Abstract
Sewage sludge processing and disposal have a significant weight on the energy and economic balances of wastewater treatment operations and contribute substantially to greenhouse gas emissions related to wastewater processing. Despite this, sewage sludge contains substantial recoverable resources in the form of energy and useful molecules. The current challenge, other than reducing the environmental and economic impacts of its disposal, is to recover energy and materials from this waste stream, implementing a biosolid-centered circular economy with the greatest possible added value. A number of options along these lines exist, and others are being investigated, ranging from biological processes, thermochemical technologies, bioelectrochemical processing, biorefineries and others. Recoverable resources comprise biogas from sludge fermentation, liquid and solid end products (e.g., biodiesel and biochar) and valuable nutrients (N and P). This paper presents a state of the art of biorefinery, with emphasis on recent developments in non-conventional resource recovery from EBSS streams for sludge-based circular economy implementation. Expectations and limitations, including technological readiness, of these technologies are discussed.
1. Introduction
Wastewater treatment generates large quantities of excess biological sewage sludge (EBSS) worldwide, estimated at >13 × 106 t/y (dry solids, DS) both in Europe [1] and in the U.S.A. [2], and in much greater amounts (>30 × 106 t/y) in China [3]. Main wastewater constituents such as organics and nutrients end up concentrated in biomass, which therefore retains an energy content comparable to that of lignite or other renewable fuels from biomass materials of approximately 11–22 MJ/kg [4], in addition to stoichiometric amounts of P and N of biological cells. In addition to these potentially exploitable resources, EBSS also embeds various types of pollutants, such as inorganic compounds (silicates, aluminates, calcium and magnesium compounds), heavy metals (Zn, Pb, Cu, Cr, Ni, Cd, Hg and As), dioxins, pesticides, nanomaterials, microplastics and a long list of other “emerging” pollutants including pharmaceutical products, hormones, endocrine disrupters, per- and poly-fluoroalkyl substances (PFASs), linear alkylbenzene sulfonate (LAS), alkylphenols (APs), phthalates (PAEs), polycyclic aromatic hydrocarbons (PAHs) and polychlorobiphenyls (PCBs), varying in concentration from less than 1 mg/kg to several g/kg [5,6,7].
Sludge processing and disposal have a significant impact on the energy and economic balances of wastewater treatment operations: it has been estimated that, despite its energy value, biosolid treatment and disposal account for over 50% of the total operational costs of conventional wastewater treatment plants (WWTPs) and may contribute to almost 40% of greenhouse gas emissions related to wastewater processing [8]. Hence, the challenge, other than reducing the environmental and economic impacts of its disposal, is to recover energy and materials from this waste stream.
A number of options along these lines have been investigated, ranging from biological processes, thermochemical technologies, bioelectrochemical processing, biorefinery and others. Recoverable resources include biogas from sludge fermentation [9], liquid and solid end products (e.g., biodiesel and biochar) [10] and valuable nutrients [11]. This paper presents a state of the art of biorefinery with emphasis on recent developments in resource recovery from EBSS streams for sludge-based circular economy implementation.
2. EBSS Composition
EBSS mass consists, for the major fraction, of non-toxic organic carbon compounds (approximately 60% on a dry basis), followed by TKN and P components. Dry matter in sludge usually varies between 7 and 12 g/L, and volatile solids between 65 and 77 g/L. Most of the organic fraction is largely composed of extracellular polymeric substances (EPSs), complex, high molecular weights, negatively charged polymers originating from the secretion and lysis of microbial sludge cells consisting of proteins, nucleic acids, humic substances, polysaccharides and lipids. Carbohydrates, proteins and humic substances form about 20% of the total EPS mass [12]. Specific sludge chemical composition and energy content vary with sludge stream, type and wastewater composition. Differences exist between primary sludge, secondary or mixed conventional activated sludge (CAS), MBR, EBPR sludge and granular sludge (GS) for similar feed streams: for example, GS usually has a higher EPS content than CAS, and primary sludge has a higher free fatty acid content than secondary. An EPS is characterized by high concentrations of charged ions, forming complex structures with biological cells that are capable of interlocking water within flocs, generating an osmotic gradient that affects water retention and attainable dry matter content, and determining the sludge’s rheologic behavior [13]. An EPS affects the formation of GS, both aerobic and anaerobic, where it is found in much higher amounts than in any other biomass aggregation [14], reaching contents that can vary between approximately 30 and 600 mg/g, depending on the specific GS type (aerobic, anaerobic or anammox) [15]. Due to the nature of their constituents, EPSs have become one of the most attractive recoverable sludge components, with high prospects to extract valuable raw materials through biorefinery.
3. Biorefinery
Biorefinery is a series of processes that, in various combinations, can produce a broad spectrum of value-added products, such as biofuels or biochemicals, from a single or a combination of biomass feedstocks (plant-based or organic waste-based), replacing non-renewable energy and chemicals and playing a key role in ensuring the closure of the biomass production and consumption cycle. Since biomass is renewable, biorefinery has also been identified as a potential solution to mitigate greenhouse gas (GHG) emissions.
The biorefinery concept includes a wide range of technologies and processes to separate biomass feedstocks into building blocks (carbohydrates, proteins, etc.) and convert them to different products, similar to oil refineries producing different fuels and raw chemicals from crude oil. The term “feedstock” indicates any biomass suitable for biorefinery use: it can be specifically grown for this purpose, as in the case of crops or microalgae converting atmospheric CO2 and H2O into sugars, or can be the residual (waste) product of a complex series of processes (i.e., wastewater treatment or food waste processing). Lately, innovative WWTPs based on the combination of microalgal consortia and bacteria have been tested in order to exploit the efficiency of microalgae in removing wastewater pollutants [16,17].
Adopting IEA’s classification, biorefineries can be described based on feedstock, intermediates, final products and conversion processes. The main four process groups include biochemical (e.g., fermentation and enzymatic), thermochemical (e.g., gasification and pyrolysis), chemical (e.g., acid hydrolysis, synthesis and esterification) and mechanical processes (e.g., fractionation, pressing and size reduction) [18]. This is summarized in Figure 1, which describes the most promising currently existing and developing biorefinery systems [19]. This general, non-sludge-specific scheme provides an indication of current possibilities; additional platforms, product groups and processes resulting from future development activities could be added as they become available.
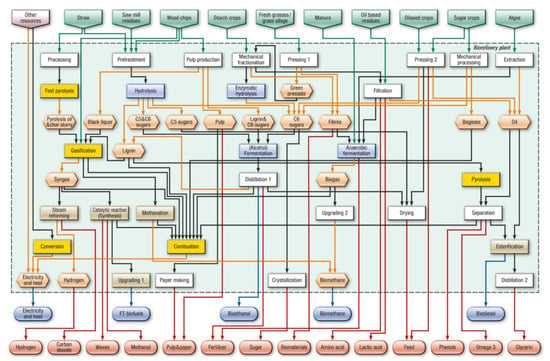
Figure 1.
Conceptual scheme describing the relationships between most promising existing and developing individual biorefinery systems (Reprinted with permission from Ref. [19]. 2019 Elsevier”).
