Feasibility of using Waste Brine/Seawater and Sea Sand for the Production of Concrete: An Experimental Investigation from Mechanical Properties and Durability Perspectives
Abstract
1. Introduction
2. Specimen Design and Preparation
2.1. Water
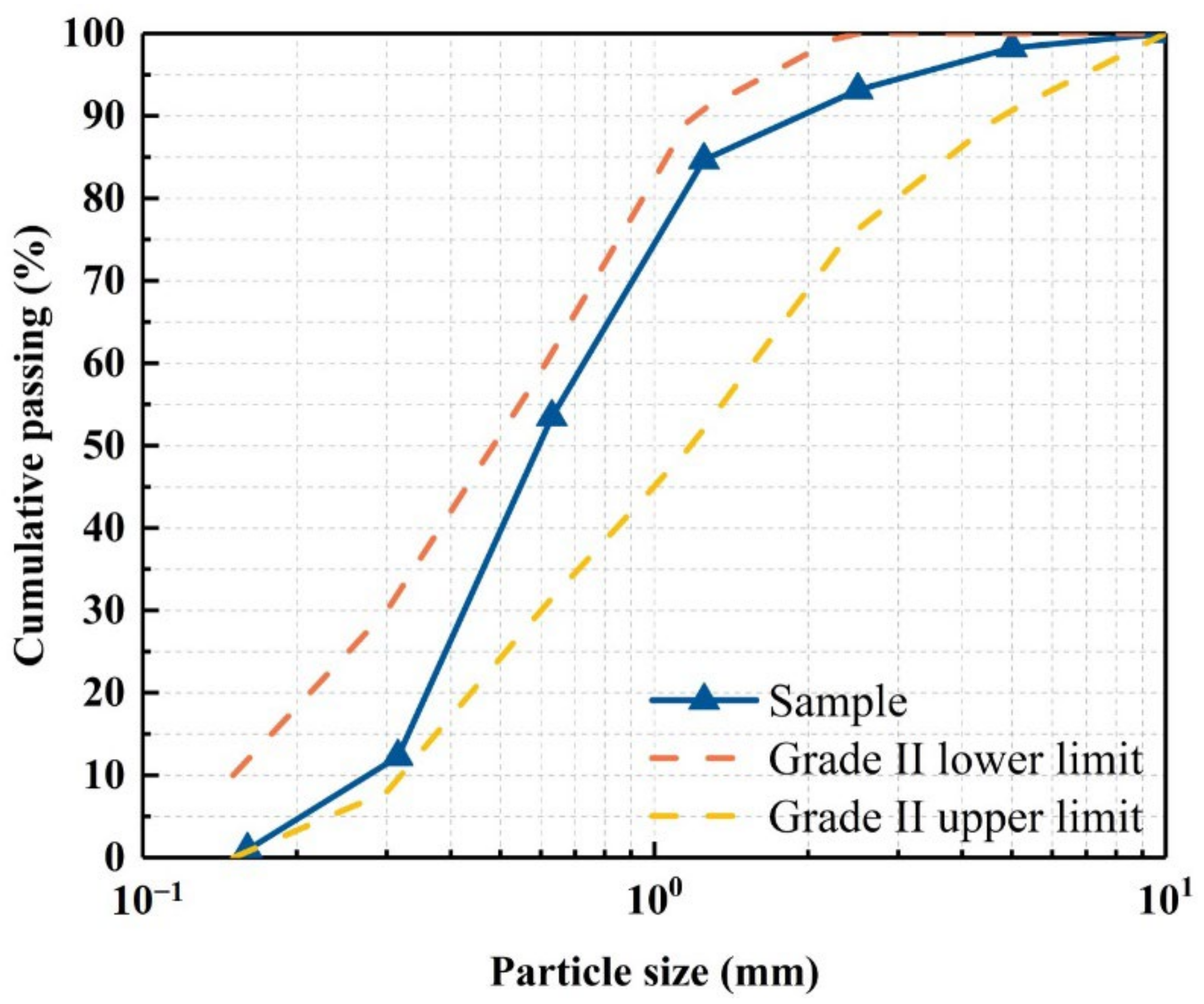
2.2. Fine Aggregates
2.3. Concrete Mixture Design
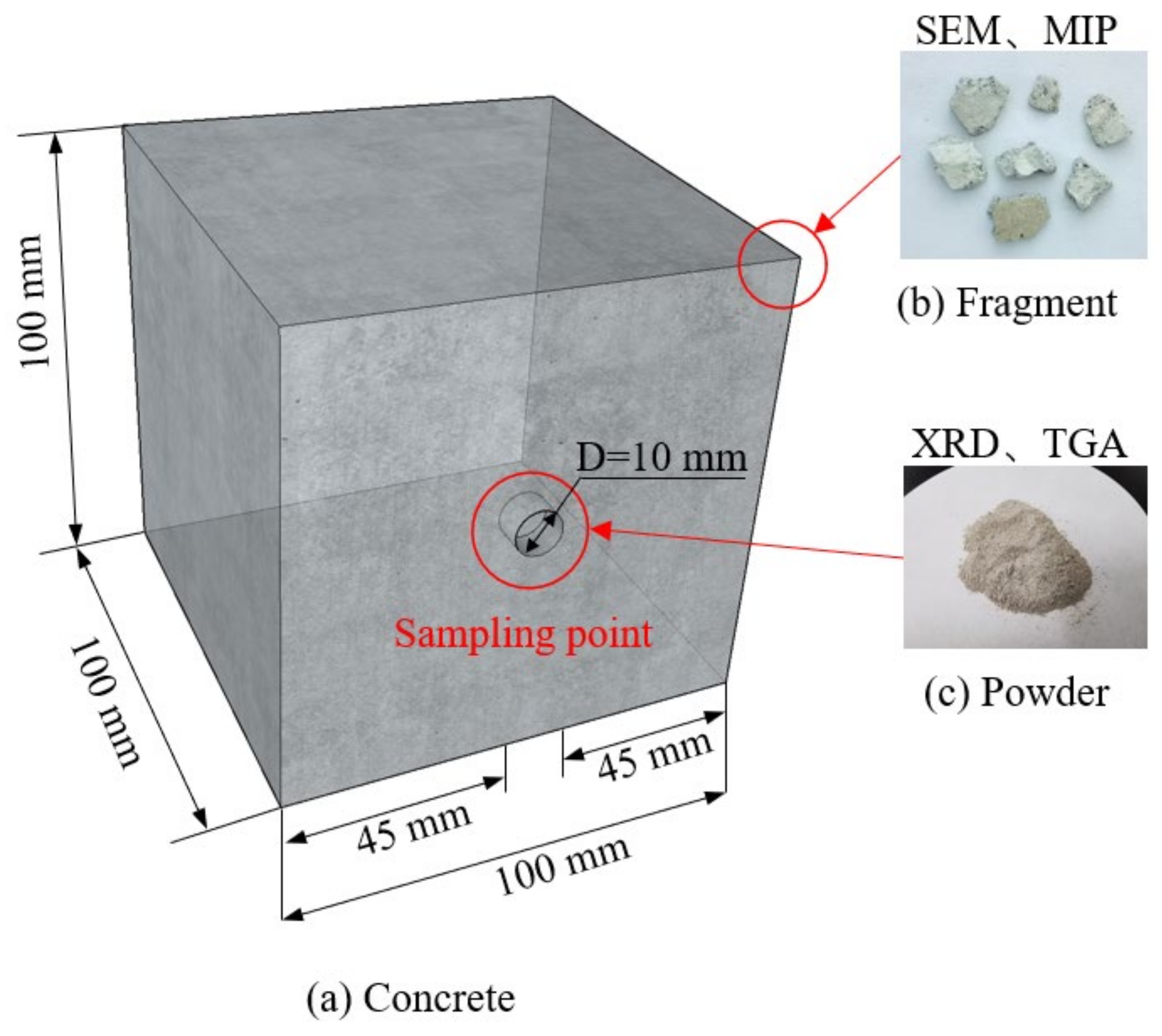
2.4. Specimen Preparation
3. Test Methods
3.1. Workability, Compressive Strength, and pH Value
3.2. Mercury Intrusion Porosimetry (MIP)
3.3. Thermogravimetric Analysis (TGA) and X-ray Diffraction (XRD)
3.4. Scanning Electron Microscopy (SEM)
4. Results and Discussion
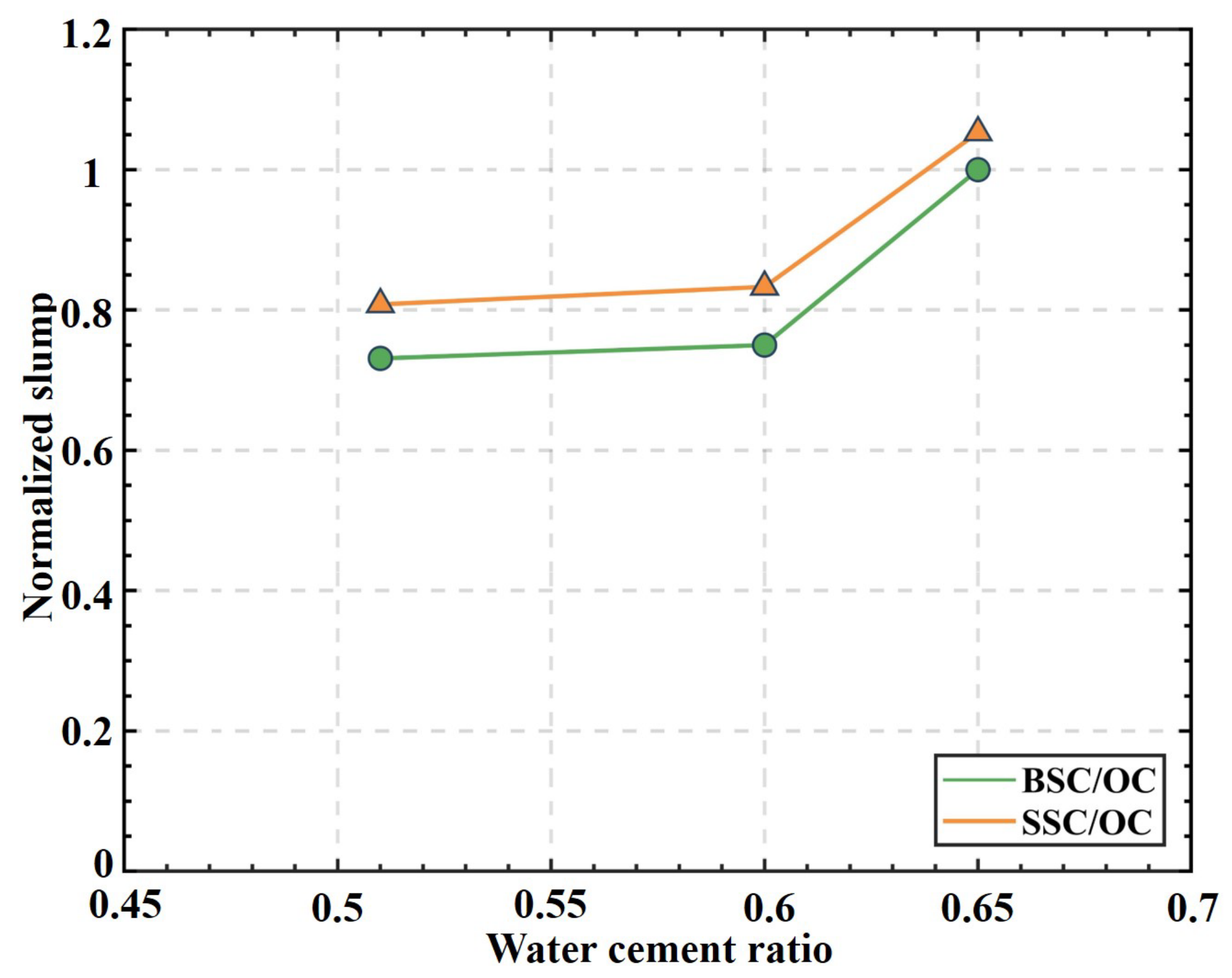
4.1. Slump
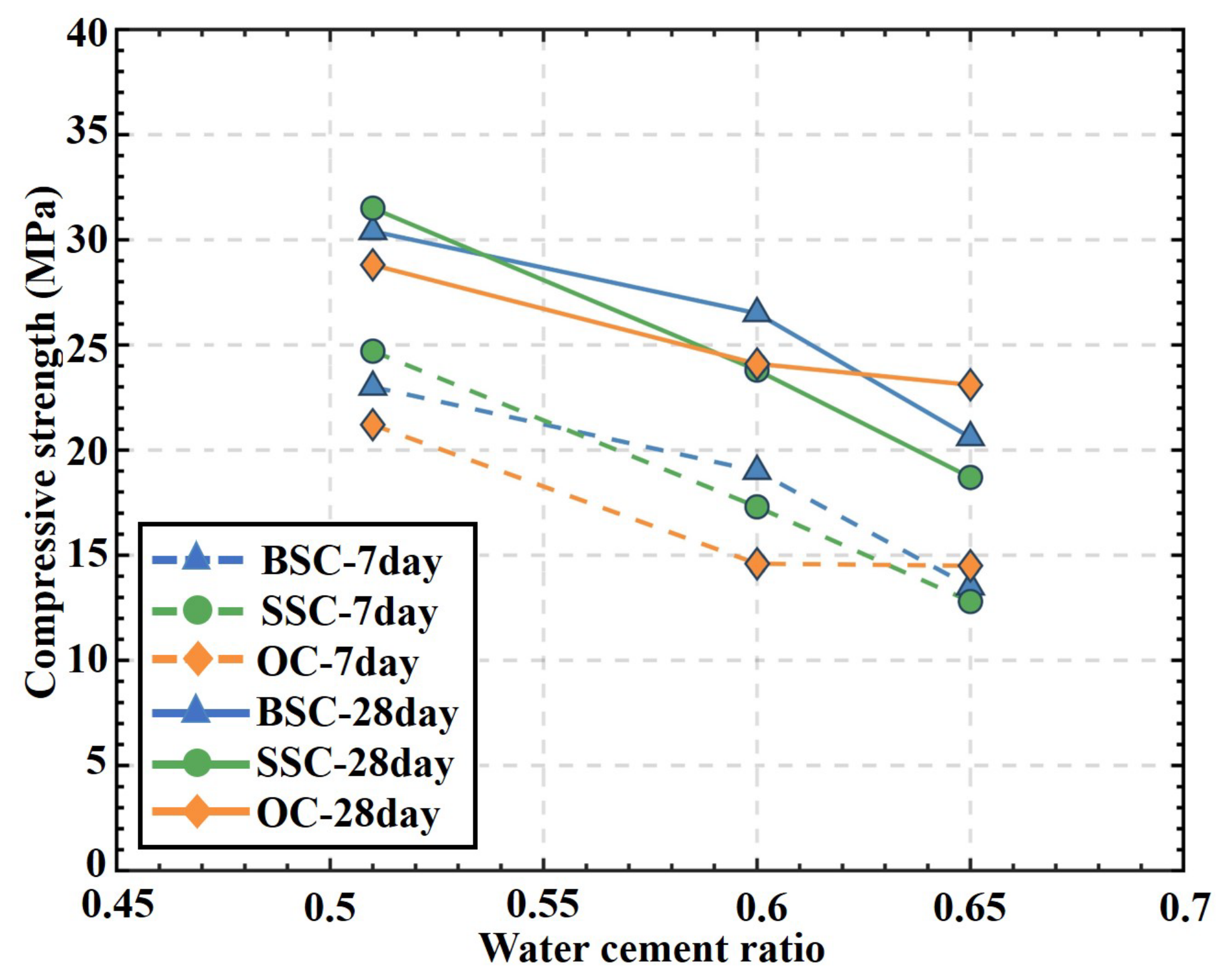
4.2. Compressive Strength
- BSC had higher 7-day compressive strength than OC. BSC had 8.5 and 30.1% higher compressive strength than the OC in the two groups with the w/c of 0.51 and 0.60, respectively. The difference between BSC and OC with a 0.65 w/c was 6.9%. The higher 7-day strength could be attributed to chloride ions in the brine which reacted with C3A and Ca(OH)2/CaSO4 to form Friedel’s and Kuzel’s salts, respectively, which accelerate the hydration reaction and optimize the microstructure of the concrete [59,60]. Therefore, at the same hydration age, BSC has more hydration products and higher compressive strength than OC.
