Abstract
To facilitate the ongoing transition towards carbon neutrality, the use of renewable materials for additive manufacturing has become increasingly important. Here, we report for the first time the fabrication of microcapsules from biobased acrylate shells using microfluidics. To select the shell, a wide range of biobased acrylates disclosed in the literature was considered according to their tensile strength, ductile transition temperature and global availability. Once acrylate epoxidised soybean oil (AESO) was selected, its viscosity was adjusted to valuables suitable for the microfluidic device using two different diluting agents. Double emulsions were successfully produced using microfluidics, followed by photopolymerisation of the shell and characterisation of the capsules. Microcapsules containing AESO and isobornyl acrylate (IBOA) were produced with an outer diameter ~490 μm, shell thickness ranging between 36 and 67 μm, and production rates around 2.4 g/h. The mechanical properties of the shell were characterised as tensile strength of 29.2 ± 7.7 MPa, Young’s modulus of 1.7 ± 0.4 GPa and the ductile transition temperature was estimated as 42 °C. To investigate physical triggering, microcapsules produced with a size of 481 ± 4 μm and with a measured shell thickness around 6 μm were embedded in the cementitious matrix. The triggered shells were observed with scanning electron microscopy (SEM) and the uniform distribution of the capsules in cement paste was confirmed using X-ray computed tomography (XCT). These advances can facilitate the wide application of biobased resins for the fabrication of microcapsules for self-healing in cementitious materials.
1. Introduction
Cementitious materials are prone to fracture, especially when subjected to tensile forces. Once these cracks have formed, they can facilitate the transport of water and other chemicals which can result in corrosion and intensify the cracking process. To minimise crack formation, traditional methods for repair and maintenance are applied, including human intervention to seal cracks. However, these actions are costly; in the United Kingdom alone, approximately 50% of the construction budget is spent on repair and maintenance [1]. An alternative strategy relies on capsule-based self-healing, which aims to distribute small capsules containing a healing agent throughout the matrix. Upon formation of a crack in the matrix, the shell is triggered to release the healing agent and heal the crack without external intervention [2]. In comparison to traditional cementitious matrices, capsule-based self-healing has a greater capacity for healing cracks [3] and may promote recovery in mechanical and transport properties [4,5]. Several protocols have been investigated to produce microcapsules, mainly focused on the encapsulation of specific healing agents, such as epoxy [6], sodium silicate [7,8] and bacterial spores [3]. Typical protocols to produce microcapsules involve the formation of emulsions followed by the deposition of a shell around the interface of droplets via processes such as coacervation [7], in situ [9], or interfacial [10] polymerisation. More recently, importance has shifted to favour selection of materials and methods which show superior cost-effectiveness [11,12], feasibility for commercial deployment [13] and sustainability [14]. For example, Garces et al., (2021) performed a cradle-to-gate life cycle assessment (LCA) investigating microcapsule based self-healing geopolymers. The solvent (toluene) and wall forming monomers (4,4′-methylenebis(phenyl diisocyanate)) were found to be the main contributors to the additional environmental impacts of self-healing geopolymers when compared with conventional geopolymer concrete [14]. Thus, greener routes for producing these microcapsules have been considered in terms of synthesis feedstocks, toxicity, and biodegradability. In this case, in situ and interfacial polymerisation must be examined with caution because toxic reagents such as formaldehyde—which is classified as a human carcinogen if unreacted—and isocyanates are used. Although these methodologies are still used on a larger scale, with the use of formaldehyde scavengers to reduce the risk of toxic exposure, the search for more benign protocols is ongoing [15]. Alternatively, complex coacervation offers a route to produce shells using renewable feedstocks with low toxicity, such as gelatin-gum arabic microcapsules containing sodium silicate. In this case, the healing agent was emulsified prior to the formation of the shell, resulting in a concentration of sodium silicate solution of approximately 42%, thus higher concentrations of healing agent could be explored [7,16]. Microfluidics offers a very controlled route to produce capsules, with excellent encapsulation efficiency and superior control over size and shell thickness [17,18]. However, the embedded carbon during the production of the polymeric shell is currently a matter of concern.
Microcapsules produced using microfluidics have so far relied on the production of double emulsion templates, using photopolymerising acrylates as a shell material [19,20,21,22]. Acrylates are formed from reacting acrylic acid with a corresponding alcohol in the presence of a catalyst [23,24], with the acrylic acid component usually derived from fossil fuel propylene. Although acrylates are a suitable shell material in terms of their photopolymerisation characteristics and material properties, the use of ever-depleting fossil fuels is unsustainable, and the production process of acrylate is also associated with a significant emission penalty. This is a particular concern, when considering the production of microcapsules in the large volumes required for applications in construction [25].
A life cycle analysis conducted by Petrescu et al. on the acrylic acid production pathway calculated the global warming potential (GWP) of acrylic acid to be 3877 kgCO2/tonne acrylic acid when natural gas was used for steam generation [26]. Even neglecting the further step of converting acrylic acid to acrylate, using microcapsules with acrylate shells carries a heavy environmental penalty, with embodied carbon per unit mass of acrylate almost 4 times that of the cementitious matrix [27]. This, combined with the use of fossil fuel feedstocks, makes microcapsules unlikely to improve the sustainability of the cement industry, even if the service life of concrete is significantly increased as a result.
To improve sustainability, a shift away from fossil fuel feedstocks is required. One way in which this can be done is to source acrylates made from a more sustainable acrylic acid, derived from biobased compounds. Both glycerol and 3-Hydroxypropanoic Acid (3-HP) can be used as feedstock for acrylic acid production, however, they pose key issues relating to scale. Crude glycerol, a naturally occurring chemical, is a by-product of biodiesel production and can be converted to acrylic acid by oxidative dehydration via intermediate acrolein [28]. Using glycerol as a feedstock for acrylic acid production has a two-fold benefit over the current standard production using propylene as it not only reduces the reliance upon fossil fuels, but it also utilises waste products from biodiesel production. However, a cradle-to-gate life cycle assessment conducted on the production of acrolein from glycerol by Cepsi et al. calculated its GWP as 4520 kg CO2/tonne of acrolein [29]. Even neglecting the conversion of acrolein to acrylic acid, GWP was still considerably higher than the 3877 kg/tonne of acrylic acid calculated for the propylene pathway [26]. An approximate calculation demonstrated that the projected biodiesel production would only be sufficient to provide enough glycerol to cover 31% of global acrylate ester demand if all available glycerol was to be used in the acrylic acid pathway [30,31,32,33,34]. However, looking more locally, a calculation was performed to find the proportion of the UK’s cementitious materials which could incorporate microcapsules from glycerol-derived acrylate, based on UK biodiesel production [31] and cement production [32] and showed the UK’s current production of biodiesel was insufficient to produce enough glycerol for microcapsule shells to be used in cement with high capsule volume fractions [35].
On the other hand, 3-HP is a valuable chemical, historically synthesized by the fermentation of either glucose or glycerol. Utilizing Levulinic acid (LA), a renewable feedstock, obtained from the dehydrative, acidic processing of raw biomass or carbohydrates, to produce 3-HP, as researched by Wu et al. [33], has the potential to lead to even higher savings in GWP if LA from waste biomass were to be used instead as 3-HP feedstock. However, this is not currently available at a large enough commercial scale for the manufacture of microcapsule shells.
Whilst obtaining acrylate produced from more sustainably sourced acrylic acid is one way to improve the sustainability of microcapsule shells, it relies upon a drastic scale-up of production industries. An alternative, perhaps more feasible, method would be to change the material of the microcapsule shells. Instead of using standard acrylates, a switch can be made to incorporate acrylates derived from biobased compounds. Many of these have been produced and characterised in the literature such as those derived from cardanol [36], camelina oil [37], soybean oil [38,39], castor oil [40,41], palm oil [42] and tung oil [43]. The broad range of properties these biobased acrylates provide, along with the potential to copolymerise them to tailor material properties, open up opportunities to improve the biobased content and sustainability of microcapsules, without potentially jeopardising their rupture properties. The different pathways for the production of acrylate are depicted in Figure 1.
