CoO, Cu, and Ag Nanoparticles on Silicon Nanowires with Photocatalytic Activity for the Degradation of Dyes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Silicon Wafers
2.2. Silicon Plate Cleaning
2.3. Synthesis of SiNWs by the MACE Method
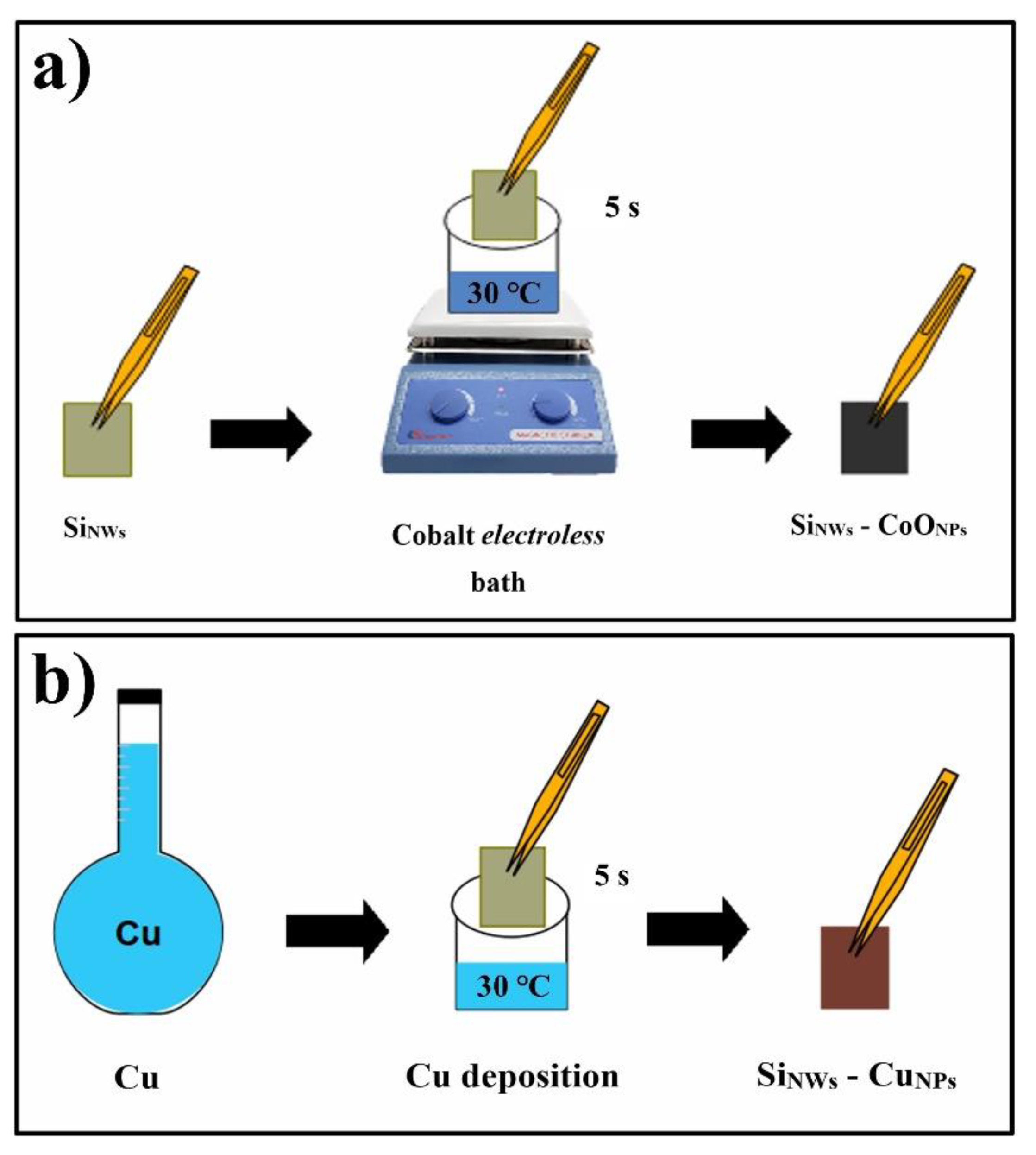
2.4. CoONPs Deposit on SiNWs (SiNWs-CoONPs)
2.5. Synthesis of SiNWs with Copper Electroless (SiNWs-CuNPs)
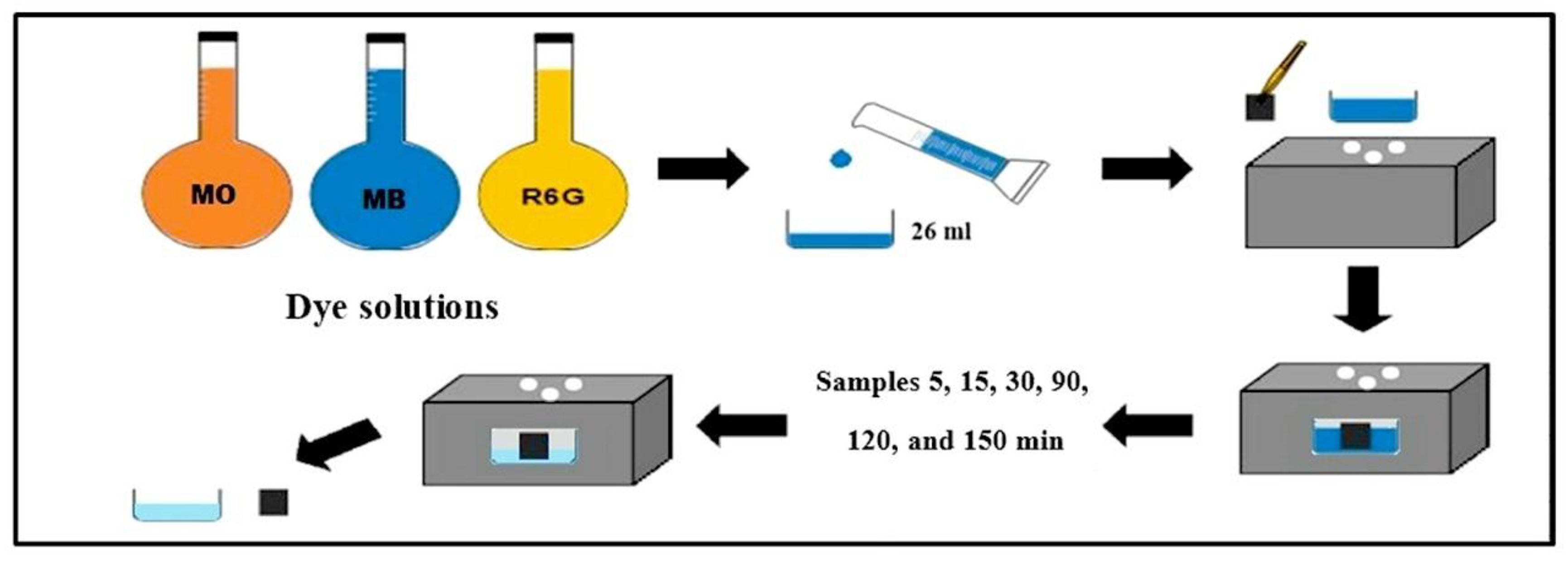
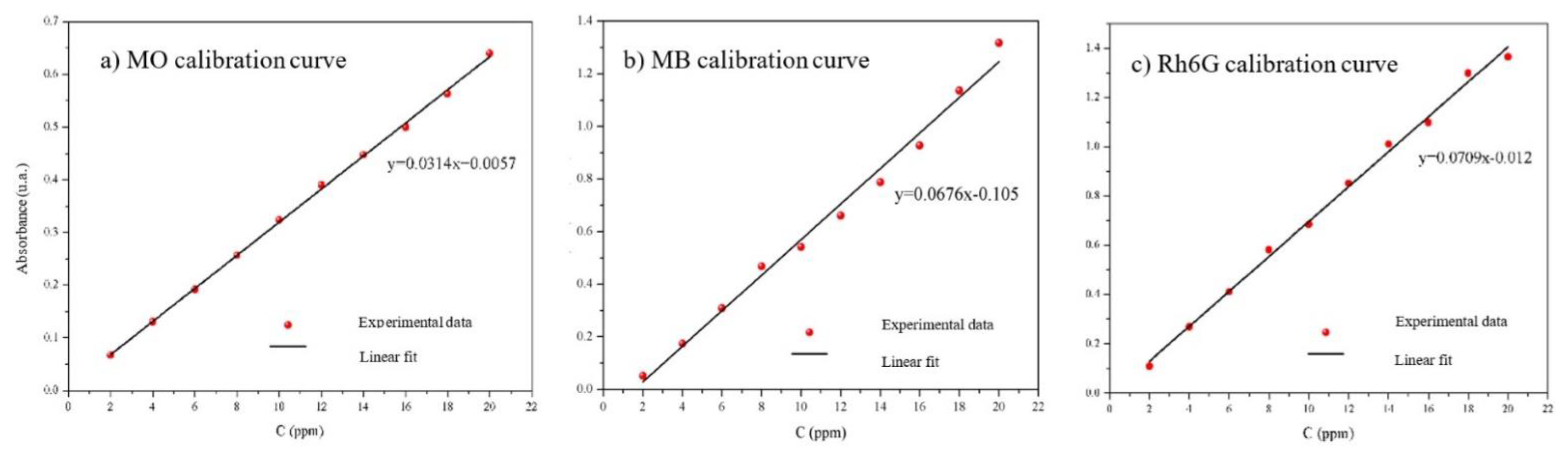
2.6. Photocatalysis with MO, MB, Rh6G
2.7. Specifications of the Characterization Techniques and Experimental Setup
2.8. Reactions of the MACE Anisotropic Wet Etching Process for SiNWs Formation and Nanometric Metallic Decoration
3. Results and Discussion
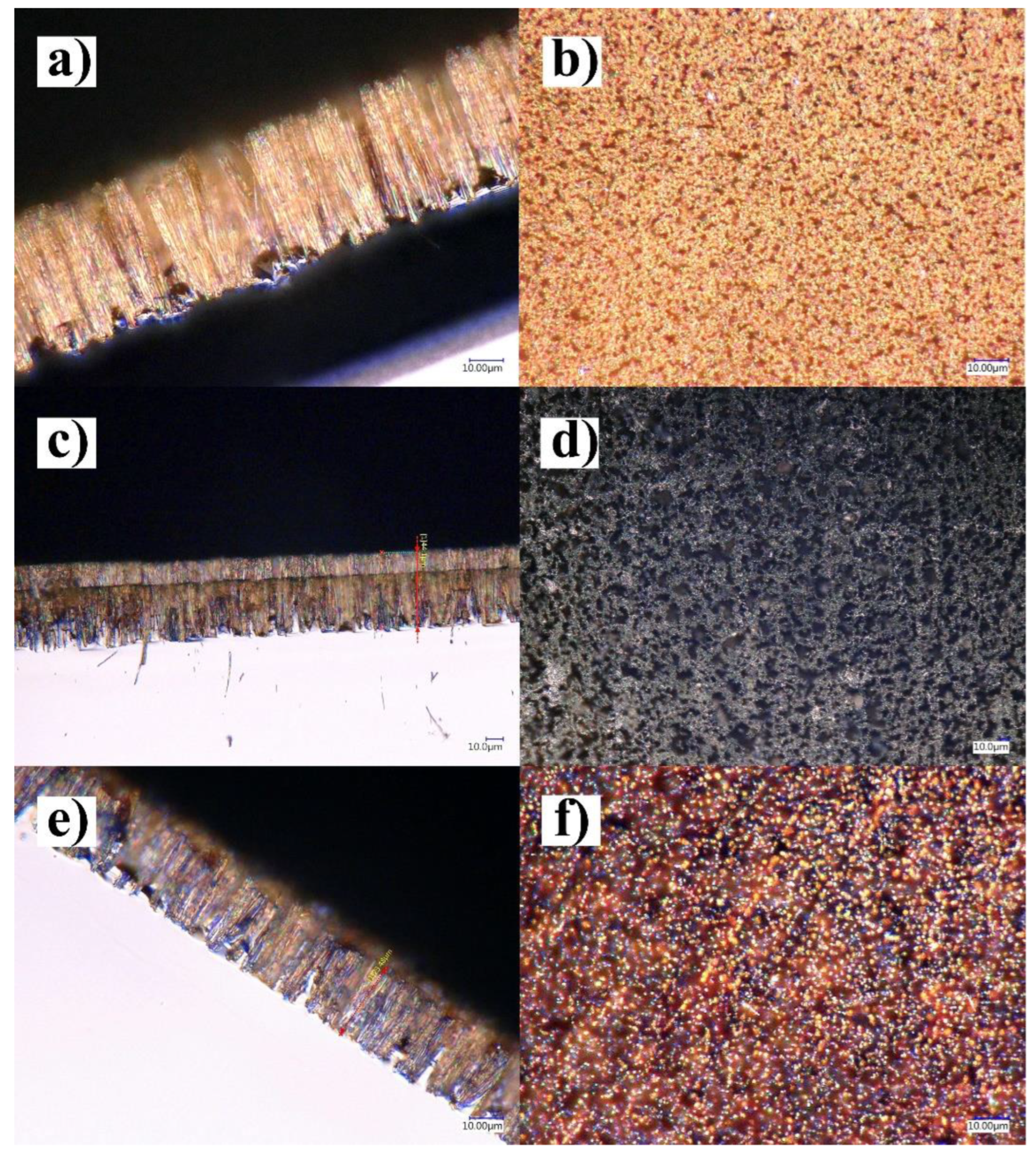
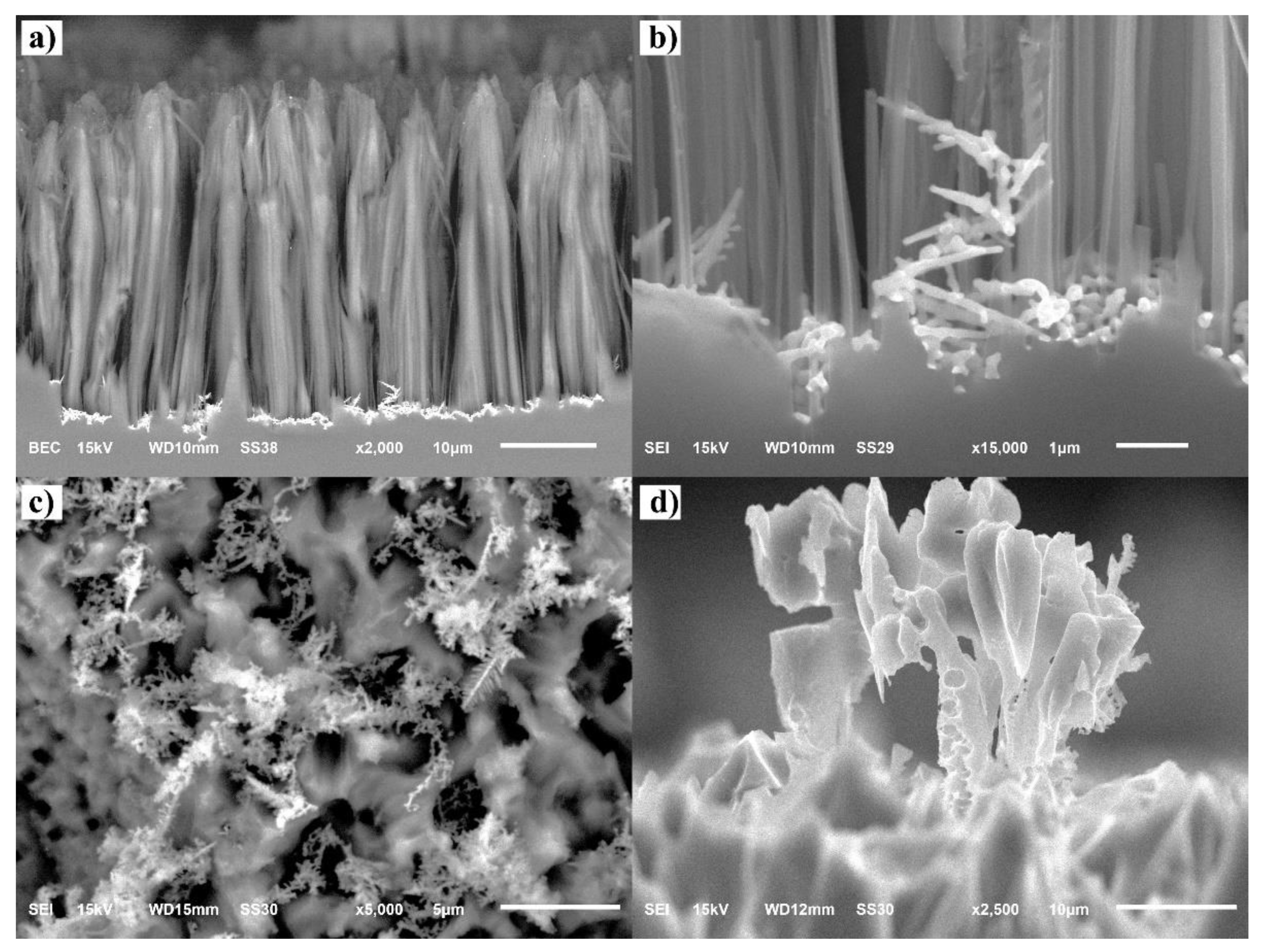
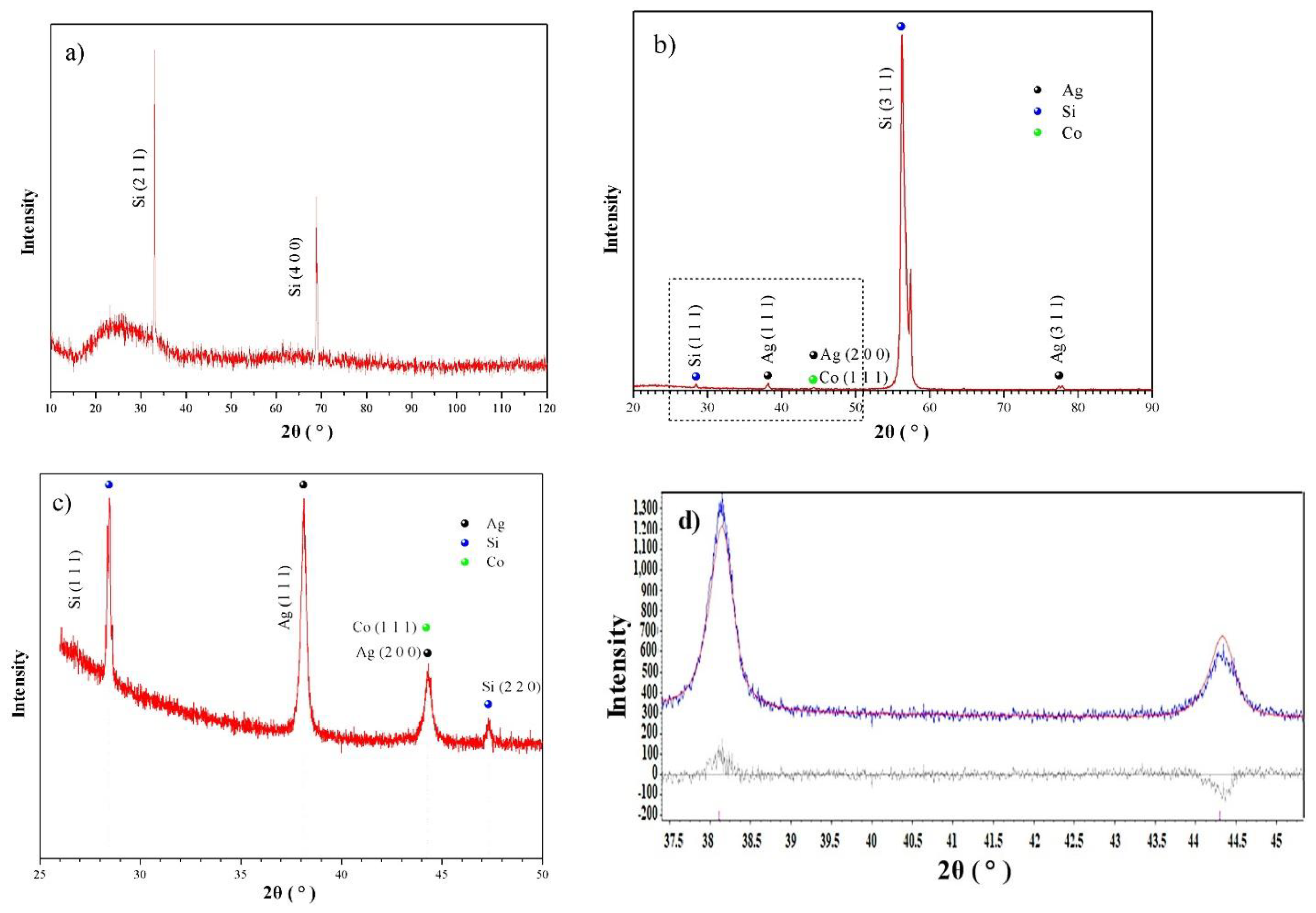
3.1. Characterization of the SiNWs-CuNPs and SiNWs-CoONPs
3.2. Heterogeneous Photocatalysis
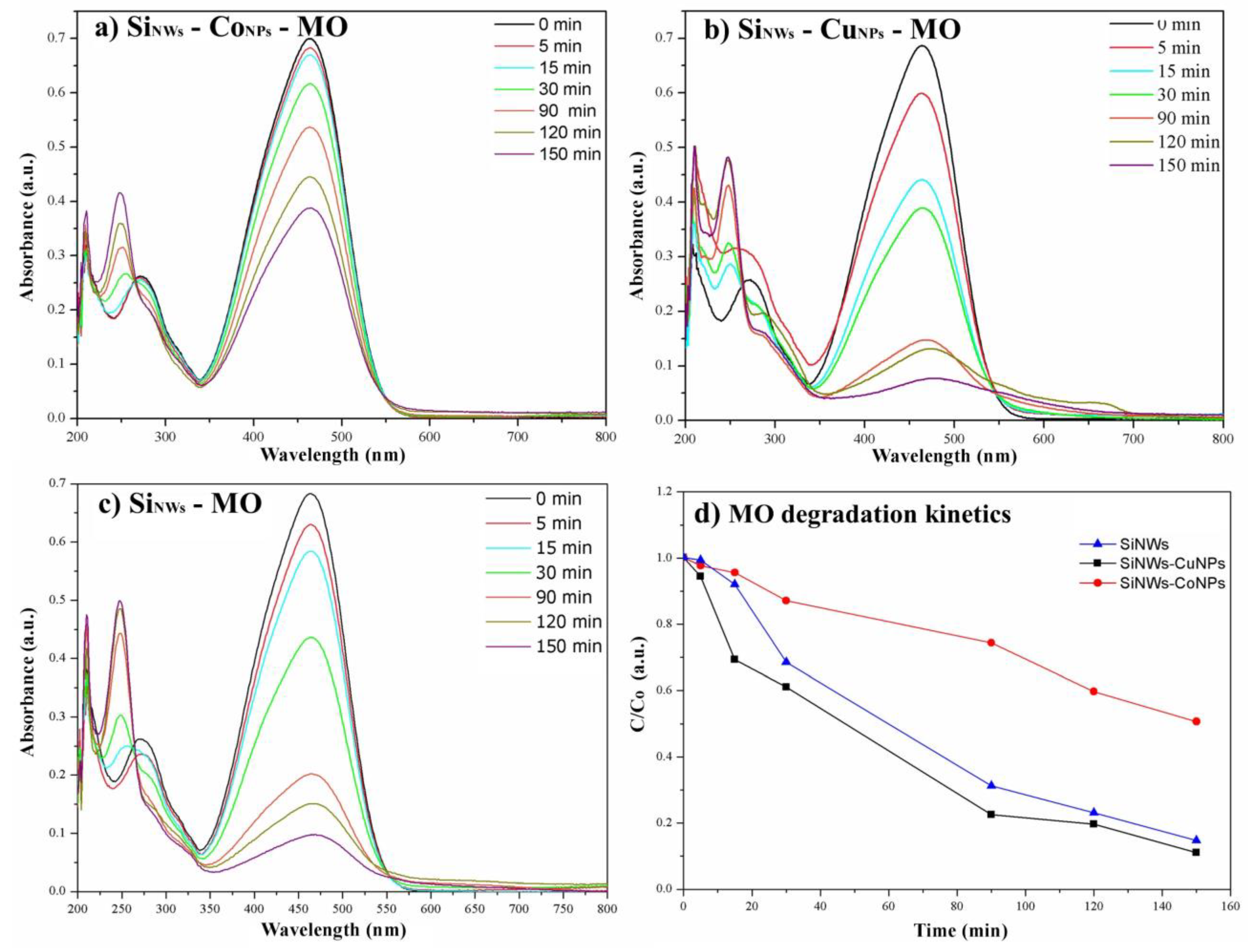
3.3. Photocatalysis with MO
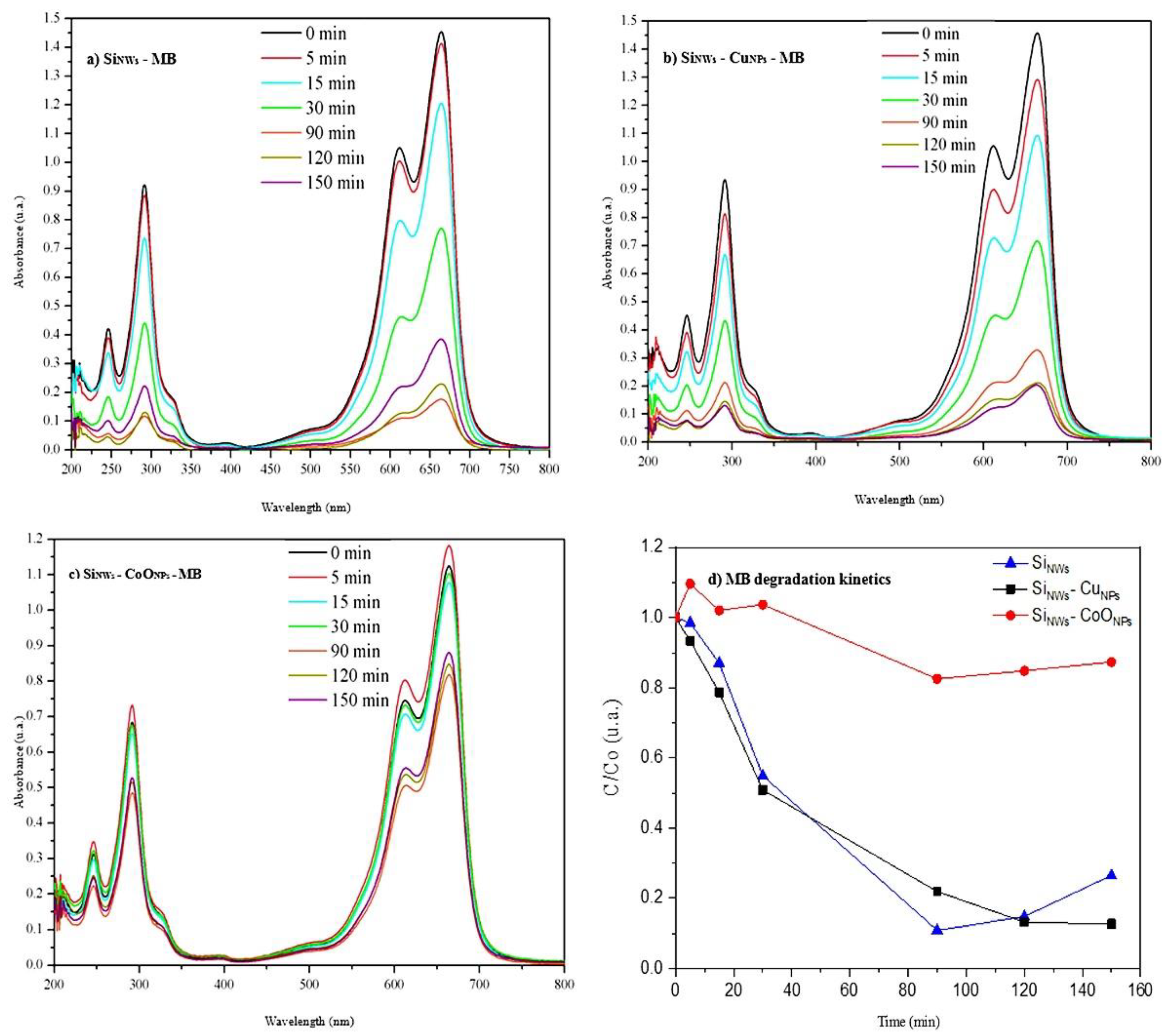
3.4. Photocatalysis with MB
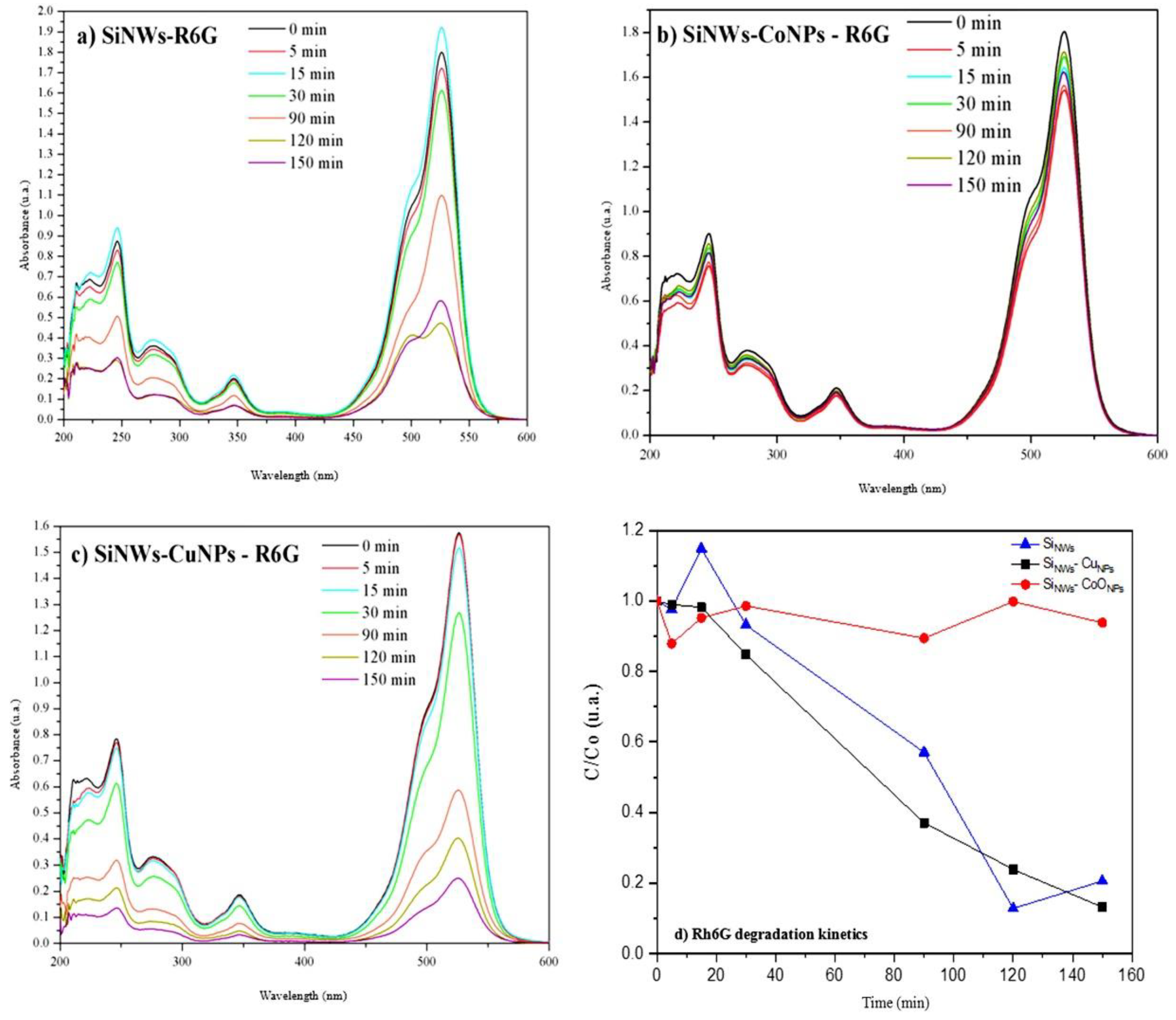
3.5. Photocatalysis with Rh6G
3.6. Review and Comparison of Photodegradations of MO, MB, and Rh B Dyes by SiNWs
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abebe, B.; Ananda Murthy, H.C.; Dessie, Y. Synthesis and Characterization of Ti–Fe Oxide Nanomaterials: Adsorption–Degradation of Methyl Orange Dye. Arab. J. Sci. Eng. 2020, 45, 4609–4620. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Z.; Xiao, K. Evaluation of Cobalt Oxide, Copper Oxide and Their Solid Solutions as Heterogeneous Catalysts for Fenton-Degradation of Dye Pollutants. RSC Adv. 2015, 5, 91846–91854. [Google Scholar] [CrossRef]
- Salierno, G.; Napoleone, S.; Maisterrena, A.; Cassanello, M.; Pellasio, M.; Doumic, L.; Ayude, M.A. Continuous Heterogeneous Fenton-Type Process for Dye Pollution Abatement Intensified by Hydrodynamic Cavitation. Ind. Eng. Chem. Res 2021, 60, 16653–16664. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs New Advanced Treatment Methods for the Removal of Contaminants of Emerging Concern from Urban Wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Johnston, A.L.; Lester, E.; Williams, O.; Gomes, R.L. Understanding Layered Double Hydroxide Properties as Sorbent Materials for Removing Organic Pollutants from Environmental Waters. J. Environ. Chem. Eng. 2021, 9, 105197. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Wang, N.; Zhao, Q.; Wang, P. Status of the Treatment of Produced Water Containing Polymer in Oilfields: A Review. J. Environ. Chem. Eng. 2021, 9, 105303. [Google Scholar] [CrossRef]
- Hernández-Morales, V.; Nava, R.; Acosta-Silva, Y.J.; MacÍas-Sánchez, S.A.; Pérez-Bueno, J.J.; Pawelec, B. Adsorption of Lead (II) on SBA-15 Mesoporous Molecular Sieve Functionalized with −NH2 Groups. Microporous Mesoporous Mater. 2012, 160, 133–142. [Google Scholar] [CrossRef]
