Nanopotassium, Nanosilicon, and Biochar Applications Improve Potato Salt Tolerance by Modulating Photosynthesis, Water Status, and Biochemical Constituents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Location and Experimental Design
2.2. Biochar Preparation
2.3. Synthesis of Potassium and Silicon Nanoparticles
2.4. Data Recorded
2.4.1. Morphological Characteristics and Yield Components
2.4.2. Leaf Chlorophyll Content and Photosynthetic Parameters
2.4.3. Activity of Antioxidant Enzymes
2.4.4. Leaf Proline, Gibberellic Acid, and Abscisic Acid Content
2.4.5. Leaf Malondialdehyde Content
2.4.6. Nutrient Content in Plant and Tuber Tissues
2.4.7. Tuber Starch and Carbohydrate Content
2.5. Statistical Analysis
3. Results
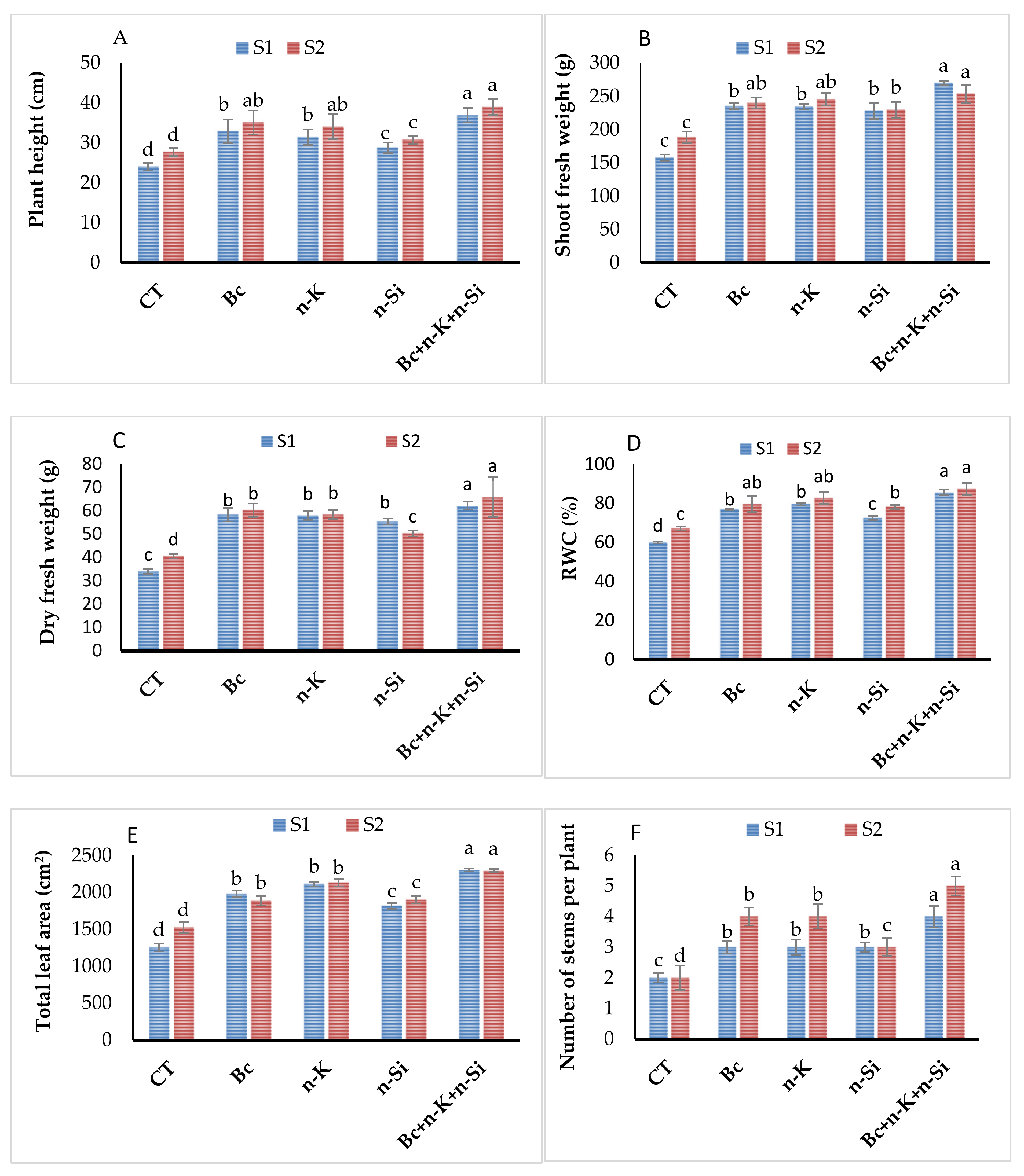
3.1. Morphological Traits and Relative Water Content
3.2. Leaf Chlorophyll and Photosynthetic Gas Exchange Parameters
3.3. Activity of Antioxidant Enzymes in Potato Leaves (CAT, POD, and PPO)
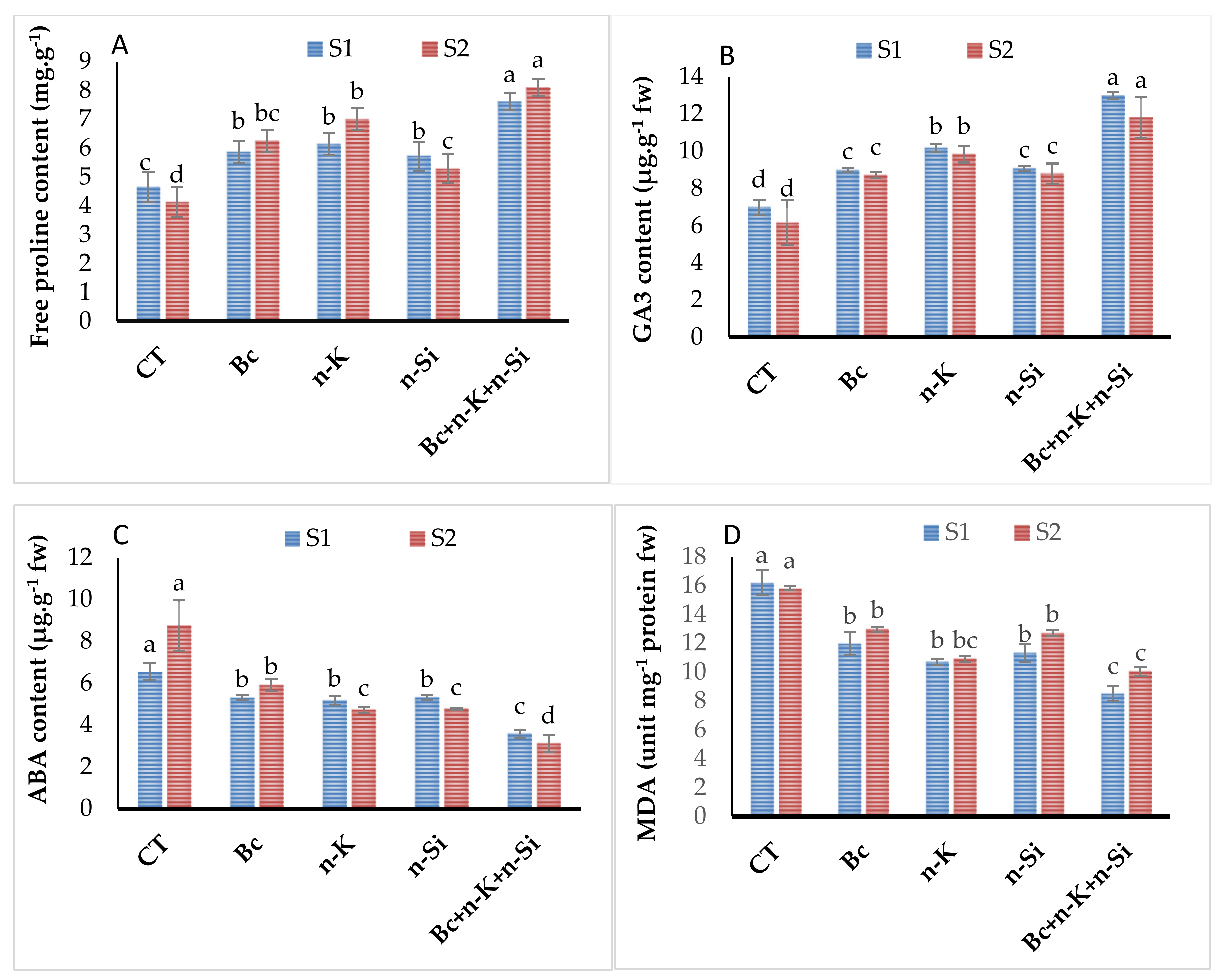
3.4. Free Proline, Lipid Peroxidation, and Plant Hormone Contents
3.5. Endogenous Nutrient Content
3.6. Tuber Yield and Quality
3.7. Correlation Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdeldaym, E.A.; Traversa, A.; Cocozza, C.; Brunetti, G. Effects of a 2-year application of different residual biomasses on soil properties and potato yield. Clean Soil Air Water 2018, 46, 1800261. [Google Scholar] [CrossRef]
- Abuarab, M.E.; El-Mogy, M.M.; Hassan, A.M.; Abdeldaym, E.A.; Abdelkader, N.H.; El-Sawy, M.B.I. The effects of root aeration and different soil conditioners on the nutritional values, yield, and water productivity of potato in clay loam soil. Agronomy 2019, 9, 418. [Google Scholar] [CrossRef] [Green Version]
- FAO. Agriculture Organization of the United Nations Statistics Division; FAO: Cairo, Egypt, 2019. [Google Scholar]
- Abdallah, I.S.; Atia, M.A.; Nasrallah, A.K.; El-Beltagi, H.S.; Kabil, F.F.; El-Mogy, M.M.; Abdeldaym, E.A. Effect of new pre-emergence herbicides on quality and yield of potato and its associated weeds. Sustainability 2021, 13, 9796. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.I.; Mottaleb, S.A. Synergetic Effects of Zinc, Boron, Silicon, and Zeolite Nanoparticles on Confer Tolerance in Potato Plants Subjected to Salinity. Agronomy 2019, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Cocozza, C.; Abdeldaym, E.A.; Brunetti, G.; Nigro, F.; Traversa, A. Synergistic effect of organic and inorganic fertilization on the soil inoculum density of the soilborne pathogens Verticillium dahliae and Phytophthora spp. under open-field conditions. Chem. Biol. Technol. Agric. 2021, 8, 24. [Google Scholar] [CrossRef]
- Abdelaziz, M.E.; Abdeldaym, E.A. Effect of grafting and different EC levels of saline irrigation water on growth, yield and fruit quality of tomato (Lycopersicon esculentum) in greenhouse. Plant Arch. 2019, 19, 3021–3027. [Google Scholar]
- Abdeldym, E.A.; El-Mogy, M.M.; Abdellateaf, H.R.L.; Atia, M.A.M. Genetic Characterization, Agro-Morphological and Physiological Evaluation of Grafted Tomato under Salinity Stress Conditions. Agronomy 2020, 10, 1948. [Google Scholar] [CrossRef]
