Material Characterization and Physical Processing of a General Type of Waste Printed Circuit Boards
Abstract
1. Introduction
- Thoroughly characterizing a general type of WPCBs by studying different sizes and densities, by SEM-EDS;
- Evaluating by particle size and density, the separation performance by elemental assay;
- Identifying the need for surfactant on finer particles for effective density separation;
- Providing information on surface elemental mapping via SEM-EDS for several select particle densities;
- Investigating thermal-reaction zones and possible reactants/products by TGA.
2. Materials and Methods
2.1. Materials
2.2. Size Reduction
2.3. Density Separation
2.4. Assaying
2.5. Characterization
3. Results and Discussion
3.1. Particle Size Distribution
3.2. Elemental Distribution in Different Size Fractions
3.3. Elemental Distribution in Various Density Fractions (Size −5 mm)
3.4. Elemental Distribution in Different Density Fractions (Size −2 mm)
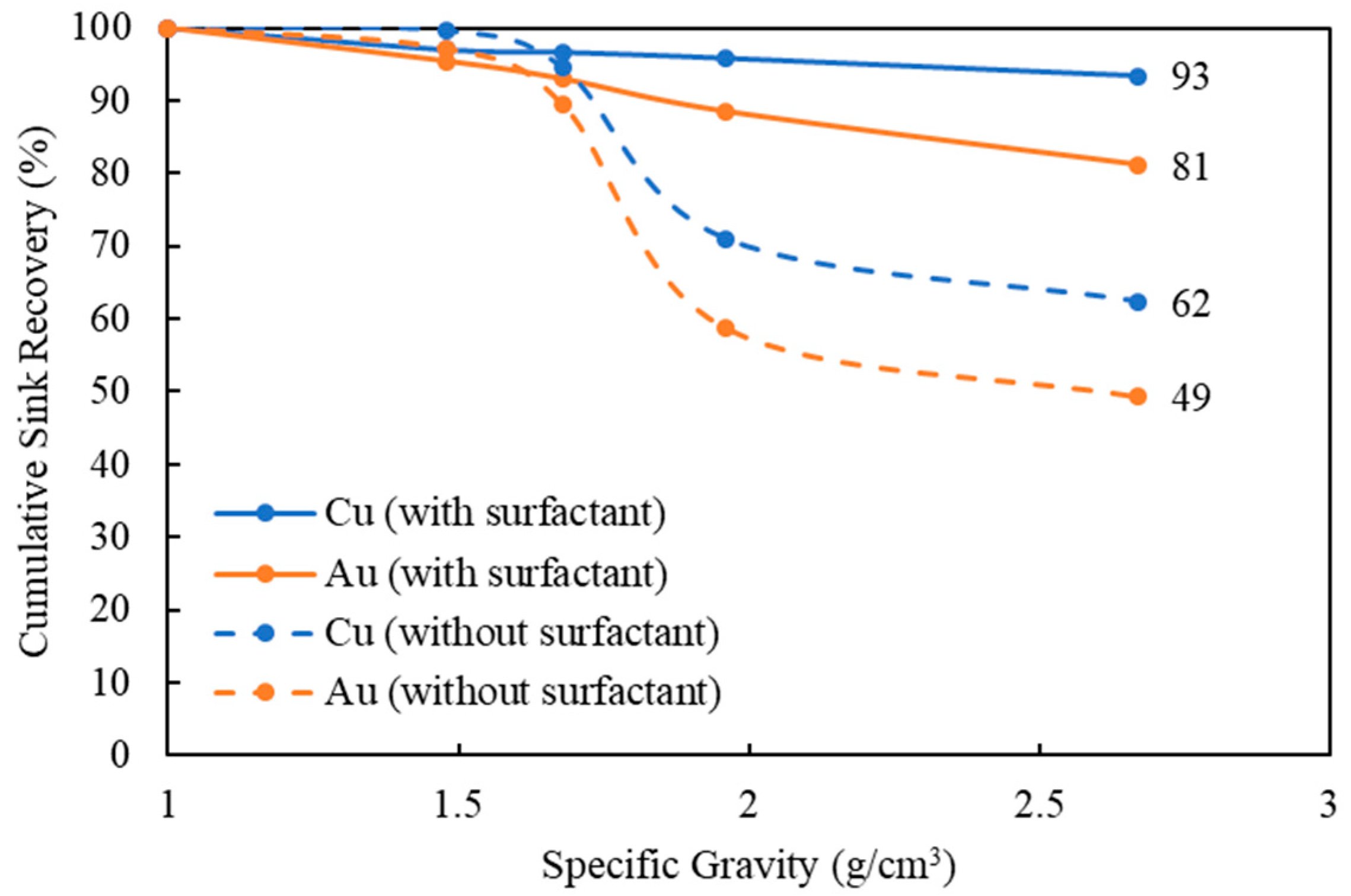
3.5. Effect of Surfactant in Finer-Particle-Size Density Separation (Size −2 mm)
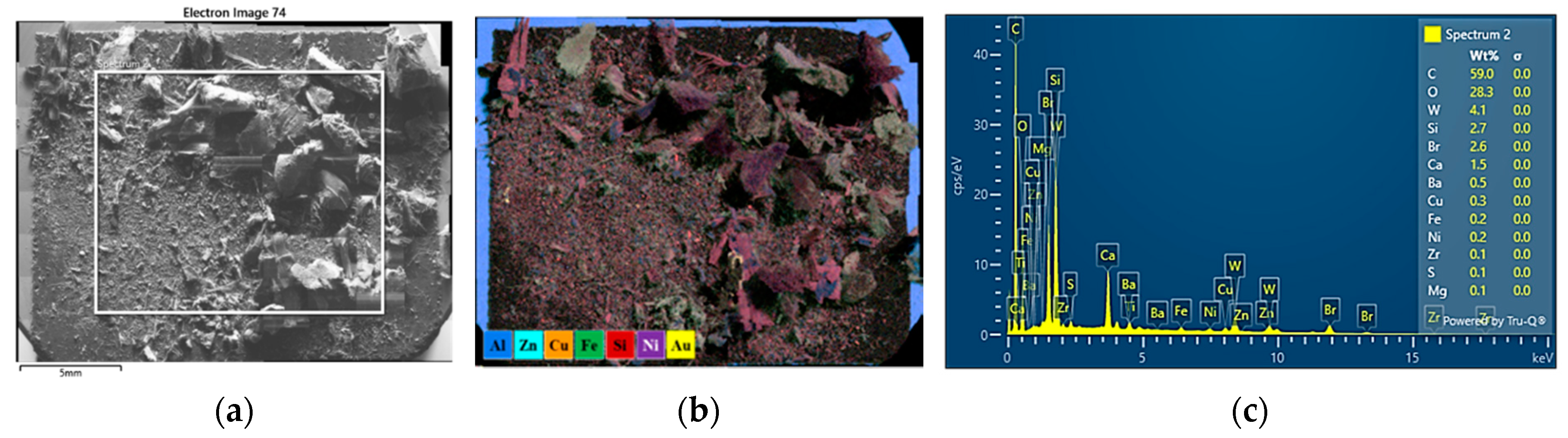
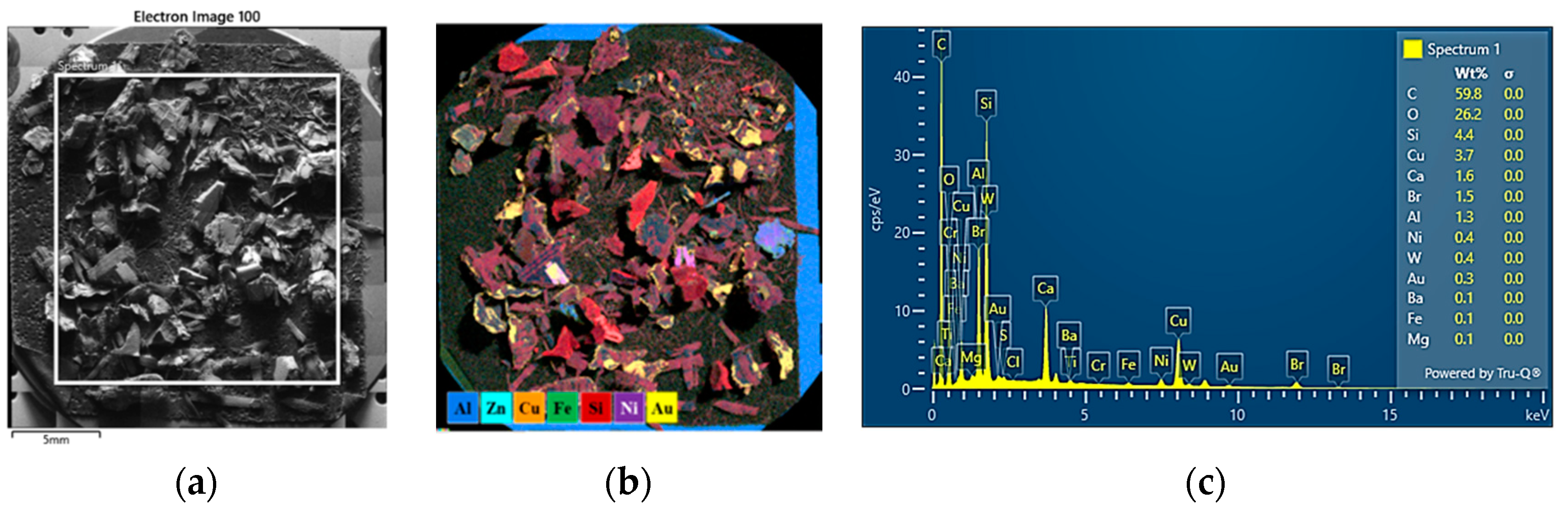
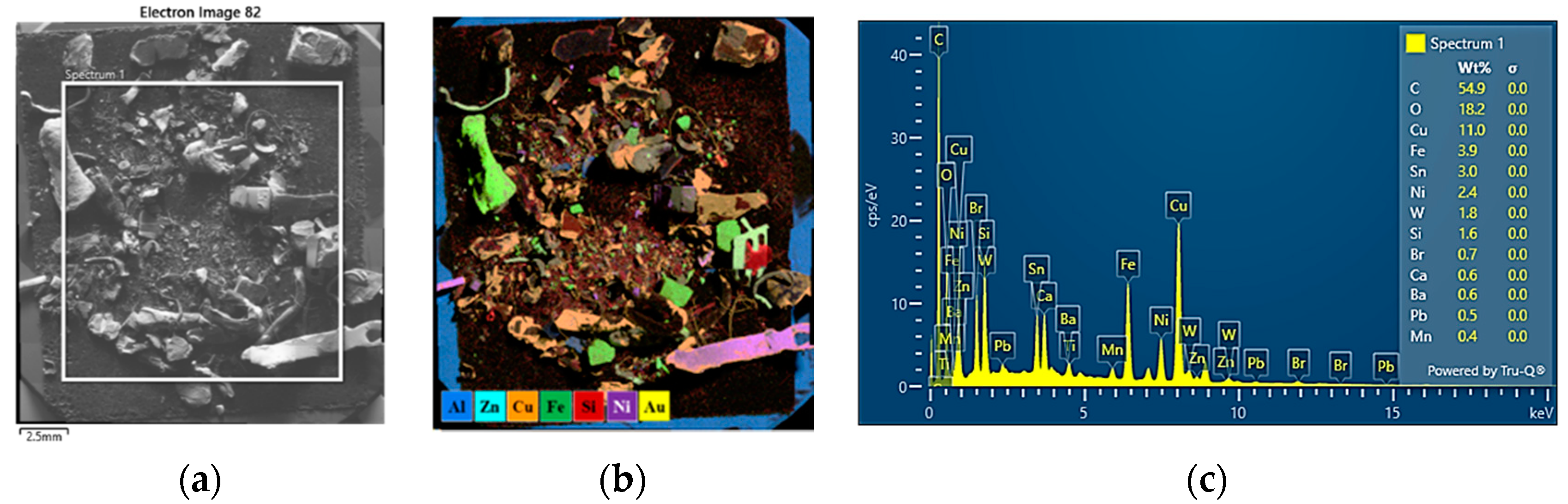
3.6. SEM-EDS Characterization of WPCBs by Density Fractions
3.7. Thermo-Gravimetric Properties of General PCBs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ning, C.; Lin, C.S.K.; Hui, D.C.W.; McKay, G. Waste Printed Circuit Board (PCB) Recycling Techniques. Top. Curr. Chem. 2017, 375, 43. [Google Scholar] [CrossRef] [PubMed]
- Forti, V.; Baldé, C.P.; Kuehr, R.; Bel, G. The Global E-Waste Monitor 2020; United Nations University: Macao, China; International Telecommunication Union: Geneva, Switzerland; International Solid Waste Association: Vienna, Austria, 2020; ISBN 9789280891140. [Google Scholar]
- Ghosh, B.; Ghosh, M.K.; Parhi, P.; Mukherjee, P.S.; Mishra, B.K. Waste Printed Circuit Boards Recycling: An Extensive Assessment of Current Status. J. Clean. Prod. 2015, 94, 5–19. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Li, B.; Pan, D.; Wu, Y.; Zuo, T. Recovery of Waste Printed Circuit Boards through Pyrometallurgical Processing: A Review. Resour. Conserv. Recycl. 2017, 126, 209–218. [Google Scholar] [CrossRef]