- Martínez, M.G.A.; de Jesus Pérez-Bueno, J.; Reza, E.M.; López, M.L.M. Lead Adsorption in Manganese Oxides as Powders and Coatings Supported on Silica Gel Beads and Tin Inverse Opal-Like Structures. Curr. Anal. Chem. 2020, 17, 831–838. [Google Scholar] [CrossRef]
- Faucher, S.; Aluru, N.; Bazant, M.Z.; Blankschtein, D.; Brozena, A.H.; Cumings, J.; Pedro De Souza, J.; Elimelech, M.; Epsztein, R.; Fourkas, J.T.; et al. Critical Knowledge Gaps in Mass Transport through Single-Digit Nanopores: A Review and Perspective. J. Phys. Chem. C 2019, 123, 21309–21326. [Google Scholar] [CrossRef]
- Márquez, E.E.; Zarazúa, G.M.S.; Bueno, J.D.J.P. Prospects for the Use of Electrooxidation and Electrocoagulation Techniques for Membrane Filtration of Irrigation Water. Environ. Process. 2020, 7, 391–420. [Google Scholar] [CrossRef]
- Olea, M.A.U.; Bueno, J.D.J.P.; Pérez, A.X.M. Nanometric and Surface Properties of Semiconductors Correlated to Photocatalysis and Photoelectrocatalysis Applied to Organic Pollutants—A Review. J. Environ. Chem. Eng. 2021, 9, 106480. [Google Scholar] [CrossRef]
- Robles, M.R.G.; Bueno, J.D.J.P.; Syllas, C.S.A.; López, M.L.M.; Guerrero, F.M. Silver/Silicon Nanowires/Copper Nanoparticles Heterojunction for Methyl Orange Degradation by Heterogeneous Photocatalysis under Visible Irradiation. MRS Adv. 2018, 3, 3933–3938. [Google Scholar] [CrossRef]
- Magallón-Cacho, L.; Pérez-Bueno, J.J.; Meas-Vong, Y.; Stremsdoerfer, G.; Espinoza-Beltrán, F.J. Surface Modification of Acrylonitrile-Butadiene-Styrene (ABS) with Heterogeneous Photocatalysis (TiO2) for the Substitution of the Etching Stage in the Electroless Process. Surf. Coat. Technol. 2011, 206, 1410–1415. [Google Scholar] [CrossRef]
- Garcia, E.M.; Santos, J.S.; Pereira, E.C.; Freitas, M.B.J.G. Electrodeposition of Cobalt from Spent Li-Ion Battery Cathodes by the Electrochemistry Quartz Crystal Microbalance Technique. J. Power Sources 2008, 185, 549–553. [Google Scholar] [CrossRef]
- Zeferino, R.S.; Pal, U.; De Anda Reues, M.E.; Rosas, E.R. Indium Doping Induced Defect Structure Evolution and Photocatalytic Activity of Hydrothermally Grown Small SnO2 Nanoparticles. Adv. Nano Res. 2019, 7, 13–24. [Google Scholar] [CrossRef]
- De Lourdes Ruiz Peralta, M.; Sánchez-Cantú, M.; Puente-López, E.; Rubio-Rosas, E.; Tzompantzi, F. Evaluation of Calcium Oxide in Rhodamine 6G Photodegradation. Catal. Today 2018, 305, 75–81. [Google Scholar] [CrossRef]
- Amdouni, S.; Cherifi, Y.; Coffinier, Y.; Addad, A.; Zaïbi, M.A.; Oueslati, M.; Boukherroub, R.; Zaibie, M.A.; Oueslati, M.; Boukherroub, R. Gold Nanoparticles Coated Silicon Nanowires for Efficient Catalytic and Photocatalytic Applications. Mater. Sci. Semicond. Process. 2018, 75, 206–213. [Google Scholar] [CrossRef]