- Gregory, P.J.; Ismail, S.; Razaq, I.B.; Wahbi, A. Soil Salinity: Current Status and in Depth Analyses for Sustainable Use; International Atomic Energy Agency: Vienna, Austria, 2018; Chapter 2. [Google Scholar]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Khan, A.L.; Muneer, S.; Kim, Y.H.; Al-Rawahi, A.; Al-Harrasi, A. Silicon and salinity: Crosstalk in crop-mediated stress tolerance mechanisms. Front. Plant Sci. 2019, 10, 1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, S.; Abbas, G.; Shahid, M.; Saqib, M.; Farooq, A.B.U.; Hussain, M.; Murtaza, B.; Amjad, M.; Naeem, M.A.; Farooq, A. Effect of salinity on cadmium tolerance, ionic homeostasis and oxidative stress responses in conocarpus exposed to cadmium stress: Implications for phytoremediation. Ecotoxicol. Environ. Saf. 2019, 171, 146–153. [Google Scholar] [CrossRef]
- Abdelaziz, M.E.; Abdelsattar, M.; Abdeldaym, E.A.; Atia, M.A.M.; Mahmoud, A.W.M.; Saad, M.M.; Hirt, H. Piriformospora indica alters Na+/K+ homeostasis, antioxidant enzymes and LeNHX1 expression of greenhouse tomato grown under salt stress. Sci. Hortic. 2019, 256, 108532. [Google Scholar] [CrossRef]
- Vwioko, E.D.; El-Esawi, M.A.; Imoni, M.E.; Al-Ghamdi, A.A.; Ali, H.M.; El-Sheekh, M.M.; Abdeldaym, E.A.; Al-Dosary, M.A. Sodium Azide Priming Enhances Waterlogging Stress Tolerance in Okra (Abelmoschus esculentus L.). Agronomy 2019, 9, 679. [Google Scholar] [CrossRef] [Green Version]
- Winterbourn, C.C. Reactive oxygen species in biological systems. In Vitamin E: Chemistry and Nutritional Benefits; Niki, E., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 98–117. [Google Scholar] [CrossRef]
- Pérez-Labrada, F.; López-Vargas, E.R.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Responses of tomato plants under saline stress to foliar application of copper nanoparticles. Plants 2019, 8, 151. [Google Scholar] [CrossRef] [Green Version]
- Irakoze, W.; Prodjinoto, H.; Nijimbere, S.; Bizimana, J.B.; Bigirimana, J.; Rufyikiri, G.; Lutts, S. NaCl-and Na2SO4-Induced Salinity Differentially Affect Clay Soil Chemical Properties and Yield Components of Two Rice Cultivars (Oryza sativa L.) in Burundi. Agronomy 2021, 11, 571. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Chen, T.; Xing, L.; Xu, K.; Ji, D. Regulatory mechanisms underlying the maintenance of homeostasis in Pyropia haitanensis under hypersaline stress conditions. Sci. Total Environ. 2019, 662, 168–179. [Google Scholar] [CrossRef]
- Macías, J.M.; Caltzontzit, M.G.L.; Martínez, E.N.R.; Ortiz, W.A.N.; Benavides Mendoza, A.; Lagunes, P.M. Enhancement to Salt Stress Tolerance in Strawberry Plants by Iodine Products Application. Agronomy 2021, 11, 602. [Google Scholar] [CrossRef]
- Manchanda, G.; Garg, N. Salinity and its effects on the functional biology of legumes. Acta Physiol. Plant. 2008, 30, 595–618. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Khodarahmi, S.; Haghighi, M. Effect of silicon nutrition on lipid peroxidation and antioxidant response of cucumber plants exposed to salinity stress. Arch. Agron. Soil Sci. 2014, 60, 639–653. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef]
- Ben Azaiez, F.E.; Ayadi, S.; Capasso, G.; Landi, S.; Paradisone, V.; Jallouli, S.; Hammami, Z.; Chamekh, Z.; Zouari, I.; Trifa, Y.; et al. Salt stress induces differentiated nitrogen uptake and antioxidant responses in two contrasting barley landraces from MENA region. Agronomy 2020, 10, 1426. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Salama, A.M.; Mohamed, H.F.; Abdelgawad, K.F.; Abdeldaym, E.A. Responding of long green pepper plants to different sources of foliar potassium fertiliser. Agriculture 2019, 65, 59–76. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; Mazrou, Y.S.; Hafez, Y.M. Silicon foliar application mitigates salt stress in sweet pepper plants by enhancing water status, photosynthesis, antioxidant enzyme activity and fruit yield. Plants 2020, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.W.M.; Abdelaziz, S.M.; El-Mogy, M.M.; Abdeldaym, E.A. Effect of foliar ZnO and FeO nanoparticles application on growth and nutritional quality of red radish and assessment of their accumulation on human health. Agriculture 2019, 65, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Haghighi, M.; Pessarakli, M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013, 161, 111–117. [Google Scholar] [CrossRef]
- Šebesta, M.; Nemček, L.; Urík, M.; Kolenčík, M.; Bujdoš, M.; Hagarová, I.; Matúš, P. Distribution of TiO2 Nanoparticles in Acidic and Alkaline Soil and Their Accumulation by Aspergillus niger. Agronomy 2020, 10, 1833. [Google Scholar] [CrossRef]
- Tondey, M.; Kalia, A.; Singh, A.; Dheri, G.S.; Taggar, M.S.; Nepovimova, E.; Krejcar, O.; Kuca, K. Seed Priming and Coating by Nano-Scale Zinc Oxide Particles Improved Vegetative Growth, Yield and Quality of Fodder Maize (Zea mays). Agronomy 2021, 11, 729. [Google Scholar] [CrossRef]
- Richards, L.S. Diagnosis and Improvement of Saline and Alkaline Soils Handbook; U.S. Department of Agriculture: Washington, DC, USA, 1954; p. 60. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis Prentice; Hall of India Private Limited: New Delhi, India, 1967; Volume 498. [Google Scholar]
- Hassan, A.Z.; Mahmoud, A.W.M.; Turky, G.M.; Safwat, G. Rice husk derived biochar as smart material loading nano nutrients and microorganisms. Bulg. J. Agric. Sci. 2020, 26, 309–322. [Google Scholar]
- Kinney, T.J.; Masiello, C.A.; Dugan, B.; Hockaday, W.C.; Dean, M.R.; Zygourakis, K. Hydrologic properties of biochars produced at different temperatures. Biomass Bioenergy 2012, 41, 34–43. [Google Scholar] [CrossRef]
- Allen, S.F.; Grimshaw, H.F.; Rowl, A.B. Chemical Analysis. In Methods in Plant Ecology; Moor, P.D., Chapman, S.B., Eds.; Blackwell: Oxford, UK, 1984; p. 185344. [Google Scholar]
- Dong, Y.; Bian, X.; Fu, Y.; Shao, Q.; Jiang, J. Simple preparation of potassium sulfate nanoparticles. Cryst. Eng. Comm. 2018, 20, 7713–7718. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F3–F4. [Google Scholar] [CrossRef]
- Polle, A.; Otter, T.; Mehne-Jakobs, B. Effect of magnesium deficiency on antioxidative systems in needles of Norway spruce [Picea abies (L.) Karst.] grown with different ratios of nitrate and ammonium as nitrogen souices. New Phytol. 1994, 128, 621–628. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–888. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Fales, T.M.; Jaouni, J.F.; Babashak, I. Simple device for preparing ethereal diazomethane without resorting to codistillation. Ann. Chem. 1973, 45, 2302–2303. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Forney, C.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- Singh, A.K.; Dubey, R.S. Changes in chlorophyll a and b contents and activities of photosystems 1 and 2 in rice seedlings induced by NaCl. Photosynthetica 1995, 31, 489–499. [Google Scholar]
- Abdeldaym, E.A.; El-Sawy, M.B.I.; El-Helaly, M.A. Combined application of different sources of nitrogen fertilizers for improvementof potato yield and quality. Plant Arch. 2019, 19, 2513–2521. [Google Scholar]
