Abstract
Improved wastewater (WW) treatment contributes to preserving human life and aquatic ecosystems and acting on climate change. The use of drinking water treatment sludges (WTS) as coagulants in the primary treatment of WW contributes, in this regard, and simultaneously enables the sustainable management of this waste. In this work, the improvement of the primary treatment of real domestic WW using unmodified WTS and chemically modified WTS with sulphuric and hydrochloric acids (reactive sludges—RSs) as coagulants was evaluated. The evaluated WTS contains a higher fraction of inorganic solids and is mainly an amorphous material. The wet WTS (W-WTS) showed a better performance in enhancing WW clarification (up to 76%), as measured by turbidity in comparison with the dry WTS (D-WTS). All RSs improved this performance considerably (up to 98%), and of these, the sulphuric reactive sludge generated from the W-WTS (SRS-W) showed the lowest costs associated with acid consumption for activation. The best treatments with W-WTS and SRS-W significantly improved the removal of solids (total suspended solids > 90% and volatile suspended solids > 80%), organic matter (total biochemical oxygen demand > 50% and total chemical oxygen demand > 55%), and total phosphorus (>75%) compared to natural sedimentation, with slight differences in favour of SRS-W, especially in the removal of phosphorus species. The reuse of WTSs in primary WW treatment becomes a valuable circular economy proposal in the water sector, which simultaneously valorises waste from the drinking water process and contributes to the fulfilment of Sustainable Development Goal 6 (Clean Water and Sanitation)
1. Introduction
Globally, more than 80% of all wastewater (over 95% in some developing countries) is released into the environment without treatment [1]. Untreated wastewater (WW) is a major source of pollution, water-related diseases, and greenhouse gases [2].
Concerning levels of domestic WW treatment, the World Health Organisation (WHO) estimated that, by 2018, 59% of domestic WW flow generated in 79 countries (mostly high- and middle-income) is collected and treated safely. Furthermore, while within Europe, the performance of wastewater treatment plants (WWTP) in biological oxygen demand (BOD) removal and BOD compliance is generally above 80%, elsewhere, this performance is as low as 20%. This low performance indicates that some treatment plants are not performing as intended due to poor operation and maintenance, unregulated industrial discharges, and overloading or underloading [3].
Therefore, improving WW treatment can help preserve human life and our water ecosystems and act on climate change [4]. Incorporating chemical coagulation and flocculation into primary treatment can improve the performance of domestic WW treatments by increasing the removal of pollutants such as solids, organic matter, and phosphorus [5].
In the implementation of chemical coagulation, in addition to conventional coagulants (aluminium and iron salts), the use of raw drinking water treatment sludge (WTS) in the treatment of domestic WW has been evaluated as a complement to primary treatment in a full-scale WWTP [6], as a coagulant prior to the ultrafiltration membrane process of secondary WW treatment [7] and as a tertiary treatment [8]. The dosing or discharge of WTSs in sewerage systems has also been evaluated at a laboratory scale [9,10] and pilot scale [11,12], and their effect on WW treatment. Similarly, WTS has been studied as a coagulant in other wastewaters, such as animal farm WW [13].
WTS is a waste generated in the conventional water purification process, a product of the coagulation and flocculation stages; finally, it accumulates in the sedimentation tanks. This waste is characterised by a high content of aluminium (Al) and iron (Fe) from the coagulant used in the water treatment process [14]. Both metals can act as coagulants in WW treatment, improving the removal of solids, organic matter, and phosphorus by sweeping and adsorption to the flocs formed during flocculation and separated during sedimentation [6].
To enhance the coagulant effect of WTS, the addition of acids to WTS has been evaluated. This process resolubilises the aluminium and iron metals in the WTS matrix, increasing the soluble fraction of these elements [15,16]. In the acidification of WTS, two phases are generated: (i) a liquid phase or supernatant containing solubilised Al and Fe, and (ii) a solid phase composed of the materials not dissolved by the acid. The first phase is called recovered coagulant, and the mixture of the two phases generates a chemically modified sludge. Coagulants recovered with WTS acidification using sulphuric acid (HSO) have been evaluated to improve the primary treatment of domestic WW [17,18,19]. With the separation and use of the recovered coagulant, the remaining solid phase becomes a waste with acidic characteristics that requires neutralisation for proper handling and disposal [16]. However, with the alternative use of the chemically modified sludge (mixture of phases i and ii), a co-treatment of the entire WTS is carried out at the WWTP, leading to sustainable management of the solid phase.
Some authors have investigated chemically modified sludge and its effect on surface water treatment [20] and dairy WW [15]. Ahmad et al. [20] acidified a WTS that was generated in the laboratory by dosing coagulant alum to Yamuna River water. The acidification was performed with different normalities and amounts of HSO to produce a sludge reagent product (SRP), which was used directly as a coagulant to remove colloids in the same river water. With the optimal SRP (1% sludge concentration, acidified with 2.5 N HSO at the rate of 0.05 mL/mL), the authors reported considerable removals of total dissolved solids (TDS), iron, turbidity, alkalinity, total hardness, BOD, and chemical oxygen demand (COD). They concluded that SRP could partially or totally replace conventional coagulants in water treatment.
Suman et al. [15] collected WTS from a drinking water treatment plant (DWTP) using PACl as a coagulant. The WTS was treated with 50 mL/L HSO at different normalities to generate conditioned water treatment sludge (CWTS), which was used as a coagulant to treat synthetic dairy WW. The CWTS generated under optimal conditions (50 mL HSO 3.5 N/L) performed comparably with the commercial coagulants alum, ferric chloride, and ferrous sulphate to remove COD, BOD, TDS and total suspended solids (TSS). Finally, the authors concluded that CWTS has the potential to be used as a coagulant in the primary treatment of dairy WW and that this use would provide sustainable WTS management and cost-effective dairy WW treatment.