- Balde, C.P.; Forti, V.; Gray, V.; Kuehr, R.; Stegmann, P. The Global E-Waste Monitor 2017; United Nations University: Macao, China; International Telecommunication Union: Geneva, Switzerland; International Solid Waste Association: Vienna, Austria, 2017; ISBN 9789280845556. [Google Scholar]
- Ogunseitan, O.A. The Basel Convention and E-Waste: Translation of Scientific Uncertainty to Protective Policy. Lancet Glob. Health 2013, 1, e313–e314. [Google Scholar] [CrossRef]
- Kumar, A.; Holuszko, M.E.; Janke, T. Characterization of the Non-Metal Fraction of the Processed Waste Printed Circuit Boards. Waste Manag. 2018, 75, 94–102. [Google Scholar] [CrossRef]
- Reuter, M.A.; Van Schaik, A.; Gutzmer, J.; Bartie, N.; Abadías-Llamas, A. Challenges of the Circular Economy: A Material, Metallurgical, and Product Design Perspective. Annu. Rev. Mater. Res. 2019, 49, 253–274. [Google Scholar] [CrossRef]
- Jawahir, I.S.; Bradley, R. Technological Elements of Circular Economy and the Principles of 6R-Based Closed-Loop Material Flow in Sustainable Manufacturing. Procedia CIRP 2016, 40, 103–108. [Google Scholar] [CrossRef]
- Reck, B.K.; Graedel, T.E. Challenges in Metal Recycling. Science 2012, 337, 690–695. [Google Scholar] [CrossRef]
- Li, Z.; Diaz, L.A.; Yang, Z.; Jin, H.; Lister, T.E.; Vahidi, E.; Zhao, F. Comparative Life Cycle Analysis for Value Recovery of Precious Metals and Rare Earth Elements from Electronic Waste. Resour. Conserv. Recycl. 2019, 149, 20–30. [Google Scholar] [CrossRef]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical Recovery of Metals from Waste Printed Circuit Boards (WPCBs): Current Status and Perspectives—A Review. Resour. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Golev, A.; Corder, G.D. Quantifying Metal Values in E-Waste in Australia: The Value Chain Perspective. Miner. Eng. 2017, 107, 81–87. [Google Scholar] [CrossRef]
- Pant, D.; Joshi, D.; Upreti, M.K.; Kotnala, R.K. Chemical and Biological Extraction of Metals Present in E Waste: A Hybrid Technology. Waste Manag. 2012, 32, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.; Barmak, K.; West, A.C.; Park, A.H.A. Advancements in the Treatment and Processing of Electronic Waste with Sustainability: A Review of Metal Extraction and Recovery Technologies. Green Chem. 2019, 21, 919–936. [Google Scholar] [CrossRef]
