The Impact of Industry 4.0 on the Medical Device Regulatory Product Life Cycle Compliance
Abstract
1. Introduction
2. Literature Review
2.1. What Is Regulatory Compliance in the MedTech Industry?
2.2. Industry 4.0 Opportunities and Challenges
3. Research Methodology
The Interview Process
4. Results
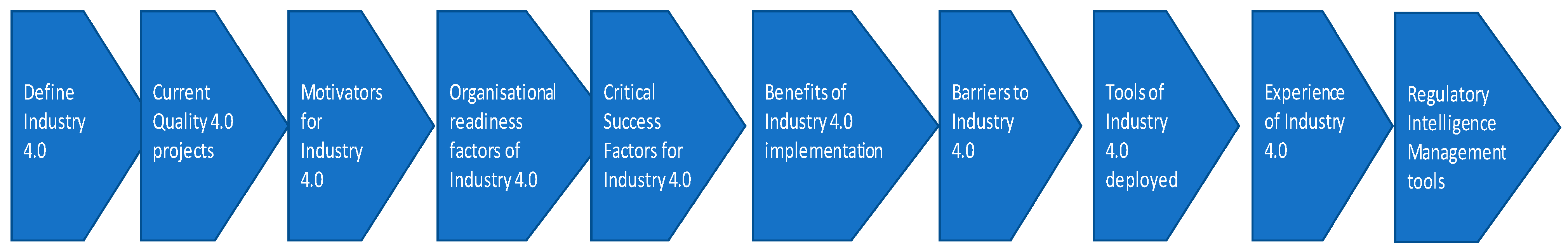
- Q1.
- How would you explain Industry 4.0 in layman’s terms?
- Q2.
- Do you currently have any Industry 4.0 type projects or initiatives within the organisation/department?
- Q3.
- What are the motivating factors for the adoption of Industry 4.0 within the organisation department/function?
- Q4.
- What organisational readiness factors would need to be considered for you to embrace and adopt Industry 4.0 within the organisation/department?
- Q5.
- What are the Critical Success Factors for the implementation of Industry 4.0? (present or future)
- Q6.
- What do you see are the benefits and opportunities of implementing Industry 4.0?
- Q7.
- What are the challenges or barriers to adopting Industry 4.0 within the organisation/department?
- Q8.
- What tools of Industry 4.0 (e.g., cybersecurity, CPS, cloud computing, mobile technologies, machine to machine, 3D printing, also referred to as additive manufacturing, advanced robotics, big data/analytics, Internet of Things (IoT), RFID technologies, and cognitive computing, AI etc.) do you think might help the organisation/department?
- Q9.
- Has the implementation of Industry 4.0 positively or negatively impacted regulatory compliance within the organisation?
- Q10.
- Are you planning on implementing any Regulatory Intelligence tools or RIMs? If so, why do you need to do this, and what benefits can be achieved from this implementation? What might the potential benefits be from implementing such a system within the organisation?
5. Discussion
5.1. Theme 1: Industry 4.0–Understanding of the Concept
5.2. Theme 2: Industry 4.0–State of Implementation
5.3. Theme 3: Industry 4.0–Reasons for Implementation
5.4. Theme 4: Industry 4.0–Benefits and Opportunities
5.5. Theme 5: Industry 4.0–Challenges and Barriers
5.6. Theme 6: Industry 4.0–Impact on Regulatory Compliance
5.7. Recommendations from the Research
5.8. Limitations of the Study & Future Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhamra, R.; Hicks, C.; Small, A.; García-Villarreal, E. Value, product delivery strategies and operational performance in the medical technology industry. Int. J. Prod. Econ. 2022, 245, 108399. [Google Scholar] [CrossRef]
- Groumpos, P.P. A Critical Historical and Scientific Overview of all Industrial Revolutions. IFAC-PapersOnLine 2021, 54, 464–471. [Google Scholar] [CrossRef]
- Iyamu, I.; Xu, A.X.T.; Gómez-Ramírez, O.; Ablona, A.; Chang, H.-J.; Mckee, G.; Gilbert, M. Defining Digital Public Health and the Role of Digitization, Digitalization, and Digital Transformation: Scoping Review. JMIR Public Health Surveill. 2021, 7, e30399. [Google Scholar] [CrossRef]
- Sony, M.; Antony, J.; Mc Dermott, O.; Garza-Reyes, J.A. An empirical examination of benefits, challenges, and critical success factors of industry 4.0 in manufacturing and service sector. Technol. Soc. 2021, 67, 101754. [Google Scholar] [CrossRef]
- Antony, J.; McDermott, O.; Sony, M. Quality 4.0 conceptualisation and theoretical understanding: A global exploratory qualitative study. TQM J. 2021. [Google Scholar] [CrossRef]
- Bianchini, E.; Francesconi, M.; Testa, M.; Tanase, M.; Gemignani, V. Unique device identification and traceability for medical software: A major challenge for manufacturers in an ever-evolving marketplace. J. Biomed. Inform. 2019, 93, 103150. [Google Scholar] [CrossRef]
- Gerke, S.; Babic, B.; Evgeniou, T.; Cohen, I.G. The need for a system view to regulate artificial intelligence/machine learning-based software as medical device. NPJ Digit. Med. 2020, 3, 53. [Google Scholar] [CrossRef]