Initially, the driving force for the development of biorefinery was the replacement of fossil fuels with biomass-derived products [20]; however, biorefinery may generate other interesting products such as food and feed products or bioproducts of industrial interest, i.e., substitutes to traditional raw materials. Biorefinery was originally applied to waste biomass from forestry, agriculture and the food industry. EBSS was not one of the originally targeted feedstocks, and therefore is not explicitly indicated in Figure 1; a detailed discussion of EBSS biorefinery is contained in the following section. Since almost all types of biomasses can be converted to biorefined products, feedstock streams may be combined according to seasonal availability and market demand for specific products. An important aspect of establishing a successful biorefinery chain is a reliable, consistent and regular supply of feedstock that can provide continuous input to the process. In this sense, sewage sludge certainly fulfills this precondition, since it is produced at an almost constant rate throughout the year by WWTP operations and can hence be used either as a mono feedstock or in combination with other, seasonably available sources.
Sewage Sludge Biorefinery
EBSS biorefinery may lead to the recovery of different resources: process integration can contribute to the recovery of multiple final products (both energy and material), as illustrated in Figure 2.
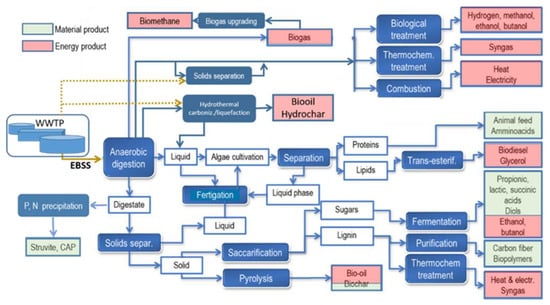
Figure 2.
Possible biorefinery full-cycle implementation from EBSS feedstock.
Some of the processes illustrated in Figure 2 have been part of traditional sludge management practices for years and have been described in detail in the existing literature. Anaerobic digestion for the production of methane-rich biogas is the earliest example of a resource recovery process combining sludge stabilization with onsite production of a valuable product [9] and, according to the definition, can be considered as part of a biorefinery scheme. Several other higher-value end products could, however, be obtained via biorefinery. Well-known thermochemical and combustion processes could also be considered part of sludge biorefinery schemes [21,22,23,24,25,26,27]. More recently, alternative approaches to sludge biorefinery have aimed at the direct recovery of specific biomass components, such as organic acids and/or alcohols, or of industrially relevant molecules, such as PHAs and VFAs, considered more industrially valuable than biogas. In this paper, the focus is directed toward innovative conversion products and processes within the EBSS biorefinery universe.
4. Resources Recovery from EBSS Biorefinery
Valuable macromolecules such as carbohydrates, proteins and lipids represent approximately 80% of the organic matter in sewage sludge, mostly in the form of EPSs, which are lost in thermochemical processes. Suitable biorefinery approaches in which the extraction and production of biomolecules have a greater priority than bioenergy production can address the recovery of these components.
4.1. Anaerobic Fermentation as the First Step in Biomolecule Recovery
Anaerobic digestion (fermentation) is considered an efficient solution for managing large amounts of organic substrates, including EBSS, reducing their volume and allowing the production of valuable methane (and/or hydrogen), contributing to the renewable energy portfolio in the EU [28]. However, this process does not necessarily need to be brought to the final biogas production stage: several biorefinery chains aimed at producing high-value end products are based on an initial anaerobic fermentation step, yielding short-chain organic acids and alcohols, such as volatile fatty acids (VFAs, mainly acetate, propionate and butyrate) and lactate, usually referred to as carboxylates. These organic acids play a significant role in many applications in the fields of medicine, agriculture, pharmaceuticals, food and other industries. With their derivatives, carboxylates are used in the production of polymers, biopolymers, coatings, adhesives and pharmaceutical drugs, and can be used as solvents, food additives, antimicrobials and flavorings [29]. Currently, carboxylates are normally produced from petroleum, but, as they derive from carbohydrate and amino acid fermentation, they can also be generated by organic matter fermentation, pending inhibition of the final conversion step of organic acids to methane and CO2 (Figure 3).
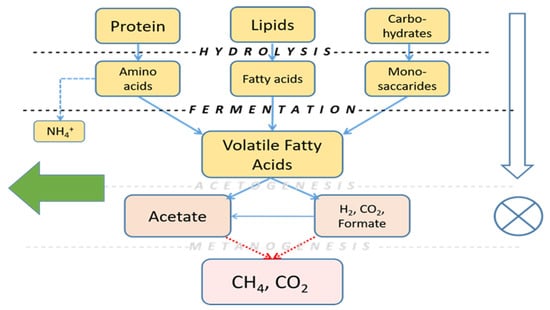
Figure 3.
Anaerobic fermentation. By suppressing the final process steps, VFAs could be recovered from the process in greater amounts.
Acetate and butyrate are possible raw materials for the production of polyhydroxybutyrate (PHB), while propionate can be used for polyhydroxyvalerate (PHV) production [30]. Due to the high O:C ratio (from 1:1 to 2:5) and low energy density, VFAs as such are unsuitable as fuels, but they can serve as a base for the production of liquid biofuels such as alcohols that are blended with gasoline [31].
Short-chain VFA production from sludge fermentation may be limited by slow hydrolysis rates and substrate availability; process control is essential for successful VFA recovery. Fermentation is best conducted under slightly acidic pH (6–7) conditions, short HRT (1–7 days) and high OLR (>10 gTS/L-d). Adequate sludge pretreatment can enhance short-chain VFA production, as summarized in Table 1. Furthermore, acid accumulation in the digester significantly reduces methanation [32].

Table 1.
Sludge pretreatment to increase fermentation VFA yield.
All the pretreatments listed in Table 1 also enhance digested sludge dewaterability, thereby possibly reducing WWTPs’ operation costs for subsequent sludge disposal. VFAs have a high economic value in several production processes (e.g., bioplastics and biotextiles) due to their characteristics [40]. The economic benefits from enhanced VFA production should be evaluated considering additional pretreatment costs and the missed economic benefits from methane recovery.
Short-chain carboxylates can be concentrated from the fermentation liquor through nanofiltration, liquid extraction or anion exchange [41], and can be further processed into medium-chain fatty acids (MCFAs), with molecular C atom content ranging from 6 to 12, which lower water solubility and provide easier extraction. Due to their properties, MCFAs are considered more valuable than short-chain VFAs: compared to the latter, MCFAs (with O:C ratios between 1:3–1:6) possess a higher energy density (3500–4800 KJ/mol) than short-chain VFAs, with possible uses as biofuel precursors and raw materials for various industries, such as fragrance, food additive, antimicrobial agent, and others [42].
Formate (COOH-) is produced from VFAs during the fermentation process (Figure 3) and can constitute a carbon source for formatotrophic organisms for the production of value-added chemicals, such as fuels, solvents, plastic monomers, pigments and even protein meal for animal and human consumption, potentially originating a formate-based circular economy [43].
The final product of anaerobic fermentation, biogas, is a valuable energy product with a high (40–75%) CH4 content, depending on feedstock. In addition to traditional uses, including biofuel [44], biogas can be converted into valuable bioproducts such as PHA, protein or ectoine [45], and polyhydroxybutyrate (PHB: a biodegradable biocompatible thermoplastic synthesized from C1 carbon sources CO2 and CH4) [46].
4.2. Materials Extraction from Raw Sludge: Resources, Uses and Techniques
4.2.1. Polyhydroxyalkanoates
Polyhydroxyalkanoates (PHAs) are biodegradable, biologically produced polymers that are internally accumulated as energy and carbon reserves by different microorganisms in variable proportions from 1 to 90% of the cell’s dry weight. More than 150 different PHA types have been identified so far. They consist of monomeric units of I-3-hydroxyalkanoic acids: each monomeric unit has a side chain R group (saturated alkyl group or unsaturated alkyl groups, substituted alkyl groups and branched alkyl groups). Common examples are poly-beta-hydroxybutyric acid and its co-polymer poly (3-hydroxybutyrate-co-hydroxyvalerate [P(3HB-co-HV)]). PHAs are classified on the basis of their structural chain length, as summarized in Table 2 [47].