- Brahiti, N.; Hadjersi, T.; Amirouche, S.; Menari, H.; ElKechai, O. Photocatalytic Degradation of Cationic and Anionic Dyes in Water Using Hydrogen-Terminated Silicon Nanowires as Catalyst. Int. J. Hydrog. Energy 2018, 43, 11411–11421. [Google Scholar] [CrossRef]
- Hamdi, A.; Boussekey, L.; Roussel, P.; Addad, A.; Ezzaouia, H.; Boukherroub, R.; Coffinier, Y. Hydrothermal Preparation of MoS2/TiO2/Si Nanowires Composite with Enhanced Photocatalytic Performance under Visible Light. Mater. Des. 2016, 109, 634–643. [Google Scholar] [CrossRef]
- Ifires, M.; Hadjersi, T.; Chegroune, R.; Lamrani, S.; Moulai, F.; Mebarki, M.; Manseri, A. One-Step Electrodeposition of Superhydrophobic NiO-Co(OH)2 Urchin-like Structures on Si Nanowires as Photocatalyst for RhB Degradation under Visible Light. J. Alloys Compd. 2019, 774, 908–917. [Google Scholar] [CrossRef]
- Busra, U.; Omer, C. Free Vibration Analysis Silicon Nanowires Surrounded by Elastic Matrix by Nonlocal Finite Element Method. Adv. Nano Res. 2019, 7, 99–108. [Google Scholar] [CrossRef]
- Arciga-Duran, E.; Meas, Y.; Pérez-Bueno, J.J.; Ballesteros, J.C.; Trejo, G. Electrochemical Synthesis of Co3O4−x Films for Their Application as Oxygen Evolution Reaction Electrocatalysts: Role of Oxygen Vacancies. J. Electrochem. Soc. 2018, 165, H3178–H3186. [Google Scholar] [CrossRef]
- Lu, K.-Q.Q.; Lin, X.; Tang, Z.-R.R.; Xu, Y.-J.J. Silicon Nanowires@Co3O4 Arrays Film with Z-scheme Band Alignment for Hydrogen Evolution. Catal. Today 2019, 335, 294–299. [Google Scholar] [CrossRef]
- Yang, R.Q.; Ji, Y.C.; Li, Q.; Zhao, Z.H.; Zhang, R.T.; Liang, L.L.; Liu, F.; Chen, Y.K.; Han, S.W.; Yu, X.; et al. Ultrafine Si Nanowires/Sn3O4 Nanosheets 3D Hierarchical Heterostructured Array as a Photoanode with High-Efficient Photoelectrocatalytic Performance. Appl. Catal. B Environ. 2019, 256, 117798. [Google Scholar] [CrossRef]
- Ghosh, P.; Kar, A.; Khandelwal, S.; Vyas, D.; Mir, A.Q.; Chakraborty, A.L.; Hegde, R.S.; Sharma, S.; Dutta, A.; Khatua, S. Plasmonic CoO-Decorated Au Nanorods for Photoelectrocatalytic Water Oxidation. ACS Appl. Nano Mater. 2019, 2, 5795–5803. [Google Scholar] [CrossRef]
- Brieño-Enriquez, K.M.; Ledesma-García, J.; Perez-Bueno, J.J.; Godinez, L.A.; Terrones, H.; Ángeles-Chavez, C. Bonding Titanium on Multi-Walled Carbon Nanotubes for Hydrogen Storage: An Electrochemical Approach. Mater. Chem. Phys. 2009, 115, 521–525. [Google Scholar] [CrossRef]
- Ildefonso, Z.T.; José de Jesús, P.B.; Celeste Yunueth, T.L.; Luis, L.R.; Maria Luisa, M.L.; Yunny, M.V. A Phenomenon of Degradation of Methyl Orange Observed during the Reaction of NH4TiOF3 Nanotubes with the Aqueous Medium to Produce TiO2 Anatase Nanoparticles. RSC Adv. 2016, 6, 76167–76173. [Google Scholar] [CrossRef]