- Hassan, S.; Dhali, M.; Zaman, F.; Tanveer, M. Big data and predictive analytics in healthcare in Bangladesh: Regulatory challenges. Heliyon 2021, 7, e07179. [Google Scholar] [CrossRef]
- Varghese, J. Artificial Intelligence in Medicine: Chances and Challenges for Wide Clinical Adoption. Visc. Med. 2020, 36, 443–449. [Google Scholar] [CrossRef]
- Popov, V.V.; Kudryavtseva, E.V.; Katiyar, N.K.; Shishkin, A.; Stepanov, S.I.; Goel, S. Industry 4.0 and Digitalisation in Healthcare. Materials 2022, 15, 2140. [Google Scholar] [CrossRef]
- Duan, L.; Da Xu, L. Data Analytics in Industry 4.0: A Survey. Inf. Syst. Front. 2021, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Maci, J.; Marešová, P. Critical Factors and Economic Methods for Regulatory Impact Assessment in the Medical Device Industry. Risk Manag. Healthc. Policy 2022, 15, 71–91. [Google Scholar] [CrossRef]
- Maganga, D.P.; Taifa, I.W. Quality 4.0 transition framework for Tanzanian manufacturing industries. TQM J. 2022. [Google Scholar] [CrossRef]
- Fonseca, L.; Amaral, A.; Oliveira, J. Quality 4.0: The EFQM 2020 Model and Industry 4.0 Relationships and Implications. Sustainability 2021, 13, 3107. [Google Scholar] [CrossRef]
- Institute of Medicine (US); Committee on Technological Innovation in Medicine. Sources of Medical Technology: Universities and Industry; Rosenberg, N., Gelijns, A.C., Dawkins, H., Eds.; National Academies Press: Cambridge, MA, USA, 1995. Available online: https://www.ncbi.nlm.nih.gov/books/NBK232043/ (accessed on 2 October 2022).
- World Health Organization. Medical Device Regulations: Global Overview and Guiding Principles; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Bolislis, W.R.; de Lucia, M.L.; Dolz, F.; Mo, R.; Nagaoka, M.; Rodriguez, H.; Woon, M.L.; Yu, W.; Kühler, T.C. Regulatory Agilities in the Time of COVID-19: Overview, Trends, and Opportunities. Clin. Ther. 2021, 43, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Melvin, T.; Torre, M. New medical device regulations: The regulator’s view. EFORT Open Rev. 2019, 4, 351–356. [Google Scholar] [CrossRef]
- Coughlan, J.; Shah, S. The impact of Brexit on oral health. Br. Dent. J. 2020, 229, 622–626. [Google Scholar] [CrossRef] [PubMed]
- van der Walt, A.; Butzkueven, H.; Shin, R.K.; Midaglia, L.; Capezzuto, L.; Lindemann, M.; Davies, G.; Butler, L.M.; Costantino, C.; Montalban, X. Developing a Digital Solution for Remote Assessment in Multiple Sclerosis: From Concept to Software as a Medical Device. Brain Sci. 2021, 11, 1247. [Google Scholar] [CrossRef]
- Pesapane, F.; Volonté, C.; Codari, M.; Sardanelli, F. Artificial intelligence as a medical device in radiology: Ethical and regulatory issues in Europe and the United States. Insights Imaging 2018, 9, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Woodward, A. Cybersecurity vulnerabilities in medical devices: A complex environment and multifaceted problem. Med Devices Évid. Res. 2015, 8, 305–316. [Google Scholar] [CrossRef]
- Arden, N.S.; Fisher, A.C.; Tyner, K.; Yu, L.X.; Lee, S.L.; Kopcha, M. Industry 4.0 for pharmaceutical manufacturing: Preparing for the smart factories of the future. Int. J. Pharm. 2021, 602, 120554. [Google Scholar] [CrossRef] [PubMed]
- Reedy, S. A Pulse on Quality 4.0 for Medical Device Manufacturing. Quality 2019, 58, 34–36. [Google Scholar]
- Antony, J.; Sony, M.; McDermott, O.; Jayaraman, R.; Flynn, D. An exploration of organisational readiness factors for Quality 4.0: An intercontinental study and future research directions. Int. J. Qual. Reliab. Manag. 2021. [Google Scholar] [CrossRef]
- Veeva.com. Modernizing Regulatory Affairs: Industry Benchmark Study Findings. 2022. Available online: https://www.veeva.com/medtech/resources/modernizing-regulatory-affairs-veeva-medtech-regulatory-benchmark-study/ (accessed on 20 October 2022).