Previous research shows the potential of chemically modified sludge in the treatment of surface water [20] and synthetic WW from the dairy industry [15]. However, to the authors’ knowledge, chemically modified WTS has not been studied in the primary treatment of real domestic WW. This application can be an alternative for the sustainable management of the WTS that aims to transition to the circular economy in the water sector [21] with a great potential for application since the waste of the DWTP can be used in the WWTP. The transition to a circular economy provides an opportunity to achieve the Sustainable Development Goals (SDG) through accelerating and scaling up recent scientific and technological advances that support greater efficiency in the water sector [22].
In the present study, modified or chemically activated WTSs were generated by adding different concentrations of sulphuric acid (HSO) and hydrochloric acid (HCl) to a raw WTS. These modified WTSs were named reactive sludges (RSs). Unmodified sludge (raw WTS) and RSs were evaluated as coagulants in improving primary water clarification in real domestic WW at natural pH. The improvement in clarification was calculated as the additional turbidity removal that occurs in primary sedimentation with the RSs and raw WTS dosages. Finally, the optimal treatments with the RSs and raw WTS were selected to study their performance in removing solids, organic matter, Fe, Al, and phosphorus in real domestic WW. These results were compared to the removals obtained in the natural sedimentation process.
2. Methodology
2.1. Water Treatment Sludge (WTS) Characterisation
The sludge evaluated was obtained from a municipal DWTP in Colombia, which results from the coagulation, flocculation, and sedimentation processes. Polyaluminium chloride (PACl) is used as a coagulant in this plant. Wet sludge samples (W-WTS) were taken directly from the settler, and the following analyses were performed: total solids (TS), total volatile solids (TVS), total fixed solids (TFS), total iron (Fe), aluminium (Al), pH, and electrical conductivity (EC). These tests were performed following the Standard Methods for Analysis of Water and Wastewater of the American Public Health Association [23].
Likewise, a dried sludge (D-WTS) was obtained from the oven drying of the W-WTS at a temperature of 110 ± 5 °C until a constant mass was reached. The D-WTS was crushed and then sieved to obtain samples with different particle sizes with passing sizes of 38, 75, 150, 300, 600 and 1180 m. The D-WTS surface chemical composition and morphology were analysed using a JEOL JSM 6490 LV (Tokyo, Japan) scanning electron microscope (SEM) equipped with an Oxford energy-dispersive spectroscopy (EDS) system (Abingdon, UK). The main chemical compounds present in the D-WTS were analysed by energy dispersive X-ray fluorescence (ED-XRF) with the Thermo Fisher ARL Optim’X WDXRF (Thermo Fisher Scientific, Ecublens, Switzerland), and minerals were analysed using a Malvern-PANalytical X-ray diffractometer using copper (Cu, K = 0.15406 nm (1.5406 Å)) as the radiation source. Measurements were taken in a 2 range extending from 4 to 80° with a step of 0.05°. Quantification was performed with the software HighScore Plus, Version: 3.0.5, 2012 (PANalytical, Almelo, The Netherlands) using the Rietveld method and the database: ICSD FIZ Karlsruhe 2012-1.
2.2. Wastewater (WW) Characterisation
Wastewater samples were collected at the influent of the primary settling tanks of the municipal WWTP. The WW samples were measured for turbidity with a HACH 2100P turbidity meter (Loveland, CO, USA). pH and EC were measured with HACH HQ40d multiparameter equipment (Loveland, CO, USA). Additionally, to evaluate the removal of solids, organic matter, metals and phosphorus for the optimal condition tests, the following parameters were determined in a WW sample: total suspended solids (TSS), volatile suspended solids (VSS), sedimentable solids (SedS), total chemical oxygen demand (tCOD), soluble chemical oxygen demand (sCOD), particulate chemical oxygen demand (pCOD, calculated by the difference between tCOD and sCOD), total biochemical oxygen demand (tBOD), total iron (Fe), aluminium (Al), total phosphorus (tP), soluble phosphorus (sP) and orthophosphates (PO), according to the Standard Methods for the Analysis of Water and Wastewater of the American Public Health Association [23].
2.3. WTS Activation and Clarification Enhancement Evaluation
Figure 1 shows a schematic of the process carried out to generate the different RSs used and their evaluation in WW clarification. The RSs were generated from the two types of raw sludge (D-WTS and W-WTS, Figure 1a) and the addition of HSO or HCl with concentrations between 75 and 175 meq H/L, resulting in 4 types of reactive sludge (Figure 1b). These RSs were named according to the acid used in their activation process and the type of sludge from which they were generated (wet or dry), as follows: sulphuric reactive sludge-wet (SRS-W), sulphuric reactive sludge-dry (SRS-D), hydrochloric reactive sludge-wet (HRS-W), and hydrochloric reactive sludge-dry (HRS-D). The D-WTS with different particle sizes, the W-WTS and the four RSs were applied to WW at natural pH to evaluate their effect on improving primary wastewater clarification. These treatments were performed in standard jar tests under the conditions described in Figure 1c. The improvement in clarification corresponds to the turbidity removal that occurs with the addition of the sludges (W-WTS, D-WTS and RSs), taking as a reference the turbidity of the wastewater after the natural sedimentation process carried out in the jar tests without the addition of sludge (control sample), as indicated in Equation (1).
where is calculated as the difference between the final turbidity of the control sample () and the final turbidity of the WW sample after treatment with different sludges.