- Torres, I.Z.; Bueno, J.D.J.P.; López, C.Y.T.; Rojas, L.L.; López, M.L.M.; Vong, Y.M. Nanotubes with Anatase Nanoparticulate Walls Obtained from NH4TiOF3 Nanotubes Prepared by Anodizing Ti. RSC Adv. 2016, 6, 41637–41643. [Google Scholar] [CrossRef]
- Chen, Y.J.; Zhu, P.F.; Duan, M.; Li, J.; Ren, Z.H.; Wang, P.P. Fabrication of a Magnetically Separable and Dual Z-Scheme PANI/Ag3PO4/NiFe2O4 Composite with Enhanced Visible-Light Photocatalytic Activity for Organic Pollutant Elimination. Appl. Surf. Sci. 2019, 486, 198–211. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, T.; An, T.; Zong, Y.; Lee, J.Y. Unraveling the Electrocatalytically Active Sites and Stability of Co & Co Oxides on Nanocarbon for Oxygen Evolution Reaction in Acid Solution. J. Energy Chem. 2020, 49, 8–13. [Google Scholar] [CrossRef]
- Sun, T.; Liu, P.; Zhang, Y.; Chen, Z.; Zhang, C.; Guo, X.; Ma, C.; Gao, Y.; Zhang, S. Boosting the Electrochemical Water Splitting on Co3O4 through Surface Decoration of Epitaxial S-Doped CoO Layers. Chem. Eng. J. 2020, 390, 124591. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Li, M.; Shi, Y.; Shi, M.; Yan, C. Constructing 3D Sub-Micrometer CoO Octahedrons Packed with Layered MoS2 Shell for Boosting Photocatalytic Overall Water Splitting Activity. Appl. Surf. Sci. 2019, 473, 928–933. [Google Scholar] [CrossRef]
- Zhu, S.; Liao, W.; Zhang, M.; Liang, S. Design of Spatially Separated Au and CoO Dual Cocatalysts on Hollow TiO2 for Enhanced Photocatalytic Activity towards the Reduction of CO2 to CH4. Chem. Eng. J. 2019, 361, 461–469. [Google Scholar] [CrossRef]
- Boughelout, A.; Macaluso, R.; Kechouane, M.; Trari, M. Photocatalysis of Rhodamine B and Methyl Orange Degradation under Solar Light on ZnO and Cu2O Thin Films. React. Kinet. Mech. Catal. 2020, 129, 1115–1130. [Google Scholar] [CrossRef]
- Rasheed, P.; Haq, S.; Waseem, M.; Rehman, S.U.; Rehman, W.; Bibi, N.; Shah, S.A.A. Green Synthesis of Vanadium Oxide-Zirconium Oxide Nanocomposite for the Degradation of Methyl Orange and Picloram. Mater. Res. Express 2020, 7, 025011. [Google Scholar] [CrossRef]
- Han, Y.-Q.; Lei, L.; Yang, C.; Zhang, S.-Y.; Zhao, Q.; Zhang, X.-J. Mechanism underlying photocatalyzed degradation of methyl orange by layered black phosphorus. J. Appl. Ecol. 2020, 31, 333–339. [Google Scholar] [CrossRef]
- Khatri, A.; Rana, P.S. Visible Light Assisted Photocatalysis of Methylene Blue and Rose Bengal Dyes by Iron Doped NiO Nanoparticles Prepared via Chemical Co-Precipitation. Phys. B Condens. Matter 2020, 579, 411905. [Google Scholar] [CrossRef]
- Sa-Nguanprang, S.; Phuruangrat, A.; Thongtem, T.; Thongtem, S. Preparation of Visible-Light-Driven Al-Doped ZnO Nanoparticles Used for Photodegradation of Methylene Blue. J. Electron. Mater. 2020, 49, 1841–1848. [Google Scholar] [CrossRef]