Table 2.
PHA types based on molecular chain length.
PHA accumulation has been observed in the range of 0.30 to 22.7 mg polymer/g sludge, depending on the processing conditions [48]. PHA molecules exhibit properties comparable to petroleum-based plastics and are a biocompatible substitute for conventional fossil-derived polymeric materials. Physical properties of PHAs include insolubility in water, good resistance to UV rays and temperature stability, toughness and elasticity, high degree of polymerization, low surface porosity, biodegradability and thermoplasticity, making them suitable for diverse applications in various fields (Table 3).

Table 3.
Possible industrial applications of PHAs [46].
In addition, PHAs can serve as biofuel precursors (3- hydroxybutyrate methyl ester, HAME), or for the production of specific chemicals such as acrylate and propene [48,49], or as building blocks for further synthesis of various chemicals. When industrially produced, however, their cost is several times higher than that of fossil-originated plastics, since they are made from pure microbial cultures and substrates such as glucose, fructose and propionic acid. The substitution of the former with low-value waste streams such as processed feedstock could decrease their overall production cost by up to 50% [50]. EBSS and mixed cultures could also allow for the reduction in energy (no sterilization requirement) and fermentation costs (by simpler reactor construction design), efficiently integrating this technology into traditional WWTPs [51]. Despite the potential of PHAs extraction from sludge, e.g., via alkaline fermentation [52,53], currently, the process still suffers from high production costs, at about six-fold the cost of conventional plastics [54].
4.2.2. Extracellular Polymeric Substances
EPSs consist of macromolecular substances such as polysaccharides, proteins, humic and fulvic acids, lipids and nucleic acids. In contrast to intracellular PHAs, EPSs are released through cell lysis into a solution or excreted across cell membranes and trapped in infracellular spaces of microbial aggregates. Therefore, EPSs are normally discarded in waste streams (e.g., centrate or filtrate) after sludge dewatering, unless specific recovery actions are taken. EPSs account for 10–40% of biological sludge dry weight, with 75–89% of the extracellular organic carbon made up of proteins and saccharides, which can serve as carbon or energy sources under nutrient shortage [55]. EPS content is higher in granular sludge forms than in flocculent sludge: sludge from a 100,000 P.E. UASB municipal facility could yield 150 kg EPS/d [56], slightly less than granular sludge anammox units at 185 kg-EPS/d [57].
EPSs extracted from granular sludge demonstrated a gel-forming capacity with divalent cations similar to that of alginates; the hydrating properties of EPSs make them suitable for various biotechnical uses, such as food, paints, oil drilling ‘muds’, cosmetics and pharmaceutical production. EPSs’ viscous properties are exploited to increase the viscosity of certain technical materials and food preparation and may have potential uses as biosurfactants in tertiary oil production, as biological glue, and as a component of biofilm systems, with still unexplored other biotechnological potentials, including medical applications [58]. As functional groups such as carboxyl, phosphoric, sulfhydryl, phenolic and hydroxyl groups which are present in EPSs can complex with heavy metals, EPSs have been tested for metal removal from solutions. For example, an EPS from Bacillus megaterium was applied for Cu removal, while other bacterial EPSs exhibited a high affinity for cadmium [59,60]. EPSs have also shown the capacity to adsorb organic pollutants. Spath et al. [61] reported adsorption of >60% BTX, and the adsorption of phenanthrene [62], humic acids [63] and dyes [64] was also reported. These studies highlighted that, contrary to common belief, only a small fraction of these solute pollutants was adsorbed by biological sludge cells themselves: as an EPS is always negatively charged, it strongly binds electrostatically with positively charged organics. Furthermore, a soluble EPS has a higher protein fraction than a bound EPS, and since proteins have a higher binding capability than humic substances, soluble EPSs actually capture most of the pollutants [63]. Due to EPSs’ phosphate content, positive effects of an EPS coating on the self-extinguishing properties and flame-retardation of fabrics have been reported [65].
Various EPS extraction techniques from biomass have been studied, for example, bound and soluble EPS fractions can be separated through centrifugation [66]. The soluble EPS is then contained in the centrate, while the bound fraction remains with the solids. Other physical (ultrasonication or heating) [67,68], chemical (ethylenediamine tetraacetic acid or formaldehyde plus NaOH) [66,69,70,71], biological [72], blending [73] and electroporation [74,75] techniques have proven effective in extracting EPSs from most types of sludge.
EPS composition is largely affected by the extraction method, which can result in differences in terms of yield and quality (properties and functional groups) of these extracted exopolymers. However, from a literature review, it emerged that existing extraction and recovery methods have never been specifically evaluated in terms of the usefulness of extracted materials for individual practical applications. Some authors recommend techniques that minimize cell lysis, while others, on the other hand, favor methods that can damage cell integrity in order to extract more diverse, novel biopolymers [68,71]. These aspects need detailed investigation when aimed at optimizing the EPS reuse value.
4.2.3. Proteins
The high fraction of proteins (∼50% of the dry weight of cells) in sludge makes it a very likely potential source for their recovery. Proteins are essential components of animal feed for the supply of energy and nitrogen, and their recovery from EBSS offers various benefits over traditional sources. In order to extract proteins, the dissolution of the bacterial cell wall and intracellular materials is a critical aspect. Intracellular protein recovery has been achieved via sludge disintegration through alkali treatment coupled with ultra-sonication [76] or enzymatic disintegration, with acid and base hydrolyzed processes followed by isoelectric precipitation, centrifugation and freeze-drying [77]. Protein extraction yield as high as 80% was observed, with a composition comparable to commercial protein feeds. Most metals present in the sludge were removed from the recovered products during the process [78]. Microbial cell destruction results in increased sludge dewaterability, therefore, after protein removal, treated sludge will result in considerably reduced volumes, improving final disposal operations.
4.2.4. Biopesticides
Bacillus thuringiensis (Bt), the most effective biopesticide used today in agriculture, forestry and the health sector, is conventionally obtained from industrial fermentation processes. Bt forms crystals of proteinaceous δ-endotoxin insecticides that show specificity against particular species, such as organisms of the orders Lepidoptera (moths), Diptera (flies and mosquitoes) and Coleoptera (beetles), and is regarded as environmentally friendly, with little or no effect on humans, wildlife, pollinators and most beneficial insects. The growth medium for industrial Bt production, consisting of specific nutrient broth and yeast extracts, involves a significant portion (40–60%) of the total production costs. Studies have shown that waste sewage sludge is a highly nutritional and cost-effective medium for industrial Bt production [76]. It has been estimated that the use of EBSS as a growth medium could reduce the industrial cost of Bt production by about 50% [79].
4.2.5. Enzymes
Enzymes (e.g., lipases, dehydrogenase, glycosidase, peroxidase and aminopeptidases) are a class of proteins that act as catalysts in various biological processes, with a much higher efficiency compared to inorganic chemical catalysts. For this reason, enzymes can be efficiently used in the pharmaceutical, food and fine chemical industries to increase chemical reaction rates and, thus, production yield. Enzymes are obtained from living organisms: plants, animals and microorganisms. The latter are an important source of enzymes since, due to fast growth, they can produce them in large quantities directly secreted into the fermentation broth, thus simplifying downstream processing compared to those obtained by higher organisms. Commercial enzymes are typically extracted from non-toxic and non-pathogenic microbial (bacteria, fungi—including yeast—and actinomycetes) biomass by disrupting the biomass itself after industrial fermentation processes. The global market for industrial enzymes is expected to reach USD 7.0 billion by 2023, with an annual growth rate of almost 5% in the previous 5 years [80].