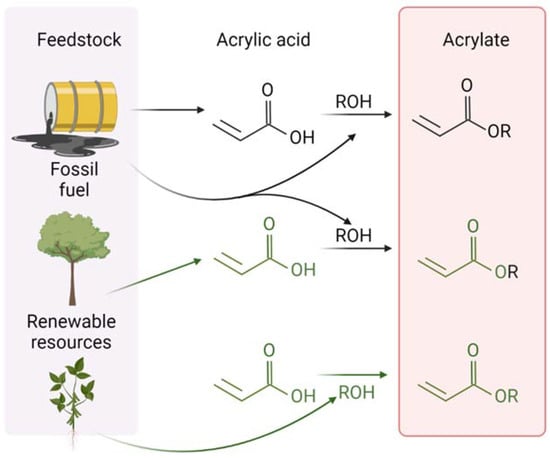
Figure 1.
Production pathway of acrylates using fossil fuel and renewable resources. Created using BioRender.com.
Thus, in this study, for the first timebiobased shells as a greener alternative to fossil fuel derived acrylates were investigated the production of microcapsules for physically triggered self-healing. Acrylated epoxidized soybean oil (AESO) was selected as a biobased acrylate based on the main properties governing the physical triggering of the capsules, namely tensile strength and ductile transition temperature, as well as global production of the material. The production of double emulsions using microfluidics was investigated to evaluate the suitability of the monomers to produce stable templates for the capsules. Due to the high viscosity of the biobased acrylate, 1,6-hexadienol diacrylate (HDDA) and isobornyl acrylate (IBOA) were used as diluting agents to achieve an adequate viscosity for the microfluidic device. Mechanical properties of the acrylates were also quantified. Furthermore, to investigate the behaviour of the microcapsules in situ, the capsules were embedded in cement paste and then observed under scanning electron microscopy (SEM) and optical microscopy (OM). This investigation into the use of greener materials for shell production complements the current goal of reducing the environmental impact of cementitious materials through self-healing.
2. Materials and Methods
2.1. Selection of Biobased Acrylates
To select a suitable shell material for testing, a literature search was conducted to find biobased material options and evaluate their potential for use within microcapsule shells. Tensile strength, ductile transition temperature and global scale were considered and once AESO was found to be the strongest contender, the following testing was completed to assess practical feasibility in microfluidic production.
The viscosities of the AESO monomers were measured with a MCR302 rheometer from Anton Paar. For this, AESO (44,779 mPa·s) was diluted with 50 and 75 wt% reactive diluents IBOA and HDDA, with resultant samples named AESO:HDDA, AESO:3HDDA, AESO:IBOA and AESO:3IBOA, respectively. The results are displayed in Figure 2.
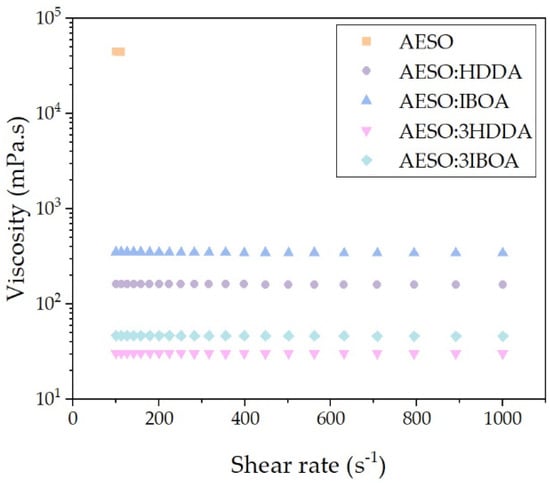
Figure 2.
Shear viscosity of AESO, AESO:HDDA, AESO:3HDDA, AESO:IBOA and AESO:3IBOA.
2.2. Production of Microcapsules
To produce the double emulsions, a microfluidic device with four flow-focusing channels in parallel was placed in a Telos device (Dolomite Microfluidics, UK), as described elsewhere [18]. The inner fluid was injected using a syringe pump (Aladdin AL-1000) at a flow rate range of 10–60 μL·min−1; the middle and outer fluids were injected using pressure pumps (Dolomite Microfluidics, UK) at typical flow rates of 10–30 μL·min−1 and 300–500 μL·min−1, respectively. Mineral oil (light, Sigma Aldrich, density of 0.838 g·mL−1, viscosity 29.3 mPa·s) was used for the inner phase and model core material. For the outer/continuous phase, an aqueous solution with 5 wt% poly(vinyl alcohol) (PVA, MW 13,000–23,000, 87–89% hydrolysed, viscosity 4.99 mPa·s) was used. To adjust the viscosity, soybean oil epoxidized acrylate (AESO, Sigma Aldrich) was diluted in 75 wt% 1,6-Hexanediol diacrylate (HDDA, Sigma Aldrich) to form sample AESO:3HDDA; and 75 wt% isobornyl acrylate (IBOA, Sigma Aldrich) to form sample AESO:3IBOA. In both cases, 0.5 wt% of photoinitiator hydroxy-2-methylpropiophenone was added to the solution. The double emulsions were collected in an aqueous solution of 5 wt% PVA solution and polymerised using a UV-lamp (Omnicure, 50% opening) exposed over the collection tube shortly after the formation of the double emulsion droplets, as represented in Figure 3.
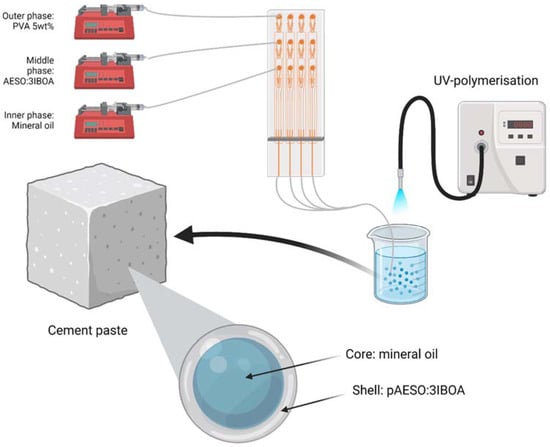
Figure 3.
Schematics of the microfluidic production of microcapsules used in this work. Created with BioRender.com.
2.3. Characterisation of the Microcapsules
The outer diameter of the produced double emulsions and microcapsules were measured with an optical microscope (OM) (DM 2700 M, Leica, Germany). The calculated shell thickness was estimated according to Equation (1):
where Douter and Dinner represent outer and inner diameter, respectively, Q1 represents inner flow rate and Q2 represents middle flow rate. To assess the thermal stability and oil content, the microcapsules, mineral oil and bulk polymerised acrylate were analysed using a thermogravimetric analysis (TGA, PerkinElmer STA6000) between 50 and 700 °C at a rate of 5 °C/min, under air atmosphere. For the investigation into the behaviour of the microcapsules in cement paste, 5% by weight of cement of microcapsules were mixed with Ordinary Portland cement (CEM I 42.5) provided by Heildelberg-UK and water (w/c = 0.45). The mixture was then cast into oiled silicone moulds with a volume of 10 × 10 × 50 mm3. After 28 days of curing, the samples were broken, and it was possible to see the oil coming out of the microcapsules and leaking onto the sample. To investigate the interfacial bonding between the capsule and cement paste, a scanning electron microscope (SEM, Evo LS15, Zeiss) was used.