- The 28-day compressive strength of BSC was comparable to that of OC. The strength of BSC with the w/c of 0.51 and 0.60 was 5.26 and 9.96% higher than the corresponding OC, respectively. The strength difference of BSC with the w/c of 0.51 and 0.60 to corresponding OC was reduced by 3.2 and 20.1%, respectively, compared to the 7-day compressive strength. Moreover, the strength of BSC with the w/c of 0.65 was 10.82% lower than that of OC. The adverse effect of additional chloride ion would be attributed to damage of microstructure due to the leaching of the hydration products and salt crystallization.
- Minor strength difference was displayed between BSC and SSC. The 7-day compressive strength of BSC with w/c of 0.60 and 0.65 was 9.83 and 5.47% higher than that of SSC, respectively. The 28-day strengths of BSC with w/c of 0.60 and 0.65 increased by 11.34 and 10.16%, respectively, compared with the corresponding SSC specimens. In the group with a w/c of 0.51, the 7-day and 28-day strength of BSC was 6.88 and 3.49% less than that of SSC, respectively. For concrete with a lower w/c, the increase in salinity slightly reduced the compressive strength. Moreover, the compressive strengths of BSC, SSC, and OC exhibited a decreasing trend with the increasing w/c in an approximately linear relationship for both 7-day and 28-day strengths.
