.Q.; Zhang, H.M. Influence of Harvesting Potamogeton crispus in A Submerged Macrophytes Community on the Growth of Submerged Aquatic Plants and Their Effects on Water Quality. J. Ecol. Environ. Sci. 2015, 24, 1222–1227. [Google Scholar]
- Pi, J.C.; Zhu, G.C.; Gong, T.T.; Lu, Y.Z. Dissolved organic matter derived from aquatic plants in constructed wetlands: Characteristics and disinfection byproducts formation. J. Environ. Chem. Eng. 2022, 10, 107991. [Google Scholar] [CrossRef]
- Huang, F.Y.; Huang, Y.B.; Jia, J.Z.; Li, Z.P.; Xu, J.F.; Ni, S.; Xiao, Y. Research and engineering application of bypass combined artificial wetlands system to improve river water quality. J. Water. Process. Eng. 2022, 48, 102905. [Google Scholar] [CrossRef]
- Paraskevi, M.; Annica, O.; Bjørg, G.H.; Torben, L.L.; Tenna, R. Investigating emergent macrophytes establishment rate and propagation towards constructed wetlands efficacy optimization. Knowl. Manag. Aquat. Ecosyst. 2021, 422, 23. [Google Scholar]
- Best, E.P.H. The Impact of Mechanical Harvesting Regimes on the Aquatic and Shore Vegetation in Water Courses of Agricultural Areas of the Netherlands. J. Plant Ecol. 1994, 112, 57–71. [Google Scholar] [CrossRef]
- King, D.L.; Burton, T.M. Efficacy of Weed Harvesting for Lake Restoration, 1st ed.; Inland Waters and Lake Restoration: Portland, OR, USA, 1980; pp. 8–12. [Google Scholar]
- Zuo, J.C. Several Ecological Problems in Manipulating Submersed Macrophytes by Harvest. Doctoral’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2006. (In Chinese with English abstract). [Google Scholar]
- Yu, H.B.; Yang, Z.J.; Xiao, R.L.; Zhang, S.N.; Liu, F.; Xiang, Z.X. Nitrogen and phosphorus uptake capacity of aquatic plants and harvesting management studies. Acta Pratacul. Sin. 2013, 22, 294–299. [Google Scholar]
- Yan, Y.T.; Gu, H.H.; Li, T.Y.; He, C.L.; Yang, Y.H.; Wang, S.; Huang, B.B. Ecological response of Potamogeton crispus management in reclaimed water river. Acta Sci. Circumstantiae 2022, 42, 59–68, (In Chinese with English abstract). [Google Scholar]
