Biochar Phosphate Fertilizer Loaded with Urea Preserves Available Nitrogen Longer than Conventional Urea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production and Characterization of Biochar-Based Fertilizers
2.2. Production of Biochar-Based Phosphate Fertilizer Enriched with Urea
2.3. Scanning Electron Microscopy (SEM), Energy-Dispersive X-ray Spectroscopy (EDX) and Fourier Transform Infrared (FTIR) Spectroscopic Characterization Analysis
2.4. Greenhouse Pot Experiment
2.4.1. Soil Samples and Preparation
2.4.2. Experimental Design and Plant Growth
2.4.3. Plant and Soil Analysis
2.5. Statistical Analysis
3. Results and Discussion
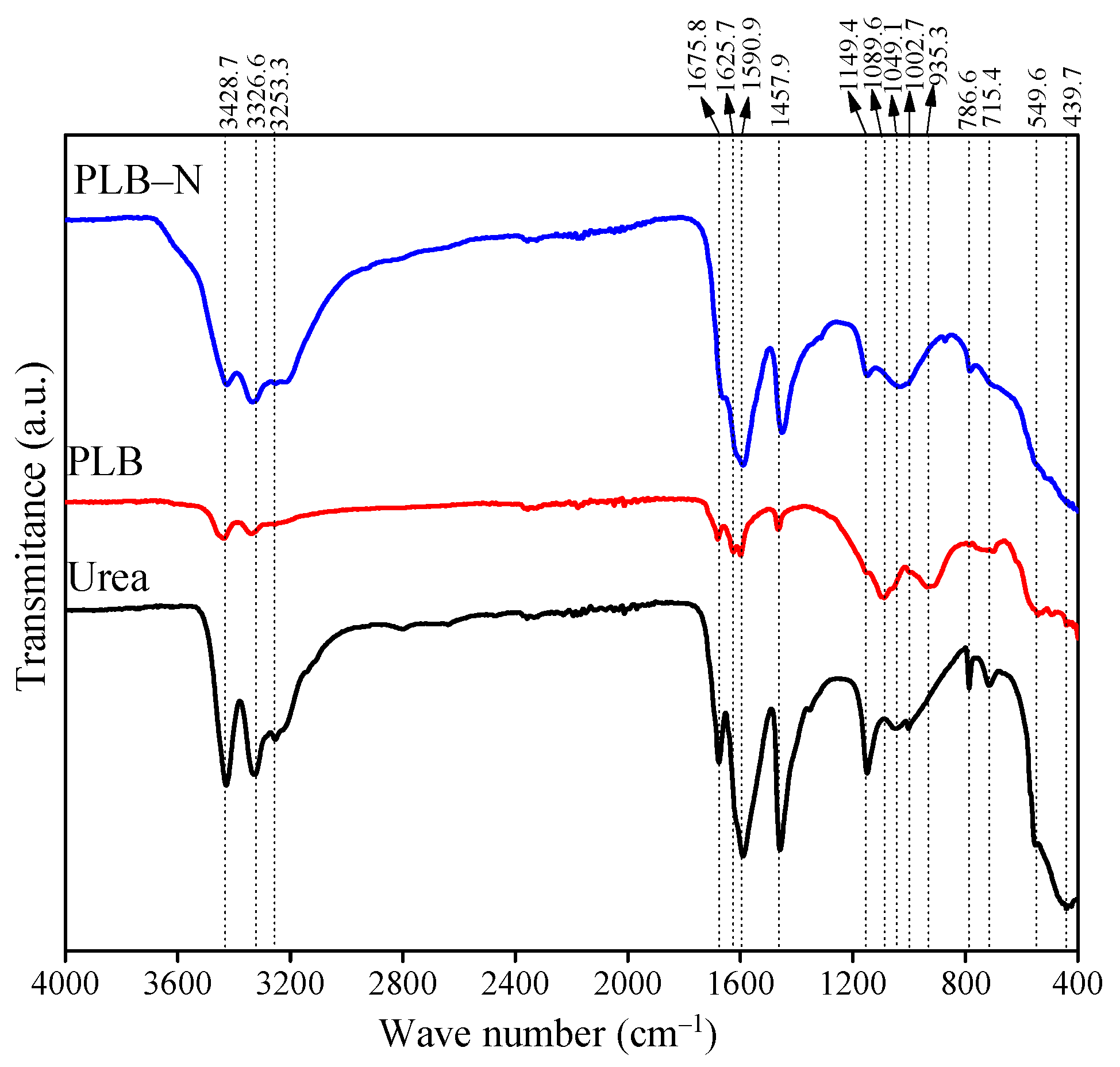
3.1. Properties of the Biochar-Based Fertilizers
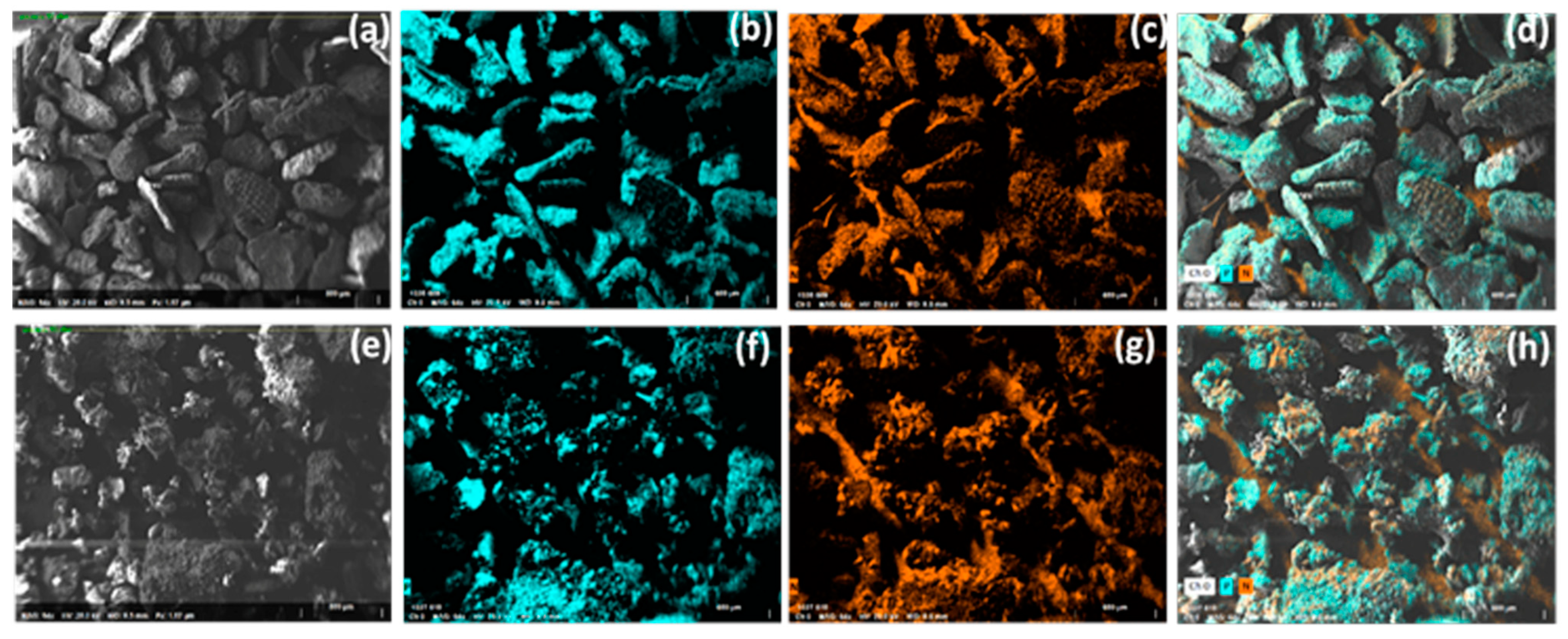
3.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX) Analysis
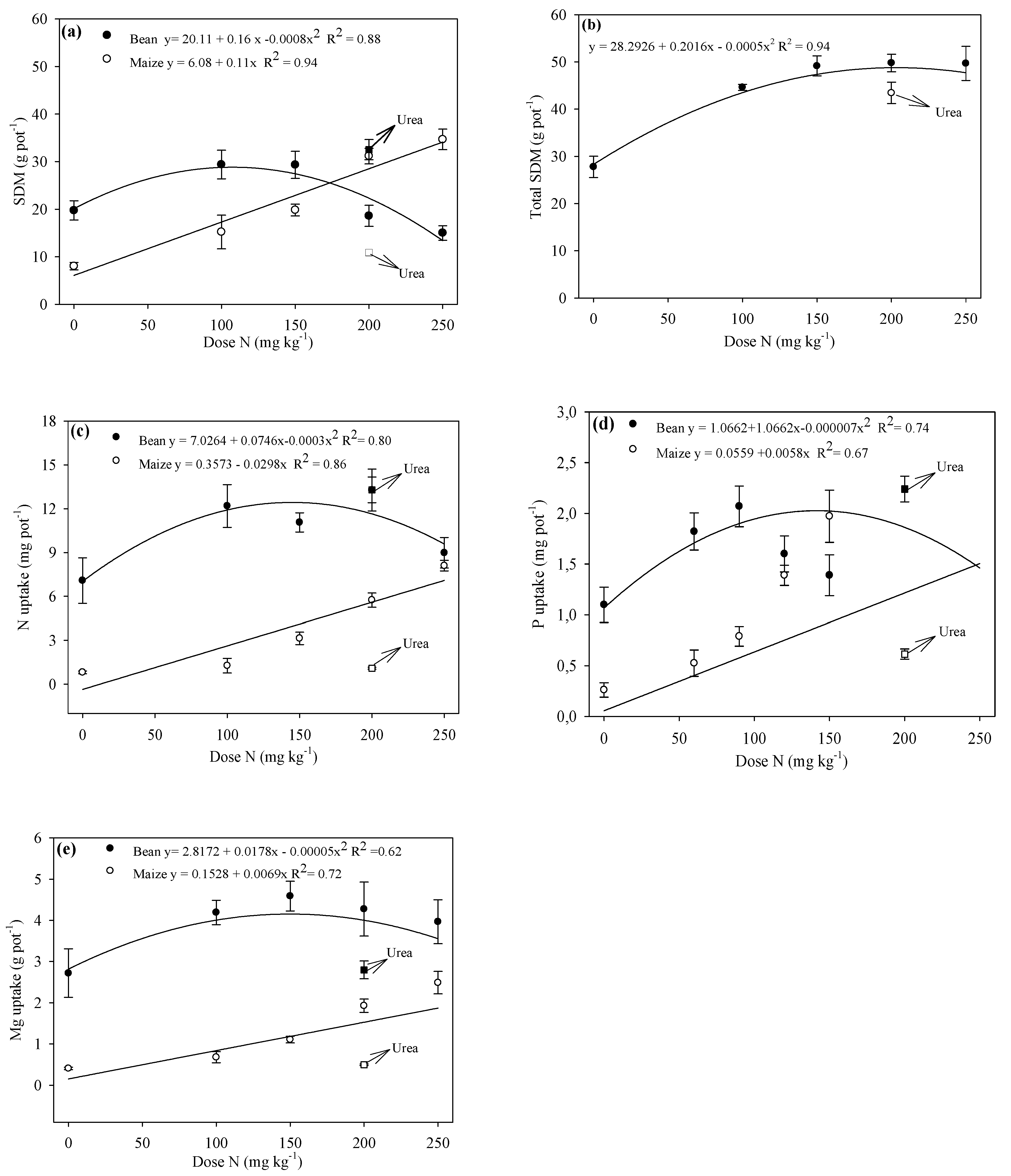
3.3. Crop Productivity and Nitrogen and Phosphorus Uptake
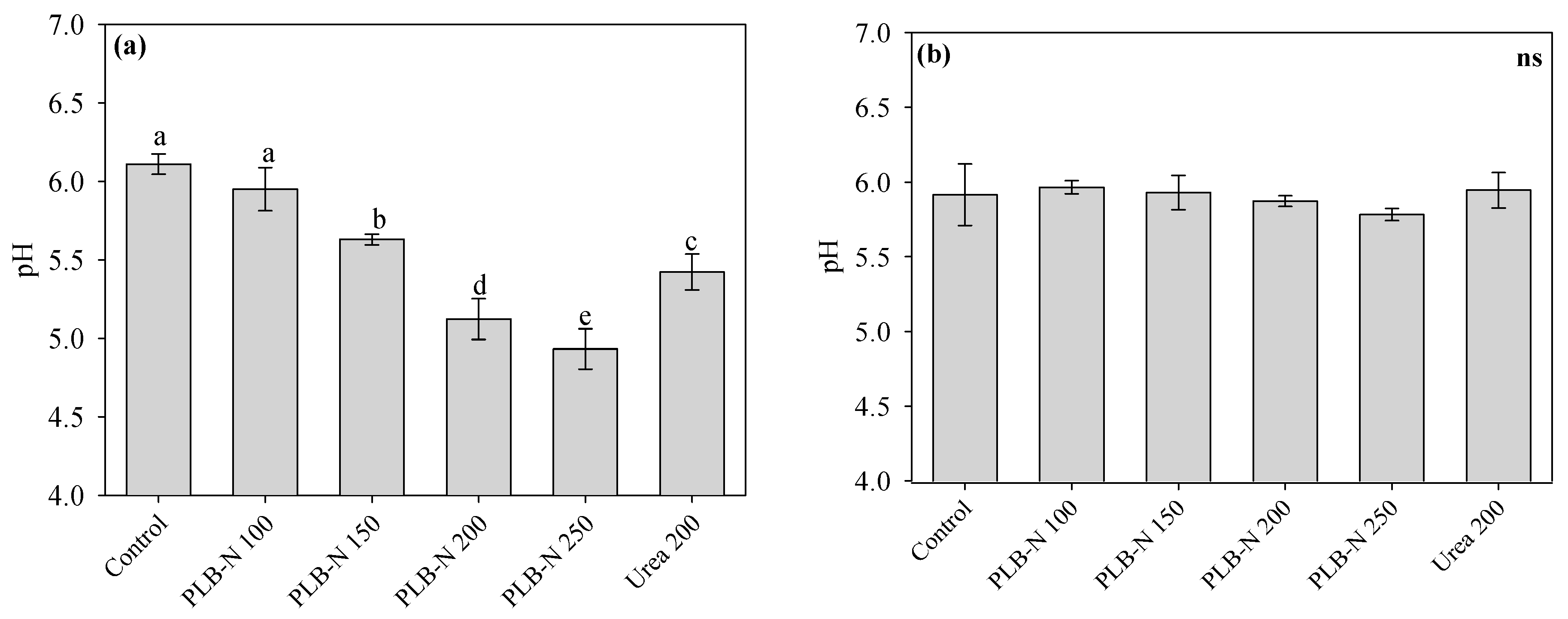
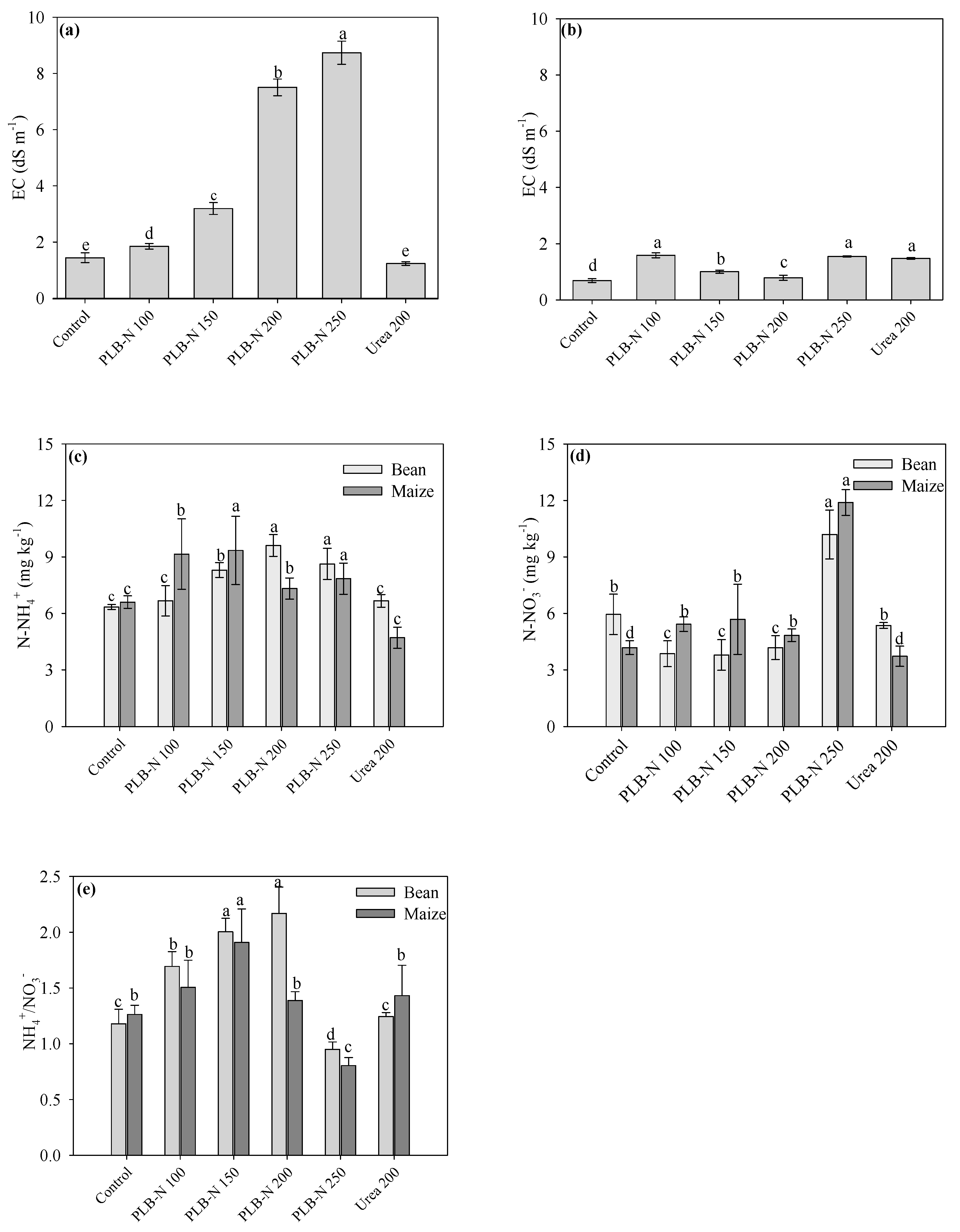
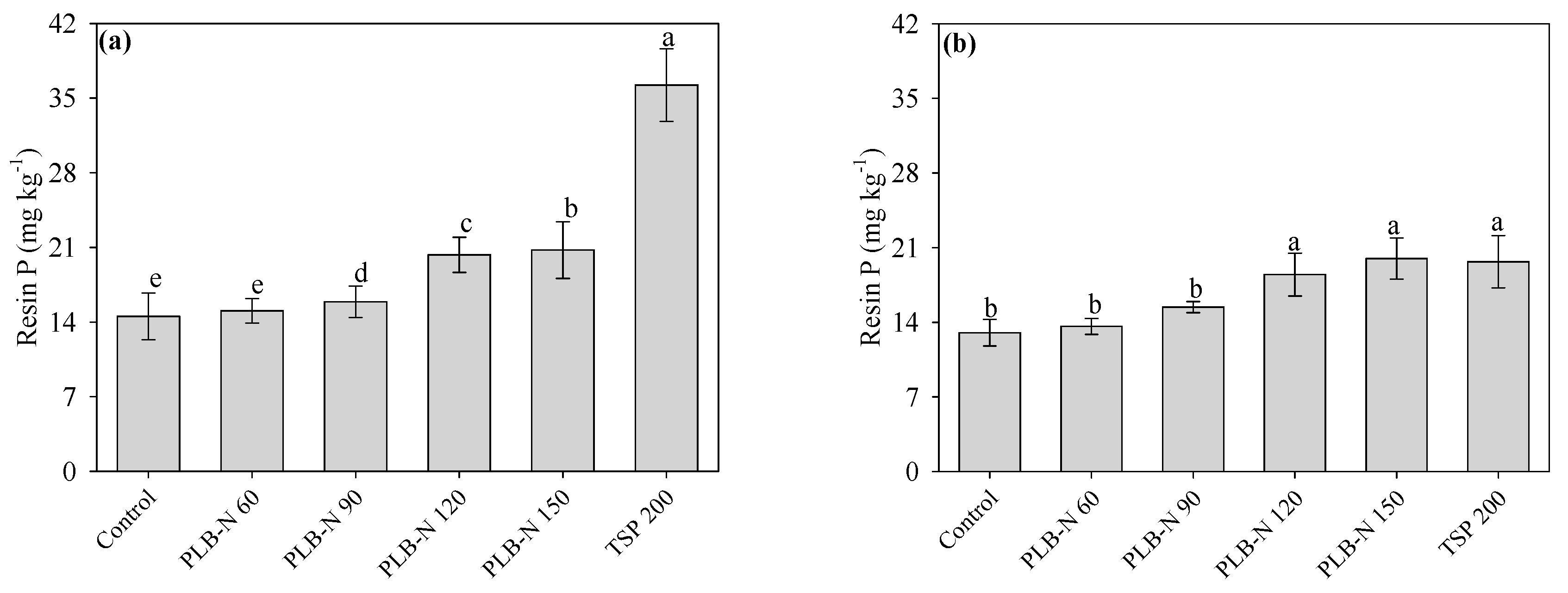
3.4. Soil Conditions after Cultivation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, P.; Wu, Z.; Han, Y.; Cravotto, G.; Wang, J.; Ye, B.C. Microwave-Assisted Synthesis of a Novel Biochar-Based Slow-Release Nitrogen Fertilizer with Enhanced Water-Retention Capacity. ACS Sustain. Chem. Eng. 2017, 5, 7374–7382. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a Century of Ammonia Synthesis Changed the World. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing Nitrogen for Sustainable Development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Tian, H. Global Nitrogen and Phosphorus Fertilizer Use for Agriculture Production in the Past Half Century: Shifted Hot Spots and Nutrient Imbalance. Earth Syst. Sci. Data 2017, 9, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Fageria, N.K.; Baligar, V.C. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar] [CrossRef]
- Bowles, T.M.; Atallah, S.S.; Campbell, E.E.; Gaudin, A.C.M.; Wieder, W.R.; Grandy, A.S. Addressing Agricultural Nitrogen Losses in a Changing Climate. Nat. Sustain. 2018, 1, 399–408. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; White, J.C.; Franklin, F.A.; Melse-Boonstra, A.; Koele, N.; Pandey, R.; Rodenburg, J.; Senthilkumar, K.; Demokritou, P.; et al. Safeguarding Human and Planetary Health Demands a Fertilizer Sector Transformation. Plants People Planet 2020, 2, 302–309. [Google Scholar] [CrossRef]
- Saha, B.K.; Rose, M.T.; Wong, V.N.L.; Cavagnaro, T.R.; Patti, A.F. Nitrogen Dynamics in Soil Fertilized with Slow Release Brown Coal-Urea Fertilizers. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.L.; Zhou, C.H.; Yang, H.M.; Ji, S.F.; Tong, D.S.; Zhong, Z.K.; Yu, W.H.; Chu, M.Q. Environmental-Friendly Montmorillonite-Biochar Composites: Facile Production and Tunable Adsorption-Release of Ammonium and Phosphate. J. Clean. Prod. 2017, 156, 648–659. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Medina, J.; González, A.; Diez, M.C.; Cartes, P.; Monreal, C.; Navia, R. Evaluation of Biodegradable Polymers as Encapsulating Agents for the Development of a Urea Controlled-Release Fertilizer Using Biochar as Support Material. Sci. Total Environ. 2015, 505, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Gwenzi, W.; Nyambishi, T.J.; Chaukura, N.; Mapope, N. Synthesis and Nutrient Release Patterns of a Biochar-Based N–P–K Slow-Release Fertilizer. Int. J. Environ. Sci. Technol. 2018, 15, 405–414. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: An Introduction. In Biochar for Environmental Management, Science, Technology and Implementation; Lehmann, J., Joseph, S., Eds.; Routledge: New York, NY, USA, 2015; pp. 1–13. [Google Scholar]
- Li, S.; Barreto, V.; Li, R.; Chen, G.; Hsieh, Y.P. Nitrogen Retention of Biochar Derived from Different Feedstocks at Variable Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2018, 133, 136–146. [Google Scholar] [CrossRef]
- Nardis, B.O.; Carneiro, J.S.S.; De Souza, I.M.G.; De Barros, R.G.; Melo, L.C.A. Phosphorus Recovery Using Magnesium-Enriched Biochar and Its Potential Use as Fertilizer. Arch. Agron. Soil Sci. 2020, 67, 1017–1033. [Google Scholar] [CrossRef]
- Puga, A.P.; de Queiroz, M.C.A.; Ligo, M.A.V.; Carvalho, C.S.; Pires, A.M.M.; Marcatto, J.d.O.S.; Andrade, C.A. Nitrogen Availability and Ammonia Volatilization in Biochar-Based Fertilizers. Arch. Agron. Soil Sci. 2019, 66, 992–1004. [Google Scholar] [CrossRef]