- Nick, G.; Kovács, T.; Kő, A.; Kádár, B. Industry 4.0 readiness in manufacturing: Company Compass 2.0, a renewed framework and solution for Industry 4.0 maturity assessment. Procedia Manuf. 2021, 54, 39–44. [Google Scholar] [CrossRef]
- Kazlovich, K.; Mishra, S.R.; Behdinan, K.; Gladman, A.; May, J.; Mashari, A. Open ventilator evaluation framework: A synstudyed database of regulatory requirements and technical standards for emergency use ventilators from Australia, Canada, UK, and US. HardwareX 2022, 11, e00260. [Google Scholar] [CrossRef]
- Kwon, J.; Johnson, M.E. Security practices and regulatory compliance in the healthcare industry. J. Am. Med. Inform. Assoc. 2013, 20, 44–51. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R. Leveraging blockchain for ensuring trust in IoT: A survey. J. King Saud Univ. Comput. Inf. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Morrison, R.J.; Kashlan, K.N.; Flanangan, C.L.; Wright, J.K.; Green, G.E.; Hollister, S.J.; Weatherwax, K.J. Regulatory Considerations in the Design and Manufacturing of Implantable 3D-Printed Medical Devices. Clin. Transl. Sci. 2015, 8, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Jandoo, T. WHO guidance for digital health: What it means for researchers. Digit. Health 2020, 6. [Google Scholar] [CrossRef]
- Coldwell, D.A.L. Negative Influences of the 4th Industrial Revolution on the Workplace: Towards a Theoretical Model of Entropic Citizen Behavior in Toxic Organisations. Int. J. Environ. Res. Public Health 2019, 16, 2670. [Google Scholar] [CrossRef]
- Tase, A.; Ni, M.Z.; Buckle, P.W.; Hanna, G.B. Current status of medical device malfunction reporting: Using end user experience to identify current problems. BMJ Open Qual. 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Kostkova, P.; Brewer, H.; De Lusignan, S.; Fottrell, E.; Goldacre, B.; Hart, G.; Koczan, P.; Knight, P.; Marsolier, C.; McKendry, R.; et al. Who Owns the Data? Open Data for Healthcare. Front. Public Health 2016, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Kruse, C.S.; Smith, B.; Vanderlinden, H.; Nealand, A. Security Techniques for the Electronic Health Records. J. Med. Syst. 2017, 41, 127. [Google Scholar] [CrossRef]
- Hasselgren, A.; Kralevska, K.; Gligoroski, D.; Pedersen, S.A.; Faxvaag, A. Blockchain in healthcare and health sciences—A scoping review. Int. J. Med. Inform. 2020, 134, 104040. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, C.; Drummond, M. Improving Medical Device Regulation: The United States and Europe in Perspective. Milbank Q. 2014, 92, 114–150. [Google Scholar] [CrossRef] [PubMed]
- Malvehy, J.; Ginsberg, R.; Sampietro-Colom, L.; Ficapal, J.; Combalia, M.; Svedenhag, P. New regulation of medical devices in the EU: Impact in dermatology. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 360–364. [Google Scholar] [CrossRef]
- Niemiec, E. Will the EU Medical Device Regulation help to improve the safety and performance of medical AI devices? Digit. Health 2022, 8, 20552076221089079. [Google Scholar] [CrossRef]
- Shojaeinasab, A.; Charter, T.; Jalayer, M.; Khadivi, M.; Ogunfowora, O.; Raiyani, N.; Yaghoubi, M.; Najjaran, H. Intelligent manufacturing execution systems: A systematic review. J. Manuf. Syst. 2022, 62, 503–522. [Google Scholar] [CrossRef]
- Ramírez-Durán, V.J.; Berges, I.; Illarramendi, A. Towards the implementation of Industry 4.0: A methodology-based approach oriented to the customer life cycle. Comput. Ind. 2021, 126, 103403. [Google Scholar] [CrossRef]
- Antony, J.; McDermott, O.; Powell, D.J.; Sony, M. Mapping the Terrain for Lean Six Sigma 4.0. In Learning in the Digital Era; Springer: Cham, Switzerland, 2021; pp. 193–204. [Google Scholar] [CrossRef]
- Antony, J.; McDermott, O.; Powell, D.; Sony, M. The evolution and future of lean Six Sigma 4.0. TQM J. 2022. [Google Scholar] [CrossRef]
- Peng, Y.; Tao, C. Can digital transformation promote enterprise performance? —From the perspective of public policy and innovation. J. Innov. Knowl. 2022, 7, 100198. [Google Scholar] [CrossRef]
- Van Veldhoven, Z.; Vanthienen, J. Digital transformation as an interaction-driven perspective between business, society, and technology. Electron. Mark. 2021, 32, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Denscombe, M. The Good Research Guide: For Small-Scale Social Research Projects; McGraw-Hill Education: London, UK, 2014. [Google Scholar]
- Moser, A.; Korstjens, I. Series: Practical guidance to qualitative research. Part 1: Introduction. Eur. J. Gen. Pract. 2017, 23, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Sefcik, J.S.; Bradway, C. Characteristics of Qualitative Descriptive Studies: A Systematic Review. Res. Nurs. Health 2017, 40, 23–42. [Google Scholar] [CrossRef]
- Salmons, J. Cases in Online Interview Research; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2012. [Google Scholar] [CrossRef]