- Zuo, J.C.; He, F.; Ma, J.M.; Zhou, Q.H.; Zeng, L.; Kong, L.W.; Hu, S.H.; Wu, Z.B. Effects of moderate-intensity harvesting on interspecific competition between Potamogeton crispus and Elodea nuttallii. Chin. J. Ecol. 2014, 33, 2414–2419, (In Chinese with English abstract). [Google Scholar]
- Zuo, J.C.; Miao, F.P.; Wang, A.Y.; Zhao, A.F.; Wang, Z.L.; Wu, Z.B. Effects of apex cutting on regrowth of Myriophyllum spicatum cultured in buckets. Chin. J. Ecol. 2009, 28, 643–647, (In Chinese with English abstract). [Google Scholar]
- Kohzu, A.; Shimotori, K.; Imai, A. Effects of macrophyte harvesting on the water quality and bottom environment of Lake Biwa, Japan. Limnology 2019, 20, 83–92. [Google Scholar] [CrossRef]
- Li, G.X.; Li, Q.Z.; Xue, P.Y.; Yan, C.Z.; Gao, Y.J. Extended Langmuir Models for Cd2+ and Cu2+ Biosorption by Hydrilla verticillata. J. Agro-Environ. Sci. 2010, 29, 145–151, (In Chinese with English abstract). [Google Scholar]
- Yan, C.Z.; Zeng, A.Y.; Jin, X.C.; Zhao, J.Z.; Xu, Q.J.; Wang, X.M. Physiological effects of ammonia nitrogen concentrations on Hydrilla verticillata. Acta Ecol. Sin 2007, 27, 1050–1055, (In Chinese with English abstract). [Google Scholar]
- Bind, A.; Kushwaha, A.; Devi, G.; Goswami, S.; Sen, B.; Prakash, V. Biosorption valorization of floating and submerged macrophytes for heavy-metal removal in a multi-component system. Appl. Water Sci. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Editional Board of Water and Wastewater Monitoring and Analysis Methods; Ministry of Environmental Protection of the People’s Republic of China (Eds.) Analytical Methods for Water and Wastewater Monitoring, 4th ed.; China Environmental Science Press: Beijing, China, 2002; pp. 107–284. (In Chinese) [Google Scholar]
- Zuo, J.C.; Liang, W.; Xu, D.; He, F.; Zhou, Q.H.; Wu, Z.B. The Vegetative Recovery of Hydrilla verticillata Under Several Harvesting Strategies. J. Agro-Environ. Sci. 2011, 30, 1391–1397, (In Chinese with English abstract). [Google Scholar]
- Fulkerson, W.J.; Donaghy, D.J. Plant-soluble carbohydrate reserves and senescence—Key criteria for developing an effective grazing management system for ryegrass-based pastures: A review. Aust. J. Exp. Agric. 2001, 41, 261–275. [Google Scholar] [CrossRef]