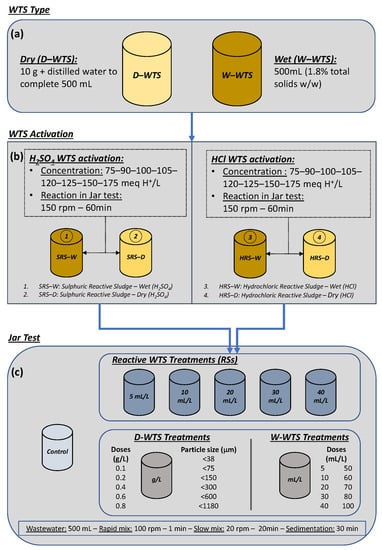
Figure 1.
Process for WTS activation and clarification enhancement evaluation. (a) WTS Type; (b) WTS Activation; (c) Jar Test.
The treatments with RS that achieved clarification enhancements higher than 80% were selected to perform a comparative analysis of the efficiency and cost of the WW clarification process associated with acid consumption. After this analysis, the treatment with RS, which improved the clarification of the WW with a 3.5 meq H/L of acid consumption per volume of WW treated, was selected as the optimal RS treatment. This acid consumption was calculated from the dose of RS added to the WW and the amount of acid added to the WTS in the activation process (e.g., a dose of RS = 20 mL of SRS-W/L activated with 175 meq H/L is equivalent to an acid consumption of 3.5 meq H/L). Additionally, the treatment with W-WTS, which showed the highest clarification enhancement of the WW, was also selected as the optimal raw WTS treatment. These two treatments were evaluated in removing solids, organic matter, Fe, Al and phosphorus from real domestic WW. These results were compared to the removals obtained in the natural sedimentation process.
3. Results and Discussion
3.1. WTS Characteristics
3.1.1. W-WTS Characteristics
Table 1 presents the results of the physicochemical characterisation of the W-WTS. The sludge presented a moisture content of 98.1%, a pH close to neutrality (6.94), a high Al content (1296 mg/L) due to the use of an Al-based coagulant (PACl) at the DWTP where the sludge was collected, and a high Fe content (837 mg/L) associated with the chemical composition of the water removed in the water treatment process. The W-WTS had a total solids concentration (TS) of 18,988 mg/L, with a higher fraction of inorganic solids (TFS/TS = 0.73). Similarly, Nair and Ahammed [8] also reported a predominance of inorganic solids in the WTS (generated with PACl) evaluated in their work.

Table 1.
Characteristics of the W-WTS.
3.1.2. D-WTS Characteristics
The chemical composition (ED-XRF) of the D-WTS is presented in Table 2. It can be observed that the main components in terms of oxides are AlO (32.55%), SiO (20.05%) and FeO (12.14%). The content of organic matter and minerals such as kaolinite that undergo dehydroxylation processes during calcination [24] is associated with loss of ignition (LOI) at 950 °C with a percentage of 32.54%.

Table 2.
Chemical composition of D-WTS.
Figure 2 (X-ray diffraction pattern) and Figure 3 (SEM) show that the D-WTS is mainly an amorphous material (81.4%), with poorly crystalline particles and sub-rounded structures. As for the crystalline material, the phases kaolinite 1A (11.9%), gibbsite (3.2%), dickite 2M1 (1.6%), quartz low (1.2%) and albite high (0.8%), which include a fraction of Si and Al from the D-WTS, were identified and quantified. This indicates that most of the Al and all the Fe present in the slurry are part of the amorphous material of the D-WTS [25,26]. The EDS spectra results (Figure 3) are consistent with the XRF results and show that Al, Si, Fe and C are the major surface elements in the analysed sludge particles, followed by Ca and Ti.
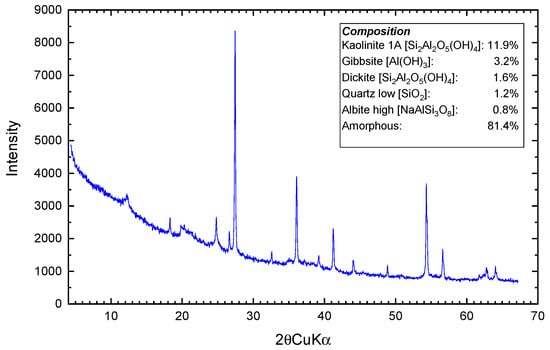
Figure 2.
XRD pattern of D-WTS.
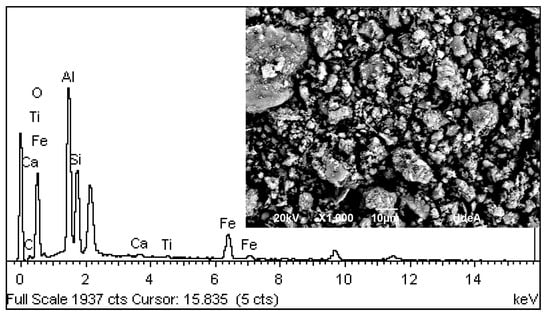
Figure 3.
SEM-EDS spectra of D-WTS.
3.2. Wastewater (WW) Characteristics
The mean values of turbidity, pH and EC for the WW samples used in the clarification enhancement evaluation were 459 NTU, 8.09 and 1080 S/cm, respectively. Turbidity presented an appreciable coefficient of variation (CV) during the sampling time (CV = 30.3%), as did EC (CV = 24.3%), while pH had less variability (CV = 7.0%) and an alkaline trend. The results of the WW characterisation for the test carried out with the optimal treatments and its comparison with the implemented treatments are presented in Section 3.4.