- Debnath, B.; Chowdhury, R.; Ghosh, S.K. Sustainability of Metal Recovery from E-Waste. Front. Environ. Sci. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Li, J.; Koh, L.; Ogunseitan, O.A. Circular Economy and Electronic Waste. Nat. Electron. 2019, 2, 86–89. [Google Scholar] [CrossRef]
- Bauer, S. Implementation of Basel Declaration on Environmentally Sound Management of Priority Waste Streams: Electronic and Electrical Wastes. In Concepts of Global Environmental Governance; Routledge: London, UK, 2020; pp. 283–286. [Google Scholar] [CrossRef]
- Bizzo, W.A.; Figueiredo, R.A.; De Andrade, V.F. Characterization of Printed Circuit Boards for Metal and Energy Recovery after Milling and Mechanical Separation. Materials 2014, 7, 4555–4566. [Google Scholar] [CrossRef]
- Veit, H.M.; de Freitas Juchneski, N.C.; Scherer, J. Use of Gravity Separation in Metals Concentration from Printed Circuit Board Scraps. Rem. Rev. Esc. De Minas 2014, 67, 73–79. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, X.; Ding, J.; Zhu, Y. Study on Recovery of Valuable Metals from Waste Mobile Phone PCB Particles Using Liquid-Solid Fluidization Technique. Chem. Eng. J. 2017, 311, 217–226. [Google Scholar] [CrossRef]
- Tatariants, M.; Yousef, S.; Denafas, G.; Bendikiene, R. Separation and Purification of Metal and Fiberglass Extracted from Waste Printed Circuit Boards Using Milling and Dissolution Techniques. Environ. Prog. Sustain. Energy 2018, 37, 2082–2092. [Google Scholar] [CrossRef]
- Zhu, X.N.; Cui, T.; Li, B.; Nie, C.C.; Zhang, H.; Lyu, X.J.; Tao, Y.J.; Qiu, J.; Li, L.; Zhang, G.W. Metal Recovery from Waste Printed Circuit Boards by Flotation Technology with Non-Ionic Renewable Collector. J. Clean. Prod. 2020, 255, 120289. [Google Scholar] [CrossRef]
- Zhu, X.N.; Nie, C.C.; Ni, Y.; Zhang, T.; Li, B.; Wang, D.Z.; Qu, S.J.; Qiao, F.M.; Lyu, X.J.; Qiu, J.; et al. Advanced Utilization of Copper in Waste Printed Circuit Boards: Synthesis of Nano-Copper Assisted by Physical Enrichment. J. Hazard. Mater. 2021, 401, 123294. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.J.; Lee, S.O.; Yang, H.S.; Ha, T.J.; Kim, M.J. Selective Leaching of Valuable Metals from Waste Printed Circuit Boards. J. Air Waste Manag. Assoc. 