- Wang, H.; Pang, Y.; Liu, S.B.; Ma, X. Research progress on influencing of environmental factors on the growth of submersed macrophytes. Acta Ecol. Sin. 2008, 28, 3958–3968, (In Chinese with English abstract). [Google Scholar]
- Li, Q. Influence Mechanism of Environment Factors on the Growth and Development of Submerged Macrophytes. Doctoral’s Thesis, Nanjing Normal University, Nanjing, China, 2007. (In Chinese with English abstract). [Google Scholar]
- Chen, W.Y.; Yuan, S.X.; Chen, Z.H. Influence of temperature on the growth of Hydrilla verticillata in the wastewater treatment. Meteorol. Environ. Res. 2011, 39, 4642–4644. [Google Scholar]
- Wu, X.D. The Effects of Water Levels and Harvesting on the Growth of Submerged Plants. Doctoral’s Thesis, Nanjing Normal University, Nanjing, China, 2012. (In Chinese with English abstract). [Google Scholar]
- Irhayyim, T.; Fehér, M.; Lelesz, J.; Bercsényi, M.; Bársony, P. Nutrient Removal Efficiency and Growth of Watercress (Nasturtium officinale) under Different Harvesting Regimes in Integrated Recirculating Aquaponic Systems for Rearing Common Carp (Cyprinus carpio L.). Water 2020, 12, 1419. [Google Scholar] [CrossRef]
- Van, T.K.; Wheeler, G.S.; Center, T.D. Competition between Hydrilla verticillata and Vallisneria americana as influenced by soil fertility. Aquat. Bot. 1999, 62, 225–233. [Google Scholar] [CrossRef]
- Denny, P. Sites of Nutrient Absorption in Aquatic Macrophytes. J. Ecol. 1972, 60, 819–829. [Google Scholar] [CrossRef]
- Li, K.Y.; Liu, Z.W.; Gu, B.H. Compensatory growth of a submerged macrophyte (Vallisneria spiralis) in response to partial leaf removal: Effects of sediment nutrient levels. Aquat. Ecol. 2010, 44, 701–707. [Google Scholar] [CrossRef]
- Gopal, B.; Goel, U. Competition and Allelopathy in Aquatic Plant Communities. Bot. Rev. 1993, 59, 155–210. [Google Scholar] [CrossRef]
- Wei, X.F. The influence of Megahbrama amblycephalato the competitive landscape of Vallisneria natans and Hydrilla verticillata. Doctoral’s Thesis, Huazhong Agricultural University, Wuhan, China, 2015. (In Chinese with English abstract). [Google Scholar]