3.3. Clarification Enhancement Evaluation
3.3.1. Reactive WTS with HSO and HCl
Figure 4a shows the clarification enhancement achieved with different doses (mL/L) of sulphuric reactive sludge-wet (SRS-W) with several acid concentrations (meq H/L). In this figure, it can be seen that to obtain a clarification enhancement greater than 80%, doses higher than or equal to 20 mL/L are required for HSO concentrations between 90 and 150 meq H/L; likewise, for the acid concentration of 75 meq H/L, doses higher than or equal to 30 mL/L are required, while for the highest concentration, doses higher than or equal to 10 mL/L are required. This implies that lower SRS-W doses are required for clarification enhancement as the HSO concentration increases. Similarly, it is observed that to achieve a clarification enhancement greater than 80%, lower doses of sulphuric reactive sludge-dry (SRS-D) are required as the HSO concentration increases in its activation (Figure 4b). However, to achieve this level of enhancement, higher doses of SRS-D (at least 30 mL/L at concentrations greater than or equal to 140 meq H/L) are required compared to SRS-W. This shows a greater effectiveness of SRS-W in the clarification enhancement when compared to the SRS-D.
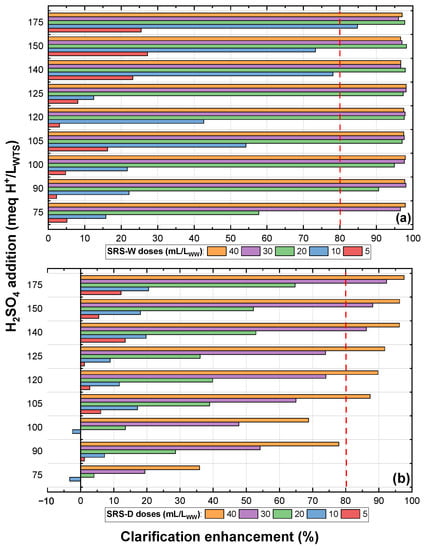
Figure 4.
Reactive sulphuric WTS: (a) wet and (b) dry.
The clarification enhancement achieved with different doses (mL/L) of hydrochloric reactive sludge-wet (HRS-W) and hydrochloric reactive sludge-dry (HRS-D) with several HCl concentrations (meq H/L) are presented in Figure 5a,b, respectively. Similar to the results obtained for SRSs, an increasing trend in clarification enhancement is shown with increasing HCl concentration applied in the sludge activation and the dose of reactive sludge applied to the WW. For both HRSs, it is observed that to obtain clarification enhancement above 80%, HCl concentrations of at least 105 meq H/L and doses between 30 and 40 mL/L are required.
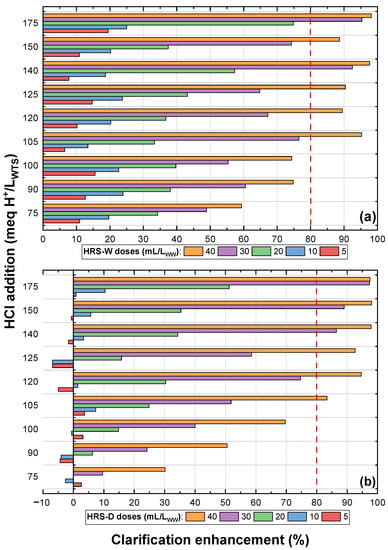
Figure 5.
Reactive hydrochloric WTS: (a) wet and (b) dry.
Figure 4 and Figure 5 show that, for the different acid additions, when the dosage of SRS and HRS is increased, the improvement in WW clarification also increases, in some cases reaching high values close to 98%. A similar trend in surface water treatment was reported by Ahmat et al. [20] on turbidity removal with HSO 2.5 N-modified WTS.
From Figure 4 and Figure 5, the RS treatments that achieved clarification enhancements higher than 80% were selected to perform a comparative analysis of the efficiency and cost of the WW clarification process associated with acid consumption. The net turbidity removal per acid consumed in the WW treatment (meq H/m) was calculated for this analysis. For example, the dose of 20 mL of SRS-W/L activated with 175 meq H/L generated an acid consumption of 3.5 meq H/L (3500 meq H/m); in turn, for this condition, the net turbidity removal was 150 NTU; therefore, the removal ratio per acid consumption was 0.043 (150 NTUremoved/3500 meq H/m). Figure 6 shows this ratio against acid consumption in the WW treatment (meq H/L), and Figure 7 shows the WW treatment cost associated with acid consumption in the WTS activation process. In Figure 6, it is observed that with lower acid consumptions (1.75–4.0 meq H/L) in the SRS-W treatments, high clarification enhancements (>80%) are achieved, whereas to achieve similar improvements with the other RSs, more significant amounts of acid (>4.0 meq H/L) are consumed. In addition, Figure 7 highlights that to achieve WW clarification enhancements higher than 80%, the WW treatment costs with HRS (between 0.35 and 0.59 USD/m) are higher than the cost of WW treatment with SRS (between 0.07 and 0.28 USD/m).