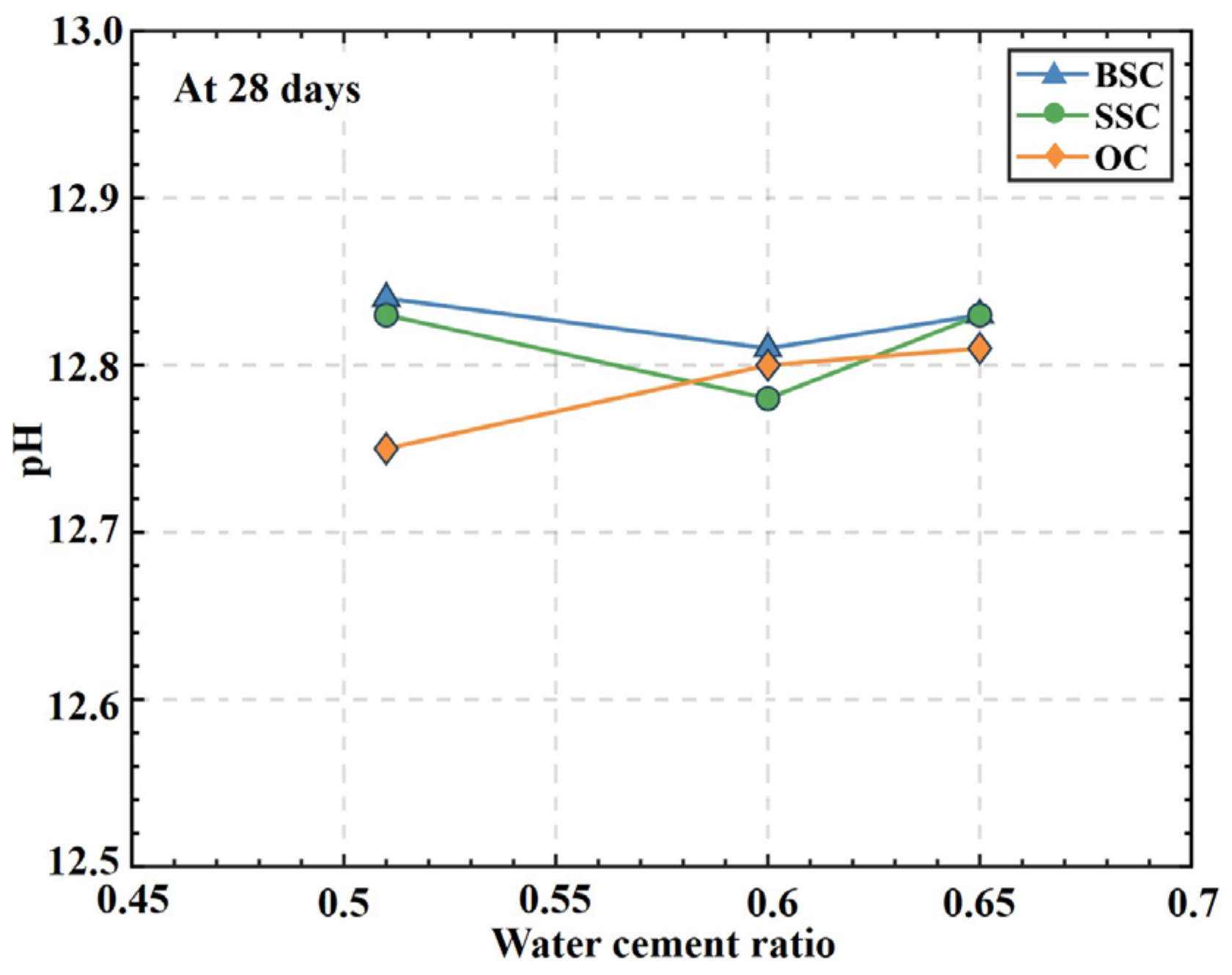
4.3. pH Value
4.4. Pore Structure Analysis and Sulfate Resistance Prediction
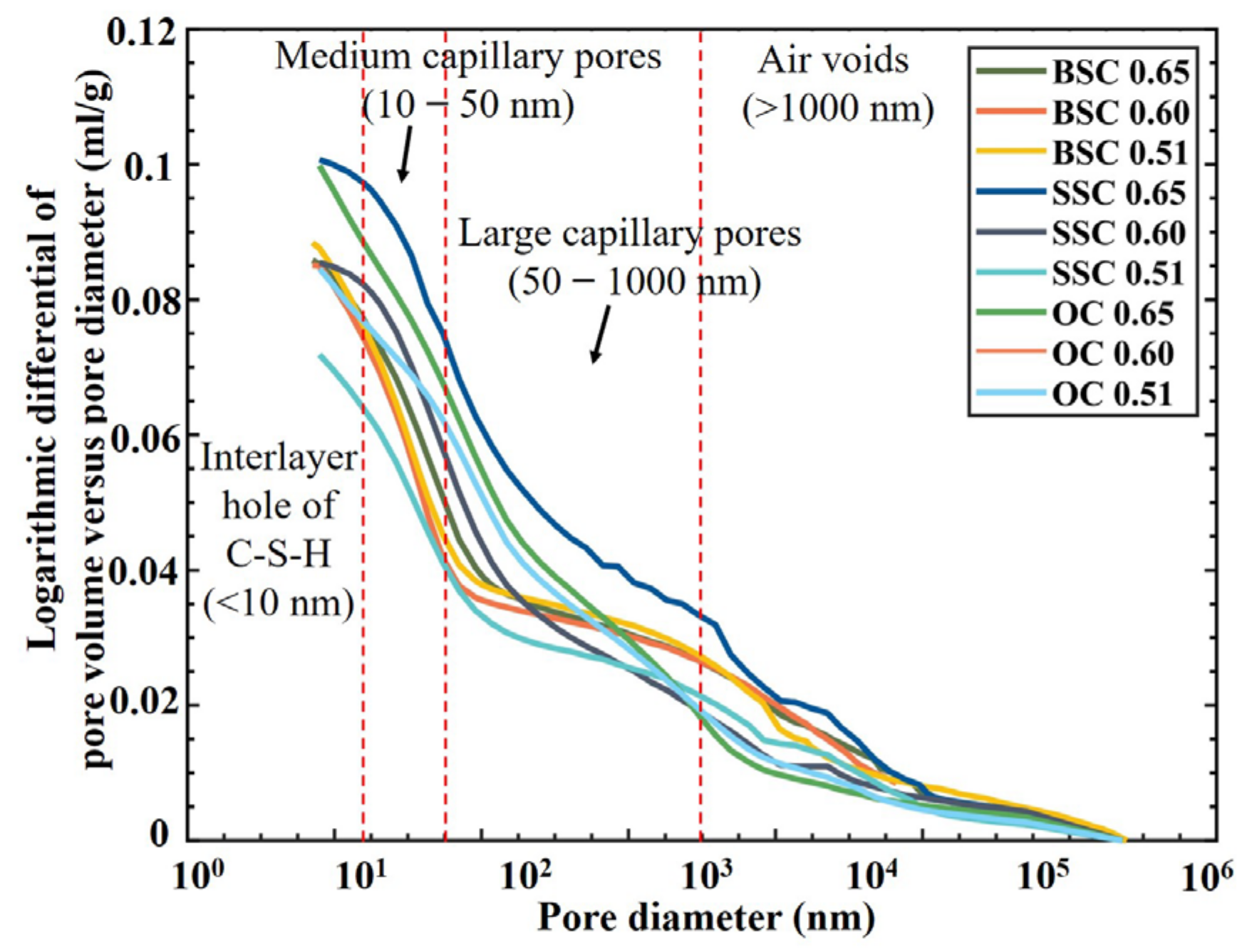
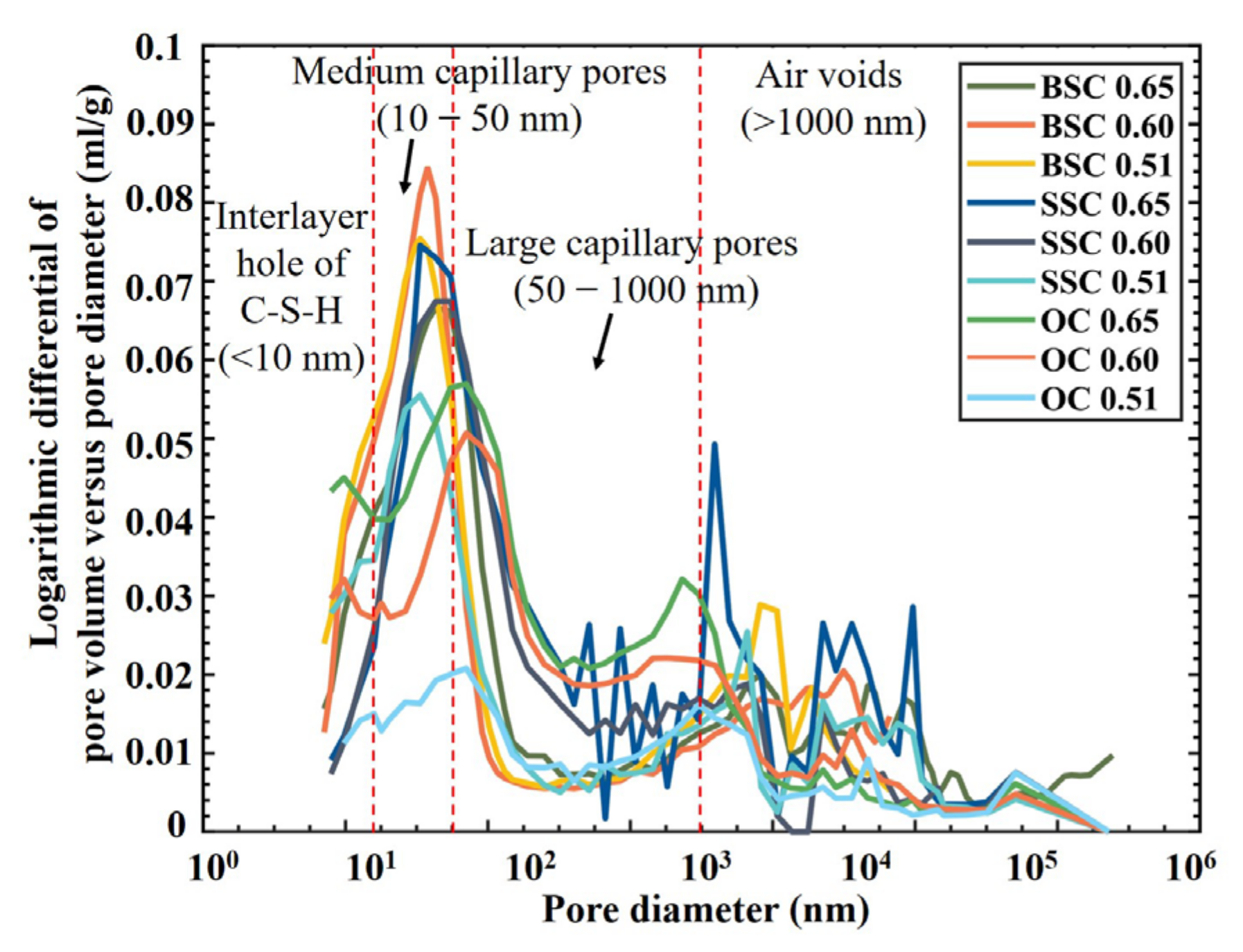
4.4.1. Cumulative Intrusion Pore Volume and Pore Size Distribution
- The pore structure was further analyzed through the pore size distribution curves, which were obtained by taking derivation of the cumulative porosity curves (Figure 8). Pore size between 1 and 10 nm (gel pores) is the size of interlayer pore of calcium silicate hydrate (C-S-H). The pore volume of BSC and SSC was larger than OC, implying that BSC and SSC may contain more interlayer pores of C-S-H than OC (the pore volume of gel pores in BSC with w/c). However, higher porosity of gel pores would not cause adverse effects on the permeability and strength of the hydrated cement paste as the gel pores were extremely tiny [60].