- Shi, W.; Ju, Y.; Bian, R.; Li, L.; Joseph, S.; Mitchell, D.R.G.; Munroe, P.; Taherymoosavi, S.; Pan, G. Science of the Total Environment Biochar Bound Urea Boosts Plant Growth and Reduces Nitrogen Leaching. Sci. Total Environ. 2020, 701, 134424. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.S.S.; Lustosa Filho, J.F.; Nardis, B.O.; Ribeiro-Soares, J.; Zinn, Y.L.; Melo, L.C.A. Carbon Stability of Engineered Biochar-Based Phosphate Fertilizers. ACS Sustain. Chem. Eng. 2018, 6, 14203–14212. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Zheng, W.; Scott, J.W.; Sharma, B.K.; Chen, X. Co-Pyrolysis of Biomass with Phosphate Fertilizers to Improve Biochar Carbon Retention, Slow Nutrient Release, and Stabilize Heavy Metals in Soil. ACS Sustain. Chem. Eng. 2016, 4, 1630–1636. [Google Scholar] [CrossRef]
- Carneiro, J.S.S.; Ribeiro, I.C.A.; Nardis, B.O.; Barbosa, C.F.; Lustosa Filho, J.F.; Melo, L.C.A. Long-Term Effect of Biochar-Based Fertilizers Application in Tropical Soil: Agronomic Efficiency and Phosphorus Availability. Sci. Total Environ. 2021, 760, 143955. [Google Scholar] [CrossRef] [PubMed]
- Filho, J.F.L.; Barbosa, C.F.; Carneiro, J.S.S.; Melo, L.C.A. Diffusion and Phosphorus Solubility of Biochar-Based Fertilizer: Visualization, Chemical Assessment and Availability to Plants. Soil Tillage Res. 2019, 194, 104298. [Google Scholar] [CrossRef]
