Study of Steel Slag Eroded by Oxalic Acid and Recovery of Leachate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
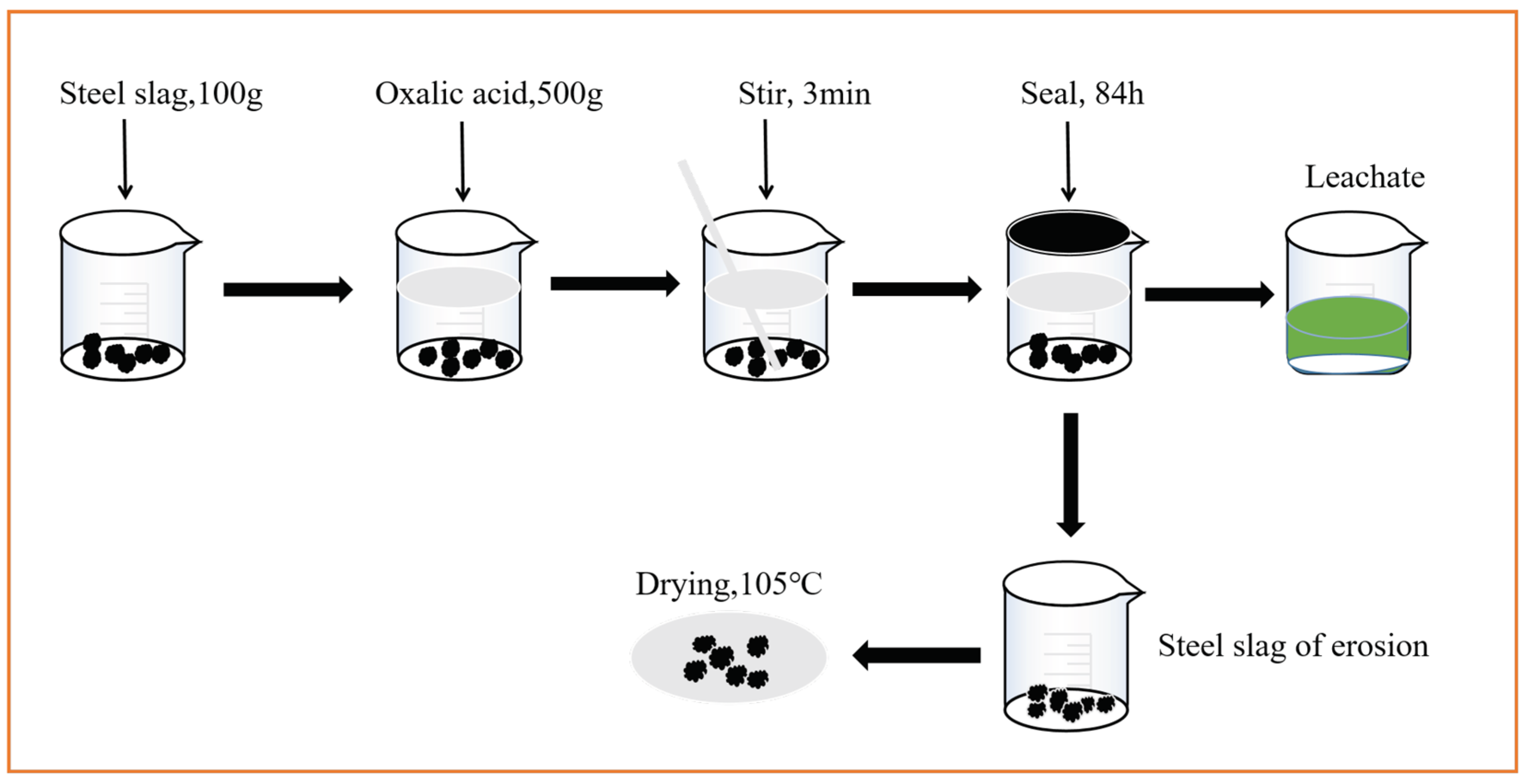
2.2. Erosion Craft of the Steel Slag
2.3. Method of Steel Slag
2.3.1. Sampling
2.3.2. Preparation
2.3.3. Characterization
- (1)
- XRD and XRF
- (2)
- SEM and EDX
- (3)
- BET and MIP
- (4)
- VK-150
2.3.4. Standards and Equipment
2.3.5. Others
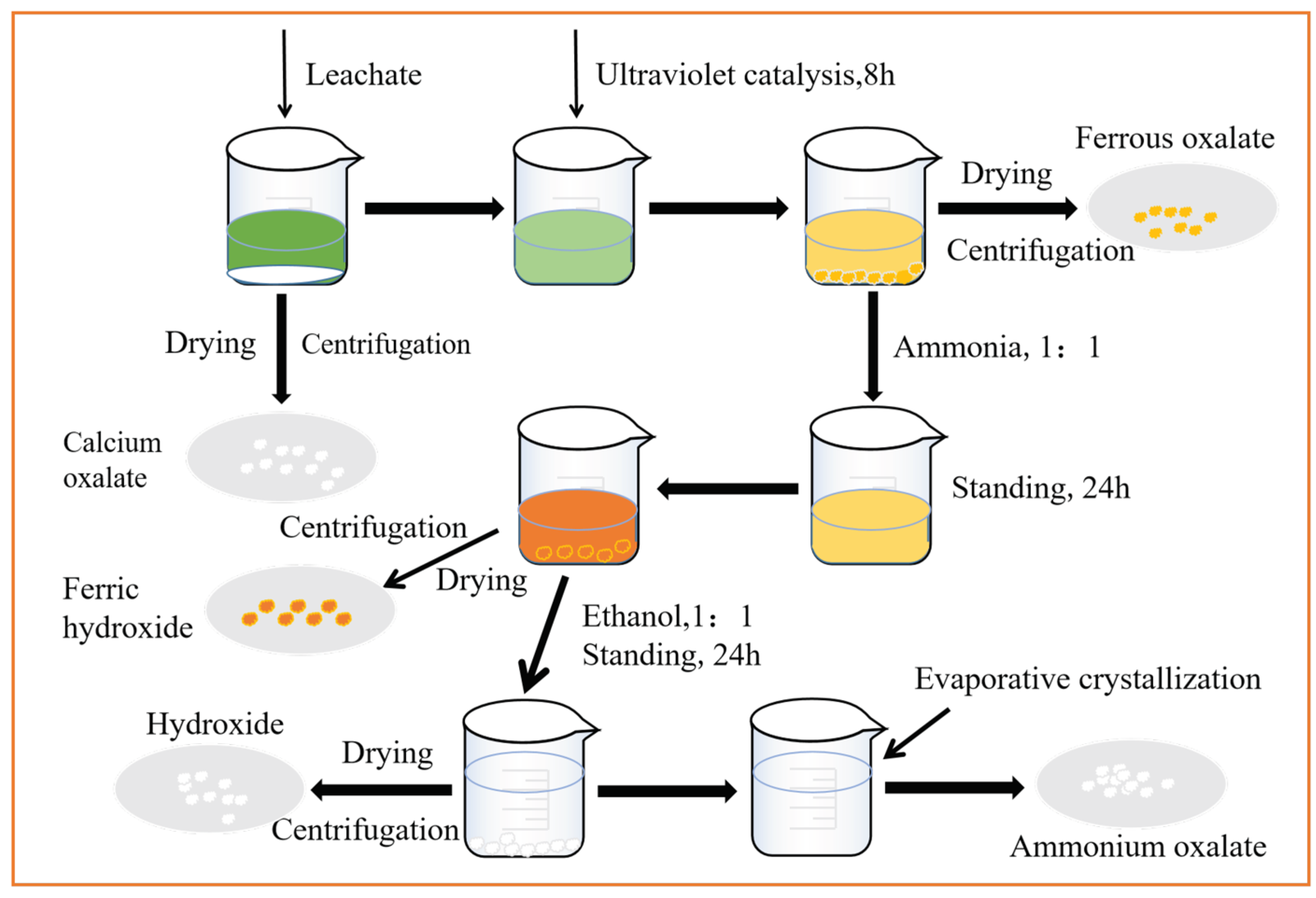
2.4. Recovery Craft of Leachate
2.5. Experimental Procedures
3. Results
3.1. Erosion of the Steel Slag
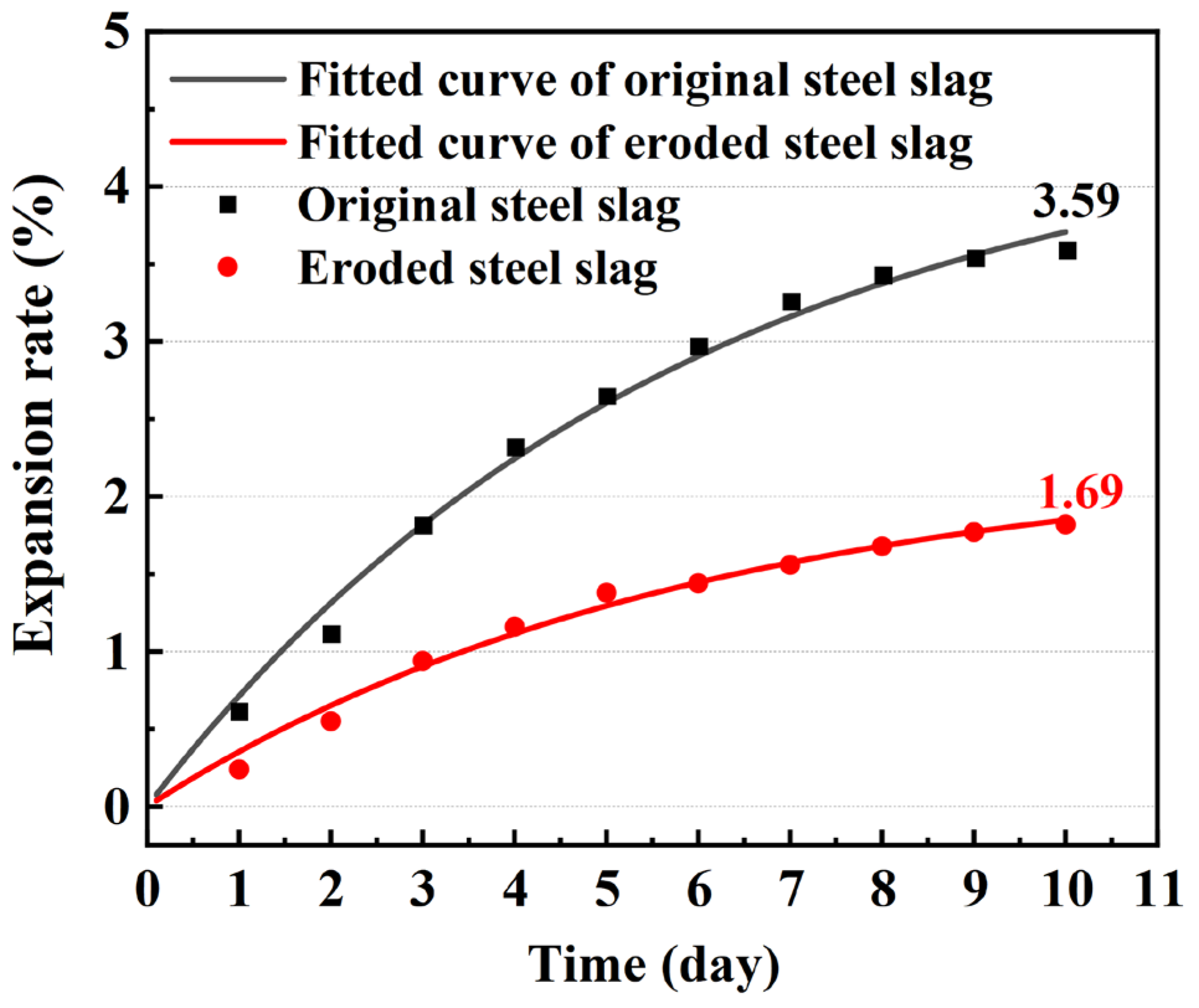
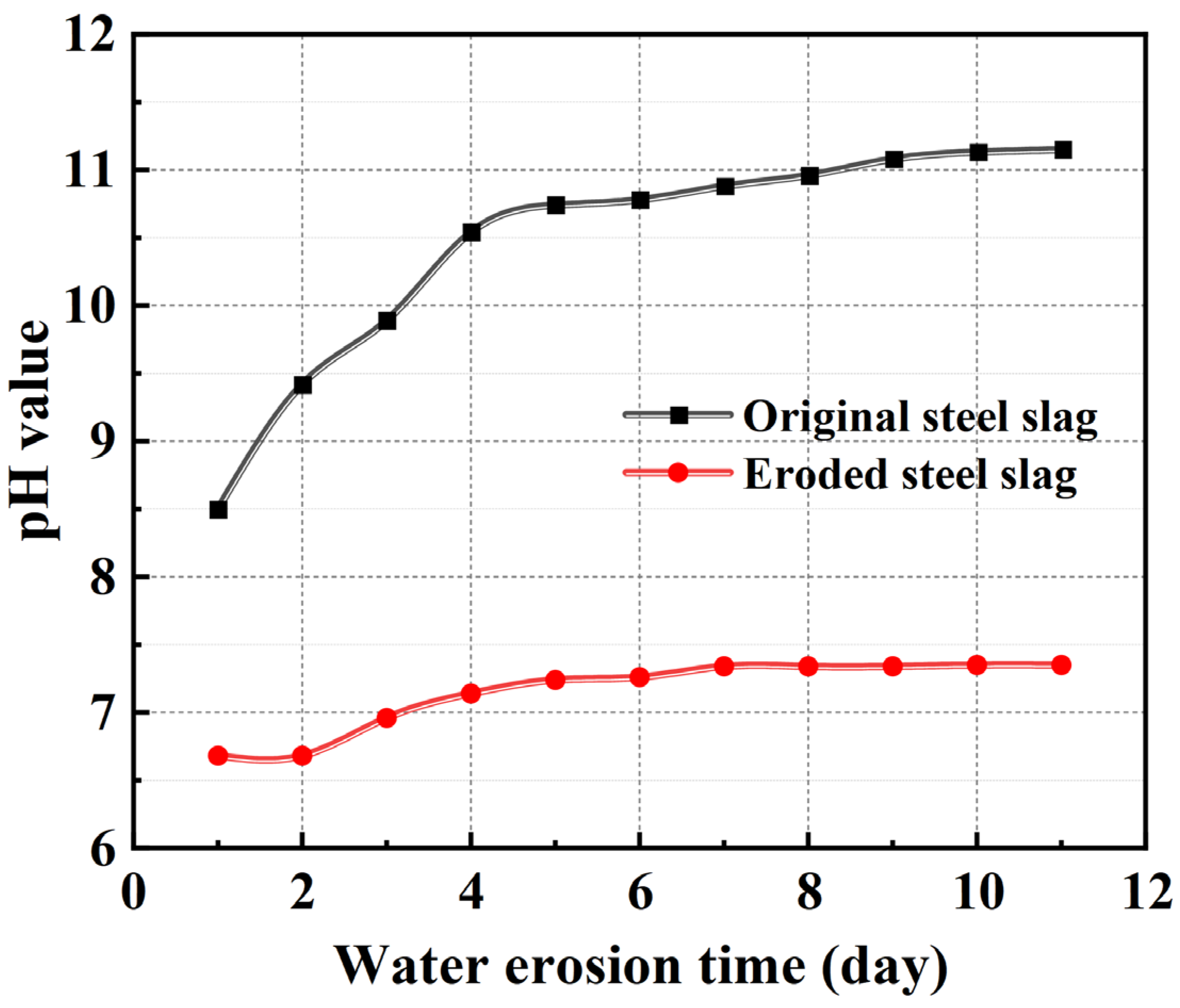
3.1.1. Volume Stability of the Steel Slag
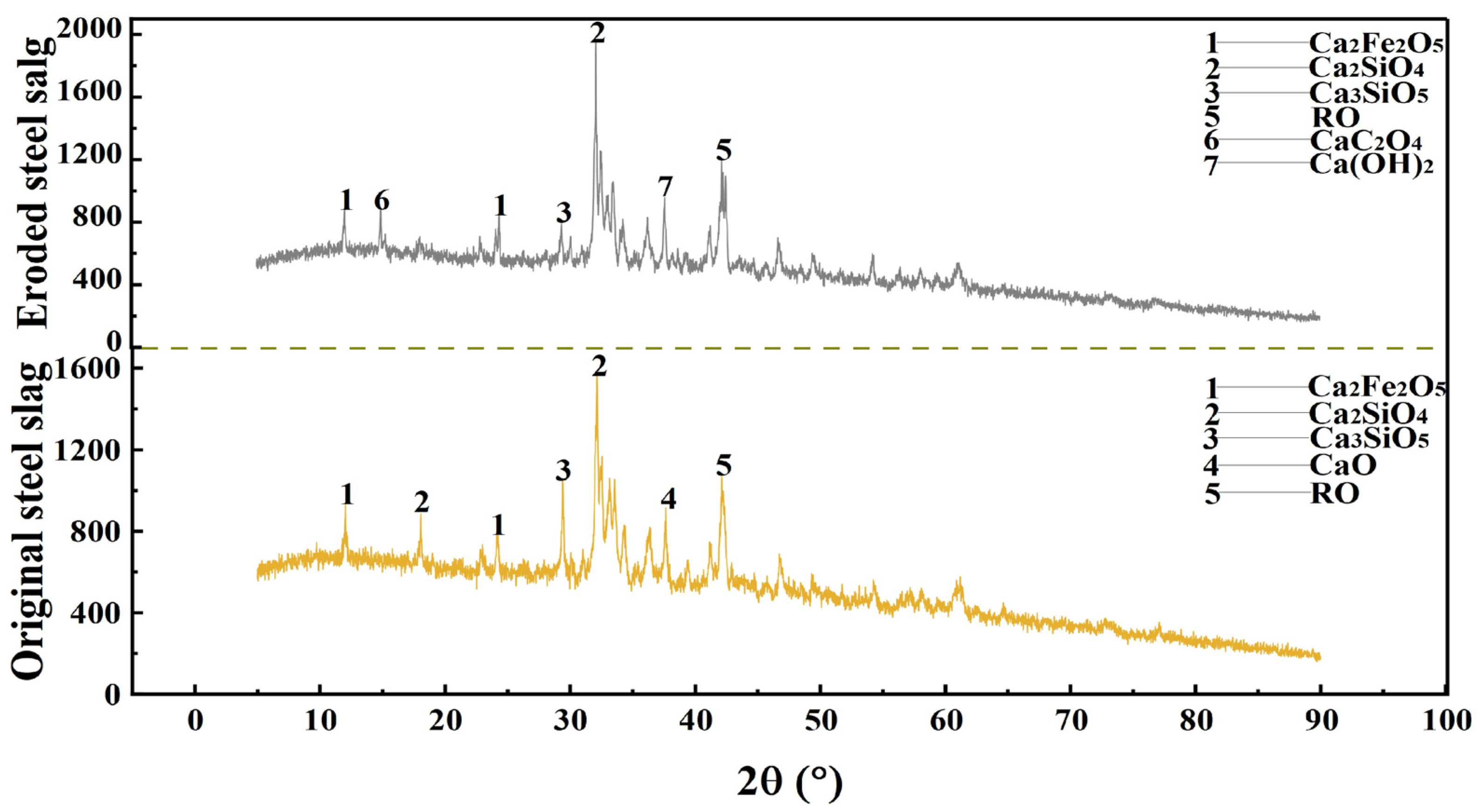
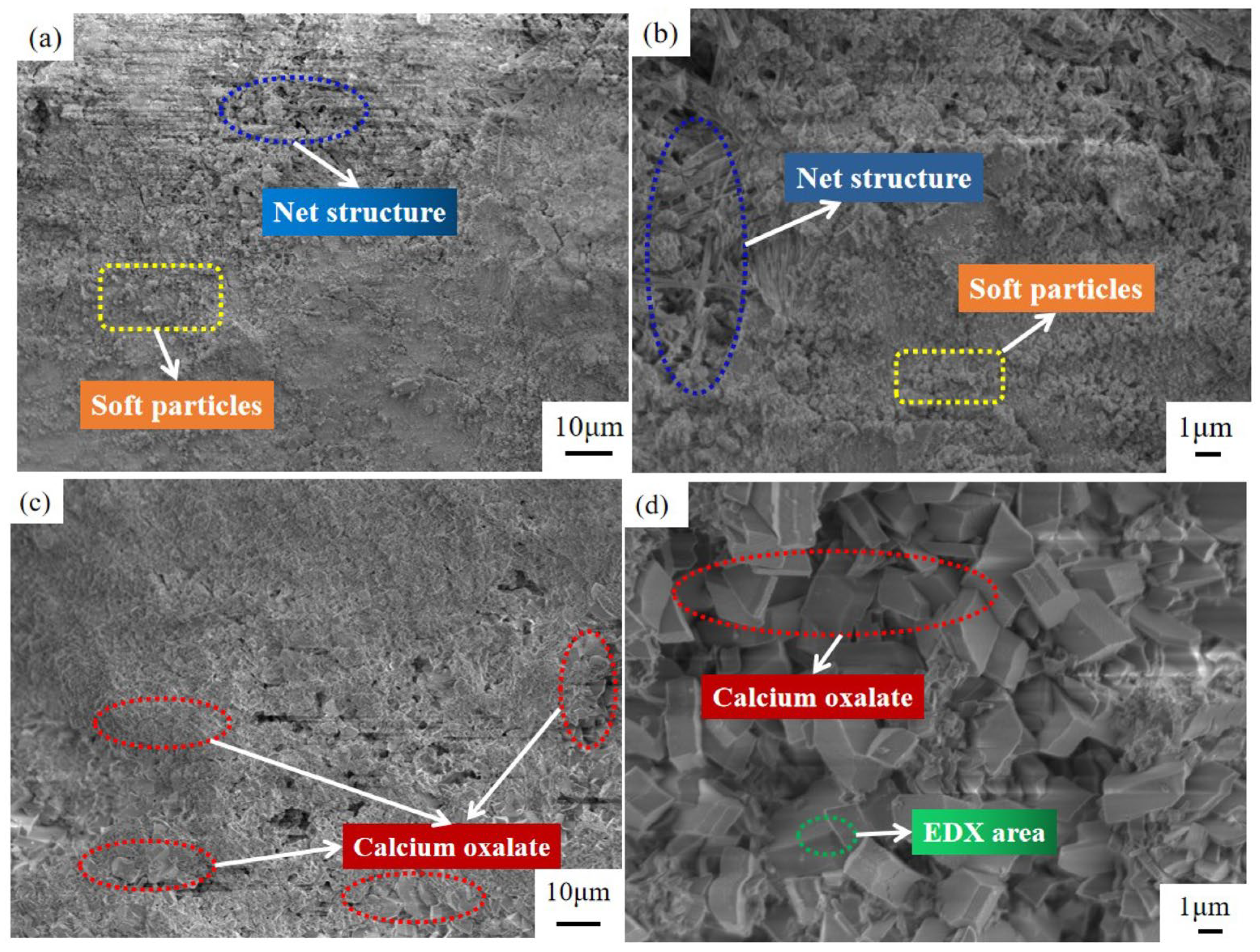
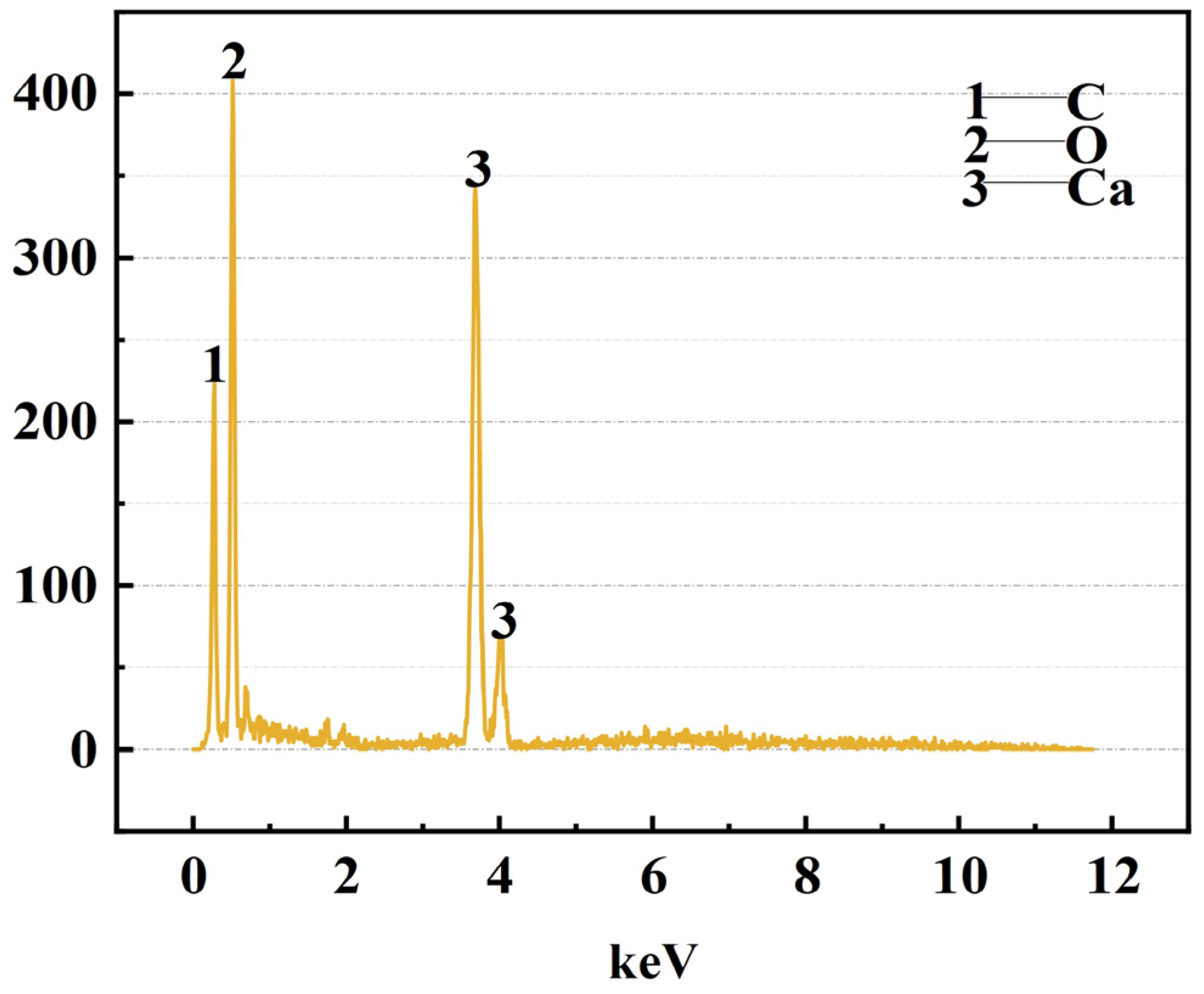
3.1.2. Phase Composition and Microstructure of the Steel Slag
3.2. Variation Law of the Hydration Activity of the Steel Slag
3.2.1. Hydration Reaction of the Steel Slag
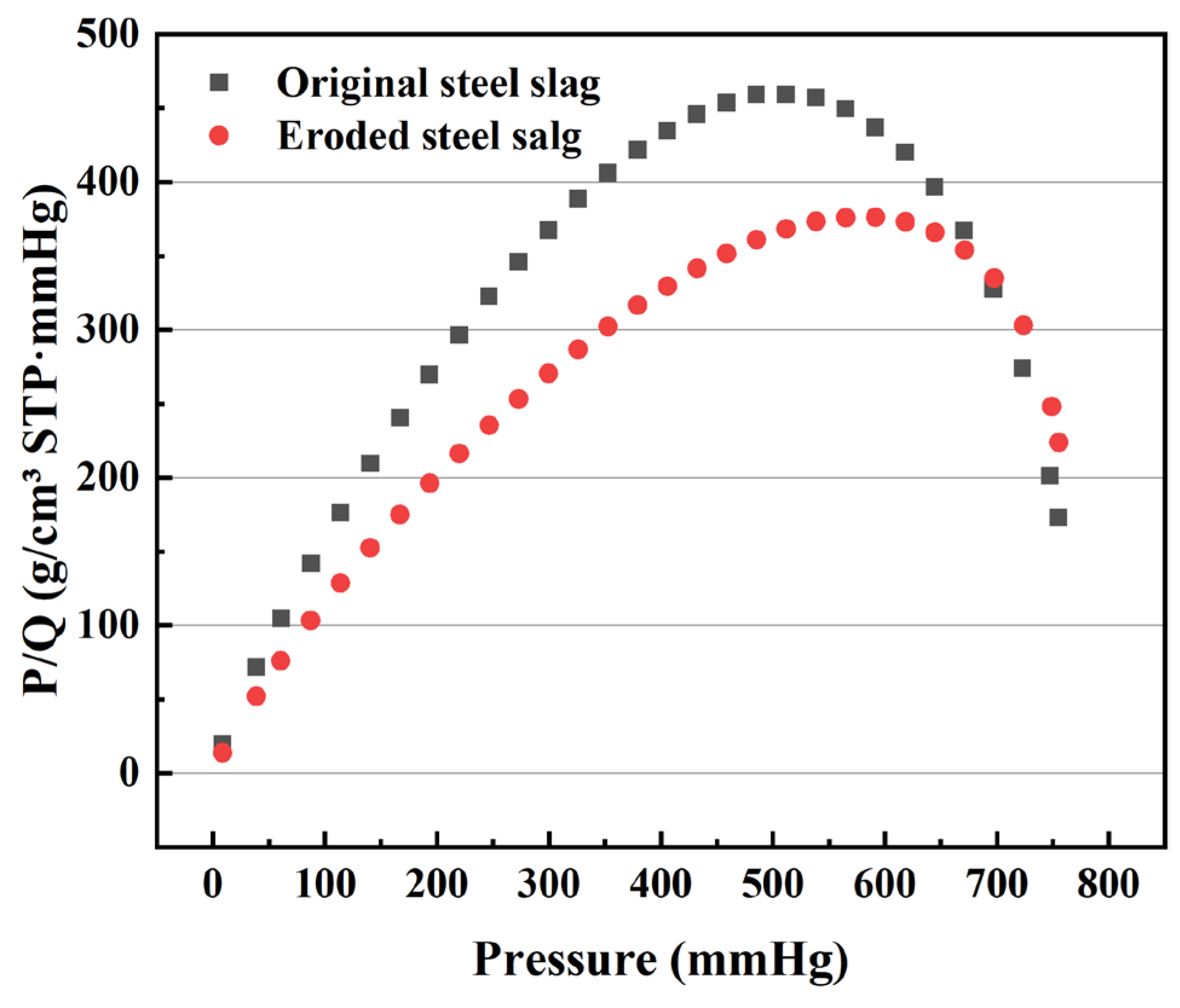