- Ashraf, M.; McNeilly, T. Improvement of Salt Tolerance in Maize by Selection and Breeding. Plant Breed. 1990, 104, 101–107. [Google Scholar] [CrossRef]
- Akram, M.; Asghar Malık, M.; Yasın Ashraf, M.; Farrukh Saleem, M.; Hussain, M. Competitive Seedling Growth And K+/Na+ Ratio in Different Maize (Zea mays L.) Hybrids under Salinity Stress. Pak. J. Bot. 2007, 39, 2553–2563. [Google Scholar]
- Akram, M.S.; Ashraf, M. Exogenous application of potassium dihydrogen phosphate can alleviate the adverse effects of salt stress on sunflower. J. Plant Nutr. 2011, 34, 1041–1057. [Google Scholar] [CrossRef]
- Dubey, R.S. Photosynthesis in plants under stressful conditions. In Handbook of Photosynthesis; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense Against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef] [PubMed]
- Arzani, A. Improving salinity tolerance in crop plants: A biotechnological view. Vitr. Cell. Dev. Biol. Plant 2008, 44, 373–383. [Google Scholar] [CrossRef]
- Liang, Y.C.; Ding, R.X. Influence of silicon on microdistribution of mineral ions in roots of salt-stressed barleyas associated with salt tolerance in plants. Sci. China C. 2002, 45, 298–308. [Google Scholar] [CrossRef]
- Li, J.; Hu, L.; Zhang, L.; Pan, X.; Hu, X. Exogenous spermidine is enhancing tomato tolerance to salinity–alkalinity stress by regulating chloroplast antioxidant system and chlorophyll metabolism. BMC Plant Biol. 2015, 15, 303. [Google Scholar] [CrossRef]
- Kosova, K.; Vıtamvas, P.; Prasil, I.T.; Renaut, J. Plant proteome changes under abiotic stress-contribution of proteomics studies to understanding plant stress response. J. Proteom. 2011, 74, 1301–1322. [Google Scholar] [CrossRef]
- Rekaby, S.A.; Awad, M.; Majrashi, A.; Ali, E.F.; Eissa, M.A. Corn Cob-Derived Biochar Improves the Growth of Saline-Irrigated Quinoa in Different Orders of Egyptian Soils. Horticulturae 2021, 7, 221. [Google Scholar] [CrossRef]
- Kafi, M.; Nabati, J.; Ahmadi-Lahijani, M.J.; Oskoueian, A. Silicon compounds and potassium sulfate improve salinity tolerance of potato plants through instigating the defense mechanisms, cell membrane stability, and accumulation of osmolytes. Commun. Soil Sci. Plant Anal. 2021, 52, 843–858. [Google Scholar] [CrossRef]
- Sharma, S.; Villamor, J.G.; Verslues, P.E. Essential role of tissue-specific proline synthesis and catabolism ingrowth and redox balance at low water potential. Plant Physiol. 2011, 157, 292–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar Mitigates Salinity Stress in Potato. J. Agron. Crop. Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Omara, R.I.; Abdelaal, K.A.A. Biochemical, histopathological and genetic analysis associated with leaf rustinfection in wheat plants (Triticum aestivum L.). Physiol. Mol. Plant Pathol. 2018, 104, 48–57. [Google Scholar] [CrossRef]
- Baiamonte, G.; Minacapilli, M.; Crescimanno, G. Effects of biochar on irrigation management and water use efficiency for three different crops in a desert sandy soil. Sustainability 2020, 12, 7678. [Google Scholar] [CrossRef]