To quantify the mechanical properties of the polymers, 1.5 mL of acrylate was poured over the negative dog bone shaped mould (70 mm length, 11 mm width, 3 mm height and 6 mm gauge width) made of PDMS. The mould was then covered with paraffin oil, to prevent the formation of a meniscus, and UV-polymerised (Omnicure, 50% opening) for at 365 nm for 5 min. After 1 week of material polymerisation, uniaxial tensile tests were performed with an Instron Testing system using a loading cell. The testing protocol used a constant pulling rate of 2 mm/min. The measured force was normalised by the undeformed cross-sectional area to obtain stress, and the extension was normalised by the undeformed segment length to calculate strain. From the resulting stress–strain curve, the elastic Young’s modulus was obtained from the slope of a line fit to the elastic loading regime, the ultimate tensile stress was obtained from the maximum stress value measured during loading, and the maximum strain was obtained from the data point just prior to failure. The dynamic mechanical analysis (DMA) tests were performed on a DMA 8000 (PerkinElmer) in single cantilever mode with an oscillating frequency of 2 Hz. The cured samples with a size of 15 × 6 × 0.09 mm3 were examined at a heating rate of 3 °C/min. To investigate the dispersion of the microcapsules embedded in cement paste, a Nikon XT H 225 ST X-ray microcomputed tomography (XCT) scanner was used. The analysis was performed with a 2.5 mm Cu filter, a 53 min scan, exposure time of 1000 ms, 3141 projections, 2000 detector pixels and a source to detector distance of 817 mm. The test was performed in a cubic sample with 10 mm side, with a source to object distance of 33 mm, acceleration voltage of 100 kV, filament current of 60 μA and voxel size of 8 μm. The X-ray images were subsequently used to reconstruct the 2D and 3D sample using the commercial software VGStudio MAX 2.2 (Volume Graphics, Heidelberg, Germany). To improve the quality of the images, the data was smoothed using a Gaussian filter before analysis. After a region of interest was selected to remove all the air surrounding the sample, a surface fit function was applied using the cementitious materials as background and the air as material. As a result, the threshold value between the cementitious matrix and the air/microcapsules creates a 3D surface used to identify the microcapsules and the pores in the matrix. The general schematic of the experimental plan is described in Figure 4.
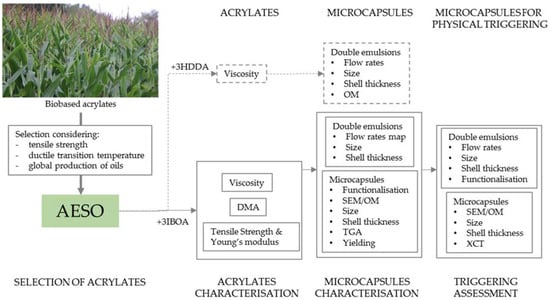
Figure 4.
Schematic of work carried out in this study. Dashed lines indicate work carried out using HDDA to decrease the viscosity.
3. Results
3.1. Selection of Biobased Acrylates
Tensile strength and ductile transition temperature were identified as key factors which impact both rupture characteristics and survival probability during the cement mixing process, and therefore success of a microcapsule. Microcapsule shells require a low enough tensile strength to facilitate rupture within the cementitious matrix upon coinciding with a propagating crack [44]. However, the capsules also need to survive the shear forces experienced during their initial mixing with cement [7]. The range of tensile strengths deemed suitable for use in microcapsule shell materials was obtained using a comparative study with 10 shell materials used in previous studies for self-healing within concrete. These were: polymethyl-methacrylate (PMMA) [45], polyurea [46]; urea formaldehyde resin [47], polylactic acid (PLA) [48], polystyrene (PS) and polymethyl methacrylate/n-butyl methacrylate (PMMA/n-BMA) [49]; phenol-formaldehyde (PF) [50], polyurethane [10] and 50 wt% 1,6-hexanediol and 50 wt% bisphenol A glycerolatedimethacrylate (BH) and 50 wt% isobornyl acrylate and 50 wt% bisphenol A glycerolatedimethacrylate (BI) produced to encapsulate mineral oil and colloidal silica [51].
A wide range of biobased acrylates were investigated, and tensile strength data, obtained from previous literature, is summarized in Figure 5, which compares the results to the 10 previously used shell materials and highlighted potential contenders for more sustainable shells. Figure 5 shows in teal, previous shell materials and in orange, biobased acrylate properties.
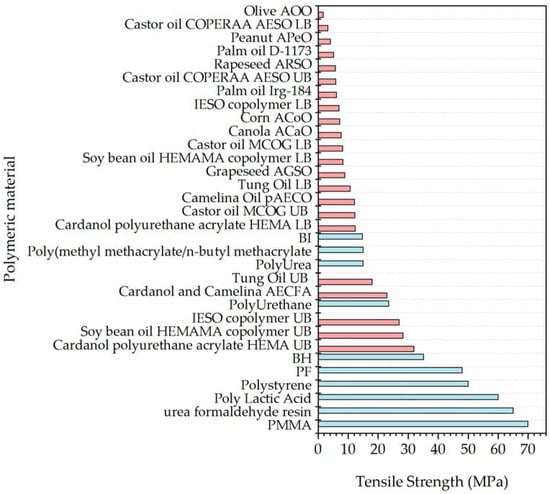
Figure 5.
Tensile strength of selected acrylates [10,17,36,37,38,39,40,41,42,43,45,46,47,49,50,52,53,54,55,56,57,58,59,60]. Teal bars indicates materials previously used whereas the orange bars represent biobased acrylates.
Tung oil-derived polymers [43], cardanol and camelina oil-derived acrylates [36,53], and soy-bean acrylates [38,39] had the highest tensile strength of all the investigated materials and were stronger than BI, Polyurea and PMMA/n-BMA, therefore demonstrating a strong likelihood of mixing survival. However, this did not rule out use of the weaker polymers, as a benefit of using a shell material with a lower tensile strength is that they are more likely to rupture under crack propagation within concrete. Therefore, as long as the capsule is strong enough to survive mixing, it has potential to be utilised in microcapsule formation.
Ductile transition can assist with mixing survival. Although it is desirable in terms of rupture properties for the capsule shell to be in a brittle regime when cast into concrete, the exothermic conditions of cement curing could utilise a ductile transition of the shells during mixing, and increase survivability [49]. Although glass transition temperature and brittle to ductile transition temperature do not always coincide, an approximation by Boyer was used in this analysis, which predicts brittle to ductile transition to occur at a temperature given by Equation (2) [61].
where Tb is the brittle to ductile transition temperature and Tg is the glass transition temperature. Figure 6 shows the ductile transition temperature for acrylates explored in this work.
Tb = 0.75 × Tg
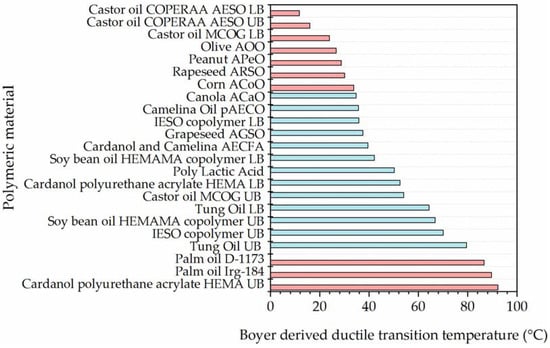
Figure 6.
Ductile transition temperature of selected acrylates [36,37,38,39,40,41,42,43,52,53]. Acrylate with glass transition temperature in the range of 35–80 °C are indicated in teal.
Many of the investigated polymers have a predicted ductile transition temperature within the range 35–80 °C, which are the range of cement temperatures during curing. This means these polymers are likely to have a transition to ductile state within this range and increases their survivability likelihood in the mixing process. Therefore, polymers with transition temperature in this range can potentially be suitable for microcapsule shell material even if tensile strength is considerably lower than previously used materials.
However, another key consideration for a shell material is the feedstock availability. These need to be produced at a high enough scale to enable production of microcapsules in cement at a commercial level. Values of global production for each oil were collated and are shown in Figure 7.
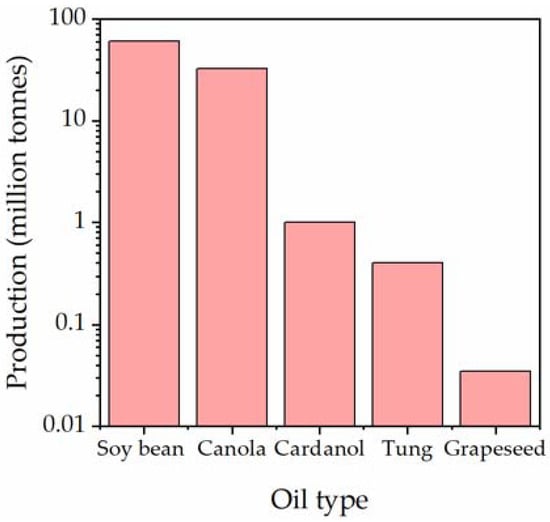
Figure 7.