2003, 53, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.A.; Martins, A.H. Recovery of Tin and Copper by Recycling of Printed Circuit Boards from Obsolete Computers. Braz. J. Chem. Eng. 2009, 26, 649–657. [Google Scholar] [CrossRef]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous Metal Recovery Techniques from E-Scrap: Hydrometallurgy in Recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Kinoshita, T.; Akita, S.; Kobayashi, N.; Nii, S.; Kawaizumi, F.; Takahashi, K. Metal Recovery from Non-Mounted Printed Wiring Boards via Hydrometallurgical Processing. Hydrometallurgy 2003, 69, 73–79. [Google Scholar] [CrossRef]
- Quan, C.; Li, A.; Gao, N.; Dan, Z. Characterization of Products Recycling from PCB Waste Pyrolysis. J. Anal. Appl. Pyrolysis 2010, 89, 102–106. [Google Scholar] [CrossRef]
- Jie, G.; Ying-Shun, L.; Mai-Xi, L. Product Characterization of Waste Printed Circuit Board by Pyrolysis. J. Anal. Appl. Pyrolysis 2008, 83, 185–189. [Google Scholar] [CrossRef]
- Fujita, T.; Ono, H.; Dodbiba, G.; Yamaguchi, K. Evaluation of a Recycling Process for Printed Circuit Board by Physical Separation and Heat Treatment. Waste Manag. 2014, 34, 1264–1273. [Google Scholar] [CrossRef]
- Tunsu, C.; Retegan, T. Hydrometallurgical Processes for the Recovery of Metals from WEEE; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128033647. [Google Scholar]
- Eng, J.A.C.; Cui, H.; Anderson, C.G. Literature Review of Hydrometallurgical Recycling of Printed Circuit Boards (PCBs). J. Adv. Chem. Eng. 2016, 6, 1. [Google Scholar] [CrossRef]
- Kumari, A.; Jha, M.K.; Singh, R.P. Recovery of Metals from Pyrolysed PCBs by Hydrometallurgical Techniques. Hydrometallurgy 2016, 165, 97–105. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Ali Kucuker, M.; Kuchta, K.; et al. Recent Advances on Hydrometallurgical Recovery of Critical and Precious Elements from End of Life Electronic Wastes-A Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, P.; Zhu, N.; Zhang, T.; Liu, W.; Wu, J.; Li, P. Bioleaching of Copper from Waste Printed Circuit Boards by Bacterial Consortium Enriched from Acid Mine Drainage. J. Hazard. Mater. 2010, 184, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, G.; Ting, Y.P. Pretreatment of E-Waste and Mutation of Alkali-Tolerant Cyanogenic Bacteria Promote Gold Biorecovery. Bioresour. Technol. 