- Varnagiris, S.; Urbonavicius, M.; Sakalauskaite, S.; Daugelavicius, R.; Pranevicius, L.; Lelis, M.; Milcius, D. Floating TiO2 Photocatalyst for Efficient Inactivation of E. Coli and Decomposition of Methylene Blue Solution. Sci. Total Environ. 2020, 720, 137600. [Google Scholar] [CrossRef]
- Haimerl, J.M.; Ghosh, I.; König, B.; Lupton, J.M.; Vogelsang, J. Chemical Photocatalysis with Rhodamine 6G: Investigation of Photoreduction by Simultaneous Fluorescence Correlation Spectroscopy and Fluorescence Lifetime Measurements. J. Phys. Chem. B 2018, 122, 10728–10735. [Google Scholar] [CrossRef]
- Tang, C.; Chen, C.; Zhang, H.; Zhang, J.; Li, Z. Enhancement of Degradation for Nitrogen Doped Zinc Oxide to Degrade Methylene Blue. Phys. B Condens. Matter 2020, 583, 412029. [Google Scholar] [CrossRef]
- Van de Linde, S.; Sauer, M. How to Switch a Fluorophore: From Undesired Blinking to Controlled Photoswitching. Chem. Soc. Rev. 2014, 43, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Bueno, J.J.; Vasquez-García, S.R.; García-González, L.; Vorobiev, Y.V.; Luna-Bárcenas, G.; González-Hernández, J. Optical Processes in PMMA, SiO2, and Hybrid Organic-Inorganic Sol-Gel Films Colored with Rhodamine 6GDN. J. Phys. Chem. B 2002, 106, 1550–1556. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kang, S.Y.; Choi, S.W.; Kwon, Y.J.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Fabrication and Gas Sensing Properties of Vertically Aligned Si Nanowires. Appl. Surf. Sci. 2018, 427, 215–226. [Google Scholar] [CrossRef]
- Martínez-Hernández, A.; Méndez-Albores, A.; Arciga-Duran, E.; Flores, J.G.; Pérez-Bueno, J.J.; Meas, Y.; Trejo, G. Effect of Heat Treatment on the Hardness and Wear Resistance of Electrodeposited Co-B Alloy Coatings. J. Mater. Res. Technol. 2019, 8, 960–968. [Google Scholar] [CrossRef]
- Huang, Z.; Geyer, N.; Werner, P.; De Boor, J.; Gösele, U. Metal-Assisted Chemical Etching of Silicon: A Review. Adv. Mater 2011, 23, 285–308. [Google Scholar] [CrossRef]
- Franz, M.; Junghans, R.; Schmitt, P.; Szeghalmi, A.; Schulz, S.E. Wafer-Level Integration of Self-Aligned High Aspect Ratio Silicon 3D Structures Using the MACE Method with Au, Pd, Pt, Cu, and Ir. Beilstein J. Nanotechnol. 2020, 11, 1439–1449. [Google Scholar] [CrossRef]
- Le Nguyen, N.; Phan, T.C.H.; Dang, T.M.D.; Dang, M.C. Formation of Silver Nanoparticles and Their Application for Suppressing Surface Reflection of N-Type Silicon. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 025014. [Google Scholar] [CrossRef]
- Leonardi, A.; Faro, M.; Irrera, A. Silicon Nanowires Synthesis by Metal-Assisted Chemical Etching: A Review. Nanomaterials 2021, 11, 383. [Google Scholar] [CrossRef]