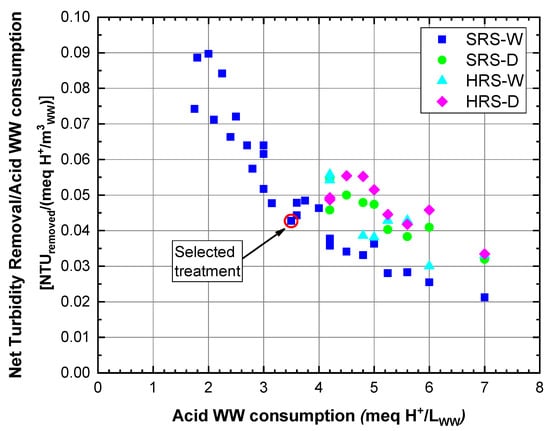
Figure 6.
Net turbidity removal in relation to acid consumption for treatment with clarification enhancement above 80%.
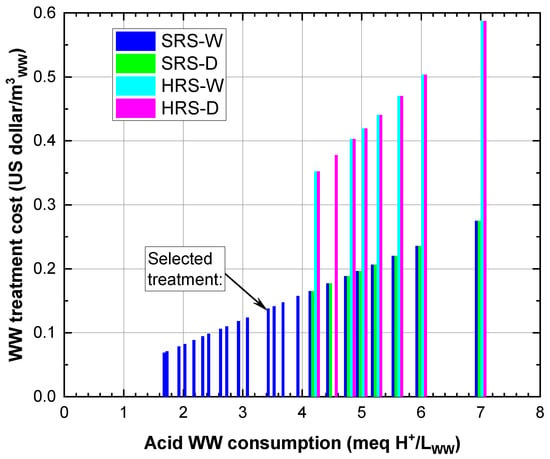
Figure 7.
WW treatment cost in relation to acid consumption for treatment with clarification enhancement above 80%.
According to the above, the best treatment alternatives for WW clarification are given by applying SRS-W with HSO consumption between 1.75 and 4.0 meq H/L, with costs less than 0.16 USD/m. In this range, a maximum net turbidity removal per acid consumed in the WW treatment of 0.090 and a minimum of 0.043 NTUremoved/(meq H/m) is obtained, corresponding to acid consumptions of 2.0 and 3.5 meq H/L, respectively. The RS dose equivalent to this minimum net turbidity removal (20 mL of SRS-W/L activated with 175 meq H/L (Figure 4a)) with a WW treatment cost of 0.14 USD/m was selected for evaluating its performance in removing solids, organic matter, Fe, Al and phosphorus from WW (Section 3.4).
3.3.2. Unmodified WTS: Wet and Dry
The clarification enhancement of WW obtained with different doses of W-WTS and different doses and particle sizes of D-WTS without activation is presented in Figure 8a,b, respectively. Figure 8a shows that as the dosage of W-WTS increases, the clarification enhancement increases, and the maximum improvement (76%) is achieved with the highest dosage (100 mL W-WTS/L). This improvement in WW clarification is more significant than the maximum reported by Nair and Ahammed [8] for the tertiary treatment of urban WW (upflow anaerobic sludge blanket (UASB) effluent) at natural pH with unmodified aluminium WTS (turbidity removal of 50%). With the application of D-WTS at different particle sizes, a contribution of turbidity was evidenced in the WW, generating negative values of clarification enhancement with all the doses evaluated. As the D-WTS dose is increased, an increase in WW turbidity is obtained.
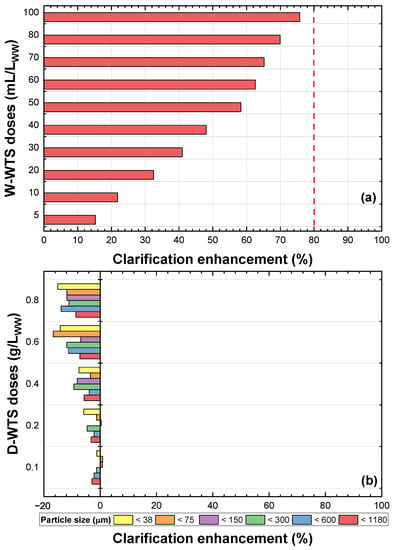
Figure 8.
Inactive WTS: (a) wet and (b) dry.
When contrasting the results obtained from the WTS activated with HSO and HCl (Figure 4 and Figure 5) and unmodified WTS (Figure 8), the positive effect on the clarification enhancement of the addition of acids to the WTS is evident. This is because the acids solubilise the aluminium and iron species present in the WTS (as shown in Table 2, Figure 2 and Figure 3), making them available to act as coagulants in the primary treatment of the WW [15,16].
It is important to note that low doses of SRS-W (10–20 mL of SRS-W/L) are required to obtain high clarification enhancement (>80%) compared to the high doses of unmodified sludge (e.g., 100 mL of W-WTS/L) required to achieve maximum clarification enhancement (76%). This implies lower residual sludge generated in the primary WW treatment when SRS-W is used. The dose of 100 mL W-WTS/L was selected as the best treatment for the unmodified sludge and was evaluated to remove solids, organic matter, Fe, Al and phosphorus from WW (Section 3.4).
3.4. Optimal Treatments Evaluation
Table 3 presents the physicochemical characteristics of the raw WW samples, the control sample, the effluent from the W-WTS (100 mL/L) and the effluent from the SRS-W (20 mL of SRS-W/L, activated with 175 meq H/L) treatments, previously selected in Section 3.3.

Table 3.
Results of the evaluation of selected treatments.
The raw WW presented a tCOD of 614 mg O/L, of which 59% corresponds to the suspended fraction (pCOD) and 41% to the dissolved fraction (sCOD). The biodegradable percentage of the tCOD presented by the tBOD was 63%. All these concentrations of the variables representing organic matter, and the relationships between them, are in the typical range of medium concentration municipal WW, which is characteristic of populations with low water consumption and significant stormwater inputs into the sewage system [27]. The TSS reported for raw WW was 377 mg/L, of which 72% corresponded to a volatile fraction (VSS). As for phosphorus, most of this nutrient was found in the water in its soluble form (61%). These concentrations of solids, phosphorus, pH, and EC reported in the raw WW are representative of the medium concentration municipal WW.