Global production of oils [62,63,64,65,66].
With a focus on the feedstocks of the candidates which showed the most promising mechanical properties, an assessment was made to rule out grapeseed oil, tung oil and cardanol as they are produced at relatively low levels which could result in problems with scale when self-healing cement is more intensely produced. On the other hand, canola oil is produced at large quantities, offering a more feasible option in terms of scale. Soy-bean oil presents the most abundant production, combining this with the well-established industry producing AESO, soy-bean oil-derived acrylates are identified here as the most suitable candidate for a sustainable microcapsule shell material.
3.2. Production of Microcapsules
Although using acrylated epoxidised soybean oil (AESO) as a shell material provides a more sustainable method of producing microcapsules, demonstrating the formation of microcapsules and physical triggering of the shell is required to establish this greener acrylate as a viable shell material. To investigate the formation of microcapsules, the first step was to use a microfluidic device to create double emulsions. To obtain a suitable viscosity for the middle phase, AESO was diluted 1:3 in 1,6-hexanediol diacrylate (HDDA), a commonly used diluent [20,67], resulting in a viscosity of 30.5 mPa·s. This viscosity was close to the values of 73.3 mPa·s reported for trimethylolpropane ethoxylate triacrylate previously used in the Telos microfluidic device [18]. Mineral oil was used as the inner phase and 5% PVA was used as the outer phase. The inner, middle, and outer phases were run with flow rates of 30, 48, and 410 µL/min, respectively. As illustrated in Figure 8, double emulsions were successfully formed. After exposure to UV-light, the shell polymerized, forming microcapsules with an outer diameter of 488 ± 22 µm and a calculated shell thickness of 56 µm. Although the encapsulation of the material was carried out successfully, the utilisation of HDDA as the main component of the shell still resulted in a high carbon content derived from fossil fuels.
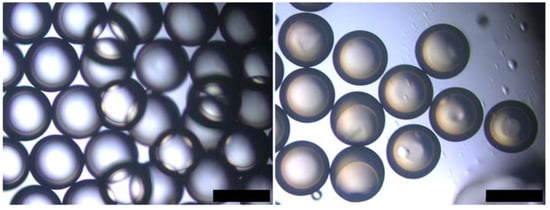
Figure 8.
Production of microcapsules with soybean oil-based shell. (left) Double emulsions produced with mineral oil, AESO:3HDDA 1:3 and PVA5% as inner, middle and outer phase; (right) Microcapsules formed after the photopolymersiation of the double emulsion template. Scale bar: 500 µm.
Aiming at increasing the proportion of biobased acrylate, isobornyl acrylate (IBOA), an acrylate produced from camphene (derived from pine trees) [68] was investigated to adjust the viscosity of AESO. To form the double emulsions, mineral oil was used as the inner phase with a flow rate varying between 10–40 μL·min−1, while the middle flow rate was kept constant at 24 μL/min with AESO:3IBOA and the outer flow rate at 370 μL·min−1 of PVA 5 wt% with 0.5 wt% of acrylic acid. The double emulsions were successfully formed at lower flow rates, typically around 10 μL/min for inner phase, as shown in Figure 9a.
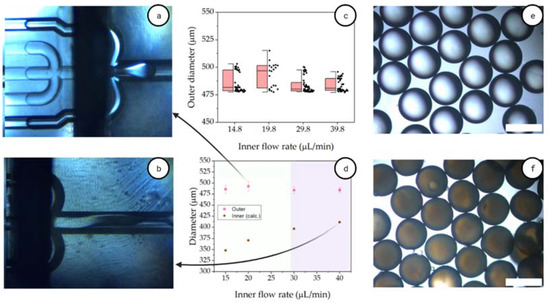
Figure 9.
Formation of microcapsules using AESO:3IBOA. Dragging (a) and dripping (b) regime where the double emulsion is formed; (c) box plot of outer diameter of the double emulsions; (d) outer diameter and calculated inner diameter for the double emulsions; Double emulsions (e) and capsules (f) formed at flow rates 29.8, 24 and 370 μL/min for inner, middle and outer fluids. Scale bar indicates 500 μm.
However, lower flow rates for the middle phase resulted in a small throughput of formed material and also thicker shells. Once there was an increase in the inner flow rate, the fluid started dragging down the channel (Figure 9a). The threshold between dripping and dragging was typically between 30 and 40 μL/min, for flows of 24 and 370 μL/min for middle and outer fluids, respectively (Figure 9d). This transition between dripping and jetting for microfluidics is a well-understood phenomenon, mainly governed by the capillary number [69,70]. When there is an increase in the capillary number, typically due to an increase in flow rate and/or increased viscosity, the phase starts dragging down the channel. Furthermore, the hydrophobicity of the second half of the channel needs to be ensured, in order to hinder this dragging. By increasing the flow rate even further, the fluid dragged even further down the channel and no double emulsion was formed.
The double emulsion outer diameter varied slightly due to the increase of inner flow rate, with average values of 486 ± 9 μm, as shown in Figure 9c. The low coefficient of variation is confirmed by the narrow distribution of measurements of double emulsion. By varying the inner flow rate between 15 and 40 μL/min, the calculated shell thickness varied between 36–67 μm (Figure 9d). At these flow rates, the throughput of material varied between 2.4 and 3.9 g/h. This is a low throughput, with previous reports of capsules being formed stable at flow rate of 13 g/h [18]. However, more chips can be placed in parallel increasing the production up to 6-fold. Figure 9e represents typical double emulsions used for outer diameter measurements, as well as microcapsules (Figure 9f), formed at flow rates of 29.8, 24 and 370 μL/min for inner, middle and outer fluids, respectively.
3.3. Characterisation of Microcapsules and Shell Materials
The core–shell structure was successfully formed and effectively retained the model core material. To examine this, capsules were formed with flow rates of 20 μL/min of mineral oil as the inner phase, 24 μL/min of AESO:3IBOA as the middle phase and 363 μL/min of PVA 5 wt% with 0.5 wt% acrylic acid as the continuous phase. This produced double emulsions (Figure 10a) and microcapsules (Figure 10b) with a size of 493 ± 11 μm and calculated shell thickness of 57 μm. Unlike other microcapsules, and the material formed with AESO:3HDDA, it was remarkably difficult to distinguish between the core and shell structure using only optical microscopy, both for the double emulsion and the microcapsules. The core shell structure was confirmed by lightly pressing the microcapsules in between two glass slides by which the capsules ruptured as shown in Figure 10c, where the shell is clearly visible. Scanning electron microscopy (Figure 10e,f) confirmed the ruptured capsules and revealed a shell thickness around 59 µm.
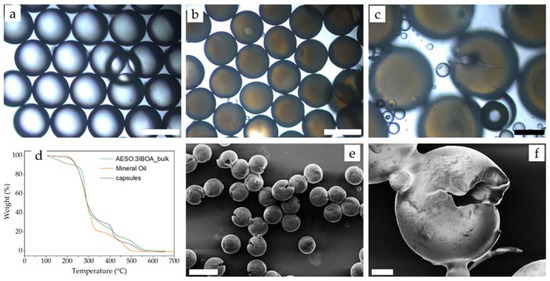
Figure 10.
Microcapsules produced at flow rates of 19.8, 24 and 363 μL/min for with mineral oil as inner, AESO:3IBOA as middle and PVA 5% as outer. Optical microscopy of (a) double emulsions (scale bar 500 μm), (b) microcapsules (scale bar 500 μm) and (c) ruptured microcapsules (scale bar 200 μm); (d) thermogravimetrical analysis of formed microcapsules; (e) SEM of ruptured microcapsules (scale bar 800 μm) and (f) SEM showing the core–shell structure of formed microcapsules (scale bar 100 μm).