- Ðordević, N.O.; Todorović, N.; Novaković, I.T.; Pezo, L.L.; Pejin, B.; Maraš, V.; Tešević, V.V.; Pajović, S.B. Antioxidant Activity of Selected Polyphenolics in Yeast Cells: The Case Study of Montenegrin Merlot Wine. Molecules 2018, 23, 1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wani, S.H.; Brajendra Singh, N.; Haribhushan, A.; Iqbal Mir, J. Compatible Solute Engineering in Plants for Abiotic Stress Tolerance—Role of Glycine Betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kafi, M.; Nabati, J.; Saadatian, B.; Oskoueian, A.; Shabahang, J. Potato response to silicone compounds (micro and nanoparticles) and potassium as affected by salinity stress. Ital. J. Agron. 2019, 14, 1182. [Google Scholar] [CrossRef] [Green Version]
- Benslima, W.; Zorrig, W.; Bagues, M.; Abdelly, C.; Hafsi, C. Silicon mitigates potassium deficiency in Hordeum vulgare by improving growth and photosynthetic activity but not through polyphenol accumulation and the related antioxidant potential. Plant Soil 2021, 1–18. [Google Scholar] [CrossRef]
- Jeong, M.J.; Park, S.C.; Byun, M.O. Improvement of salt tolerance in transgenic potato plants byglyceraldehyde-3 phosphate dehydrogenase gene transfer. Mol. Cells 2001, 12, 185–189. [Google Scholar] [CrossRef]
- Moran, R. Formulae for Determination of Chlorophyllous Pigments Extracted with N,N-Dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef] [Green Version]
- Avestan, S.; Ghasemnezhad, M.; Esfahani, M.; Byrt, C.S. Application of nano-silicon dioxide improves salt stress tolerance in strawberry plants. Agronomy 2019, 9, 246. [Google Scholar] [CrossRef] [Green Version]
- Dawood, M.G.; Taie, H.A.A.; Nassar, R.M.A.; Abdelhamid, M.T.; Schmidhalter, U. The changes induced in the physiological, biochemical and anatomical characteristics of Vicia faba by the exogenous application of proline under seawater stress. S. Afr. J. Bot. 2014, 93, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Alsaeedi, A.; El-Ramady, H.; Alshaal, T.; El-Garawany, M.; Elhawat, N.; Al-Otaibi, A. Silica nanoparticles boost growth and productivity of cucumber under water deficit and salinity stresses by balancing nutrients uptake. Plant Physiol. Biochem. 2019, 139, 1–10. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.; Ahmed, W.; Ikram, M.; Imtiaz, M.; Mahmood, S.; Tu, S.; Chen, D. Chitosan modified biochar increases soybean (Glycine max L.) resistance to salt-stress by augmenting root morphology, antioxidant defense mechanisms and the expression of stress-responsive genes. Plants 2020, 9, 1173. [Google Scholar] [CrossRef] [PubMed]
- Vighi, I.L.; Benitez, L.C.; Amaral, M.N.; Moraes, G.P.; Auler, P.A.; Rodrigues, G.S.; Deuner, S.; Maia, L.C.; Braga, E.J.B. Functional characterization of the antioxidant enzymes in rice plants exposed to salinity stress. Biol. Plant. 2017, 61, 540–550. [Google Scholar] [CrossRef]
- Zhang, Z.; Mao, B.; Li, H.; Zhou, W.; Takeuchi, Y.; Yoneyama, K. Effect of salinity on physiological characteristics, yield and quality of microtubers in vitro in potato. Acta Physiol. Plant. 2005, 27, 481–489. [Google Scholar] [CrossRef]
- Ghosh, S.C.; Asanuma, K.I.; Kusutani, A.; Toyota, M. Effect of salt stress on some chemical components and yield of potato. Soil Sci. Plant Nutr. 2001, 47, 467–475. [Google Scholar] [CrossRef]
- Balibrea, M.E.; Dell’Amico, J.; Bolarín, M.C.; Pérez-Alfocea, F. Carbon partitioning and sucrose metabolism in tomato plants growing under salinity. Physiol. Plant. 2000, 110, 503–511. [Google Scholar] [CrossRef]