In conventional processes, enzymes are microbially produced on synthetic media generally made of ground soybean, fish, glucose, yeast extract, peptone and trace elements; the cost of this substrate can account for up to 40% of the enzymes’ industrial cost [12]. EBSS could replace, as inexpensive and nutrient-rich substrates, commercial medium ingredients, with a considerable improvement in the cost/benefit balance of the process. Sewage sludge is a substrate from which different enzymes have been produced and harvested. Among these are: protease, dehydrogenase, catalase, peroxidase and o-diphenol oxidase, esterase and dehydrogenase, α-amylase, glycosidases and α-glucosidase [81,82]. Enzymes can be produced by submerged or solid-state fermentation from sludge by Bacillus licheniformis [83,84]. Furthermore, hydrolytic enzymes can be effectively extracted directly from sludge without specific modifications to standard wastewater treatment processes.
Enzymes are not evenly distributed within sludge flocs: hydrolytic enzymes are not usually in the free water phase, but are mainly found outside cells (extracellular), either attached to cell walls or embedded within the EPS phase. The degree of flocs and cell disruption required to harvest extracellular enzymes depends on the strength of their bounds to the EPS (loose, LB or tight-bound, TB), or whether they are attached to cell walls. Enzymes associated with EPSs (“exo-enzymes”) can be readily extracted by harvesting the latter, as previously described. Different exo-enzymes are distributed between LB-EPS and TB-EPS fractions and, as such, vary in extractability: exposure of the solution to turbulence is normally sufficient to separate LB-EPSs from sludge flocs via shear, whereas TB-EPSs exhibit stronger hydrophobic properties and are less affected by mechanical agitation [82]. In contrast, enzymes that are closely attached to the cell surface (“ecto-enzymes”) can be extracted from sludge only through proper disrupting techniques, which include ultrasonication, alone or supported by additives [85,86,87]; CER and nonionic detergents, alone or in combination with EDTA [82,88]; formaldehyde, alone or in combination with sodium hydroxide (NaOH) [89]; and bead milling [90].
Crude enzyme extracts still contain a large amount of water, and their conservation stability and suitability for industrial application are relatively limited. The economic value and marketability of these enzymes can be enhanced by subsequent purification, concentration and immobilization techniques. Purification is achieved through a multistep process consisting of filtration, chemical sedimentation and ultrafiltration to increase the final enzyme concentration. Immobilization by chemical or physical binding to an inert carrier (e.g., zeolites, silica, alginate, polyacrylamide, hollow fibers, acrylic resins and others) via adsorption, entrapment or encapsulation is a promising approach to improve enzyme conservation and applicability by enhancing their mechanical strength and resistance to denaturation and facilitating enzyme catalyst recycling within a single process [83].
Sludge-derived concentrated and purified enzymes could readily substitute inorganic and/or commercial bioenzyme catalysts in many applications. Alkaline protease enzymes have application potential in detergents, leather processing, silver recovery, medical purposes, food processing, feed and chemical industries, as well as in waste treatment. Enzymes have a role in environmental bioremediation, having shown potential in diethylene glycol terephthalate (DTP) and polyethylene terephthalate (PET) fiber biodegradation. Microbial enzymes may also enhance WWTP operation by improving sludge solids degradation, the hydrolysis of organic matter, and the biodegradation of toxic pollutants [91].
4.2.6. Solvents and Biofuels
Protein and lipids in sewage sludge can also be processed to yield bio-oils and chemical added-value products. Thermochemical processes, such as pyrolysis [92,93], gasification [94], hydrothermal carbonization [26] and hydrothermal liquefaction [27] have been tested at laboratory and pilot scales with the production of bio-oils. However, oil derived from proteins is usually high in N and S, which precludes its generalized use as a fuel without further preprocessing. Since lipids are considered the major precursor of “good” fuel oil, the separation of this fraction prior to pyrolysis can be applied to recover better-quality fuel. Chemical solvent-based (chloroform, toluene or methanol/chloroform/water) methods can be very effective for the selective extraction of lipids from raw sewage sludge, with the production of a pyrolysis feedstock with low N and S content. Since solvents are relatively volatile, they could then be recovered for reuse with relative ease after extraction [95].
A clean mixture of fatty acid methyl esters (FAMEs), otherwise known as biodiesel, may be obtained through direct esterification/transesterification, i.e., the process of exchanging the organic ester group with an alcohol, with or without acid or basic catalysts, or enzymes (lipases). Industrially, biodiesel can, in fact, be obtained via methanol transesterification of vegetable oils or animal fats (in this case, it is considered a “first generation” biofuel) [20]. However, the competitive potential of this type of biodiesel is limited due to the high cost of the feedstock and the competition between energy and food uses, which introduces ethical issues. On the other hand, biodiesel is one of the more promising biofuels that could be obtained from waste material streams, including lipid-rich sewage sludge. The use of sludge as a widely available and non-edible source of lipids could therefore represent a viable solution to sludge management and disposal, and an alternative for profitable biodiesel production, embracing both the circular economy principles and EU policies concerning sustainable mobility. FAME yield from primary sludge is much higher than from secondary [96]. The latter is, in fact, mainly constituted of mono-, di- and triglycerides, together with free fatty acids (FFAs), phospholipids and waxes, while primary sludge is mainly constituted of soaps and FFAs [97].
Acid transesterification is quite an efficient process, which is, however, conditioned by lipid extraction efficiency. Different alternatives have been proposed to enhance this step, including sludge sonication and mechanical disintegration, with not yet definitive results [98]. Liquid–liquid lipid extraction techniques can use an organic phase (usually hexane), pure or with methanol/acetone addition, which seems to help to disrupt the membrane cells, to extract them from the suspended sludge flocs. Overall, however, liquid/liquid extraction is usually not the most efficient method, as water negatively affects esterification/transesterification reactions and efficient lipid extraction from the sludge matrix. This affects the full-scale production of biodiesel from sludge; therefore, sludge pretreatment to remove excess water prior to lipid extraction is preferred, using hexane as solvent at a hexane:dry sludge ratio of about 10:1 [99]. Biodiesel production from these lipids can then be carried out via acid catalysis [100]. The residual biomass could then be returned to anaerobic digestion for additional biogas production [101].
The limit of using some organic solvents such as hexane is that they could be released into the environment during the extraction process, producing ozone and other photochemical oxidants, or generating potentially hazardous processing residuals. Solvents residuals in treated sludge may strongly reduce (by more than ten-fold) biogas production in a subsequent anaerobic digestion step, modifying the process’ economic balance and increasing its environmental impact [96]. This can be reduced by the use of more environmentally friendly solvents: ethylbutyrate, a bio-derived VFA ethyl ester, is a green solvent that showed a high recovery efficiency (>90%) of saponifiable lipids from primary sludge without acid addition, with a greater recovery efficiency than hexane [97]. Other organic-based green solvents and some ionic liquids (including deep eutectic solvents), or CO2, typically used in supercritical conditions have also been pointed out as potential substitutes for fossil solvents in lipid extraction processes. However, the current high costs of green organic-based solvents and ionic liquids, and the lack of industrially tested extraction protocols and extracted products’ certification, still constitute significant barriers to the industrial implementation of these techniques versus traditional methods based on fossil solvents [102].