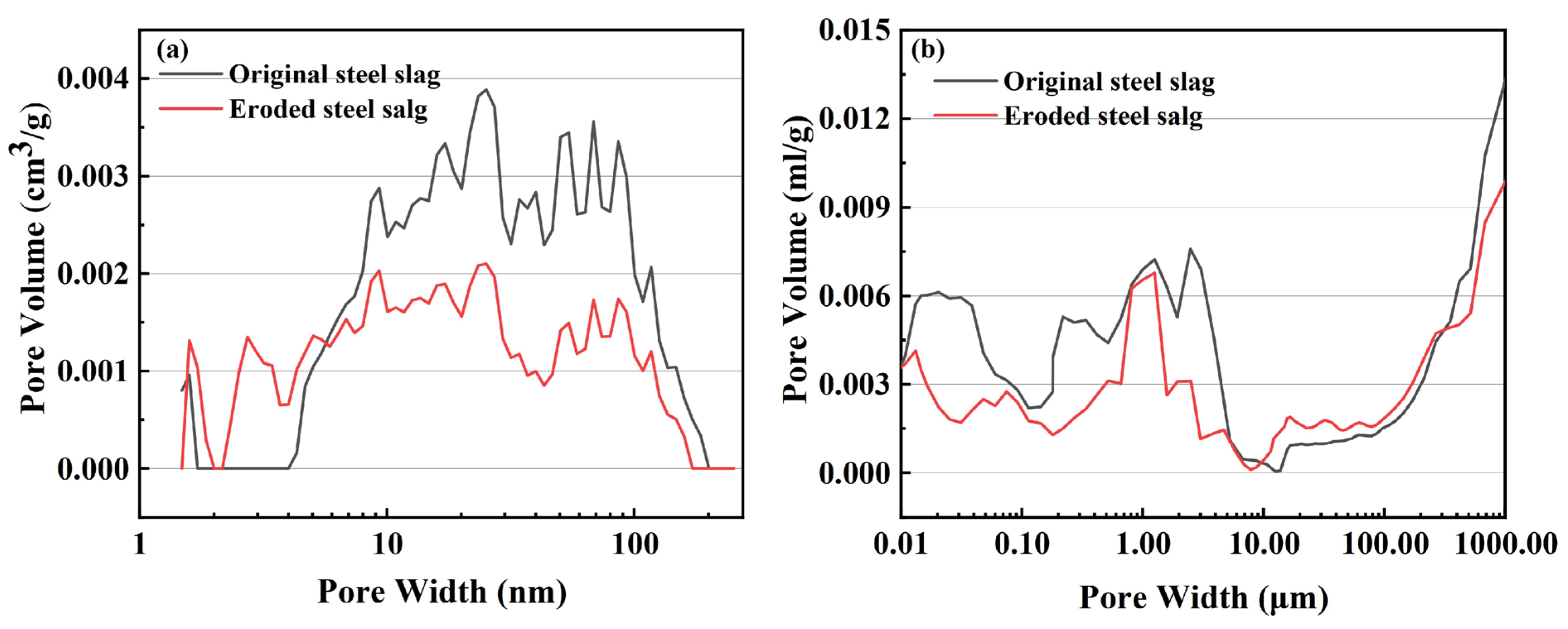
3.2.2. Surface Roughness and Pore Size Distribution of the Steel Slag
3.3. Recovery Effect of the Leachate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Term | Abbreviation |
| Activated alkali cements | AAC |
| Ordinary Portland cement | OPC |
| X-ray fluorescence spectrometer | XRF |
| X-ray diffraction | XRD |
| Scanning electron microscopy | SEM |
| 3D laser microscopic imaging system | VK-150 |
| Automatic mercury injection apparatus | MIP |
| Energy spectrum analysis | EDX |
| Automatic specific surface area and porosity analyzer | BET |
| Free calcium oxide | f-CaO |
| Free magnesium oxide | f-MgO |
| Dicalcium silicate | Ca2SiO4 |
| Calcium ferrite | Ca2Fe2O5 |
| Calcium hydroxide | Ca(OH)2 |
| Calcium carbonate | Ca(CO)3 |
| Tricalcium silicate | Ca3SiO4 |
| Ultraviolet irradiation box | XE-1 |
| Calcium oxalate | Ca2C2O4 |
| Ferrous oxalate | FeC2O4 |
| Ferric hydroxide | Fe(OH)3 |
| Ammonium oxalate | (NH4)C2O4 |
| Oxalic acid | H2C2O4 |
References