- Sah, L.; Singh, D.R.; Sah, R.K. Conducting Qualitative Interviews using Virtual Communication Tools amid COVID-19 Pandemic: A Learning Opportunity for Future Research. J. Nepal Med. Assoc. 2020, 58, 1103–1106. [Google Scholar] [CrossRef]
- Andrade, C. The Inconvenient Truth About Convenience and Purposive Samples. Indian J. Psychol. Med. 2021, 43, 86–88. [Google Scholar] [CrossRef]
- Guest, G.; Bunce, A.; Johnson, L. How Many Interviews Are Enough? An Experiment with Data Saturation and Variability. Field Methods 2006, 18, 59–82. [Google Scholar] [CrossRef]
- Crowe, S.; Cresswell, K.; Robertson, A.; Huby, G.; Avery, A.; Sheikh, A. The case study approach. BMC Med. Res. Methodol. 2011, 11, 100. [Google Scholar] [CrossRef]
- Cascio, M.A.; Lee, E.; Vaudrin, N.; Freedman, D.A. A Team-based Approach to Open Coding: Considerations for Creating Intercoder Consensus. Field Methods 2019, 31, 116–130. [Google Scholar] [CrossRef]
- Jørgensen, U. Grounded Theory: Methodology and Theory Construction. Int. Encycl. Soc. Behav. Sci. 2001, 1, 6396–6399. [Google Scholar]
- Charmaz, K. Constructing Grounded Theory; Sage: New York, NY, USA, 2014; ISBN 1-4462-9722-5. [Google Scholar]
- Parameswaran, U.D.; Ozawa-Kirk, J.L.; Latendresse, G. To live (code) or to not: A new method for coding in qualitative research. Qual. Soc. Work 2020, 19, 630–644. [Google Scholar] [CrossRef]
- CDRH. Software as a Medical Device (SaMD). FDA. Available online: https://www.fda.gov/medical-devices/digital-health-center-excellence/software-medical-device-samd (accessed on 20 September 2022).
- Frère, E.; Zureck, A.; Röhrig, K. Industry 4.0 in Germany—The Obstacles Regarding Smart Production in the Manufacturing Industry. SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
- Martínez-Gutiérrez, A.; Díez-González, J.; Ferrero-Guillén, R.; Verde, P.; Álvarez, R.; Perez, H. Digital Twin for Automatic Transportation in Industry 4.0. Sensors 2021, 21, 3344. [Google Scholar] [CrossRef] [PubMed]
- Rojko, A. Industry 4.0 Concept: Background and Overview. Int. J. Interact. Mob. Technol. 2017, 11, 77–90. [Google Scholar] [CrossRef]
- Najwa, E.; Bertrand, R.; Yassine, M.; Fernandes, G.; Abdeen, M.; Souad, S. Lean 4.0 tools and technologies to improve companies’ maturity level: The COVID-19 context. Procedia Comput. Sci. 2022, 196, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.; Sony, M.; McDermott, O. Conceptualizing Industry 4.0 readiness model dimensions: An exploratory sequential mixed-method study. TQM J. 2021. [Google Scholar] [CrossRef]
- Sony, M.; Aithal, P.S. Transforming Indian Engineering Industries through Industry 4.0: An Integrative Conceptual Analysis. Int. J. Appl. Eng. Manag. Lett. 2020, 4, 111–123. [Google Scholar] [CrossRef]
- Angelopoulos, A.; Michailidis, E.T.; Nomikos, N.; Trakadas, P.; Hatziefremidis, A.; Voliotis, S.; Zahariadis, T. Tackling Faults in the Industry 4.0 Era-A Survey of Machine- Learning Solutions and Key Aspects. Sensors 2019, 20, 109. [Google Scholar] [CrossRef]
- Huang, Z.; Shen, Y.; Li, J.; Fey, M.; Brecher, C. A Survey on AI-Driven Digital Twins in Industry 4.0: Smart Manufacturing and Advanced Robotics. Sensors 2021, 21, 6340. [Google Scholar] [CrossRef]
- Baethge-Kinsky, V. Digitized Industrial Work: Requirements, Opportunities, and Problems of Competence Development. Front. Sociol. 2020, 5, 33. [Google Scholar] [CrossRef]
- Sony, M. Implementing sustainable operational excellence in organizations: An integrative viewpoint. Prod. Manuf. Res. 2019, 7, 67–87. [Google Scholar] [CrossRef]
- Peralta, G.; Garrido, P.; Bilbao, J.; Agüero, R.; Crespo, P.M. On the Combination of Multi-Cloud and Network Coding for Cost-Efficient Storage in Industrial Applications. Sensors 2019, 19, 1673. [Google Scholar] [CrossRef] [PubMed]
- Antony, J.; Sony, M.; McDermott, O.; Furterer, S.; Pepper, M. How does performance vary between early and late adopters of Industry 4.0? A qualitative viewpoint. Int. J. Qual. Reliab. Manag. 2021. [Google Scholar] [CrossRef]
- Avis, J. Socio-technical imaginary of the fourth industrial revolution and its implications for vocational education and training: A literature review. J. Vocat. Educ. Train. 2018, 70, 337–363. [Google Scholar] [CrossRef]
- Sony, M.; Naik, S. Key ingredients for evaluating Industry 4.0 readiness for organizations: A literature review. Benchmarking Int. J. 2019, 27, 2213–2232. [Google Scholar] [CrossRef]
- Babatunde, O.K. Mapping the implications and competencies for Industry 4.0 to hard and soft total quality management. TQM J. 2020, 33, 896–914. [Google Scholar] [CrossRef]
- Watson, G. The Ascent of Quality 4.0. ASQ. Asq.Org. 2019. Available online: https://asq.org/quality-progress/articles/the-ascent-of-quality-40?id=8321f828c7c44634b996b2b1ba25a315 (accessed on 2 October 2022).