Figure 9, Figure 10 and Figure 11 show the removals of solids, Al, Fe, organic matter and phosphorus species in WW, obtained with the natural sedimentation process (control) and the W-WTS and SRS-W treatments, calculated from the data in Table 3.
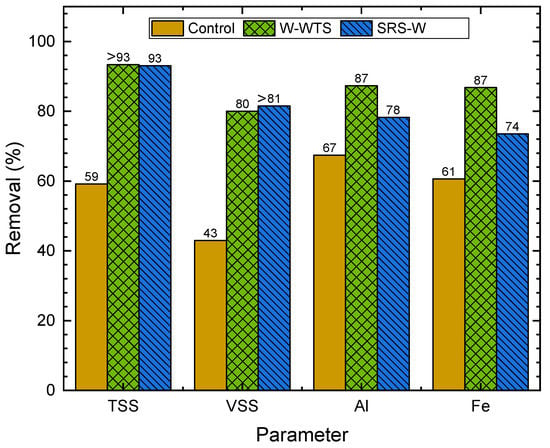
Figure 9.
Removal of solids, Fe and Al.
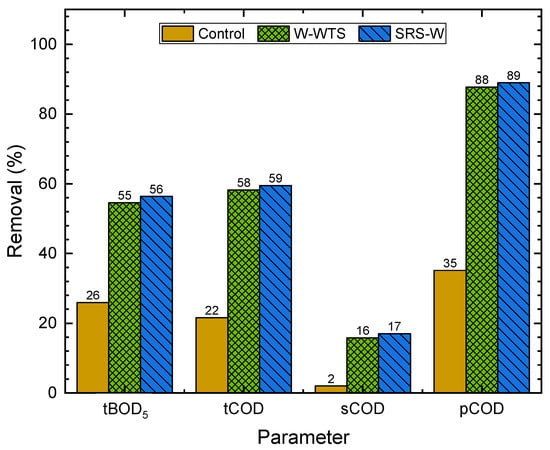
Figure 10.
Removal of organic matter parameters.
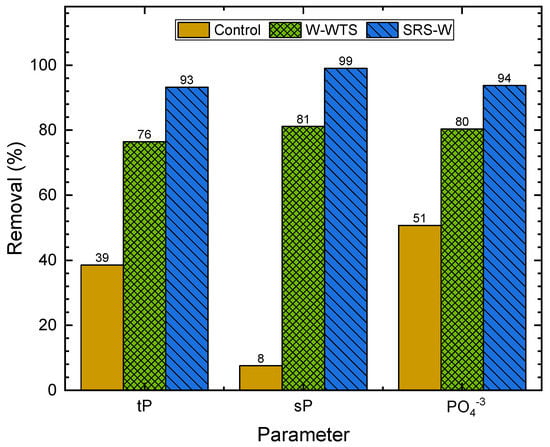
Figure 11.
Removal of phosphorus species.
Figure 9 presents the results of the percentage removal of TSS, VSS, Al and Fe from WW. Natural sedimentation significantly removes TSS (59%), which increases considerably to values greater than 93% when treatments are applied. The volatile fraction of suspended solids (VSS) shows similar behaviour to TSS, with removals of 43% for the control and greater than or equal to 80% for both treatments. With natural sedimentation and the two treatments evaluated, similar concentrations of settleable solids (SedS < 1.0 mL/L, Table 3) were achieved.
Similarly, high removals of Al (67%) and Fe (61%) are reported in the control sample due to the natural sedimentation of particles containing these elements. Although, with the W-WTS and SRS-W treatments, these metals are added, which are part of the minerals (kaolinite 1A, gibbsite, dickite and albite high) and the amorphs found in the WTS chemical characterisation, and increases in Al and Fe removals are observed. The dissolved fraction of both metals present in the W-WTS can act as coagulants forming flocs that are subsequently removed by sedimentation. With the addition of HSO in the activation of the WTS (SRS-W), the concentrations of Al and Fe available for coagulation are increased by dissolution [15]. However, the removals achieved with SRS-W for Al and Fe (78 and 74%, respectively) were higher than those obtained with the control. In the W-WTS treatment, removals of 87% were obtained for both metals. The coagulation and flocculation processes are responsible for the high solid removals achieved with these sludges and previously reported.
Figure 10 shows that, respectively, with natural sedimentation, tBOD, tCOD and pCOD removals of 26, 22 and 35% are achieved. With both treatments evaluated, a similar and significant increase in organic matter removal is achieved for natural sedimentation, reaching removals of over 55% for tBOD and tCOD. The effect of the treatments on the removal of the suspended fraction of organic matter was more significant, achieving removals close to 90% in the pCOD, which are consistent with the high removals obtained for VSS (Figure 9).
As expected, the dissolved fraction of organic matter (sCOD) was not removed in the natural sedimentation; however, removals close to 17% were achieved for this variable with the treatments. These removals can be associated with the adsorption process of the soluble organic matter on the surface of the flocs generated during coagulation and flocculation because of the addition of Al and Fe present in the W-WTS and SRS-W [12,28].