- Wu, S.P.; Cui, P.D.; Xie, J.; Liu, Q.T.; Pang, L. Expansive Inhibition Method of Steel Slag Aggregate and Volume Stability of Mixture: A Review. China J. Highw. Transp. 2021, 34, 166–179. [Google Scholar]
- He, L.; Zhan, C.Y.; Lv, S.T.; James, G.; Gao, J.; Karol, K.J.; Jan, V.; Xie, J.; Lidija, R.; Lling, T.Q. Application Status of Steel Slag Asphalt Mixture. J. Traffic Transp. Eng. 2020, 20, 15–33. [Google Scholar]
- Dong, Q.; Wang, G.T.; Chen, X.Q.; Tan, J.; Gu, X.Y. Recycling of Steel Slag Aggregate in Portland Cement Concrete: An Overview. J. Clean. Prod. 2020, 282, 124447. [Google Scholar] [CrossRef]
- Huang, S.Y.; Huo, B.B.; Chen, C.; Zhang, Y.M. The Influence of Metakaolin on the Hydration of Steam-cured Steel Slag Blended Cement. Mater. Rep. 2022, 36, 73–78. [Google Scholar]
- Qiang, W.; Yan, P.Y. Hydration Properties of Basic Oxygen Furnace Steel Slag. Constr. Build. Mater. 2010, 24, 1134–1140. [Google Scholar]
- Liang, X.J.; Ye, Z.M.; Chang, J. Early Hydration Activity of Composite with Carbonated Steel Slag. J. Chin. Ceram. Soc. 2012, 40, 226–233. [Google Scholar]