- Lustosa Filho, J.F.; Penido, E.S.; Castro, P.; Silva, C.A.; Melo, L.C.A. Co-Pyrolysis of Poultry Litter and Phosphate and Magnesium Generates Alternative Slow-Release Fertilizer Suitable for Tropical Soils. ACS Sustain. Chem. Eng. 2017, 5, 9043–9052. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn Growth and Nitrogen Nutrition after Additions of Biochars with Varying Properties to a Temperate Soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of Biochars to Evaluate Recalcitrance and Agronomic Performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Manual de Métodos Analíticos Oficiais Para Fertilizantes e Corretivos; MAPA, Ministério da Agricultura Pecuária e Abastecimento/SDA, Secretaria de Defesa Agropecuária: Brasília, Brasil, 2017.
- Trenkel, M.E. Slow and Controlled-Release and Stabilized Fertilizers; International Fertilizer Industry Association: France, Paris, 2013; Volume 53. [Google Scholar]
- Da Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes Manual de Análises Químicas de Solos, Plantas e Fertilizantes, 2nd ed.; Embrapa Informação Tecnológica: Brasília, Brasil, 2009. [Google Scholar]
- Novais, R.F.; Neves, J.C.L.; Barros, N.F. Ensaio Em Ambiente Controlado. In Métodos de Pesquisa Em Fertilidade Do Solo; Embrapa-SE: Brasília, Brasil, 1991. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen -Total. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; John Wiley & Sons; Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Avaliação Do Estado Nutricional Das Plantas—Princípios e Aplicações, 2nd ed.; Associação Brasileira para Pesquisa da Potassa e do Fósforo: Piracicaba, Brasil, 1997. [Google Scholar]
