Green Nanoparticle-Aided Biosorption of Nickel Ions Using Four Dry Residual Biomasses: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Preparation
2.2. Modification with TiO2 Nanoparticles
2.3. Characterization Techniques
2.4. Adsorption Study
3. Results and Discussion
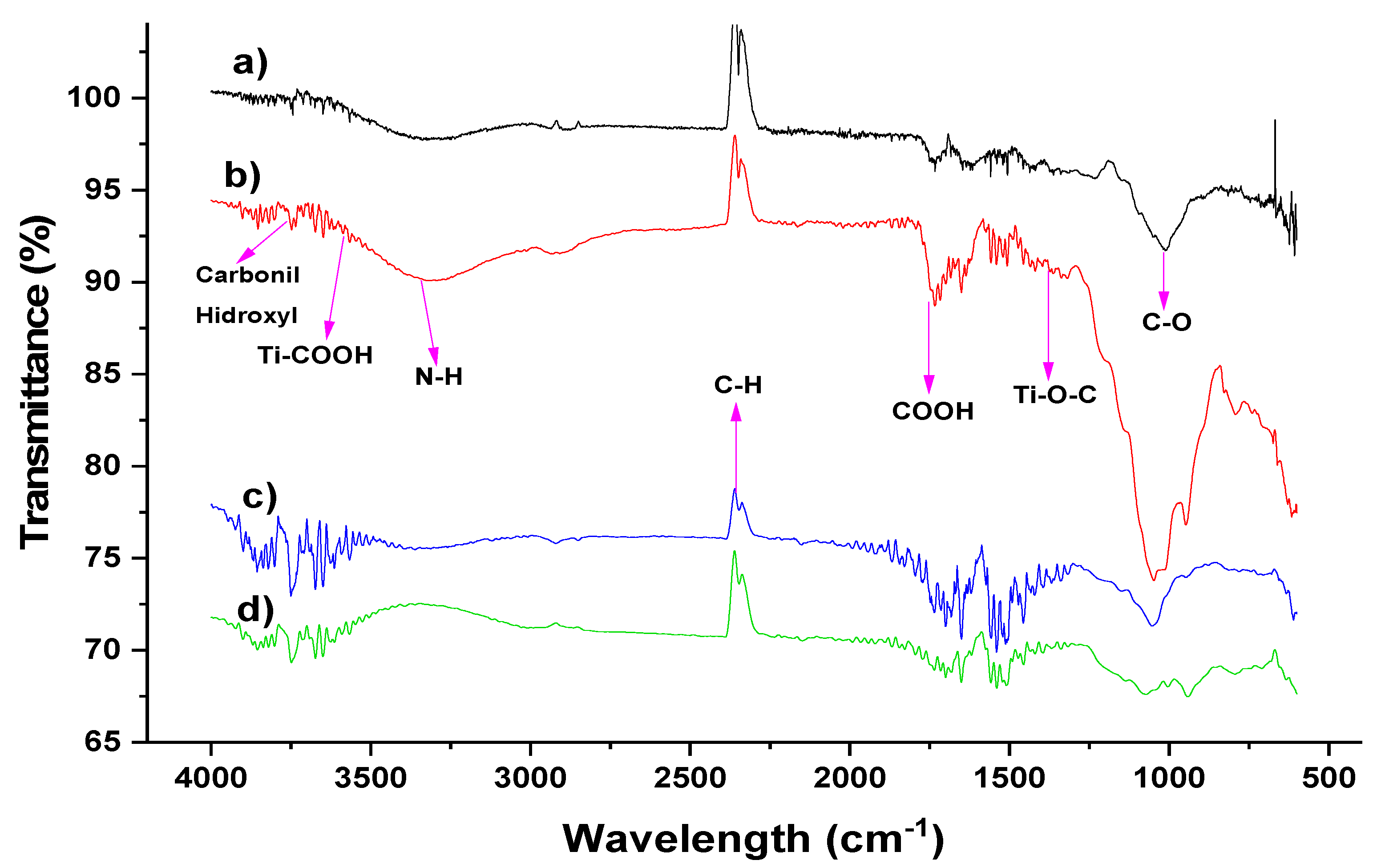
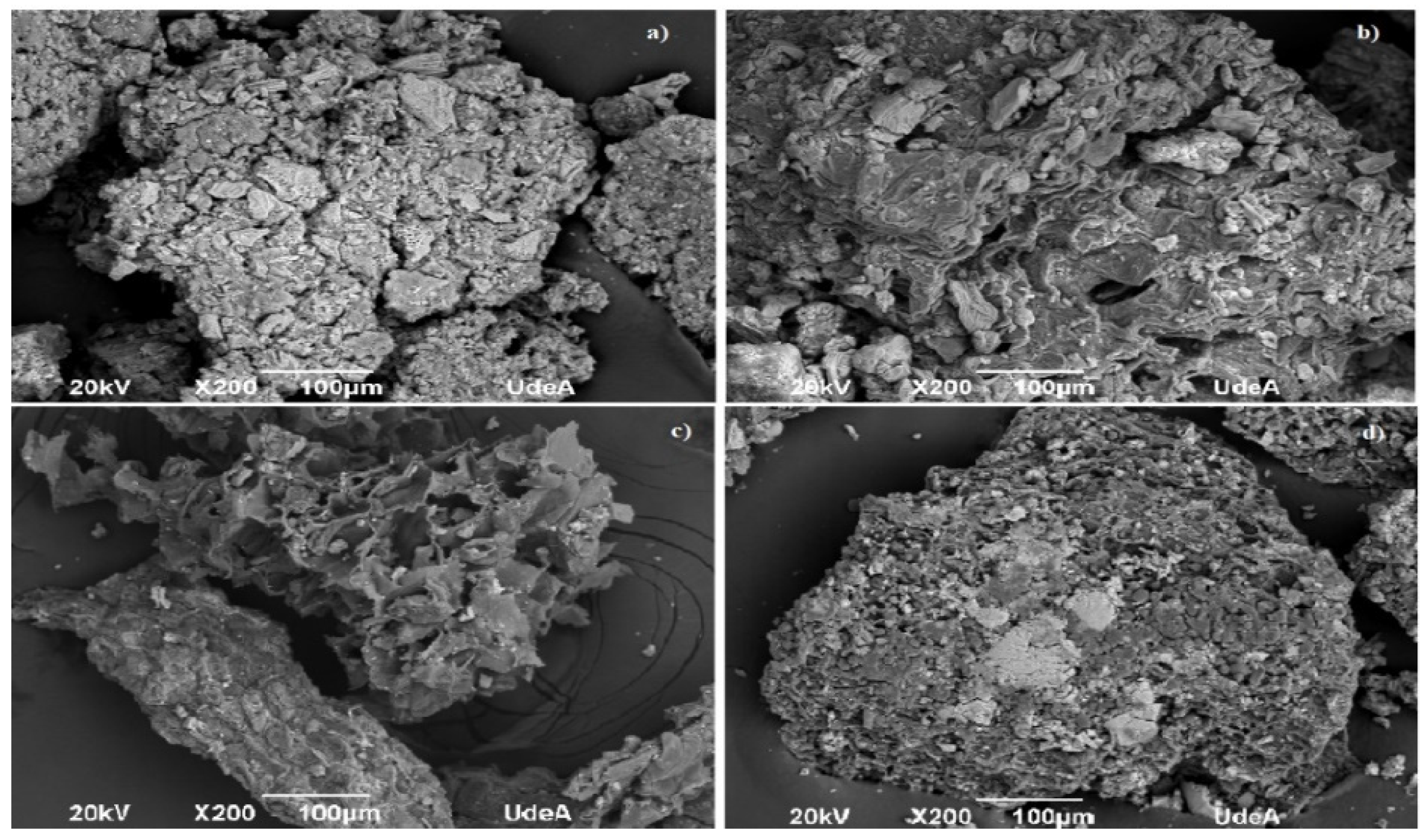
3.1. Biomass Characterization
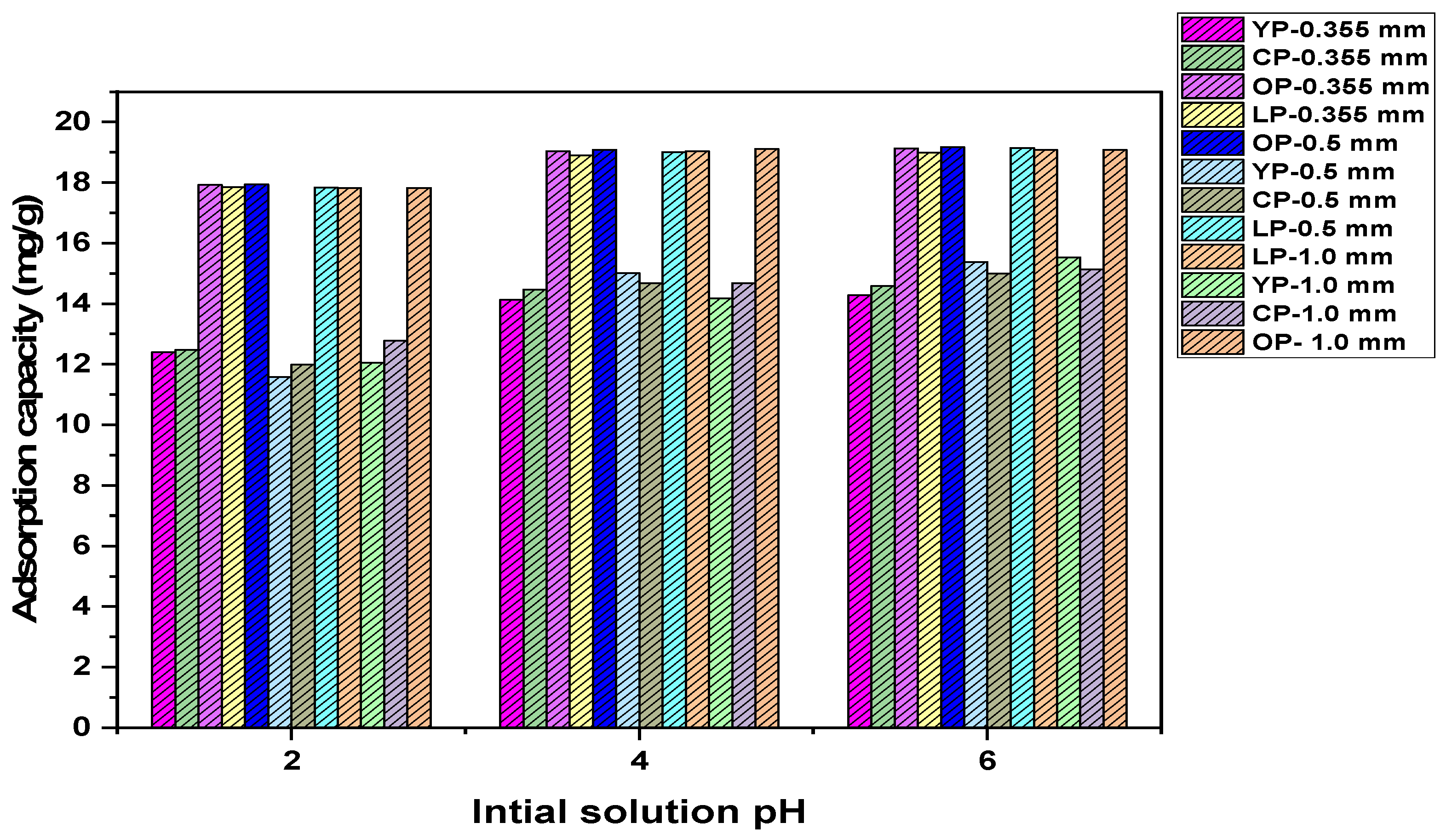
3.2. Adsorption Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, D.; Pingul-Ong, S.; Kan, C.-C. Removal of nickel ions from aqueous solutions by manganese dioxide derived from groundwater treatment sludge. J. Clean. Prod. 2018, 190, 443–451. [Google Scholar] [CrossRef]
- Li, C.; Zhao, J.; Zhang, Y. Study on Adsorption Behavior of Nickel Ions Using Silica-Based Sandwich Layered Zirconium-Titanium Phosphate Prepared by Layer-by-Layer Grafting Method. Nanomaterials 2021, 11, 2314. [Google Scholar] [CrossRef] [PubMed]
- Bulgariu, L.; Bulgariu, D. Functionalized soy waste biomass-A novel environmental-friendly biosorbent for the removal of heavy metals from aqueous solution. J. Clean. Prod. 2018, 197, 875–885. [Google Scholar] [CrossRef]
- World-Health-Organization. Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Ministerio de Ambiente y Desarrollo Sostenible. Parameters and Maximum Permissible Limit Values in Specific Discharges (Parámetros y Los Valores Límites Máximos Permisibles en Vertimientos Puntuales a Cuerpos de Aguas Superficiales y a Sistemas de Alcantarillado Público); Ministerio de Ambiente y Desarrollo Sostenible: Bogotá, Colombia, 2012; pp. 1–5.