2014, 152, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; du Laing, G.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological Strategies for the Recovery of Valuable and Critical Raw Materials from Waste Electrical and Electronic Equipment (WEEE)—A Review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, A.; la Mensbruge, L.D.; King, A.; Serranti, S.; Fiore, L.; Bonifazi, G. Non-Destructive Characterization of Mechanically Processed Waste Printed Circuit Boards-Particle Liberation Analysis. Waste Manag. 2020, 102, 510–519. [Google Scholar] [CrossRef]
- Sarvar, M.; Salarirad, M.M.; Shabani, M.A. Characterization and Mechanical Separation of Metals from Computer Printed Circuit Boards (PCBs) Based on Mineral Processing Methods. Waste Manag. 2015, 45, 246–257. [Google Scholar] [CrossRef]
- Tan, Z.; He, Y.; Xie, W.; Duan, C.; Zhou, E.; Yu, Z. Size Distribution of Wet Crushed Waste Printed Circuit Boards. Min. Sci. Technol. 2011, 21, 359–363. [Google Scholar] [CrossRef]
- Eswaraiah, C.; Kavitha, T.; Vidyasagar, S.; Narayanan, S.S. Classification of Metals and Plastics from Printed Circuit Boards (PCB) Using Air Classifier. Chem. Eng. Process. Process Intensif. 2008, 47, 565–576. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Zhou, Y. Application of Corona Discharge and Electrostatic Force to Separate Metals and Nonmetals from Crushed Particles of Waste Printed Circuit Boards. J. Electrost. 2007, 65, 233–238. [Google Scholar] [CrossRef]
- Duan, C.; Wen, X.; Shi, C.; Zhao, Y.; Wen, B.; He, Y. Recovery of Metals from Waste Printed Circuit Boards by a Mechanical Method Using a Water Medium. J. Hazard. Mater. 2009, 166, 478–482. [Google Scholar] [CrossRef]
- Zhou, C.; Pan, Y.; Lu, M.; Yang, C. Liberation Characteristics after Cryogenic Modification and Air Table Separation of Discarded Printed Circuit Boards. J. Hazard. Mater. 2016, 311, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, Z. Environmental Friendly Automatic Line for Recovering Metal from Waste Printed Circuit Boards. Environ. Sci. Technol. 2010, 44, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Ruiz, R.H.; Flores-Campos, R.; Gámez-Altamirano, H.A.; Velarde-Sánchez, E.J. Separation of the Metallic and Non-Metallic Fraction from Printed Circuit Boards Employing Green Technology. J. Hazard. Mater. 