5. Biorefinery, Sustainability and Circular Economy
Wastewater treatment facility operators are mainly focused on their mission to meet their statutory responsibilities for sewage treatment and pollution control. However, WWTPs now face a myriad of challenges, including aging infrastructure, increasing service demands and regulatory requirements, rising utility and chemical costs, and social and environmental concerns about biosolid disposal practices. The undergoing paradigmatic transformation from WWTP to WRRF (Water and Resources Recovery Facilities) expands the scope of wastewater treatment to include materials recovery [103], carbon management [104], emissions reduction and water reuse [105].
Biorefining can therefore be regarded as the sustainable processing of waste sludge biomass, alone or in combination with biomasses of other origins, towards the implementation of a sludge-based circular economy: an integrated concept encompassing the circular economy and bioeconomy, also known as the circular bioeconomy (CBE). While the key goal of the CE is to slow, streamline and close material resource loops, the CBE goes beyond the simple substitution of fossil resources with renewable resources. The CBE entails low-carbon energy inputs, sustainable supply chains and disruptive conversion technologies for the sustainable transformation of renewable bioresources to high-value bio-based products, materials and fuels, i.e., the cascading use of biomass from biological resources into a systemic economic development approach.
Sewage sludge is a steady and growing global waste stream that, under current approaches, is both costly to manage both in monetary and energy terms and largely undervalued regarding recovery possibilities. Biorefinery, as discussed in the previous sections, can exploit EBSS streams to extract useful materials and energy; the quality of EBSS-derived products, their market value and appeal are important factors with respect to the future sustainability of biorefinery. Challenges associated with sludge biorefinery include:
- Technological readiness level (TRL): most of the proposed technologies, although tested and validated at a laboratory or pilot scale, still remain in their infancy from the industrial application aspect, and further operational and cost optimization is needed;
- Biorefinery products such as proteins and enzymes still demand high production costs and raise some concerns in terms of pathogenic and metal toxicity, which effectively stifle technological scaling-up developments; efficient purification procedures for biorefined products (in line with the required purity standards for specific applications) still need further development to improve the overall economics of biorefinery approaches;
- The scale-up of production technology is an important issue since much of the research so far was performed at a laboratory scale. Scaling-up requires the optimization of each biorefinery process step and its integration with the local feedstock supply. The integration of compatible biomass sources (e.g., food and organic fraction of municipal solid waste) would contribute to the overall economics of biorefinery approaches;
- Existing non-technological barriers (i.e., unrelated to technological processes but limiting the subsequent diffusion of the product’s use) should also be taken into account. These can be summarily divided into economic, administrative and market-related barriers [106]. Examples of the first category are: the need for large investments, low profitability (compared to traditional production) and logistic difficulties for feedstock procurement. Administrative barriers may include final products’/materials’ certifications (when needed);
- Public and consumer acceptance: the ultimate success of a CBE approach depends on the consumer acceptance of recovered waste-based products. Consumers’ perception of CBE schemes has been largely overlooked in the scientific literature since generic ethical intentions by citizens do not necessarily translate into virtuous behavior [107].
6. Conclusions
Sludge contains a tremendous amount of renewable organics and can be deemed a sustainable resource with considerable economic potential, whether in the form of nutrients, energy or emerging materials recovery. Among various technologies for sewage sludge-embedded resource exploitation, biorefinery is an approach that integrates existing and developing processes to optimize resource recovery and reduce the environmental impacts of disposal. Possible sludge biorefinery’s added-value final products are varied, renewable and sustainable, and could open unique new perspectives for a lesser fossil fuel-dependent economy.
Some of the applicable technologies are still at the bench/pilot scale level, and industrial optimization is needed. Although showing great potential for industrial application, issues such as feedstock volume supply reliability, product certification and competitiveness must still be fully addressed. Capital investment into biorefinery facilities and related operational expenses are largely dependent on the production scale and applied technologies. A comprehensive approach to evaluate the whole process in terms of environmental and economic impacts and benefits, with the development of local synergies from additional compatible feedstock sources, is still largely lacking.
Sustainable biorefinery design must include an assessment of environmental impacts and carbon footprint reduction compared to traditional disposal practices. These facilities should be flexible in design to sustain short- and long-term variations in sludge characteristics and final product outlets, as requested by market conditions. Co-processing of sewage sludge with other municipal organic waste streams (i.e., food waste and municipal solid waste organic fraction) may improve the long-term sustainability of these facilities.
Author Contributions
Conceptualization, A.G.C. and D.C.; methodology, A.G.C.; formal analysis and investigation, D.C.; writing—original draft preparation, D.C.; writing—review and editing, A.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- EC. Solving Treatment of Wastewater Sewage Sludge with New HTL Technology to Produce Hydrocarbons, Asphalts and Fertilizers; Life Public Databases Reference: LIFE19 ENV/IT/000165U. 2020. Available online: https://webgate.ec.europa.eu/life/publicWebsite/index.cfm?fuseaction=search.dspPage&n_proj_id=7687 (accessed on 26 September 2022).
- University of Michigan. US Wastewater Treatment—Factsheet Water. 2021. Available online: https://css.umich.edu/sites/default/files/Wastewater%20Treatment_CSS04-14_e2021.pdf (accessed on 26 September 2022).
- Wei, L.; Zhu, F.; Lia, Q.; Xue, C.; Xia, X.; Yu, H.; Zhao, Q.; Jiang, J.; Bai, B. Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivaient emissions analysis. Environ. Int. 2020, 144, 106093. [Google Scholar] [CrossRef] [PubMed]