- Herrera-Barros, A.; Tejada-Tovar, C.; Villabona-Ortíz, A.; Gonzalez-Delgado, A.D.; Benitez-Monroy, J. Cd (II) and Ni (II) uptake by novel biosorbent prepared from oil palm residual biomass and Al2O3 nanoparticles. Sustain. Chem. Pharm. 2020, 15, 100216. [Google Scholar] [CrossRef]
- Tran, T.P.A.; Cho, H.; Cho, G.C.; Han, J.I.; Chang, I. Nickel (Ni2+) Removal from Water Using Gellan Gum–Sand Mixture as a Filter Material. Appl. Sci. 2021, 11, 7884. [Google Scholar] [CrossRef]
- Muhammad, A.; Shah, A.U.H.A.; Bilal, S. Effective Adsorption of Hexavalent Chromium and Divalent Nickel Ions from Water through Polyaniline, Iron Oxide, and Their Composites. Appl. Sci. 2020, 10, 2882. [Google Scholar] [CrossRef] [Green Version]
- Do, Q.C.; Choi, S.; Kim, H.; Kang, S. Adsorption of Lead and Nickel on to Expanded Graphite Decorated with Manganese Oxide Nanoparticles. Appl. Sci. 2019, 9, 5375. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Sheng, L.; Wang, Y.; Wyckoff, K.N.; He, C. Characteristics of Cadmium Sorption by Heat-Activated Red Mud in Aqueous Solution. Sci. Rep. 2018, 8, 13558. [Google Scholar] [CrossRef]
- Liu, X.; Pang, H.; Liu, X.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M.; Hu, B.; Yang, H.; Wang, X. Orderly Porous Covalent Organic Frameworks-based Materials: Superior Adsorbents for Pollutants Removal from Aqueous Solutions. Innovation 2021, 2, 100076. [Google Scholar] [CrossRef]
- Abegunde, S.M.; Idowu, K.S.; Adejuwon, O.M.; Adeyemi-Adejolu, T. A review on the influence of chemical modification on the performance of adsorbents. Resour. Environ. Sustain. 2020, 1, 100001. [Google Scholar] [CrossRef]
- Thangavelu, K.; Annamalai, R.; Arulnandhi, D. Preparation and Characterization of Nanosized Tio2 Powder by Sol-Gel Precipitation Route. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 636–639. [Google Scholar]
- Ajith, M.P.; Aswathi, M.; Priyadarshini, E.; Rajamani, P. Recent innovations of nanotechnology in water treatment: A comprehensive review. Bioresour. Technol. 2021, 342, 126000. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, H.; Guo, H.; Ling, C.; Yuan, X.; Li, P. Hydrated titanium oxide nanoparticles supported on natural rice straw for Cu (II) removal from water. Environ. Technol. Innov. 2020, 20, 101143. [Google Scholar] [CrossRef]
- Janani, R.; Gurunathan, B.K.S.; Varjani, S.; Ngo, H.H.; Gnansounou, E. Advancements in heavy metals removal from effluents employing nano-adsorbents: Way towards cleaner production. Environ. Res. 2022, 203, 111815. [Google Scholar] [CrossRef]
- El-Sheikh, A.H.; Shudayfat, A.M.; Fasfous, I.I. Preparation of magnetic biosorbents based on cypress wood that was pretreated by heating or TiO2 deposition. Ind. Crops Prod. 2019, 129, 105–113. [Google Scholar] [CrossRef]
- Herrera-Barros, A.; Tejada-Tovar, C.; Villabona-Ortíz, Á.; González-Delgado, Á.; Reyes-Ramos, A. Adsorption study of Ni (II) and Pb (II) onto low-cost agricultural biomasses chemically modified with TiO2 nanoparticles. Indian J. Sci. Technol. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Dou, B.; Dupont, V.; Pan, W.; Chen, B. Removal of aqueous toxic Hg(II) by synthesized TiO2 nanoparticles and TiO2/montmorillonite. Chem. Eng. J. 2011, 166, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Guerrero, A.; Cortés-Martínez, R.; Alfaro-Cuevas-villanueva, R.; Rivera-Muñoz, E.M.; Huirache-Acuña, R. Cd(II) and Pb(II) Adsorption Using a Composite Obtained from Moringa oleifera Lam. Cellulose Nanofibrils Impregnated with Iron Nanoparticles. Water 2021, 13, 89. [Google Scholar] [CrossRef]