- Barko, J.W.; Michael, S.R.; McFarland, D.G.; Chen, R.L. Interrelationships between the growth of Hydrilla verticillata (L.f.) Royle and sediment nutrient availability. Aquat. Bot. 1988, 32, 205–216. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Liu, W.; Zhao, J.; Liu, Y.L. Purification and Influence of Submerged Macrophytes on Water Quality of Landscape Water Body Supplied by Reclaimed Water. J. Soil Water Conserv. 2012, 26, 214–219, (In Chinese with English abstract). [Google Scholar]
- Grace, J.B. The adaptive significance of clonal reproduction in angiosperms: An aquatic perspective. Aquat. Bot. 1993, 44, 159–180. [Google Scholar] [CrossRef]
- Cheng, N.N.; Zhu, W.; Zhang, J. Propagation and transplanting techniques for submerged macrophytes in heavily polluted water bodies. Water Resour. Prot. 2004, 20, 8–11. [Google Scholar]
- Yu, S.Q. Growth and reproductive strategies of Vallisneria natans and Hydrilla verticillata under eutrophic water. Doctoral’s Thesis, Wuhan University, Wuhan, China, 2019. (In Chinese with English abstract). [Google Scholar]
- Ge, X.G.; Wang, G.X.; Lu, Y.C. A study on the regenerative capacity of four submerged plant species with broken branches. J. Hydro. 2009, 30, 23–28. [Google Scholar]
- Cui, X.H.; Xiong, B.H.; Pu, Y.H.; Li, W.; Chen, J.K.; He, G.Q. Comparative study of regeneration and colonization ability in five submersed macrophytes. Acta Phytoecol. Sin. 2000, 24, 502–505. [Google Scholar]
- Ding, L.; Li, L.J.; Li, J.F.; Li, W.; Chen, D.Z.; Chen, X.Q. Experimental studies on purification of nitrogen, phosphorus and suspended solids in raw water from an artificial source lake by submerged macrophytes. J. Ecol. Environ. Sci. 2018, 27, 122–129. [Google Scholar]
- Li, J.H.; Yang, X.Y.; Wang, Z.F.; Shan, Y.; Zheng, Z. Comparison of four aquatic plant treatment systems for nutrient removal from eutrophied water. Bioresour. Technol. 2015, 179, 1–7. [Google Scholar] [CrossRef]
- Wang, S.R. The distribution pattern and ecological restoration technology of aquatic plants in a eutrophic water landscape belt. Water Supply 2022, 22, 860–873. [Google Scholar] [CrossRef]