- Canevali, C.; Alia, M.; Fanciulli, M.; Longo, M.; Ruffo, R.; Mari, C.M. Influence of Doping Elements on the Formation Rate of Silicon Nanowires by Silver-Assisted Chemical Etching. Surf. Coat. Technol. 2015, 280, 37–42. [Google Scholar] [CrossRef]
- Peng, K.Q.; Hu, J.J.; Yan, Y.J.; Wu, Y.; Fang, H.; Xu, Y.; Lee, S.T.; Zhu, J. Fabrication of Single-Crystalline Silicon Nanowires by Scratching a Silicon Surface with Catalytic Metal Particles. Adv. Funct. Mater. 2006, 16, 387–394. [Google Scholar] [CrossRef]
- Megouda, N.; Hadjersi, T.; Coffinier, Y.; Szunerits, S.; Boukherroub, R. Investigation of Morphology, Reflectance and Photocatalytic Activity of Nanostructured Silicon Surfaces. Microelectron. Eng. 2016, 159, 94–101. [Google Scholar] [CrossRef]
- Hsiao, P.H.; Li, T.C.; Chen, C.Y. ZnO/Cu2O/Si Nanowire Arrays as Ternary Heterostructure-Based Photocatalysts with Enhanced Photodegradation Performances. Nanoscale Res. Lett. 2019, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Santoyo, L.D.D.; Pérez-Bueno, J.J.J.; Manzano-Ramírez, A.; Gonzalez-Hernández, J.; Pérez-Robles, J.F.F.; Vorobiev, Y.V.V. Origin of Interference Colors on Austenitic Stainless Steel. Inorg. Mater. 2005, 41, 955–960. [Google Scholar] [CrossRef]
- Akram, N.; Guo, J.; Ma, W.; Guo, Y.; Hassan, A.; Wang, J. Synergistic Catalysis of Co(OH)2/CuO for the Degradation of Organic Pollutant Under Visible Light Irradiation. Sci. Rep. 2020, 10, 1939. [Google Scholar] [CrossRef]
- Sivakov, V.; Kaniukov, E.Y.; Petrov, A.V.; Korolik, O.V.; Mazanik, A.V.; Bochmann, A.; Teichert, S.; Hidi, I.J.; Schleusener, A.; Cialla, D.; et al. Silver Nanostructures Formation in Porous Si/SiO2 Matrix. J. Cryst. Growth 2014, 400, 21–26. [Google Scholar] [CrossRef]
- Chiou, A.-H.; Chien, T.-C.; Su, C.-K.; Lin, J.-F.; Hsu, C.-Y. The Effect of Differently Sized Ag Catalysts on the Fabrication of a Silicon Nanowire Array Using Ag-Assisted Electroless Etching. Curr. Appl. Phys. 2013, 13, 717–724. [Google Scholar] [CrossRef]
- Fewster, P.F. A New Theory for X-Ray Diffraction. Acta Crystallogr. Sect. A Found. Adv. 2014, 70, 257–282. [Google Scholar] [CrossRef]
- Fraser, J.T.; Wark, J.S. Comments on a New Theory for X-Ray Diffraction. Acta Crystallogr. Sect. A Found. Adv. 2018, 74, 447–465. [Google Scholar] [CrossRef]
- Sha, Y.; Mathew, I.; Cui, Q.; Clay, M.; Gao, F.; Zhang, X.J.; Gu, Z. Rapid Degradation of Azo Dye Methyl Orange Using Hollow Cobalt Nanoparticles. Chemosphere 2016, 144, 1530–1535. [Google Scholar] [CrossRef]
- Brik, A.; Naama, S.; Hadjersi, T.; Benamar, M.E.A.; Bouanik, S.; Manseri, A. Photodegradation of Methylene Blue under UV and Visible Light Irradiation by Er2O3-Coated Silicon Nanowires as Photocatalyst. React. Kinet. Mech. Catal. 2020, 131, 525–536. [Google Scholar] [CrossRef]