- A pore size between 10 and 1000 nm is a capillary pore. It can be seen that the large capillary pores volume (diameters between 50 and 1000 nm) of concrete with a lower w/c was smaller than that of concrete with a higher w/c. A similar conclusion can be found in the other studies [20,62,63]. It demonstrated that the large capillary pores had a more significant impact on the strength and permeability of concrete [64]. Moreover, it can be observed that the critical pore size was located in this range. For all types of w/c, the critical pore sizes of BSC and SSC are less than OC. The volume of capillary pores less than 50 nm increased with increasing content of chloride ions, i.e., BSC > SSC > OC. The volume of large capillary pores decreased significantly with the increasing concentration of chloride ions. In general, the large capillary pore structure of BSC and SSC was more compact than that of OC. It indicated that the addition of chloride ions is beneficial for lowering the content of large capillary pores. It could be attributed to the presence of chloride ions combined with sodium ions to form sodium chloride crystals which fill the large capillary pores in BSC and SSC [65].
- Pore size greater than 1000 nm is the air voids. The relative pore volume of BSC, SSC, and OC was in the order of BSC > SSC > OC, indicating that introducing the chemical ions from seawater could increase the volume of air voids in the concrete.
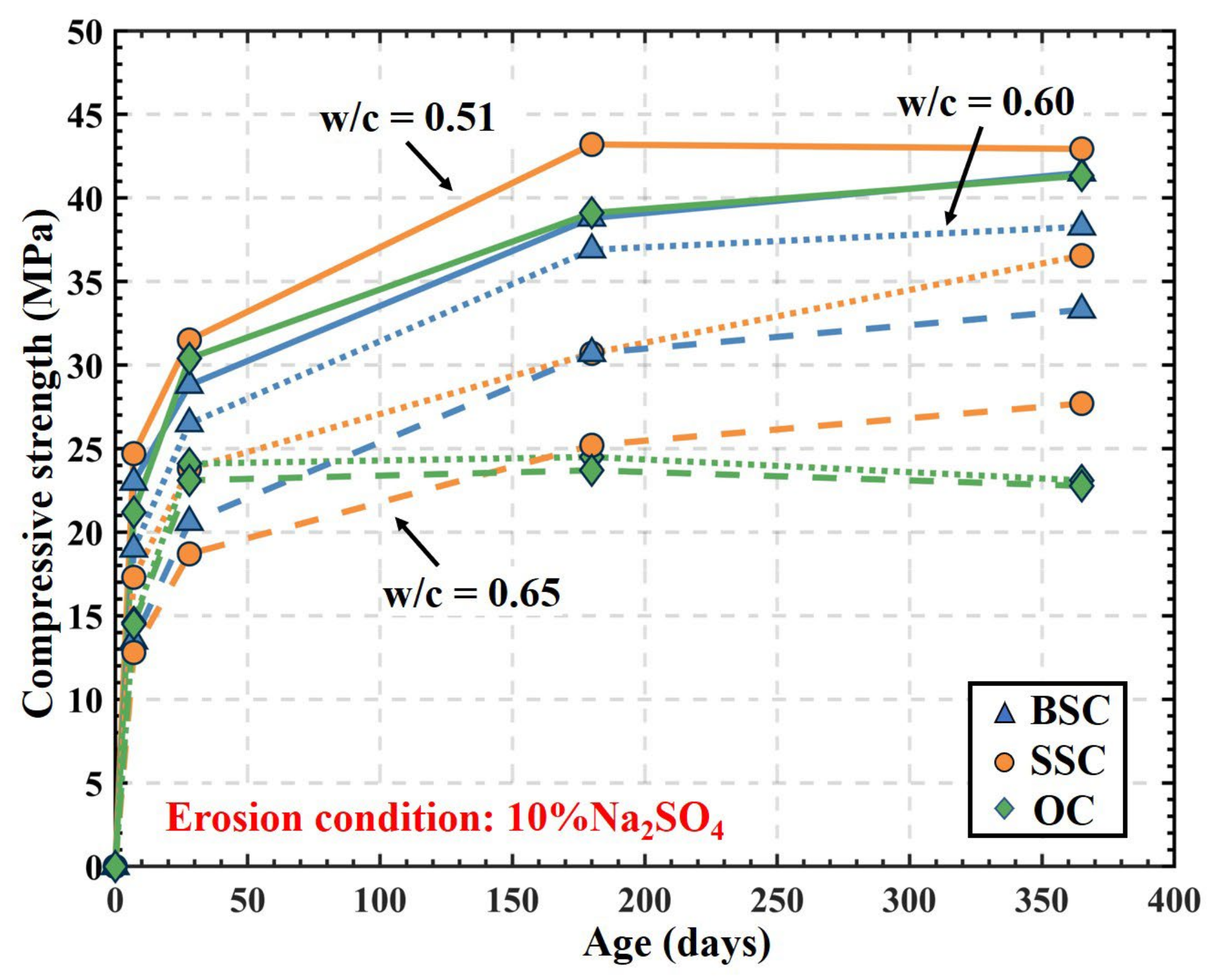
4.4.2. Sulfate Resistance Prediction
4.5. Hydration Product Analysis and Microstructures
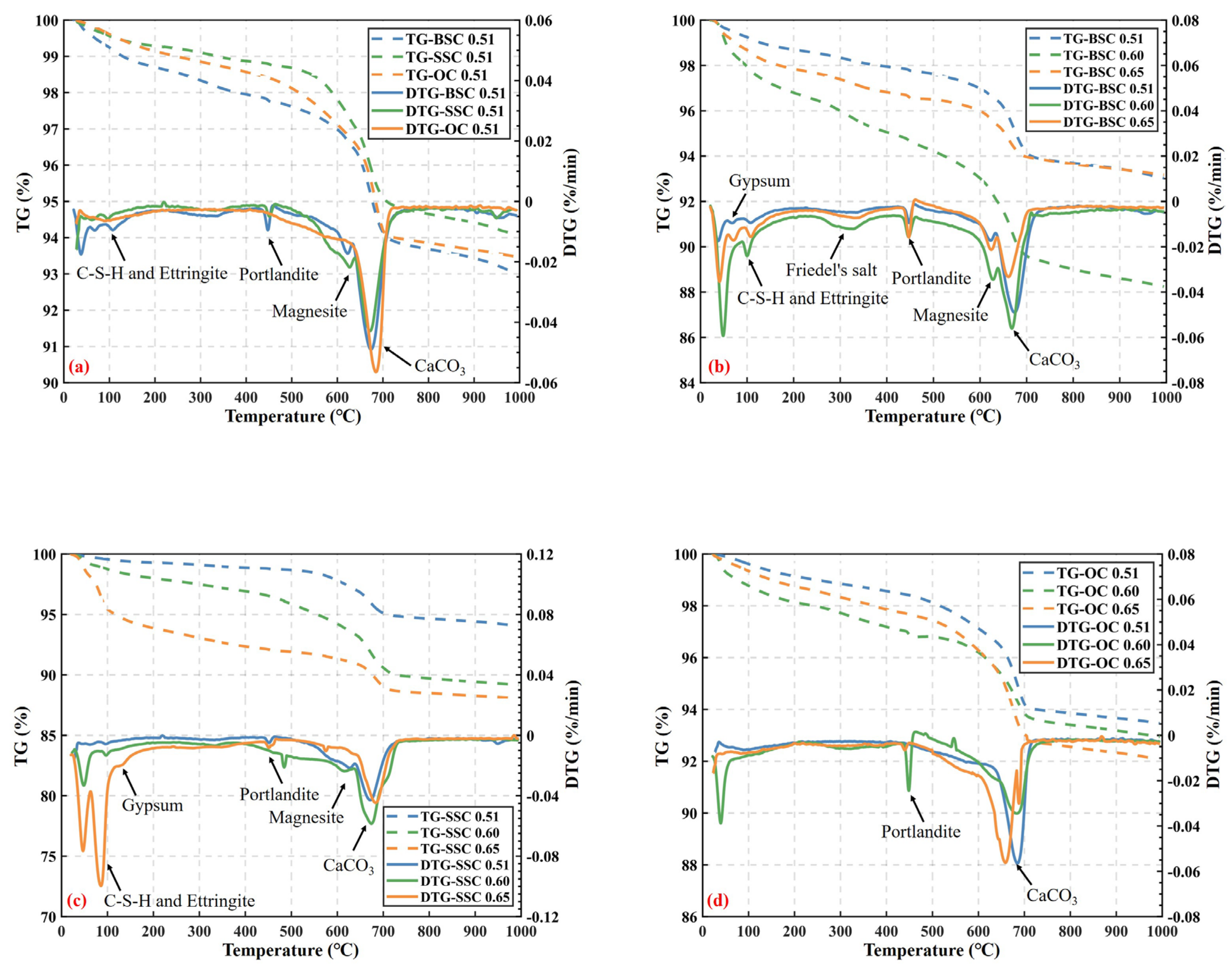
4.5.1. Thermogravimetric Analysis (TGA)
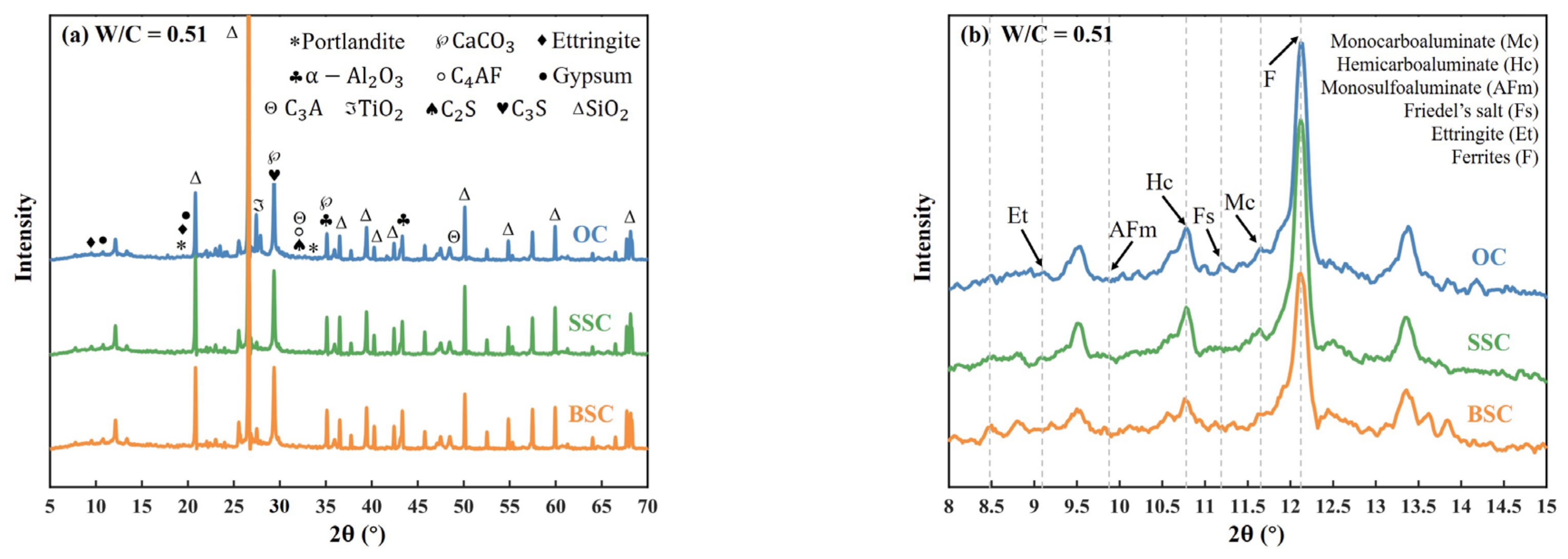
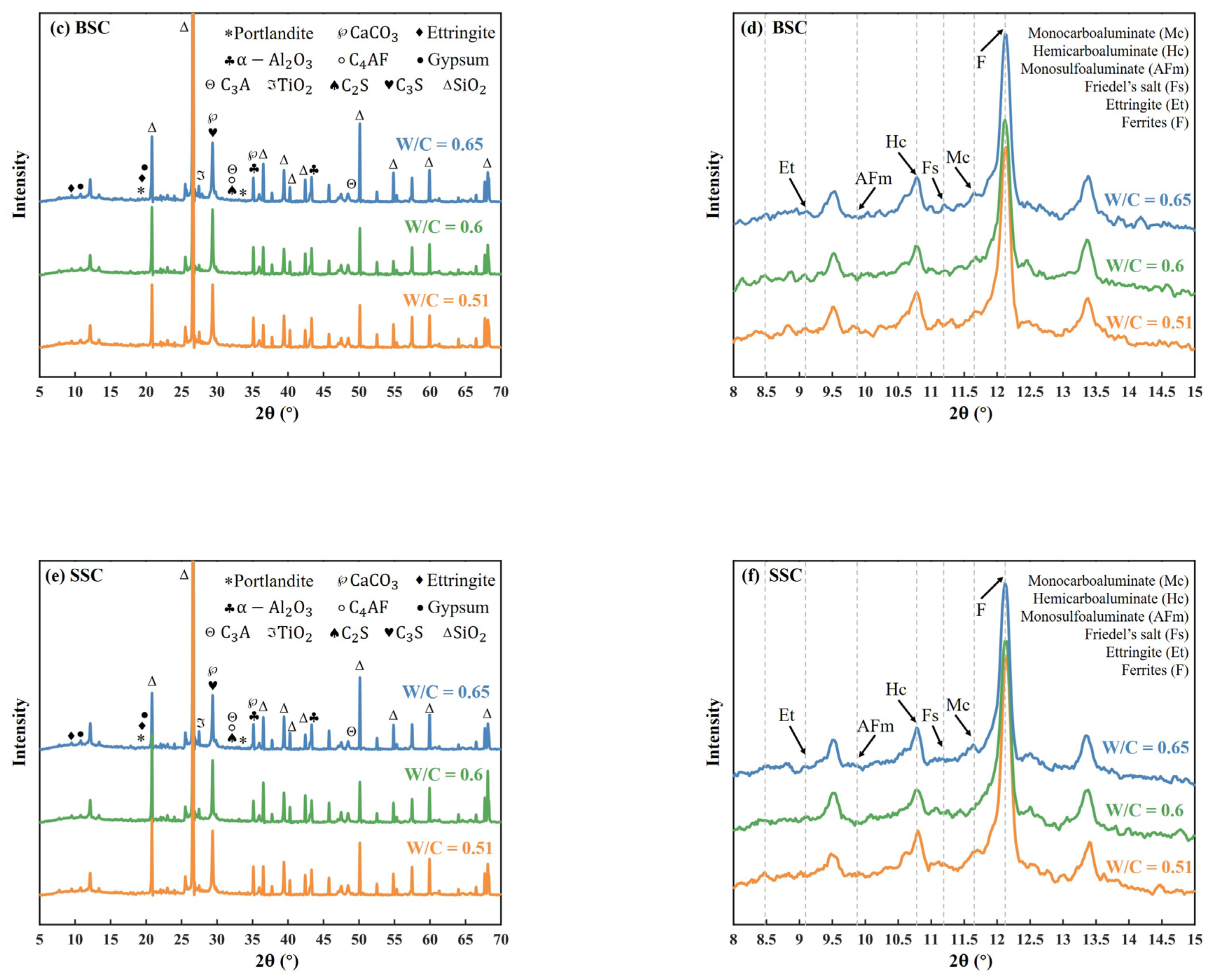
4.5.2. X-ray Diffraction (XRD)
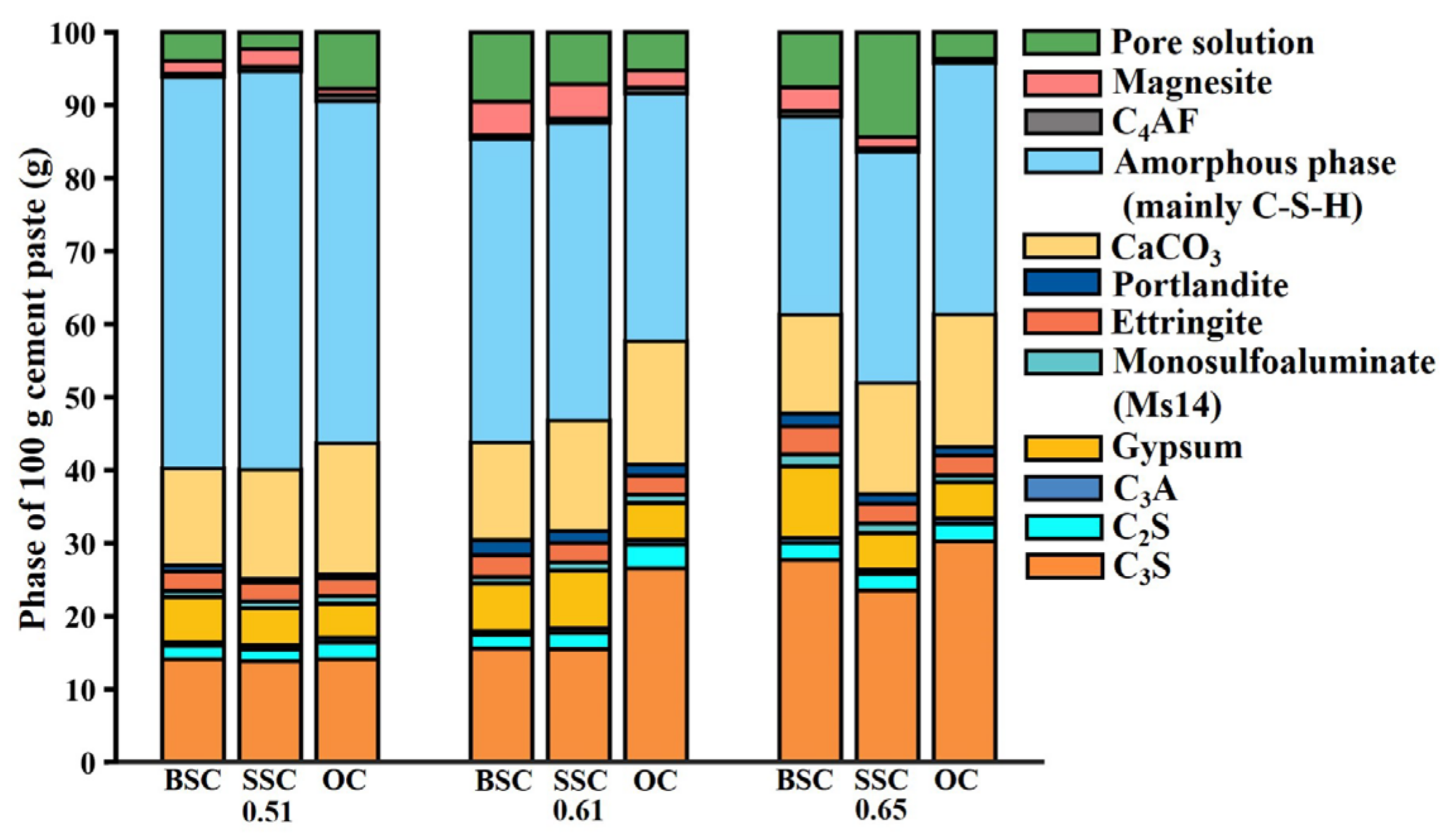
4.6. Semi-Quantitative Analysis of Concrete Phase
4.6.1. Amorphous Phase
4.6.2. AFm, Ettringite, and Gypsum
4.6.3. Calcium Carbonate and Portlandite
4.6.4. C3S, C2S, C3A and C4AF
4.7. Performance Evaluation of BSC and SSC
- In short-term performance, the difference in compressive strength between BSC/SSC and OC was minor when w/c was 0.51 and 0.6, while the compressive strengths of BSC and SSC with w/c of 0.65 were reduced by 10.82 and 19.05% compared to OC, respectively. The strength-based design for ordinary concrete applies to BSC and SSC.
- Although the workability of all concrete satisfied the requirement of infrastructural construction in the code GB50164-2011 [51], the reduction effect on the slump could be observed with the increase in salinity. Therefore, the water-consuming design should be re-examined for BSC and SSC, especially for BSC and SSC with a low w/c and high workability demand.
- Regarding the long-term performance, the pH value of BSC and SSC was slightly larger than that of OC but within the conventional range of concrete. Therefore, it demonstrated no significant effect of the introduction of chemical ions in brine/seawater or sea sand on the pH value in concrete. This is valuable information for the application of FRP bars in BSC and SSC as the mechanical properties of FRP materials are more sensitive to alkali environments.
- By comparing the quantity of carbonization product, it could be found that the index of carbonization resistance was the largest for BSC and the lowest for OC. The w/c variation had a minor effect on the index difference among different concrete mixtures with the same w/c.
- The effect of the w/c on the pore volume of large capillary pores in OC and SSC was more significant than in BSC, while the reduction effect on the volume of large capillary pores in BSC by reducing the w/c was limited. It indicated that the sulfate resistance difference between BSC and OC notably turned smaller with the decrease of the w/c. In general, among three different concrete mixtures with a w/c between 0.51 and 0.65, the sulfate resistance of BSC was the best, and that of SSC was next.
5. Conclusions