The removal of phosphorus species achieved is shown in Figure 11. With natural sedimentation, removal of 39% of tP, 8% of sP and 51% of PO is achieved. The removal of these three variables increased to at least 76% when using the W-WTS by sweeping and adsorption to the flocs formed with the Al and Fe present in the WTS [6] (Table 1). Maximum phosphorus removals (>93%) are achieved using SRS-W due to the solubilisation of Fe and Al in the WTS activation, which contributes to the coagulation process [20]. High removals of the sP (81% for W-WTS and 99% for SRS-W) and PO (80% for W-WTS and 94% for SRS-W) achieved with the evaluated treatments were favoured by the additional mechanism of scavenging or chemical precipitation [6,29].
The increase in the electrical conductivity of the raw WW obtained with the SRS-W treatment (from 948 to 1281 S/cm, Table 3) is related to the solubilisation of solids occurring in the activation process. On the contrary, the SRS-W treatment generated a decrease in the pH of the raw WW (from 7.47 to 6.59, Table 3) due to the acidic condition of SRS-W reached in the activation process (pH = 2.41).
Finally, the W-WTS treatment results in a higher amount of primary sewage sludge compared to the SRS-W treatment because the SRS-W treatment requires five times fewer sludge doses to obtain similar removal results. However, the advantage of the W-WTS alternative is that it does not require the use of chemicals and the activation process.
4. Conclusions
The water treatment sludge evaluated in the primary treatment of domestic WW was effective in clarification enhancement compared to natural sedimentation. The clarification enhancement achieved with the addition of raw sludge (W-WTS) and reactive sludges (RSs) is due to the contribution of aluminium and iron in both sludges, which act as coagulants favouring the agglomeration of particles and their subsequent removal by sedimentation.
The activating process of dry and wet sludge with HSO generated reactive sludge that allowed high clarification enhancement with low acid consumptions compared to HCl-activated sludge. Additionally, the more considerable acquisition cost (2.1 times) of HCl with respect to HS and the drying and size reduction processes necessary for the use of dry sludge make sulphuric reactive sludge-wet (SRS-W) the best technical-economic alternative for the use of reactive sludge in the clarification enhancement of domestic WW.
In addition to the improvement in solids removal (TSS, VSS and SSed), the application of W-WTS and SRS-W considerably improved the primary treatment of domestic WW, with a significant increase in the removal of organic matter, represented by the variables tBOD and COD, in its different fractions. Similarly, phosphorus removal was increased, with the most significant impact on its soluble fraction (sP).
The dosage of W-WTS (100 mL/L) required to achieve the positive effect on WW treatment was higher than that required for SRS-W (20 mL/L), which implies a lower production of primary sewage sludge in the latter alternative. However, it is essential to note that the use of W-WTS is a simple application that does not require the previous stages of chemical activation, which entail additional costs in infrastructure and operation.
The application of unmodified sludge (W-WTS) and sulphuric reactive sludge-wet (SRS-W) in the primary treatment of WW is a valuable circular economy proposal, which simultaneously valorises waste from the drinking water process and contributes to the fulfilment of SDG 6 (Clean Water and Sanitation) associated with the reduction of pollution in WW discharges, mainly in developing countries that have high deficiencies in their WW treatment systems.
Author Contributions
Conceptualization, C.C.C.-J., J.C.S.-M., E.F.G. and M.A.C.-O.; methodology, C.C.C.-J., J.C.S.-M., E.F.G. and M.A.C.-O.; software, C.C.C.-J. and E.F.G.; formal analysis, C.C.C.-J., J.C.S.-M. and E.F.G.; writing—original draft preparation, C.C.C.-J. and E.F.G.; writing—review and editing, C.C.C.-J. and E.F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by Universidad de Antioquia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are within the submitted manuscript.
Acknowledgments
The authors would like to thank the DWTP and WWTP of the municipality of El Santuario—Antioquia, Colombia, for facilitating the collection of samples for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- UNESCO; UN-Water. The United Nations World Water Development Report 2017. Wastewater: The Untapped Resource. 2017. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000247153 (accessed on 20 May 2022).
- UNESCO; UN-Water. The United Nations World Water Development Report 2020: Water and Climate Change. 2020. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000372985.locale=en (accessed on 20 May 2022).
- WHO; UN-Habitat. Progress on Safe Treatment and Use of Wastewater. Piloting the Monitoring Methodology and Initial Findings for SDG Indicator 6.3.1. 2018. Available online: http://www.unwater.org/app/uploads/2018/08/631-progress-on-wastewater-treatment-2018.pdf (accessed on 23 May 2022).
- United Nations. Sustainable Development Goals. 2018. Available online: https://www.un.org/sustainabledevelopment/es/wp-content/uploads/sites/3/2016/10/6_Spanish_Why_it_Matters.pdf (accessed on 3 June 2022).