- Ameen, S.; Park, D.R.; Shin, H.S. Silicon Nanowires Arrays for Visible Light Driven Photocatalytic Degradation of Rose Bengal Dye. J. Mater. Sci. Mater. Electron. 2016, 27, 10460–10467. [Google Scholar] [CrossRef]
- Han, H.X.; Shi, C.; Yuan, L.; Sheng, G.P. Enhancement of Methyl Orange Degradation and Power Generation in a Photoelectrocatalytic Microbial Fuel Cell. Appl. Energy 2017, 204, 382–389. [Google Scholar] [CrossRef]
- Gaidi, M.; Daoudi, K.; Columbus, S.; Hajjaji, A.; El Khakani, M.A.; Bessais, B. Enhanced Photocatalytic Activities of Silicon Nanowires/Graphene Oxide Nanocomposite: Effect of Etching Parameters. J. Environ. Sci. 2021, 101, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Ghosh, J.; Das, R.; Mawlong, L.P.L.L.; Paul, K.K.; Giri, P.K. Multifunctional Ag Nanoparticle Decorated Si Nanowires for Sensing, Photocatalysis and Light Emission Applications. J. Colloid Interface Sci. 2018, 532, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhong, H.; Zhu, Y.; Jiang, H.; Shen, J.; Huang, J.; Li, C. Highly Efficient Reusable Catalyst Based on Silicon Nanowire Arrays Decorated with Copper Nanoparticles. J. Mater. Chem. A 2014, 2, 9040–9047. [Google Scholar] [CrossRef]
- Tang, C.H.; Hsiao, P.H.; Chen, C.Y. Efficient Photocatalysts Made by Uniform Decoration of Cu2O Nanoparticles on Si Nanowire Arrays with Low Visible Reflectivity. Nanoscale Res. Lett. 2018, 13, 312–315. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, G.; Wang, J.; Liang, X.; Zuo, Z.; Shi, Y. Fabrication and Photocatalytic Properties of Silicon Nanowires by Metal-Assisted Chemical Etching: Effect of H2O2 Concentration. Nanoscale Res. Lett. 2012, 7, 663. [Google Scholar] [CrossRef]



| Methyl Orange | Methylene Blue | Rhodamine 6G | |||||
|---|---|---|---|---|---|---|---|
| Order | R2 | k | R2 | k | R2 | k | |
| SiNWs-CuNPs | 1 | 0.983 | −0.01416 | 0.9719 | −0.014 | 0.983 | −0.0134 |
| 2 | 0.927 | 774.45 | 0.9738 | 820.43 | 0.8706 | 623.185 | |
| SiNWs | 1 | 0.997 | −0.0129 | 0.694 | −0.0129 | 0.829 | −0.013 |
| 2 | 0.94 | 583.75 | 0.4742 | 625.78 | 0.655 | 570.47 | |
| SiNWs-CoNPs | 1 | 0.978 | −0.00435 | 0.746 | −0.0016 | 6.88 × 10−5 | −7.26 × 10−6 |
| 2 | 0.953 | 99.238 | 0.743 | 27.80 | 6.99 × 10−6 | 0.040 | |
| Silicon Type and Orientation | Photocatalyst Composite | Light Source | Target Molecule | Concentration (mg/L) | Degradation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| p | Cu- SiNWs | Visible light LEDs | MO | 20.0 | 92 (150 min) | [12] |
| p (100) | Pd- SiNWs | Visible light | MO | 25.0 | 84.5 (36 h in a fuel cell) | [63] |
| n (100) | H-SiNWs | UV | MO | 0.327 | 93 (200 min) | [18] |
| p (111) | H-SiNWs | UV | MB | 0.32 | 49.35 (200 min) | [18] |
| p (100) | SiNWs/GO | UV | MB | 6.4 | 93 (280 min) | [64] |
| p (100) | Ag-SiNWs | Visible | MB | 3.199 | ~98 (200 min) | [65] |
| SiNWs | 580 nm | MB | 31.985 | 18.4 (120 min) | [53] | |