2016, 311, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Yamane, L.H.; de Moraes, V.T.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of WEEE: Characterization of Spent Printed Circuit Boards from Mobile Phones and Computers. Waste Manag. 2011, 31, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.H.I.; Xiao, Y.; Sietsma, J.; Agterhuis, H.; Visser, G.; Yang, Y. Characterisation of Metals in the Electronic Waste of Complex Mixtures of End-of-Life ICT Products for Development of Cleaner Recovery Technology. Waste Manag. 2015, 35, 227–235. [Google Scholar] [CrossRef]
- Guo, C.; Wang, H.; Liang, W.; Fu, J.; Yi, X. Liberation Characteristic and Physical Separation of Printed Circuit Board (PCB). Waste Manag. 2011, 31, 2161–2166. [Google Scholar] [CrossRef]
- Ogunniyi, I.O.; Vermaak, M.K.G.; Groot, D.R. Chemical Composition and Liberation Characterization of Printed Circuit Board Comminution Fines for Beneficiation Investigations. Waste Manag. 2009, 29, 2140–2146. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Rajarao, R.; Golmohammadzadeh, R.; Sahajwalla, V. Two-Step Pre-Processing Enrichment of Waste Printed Circuit Boards: Mechanical Milling and Physical Separation. J. Clean. Prod. 2018, 184, 1113–1124. [Google Scholar] [CrossRef]
- Gonçalves, P.P.; Otsuki, A. Determination of Liberation Degree of Mechanically Processed Waste Printed Circuit Boards by Using the Digital Microscope and SEM-EDS Analysis. Electronics 2019, 8, 1202. [Google Scholar] [CrossRef]
- Schuhmann, R., Jr. Energy Input and Size Distribution in Comminution. Trans. SME/AIME 1960, 217, 22–25. [Google Scholar]
- Rosin, P. Laws Governing the Fineness of Powdered Coal. J. Inst. Fuel 1933, 7, 29–36. [Google Scholar]
- Colorado-Arango, L.; Menéndez-Aguado, J.M.; Osorio-Correa, A. Particle Size Distribution Models for Metallurgical Coke Grinding Products. Metals 2021, 11, 1288. [Google Scholar] [CrossRef]
- Kim, Y.M.; Han, T.U.; Watanabe, C.; Teramae, N.; Park, Y.K.; Kim, S.; Hwang, B. Analytical Pyrolysis of Waste Paper Laminated Phenolic-Printed Circuit Board (PLP-PCB). J. Anal. Appl. Pyrolysis 2015, 115, 87–95. [Google Scholar] [CrossRef]