- Carpintero, A.; Foster, T.; Makarova, E.; Telpis, V. Reimagining Smart Quality Approach|McKinsey. Available online: https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/smart-quality-reimagining-the-way-quality-works (accessed on 23 June 2021).
- Radziwill, N.M. Let’s Get Digital|ASQ. Available online: https://asq.org/quality-progress/articles/lets-get-digital?id=526b64168f1f4f2c80648300336bad1a (accessed on 24 June 2021).
- Park, S.H.; Shin, W.S.; Park, Y.H.; Lee, Y. Building a new culture for quality management in the era of the Fourth Industrial Revolution. Total Qual. Manag. Bus. Excel. 2017, 28, 934–945. [Google Scholar] [CrossRef]
- Wei, B.; Alius, K. Industry 4.0: Analysis of the Implementation of Industry 4.0 in a Medical Technology Enterprise with a Comparison with Automotive Enterprises and Options for Improvement. Master’s Thesis, Blekinge Institute of Technology, Karlskrona, Sweden, 2021. [Google Scholar]
| No. | Question |
|---|---|
| Q1 | How would you explain Industry 4.0 in layman’s terms? |
| Q2 | Do you currently have any Industry 4.0 type projects or initiatives within the organisation/department/function? |
| Q3 | What are the motivating factors for adopting Industry 4.0 within the organisation/department? |
| Q4 | What organisational readiness factors would need to be considered for you to embrace and adopt Industry 4.0 within the organisation/department? |
| Q5 | What are the Critical Success Factors for the implementation of Industry 4.0? (present or future) |
| Q6 | What do you see are the benefits and opportunities of implementing Industry 4.0? |
| Q7 | What are the challenges or barriers to adopting Industry 4.0 within the organisation/department? |
| Q8 | What tools of Industry 4.0 (e.g., cybersecurity, Cyber physical systems (CPS), cloud computing, mobile technologies, machine to machine, 3D printing, also referred to as additive manufacturing, advanced robotics, big data/analytics, IoT, RFID technologies, and cognitive computing, AI, etc.) do you think might help the organisation/department? |
| Q9 | Has the implementation of Industry 4.0 positively or negatively impacted regulatory compliance within the organisation? |
| Q10 | Are you planning on implementing any Regulatory Intelligence tools or RIMs? If so, why do you need to do this, and what benefits can be achieved from this implementation? What might the potential benefits be from implementing such a system within the organisation? |
| No. | Interviewee | Function | Experience in Leadership Role |
|---|---|---|---|
| 1 | Corporate Vice President (VP) Quality Assurance & Regulatory Affairs | QARA | >10 years |
| 2 | Corporate VP Manufacturing and Supply Chain | Operations | >10 years |
| 3 | Corporate VP of Strategic Projects | Strategic Projects | >10 years |
| 4 | VP of Quality Assurance (QA) (Manufacturing) | QA | >10 years |
| 5 | VP of Regulatory Affairs (RA) | RA | >10 years |
| 6 | VP of Human Resources Global Ops | HR | >10 years |
| 7 | VP Global Quality Engineering (QE) | QE | >10 years |
| 8 | Senior Director of Quality Systems (QS) | QS | >10 years |
| 9 | Senior Director Operations Excellence (OE) | OE | >10 years |
| 10 | Senior Director QA, Global Supplier Quality, Logistics and Distribution (SQLD) | SQLD | >10 years |
| 11 | Senior Director, Global Scientific Affairs | Clinical Medical Affairs | >10 years |
| 12 | Senior Director of Shared Services Europe Middle East & Africa (EMEA) Controller and Site Director | Finance | >10 years |
| 13 | Director, IT–Global Operations & Quality Assurance & Regulatory Assurance (QARA/Global Information Technology (IT) Applications | IT | >10 years |
| “I would probably describe it as the next step in computerization and digitalization, where now you’ve got more direct connectivity between systems. And I suppose it’s probably getting to a lesser human interface between systems, more of a system-to-system connection.” |
| “Really all-around digitalization in terms of the industry, you know, obviously we’ve gone through a number of different phases in the industry over many years. But you know I think 4.0 is really about digitalization and making sure our systems and processes are all connected and from a digital perspective, but it’s more than just, let’s say having your computer systems in place, it’s really about making sure things are connected and in terms of reporting of metrics from a manufacturing perspective that it’s the collection and the reporting of data is all digital. |