| Cu2O-SiNWs | 580 nm | MB | 15.99 | 53.8 (120 min) | [53] | |
| p (100) | Cu-SiNWs/NaBH4 | None | MB | 15.99 | ~96 (10 min) | [66] |
| SiNWs/GO + H2O2 | 6.4 | 96 (120 min) | [64] | |||
| SiNWs/GO | 6.4 | 93 (280 min) | [64] | |||
| p (100) | CuO2-SiNWs | 580 nm | MB | 0.2 × 10−3 M (5 mL) | (100 min) | [67] |
| p & n (100), (111), (110); 0.7 × 1.5 cm2; 0.02, 5–10, 100 Ω cm | H-SiNWs | >420 nm | MB MO | 10−6 M (4 mL) | (200 min) | [18] |
| p (100), 1–10 Ω cm | SiNWs—AuNPS | >420 nm | Rh B | 0.5 × 10−6 M (3 mL) | (240 min) | [17] |
| p (100), 1.2 × 0.8 cm2, 0.009–0.01 Ω cm | SiNWs—AgNPS SiNWs—CuNPS | >420 nm | Rh B | 0.5 × 10−5 M (2 mL) | (120 min) | [52] |
| p (100), 2 × 2 cm2, 5–10 Ω cm | SiNWs | 400 nm | Rh B | 10 | (90 min) | [62] |
| p (100) 2 × 2 cm2 | SiNWs | >420 nm | Rh B | 1 (50 mL) | (300 min) | [68] |
| p (111) | SiNWs | Visible light LEDs | MO, MB, Rh6G | 20.0 | 85.3 (150 min), 86 (90 min), 87 (120 min) | This work |
| Cu-SiNWs | 88.9%, 87.2%, 86.88 (150 min) | |||||
| CoO-SiNWs | 49.3 (150 min), 17.3 (90 min), 12 (5 min) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas Cortez, O.A.; de Jesús Pérez Bueno, J.; Casados Mexicano, Y.; Mendoza López, M.L.; Rodríguez, C.H.; Maldonado Pérez, A.X.; Cruz Alejandre, D.; Magdaleno López, C.; García Robles, M.R.; Oza, G.; et al. CoO, Cu, and Ag Nanoparticles on Silicon Nanowires with Photocatalytic Activity for the Degradation of Dyes. Sustainability 2022, 14, 13361. https://doi.org/10.3390/su142013361
Cárdenas Cortez OA, de Jesús Pérez Bueno J, Casados Mexicano Y, Mendoza López ML, Rodríguez CH, Maldonado Pérez AX, Cruz Alejandre D, Magdaleno López C, García Robles MR, Oza G, et al. CoO, Cu, and Ag Nanoparticles on Silicon Nanowires with Photocatalytic Activity for the Degradation of Dyes. Sustainability. 2022; 14(20):13361. https://doi.org/10.3390/su142013361
Chicago/Turabian StyleCárdenas Cortez, Olda Alexia, José de Jesús Pérez Bueno, Yolanda Casados Mexicano, Maria Luisa Mendoza López, Carlos Hernández Rodríguez, Alejandra Xochitl Maldonado Pérez, David Cruz Alejandre, Coraquetzali Magdaleno López, María Reina García Robles, Goldie Oza, and et al. 2022. "CoO, Cu, and Ag Nanoparticles on Silicon Nanowires with Photocatalytic Activity for the Degradation of Dyes" Sustainability 14, no. 20: 13361. https://doi.org/10.3390/su142013361
APA StyleCárdenas Cortez, O. A., de Jesús Pérez Bueno, J., Casados Mexicano, Y., Mendoza López, M. L., Rodríguez, C. H., Maldonado Pérez, A. X., Cruz Alejandre, D., Magdaleno López, C., García Robles, M. R., Oza, G., Flores López, J. G., & Silva, H. R. (2022). CoO, Cu, and Ag Nanoparticles on Silicon Nanowires with Photocatalytic Activity for the Degradation of Dyes. Sustainability, 14(20), 13361. https://doi.org/10.3390/su142013361