Furthermore, thermogravimetric analysis (TGA) was used to investigate the behaviour of the microcapsules at elevated temperatures, as shown in Figure 10d. In this case, three distinct peaks were observed: (i) the first, from 195–325 °C, where 66% of weight was lost, associated with the loss of core and shell material; (ii) the second, peaking around 465 °C, associated with decomposition of mineral oil (orange line); and (iii) the third from 465 °C onwards, associated with the decomposition of only the acrylate shell. Thus, considering the weight of the capsules and the bulk shell at 518 °C, it is estimated that 62% of the weight was associated with the shell material and 38% was attributed to the core. Combining this with the inner and middle flow rates, and assuming the density of the middle layer was the same as the density of IBOA, resulted in an encapsulation efficiency of 92%. During the production of the capsules, virtually no density mismatch deformation of the shell was observed, resulting in no loss of the core. This was attributed to the low interfacial tension in between the core and the acrylates, as well as the similar density of the shell and core. As a result, the production rate was 2.38 g/h, measured by collecting the capsules for 30 min and followed by filtering and drying.
Beyond effectively retaining the core, another relevant characteristic of the microcapsules was the timely release of its core when triggered. In the case of physically triggered self-healing, the formation of the crack in the matrix also triggers the shell, resulting in the release of the core. The likelihood of a crack encountering microcapsules and triggering self-healing is largely dependent on three factors: (i) the Young’s modulus of the capsules, (ii) the interfacial bonding between the capsule and matrix, and (iii) the tensile strength of the shell [44,71]. For this, the Young’s modulus of the shell, as well as its tensile strength, play a key role. For AESO:3IBOA, i.e., the sample with 75 wt% IBOA, the Young’s modulus was 1.7 ± 0.4 GPa and the ultimate tensile strength was 29.2 ± 7.7 MPa with a strain of 2.1 ± 0.8%, as depicted in Figure 11a. Results with a similar order of magnitude have previously been reported for similar mixtures of AESO and isobornyl acrylates [72,73]. The mismatch in elastic modulus between the capsules’ shell and the cementitious matrix, contributes to the crack being attracted by the microcapsules [44,71]. Although the shell material presents a tensile strength higher than typical values for cementitious systems, and higher than some capsules described in Figure 4, by adjusting the interfacial bonding and the shell thickness of the capsules, physical triggering can be promoted. Furthermore, other reactive diluents [73] could be explored with the aim of adjusting the viscosity while enabling suitable mechanical properties. Figure 11b demonstrates DMA curves of the AESO:3IBOA with the glass transition temperature (Tg) values determined from the peak temperatures of tan δ curve. The acrylate showed a storage modulus at 25 °C of 1 × 1013 Pa and Tg of 56 °C. Using Equation (2), the ductile transition temperature for the AESO:3IBOA shell can be estimated as 42 °C, precisely in the range of cement temperature during curing.
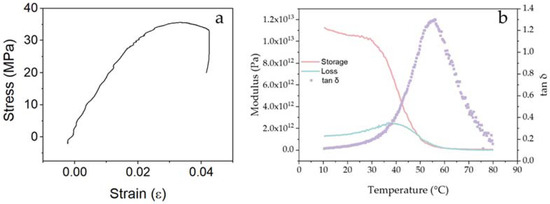
Figure 11.
Mechanical characterisation of AESO:3IBOA. (a) tensile stress–strain curve and (b) Modulus–temperature and tangent delta–temperature curves gathered by DMA.
3.4. Production of Microcapsules for Physically Triggered Self-Healing
The physical triggering of microcapsules is facilitated by increasing the interfacial bonding between the capsules and the cementitious matrix and decreasing the shell thickness. However, the hydrophobic nature of the acrylate layer hinders the formation of polar and covalent bonds with the cement mix. In order to enhance the bonding between the capsules and the cement paste, the shell surface was functionalised with polar groups, increasing the likelihood of the capsules bonding with the matrix. To demonstrate this, double emulsions (Figure 12a) and microcapsules (Figure 12b) were produced with flow rates of 49.8, 19 and 432 μL·min−1 using mineral oil as the inner phase, AESO:3IBOA as the middle phase and PVA 5% with 1 wt% acrylic acid as the outer phase. During the polymerisation, those acrylic groups were added to the surface of the capsules [17]. Despite the presence of acrylic acid in the outer phase, no clustering of the shells was observed during the production of microcapsules, as shown in Figure 12b,c. Double emulsions (Figure 12a) and microcapsules (Figure 12b) with sizes of 481 ± 4 µm were produced with a calculated shell thickness of 25 µm. After rupturing a subset of the microcapsules in-between glass slides, a thin shell was observed, as well as core release, as depicted in Figure 12c. These capsules were cast in cement paste, (w/c = 0.45) and SEM was used to examine shell rupture. Multiple broken microcapsules were observed on the crack surface, confirming the likelihood of the timely release of the core, appropriated for physical triggering (Figure 12d–f). Furthermore, a shell thickness of around 6 μm (Figure 12e,f) was observed, indicating a possible miscalibration of the pressure pump. The formation of hydrating products on the surface of the acrylate shell (Figure 12d) indicated the effective functionalisation of the shell and enhanced interfacial bond strength. A nondestructive XCT scan was performed in order to examine the uniform distribution of microcapsules throughout the cement paste sample. Figure 12g–i depicts the 2D reconstruction of the samples, where the light grey represents the cement paste and the dark grey circular dots around 500 μm represent the microcapsule distribution. Small dark grey circles ~100 μm are also observed and associated with air bubbles [74]. A 3D reconstruction of the sample (Figure 12h) with a green shell surface demonstrates the uniform distribution of microcapsules throughout the sample. According to the literature, capsules can agglomerate within the matrix through two distinct mechanisms: if the capsules cluster during its production, during the photopolymerisation or filtering step, it is possible that they do not separate during mixing and therefore remain in clumps [51]. In this study, however, agglomeration was prevented by collecting the capsules in PVA solution, as shown in Figure 12b, which depicts clearly dispersed capsules. Alternatively, the density differences accentuated by the shaking table may cause the capsules to float and concentrate closer to the cementitious matrix’s surface [75]. Adjusting the material’s viscosity and/or the capsules’ density can reduce this anisotropy. Consequently, a number of authors have reported the uniform distribution of capsules in cementitious matrices for both laboratory tests and in situ applications [50,76,77]. To further understand the performance of these new microcapsules, the encapsulation of healing agents, instead of model mineral oil as inner fluid, followed by quantification of self-healing performance is recommended.
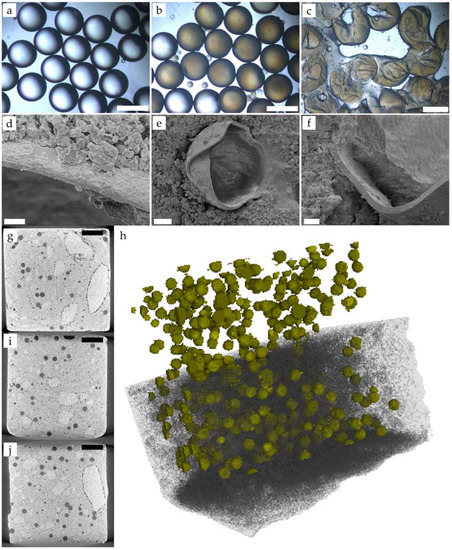
Figure 12.
Microcapsules with functionalised shell produced at flow rates of 49.8, 19 and 432 μL/min for with mineral oil as inner phase, AESO:3IBOA as middle phase and PVA 5% with 1 wt% acrylic acid as outer phase. Optical microscopy of (a) double emulsions, (b) microcapsules and (c) ruptured microcapsules (scale bar 500 μm); SEM showing the microcapsules embedded in cement paste ((d,e): scale bar 10 μm; (f): scale bar 20 μm); XCT of cement paste showing the dispersion of microcapsules in 2D ((g,i,j); scale bar 2 mm) and 3D (h).