- Zhao, J.; Boada, R.; Cibin, G.; Palet, C. Enhancement of selective adsorption of Cr species via modification of pine biomass. Sci. Total Environ. 2021, 756, 143816. [Google Scholar] [CrossRef]
- Ouma, I.L.A.; Naidoo, E.B.; Ofomaja, A.E. Iron oxide nanoparticles stabilized by lignocellulosic waste as green adsorbent for Cr(VI) removal from wastewater. Eur. Phys. J. Appl. Phys. 2017, 79, 30401. [Google Scholar] [CrossRef]
- Herrera-Barros, A.; Tejada-Tovar, C.; Villabona-Ortiz, A.; Gonzalez-Delgado, A.D.; Fornaris-Lozada, L. Effect of pH and Particle Size for Lead and Nickel Uptake from Aqueous Solution using Cassava (Manihot esculenta) and yam (Dioscorea alata) Residual Biomasses Modified with Titanium Dioxide Nanoparticles. Indian J. Sci. Technol. 2018, 11, 1–7. [Google Scholar] [CrossRef]
- Herrera-Barros, A.; Tejada-Tovar, C.; Villabona-Ortíz, A.; Gonzalez-Delgado, Á.; Mejia-Meza, R. Assessment of the Effect of Al2O3 and TiO2 Nanoparticles on Orange Peel Biomass and its Application for Cd (II) and Ni (II) Uptake. Trans. ASABE 2019, 62, 139–147. [Google Scholar] [CrossRef]
- Rhee, K.C. Determination of Total Nitrogen. Curr. Protoc. Food Anal. Chem. 2001, 00, B1.2.1–B1.2.9. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists AOAC 972.43-1975. Microchemical Determination of Carbon, Hydrogen; Association of Official Agricultural Chemists: Rockville, MD, USA, 1975. [Google Scholar]
- Kumar, M.; Turner, S. Protocol: A medium-throughput method for determination of cellulose content from single stem pieces of Arabidopsis thaliana. Plant Methods 2015, 11, 46. [Google Scholar] [CrossRef]
- Moreno-Sader, K.; García-Padilla, A.; Realpe, A.; Acevedo-Morantes, M.; Soares, J. Removal of Heavy Metal Water Pollutants (Co2+ and Ni2+) Using Polyacrylamide/Sodium Montmorillonite (PAM/Na-MMT) Nanocomposites. ACS Omega 2019, 4, 10834–10844. [Google Scholar] [CrossRef] [Green Version]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Fruit peel waste: Characterization and its potential uses. Curr. Sci. 2017, 113, 444–454. [Google Scholar] [CrossRef]
- Wan, L.; Chen, D.; Liu, J.; Zhang, Y.; Chen, J.; Du, C.; Xie, M. Facile preparation of porous carbons derived from orange peel via basic copper carbonate activation for supercapacitors. J. Alloys Compd. 2020, 823, 153747. [Google Scholar] [CrossRef]
- Asuquo, E.; Martin, A.; Nzerem, P. Evaluation of Cd (II) Ion Removal from Aqueous Solution by a Low-Cost Adsorbent Prepared from White Yam (Dioscorea rotundata) Waste Using Batch Sorption. Chemengineering 2018, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Ibeto, C.; Anisha, M.; Anyanwu, C. Evaluation of the Fuel Properties and Pollution Potentials of Lignite Coal and Pellets of its Blends with Different Biowastes. Am. Chem. Sci. J. 2016, 14, 1–12. [Google Scholar] [CrossRef]
- Mamma, D.; Christakopoulos, P. Biotransformation of Citrus By-Products into Value Added Products. Waste Biomass Valorization 2014, 5, 529–549. [Google Scholar] [CrossRef]
- Fakhre, N.A.; Ibrahim, B.M. The use of new chemically modified cellulose for heavy metal ion adsorption. J. Hazard. Mater. 2018, 343, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Duo, H.; Li, S.; An, Y.; Chen, Z.; Liu, Z.; Ren, Y.; Wang, S.; Zhang, X.; Wang, X. An overview of the recent advances in functionalization biomass adsorbents for toxic metals removal. Colloid Interface Sci. Commun. 2020, 38, 100308. [Google Scholar] [CrossRef]
- Ba-Abbad, M.; Kahum, A.; Mohamad, A.; Takriff, M.; Sopian, K. Synthesis and Catalytic Activity of TiO2 Nanoparticles for Photochemical Oxidation of Concentrated Chlorophenols under Direct Solar Radiation. Int. J. Electrochem. Sci. 2012, 7, 4871–4888. [Google Scholar]