| “I think it’s just embracing the whole digital revolution and applying that to the way we go about running our manufacturing plants, our supply chains from a smart factory perspective. So really looking at autonomous systems, the whole Internet of Things as well and AI machine learning. It’s the whole digital revolution that’s taking place and applying that then to how we can do things smarter more efficiently, virtually and in real time.” |
| “You know industry 4.0. I think people’s understanding of this has evolved. It’s a buzzword that’s been around 10 or 15 years for sure. You know from my perspective how it interprets in terms of applications for Company X rather than what its broader meaning is, it’s really how we can best optimize all elements of our manufacturing and supply chain right from an intent, and you’ve heard me use the phrase end-to-end supply chain perspective and Factory 4.0. If you’ve been here a couple of years ago prior to the early days of our command centre. It was Excel files; people were doing data extraction. Nothing was real time. You were depending on someone then doing analysis. Whereas what we’ve now developed in the command centre is a much more real time automated reporting and so on so forth. So that certainly from our Company X perspective, I think that’s our real focus. I’m more into the supply chain rather than the intricacies of manufacturing per se. But when I’m thinking about 4.0 from our perspective, I really think about it more in the context of the control tower, the command centre. |
| “I suppose it is always efficiencies, isn’t it? You are always looking to see if we can do things better, smarter, quicker, and can we reduce errors.”. |
| “I suppose the motivational factor is the quickest, easiest, most reliable way to assess product data and change management; it is also a huge part of that. So again, with us making products that are the same in different locations, making products labelled differently but the same from different locations”. The change management activities to keep changes aligned is critical and trying to do that with a human interface to say, you know Mary Jane has her checklist, so she will remember to check with Mexico when making this change in Europe. It is just not, and we know this–that is not a robust process. So, for me, that is a huge motivational factor.” |
| “I mean, like most things in business, time, money, resources, that is what a lot of it comes down to and the transparency and the visibility to global data”. |
| “I think it is like any program; you know it has to come from the top down, bottom-up discussion. It has to be seen as a strategic intent. It has to be driven as “you know this is what we want to do”, “These are the gains”, “These are the short-term gains”, –”These are the things we are going after”. |
| “I would say definitely willingness. The change is hard and ensuring that we have an understanding within the organisation to understand what we are looking at in terms of infrastructure support. You cannot go digital without IT support without servers, safety measures, capability, and security; it is truly an investment. Human investment and infrastructure and then the ability to maintain it because digital transformation occurs so quickly in the regulatory world, you cannot leave the information static; there is a footprint there forever. So you need to be able to manage, update and obsolete information and have the organisation ready to do that throughout the life cycle of the product development process and having the infrastructure in place to drive it.”. |
| “So you have got to have people strategically looking forward, hiring the right person to educate us and help us get there, you know, it is not an easy journey.”. |
| “Listen, you know as well as anyone that culture is always one of the hardest things to change and getting folks ready for something this massive is always challenging.”. |
| “You know, ultimately; I would say the success is that we have systems that are interconnected speaking to each other, and we can realise the benefits and the efficiencies from the systems that we move to.” |