- Grause, G.; Furusawa, M.; Okuwaki, A.; Yoshioka, T. Pyrolysis of Tetrabromobisphenol-A Containing Paper Laminated Printed Circuit Boards. Chemosphere 2008, 71, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Evangelopoulos, P.; Kantarelis, E.; Yang, W. Investigation of the Thermal Decomposition of Printed Circuit Boards (PCBs) via Thermogravimetric Analysis (TGA) and Analytical Pyrolysis (Py-GC/MS). J. Anal. Appl. Pyrolysis 2015, 115, 337–343. [Google Scholar] [CrossRef]
- Li, J.; Duan, H.; Yu, K.; Liu, L.; Wang, S. Characteristic of Low-Temperature Pyrolysis of Printed Circuit Boards Subjected to Various Atmosphere. Resour. Conserv. Recycl. 2010, 54, 810–815. [Google Scholar] [CrossRef]
- Ortuño, N.; Conesa, J.A.; Moltó, J.; Font, R. Pollutant Emissions during Pyrolysis and Combustion of Waste Printed Circuit Boards, before and after Metal Removal. Sci. Total Environ. 2014, 499, 27–35. [Google Scholar] [CrossRef]
- Ortuño, N.; Moltó, J.; Egea, S.; Font, R.; Conesa, J.A. Thermogravimetric Study of the Decomposition of Printed Circuit Boards from Mobile Phones. J. Anal. Appl. Pyrolysis 2013, 103, 189–200. [Google Scholar] [CrossRef]
- Diaz, F.; Flerus, B.; Nagraj, S.; Bokelmann, K.; Stauber, R.; Friedrich, B. Comparative Analysis About Degradation Mechanisms of Printed Circuit Boards (PCBs) in Slow and Fast Pyrolysis: The Influence of Heating Speed. J. Sustain. Metall. 2018, 4, 205–221. [Google Scholar] [CrossRef]
- Jin, Y.Q.; Tao, L.; Chi, Y.; Yan, J.H. Conversion of Bromine during Thermal Decomposition of Printed Circuit Boards at High Temperature. J. Hazard. Mater. 2011, 186, 707–712. [Google Scholar] [CrossRef]




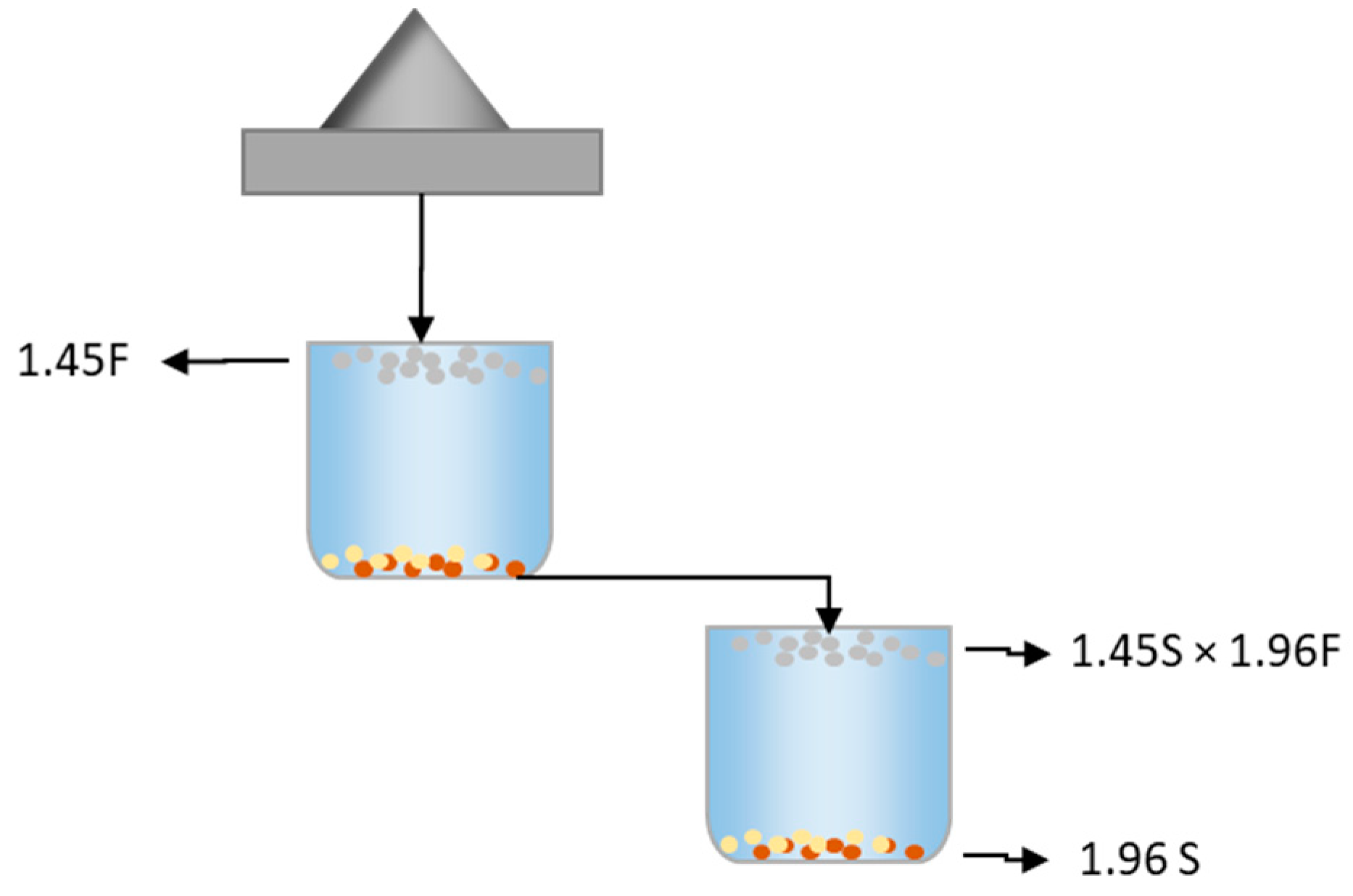




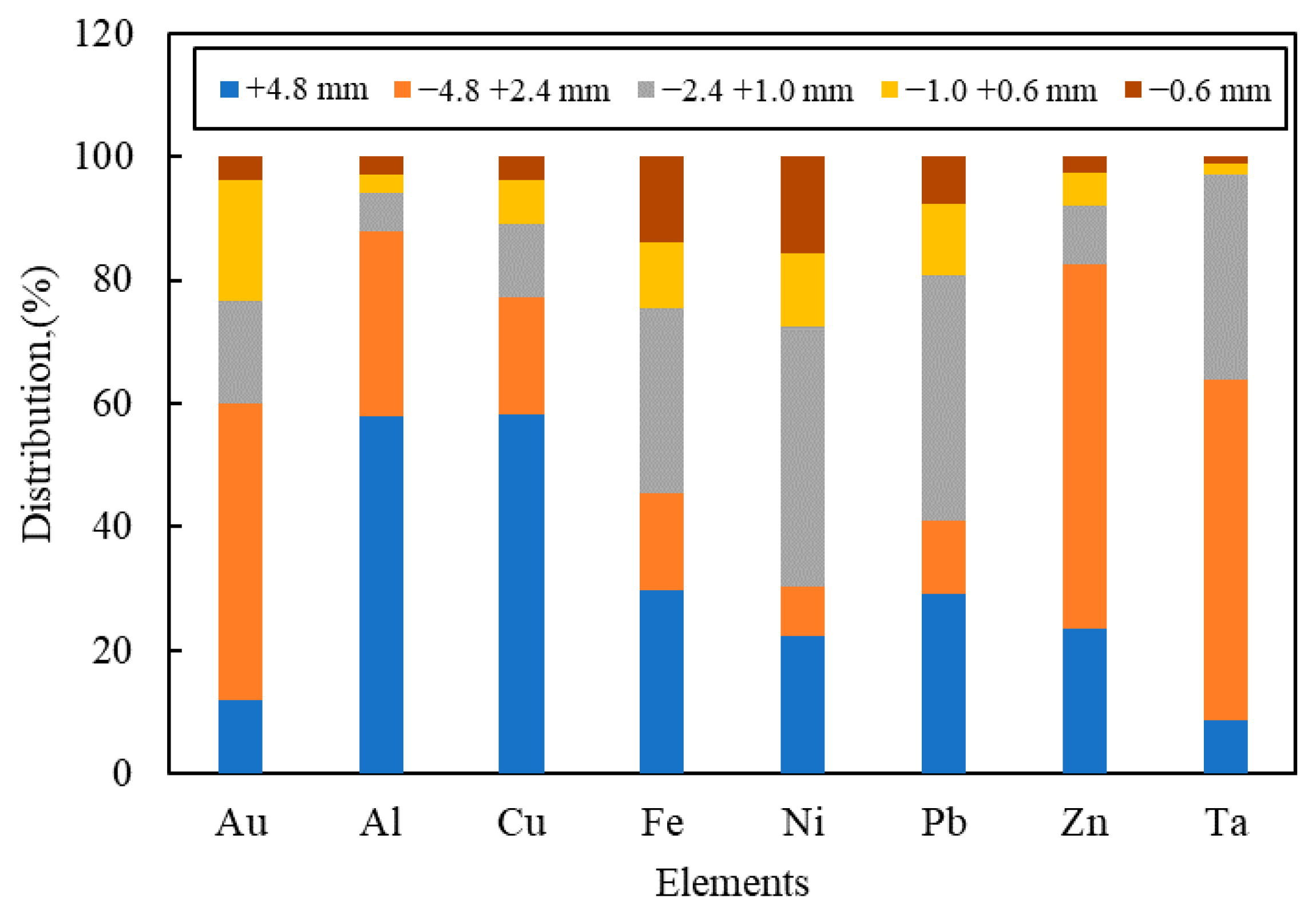





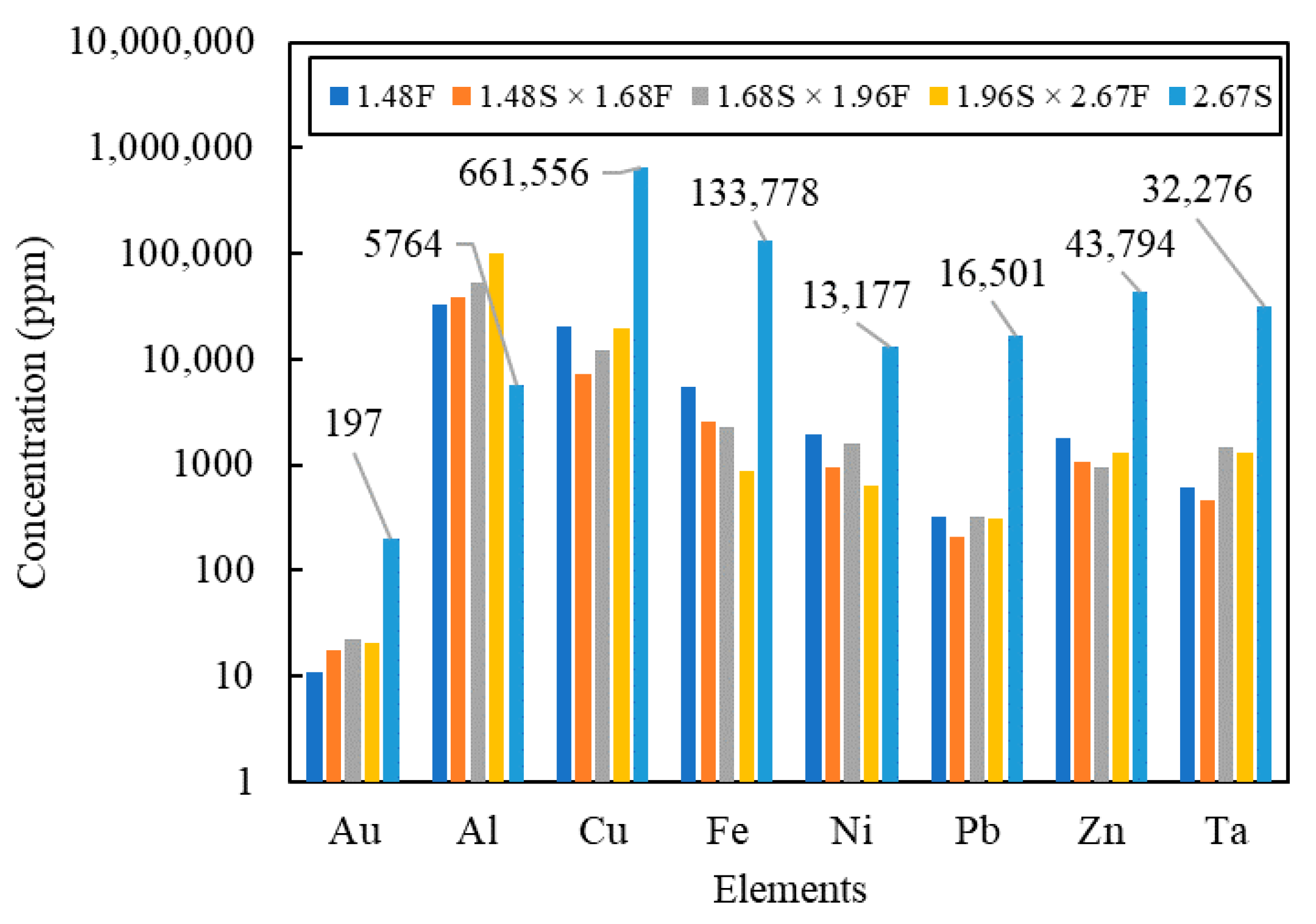

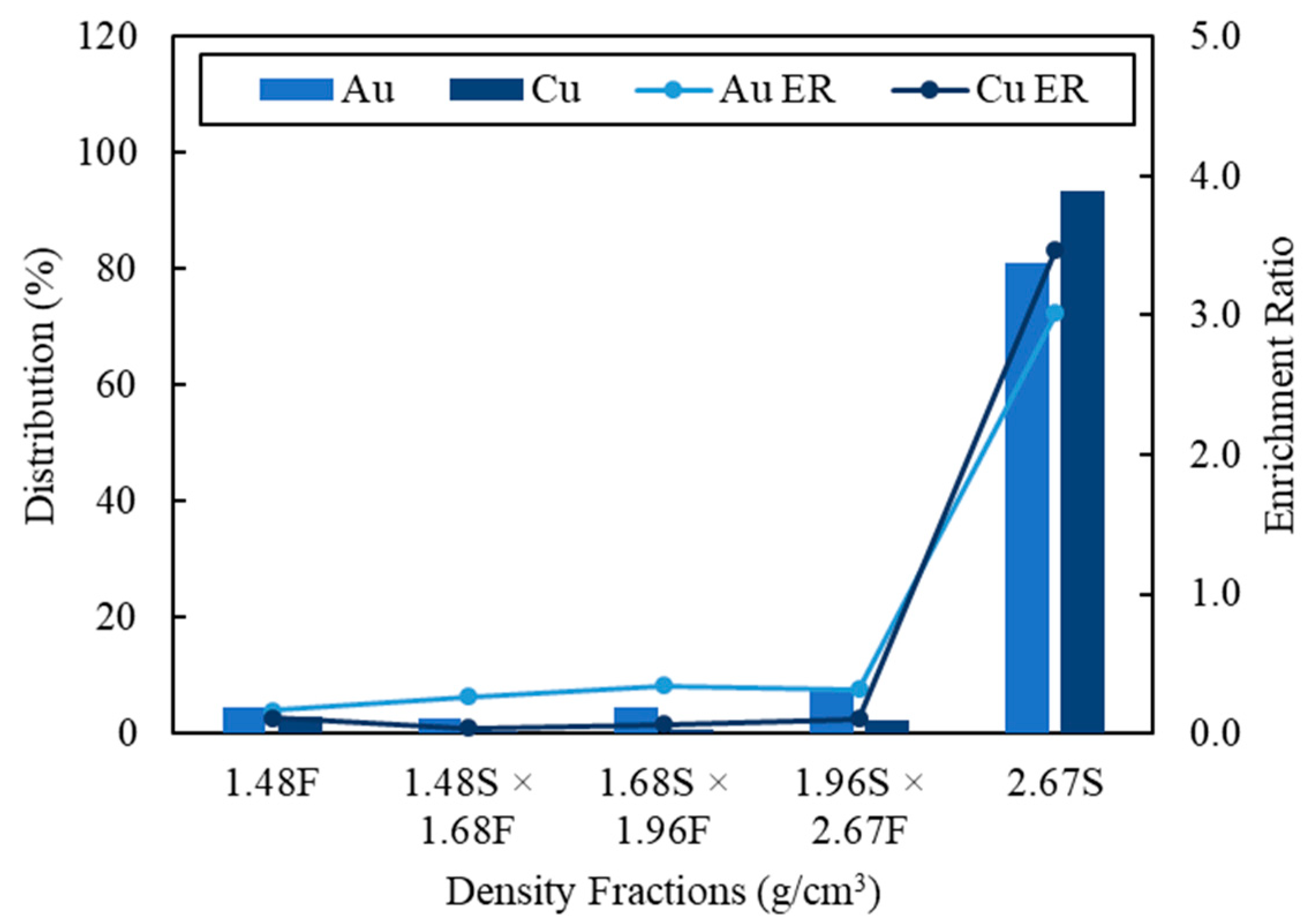

| Type of Study | Particle Size (mm) | Separation Methods | Elemental Assay | Thermo-Gravimetry | Spectrum Characterization | Reference |
|---|---|---|---|---|---|---|
| Physical Processing | −2.8 | Air table | N/A | N/A | N/A | [45] |
| −0.25 | Flotation (Superficial air) | N/A | N/A | SEM-EDS (Al, Si, Sn, Pb) | [47] | |
| −2 | N/A | N/A | N/A | SEM-EDS (Cu and Al) | [53] | |
| Physical Processing and Characterization | −4, −2 | Magnetic and electrostatic | Ag, Au, Al, Cu, Fe, Ni, Pb, Zn, Sn | N/A | N/A | [48] |