- Abatal, M.; Lima, E.C.; Giannakoudakis, D.A.; Vargas, J.; Anastopoulos, I.; Olguin, M.T.; Alfonso, I. Pitahaya Fruit (Hylocereus spp.) Peels Evaluation for Removal of Pb(II), Cd(II), Co(II), and Ni(II) from the Waters. Sustainability 2022, 14, 1685. [Google Scholar] [CrossRef]
- Singh, R.J.; Martin, C.E.; Barr, D.; Rosengren, R.J. Immobilised apple peel bead biosorbent for the simultaneous removal of heavy metals from cocktail solution. Cogent Environ. Sci. 2019, 5, 1673116. [Google Scholar] [CrossRef]
- Nithya, K.; Sathish, A.; Kumar, P.S. Packed bed column optimization and modeling studies for removal of chromium ions using chemically modified Lantana camara adsorbent. J. Water Process Eng. 2020, 33, 101069. [Google Scholar] [CrossRef]
- Herrera-Barros, A.; Tejada-Tovar, C.; Villabona-Ortíz, Á.; González-Delgado, Á.; Reyes-Ramos, A. Synthesis and Characterization of Cassava, Yam and Lemon Peels Modified with TiO2 Nanoparticles. Contemp. Eng. Sci. 2018, 11, 1863–1871. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, S.; Laird, D.A.; Wang, X.; Meng, Z. Adsorption behaviour and mechanisms of cadmium and nickel on rice straw biochars in single- and binary-metal systems. Chemosphere 2019, 218, 218–308. [Google Scholar] [CrossRef]
- El-Azazy, M.; El-Shafie, A.S.; Issa, A.A.; Al-Sulaiti, M.; Al-Yafie, J.; Shomar, B.; Al-Saad, K. Potato Peels as an Adsorbent for Heavy Metals from Aqueous Solutions: Eco-Structuring of a Green Adsorbent Operating Plackett-Burman Design. J. Chem. 2019, 2019, 4926240. [Google Scholar] [CrossRef] [Green Version]
- Mende, M.; Schwarz, D.; Steinbach, C.; Boldt, R.; Schwarz, S. The Influence of Salt Anions on Heavy Metal Ion Adsorption on the Example of Nickel. Materials 2018, 11, 373. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Xu, X.; Wang, X.; Gao, B.; Yue, Q.; Song, W.; Zhang, L.; Wang, H. FTIR, Raman, and XPS analysis during phosphate, nitrate and Cr(VI) removal by amine cross-linking biosorbent. J. Colloid Interface Sci. 2016, 468, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, H.; Farooqi, Z.H.; Ahmed, E.; Sharif, A.; Din, M.I.; Arshad, M.; Nisar, J.; Zhou, L.; Yun, W.; Nawaz, I.; et al. Fabrication of a novel hybrid biocomposite based on amino-thiocarbamate derivative of alginate/carboxymethyl chitosan/TiO2 for Ni(II) recovery. Int. J. Biol. Macromol. 2020, 152, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Karapinar, H.S.; Kilicel, F.; Ozel, F.; Sarilmaz, A. Fast and effective removal of Pb(II), Cu(II) and Ni(II) ions from aqueous solutions with TiO2 nanofibers: Synthesis, adsorption-desorption process and kinetic studies. Int. J. Environ. Anal. Chem. 2021, 1–21. [Google Scholar] [CrossRef]
- Shehzad, H.; Ahmed, E.; Sharif, A.; Farooqi, Z.H.; Din, M.I.; Begum, R.; Liu, Z.; Zhou, L.; Ouyang, J.; Irfan, A.; et al. Modified alginate-chitosan-TiO2 composites for adsorptive removal of Ni(II) ions from aqueous medium. Int. J. Biol. Macromol. 2022, 194, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, M.; Keshtkar, A.R. Removal of Th(IV), Ni(II)and Fe(II) from aqueous solutions by a novel PAN–TiO2 nanofiber adsorbent modified with aminopropyltriethoxysilane. Res. Chem. Intermed. 2016, 42, 4055–4076. [Google Scholar] [CrossRef]
- Mahdavi, S. Nano-TiO2 modified with natural and chemical compounds as efficient adsorbents for the removal of Cd+2, Cu+2, and Ni+2 from water. Clean Technol. Environ. Policy 2016, 18, 81–94. [Google Scholar] [CrossRef]


| Parameter | Method | Description | |
|---|---|---|---|