- Huo, B.B.; Li, B.L.; Chen, C.; Zhang, Y.M. Surface Etching and Early Age Hydration Mechanisms of Steel Slag Powder with Formic Acid. Constr. Build. Mater. 2021, 280, 122500. [Google Scholar] [CrossRef]
- Huo, B.B.; Li, B.L.; Chen, C.; Zhang, Y.M. Mechanism and Properties of Glacial Acetic Acid Modified Steel Slag Powder via Dry Chemical Modification Method. J. Chin. Ceram. Soc. 2021, 49, 948–954. [Google Scholar]
- Ding, Y.C.; Cheng, T.W.; Liu, P.C.; Lee, W.H. Study on the Treatment of BOF Slag to Replace Fine Aggregate in Concrete. Constr. Build. Mater. 2017, 146, 644–651. [Google Scholar] [CrossRef]
- Chen, Z.W.; Gong, Z.L.; Jiao, Y.Y.; Wang, Y.; Shi, K.; Wu, J.C. Moisture Stability Improvement of Asphalt Mixture Considering the Surface Characteristics of Steel Slag Coarse Aggregate. Constr. Build. Mater. 2020, 251, 118987. [Google Scholar] [CrossRef]
- Ma, L.L.; Xu, D.B.; Wang, S.Y.; Gu, X.Y. Expansion Inhibition of Steel Slag in Asphalt Mixture by a Surface Water Isolation Structure. Road Mater. Pavement 2020, 21, 2215–2229. [Google Scholar] [CrossRef]
- Zhu, B.R.; Yang, Q.B.; Yao, D.W.; Yang, G.L. Study on Extraction of Calcium from Steel Slag with Acetic Acid. J. Tongji Univ. (Nat. Sci.) 2009, 37, 99–102. [Google Scholar]
- Ragipani, R.; Bhattacharya, S.P.; Suresh, A.K. Understanding Dissolution Characteristics of Steel Slag for Resource Recovery. Waste Manag. 2020, 117, 179–187. [Google Scholar] [CrossRef]
- Ju, J.R.; Feng, Y.L.; Li, H.R.; Xu, C.L.; Yang, Y. Efficient Separation and Recovery of Vanadium, Titanium, Iron, Magnesium, and Synthesizing Anhydrite from Steel Slag. Mining. Metall. Explor. 2022, 39, 733–748. [Google Scholar] [CrossRef]
- Markssuel, T.M.; Afonso, R.G.A.; Leandro, B.O.; Gustavo, C.X.; Carlos, M.F.V. Mechanical, Physical and Durability Properties of Activated Alkali Cement Based on Blast Furnace Slag as a Function of %Na2O. CaseStud. Constr. Mater. 2021, 15, e00723. [Google Scholar]