- Szabo-Nagi, A.; Galiba, G.; Erdei, L. Induction of soluble phosphatases under ionic and non-ionic osmotic stresses in wheat. J. Plant Physiol. 1992, 140, 629–633. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Abdelaziz, M.E.; Atia, M.A.; Abdelsattar, M.; Abdelaziz, S.M.; Ibrahim, T.A.; Abdeldaym, E.A. Unravelling the Role of Piriformospora indica in combating water deficiency by modulating physiological performance and chlorophyll metabolism-related genes in Cucumis sativus. Horticulturae 2021, 7, 399. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Parmar, A.; Ali, M.R.; Abdel-Aziz, M.E.; Abdeldaym, E.A. Improving postharvest storage of fresh artichoke bottoms by an edible coating of Cordia myxa gum. Postharvest Biol. Technol. 2020, 163, 111143. [Google Scholar] [CrossRef]
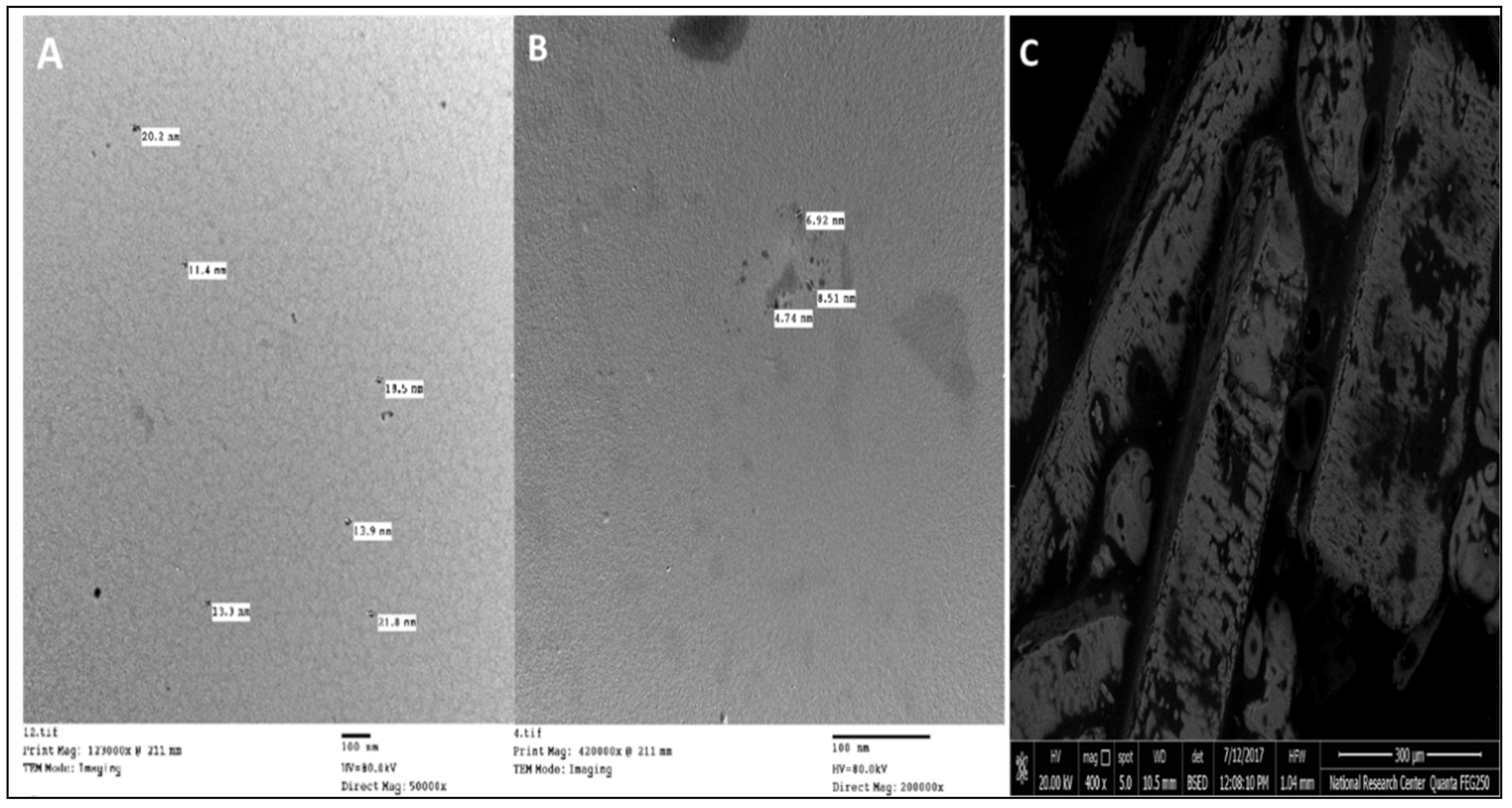



| Particle Size of Soil (%) | Value |
|---|---|
| Sand | 89.6 |
| Silt | 7.1 |
| Clay | 3.3 |
| Textural class | Sand |
| Organic matter (%) | 0.28 |
| pH | 7.8 |
| EC (dS·m−1) | 4.3 |
| Calcium carbonates (%) | 4.14 |
| Soluble Anions (Cmole·Kg−1 soil) | |
| Cl− | 10.8 |
| SO4− | 12.3 |
| HCO3− | 18.2 |
| Soluble Cations(Cmole·Kg−1 soil) | |
| Mg2+ | 11.4 |
| Na+ | 9.2 |
| K+ | 6.1 |
| Available nutrients (ppm) | |
| N | 14.7 |
| P | 7.01 |
| K | 46.2 |
| Fe | 10.60 |
| Mn | 1.60 |
| B | 0.22 |
| Properties | Rice-Husk-Derived Biochar |
|---|---|
| Moisture content (%) | 3.88 |
| Ash (%) | 47.90 |
| pH (1:1, w:v) | 7.65 |
| C (mg) | 46.35 |
| H (mg) | 2.64 |
| N (mg) (after soaking in ammonium) | 3.65 |
| Sulphate (mg) | 0.22 |
| Oxygen (mg) | 2.74 |
| C:H | 0.05 |
| C:N (after soaking in ammonium sulphate) | 12.92 |
| EC (dS·m−1) | 0.14 |
| Si (mg·kg−1) | 179 |
| Ca (mg·kg−1) | 213 |
| K (mg·kg−1) | 199 |
| Mg (mg·kg−1) | 179 |
| Zeta potential (mV) | −26.6 |
| Treatments | CT | Bc | n-K | n-Si | Bc+n-K+n-Si | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |
| N (%) | 2.04 ± 0.14 d | 1.75 ± 0.21 d | 2.88 ± 0.18 b | 2.73 ± 0.41 b | 2.89 ± 0.23 b | 3.04 ± 0.35 ab | 2.50 ± 0.21 c | 2.32 ± 0.17 c | 3.34 ± 0.25 a | 3.41 ± 0.95 a |
| P (%) | 0.15 ± 0.02 d | 0.19 ± 0.02 d | 0.23 ± 0.011 c | 0.26 ± 0.009 c | 0.25 ± 0.01 b | 0.28 ± 0.01 b | 0.24 ± 0.003 cb | 0.27 ± 0.001 b | 0.32 ± 0.011 a | 0.36 ± 0.011 a |
| K (%) | 3.42 ± 0.27 d | 3.32 ± 0.32 d | 4.56 ± 0.18 c | 5.10 ± 0.44 c | 5.37 ± 0.18 b | 5.68 ± 0.62 b | 4.60 ± 0.28 c | 5.16 ± 0.21 c | 6.08 ± 0.21 a | 6.45 ± 0.52 a |
| Na (%) | 5.92 ± 0.9 a | 6.11 ± 1.21 a | 4.28 ± 0.42 c | 4.58 ± 0.56 b | 4.15 ± 0.9 cd | 4.38 ± 0.89 c | 4.40 ± 0.31 b | 4.78 ± 0.27 b | 3.21 ± 0.51 d | 3.87 ± 0.76 d |
| Mg (%) | 0.41 ± 0.03 d | 0.40 ± 0.074 e | 0.76 ± 0.86 b | 0.63 ± 0.23 c | 0.75 ± 0.05 b | 0.80 ± 0.08 ab | 0.59 ± 0.05 d | 0.63 ± 0.021 c | 0.88 ± 0.25 a | 0.83 ± 0.13 a |
| Fe (ppm) | 30.2 ± 1.1 d | 29.50 ± 2.3d | 67.80 ± 1.34 b | 69.20 ± 1.91 b | 66.30 ± 1.9 b | 70.60 ± 2.1 b | 60.70 ± 3.01 c | 59.10 ± 1.55 c | 88.50 ± 3.43 a | 90.2 ± 4.17 a |
| Mn (ppm) | 42.60 ± 2.30 d | 50.10 ± 1.25 e | 89.60 ± 3.14 b | 95.50 ± 3.23 b | 90.80 ± 2.9 b | 86.50 ± 3.56 c | 78.40 ± 1.26 c | 74.60 ± 1.95 d | 109.60 ± 4.32 a | 100.7 ± 4.2 a |
| B (ppm) | 17.60 ± 0.95 e | 19.50 ± 1.32 e | 22.50 ± 2.05 d | 25.70 ± 2.45 d | 31.20 ± 1.6 b | 35.40 ± 1.85 b | 26.96 ± 2.01 c | 28.10 ± 2.05 c | 37.30 ± 2.45 a | 41.2 ± 2.09 a |
| Treatments | CT | Bc | n-K | n-Si | Bc+n-K+n-Si | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |
| Total Yield (t.ha−1) | 22.3 ± 2.1c | 25.75 ± 2.3 e | 32.4 ± 3.21 b | 34.75 ± 2.09 d | 33.5 ± 1.9 b | 40.75 ± 3.4 c | 32.8 ± 1.44 b | 39.25 ± 3.15 b | 39.1 ± 0.98 a | 44.5 ± 2.77 a |