- Van Raij, B.; Quaggio, J.A.; da Silva, N.M. Extraction of Phosphorus, Potassium, Calcium, and Magnesium from Soils by an Ion-exchange Resin Procedure. Commun. Soil Sci. Plant Anal. 1986, 17, 547–566. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Cantarella, H.; Trivelin, P.O. Determinacão de Nitrogênio Inorgânico Em Solo Pelo Método Da Destilacão a Vapor. In Análise Química Para Avaliacão Da Fertilidade de Solos Tropicais; Instituto Agronômico: Campinas, Brazil, 2001. [Google Scholar]
- Ferreira, D.F. Sisvar: A Guide for Its Bootstrap Procedures in Multiple Comparisons Sisvar: Um Guia Dos Seus Procedimentos de Comparações Múltiplas Bootstrap. Ciência Agrotecnologia 2014, 38, 109–112. [Google Scholar] [CrossRef]
- Leite, A.d.A.; Cardoso, A.A.d.S.; Leite, R.d.A.; Oliveira-Longatti, S.M.d.; Lustosa Filho, J.F.; de Moreira, F.M.S.; Melo, L.C.A. Selected Bacterial Strains Enhance Phosphorus Availability from Biochar-Based Rock Phosphate Fertilizer. Ann. Microbiol. 2020, 70, 1–13. [Google Scholar] [CrossRef]
- Abdala, D.B.; Moore, P.A.; Rodrigues, M.; Herrera, W.F.; Pavinato, P.S. Long-Term Effects of Alum-Treated Litter, Untreated Litter and NH4NO3application on Phosphorus Speciation, Distribution and Reactivity in Soils Using K-Edge XANES and Chemical Fractionation. J. Environ. Manag. 2018, 213, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Novais, R.F.d.; Smyth, T.J. Fósforo Em Solo e Planta Em Condições Tropicais. Inf. Agronômicas 1999, 87, 10–11. [Google Scholar]