- Fu, G.X.; Cao, W.Z.; Tao, J. Purification Effect of 12 Aquatic Plants on Eutrophic Water. Environ. Sci. Technol. 2021, 44, 308–315, (In Chinese with English abstract). [Google Scholar]
- Zhao, M.Y.; Xiong, J.Q.; Zheng, Y.C.; Ren, S.H.; Wang, X.C. Long-term effects of plant harvesting on pollutant removal in artificial wetlands. Technol. Water Treat. 2019, 45, 112–116. [Google Scholar]
- Zheng, Y.; Dzakpasu, M.; Wang, X.; Zhang, L.; Ngo, H.H.; Guo, W.; Zhao, Y. Molecular characterization of long-term impacts of macrophytes harvest management in constructed wetlands. Bioresour. Technol. 2018, 268, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.J. Study on Water Improvement Effect on Polluted Water by Submerged Macrophytes. Master’s Thesis, Hohai University, Nanjing, China, 2005. (In Chinese with English abstract). [Google Scholar]
- Yao, J.; Yang, F.; Zhang, Y.M.; Zhu, Y.M.; Gao, Y.X.; Yin, J.; Du, C.; Ba, C.C.; Li, D.L. Research on the dissolved organic matter of Hydrilla verticillata’s leaf and stem decomposition. China Environ. Sci. 2017, 37, 4294–4303, (In Chinese with English abstract). [Google Scholar]
- Yu, Z.F.; Xu, D.P.; Wang, G.X.; Zhou, Y.F.; Wu, X.D.; Zhang, M.Y. Effects of decline phase of Vallisneria natans on phosphorus transportation between water and sediment. J. Lake Sci. 2016, 28, 94–104, (In Chinese with English abstract). [Google Scholar]
- Nakamura, K.; Kayaba, Y.; Nishihiro, J.; Takamura, N. Effects of submerged macrophytes on water quality and biota in large-scale experimental ponds. Landsc. Ecol. Eng. 2008, 4, 1–9. [Google Scholar] [CrossRef]
- Verhofstad, M.J.J.M.; Poelen, M.D.M.; Van Kempen, M.M.L.; Bakker, E.S.; Smolders, A.J.P. Finding the harvesting frequency to maximize nutrient removal in a constructed wetland dominated by submerged aquatic plants. Ecol. Eng. 2017, 106, 423–430. [Google Scholar] [CrossRef]
- Xiao, X.; Lou, L.P.; Li, H.; Chen, Y.X. Algal control ability of allelopathically active submerged macrophytes: A review. Chin. J. Appl. Ecol. 2009, 20, 705–712, (In Chinese with English abstract). [Google Scholar]