| No. tuber | 11.2 ± 0.4 c | 10.1 ± 1.55 c | 15.1 ± 1.61 a | 16.21 ± 2.2a b | 16.6 ± 1.04 a | 15.5 ± 0.2 ab | 15.41 ± 1.5 ab | 15.52 ± 2.4 a | 16.4 ± 1.24 a | 17.09 ± 1.08 a |
| Tuber weight (g) | 97.3 ± 4.6 c | 101.7 ± 6.3 c | 136.3 ± 6.7 b | 135.68 ± 8.5b | 135.92 ± 4.7 b | 136.8 ± 3.8 b | 131.83 ± 4.6 b | 135.2 ± 4.7 b | 139.6 ± 4.01 a | 141.05 ± 6.1 a |
| Tubers Hardness (kg.m2) | 11 ± 1.24 b | 12.1 ± 2.01c | 14.9 ± 2.1ab | 15.01 ± 2.8ab | 14.97 ± 0.88 a | 15.05 ± 1.8 a | 13.67 ± 1.43 b | 13.81 ± 1.7 b | 16.91 ± 1.67 a | 17.08 ± 1.5 a |
| Tuber diameter (cm) | 3.5 ± 0.77 c | 3.12 ± 0.32 c | 5.3 ± 0.3 ab | 5.54 ± 0.47 ab | 5.34 ± 0.86 ab | 5.51 ± 1.02 ab | 4.28 ± 0.85 b | 5.20 ± 0.9 ab | 5.96 ± 0.74 a | 6.21 ± 0.59 a |
| Tuber length (cm) | 6.1 ± 1.62 c | 6.56 ± 0.89 c | 7.3 ± 1.39 b | 8.07 ± 1.98 ab | 7.89 ± 0.99 ab | 8.01 ± 1.15 ab | 7.11 ± 0.65 b | 7.17 ± 1.77 b | 8.65 ± 1.86 a | 8.77 ± 1.32 a |
| Treatments | CT | Bc | n-K | n-Si | Bc+n-K+n-Si | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |
| Carbohydrates (%) | 66.11 ± 1.15 c | 70.14 ± 2.9 c | 74.13 ± 1.9 ab | 75.37 ± 1.3 b | 76.16 ± 1.8 ab | 74.22 ± 2.1 b | 73.10 ± 1.06 b | 75.35 ± 3.1 ab | 77.58 ± 2.02 a | 79.50 ± 2.5 a |
| Starch content (%) | 78.5 ± 2.11 a | 80.4 ± 3.01 a | 70.5 ± 2.3 b | 69.7 ± 1.98 c | 63.8 ± 0.78 c | 65.9 ± 1.76 d | 68.8 ± 2.85 cb | 74.5 ± 2.11 b | 57.4 ± 1.02 d | 55.7 ± 1.23 e |
| Protein content (%) | 6.31 ± 1.62 d | 6.25 ± 1.82 c | 7.50 ± 1.02 c | 7.69 ± 2.91 b | 7.81 ± 1.90 ab | 7.87 ± 2.83 b | 7.44 ± 1.85 c | 7.75 ± 1.41 b | 8.11 ± 1.55 a | 8.35 ± 1.45 a |
| N (%) | 1.01 ± 0.83 d | 1.0 ± 0.90 c | 1.2 ± 0.16 c | 1.20 ± 0.18 b | 1.25 ± 0.31 ab | 1.26 ± 0.45 b | 1.19 ± 0.30 c | 1.24 ± 0.13 b | 1.29 ± 0.25 a | 1.4 ± 0.23 a |
| P (%) | 0.14 ± 0.005 c | 0.12 ± 0.01 c | 0.17 ± 0.02 b | 0.19 ± 0.006 ab | 0.19 ± 0.005 ab | 0.20 ± 0.01 ab | 0.17 ± 0.003 b | 0.18 ± 0.005 b | 0.23 ± 0.007a | 0.22 ± 0.01 a |
| K (%) | 1.88 ± 0.26 d | 1.95 ± 0.16 d | 2.16 ± 0.10 c | 2.18 ± 0.18 c | 2.59 ± 0.92 b | 2.71 ± 0.24 ab | 2.15 ± 0.32 c | 2.17 ± 0.17 c | 2.8 ± 0.41 a | 2.93 ± 0.38 a |
| Variables | Plant Height | Shoot FW | Shoot DW | RWC | Leaf Area | No. Stems | Leaf-Na | Pn | Gc | Inter. CO2 | Tr | WUE | Chl | Proline | GA3 | ABA | POD | PPO | CAT | MDA | Yield | No. Tubers | Tuber Weight | Carboh. | Starch | Protein |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant height | 1 | |||||||||||||||||||||||||
| Shoot FW | 0.93 | 1 | ||||||||||||||||||||||||
| Shoot DW | 0.87 | 0.91 | 1 | |||||||||||||||||||||||
| RWC | 0.90 | 0.89 | 0.80 | 1 | ||||||||||||||||||||||
| Leaf area (tot.) | 0.93 | 0.93 | 0.90 | 0.95 | 1 | |||||||||||||||||||||
| No. stems | 0.02 | 0.91 | 0.91 | 0.91 | 0.92 | 1 | ||||||||||||||||||||
| leaf-Na | −0.91 | −0.85 | −0.90 | −0.79 | −0.88 | −0.92 | 1 | |||||||||||||||||||
| Pn | 0.40 | 0.90 | 0.83 | 0.94 | 0.49 | 0.29 | −0.93 | 1 | ||||||||||||||||||
| Gc | 0.36 | 0.93 | 0.81 | 0.86 | 0.91 | 0.91 | −0.87 | 0.94 | 1 | |||||||||||||||||
| Inter. CO2 | 0.11 | 0.88 | 0.94 | 0.70 | 0.32 | 0.30 | −0.45 | 0.86 | 0.91 | 1 | ||||||||||||||||
| Tr | −0.33 | −0.95 | −0.95 | −0.81 | −0.86 | −0.88 | 0.88 | −0.96 | −0.59 | −0.87 | 1 | |||||||||||||||
| WUE | 0.89 | 0.88 | 0.92 | 0.97 | 0.87 | 0.92 | −0.92 | 0.73 | 0.88 | 0.90 | −0.83 | 1 | ||||||||||||||
| Chl | 0.87 | 0.93 | 0.85 | 0.95 | 0.73 | 0.28 | −0.39 | 0.89 | 0.75 | 0.96 | −0.80 | 0.98 | 1 | |||||||||||||