| “There are a lot of different answers with this, but you know, its success in this situation is really about getting a system implemented that allows us to make decisions in real-time. That is the ultimate goal.”. |
| “It (Industry 4.0) would eliminate the need for us to do all this manually. We are doing a lot of work manually using email, contacting people in the particular location who have to go through the requirements and see if the requirements apply to the particular product group that we want to ship and very often, we experience errors, so I can see a certain reduction in workload and the amount of resources involved in this process and a reduction of errors which are very consequential where we have field safety corrective actions that resulted in shipping product that should not have been shipped to various countries. Moreover, if you automate this process, it will be robust enough to eliminate or reduce the majority of such incidents. This is a huge benefit.” |
| “Faster, quicker, more reliable, more robustness there, and I think, you know, even with things like the regulatory database, you can get more data from different places more quickly. So again, it is efficiency; more information is coming in.” |
| “First and foremost, it is quicker to market, better regulatory compliance, a centralised data system that is managing and hopefully at some point self-learning.” |
| “It has accessible, readily available data faster, quicker, more compliant, transparent. I mean, in the end, it is also cost savings, and in the grand scheme of things, right, I mean from several different areas, you can help prevent repeat root cause recalls, you can react to issues faster and theoretically, you are saving some workforce, right? Anytime you do not have to crunch the data yourself and have a system doing it, which is also one of the challenges that come with it, is what? What do you then do with the people? Because you do not want just to let your entire workforce go. I think it transforms what the workers are doing because someone is got to maintain the system, right?” |
| “right first time improved, reduced, Non-conformances, reduced recalls, and transparency.” |
| “The challenge is you still need somebody sitting there going; what does this mean for us? However, you know, whereas again, a manufacturing system or even XXX, you know it will be data in and data out if the data does not meet the rules, it will go, hello, it does not meet the rules! So somebody needs to fix this, and so again, it depends on the type of system.” |
| “One of the challenges is an investment. Many of these systems do not come cheap, especially when you want to roll it out across a sector of up to 20 sites, including manufacturing sites, offices, etcetera. So, it is investments; there is a big investment needed. It is challenging to state upfront what is going to be the benefit and the payback for this investment, especially when we are talking about QARA because many of our dollars are our headcount.”. |
| “If you are fully digital, digitalised and your production systems or your systems go down, your quality management system, electronic goes down for a week or 10 days or two weeks because of a security attack, then your company pretty much stops, you know? So that is a risk.”. |
| “We have got to pick one. However, moreover, that is just getting a good technical answer before you deal with having to change people and change people’s way of working into that new standard.” |
| “You know, in terms of Industry 4.0 implementation, the biggest challenge you have is people do not know what they want or when to start”. |
| “The biggest challenge we have right now is probably resource constraints, and that is across the board, and it is the availability of skilled resources.”. |
| “And the challenge you will always get is why? Why would I change it? I am not going to get anything out of it. |
| “And do you know that would certainly help us, you know, even yesterday we had a conversation about ship holds and current shipments that are live and authorised.. Where the regulatory shipments list right now does not stop product shipping from a site, everything is hoarded in the DCs, and we are hoping that the ship list holds that. So, in reality, where it would be hugely beneficial is if we can push back to the sites and say, right, there is a trigger here within the system that says you can or cannot ship to that DC because it has been approved to go to that market or it is not approved for that market. So if the sites are not approved to supply product to that market, it cannot go anywhere.”. |