4. Conclusions
This study demonstrates for the first time the production of self-healing microcap-sules with biobased acrylate shells using microfluidics. A throughout review of the literature was performed, considering a wide range of biobased acrylates according to global production and parameters associated with physical triggering and survival during mixing. According to those parameters, soybean oil was the most suitable contender for the production of acrylated microcapsules. The viscosity is another key parameter governing the applicability of these acrylates for microcapsules production using microfluidics, thus the acrylated epoxidised soybean oil (AESO) viscosity was adjusted to the range of 30–50 mPa·s with 1,6-hexanediol acrylate (HDDA) and biobased isobornyl acrylate (IBOA). However, the use of 75 wt% of the shell as HDDA remains an issue due to the high carbon content derived from fossil fuels. Capsules were successfully produced using IBOA and AESO as shell, with outer diameter of 486 ± 9 μm with calculated shell thickness ranging between 36 and 67 μm. SEM and OM demonstrated a clear core–shell structure and confirmed the calculated shell thickness of the capsules. The retention of mineral oil was confirmed by thermogravimetric analysis of dried capsules and indicated that ~50% of the capsules consisted of the core material. Furthermore, production rates of 2.38 g/h were confirmed, resulting in an 92% encapsulation efficiency. The uniaxial tension test of AESO with 75 wt% IBOA was conducted, resulting in a Young’s modulus of 1.7 ± 0.4 GPa, ultimate tensile strength of 29.2 ± 7.7 MPa and a strain at failure of 2.1 ± 0.8%. Using DMA, the ductile transition temperature was estimated as 42 °C, increasing the likelihood of the capsules surviving mixing. Microcapsules were produced with a size of 481 ± 4 μm and functionalised shells’ surface and embedded in cement paste; upon introducing a crack in the cementitious matrix, several broken capsules were observed, with a measured shell thickness around 6 μm. Furthermore, good interfacial bonds between the capsules and the cement paste were observed, facilitating the rupture, and XCT confirmed a good distribution of the capsules throughout the samples. These results support the extensive application of biobased resins for the creation of novel sustainable microcapsules for self-healing in cementitious materials.
Author Contributions
Conceptualization, L.R.d.S., B.W. and A.A.-T.; methodology, L.R.d.S. and B.W.; validation, L.R.d.S.; formal analysis, L.R.d.S. and B.W.; investigation, L.R.d.S.; resources, A.A.-T.; data curation, L.R.d.S. and B.W.; writing—original draft preparation, L.R.d.S. and B.W.; writing—review and editing, L.R.d.S., B.W. and A.A.-T.; visualization, L.R.d.S.; supervision, project administration, funding acquisition, A.A.-T. All authors have read and agreed to the published version of the manuscript.
Funding
Aspects of this work reported here was carried out as part of an MEng research project by the second author [35]. The authors also acknowledge the financial support provided to the first author from the UKRI-EPSRC, grant number EP/P02081X/1 (Resilient Materials 4 Life, RM4L).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due being part of an ongoing study.
Acknowledgments
The viscosity measurements executed by Niamh Fox and the SEM analysis performed by Graham Smith are kindly acknowledged. The authors also acknowledge the technical support provided by Chris Knight. The authors would like to thank Andrew Worton for his contribution with the schematics representation (Figure 3).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Office for National Statistics Construction Statistics Annual Tables. Available online: https://www.ons.gov.uk/businessindustryandtrade/constructionindustry/datalist (accessed on 29 September 2022).
- De Belie, N.; Gruyaert, E.; Al-Tabbaa, A.; Antonaci, P.; Baera, C.; Bajare, D.; Darquennes, A.; Davies, R.; Ferrara, L.; Jefferson, T.; et al. A Review of Self-Healing Concrete for Damage Management of Structures. Adv. Mater. Interfaces 2018, 5, 1800074. [Google Scholar] [CrossRef]
- Wang, J.Y.; Soens, H.; Verstraete, W.; De Belie, N. Self-Healing Concrete by Use of Microencapsulated Bacterial Spores. Cem. Concr. Res. 2014, 56, 139–152. [Google Scholar] [CrossRef]
- Kanellopoulos, A.; Giannaros, P.; Al-Tabbaa, A. The Effect of Varying Volume Fraction of Microcapsules on Fresh, Mechanical and Self-Healing Properties of Mortars. Constr. Build. Mater. 2016, 122, 577–593. [Google Scholar] [CrossRef]
- Alghamri, R.; Kanellopoulos, A.; Al-Tabbaa, A. Impregnation and Encapsulation of Lightweight Aggregates for Self-Healing Concrete. Constr. Build. Mater. 2016, 124, 910–921. [Google Scholar] [CrossRef]
- Dong, B.; Fang, G.; Wang, Y.; Liu, Y.; Hong, S.; Zhang, J.; Lin, S.; Xing, F. Performance Recovery Concerning the Permeability of Concrete by Means of a Microcapsule Based Self-Healing System. Cem. Concr. Compos. 2017, 78, 84–96. [Google Scholar] [CrossRef]
- Kanellopoulos, A.; Giannaros, P.; Palmer, D.; Kerr, A.; Al-Tabbaa, A. Polymeric Microcapsules with Switchable Mechanical Properties for Self-Healing Concrete: Synthesis, Characterisation and Proof of Concept. Smart Mater. Struct. 2017, 26, 045025. [Google Scholar] [CrossRef]
- Beglarigale, A.; Eyice, D.; Seki, Y.; Yalçınkaya, Ç.; Çopuroğlu, O.; Yazıcı, H. Sodium Silicate/Polyurethane Microcapsules Synthesized for Enhancing Self-Healing Ability of Cementitious Materials: Optimization of Stirring Speeds and Evaluation of Self-Healing Efficiency. J. Build. Eng. 2021, 39, 102279. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Y.; Huang, Y.; Zhang, J.; Fang, C.; Yu, K.; Chen, Q.; Li, T.; Han, R.; Yang, Z.; et al. Laboratory and Field Study on the Performance of Microcapsule-Based Self-Healing Concrete in Tunnel Engineering. Constr. Build. Mater. 2019, 220, 90–101. [Google Scholar] [CrossRef]
- Beglarigale, A.; Seki, Y.; Demir, N.Y.; Yazıcı, H. Sodium Silicate/Polyurethane Microcapsules Used for Self-Healing in Cementitious Materials: Monomer Optimization, Characterization, and Fracture Behavior. Constr. Build. Mater. 2018, 162, 57–64. [Google Scholar] [CrossRef]
- Al-Tabbaa, A.; Litina, C.; Giannaros, P.; Kanellopoulos, A.; Souza, L. First UK Field Application and Performance of Microcapsule-Based Self-Healing Concrete. Constr. Build. Mater. 2019, 208, 669–685. [Google Scholar] [CrossRef]
- Davies, R.; Teall, O.; Pilegis, M.; Kanellopoulos, A.; Sharma, T.; Jefferson, A.; Gardner, D.; Al-Tabbaa, A.; Paine, K.; Lark, R. Large Scale Application of Self-Healing Concrete: Design, Construction, and Testing. Front. Mater. 2018, 5, 51. [Google Scholar] [CrossRef]