- Under the same w/c, the 7-day compressive strengths of BSC and SSC were higher than that of OC (except for a w/c of 0.65), and the 28-day compressive strengths of BSC, SSC, and OC had a difference within 5.56–9.36%. From the short-term strength perspective, the salinity had a minor effect on the mechanical properties of concrete.
- The increasing salinity had a reducing effect on the slump of concrete. For the concrete with a w/c between 0.51 and 0.65, the reduction effect could be neglected. However, for high-performance concrete with a lower w/c, it is necessary to add admixtures to enhance the workability.
- Regardless of whether it is BSC, SSC, or OC, the volume of porosity increases with the w/c. The pore volume of large capillary pores in BSC and SSC was smaller than that of OC, especially for the concrete with a higher w/c, which implied the lower permeability of concrete. The analysis based on the difference in pore structure shows that BSC and SSC had better sulfate resistance than OC. In addition, the experimental results showed that the carbonization resistance of BSC and SSC was higher than that of OC. Therefore, the BSC and SSC’s durability could be better than the OC’s.
- It was found that the phase difference was the presence of Friedel’s salt in BSC and SSC, but not in OC. Among the same phases in three types of concrete, the mass fraction of the amorphous phase, ettringite, and gypsum in BSC and SSC were greater than that of OC. In contrast, the mass fraction of AFm, C3S, and C2S in BSC and SSC was lower than in OC. The difference could be well explained by the increasing salinity and the resulting pore structural difference.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, E.; Qadir, M.; van Vliet, M.T.H.; Smakhtin, V.; Kang, S. The State of Desalination and Brine Production: A Global Outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Eke, J.; Yusuf, A.; Giwa, A.; Sodiq, A. The Global Status of Desalination: An Assessment of Current Desalination Technologies, Plants and Capacity. Desalination 2020, 495, 114633. [Google Scholar] [CrossRef]
- Virgili, F.; Brown, H.; Pankratz, T. IDA Desalination Yearbook 2017–2018; Media Analytics Ltd.: Oxford, UK, 2019. [Google Scholar]
- Huang, L.; Wang, D.; He, C.; Pan, M.; Zhang, B.; Chen, Q.; Ren, J. Industrial Wastewater Desalination under Uncertainty in Coal-Chemical Eco-Industrial Parks. Resour. Conserv. Recycl. 2019, 145, 370–378. [Google Scholar] [CrossRef]
- Van Wyk, S.; van der Ham, A.G.J.; Kersten, S.R.A. Analysis of the Energy Consumption of Supercritical Water Desalination (SCWD). Desalination 2020, 474, 114189. [Google Scholar] [CrossRef]
- Sánchez-Lizaso, J.L.; Romero, J.; Ruiz, J.; Gacia, E.; Buceta, J.L.; Invers, O.; Fernández Torquemada, Y.; Mas, J.; Ruiz-Mateo, A.; Manzanera, M. Salinity Tolerance of the Mediterranean Seagrass Posidonia Oceanica: Recommendations to Minimize the Impact of Brine Discharges from Desalination Plants. Desalination 2008, 221, 602–607. [Google Scholar] [CrossRef]
- Hancock, N.T.; Black, N.D.; Cath, T.Y. A Comparative Life Cycle Assessment of Hybrid Osmotic Dilution Desalination and Established Seawater Desalination and Wastewater Reclamation Processes. Water Res. 2012, 46, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Backer, S.N.; Bouaziz, I.; Kallayi, N.; Thomas, R.T.; Preethikumar, G.; Takriff, M.S.; Laoui, T.; Atieh, M.A. Review: Brine Solution: Current Status, Future Management and Technology Development. Sustainability 2022, 14, 6752. [Google Scholar] [CrossRef]
- Miller, S.A.; Horvath, A.; Monteiro, P.J.M. Impacts of Booming Concrete Production on Water Resources Worldwide. Nat. Sustain. 2018, 1, 69–76. [Google Scholar] [CrossRef]
- Wang, X. Study on Legal Issues of Mining and Utilization and Protection of River Gravel Resources in China. Master’s Thesis, China University of Geosciences, Beijing, China, 2020. [Google Scholar]
- Da, B.; Chen, Y.; Yu, H.; Ma, H.; Chen, D.; Wu, Z.; Liu, J.; Li, Y. Preparation Technology, Mechanical Properties and Durability of Coral Aggregate Seawater Concrete in the Island-Reef Environment. J. Clean. Prod. 2022, 339, 130572. [Google Scholar] [CrossRef]