| “So as much as we can manage our data without the subjectivity of a human, the better and when I talk about that data, I mean the binary data, is the product made with everything on the bottom, yes or no is the product made to this spec, yes or no, that is critical.” |
| “We have also looked at optical scanners for label verification during production shipment and creation. So, using sensors to scan barcodes, package tracing for biologics, temperature, real-time, temperature, and excursions to alert it for stability.” |
| “it is definitely more positive. However, on the other hand, it cannot be more negative because it allows people to do things they were not able to do before.” |
| “I think it definitely has a positive impact. There is no way I could see it will have a negative impact.” |
| “Absolutely positive without question, it gives us quicker, faster access, better understanding, better management of our data, better communication with regulators and patients. Absolutely one of the biggest opportunities that we have had.” |
| “it offers huge potential for Company X” |
| “I think it is very clear that this tool Veeva, that we have used has, you know, a positive impact.” |
| “absolutely positive” |
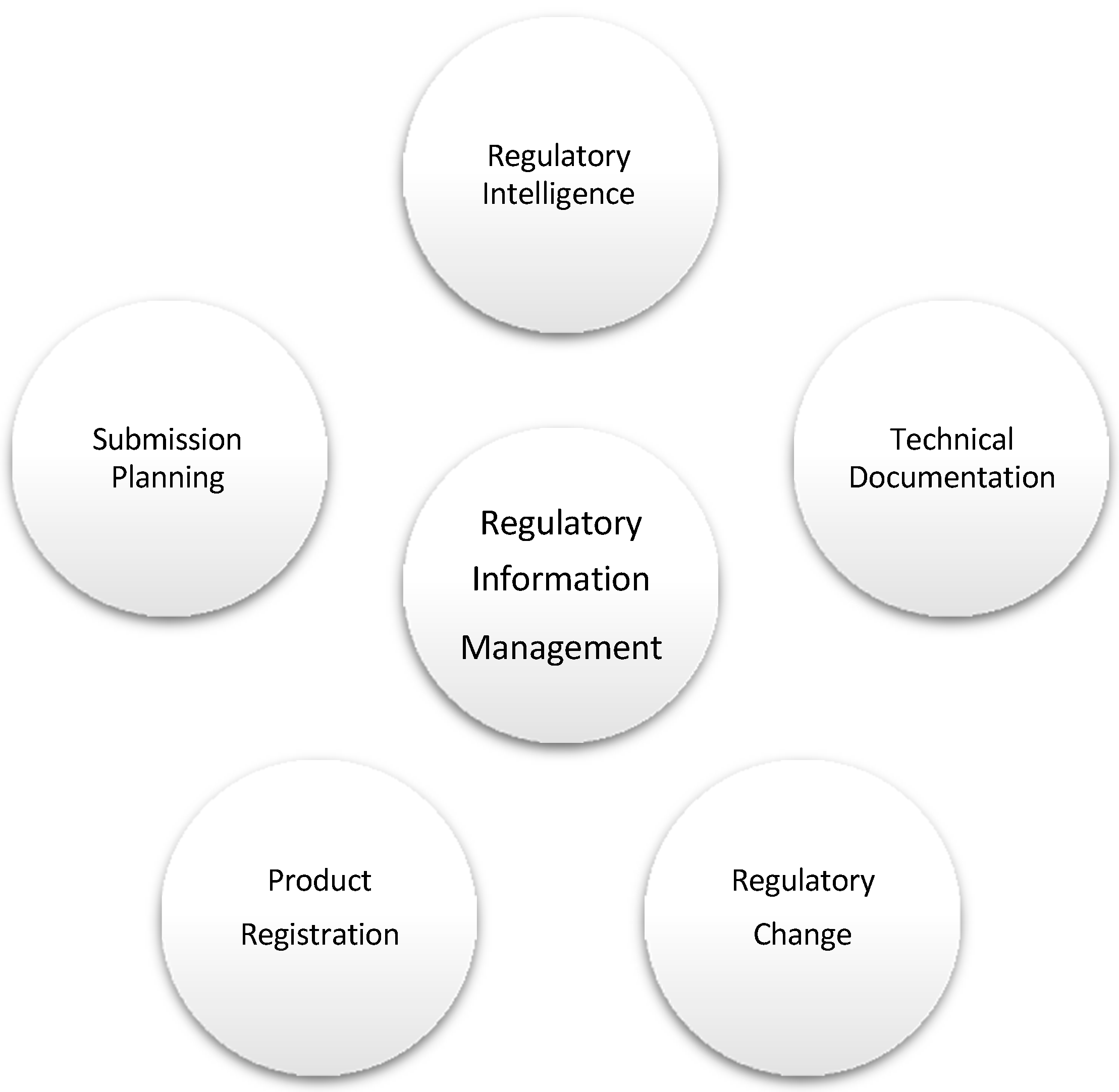
| “The RIMs project will essentially move in the direction and be a foundation for Industry 4.0 and for RA. The main elements of it as a tool that allows us to control our ship tables, and we want to automate this process as much as possible, but it will only be as good as the data that contains information about regulatory approvals globally. Regulatory approvals are not given forever, and they depend on a lot of things; RA and Regulations evolve. Moreover, we are looking for an automated way of looking at those changes that would influence whether we can or cannot ship product into a particular region”. |
| “So, it’s an automated regulatory intelligence tool that basically monitors changes in the regulatory requirements around the world and helps us decide whether they are applicable or not to our portfolio. to a large extent; it will happen in the background and allow us to identify where there may be an impact to existing registrations or to the new products entering the marketing.” |
| “The Unique Device Identifier (UDI) will give us much greater control over our products because UDI allows you to monitor the product throughout its lifecycle, including all the actors and touch points with the product, and I think having this information automated and having it available to us allowing us to search at the product and whatever happened with it will be invaluable for traceability of the products on the market. This obviously translates into safety when you have a safety issue or a product quality-related issue; you are quickly able to identify where those products are, whether they have been utilised already, what is happening with them and whether you can pull them off the market and also you can differentiate between good products and bad products. Hence, there are multiple benefits of having that system automated. So, the main two benefits are reduction in workload and resources and the reduction in potential errors”. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDermott, O.; Foley, I.; Antony, J.; Sony, M.; Butler, M. The Impact of Industry 4.0 on the Medical Device Regulatory Product Life Cycle Compliance. Sustainability 2022, 14, 14650. https://doi.org/10.3390/su142114650
McDermott O, Foley I, Antony J, Sony M, Butler M. The Impact of Industry 4.0 on the Medical Device Regulatory Product Life Cycle Compliance. Sustainability. 2022; 14(21):14650. https://doi.org/10.3390/su142114650
Chicago/Turabian StyleMcDermott, Olivia, Ida Foley, Jiju Antony, Michael Sony, and Mary Butler. 2022. "The Impact of Industry 4.0 on the Medical Device Regulatory Product Life Cycle Compliance" Sustainability 14, no. 21: 14650. https://doi.org/10.3390/su142114650
APA StyleMcDermott, O., Foley, I., Antony, J., Sony, M., & Butler, M. (2022). The Impact of Industry 4.0 on the Medical Device Regulatory Product Life Cycle Compliance. Sustainability, 14(21), 14650. https://doi.org/10.3390/su142114650