| Proline | 0.91 | 0.85 | 0.90 | 0.90 | 0.83 | 0.89 | −0.97 | 0.88 | 0.64 | 0.93 | −0.95 | 0.97 | 0.93 | 1 | ||||||||||||
| GA3 | 0.91 | 0.82 | 0.94 | 0.94 | 0.93 | 0.95 | −0.82 | 0.17 | 0.92 | 0.91 | −0.94 | 0.94 | 0.91 | 0.83 | 1 | |||||||||||
| ABA | −0.89 | −0.97 | −0.96 | −0.95 | −0.21 | −0.90 | 0.84 | −0.96 | −0.93 | −0.77 | 0.97 | −0.89 | −0.88 | −0.74 | −0.90 | 1 | ||||||||||
| POD | −0.34 | -0.27 | -0.26 | −0.34 | −0.11 | −0.35 | 0.19 | −0.06 | -0.36 | -0.15 | 0.30 | −0.36 | −0.37 | −0.38 | −0.45 | 0.16 | 1 | |||||||||
| PPO | −0.10 | −0.70 | −0.76 | v0.87 | −0.08 | −0.41 | 0.20 | −0.77 | −0.93 | −0.89 | 0.82 | −0.80 | −0.84 | −0.17 | −0.12 | 0.94 | 0.07 | 1 | ||||||||
| CAT | −0.90 | −0.76 | −0.86 | −0.81 | −0.88 | −0.89 | 0.87 | −0.83 | −0.95 | −0.82 | 0.76 | −0.96 | −0.76 | −0.89 | −0.91 | 0.93 | 0.16 | 0.98 | 1 | |||||||
| MDA | −0.15 | −0.86 | −0.90 | −0.90 | −0.91 | −0.66 | 0.83 | −0.24 | −0.72 | −0.90 | 0.93 | −0.66 | −0.91 | −0.93 | −0.86 | 0.92 | 0.46 | 0.20 | 0.42 | 1 | ||||||
| Yield | 0.33 | 0.95 | 0.92 | 0.85 | 0.84 | 0.88 | −0.93 | 0.89 | 0.97 | 0.88 | −0.84 | 0.76 | 0.70 | 0.94 | 0.93 | −0.90 | −0.14 | −0.79 | −0.68 | −0.91 | 1 | |||||
| No. tubers | 0.89 | 0.94 | 0.90 | 0.87 | 0.97 | 0.95 | −0.61 | 0.90 | 0.95 | 0.77 | −0.90 | 0.87 | 0.89 | 0.88 | 0.95 | −0.94 | −0.38 | −0.90 | −0.59 | −0.92 | 0.73 | 1 | ||||
| Tuber weight | 0.32 | 0.88 | 0.89 | 0.92 | 0.29 | 0.27 | −0.45 | 0.88 | 0.88 | 0.68 | −0.93 | 0.89 | 0.90 | 0.01 | 0.05 | −0.83 | 0.07 | −0.73 | −0.66 | −0.09 | 0.76 | 0.06 | 1 | |||
| Carboh. | 0.89 | 0.95 | 0.91 | 0.94 | 0.75 | 0.89 | −0.89 | 0.90 | 0.92 | 0.83 | −0.92 | 0.96 | 0.87 | 0.89 | 0.88 | −0.71 | −0.40 | −0.68 | −0.96 | −0.51 | 0.96 | 0.86 | 0.88 | 1 | ||
| Starch | −0.27 | −0.89 | −0.87 | −0.85 | −0.91 | −0.92 | 0.77 | −0.80 | −0.84 | −0.96 | 0.84 | −0.87 | −0.92 | −0.79 | −0.90 | 0.87 | 0.36 | 0.55 | 0.81 | 0.82 | −0.91 | −0.84 | −0.38 | −0.99 | 1 | |
| Protein | 0.90 | 0.91 | 0.85 | 0.82 | 0.94 | 0.93 | −0.73 | 0.77 | 0.94 | 0.56 | −0.76 | 0.78 | 0.82 | 0.95 | 0.90 | −0.82 | −0.40 | −0.78 | −0.86 | −0.83 | 0.60 | 0.90 | 0.04 | 0.99 | −0.77 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.W.M.; Samy, M.M.; Sany, H.; Eid, R.R.; Rashad, H.M.; Abdeldaym, E.A. Nanopotassium, Nanosilicon, and Biochar Applications Improve Potato Salt Tolerance by Modulating Photosynthesis, Water Status, and Biochemical Constituents. Sustainability 2022, 14, 723. https://doi.org/10.3390/su14020723
Mahmoud AWM, Samy MM, Sany H, Eid RR, Rashad HM, Abdeldaym EA. Nanopotassium, Nanosilicon, and Biochar Applications Improve Potato Salt Tolerance by Modulating Photosynthesis, Water Status, and Biochemical Constituents. Sustainability. 2022; 14(2):723. https://doi.org/10.3390/su14020723
Chicago/Turabian StyleMahmoud, Abdel Wahab M., Mahmoud M. Samy, Hoda Sany, Rasha R. Eid, Hassan M. Rashad, and Emad A. Abdeldaym. 2022. "Nanopotassium, Nanosilicon, and Biochar Applications Improve Potato Salt Tolerance by Modulating Photosynthesis, Water Status, and Biochemical Constituents" Sustainability 14, no. 2: 723. https://doi.org/10.3390/su14020723
APA StyleMahmoud, A. W. M., Samy, M. M., Sany, H., Eid, R. R., Rashad, H. M., & Abdeldaym, E. A. (2022). Nanopotassium, Nanosilicon, and Biochar Applications Improve Potato Salt Tolerance by Modulating Photosynthesis, Water Status, and Biochemical Constituents. Sustainability, 14(2), 723. https://doi.org/10.3390/su14020723