- Jackson, M.D.; Chae, S.R.; Mulcahy, S.R.; Meral, C.; Taylor, R.; Li, P.; Emwas, A.-H.; Moon, J.; Yoon, S.; Vola, G.; et al. Unlocking the Secrets of Al-Tobermorite in Roman Seawater Concrete. Am. Mineral. 2013, 98, 1669–1687. [Google Scholar] [CrossRef]
- Younis, A.; Ebead, U.; Suraneni, P.; Nanni, A. Fresh and Hardened Properties of Seawater-Mixed Concrete. Constr. Build. Mater. 2018, 190, 276–286. [Google Scholar] [CrossRef]
- Yang, E.-I.; Yi, S.-T.; Leem, Y.-M. Effect of Oyster Shell Substituted for Fine Aggregate on Concrete Characteristics: Part I. Fundamental Properties. Cem. Concr. Res. 2005, 35, 2175–2182. [Google Scholar] [CrossRef]
- Safi, B.; Saidi, M.; Daoui, A.; Bellal, A.; Mechekak, A.; Toumi, K. The Use of Seashells as a Fine Aggregate (by Sand Substitution) in Self-Compacting Mortar (SCM). Constr. Build. Mater. 2015, 78, 430–438. [Google Scholar] [CrossRef]
- Pan, D.; Yaseen, S.A.; Chen, K.; Niu, D.; Ying Leung, C.K.; Li, Z. Study of the Influence of Seawater and Sea Sand on the Mechanical and Microstructural Properties of Concrete. J. Build. Eng. 2021, 42, 103006. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Yu, T.; Qu, F.; Tam, V.W.Y. Investigation on Early-Age Hydration, Mechanical Properties and Microstructure of Seawater Sea Sand Cement Mortar. Constr. Build. Mater. 2020, 249, 118776. [Google Scholar] [CrossRef]
- Xu, Q.; Ji, T.; Yang, Z.; Ye, Y. Preliminary Investigation of Artificial Reef Concrete with Sulphoaluminate Cement, Marine Sand and Sea Water. Constr. Build. Mater. 2019, 211, 837–846. [Google Scholar] [CrossRef]
- Xiao, J.; Qiang, C.; Nanni, A.; Zhang, K. Use of Sea-Sand and Seawater in Concrete Construction: Current Status and Future Opportunities. Constr. Build. Mater. 2017, 155, 1101–1111. [Google Scholar] [CrossRef]
- Wang, J.; Liu, E.; Li, L. Multiscale Investigations on Hydration Mechanisms in Seawater OPC Paste. Constr. Build. Mater. 2018, 191, 891–903. [Google Scholar] [CrossRef]
- Liu, W.; Cui, H.; Dong, Z.; Xing, F.; Zhang, H.; Lo, T.Y. Carbonation of Concrete Made with Dredged Marine Sand and Its Effect on Chloride Binding. Constr. Build. Mater. 2016, 120, 1–9. [Google Scholar] [CrossRef]
- Li, Q.; Geng, H.; Shui, Z.; Huang, Y. Effect of Metakaolin Addition and Seawater Mixing on the Properties and Hydration of Concrete. Appl. Clay Sci. 2015, 115, 51–60. [Google Scholar] [CrossRef]
- Etxeberria, M.; Fernandez, J.M.; Limeira, J. Secondary Aggregates and Seawater Employment for Sustainable Concrete Dyke Blocks Production: Case Study. Constr. Build. Mater. 2016, 113, 586–595. [Google Scholar] [CrossRef]
- González, J.A.; Ramírez, E.; Bautista, A. Protection of Steel Embedded in Chloride-Containing Concrete by Means of Inhibitors. Cem. Concr. Res. 1998, 28, 577–589. [Google Scholar] [CrossRef]
- Kobayashi, K. Deterioration Mechanism of Concrete Structures Due to Steel Corrosion. Dobokuseko 1984, 25, 20–26. [Google Scholar]
- Ebead, U.; Lau, D.; Lollini, F.; Nanni, A.; Suraneni, P.; Yu, T. A Review of Recent Advances in the Science and Technology of Seawater-Mixed Concrete. Cem. Concr. Res. 2022, 152, 106666. [Google Scholar] [CrossRef]
- Teng, J.; Yu, T.; Dai, J.; Chen, G. FRP Composites in New Construction: Current Status and Opportunities. In Proceedings of the 7th National Conference on FRP Composites in Infrastructure, Hangzhou, China, 14 October 2011. [Google Scholar]
- Zhang, Y.; Wei, Y.; Bai, J.; Wu, G.; Dong, Z. A Novel Seawater and Sea Sand Concrete Filled FRP-Carbon Steel Composite Tube Column: Concept and Behaviour. Compos. Struct. 2020, 246, 112421. [Google Scholar] [CrossRef]
- Mirdarsoltany, M.; Abed, F.; Homayoonmehr, R.; Alavi Nezhad Khalil Abad, S.V. A Comprehensive Review of the Effects of Different Simulated Environmental Conditions and Hybridization Processes on the Mechanical Behavior of Different FRP Bars. Sustainability 2022, 14, 8834. [Google Scholar] [CrossRef]