| Carbon (%) | AOAC 972.43 | A Thermo Scientific FLASH 2000 Analyzer, Organic Elemental Analyzer was used; 5 g of the sample was burnt in excess of oxygen, and the carbon and hydrogen present in it were oxidized to carbon dioxide and water. For this, the sample was dropped into a combustion tube containing chromium (III) oxide and silvered cobaltous/cobaltic oxide catalysts. An aliquot of purified oxygen was added to the quartz tube, generating a flash combustion reaction and increasing the reaction temperature from 1020 °C to 1800–2000 °C. | |
| Hydrogen (%) | AOAC 972.43 | ||
| Nitrogen (%) | AOAC 984.13 KJELDAHL | Two grams of the sample with particle size <1 mm was placed into a digestion flask along with 40 mL of sulphuric acid at 98%, adding 7 g of potassium sulfate and copper. The mixture was heated at 370 °C during 180 min until white fumes could be seen, continuing the heating for about 60–90 min. The digestion is finished when the sample will be totally transparent with a slightly blue color due to the Cu from the catalyst. The tube flask was cooled at room temperature, and 250 mL of water was added cautiously. During the distillation, 50 mL of sodium hydroxide 50% w/w solution was added to the sample to neutralize the pH and to convert NH4+ into NH3−; NH3− was captured in a 50 mL of boric acid solution 4% that contains 6–7 drops of Tashiro’s indicator, and the solution turns from red violet to green (pH 4.4–5.8) due to the color change of the indicator from acid to basic medium. Then, the solution was titrated with HCl 0.25 mol/L until the solution had a slightly violet color. | |
| Ashes (%) | Thermogravimetry | Five grams of dry material (M1) was placed at 700 °C and left for 1 h to determine the ash content (M4). The percentage of ash was determined using the following equation: | |
| (2) | |||
| Pectin (%) | Digestion-thermogravimetry | Ten grams of sample was degraded with a mixture of nitric acid and acetic acid and boiled at 80 °C for 30 min in an apparatus that contained a condenser. The solution was then filtered through a funnel. Then the filter paper containing an insoluble residue was dried in the oven and measured for determining the cellulose content. The extraction process of pectin was carried out under reflux using acidified water at 97 °C for 30 min. The hot acid extract was then filtered using a cheese cloth to remove the pulp. The filtrate was then cooled to 4 °C and precipitated using double the volume of ethanol. The solvent precipitate mixture was then mixed until the pectin floated and removed by using cheese cloth followed by drying. Separation of hemicelluloces was performed using a two-stage separation process based on a prehydrolysis step with a weak acid dilution (H2SO4+ water) where the hemicellulose was dissolved in water, followed by an organosolv process with ethanol. | |
| Cellulose (%) | Digestion-thermogravimetry | ||
| Hemicellulose (%) | Digestion-thermogravimetry | ||
| Lignin (%) | Photocalorimetry | Five grams of biomass oven-dry mass was placed into a flask, where a potassium hydroxide 0.2% water solution of 2,3,5-triphenyltetrazolium chloride was added. The flask was heated in a water bath (during this time the sample obtains red color) and cooled afterwards. Then, the sample was filtered and washed with ethanol unless formed dye transferred to the solution | |
| Biomass | Carbon (%) | Hydrogen (%) | Nitrogen (%) |
|---|---|---|---|
| OP | 44.43 ± 0.39 | 6.21 ± 0.18 | 0.81 ± 0.03 |
| LP | 38.48 ± 0.28 | 4.98 ± 0.09 | 1.21 ± 0.06 |
| YP | 48.14 ± 0.47 | 5.44 ± 0.12 | 0.18 ± 0.05 |
| CP | 39.96 ± 0.36 | 3.98 ± 0.08 | 0.26 ± 0.03 |
| OP | 44.43 ± 0.93 | 6.21 ± 1.05 | 0.81 ± 0.02 |
| Biomass | Pectin | Cellulose | Ashes | Lignin | Hemicellulose |
|---|---|---|---|---|---|