- Ashraf, M.H.; Alsaeed, A.E.S.M.; Ibrahim, S.A. Simulation of the Behavior of Pressurized Underwater Concrete. Alex. Eng. J. 2015, 54, 183–195. [Google Scholar]
- Heniegal, A.M.A.; Maaty, A.A.E.S.; Agwa, I.S. Influence of Anti Washout Admixtures and Coarse Aggregate Types on Self-flowing Underwater Concrete Properties. Int. J. Civ. Eng. 2016, 6, 245–262. [Google Scholar]
- Mohamed, A.; Abdullah, M.Z.; Bassam, A.T.; Ibrahim, S.A. Effect of High Temperatures on Mechanical, Radiation Attenuation and Microstructure Properties of Heavyweight Geopolymer Concrete. Struct. Eng. Mech. 2021, 80, 181–199. [Google Scholar]
- JTG E42-2005; Test Methods for Aggregate of Highway Engineering. China Standards Press: Beijing, China, 2005.
- JGJ 52-2006; Standard for Technical Requirements and Test Method of Sand and Crushed, Stone (or Gravel) for Ordinary Concrete. China Standards Press: Beijing, China, 2006.
- EJ/T 553-1991; Determination of Unit Cell Parameters of Minerals-Powder X-ray Diffraction Method. China Standards Press: Beijing, China, 1991.
- GB/T 6730.62-2005; Iron Ores-Determination of Calcium, Silicon, Manganese, Titanium, Phosphorusmagnesium, Aluminium and Barium Content-Wavelength Dispersive X-ray Fluorescence Spectrometric Method. China Standards Press: Beijing, China, 2005.
- GB/T 16594-2008; General Rules for Measurement of Length in Micron Scale by SEM. China Standards Press: Beijing, China, 2008.
- GB/T 21650.3-2011; Pore Size Distribution and Porosity of Solid Materials by Mercury Porosimetry and Gas Adsorption-Part 3:Analysis of Micropores by Gas Adsorption. China Standards Press: Beijing, China, 2011.
- GB/T 3505-2000; Geometrical Product Specfications (GPs)—Surface Texture: Profile method—Terms, Definitions and Surface Texture Parameters. China Standards Press: Beijing, China, 2000.
- YB/T 4328-2012; Method for the Determination of Free Calcium Oxide Content in Steel Slag. China Standards Press: Beijing, China, 2012.
- GB/T 24175-2009; Test Method for Stability of Steel Slag. China Standards Press: Beijing, China, 2009.