- Ejraei, A.; Aroon, M.A.; Ziarati Saravani, A. Wastewater Treatment Using a Hybrid System Combining Adsorption, Photocatalytic Degradation and Membrane Filtration Processes. J. Water Process Eng. 2019, 28, 45–53. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, X.; Zhang, Q.; Wei, Q.; Liu, X.; Deng, W.; Wang, J.; Yang, W.; Deng, B.; Zhang, L. Biochar Derived from Spent Mushroom Substrate Reduced N2O Emissions with Lower Water Content but Increased CH4 Emissions under Flooded Condition from Fertilized Soils in Camellia Oleifera Plantations. Chemosphere 2022, 287, 132110. [Google Scholar] [CrossRef] [PubMed]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introdução à Espectroscopia, 4th ed.; Cengage Learning: Sao Paulo, Brazil, 2010. [Google Scholar]
- Pan, W.L.; Madsen, I.J.; Bolton, R.P.; Graves, L.; Sistrunk, T. Ammonia/Ammonium Toxicity Root Symptoms Induced by Inorganic and Organic Fertilizers and Placement. Agron. J. 2016, 108, 2485–2492. [Google Scholar] [CrossRef] [Green Version]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of Ammonium Toxicity and the Quest for Tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.K.; Rose, M.T.; Wong, V.N.L.; Cavagnaro, T.R.; Patti, A.F. A Slow Release Brown Coal-Urea Fertiliser Reduced Gaseous N Loss from Soil and Increased Silver Beet Yield and N Uptake. Sci. Total Environ. 2019, 649, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Bornø, M.L.; Müller-Stöver, D.S.; Liu, F. Contrasting Effects of Biochar on Phosphorus Dynamics and Bioavailability in Different Soil Types. Sci. Total Environ. 