| OP | 18.15 ± 0.06 | 18.49 ± 0.05 | 2.08 ± 0.03 | 7.22 ± 0.15 | 7.02 ± 0.23 |
| LP | 5.41 ± 0.01 | 14.28 ± 0.07 | 3.68 ± 0.06 | 7.14 ± 0.11 | 6.07 ± 0.18 |
| YP | 10.98 ± 0.01 | 13.08 ± 0.04 | 4.85 ± 0.08 | 27.73 ± 0.07 | 6.47 ± 0.28 |
| CP | 2.84 ± 0.04 | 18.47 ± 0.06 | 1.58 ± 0.04 | 2.2 ± 0.15 | 6.01 ± 0.19 |
| Parameter | OP | LP | YP | CP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sum of Squares | Factor-F | Value-p | Sum of Squares | Factor-F | Value-p | Sum of Squares | Factor-F | Value-p | Sum of Squares | Factor-F | Value-p | |
| A: pH | 4117.41 | 3701.25 | 0.00 | 2.10 | 10.86 | 0.05 | 357.34 | 36.20 | 0.009 | 110.34 | 9.43 | 0.05 |
| B: Particle size | 2.12 | 1.90 | 0.18 | 0.070 | 0.36 | 0.59 | 3.86 | 0.39 | 0.58 | 0.07 | 0.01 | 0.94 |
| AA | 1195.35 | 1074.54 | 0.00 | 2.24 | 11.58 | 0.04 | 40.98 | 4.15 | 0.13 | 10.02 | 0.86 | 0.42 |
| AB | 0.79 | 0.71 | 0.41 | 0.00 | 0.01 | 0.92 | 8.11 | 0.82 | 0.43 | 0.12 | 0.01 | 0.23 |
| BB | 5.31 | 4.77 | 0.04 | 1.25 | 6.48 | 0.08 | 4.73 | 0.48 | 0.54 | 19.26 | 1.65 | 0.29 |
| Error total | 21.14 | 0.06 | 0.95 | 0.58 | 29.61 | 35.11 | ||||||
| Total (corr.) | 5509.17 | 6.25 | 435.83 | 174.91 | ||||||||
| Biomass | Optimum Initial Solution pH | Optimum Particle Size (mm) | Removal Yield (%) |
|---|---|---|---|
| OP | 6 | 0.5 | 79 |
| LP | 6 | 0.5 | 79 |
| YP | 6 | 1 | 78 |
| CP | 6 | 1 | 76 |
| Contaminant | Adsorbent | Experimental Conditions | qmax (mg/g) | References |
|---|---|---|---|---|
| Ni(II) | Hybrid biocomposite based on amino-thiocarbamate derivative of alginate/carboxymethyl chitosan/TiO2 | pH 6, 25 °C, 30 % TiO2/TSC-CMC mass ratio, 750 rpm, 5 h, 6 g/L of adsorbent, 100 mg/L | 91 | [45] |
| TiO2 nanofibers | pH 6, 100 mg of adsorbent, 25 °C, 28 min of contact time, 1000 mg/L and 100 mL of solution | 80 | [46] | |
| Alginate-chitosan-TiO2 composites | pH 6, 25 mL of solution, 180 mg of adsorbent, 3 h of contact time, 100 mg/L | 76 | [47] | |
| Orange peels modified with TiO2 | pH 6, 100 mg/L, 5 g/L of adsorbent dose, 0.5 mm of particle size and 24 h of contact time | 21.3 | This study | |
| Lemon peels modified with TiO2 | 20 | |||
| Yam peels modified with TiO2 | 18.01 | |||
| Cassava peels modified with TiO2 | 17.33 | |||
| Polyacrylonitrile-TiO2 nanofiber | pH 6, 0.05 g of adsorbent., 50 mL of solution, 25 °C, 100 mg/L | 11 | [48] | |
| TiO2 NPs modified with humic acid | 25 mL of solution, 25 mg of adsorbent, pH 8, 50 mg/L, 22 h of contact time | 20 | [49] | |
| TiO2 NPs modified with extractant of Walnut shell | 18.5 | |||
| TiO2 NPs modified with 1, 5 diphenyl-Carbazon | 10.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Barros, A.; Tejada-Tovar, C.; González-Delgado, Á.D. Green Nanoparticle-Aided Biosorption of Nickel Ions Using Four Dry Residual Biomasses: A Comparative Study. Sustainability 2022, 14, 7250. https://doi.org/10.3390/su14127250
Herrera-Barros A, Tejada-Tovar C, González-Delgado ÁD. Green Nanoparticle-Aided Biosorption of Nickel Ions Using Four Dry Residual Biomasses: A Comparative Study. Sustainability. 2022; 14(12):7250. https://doi.org/10.3390/su14127250
Chicago/Turabian StyleHerrera-Barros, Adriana, Candelaria Tejada-Tovar, and Ángel Darío González-Delgado. 2022. "Green Nanoparticle-Aided Biosorption of Nickel Ions Using Four Dry Residual Biomasses: A Comparative Study" Sustainability 14, no. 12: 7250. https://doi.org/10.3390/su14127250
APA StyleHerrera-Barros, A., Tejada-Tovar, C., & González-Delgado, Á. D. (2022). Green Nanoparticle-Aided Biosorption of Nickel Ions Using Four Dry Residual Biomasses: A Comparative Study. Sustainability, 14(12), 7250. https://doi.org/10.3390/su14127250