- Yao, Y.; He, F.; Hu, S.H.; Kong, L.W.; Liu, B.Y.; Zeng, L.; Zhang, L.P.; Wu, Z.B. Effects of allelopathy of submerged macrophytes on the phytoplankton community collected from the west part of the West Lake wetland in Hangzhou, China. Acta Ecol. Sin. 2016, 36, 971–978, (In Chinese with English abstract). [Google Scholar]
- Lv, J. Study on the Influential Factors of phytoplankton in Shallow Lakes and Restoration Assessment for Aquatic Ecosystem. Doctoral’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2012. (In Chinese with English abstract). [Google Scholar]
- Deng, P. Studies on Allelopathic Effects of Three Submerged Macrophytes on Phytoplankton. Doctoral’s Thesis, University of Chinese Academy of Sciences, Beijing, China, 2007. (In Chinese with English abstract). [Google Scholar]



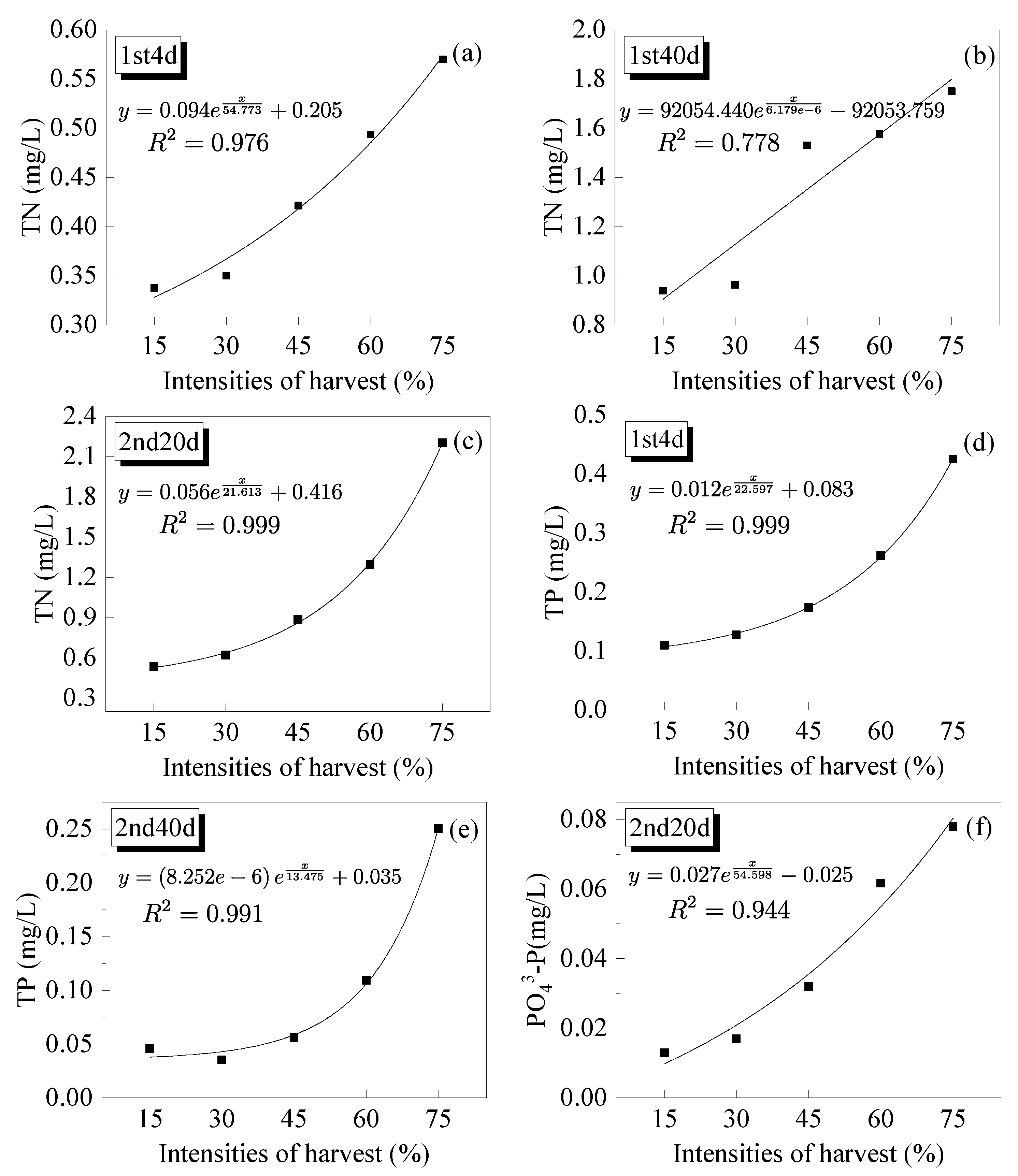

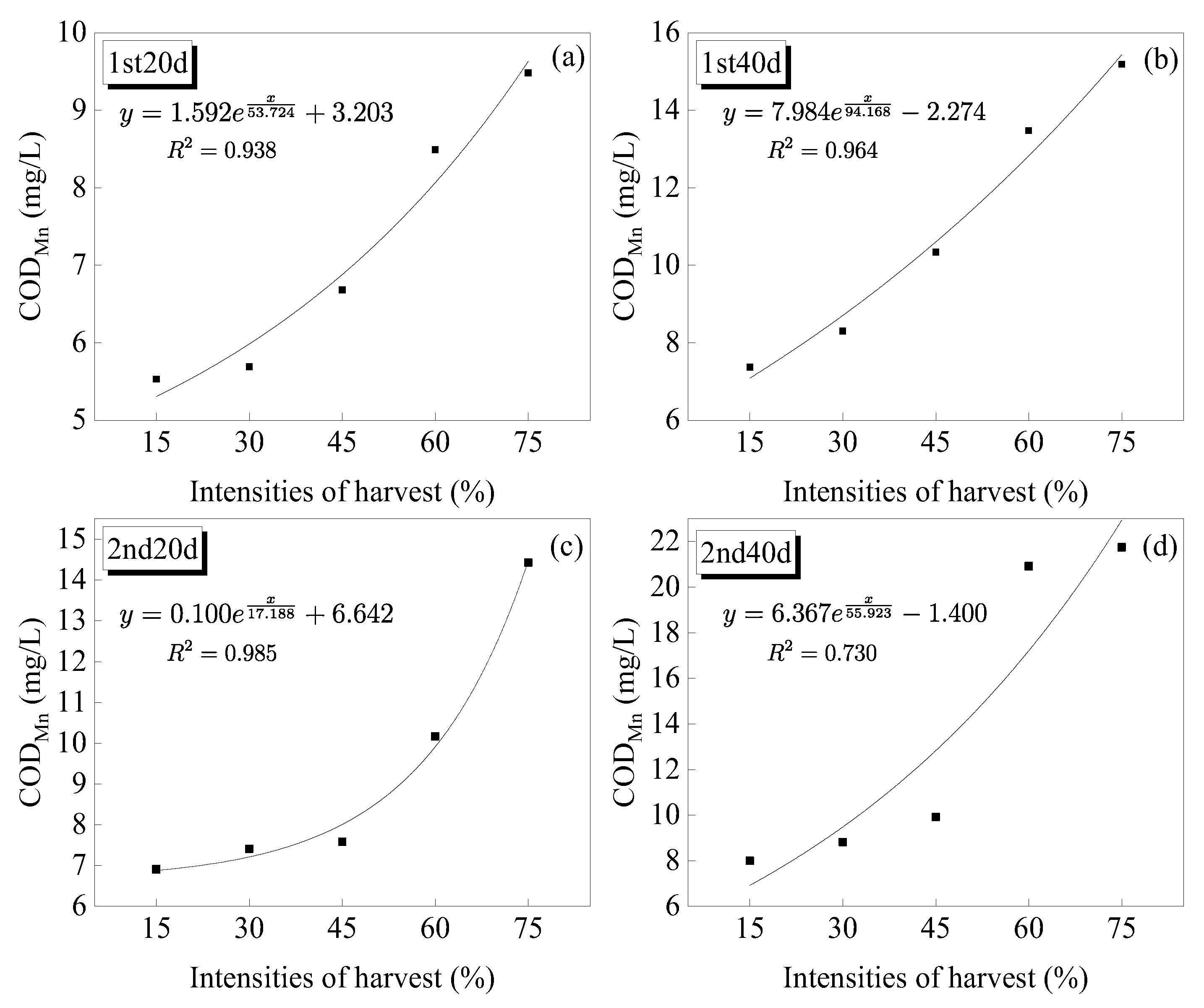
| Measurement Indicators | Measurement Methods |
|---|---|
| TN | Alkaline potassium persulfate oxidation-UV spectro photo metric method |
| TP | Potassium persulfate digestion method |
| PO43-P | Ammonium molybdate spectrophotometry |
| CODMn | Acidic method |
| Chl-a | 90% acetone extraction method |
| CDOM | Spectral coefficient absorption method |
| SS | Gravimetric method |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Wu, X.; Zhou, M.; Ge, X.; Yang, X.; Wang, N.; Lin, X.; Li, Z. Effects of Harvesting Intensity on the Growth of Hydrilla verticillata and Water Quality. Sustainability 2022, 14, 15390. https://doi.org/10.3390/su142215390
Zhu S, Wu X, Zhou M, Ge X, Yang X, Wang N, Lin X, Li Z. Effects of Harvesting Intensity on the Growth of Hydrilla verticillata and Water Quality. Sustainability. 2022; 14(22):15390. https://doi.org/10.3390/su142215390
Chicago/Turabian StyleZhu, Shunmei, Xiaodong Wu, Mengdie Zhou, Xuguang Ge, Xingqiang Yang, Nuoxi Wang, Xiaowen Lin, and Zhenguo Li. 2022. "Effects of Harvesting Intensity on the Growth of Hydrilla verticillata and Water Quality" Sustainability 14, no. 22: 15390. https://doi.org/10.3390/su142215390
APA StyleZhu, S., Wu, X., Zhou, M., Ge, X., Yang, X., Wang, N., Lin, X., & Li, Z. (2022). Effects of Harvesting Intensity on the Growth of Hydrilla verticillata and Water Quality. Sustainability, 14(22), 15390. https://doi.org/10.3390/su142215390