- Shewa, W.A.; Dagnew, M. Revisiting chemically enhanced primary treatment of wastewater: A review. Sustainability 2020, 12, 5928. [Google Scholar] [CrossRef]
- Marguti, A.L.; Ferreira, S.S.; Piveli, R.P. Full-scale effects of addition of sludge from water treatment stations into processes of sewage treatment by conventional activated sludge. J. Environ. Manag. 2018, 215, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Mazari, L.; Abdessemed, D.; Szymczyk, A. Evaluating Reuse of Alum Sludge as Coagulant for Tertiary Wastewater Treatment. J. Environ. Eng. 2018, 144, 04018119. [Google Scholar] [CrossRef]
- Nair, A.T.; Ahammed, M.M. The reuse of water treatment sludge as a coagulant for post-treatment of UASB reactor treating urban wastewater. J. Clean. Prod. 2015, 96, 272–281. [Google Scholar] [CrossRef]
- Rebosura, M.; Salehin, S.; Pikaar, I.; Kulandaivelu, J.; Jiang, G.; Keller, J.; Sharma, K.; Yuan, Z. Effects of in-sewer dosing of iron-rich drinking water sludge on wastewater collection and treatment systems. Water Res. 2020, 171, 115396. [Google Scholar] [CrossRef]
- Rebosura, M.; Salehin, S.; Pikaar, I.; Keller, J.; Sharma, K.; Yuan, Z. The impact of primary sedimentation on the use of iron-rich drinking water sludge on the urban wastewater system. J. Hazard Mater. 2021, 402, 124051. [Google Scholar] [CrossRef]
- Kulandaivelu, J.; Choi, P.M.; Shrestha, S.; Li, X.; Song, Y.; Li, J.; Sharma, K.; Yuan, Z.; Mueller, J.F.; Wang, C.; et al. Assessing the removal of organic micropollutants from wastewater by discharging drinking water sludge to sewers. Water Res. 2020, 181, 115945. [Google Scholar] [CrossRef]
- Shrestha, S.; Kulandaivelu, J.; Sharma, K.; Jiang, G.; Yuan, Z. Effects of dosing iron- and alum-containing waterworks sludge on sulfide and phosphate removal in a pilot sewer. Chem. Eng. J. 2020, 387, 124073. [Google Scholar] [CrossRef]
- Kang, C.; Zhao, Y.; Tang, C.; Addo-Bankas, O. Use of aluminum-based water treatment sludge as coagulant for animal farm wastewater treatment. J. Water Proc. Eng. 2022, 46, 102645. [Google Scholar] [CrossRef]
- de Oliveira Andrade, J.J.; Wenzel, M.C.; Rocha, G.H.; Silva, S.R. Performance of rendering mortars containing sludge from water treatment plants as fine recycled aggregate. J. Clean. Prod. 2018, 196, 159–168. [Google Scholar] [CrossRef]
- Suman, A.; Ahmad, T.; Ahmad, K. Dairy wastewater treatment using water treatment sludge as coagulant: A novel treatment approach. J. Environ. Dev. Sustain. 2018, 20, 1615–1625. [Google Scholar] [CrossRef]
- Keeley, J.; Jarvis, P.; Judd, S.J. Coagulant recovery from water treatment residuals: A review of applicable technologies. J. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2675–2719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayoub, M.; Afify, H.; Abdelfattah, A. Chemically enhanced primary treatment of sewage using the recovered alum from water treatment sludge in a model of hydraulic clariflocculator. J. Water Proc. Eng. 2017, 19, 133–138. [Google Scholar] [CrossRef]
- Ishikawa, S.; Ueda, N.; Okumura, Y.; Iida, Y.; Baba, K. Recovery of coagulant from water supply plant sludge and its effect on clarification. J. Mater. Cycles Waste Manag. 2007, 9, 167–172. [Google Scholar] [CrossRef]
- Xu, G.R.; Yan, Z.C.; Wang, Y.C.; Wang, N. Recycle of Alum recovered from water treatment sludge in chemically enhanced primary treatment. J. Hazard Mater. 2009, 161, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Ahmad, K.; Ahad, A.; Alam, M. Characterization of water treatment sludge and its reuse as coagulant. J. Environ. Manag. 2016, 182, 606–611. [Google Scholar] [CrossRef]
- Dias, R.; Sousa, D.; Bernardo, M.; Matos, I.; Fonseca, I.; Cardoso, V.V.; Carneiro, R.N.; Silva, S.; Fontes, P.; Daam, M.A.; et al. Study of the potential of water treatment sludges in the removal of emerging pollutants. Molecules 2021, 26, 1010. [Google Scholar] [CrossRef] [PubMed]
- IWA. Wastewater Report 2018: The Reuse Opportunity. Available online: https://www.iwa-network.org/wp-content/uploads/2018/02/OFID-Wastewater-report-2018.pdf (accessed on 24 May 2022).
- APHA; AWWA; WFE. Standard Methods for Examination of Water and Wastewater, 23th ed.; AWWA Government Affairs Office: Washington, DC, USA, 2017. [Google Scholar]
- Santos, G.Z.B.; Melo Filho, J.A.; Manzato, L. Proposta de uma cerâmica obtida por meio de geopolimerizaç~ao de lodo de ETA calcinado. Cerâmica 2018, 64, 276–283. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhao, Y.Q.; Babatunde, A.O.; Wang, L.; Ren, Y.X.; Han, Y. Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Separ. Purif. Technol. 2006, 51, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Santos, G.Z.B.; de Almeida Melo Filho, J.; Manzato, L. Perspectivas de aplicações tecnológicas de lodo gerado no processo de tratamento de água dos rios Negro e Solimões. Materia 2018, 23, e-12167. [Google Scholar] [CrossRef]
- Lopez-Vásquez, C.M.; Buitrón-Mendez, G.; García, H.A.; Cervantes-Carrillo, F.J. Tratamiento Biológico de Aguas Residuales: Principios, Modelación y Diseño, 1st ed.; IWA Publishing: London, UK, 2017. [Google Scholar]
- Chakraborty, T.; Gabriel, M.; Amiri, A.S.; Santoro, D.; Walton, J.; Smith, S.; Ray, M.B.; Nakhla, G. Carbon and phosphorus removal from primary municipal wastewater using recovered aluminum. Environ. Sci. Technol. 2017, 51, 12302–12309. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, E. Wastewater Engineering: Treatment and Reuse, 5th ed.; McGraw-Hill: New York, NY, USA, 2014. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).