- Huo, B.B.; Li, B.L.; Luo, Y.L.; Zhang, Y.M.; Wang, D.M. Morphological and Mineralogical Characteristics of Phosphoric Acid Etched Steel Slag. J. Build. Mater. 2022, 25, 591–597. [Google Scholar]
- Dorian, P.G.; Rubén, V.M.; Margarita, H.E. Solar Photoassisted Advanced Oxidation of Synthetic Phenolic Wastewaters Using Ferrioxalate Complexes. Sol. Energy 2008, 83, 306–315. [Google Scholar]
- Ambikadevi, V.R.; Lalithambika, M. Effect of Organic Acids on Ferric Iron removal from Iron-Stained Kaolinite. Appl. Clay. Sci. 2000, 16, 133–145. [Google Scholar] [CrossRef]
- George, W. Determination of the Expansion Force of Coarse Steel Slag Aggregate-ScienceDirect. Constr. Build. Mater. 2010, 24, 1961–1966. [Google Scholar]
- Dong, C.L. Expansion Inhibition Mechanism of Steel Slag with Hydrophobic Surface and Properties of Its Asphalt Mixture. Master’s Thesis, Southeast University, Nanjing, China, 2019. (In Chinese). [Google Scholar]
- Xie, J. Research on the Preparation, Performance and Application of Basic Oxygen Slag Based Asphalt Concrete. Master’s thesis, Wuhan University of Technology, Wuhan, China, 2013. (In Chinese). [Google Scholar]
- Zhao, J.H.; Yan, P.Y. Volume stability and stabilization treatment of steel slag in China. Bull. Chin. Ceram. Soc. 2017, 36, 477. [Google Scholar]
- Doherty, B.; Pamplona, M.; Selvaggi, R.; Miliani, C.; Matteini, M.; Sgamellotti, A.; Brunetti, B. Efficiency and Resistance of the Artificial Oxalate Protection Treatment on Marble Against Chemical Weathering. Appl. Surf. Sci. 2007, 253, 4477. [Google Scholar] [CrossRef]
- Tang, M.S.; Yuan, M.X.; Han, S.F.; Shen, X. The Crystalline State of MgO, FeO and MnO in Steel Slag and the Soundness of Steel Slag Cement. J. Chin. Ceram. Soc. 1979, 7, 35–46. [Google Scholar]
- Arvaniti, E.C.; Lioliou, M.G.; Paraskeva, C.A.; Ostvold, T.; Koutsoukos, P.G. Calcium Oxalate Crystallization on Concrete Heterogeneities. Chem. Eng. Res. Des. 2010, 88, 1455. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Gao, Z. Use of Steel Slag as a Granular Material: Volume Expansion Prediction and Usability Criteria. J. Hazard. Mater. 2010, 184, 555. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Xie, J.; Xiao, Y.; Chen, J.Y.; Wu, S.P. Characteristics of Bonding Behavior between Basic Oxygen Furnace Slag and Asphalt Binder. Constr. Build. Mater. 2014, 64, 60–66. [Google Scholar] [CrossRef]
- Huo, B.B.; Li, B.L.; Huang, S.Y.; Chen, C.; Zhang, Y.M.; Banthia, N. Hydration and Soundness of Phosphoric Acid Modified Steel Slag Powder. Constr. Build. Mater. 2020, 254, 119319. [Google Scholar] [CrossRef]
- Ramalingam, M.; Masilamani, A.; Illmin, C.; Mayakrishnan, P. Effect of Surface-Treated Energy Optimized Furnace Steel Slag as Coarse Aggregate in the Performance of Concrete under Corrosive Environment. Constr. Build. Mater. 2021, 284, 122840. [Google Scholar]
- Zhang, H.; Zhang, X.Y.; Long, H.M. Spectroscopic Analysis of Weak Acid Modified Steel Slag Powder. Spectrosc. Spectr. Anal. 2018, 38, 3502–3506. [Google Scholar]






| CaO | Fe2O3 | SiO2 | MgO | Al2O3 | P2O5 | MnO | |
|---|---|---|---|---|---|---|---|
| Steel Slag | 43.49 | 28.36 | 12.99 | 6.75 | 2.33 | 2.17 | 1.34 |
| Test Project | Unit | Technical Indicators | Test Value |
|---|---|---|---|
| Crush Value | % | ≤26 | 17.8 |
| Los Angeles Wear (C) | % | ≤28 | 16.9 |
| Bibulous Rate | % | ≤2.0 | 1.91 |
| Gross Volume Relative Density | g/cm3 | - | 3.465 |
| Apparent Relative Density | g/cm3 | ≥2.60 | 3.586 |
| Adhesiveness | level | ≥5 | 5 |
| Free Calcium | Ca(OH)2 | f-CaO | |
|---|---|---|---|
| Original Steel Slag | 4.851 | 0.01 | 4.841 |
| Eroded Steel Slag | 2.348 | 0.023 | 2.325 |
| Fitting Equation | Final Expansion Rate | A Derivative | |
|---|---|---|---|
| Original Steel Slag | 3.59% | ||
| Eroded Steel Slag | 1.69% |
| Ca | Fe | Si | Mg | Al | P | Mn | |
|---|---|---|---|---|---|---|---|
| Original Steel Slag | 28.13 | 17.30 | 5.38 | 3.65 | 1.14 | 0.832 | 0.836 |
| Eroded Steel Slag | 26.30 | 15.95 | 5.28 | 3.63 | 1.099 | 0.815 | 0.788 |
| O | Ca | C | Others | Si | |
|---|---|---|---|---|---|
| EDX Area | 57.07 | 31.53 | 9.06 | 2.01 | 0.33 |
| Blue Area (%) | Red Area (%) | Roughness (μm) | |
|---|---|---|---|
| Original Steel Slag | 8.68 | 33.74 | 10.089 |
| Eroded Steel Slag | 5.43 | 29.5 | 7.531 |
| Specific Surface Area (m2/g) | Porosity (%) | Total Pore Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | Average Pore Diameter (μm) | |
|---|---|---|---|---|---|---|
| Original Steel Slag | 14.4653 | 7.0 | 1.5746 | 0.00419 | 6.5903 | 1.717 |
| Eroded Steel Slag | 11.9262 | 5.8 | 1.2214 | 0.00319 | 4.3653 | 0.208 |
| Fe | Ca | Si | Al | Mg | P | Mn | |
|---|---|---|---|---|---|---|---|
| Leachate | 35.43 | 17.17 | 17.05 | 7.54 | 7.42 | 2.92 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Yan, F.; Guo, R.; He, H.; Li, H. Study of Steel Slag Eroded by Oxalic Acid and Recovery of Leachate. Sustainability 2022, 14, 13598. https://doi.org/10.3390/su142013598
Huang X, Yan F, Guo R, He H, Li H. Study of Steel Slag Eroded by Oxalic Acid and Recovery of Leachate. Sustainability. 2022; 14(20):13598. https://doi.org/10.3390/su142013598
Chicago/Turabian StyleHuang, Xiaoming, Feng Yan, Rongxin Guo, Huan He, and Hao Li. 2022. "Study of Steel Slag Eroded by Oxalic Acid and Recovery of Leachate" Sustainability 14, no. 20: 13598. https://doi.org/10.3390/su142013598
APA StyleHuang, X., Yan, F., Guo, R., He, H., & Li, H. (2022). Study of Steel Slag Eroded by Oxalic Acid and Recovery of Leachate. Sustainability, 14(20), 13598. https://doi.org/10.3390/su142013598