- Shizas, I.; Bagley, D.M. Experimental determination of energy content of unknown organics in municipal wastewater streams. J. Energy Eng. 2004, 130, 45–53. [Google Scholar] [CrossRef]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwang, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Hennebert, P.; Anderson, A.; Merdy, P. Mineral Nanoparticles in Waste: Potential Sources, Occurrence in Some Engineered Nanomaterials Leachates, Municipal Sewage Sludges and Municipal Landfill Sludges. J. Biotechnol. Biomater. 2017, 7, 2. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Patureau, D.; Vulliet, E.; Delgenes, N.; Danel, N.; Deshayesa, S.; Eudes, V.; Guerine, S.; Moilleron, R.; et al. Fate of emerging and priority micropollutants during the sewage sludge treatment: Case study of Paris conurbation—Part 1: Contamination of the different types of sewage sludge. Waste Manag. 2017, 59, 379–393. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Olsson, G. Energy issues in sustainable urban wastewater management: Use, demand reduction and recovery in the urban water cycle. Sustainability 2020, 12, 266. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degreve, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Callegari, A.; Hlavinek, P.; Capodaglio, A.G. Production of energy (biodiesel) and recovery of materials (biochar) from pyrolysis of urban waste sludge. Rev. Ambiente Água 2018, 13, e2128. [Google Scholar] [CrossRef]
- Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A.G. Economic and energetic assessment of different phosphorus recovery options from aerobic sludge. J. Clean. Prod. 2019, 223, 729–738. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Sludge: A waste or renewable source for energy and resources recovery? Renew. Sustain. Energy Rev. 2013, 25, 708–728. [Google Scholar] [CrossRef]
- Keiding, K.; Wybrandt, L.; Nielsen, P.H. Remember the water: A comment on EPS colligative properties. Water Sci. Technol. 2001, 43, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Bourven, I.; Guibaud, G.; van Hullebusch, E.D.; Panico, A.; Pirozzi, F.; Esposito, G. Role of extracellular polymeric substances (EPS) production in bioaggregation: Application to wastewater treatment. Appl. Microbiol. Biotechnol. 2015, 99, 9883–9905. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Lotti, T.; Canziani, R.; Lin, Y.; Tagliabue, C.; Malpei, F. Extracellular biopolymers recovered as raw biomaterials from waste granular sludge and potential applications: A critical review. Sci. Total Environ. 2021, 753, 142051. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Bolognesi, S.; Cecconet, D. Sustainable, decentralized sanitation and reuse with hybrid nature-based systems. Water 2021, 13, 1583. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fan, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, F.; Jungmeier, G.; Wellisch, M.; Wilke, T.; Skiadas, I.; Ree, V.R.; Jong, D.E. Toward a common classification approach for biorefinery systems. Biofuels Bioprod. Biorefining 2009, 3, 534–546. [Google Scholar] [CrossRef]
- Hingsamer, M.; Jungmeier, G. Biorefineries. In The Role of Bioenergy in the Bioeconomy; Lago, C., Caldés, N., Lechón, Y., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 5; pp. 179–222. [Google Scholar] [CrossRef]
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role and future prospects of biofuels feedstocks: A state-of-the-art review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 384–436. [Google Scholar] [CrossRef]
- Bora, R.R.; Richardson, R.E.; You, F. Resource recovery and waste-to-energy from wastewater sludge via thermochemical conversion technologies in support of circular economy: A comprehensive review. BMC Chem. Eng. 2020, 2, 8. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Hu, M.; Ye, Z.; Zhang, H.; Chen, B.; Pan, Z.; Wang, J. Thermochemical conversion of sewage sludge for energy and resource recovery: Technical challenges and prospects. Environ. Pollut. Bioavailab. 2021, 33, 145–163. [Google Scholar] [CrossRef]
- Bolognesi, S.; Bernardi, G.; Callegari, A.; Dondi, D.; Capodaglio, A.G. Biochar production from sewage sludge and microalgae mixtures: Properties, sustainability and possible role in circular economy. Biomass-Convers. Biorefinery 2021, 11, 289–299. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Dondi, D. Microwave-Induced Pyrolysis for Production of Sustainable Biodiesel from Waste Sludges. Waste Biomass Valoriz. 2016, 7, 703–709. [Google Scholar] [CrossRef]
- Tasca, A.L.; Puccini, M.; Gori, R.; Corsi, I.; Galletti, A.M.R.; Vitolo, S. Hydrothermal carbonization of sewage sludge: A critical analysis of process severity, hydrochar properties and environmental implications. Waste Manag. 2019, 93, 1–13. [Google Scholar] [CrossRef]
- Thomsen, L.B.S.; Carvalho, P.N.; Dos Passos, J.S.; Anastasakis, K.; Bester, K.; Biller, P. Hydrothermal liquefaction of sewage sludge; energy considerations and fate of micropollutants during pilot scale processing. Water Res. 2020, 183, 116101. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Callegari, A.; Lopez, M.V. European Framework for the Diffusion of Biogas Uses: Emerging Technologies, Acceptance, Incentive Strategies and Institutional-Regulatory Support. Sustainability 2016, 8, 298. [Google Scholar] [CrossRef]
- Badea, G.I.; Radu, G.L. Introductory Chapter: Carboxylic Acids—Key Role in Life Sciences. In Carboxylic Acid—Key Role in Life Sciences; Badea, G.I., Radu, G.L., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Shen, L.; Hu, H.; Ji, H.; Cai, J.; He, N.; Li, Q.; Wang, Y. Production of poly(hydroxybutyrate-hydroxyvalerate) from waste organics by the two-stage process: Focus on the intermediate volatile fatty acids. Bioresour. Technol. 2014, 166, 194–200. [Google Scholar] [CrossRef]
- Levy, P.F.; Sanderson, J.E.; Kispert, R.G.; Wise, D.L. Biorefining of biomass to liquid fuels and organic chemicals. Enzym. Microb. Technol. 1981, 3, 207–215. [Google Scholar] [CrossRef]
- Cord-Ruwisch, R. Thermodynamics of anaerobic digestion: Mechanism of suppression on biogas production during acidogenesis. INMATEH Agric. Eng. 2019, 57, 287–301. [Google Scholar]
- Li, X.; Zhao, J.; Wang, D.; Yang, Q.; Zeng, G. An efficient and green pretreatment to stimulate short-chain fatty acids production from waste activated sludge anaerobic fermentation using free nitrous acid. Chemosphere 2016, 144, 160–167. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, Y.; Xu, Q.; Liu, X.; Liu, Y.; Ni, B.J.; Yang, Q.; Wang, D.; Li, X.; Wang, Q. Free ammonia-based pretreatment promotes short-chain fatty acid production from waste activated sludge. ACS Sustain. Chem. Eng. 2018, 6, 9120–9129. [Google Scholar] [CrossRef]
- Liu, X.; Du, M.; Yang, J.; Wu, X.; Xu, Q.; Wang, D.; Yang, Q.; Yang, G.; Li, X. Sulfite serving as a pretreatment method for alkaline fermentation to enhance short-chain fatty acid production from waste activated sludge. Chem. Eng. J. 2020, 385, 123991. [Google Scholar] [CrossRef]
- He, Z.W.; Liu, W.Z.; Gao, Q.; Tang, C.C.; Wang, L.; Guo, Z.C.; Zhou, A.J.; Wang, A.J. Potassium ferrate addition as an alternative pre-treatment to enhance short-chain fatty acids production from waste activated sludge. Bioresour. Technol. 2018, 247, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, Y.; Xu, Q.; Liu, X.; Yang, Q.; Li, X. Free ammonia aids ultrasound pretreatment to enhance short-chain fatty acids production from waste activated sludge. Bioresour. Technol. 2019, 275, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Li, L.; He, J.; Yan, Z.; Ma, Z.; Nan, J.; Liu, Y. New insight into enhanced production of short-chain fatty acids from waste activated sludge by cation exchange resin-induced hydrolysis. Chem. Eng. J. 2020, 388, 124235. [Google Scholar] [CrossRef]