- Younis, A.; Ebead, U.; Judd, S. Life Cycle Cost Analysis of Structural Concrete Using Seawater, Recycled Concrete Aggregate, and GFRP Reinforcement. Constr. Build. Mater. 2018, 175, 152–160. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J.; Loizidou, M. Desalination Brine Disposal Methods and Treatment Technologies—A Review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- GB175-2020; Common Portland Cement. China Planning Press: Beijing, China, 2020.
- ASTM D1141–2021; Standard Practice for the Preparation of Substitute Ocean Water. ASTM International: West Conshohocken, PA, USA, 2021.
- Thabit, M.S.; Hawari, A.H.; Ammar, M.H.; Zaidi, S.; Zaragoza, G.; Altaee, A. Evaluation of Forward Osmosis as a Pretreatment Process for Multi Stage Flash Seawater Desalination. Desalination 2019, 461, 22–29. [Google Scholar] [CrossRef]
- Ji, X.S.; Curcio, E.; Al Obaidani, S.; Di Profio, G.; Fontananova, E.; Drioli, E. Membrane Distillation-Crystallization of Seawater Reverse Osmosis Brines. Sep. Purif. Technol. 2010, 71, 76–82. [Google Scholar] [CrossRef]
- Loizides, L. The Cost of Environmental and Social Sustainability of Desalination. In Proceedings of the International Conference on Desalination Costing, Limassol, Cyprus, December 2004. [Google Scholar]
- Ahmed, M.; Shayya, W.H.; Hoey, D.; Al-Handaly, J. Brine Disposal from Reverse Osmosis Desalination Plants in Oman and the United Arab Emirates. Desalination 2001, 133, 135–147. [Google Scholar] [CrossRef]
- Sadhwani, J.J.; Veza, J.M.; Santana, C. Case Studies on Environmental Impact of Seawater Desalination. Desalination 2005, 185, 1–8. [Google Scholar] [CrossRef]
- Al Mutaz, I.S.; Wagialia, K.M. Production of Magnesium from Desalination Brines. Resour. Conserv. Recycl. 1990, 3, 231–239. [Google Scholar] [CrossRef]
- Melián-Martel, N.; Sadhwani Alonso, J.J.; Pérez Báez, S.O. Reuse and Management of Brine in Sustainable SWRO Desalination Plants. Desalin. Water Treat. 2013, 51, 560–566. [Google Scholar] [CrossRef]
- Sanmartino, J.A.; Khayet, M.; García-Payo, M.C.; El-Bakouri, H.; Riaza, A. Treatment of Reverse Osmosis Brine by Direct Contact Membrane Distillation: Chemical Pretreatment Approach. Desalination 2017, 420, 79–90. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Hilal, N. Emerging Technologies for Sustainable Desalination Handbook; Butterworth-Heinemann: Oxford, UK, 2018. [Google Scholar]
- Lior, N.; Kim, D. Quantitative Sustainability Analysis of Water Desalination—A Didactic Example for Reverse Osmosis. Desalination 2018, 431, 157–170. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; Rhadfi, T.; Khraisheh, M.; Atieh, M.A.; Khraisheh, M.; Hilal, N. Reducing Flux Decline and Fouling of Direct Contact Membrane Distillation by Utilizing Thermal Brine from MSF Desalination Plant. Desalination 2016, 379, 172–181. [Google Scholar] [CrossRef]
- JGJ 52; Standard for Technical Requirements and Test Method of Sand and Crushed Stone (or Gravel) for Ordinary Concrete. China Architecture & Building Press: Beijing, China, 2006.
- JGJ206-2010; Technical Code for Application of Sea Sand Concrete. China Architecture & Building Press: Beijing, China, 2010.
- Zhang, Q.; Xiao, J.; Zhang, P.; Zhang, K. Mechanical Behaviour of Seawater Sea-Sand Recycled Coarse Aggregate Concrete Columns under Axial Compressive Loading. Constr. Build. Mater. 2019, 229, 117050. [Google Scholar] [CrossRef]
- Liu, W.; Pu, Z.L.; Sun, H.F.; He, Z.M.; Xing, F. Study on the influencing factors of chloride ion content in sea sand. J. Build. Mater. 2016, 19, 921–925+932. [Google Scholar]
- Dong, Z.Q.; Wu, G.; Xu, Y.Q. Experimental Study on the Bond Durability between Steel-FRP Composite Bars (SFCBs) and Sea Sand Concrete in Ocean Environment. Constr. Build. Mater. 2016, 115, 277–284. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Aziz, M.; Murthy, C. Sea Dredged Sand for Concrete. In Extending Aggregate Resources; Hotaling, W., Ed.; ASTM International: West Conshohocken, PA, USA, 1982; pp. 167–177. ISBN 978-0-8031-0611-6. [Google Scholar]
- GB50164-2011; Standard of Quality Control of Concrete. China Planning Press: Beijing, China, 2011.
- JGJ55-2011; Specification for Mix Proportion Design of Ordinary Concrete. China Architecture & Building Press: Beijing, China, 2011.