- Litina, C.; Cao, B.; Chen, J.; Li, Z.; Papanikolaou, I.; Al-Tabbaa, A. First UK Commercial Deployment of Microcapsule-Based Self-Healing Reinforced Concrete. J. Mater. Civ. Eng. 2021, 33, 04021095. [Google Scholar] [CrossRef]
- Garces, J.I.T.; Dollente, I.J.; Beltran, A.B.; Tan, R.R.; Promentilla, M.A.B. Life Cycle Assessment of Self-Healing Geopolymer Concrete. Clean. Eng. Technol. 2021, 4, 100147. [Google Scholar] [CrossRef]
- Bruyninckx, K.; Dusselier, M. Sustainable Chemistry Considerations for the Encapsulation of Volatile Compounds in Laundry-Type Applications. ACS Sustain. Chem. Eng. 2019, 7, 8041–8054. [Google Scholar] [CrossRef]
- Giannaros, P.; Kanellopoulos, A.; Al-Tabbaa, A. Sealing of Cracks in Cement Using Microencapsulated Sodium Silicate. Smart Mater. Struct. 2016, 25, 084005. [Google Scholar] [CrossRef]
- Souza, L.; Al-Tabbaa, A. Microfluidic Fabrication of Microcapsules Tailored for Self-Healing in Cementitious Materials. Constr. Build. Mater. 2018, 184, 713–722. [Google Scholar] [CrossRef]
- de Souza, L.R.; Al-Tabbaa, A. High Throughput Production of Microcapsules Using Microfluidics for Self-Healing of Cementitious Materials. Lab Chip 2021, 21, 4652–4659. [Google Scholar] [CrossRef]
- Utada, A.S.; Lorenceau, E.; Link, D.R.; Kaplan, P.D.; Stone, H.A.; Weitz, D.A. Monodisperse Double Emulsions Generated from a Microcapillary Device. Science 2005, 308, 537–541. [Google Scholar] [CrossRef]
- Chen, P.W.; Cadisch, G.; Studart, A.R. Encapsulation of Aliphatic Amines Using Microfluidics. Langmuir 2014, 30, 2346–2350. [Google Scholar] [CrossRef]
- Arriaga, L.R.; Amstad, E.; Weitz, D.A. Scalable Single-Step Microfluidic Production of Single-Core Double Emulsions with Ultra-Thin Shells. Lab Chip 2015, 15, 3335–3340. [Google Scholar] [CrossRef]
- Xu, S.; Nisisako, T. Polymer Capsules with Tunable Shell Thickness Synthesized via Janus-to-Core Shell Transition of Biphasic Droplets Produced in a Microfluidic Flow-Focusing Device. Sci. Rep. 2020, 10, 4549. [Google Scholar] [CrossRef]
- Pirman, T.; Ocepek, M.; Likozar, B. Radical Polymerization of Acrylates, Methacrylates, and Styrene: Biobased Approaches, Mechanism, Kinetics, Secondary Reactions, and Modeling. Ind. Eng. Chem. Res. 2021, 60, 9347–9367. [Google Scholar] [CrossRef]
- Bauer, F.; Mehnert, R. UV Curable Acrylate Nanocomposites: Properties and Applications. J. Polym. Res. 2005, 12, 483–491. [Google Scholar] [CrossRef]
- Litina, C.; Palmer, D.; Al-Tabbaa, A. A Novel Membrane Emulsification Technique for Microencapsulation in Self-Healing Concrete: Development and Proof of Concept. Eng. Res. Express 2021, 3, 025015. [Google Scholar] [CrossRef]
- Petrescu, L.; Fermeglia, M.; Cormos, C.-C. Life Cycle Analysis Applied to Acrylic Acid Production Process with Different Fuels for Steam Generation. J. Clean. Prod. 2016, 133, 294–303. [Google Scholar] [CrossRef]
- Fischedick, M.; Roy, J.; Abdel-Aziz, A.; Acquaye, A.; Allwood, J.M.; Ceron, J.-P.; Geng, Y.; Kheshgi, H.; Lanza, A.; Perczyk, D.; et al. 2014: Industry. In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Mallesham, B.; Babu, P.S.; Li, H.; Sudarsanam, P. Catalytic Conversion of Acrolein and Acrylic Acid Drop-Ins for Added-Value Chemicals. Adv. Catal. Drop Chem. 2022, 47–62. [Google Scholar] [CrossRef]
- Cespi, D.; Passarini, F.; Mastragostino, G.; Vassura, I.; Larocca, S.; Iaconi, A.; Chieregato, A.; Dubois, J.L.; Cavani, F. Glycerol as Feedstock in the Synthesis of Chemicals: A Life Cycle Analysis for Acrolein Production. Green Chem. 2014, 17, 343–355. [Google Scholar] [CrossRef]
- Liu, L.; Ye, X.P.; Bozell, J.J. A Comparative Review of Petroleum-Based and Bio-Based Acrolein Production. Chem. Sus. Chem. 2012, 5, 1162–1180. [Google Scholar] [CrossRef]
- Alberici, G. Toop Overview of UK Biofuel Producers. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/308142/uk-biofuel-producer.pdf (accessed on 14 October 2022).
- MPA Cement Industry Leaflet. Available online: https://cement.mineralproducts.org/documents/DL-Cement-Industry-leaflet-29-05-14web.pdf (accessed on 29 September 2022).
- Wu, L.; Dutta, S.; Mascal, M. Efficient, Chemical-Catalytic Approach to the Production of 3-Hydroxypropanoic Acid by Oxidation of Biomass-Derived Levulinic Acid with Hydrogen Peroxide. ChemSusChem 2015, 8, 1167–1169. [Google Scholar] [CrossRef]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fitzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of Levulinic Acid and Use as a Platform Chemical for Derived Products. Resour. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Whitfield, B. Sustainable Scale-up of Microfuildics-Based Production of Self-Healing Microcapsules. Master’s Thesis, University of Cambridge, Cambridge, UK, 2021. [Google Scholar]
- Hu, Y.; Shang, Q.; Bo, C.; Jia, P.; Feng, G.; Zhang, F.; Liu, C.; Zhou, Y. Synthesis and Properties of UV-Curable Polyfunctional Polyurethane Acrylate Resins from Cardanol. ACS Omega 2019, 4, 12505–12511. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Sun, X.S. Epoxidized and Acrylated Epoxidized Camelina Oils for Ultraviolet-Curable Wood Coatings. J. Am. Oil Chem. Soc. 2018, 95, 1307–1318. [Google Scholar] [CrossRef]
- Wu, Q.; Hu, Y.; Tang, J.; Zhang, J.; Wang, C.; Shang, Q.; Feng, G.; Liu, C.; Zhou, Y.; Lei, W. High-Performance Soybean-Oil-Based Epoxy Acrylate Resins: “Green” Synthesis and Application in UV-Curable Coatings. ACS Sustain. Chem. Eng. 2018, 6, 8340–8349. [Google Scholar] [CrossRef]
- Li, P.; Ma, S.; Dai, J.; Liu, X.; Jiang, Y.; Wang, S.; Wei, J.; Chen, J.; Zhu, J. Itaconic Acid as a Green Alternative to Acrylic Acid for Producing a Soybean Oil-Based Thermoset: Synthesis and Properties. ACS Sustain. Chem. Eng. 2017, 5, 1228–1236. [Google Scholar] [CrossRef]
- Liu, C.; Wang, C.; Hu, Y.; Zhang, F.; Shang, Q.; Lei, W.; Zhou, Y.; Cai, Z. Castor Oil-Based Polyfunctional Acrylate Monomers: Synthesis and Utilization in UV-Curable Materials. Prog. Org. Coat. 2018, 121, 236–246. [Google Scholar] [CrossRef]
- Liang, B.; Li, R.; Zhang, C.; Yang, Z.; Yuan, T. Synthesis and Characterization of a Novel Tri-Functional Bio-Based Methacrylate Prepolymer from Castor Oil and Its Application in UV-Curable Coatings. Ind. Crops Prod. 2019, 135, 170–178. [Google Scholar] [CrossRef]
- Salih, A.M.; Ahmad, M.B.; Ibrahim, N.A.; Dahlan, K.Z.H.M.; Tajau, R.; Mahmood, M.H.; Yunus, W.M.Z.W. Molecules Synthesis of Radiation Curable Palm Oil-Based Epoxy Acrylate: NMR and FTIR Spectroscopic Investigations. Molecules 2015, 20, 14191–14211. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, J.; Li, G.; Huang, Y.; Yang, Z.; Yuan, T. Facile Synthesis and Characterization of Novel Multi-Functional Bio-Based Acrylate Prepolymers Derived from Tung Oil and Its Application in UV-Curable Coatings. Ind. Crops Prod. 2019, 138, 111585. [Google Scholar] [CrossRef]
- Mauludin, L.M.; Oucif, C.; Rabczuk, T. The Effects of Mismatch Fracture Properties in Encapsulation-Based Self-Healing Concrete Using Cohesive-Zone Model. Front. Struct. Civ. Eng. 2020, 14, 792–801. [Google Scholar] [CrossRef]