- Wu, Y.; Song, K. Effect of thermal activated peroxydisulfate pretreatment on short-chain fatty acids production from waste activated sludge anaerobic fermentation. Bioresour. Technol. 2019, 292, 121977. [Google Scholar] [CrossRef]
- Frison, N.; Katsou, E.; Malamis, S.; Oehmen, A.; Fatone, F. Development of a novel process integrating the treatment of sludge reject water and the production of polyhydroxyalkanoates (PHAs). Environ. Sci. Technol. 2015, 49, 10877–10885. [Google Scholar] [CrossRef]
- Zacharof, M.P.; Lovitt, R.W. Complex effluent streams as a potential source of volatile fatty acids. Waste Biomass Valoriz. 2013, 4, 557–581. [Google Scholar] [CrossRef]
- Wu, S.L.; Wei, W.; Sun, J.; Xu, Q.; Dai, X.; Ni, B.J. Medium-Chain fatty acids and long-chain alcohols production from waste activated sludge via two-stage anaerobic fermentation. Water. Res. 2020, 186, 116381. [Google Scholar] [CrossRef]
- Yishai, O.; Lindner, S.N.; de la Cruz, J.G.; Tenenboim, H.; Bar-Even, A. The formate bio-economy. Curr. Opin. Chem. Biol. 2016, 35, 1–9. [Google Scholar] [CrossRef]
- Raboni, M.; Viotti, P.; Capodaglio, A.G. A comprehensive analysis of the current and future role of biofuels for transport in the European Union (EU). Rev. Ambiente Agua 2015, 10, 9. [Google Scholar] [CrossRef]
- Mühlemeier, I.M.; Speight, R.; Strong, P.J. Biogas, bioreactors and bacterial methane oxidation. In Methane Biocatalysis: Paving the Way to Sustainability; Kalyuzhnaya, M.G., Xing, X., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 213–235. [Google Scholar]
- Zhang, T.; Zhou, J.; Wang, X.; Zhang, Y. Coupled effects of methane monooxygenase and nitrogen source on growth and poly-β-hydroxybutyrate (PHB) production of Methylosinus trichosporium OB3b. J. Env. Sci. 2017, 52, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and Modifications. Polymer 2021, 212, 123161. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.C.; Wu, Q.; Chen, W. Polyhydroxyalkanoates as a source of chemicals, polymers and biofuels. Curr. Opin. Biotechnol. 2011, 22, 768–774. [Google Scholar] [CrossRef]
- Spekreijse, J.; Le Nôtre, J.; van Haveren, J.; Scott, E.L.; Sanders, J.P.M. Simultaneous production of biobased styrene and acrylates using ethenolysis. Green Chem. 2012, 14, 2747–2751. [Google Scholar] [CrossRef]
- Yadav, B.; Talan, A.; Tyagi, R.D.; Drogui, P. Concomitant production of value-added products with polyhydroxyalkanoate (PHA) synthesis: A review. Biores. Technol. 2021, 337, 125419. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Sagastume, F.; Hjort, M.; Cirne, D.; Gérardin, F.; Lacroix, S.; Gaval, G.; Karabegovic, L.; Alexandersson, T.; Johansson, P.; Karlsson, A.; et al. Integrated production of polyhydroxyalkanoates (PHAs) with municipal wastewater and sludge treatment at pilot scale. Bioresour. Technol. 2015, 181, 78–89. [Google Scholar] [CrossRef]
- Battista, F.; Frison, N.; Pavan, P.; Cavinato, C.; Gottardo, M.; Fatone, F.; Eusebi, A.L.; Majone, M.; Zeppilli, M.; Valentino, F.; et al. Food wastes and sewage sludge as feedstock for an urban biorefinery producing biofuels and added-value bioproducts. J. Chem. Technol. Biotechnol. 2020, 95, 328–338. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Karlsson, A.; Johansson, P.; Pratt, S.; Boon, N.; Lant, P.; Werker, A. Production of polyhydroxyalkanoates in open, mixed cultures from a waste sludge stream containing high levels of soluble organics, nitrogen and phosphorus. Water Res. 2010, 44, 5196–5211. [Google Scholar] [CrossRef]
- Bluemink, E.; van Nieuwenhuijzen, A.; Wypkema, E.; Uijterlinde, C. Bio-plastic (poly-hydroxy-alkanoate) production from municipal sewage sludge in the Netherlands: A technology push or a demand driven process? Water Sci. Technol. 2016, 74, 353–358. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Bishop, P.L. Biodegradability of biofilm extracellular polymeric substances. Chemosphere 2003, 50, 63–69. [Google Scholar] [CrossRef]
- Andreoli, C.V.; Von Sperling, M.; Fernandes, F.; Ronteltap, M. Sludge Treatment and Disposal; IWA Publishing: London, UK, 2007. [Google Scholar]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C.M. Full-scale partial nitritation/anammox experiences–an application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)—Part II: Technical aspects. Water Sci. Technol. 2001, 43, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Hohapatra, S.P.; Siebel, M.A.; Alaerts, G.J. Effect of Bacillus megaterium on removal of copper from aqueous solutions by activated carbon. J. Environ. Sci. Health Part A 1993, 28, 615–629. [Google Scholar]
- Schlekat, C.E.; Decho, A.W.; Chandler, G.T. Sorption of cadmium to bacterial extracellular polymeric sediment coatings under estuarine conditions. Environ. Toxicol. Chem. Int. J. 1998, 17, 1867–1874. [Google Scholar] [CrossRef]
- Spath, R.; Flemming, H.C.; Wuertz, S. Sorption properties of biofilms. Water Sci. Technol. 1998, 37, 207–210. [Google Scholar] [CrossRef]
- Liu, A.B.; Ahn, I.S.; Mansfield, C.; Lion, L.W.; Shuler, M.L.; Ghiorse, W.C. Phenanthrene desorption from soil in the presence of bacterial extracellular polymer: Observations and model predictions of dynamic behaviour. Water Res. 2000, 35, 835–843. [Google Scholar] [CrossRef]
- Esparza-Soto, M.; Westerhoff, P. Biosorption of humic and fulvic acids to live activated sludge biomass. Water Res. 2003, 37, 2301–2310. [Google Scholar] [CrossRef]
- Sheng, G.P.; Zhang, M.L.; Yu, H.Q. Characterization of adsorption properties of extracellular polymeric substances (EPS) extracted from sludge. Colloids Surf. B Biointerface 2008, 62, 83–90. [Google Scholar] [CrossRef]
- Kim, N.K.; Mao, N.; Lin, R.; Bhattacharyya, D.; van Loosdrecht, M.C.M.; Lin, Y. Flame retardant property of flax fabrics coated by extracellular polymeric substances recovered from both activated sludge and aerobic granular sludge. Water Res. 2020, 170, 115344. [Google Scholar] [CrossRef]
- Liu, H.; Fang, H.H. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef]
- Dubé, C.D.; Guiot, S.R. Characterization of the protein fraction of the extracellular polymeric substances of three anaerobic granular sludges. AMB Express 2019, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Felz, S.; Al-Zuhairy, S.; Aarstad, O.A.; van Loosdrecht, M.C.M.; Lin, Y.M. Extraction of structural extracellular polymeric substances from aerobic granular sludge. J. Vis. Exp. 2016, 115, e54534. [Google Scholar] [CrossRef] [PubMed]
- Boleij, M.; Seviour, T.; Wong, L.L.; van Loosdrecht, M.C.M.; Lin, Y. Solubilization and characterization of extracellular proteins from anammox granular sludge. Water Res. 2019, 164, 114952. [Google Scholar] [CrossRef] [PubMed]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Sheng, G.; Yu, H.; Li, X. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Sesay, M.L.; Özcengiz, G.; Sanin, F.D. Enzymatic extraction of activated sludge extracellular polymers and implications on bioflocculation. Water Res. 2006, 40, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Gehr, R.; Henry, J. Removal of extracellular material techniques and pitfalls. Water Res. 1983, 17, 1743–1748. [Google Scholar] [CrossRef]
- Seviour, T.; Donose, B.C.; Pijuan, M.; Yuan, Z. Purification and conformational analysis of a key exopolysaccharide component of mixed culture aerobic sludge granules. Environ. Sci. Technol. 2010, 44, 4729–4734. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Pulse electric field technology for wastewater and biomass residues’ improved valorization. Processes 2021, 9, 736. [Google Scholar] [CrossRef]