- GB/T 50081; Standard for Test Methods of Concrete Physical and Mechanical Properties. China Architecture & Building Press: Beijing, China, 2019.
- ASTM C143/C143M-15a; Standard Test Method for Slump of Hydraulic-Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2015.
- NYT 1377; Determination of Ph in Soil. Beijing, China, 2007. (In Chinese)
- Pan, F.; Wang, Y.H.; Chen, C. X-ray Diffraction Technique; Chemical industry Press: Beijing, China, 2016. (In Chinese) [Google Scholar]
- Geng, J.; Easterbrook, D.; Liu, Q.-F.; Li, L.-Y. Effect of Carbonation on Release of Bound Chlorides in Chloride-Contaminated Concrete. Mag. Concr. Res. 2016, 68, 353–363. [Google Scholar] [CrossRef]
- Kishar, E.A.; Ahmed, D.A.; Mohammed, M.R.; Noury, R. Effect of Calcium Chloride on the Hydration Characteristics of Ground Clay Bricks Cement Pastes. Beni-Suef Univ. J. Basic Appl. Sci. 2013, 2, 20–30. [Google Scholar] [CrossRef]
- Qu, F.; Li, W.; Wang, K.; Tam, V.W.Y.; Zhang, S. Effects of Seawater and Undesalted Sea Sand on the Hydration Products, Mechanical Properties and Microstructures of Cement Mortar. Constr. Build. Mater. 2021, 310, 125229. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Morales, C.N.; Claure, G.; Emparanza, A.R.; Nanni, A. Durability of GFRP Reinforcing Bars in Seawater Concrete. Constr. Build. Mater. 2021, 270, 121492. [Google Scholar] [CrossRef]
- Montanari, L.; Suraneni, P.; Tsui Chang, M.; Khatib, M.; Ebead, U.; Weiss, W.; Nanni, A. Hydration, Pore Solution, and Porosity of Cementitious Pastes Made with Seawater. J. Mater. Civ. Eng. 2019, 31, 04019154. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, X.; Shi, C.; Zhang, Z.; Zhu, D. A Review on Seawater Sea-Sand Concrete: Mixture Proportion, Hydration, Microstructure and Properties. Constr. Build. Mater. 2021, 295, 123602. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Bian, F.; Zhang, Y.R.; Fang, Z.F.; Fu, C.Q.; Guo, J. Effect of Pore Structures on Gas Permeability and Chloride Diffusivity of Concrete. Constr. Build. Mater. 2018, 163, 402–413. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in Pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Ikumi, T.; Segura, I. Numerical Assessment of External Sulfate Attack in Concrete Structures. A Review. Cem. Concr. Res. 2019, 121, 91–105. [Google Scholar] [CrossRef]
- Taylor, H.F.W.; Famy, C.; Scrivener, K.L. Delayed Ettringite Formation. Cem. Concr. Res. 2001, 31, 683–693. [Google Scholar] [CrossRef]
- Sánchez, I.; Nóvoa, X.R.; de Vera, G.; Climent, M.A. Microstructural Modifications in Portland Cement Concrete Due to Forced Ionic Migration Tests. Study by Impedance Spectroscopy. Cem. Concr. Res. 2008, 38, 1015–1025. [Google Scholar] [CrossRef]
- Mehta, P.K. Mechanism of Sulfate Attack on Portland Cement Concrete—Another Look. Cem. Concr. Res. 1983, 13, 401–406. [Google Scholar] [CrossRef]
- Müllauer, W.; Beddoe, R.E.; Heinz, D. Sulfate Attack Expansion Mechanisms. Cem. Concr. Res. 2013, 52, 208–215. [Google Scholar] [CrossRef]
- Cui, Y.X.; Xiong, H.B.; Liu, S.F.; Jiang, J.F.; Xiao, J.Z. Basic Mechanical Performances of Concentration Seawater-Sea Sand Concrete. In Proceedings of the Fib Shanghai Symposium, Shanghai, China, 22–24 November 2020. [Google Scholar]


| Type | Technology/Source | TDS (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | pH | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Seawater | Artificial seawater | 36,032 | 418 | 11,026 | 1314 | 364 | 19,856 | 2765 | 8.2 | This study [33] |
| The west bay in Qatar | 43,474 | 486 | 12,952 | 1535 | 459 | 22,184 | 3154 | 8.4 | [34] | |
| Tyrrhenian Sea | 35,300 | 440 | 10,900 | 1420 | - | 20,250 | 2150 | - | [35] | |
| The Mediterranean Sea | 40,498 | 450 | 12,480 | 1452 | 450 | 22,099 | 3406 | 8.1 | [36] | |
| The United Arab Emirates | 40,592 | 464 | 11,900 | 1640 | 574 | 23,149 | 2787 | 7.9 | [37] | |
| The Gran Canaria | 38,000 | 450 | 11,415 | 1520 | 450 | 20,800 | 3110 | - | [38] | |
| Arabian Gulf | 45,000 | 600 | 14,800 | 1600 | - | 25,000 | 3450 | 8.2 | [39] | |
| Brine | Artificial brine | 72,064 | 836 | 22,052 | 2628 | 728 | 39,712 | 5530 | - | This study |
| RO | 79,660 | 960 | 25,237 | 2867 | 781 | 41,890 | 6050 | - | [40] | |
| RO | 55,000 | 879 | 15,270 | 1864 | - | 31,150 | 5264 | 7.9 | [41] | |
| RO | 70,488 | 790 | 21,921 | 2479 | 743 | 38,886 | 5316 | - | [42] | |
| RO | 68,967 | 845 | 21,070 | 2550 | 784 | 38,014 | 5342 | - | [42] | |
| RO | 80,028 | 891 | 24,649 | 2878 | 888 | 43,661 | 6745 | 7.8 | [43] | |
| MSF | 57,400 | 521 | 18,434 | 1738 | 491 | 32,127 | 4025 | 8.3 | [44] | |
| MSF | 81,492 | 725 | 20,993 | 2505 | 740 | 35,378 | 5041 | 9.1 | [34] |
| Sea Sand in This Study (Zhoushan) | Fujian | The Pearl River | Yantian | The Yellow Sea | Changi | |
|---|---|---|---|---|---|---|
| Chloride ion (%) | 0.238 | 0.057 | 0.21 | 0.21 | 0.082 | 0.286 |
| Reference | This study | [47] | [21] | [48] | [49] | [50] |
| w/c | Water | Cement | Coarse Aggregate | Fine Aggregate |
|---|---|---|---|---|
| 0.65 | 197.36 | 304.50 | 1129.41 | 752.94 |
| 0.60 | 197.66 | 327.92 | 1152.54 | 706.39 |
| 0.51 | 198.26 | 387.55 | 1151.55 | 647.75 |
| Classification of Pore Size | BSC (0.65) | SSC (0.65) | OC (0.65) | BSC (0.60) | SSC (0.60) | OC (0.60) | BSC (0.51) | SSC (0.51) | OC (0.51) |
|---|---|---|---|---|---|---|---|---|---|
| <10 | 1.44 | 0.73 | 2.30 | 1.22 | 0.72 | 1.64 | 1.79 | 1.73 | 0.55 |
| 10–50 | 8.58 | 8.11 | 7.55 | 7.66 | 8.88 | 6.03 | 8.44 | 6.99 | 3.33 |
| 50–1000 | 1.34 | 4.92 | 6.20 | 2.11 | 4.45 | 5.59 | 1.59 | 2.10 | 2.79 |
| >1000 | 6.17 | 6.02 | 2.90 | 5.95 | 3.19 | 3.32 | 5.75 | 3.99 | 2.80 |
| Phase | C3S | C2S | C3A | Gypsum | Ms 14 | Ettringite | Portlandite | CaCO3 | C4AF | α-Al203 |
|---|---|---|---|---|---|---|---|---|---|---|
| 2-Theta (°) | 32.28 | 32.15 | 47.63 | 20.72 | 9.89 | 22.94 | 34.08 | 29.42 | 33.718 | 35.14 |
| K Value | 0.8 | 0.76 | 3.16 | 1.83 | 2.97 | 0.45 | 1.4 | 2 | 2.24 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Jiang, J.; Fu, T.; Liu, S. Feasibility of using Waste Brine/Seawater and Sea Sand for the Production of Concrete: An Experimental Investigation from Mechanical Properties and Durability Perspectives. Sustainability 2022, 14, 13340. https://doi.org/10.3390/su142013340
Cui Y, Jiang J, Fu T, Liu S. Feasibility of using Waste Brine/Seawater and Sea Sand for the Production of Concrete: An Experimental Investigation from Mechanical Properties and Durability Perspectives. Sustainability. 2022; 14(20):13340. https://doi.org/10.3390/su142013340
Chicago/Turabian StyleCui, Yongxiang, Jiafei Jiang, Tengfei Fu, and Sifeng Liu. 2022. "Feasibility of using Waste Brine/Seawater and Sea Sand for the Production of Concrete: An Experimental Investigation from Mechanical Properties and Durability Perspectives" Sustainability 14, no. 20: 13340. https://doi.org/10.3390/su142013340
APA StyleCui, Y., Jiang, J., Fu, T., & Liu, S. (2022). Feasibility of using Waste Brine/Seawater and Sea Sand for the Production of Concrete: An Experimental Investigation from Mechanical Properties and Durability Perspectives. Sustainability, 14(20), 13340. https://doi.org/10.3390/su142013340