| −4 | Density (S.G. 2.89) | Au, Ag, Cu, Fe, Ni, Pb, Zn, etc. | GCV * | N/A | [19] | |
| −4 | N/A | Au, Al, Cu, Fe, Ni, Pb, Sn | TG-DTA * | N/A | [31] | |
| +1.68, +0.59–1.68, −0.59 | Wet jigging, flotation | Cu, Au | N/A | N/A | [40] | |
| −4 | N/A | Ag, Au, Al, Cu, Fe, Ni, Pb, Zn, Sn, Nd | TGA-DSC * | SEM-EDS (Al, Pb, Zn, Cu, Au, Fe, Si, etc.) | [49] | |
| −0.8, −0.45 | Density (S.G. 2.82) | Ag, Au, Al, Cu, Fe, Ni, Pb, Sn, Ti, Zn | N/A | SEM-EDS FTIR XRD and XRF | [52] | |
| −9, −5, −2 | Density (S.G. 1.45 to 2.67) | Au, Al, Cu, Fe, Ni, Pb, Zn, Ta | TGA * | SEM-EDS (Al, Zn, Cu, Fe, Si, Ni, Au) | This Study |
| PSD Model | Expression | Variables | RRS |
|---|---|---|---|
| GGS | k = 4.79; m = 0.94 | 14.67 | |
| RR | R = 2.57; b = 1.48 | 144.45 |
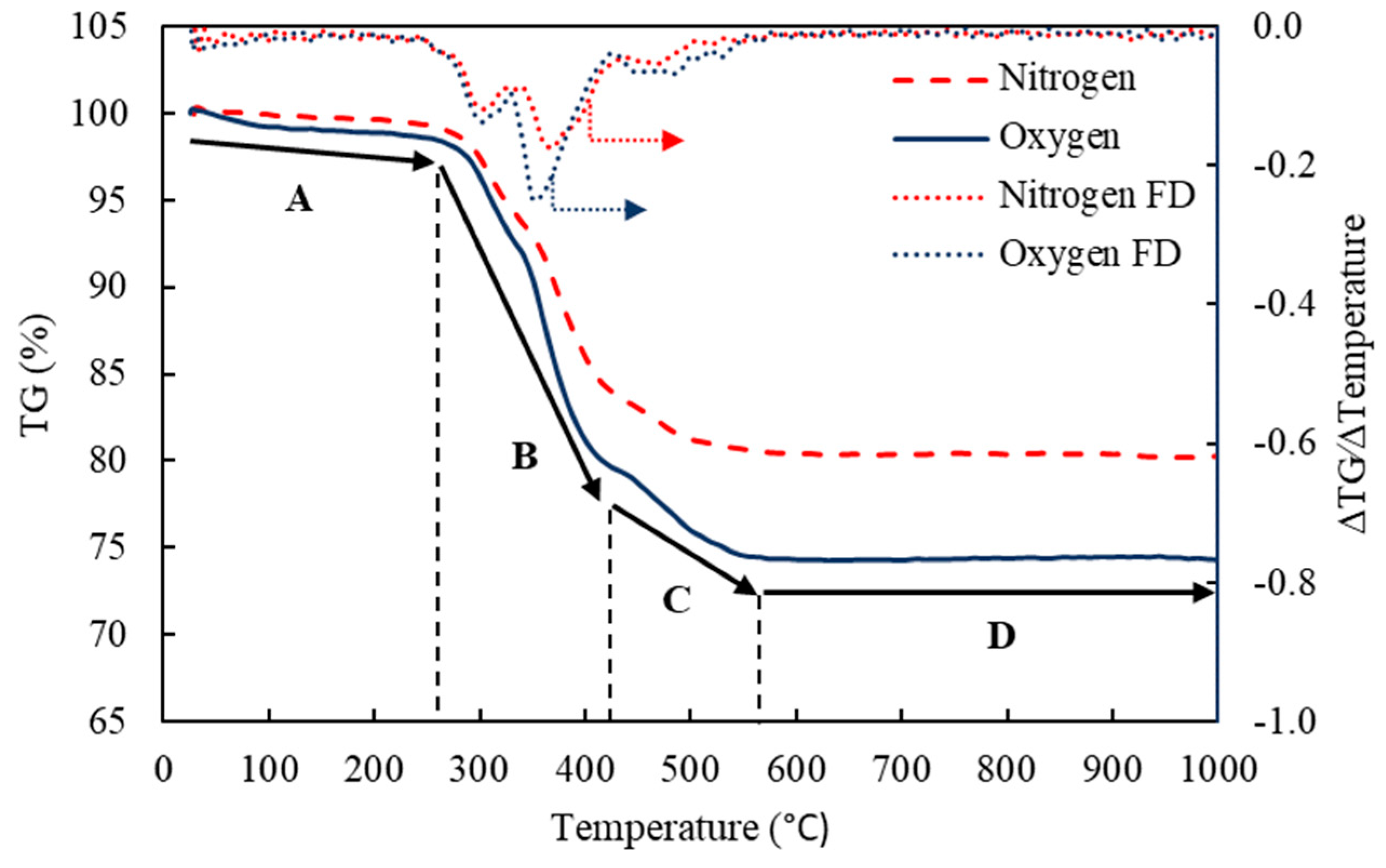
| Stage | Temperature (°C) | Type of Reaction | Major Compounds | %Wt. Loss (N2) | %Wt. Loss (O2) | Similar Observations |
|---|---|---|---|---|---|---|
| A | 25–270 | Evaporation of moisture and volatile organics | H2O and VOCs | 1.40 | 2.25 | [57] |
| B | 270–410 | Pyrolysis of brominated resins | TBBA and ERs/BERs | 13.40 | 17.17 | [60] |
| C | 410–570 | Decomposition and combustion of polymers | Polymer resins | 4.76 | 6.28 | [61] |
| D | >570 | Stabilization | Ash of general PCBs | 0.20 | 0.01 | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, P.; Werner, J.; Groppo, J.; Yang, X. Material Characterization and Physical Processing of a General Type of Waste Printed Circuit Boards. Sustainability 2022, 14, 13479. https://doi.org/10.3390/su142013479
Lin P, Werner J, Groppo J, Yang X. Material Characterization and Physical Processing of a General Type of Waste Printed Circuit Boards. Sustainability. 2022; 14(20):13479. https://doi.org/10.3390/su142013479
Chicago/Turabian StyleLin, Peijia, Joshua Werner, Jack Groppo, and Xinbo Yang. 2022. "Material Characterization and Physical Processing of a General Type of Waste Printed Circuit Boards" Sustainability 14, no. 20: 13479. https://doi.org/10.3390/su142013479
APA StyleLin, P., Werner, J., Groppo, J., & Yang, X. (2022). Material Characterization and Physical Processing of a General Type of Waste Printed Circuit Boards. Sustainability, 14(20), 13479. https://doi.org/10.3390/su142013479