- Li, Q.; Mishra, A.K.; Kim, N.H.; Kuila, T.; Lau, K.T.; Lee, J.H. Effects of Processing Conditions of Poly(Methylmethacrylate) Encapsulated Liquid Curing Agent on the Properties of Self-Healing Composites. Compos. Part B Eng. 2013, 49, 6–15. [Google Scholar] [CrossRef]
- Zamani, M.; Nikafshar, S.; Mousa, A.; Behnia, A. Bacteria Encapsulation Using Synthesized Polyurea for Self-Healing of Cement Paste. Constr. Build. Mater. 2020, 249, 118556. [Google Scholar] [CrossRef]
- Kumar, C.V.; Prakash, S. Investigation Study on the Use of Urea-Formaldehyde in Self-Healing Concrete. In Proceedings of the International Conference on Innovation Research and Solutions, (ICIRS-2014) at Pondicherry, Pondicherry, India, 20 April 2014; pp. 237–244. [Google Scholar]
- Sinha, A.; Wang, Q.; Wei, J. Feasibility and Compatibility of a Biomass Capsule System in Self-Healing Concrete. Materials 2021, 14, 958. [Google Scholar] [CrossRef]
- Hilloulin, B.; Van Tittelboom, K.; Gruyaert, E.; De Belie, N.; Loukili, A. Design of Polymeric Capsules for Self-Healing Concrete. Cem. Concr. Compos. 2015, 55, 298–307. [Google Scholar] [CrossRef]
- Lv, L.; Yang, Z.; Chen, G.; Zhu, G.; Han, N.; Schlangen, E.; Xing, F. Synthesis and Characterization of a New Polymeric Microcapsule and Feasibility Investigation in Self-Healing Cementitious Materials. Constr. Build. Mater. 2016, 105, 487–495. [Google Scholar] [CrossRef]
- de Souza, L.R. Design and Synthesis of Microcapsules Using Microfluidics for Autonomic Self-Healing in Cementitious Materials. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2017. [Google Scholar]
- Su, Y.; Lin, H.; Zhang, S.; Yang, Z.; Yuan, T. One-Step Synthesis of Novel Renewable Vegetable Oil-Based Acrylate Prepolymers and Their Application in UV-Curable Coatings. Polymers 2020, 12, 1165. [Google Scholar] [CrossRef]
- Sung, J.; Sun, X.S. Cardanol Modified Fatty Acids from Camelina Oils for Flexible Bio-Based Acrylates Coatings. Prog. Org. Coat. 2018, 123, 242–253. [Google Scholar] [CrossRef]
- Paesano, A. Handbook of Sustainable Polymers for Additive Manufacturing, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; ISBN 9781003221210. [Google Scholar]
- Phenol Formaldehyde (PF, Phenolic): MakeItFrom.com. Available online: https://www.makeitfrom.com/material-properties/Phenol-Formaldehyde-PF-Phenolic (accessed on 14 May 2022).
- Polymethylmethacrylate-Acrylic-PMMA General Purpose. Available online: https://www.azom.com/article.aspx?ArticleID=788 (accessed on 14 May 2022).
- Yuan, J.; Zhao, X.; Ye, L. Structure and Properties of Urea-Formaldehyde Resin/Polyurethane Blend Prepared via in-Situ Polymerization. RSC Adv. 2015, 5, 53700–53707. [Google Scholar] [CrossRef]
- Teng, H.; Koike, K.; Zhou, D.; Satoh, Z.; Koike, Y.; Okamoto, Y. High Glass Transition Temperatures of Poly(Methyl Methacrylate) Prepared by Free Radical Initiators. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 315–317. [Google Scholar] [CrossRef]
- Reinecker, M.; Soprunyuk, V.; Fally, M.; Sánchez-Ferrer, A.; Schranz, W. Two Glass Transitions of Polyurea Networks: Effect of the Segmental Molecular Weight. Soft Matter 2014, 10, 5729–5738. [Google Scholar] [CrossRef]
- Arunkumar, T.; Anand, G.; Venkatachalam, P.; Anish, M.; Jayaprabakar, J.; Sajin, J.B. Study on Mechanical Properties of Polyurea Coating with Various Process Parameters. Mater. Res. Innov. 2020, 25, 257–263. [Google Scholar] [CrossRef]
- Boyer, R.F. Mechanical Motions in Amorphous and Semi-Crystalline Polymers. Polymer 1976, 17, 996–1008. [Google Scholar] [CrossRef]
- Production Volume Soybean Oil Worldwide 2021/22|Statista. Available online: https://www.statista.com/statistics/620477/soybean-oil-production-volume-worldwide/ (accessed on 24 May 2022).
- Global Canola Oil Industry. Available online: https://www.prnewswire.com/news-releases/global-canola-oil-industry-301110831.html (accessed on 24 May 2022).
- Caillol, S. Cardanol: A Promising Building Block for Biobased Polymers and Additives. Curr. Opin. Green Sustain. Chem. 2018, 14, 26–32. [Google Scholar] [CrossRef]
- Mielke, I. Fats and Oils Handbook; Elsevier: Amsterdam, The Netherlands, 1998; ISBN 9780981893600. [Google Scholar]
- Minor Oils|Cyberlipid. Available online: http://cyberlipid.gerli.com/description/simple-lipids/triacylglycerols/plants-oils-and-fats/minor-oils/ (accessed on 24 May 2022).
- Barkane, A.; Platnieks, O.; Jurinovs, M.; Kasetaite, S.; Ostrauskaite, J.; Gaidukovs, S.; Habibi, Y. Uv-Light Curing of 3d Printing Inks from Vegetable Oils for Stereolithography. Polymers 2021, 13, 1195. [Google Scholar] [CrossRef]
- Voet, V.S.D.; Strating, T.; Schnelting, G.H.M.; Dijkstra, P.; Tietema, M.; Xu, J.; Woortman, A.J.J.; Loos, K.; Jager, J.; Folkersma, R. Biobased Acrylate Photocurable Resin Formulation for Stereolithography 3D Printing. Mater. Res. Innov. 2018, 3, 1403–1408. [Google Scholar] [CrossRef]
- Michelon, M.; Leopércio, B.C.; Carvalho, M.S. Microfluidic Production of Aqueous Suspensions of Gellan-Based Microcapsules Containing Hydrophobic Compounds. Chem. Eng. Sci. 2020, 211, 115314. [Google Scholar] [CrossRef]
- Cubaud, T.; Mason, T.G. Capillary Threads and Viscous Droplets in Square Microchannels. Phys. Fluids 2008, 20, 053302. [Google Scholar] [CrossRef]
- Ponnusami, S.A.; Turteltaub, S.; van der Zwaag, S. Cohesive-Zone Modelling of Crack Nucleation and Propagation in Particulate Composites. Eng. Fract. Mech. 2015, 149, 170–190. [Google Scholar] [CrossRef]
- Voet, V.S.D.; Guit, J.; Loos, K. Sustainable Photopolymers in 3D Printing: A Review on Biobased, Biodegradable, and Recyclable Alternatives. Macromol. Rapid Commun. 2021, 42, 2000475. [Google Scholar] [CrossRef]
- Lebedevaite, M.; Talacka, V.; Ostrauskaite, J. High Biorenewable Content Acrylate Photocurable Resins for DLP 3D Printing. J. Appl. Polym. Sci. 2021, 138, 50233. [Google Scholar] [CrossRef]
- du Plessis, A.; Olawuyi, B.J.; Boshoff, W.P.; le Roux, S.G. Simple and Fast Porosity Analysis of Concrete Using X-Ray Computed Tomography. Mater. Struct. 2016, 49, 553–562. [Google Scholar] [CrossRef]
- Gao, J.; Jin, P.; Zhang, Y.; Dong, H.; Wang, R. Fast-Responsive Capsule Based on Two Soluble Components for Self-Healing Concrete. Cem. Concr. Compos. 2022, 133, 104711. [Google Scholar] [CrossRef]
- Cao, B.; Souza, L.; Xu, J.; Mao, W.; Wang, F.; Al-Tabbaa, A. Soil Mix Cutoff Wall Materials with Microcapsule-Based Self-Healing Grout. J. Geotech. Geoenviron. Eng. 2021, 147, 04021124. [Google Scholar] [CrossRef]
- Cao, B.; Souza, L.; Wang, F.; Xu, J.; Litina, C.; Al-Tabbaa, A. The First Microcapsule-Based Self-Healing Cement–Bentonite Cut-off Wall Materials. In Géotechnique; Thomas Telford: London, UK, 2021; pp. 1–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).