- Hwang, J.; Zhang, L.; Seo, S.; Lee, Y.W.; Jahng, D. Protein recovery from excess sludge for its use as animal feed. Bioresour. Technol. 2008, 99, 8949–8954. [Google Scholar] [CrossRef]
- Su, R.; Hussain, A.; Guo, J.; Guan, J.; He, Q.; Yan, X.; Li, D.; Guo, Z. Animal feeds extracted from excess sludge by enzyme, acid and base hydrolysis processes. ACS Sust. Chem. Eng. 2015, 3, 2084–2091. [Google Scholar]
- Zhuang, L.; Zhou, S.; Wang, Y.; Liu, Z.; Xu, R. Cost-effective production of Bacillus thuringiensis biopesticides by solid-state fermentation using wastewater sludge: Effects of heavy metals. Bioresour. Technol. 2011, 102, 4820–4826. [Google Scholar] [CrossRef] [PubMed]
- Montiel, M.T.; Tyagi, R.; Valero, J.; Surampalli, R. Production of biopesticides using wastewater sludge as a raw material-effect of process parameters. Water Sci. Technol. 2003, 48, 239–246. [Google Scholar] [CrossRef]
- Liu, Z.; Smith, S.R. Enzyme Recovery from Biological Wastewater Treatment. Waste Biomass Valoriz. 2021, 12, 4185–4211. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Tyagi, R.D. Value-Added Bio-Products from Sewage Sludge. In Current Developments in Biotechnology and Bioengineering; Wong, J.W.-C., Tyagi, R.D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–42. [Google Scholar]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Enzymatic activity in the activated sludge floc matrix. Appl. Microbiol. Biotechnol. 1995, 43, 708–716. [Google Scholar] [CrossRef]
- Drouin, M.; Lai, C.K.; Tyagi, R.D.; Surampalli, R.Y. Bacillus licheniformis proteases as high value added products from fermentation of wastewater sludge: Pre-treatment of sludge to increase the performance of the process. In Proceedings of the IWA Specialist Conference on Moving Forward Wastewater Biosolids Sustainability: Technical, Managerial and Public Synergy, Moncton, NB, Canada, 24–27 June 2007; pp. 599–605. [Google Scholar]
- Subramanian, S.B.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Enzymes: Production and Extraction. In Sustainable Sludge Management: Production of Value Added Products; Tyagi, R.D., Surampalli, R.Y., Yan, S., Zhang, T.C., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2013. [Google Scholar]
- Guanghui, Y.; Pinjing, H.; Liming, S.; Yishu, Z. Enzyme extraction by ultrasound from sludge flocs. J. Environ. Sci. 2009, 21, 204–210. [Google Scholar]
- Jung, J.; Xing, X.H.; Matsumoto, K. Recoverability of protease released from disrupted excess sludge and its potential application to enhanced hydrolysis of proteins in wastewater. Biochem. Eng. J. 2002, 10, 67–72. [Google Scholar] [CrossRef]
- Nabarlatz, D.; Vondrysova, J.; Jenicek, P.; Stüber, F.; Font, J.; Fortuny, A.; Fabregat, A.; Bengoa, C. Hydrolytic enzymes in activated sludge: Extraction of protease and lipase by stirring and ultrasonication. Ultrason. Sonochem. 2010, 17, 923–931. [Google Scholar] [CrossRef]
- Gessesse, A.; Dueholm, T. Lipase and protease extraction from activated sludge. Water Res. 2011, 37, 3652–3657. [Google Scholar] [CrossRef]
- Yu, G.H.; He, P.J.; Shao, L.M.; Lee, D.J. Enzyme activities in activated sludge flocs. Appl. Microbiol. Biotechnol. 2007, 77, 605–612. [Google Scholar] [CrossRef]
- Clavijo Rivera, E.; Montalescot, V.; Viau, M.; Drouin, D.; Bourseau, P.; Frappart, M.; Monteux, C.; Couallier, E. Mechanical cell disruption of Parachlorella kessleri microalgae: Impact on lipid fraction composition. Bioresour. Technol. 2018, 256, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Cheng, D.; Varjani, S.; Lei, Z.; Liu, Y. Roles and applications of enzymes for resistant pollutants removal in wastewater treatment. Bioresour. Technol. 2021, 335, 125278. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Callegari, A. Feedstock and process influence on biodiesel produced from waste sewage sludge. J. Environ. Manag. 2018, 216, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Djandja, O.S.; Wang, Z.C.; Wang, F.; Xu, Y.P.; Duan, P.G. Pyrolysis of Municipal Sewage Sludge for Biofuel Production: A Review. Ind. Eng. Chem. Res. 2020, 59, 16939–16956. [Google Scholar] [CrossRef]
- Quan, L.M.; Kamyab, H.; Yuzir, A.; Ashokkumar, V.; Hosseini, S.E.; Balasubramanian, B.; Kirpichnikova, I. Review of the application of gasification and combustion technology and waste-to-energy technologies in sewage sludge treatment. Fuel 2022, 316, 123199. [Google Scholar] [CrossRef]
- Boocock, D.G.B.; Konar, S.K.; Leung, A.; Ly, L.D. Fuels and chemicals from sewage sludge: 1. The solvent extraction and composition of a lipid from a raw sewage sludge. Fuel 1992, 71, 1283–1289. [Google Scholar] [CrossRef]
- Olkiewicz, M.; Caporgno, M.P.; Fortuny, P.; Stüber, F.; Fabregat, A.; Font, J.; Bengoa, C.M. Direct liquid-liquid extraction of lipid from municipal sewage sludge for biodiesel production. Fuel Process. Technol. 2014, 128, 331–338. [Google Scholar] [CrossRef]
- D’Ambrosio, V.; di Bitonto, L.; Angelini, A.; Gallipoli, A.; Braguglia, C.M.; Pastore, C. Lipid extraction from sewage sludge using green biosolvent for sustainable biodiesel production. J. Clean. Prod. 2021, 329, 129643. [Google Scholar] [CrossRef]
- Olkiewicz, M.; Fortuny, P.; Stüber, F.; Fabregat, A.; Font, J.; Bengoa, C. Effects of pre-treatments on the lipid extraction and biodiesel production from municipal WWTP sludge. Fuel 2015, 141, 250–257. [Google Scholar] [CrossRef]
- Siddiquee, M.N.; Rohani, S. Lipid extraction and biodiesel production from municipal sewage sludges: A review. Renew. Sustain. Energy Rev. 2011, 15, 1067–1072. [Google Scholar] [CrossRef]
- Patiño, Y.; Mantecón, L.G.; Polo, S.; Faba, L.; Díaz, E.; Ordóñez, S. Effect of sludge features and extraction-esterification technology on the synthesis of biodiesel from secondary wastewater treatment sludges. Bioresour. Technol. 2018, 247, 209–216. [Google Scholar] [CrossRef]
- Sakaveli, F.; Petala, M.; Tsiridis, V.; Darakas, E. Enhanced mesophilic anaerobic digestion of primary sewage sludge. Water 2021, 13, 348. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Maciel Filho, R. Recent advances in lipid extraction using green solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Faragò, M.; Damgaard, A.; Madsen, J.A.; Andersen, J.K.; Thornberg, D.; Andersen, M.H.; Rygaard, M. From wastewater treatment to water resource recovery: Environmental and economic impacts of full-scale implementation. Water Res. 2021, 204, 117554. [Google Scholar] [CrossRef]
- Qandil, M.D.; Abbas, A.I.; Abdelhadi, A.I.; Salem, A.R.; Amano, R.S. Energy Analysis: Ways to Save Energy and Reduce the Emissions in Wastewater Treatment Plants. Int. J. Energy Clean Environ. 2021, 22, 91–112. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Fit-for-purpose urban wastewater reuse: Analysis of issues and available technologies for sustainable multiple barrier approaches. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1619–1666. [Google Scholar] [CrossRef]
- McCormick, K.; Kaberger, T. Key barriers for bioenergy in Europe: Economic conditions, know-how and institutional capacity and supply chain co-ordination. Biomass Bioenergy 2007, 31, 443–452. [Google Scholar] [CrossRef]
- Russo, I.; Confente, I.; Scarpi, D.; Hazen, B.T. From trash to treasure: The impact of consumer perception of bio-waste products in closed-loop supply chains. J. Clean. Prod. 2019, 218, 966–974. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).