2018, 627, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K.; Gheyi, H.R.; Moreira, A. Nutrient Bioavailability in Salt Affected Soils. J. Plant Nutr. 2011, 34, 945–962. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Review NH4+ Toxicity in Higher Plants: A Critical Review I. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef] [Green Version]
- Tian, D.; Niu, S. A Global Analysis of Soil Acidification Caused by Nitrogen Addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Hao, T.; Zhu, Q.; Zeng, M.; Shen, J.; Shi, X.; Liu, X.; Zhang, F.; de Vries, W. Impacts of Nitrogen Fertilizer Type and Application Rate on Soil Acidification Rate under a Wheat-Maize Double Cropping System. J. Environ. Manag. 2020, 270, 110888. [Google Scholar] [CrossRef] [PubMed]
- De Vries, W.; Breeuwsma, A. The Relation between Soil Acidification and Element Cycling. Water. Air Soil Pollut. 1987, 35, 293–310. [Google Scholar] [CrossRef]
- Han, J.; Shi, J.; Zeng, L.; Xu, J.; Wu, L. Effects of Nitrogen Fertilization on the Acidity and Salinity of Greenhouse Soils. Environ. Sci. Pollut. Res. 2015, 22, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Ming, G.; Lian, D.; Ling, C.; Hu, S.; Hua, M. Use of Iron Oxide Nanomaterials in Wastewater Treatment: A Review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M.; Nandillon, R.; Miard, F.; Bourgerie, S.; Visser, R.; Morabito, D. Biochar Application Modifies Soil Properties of a Former Mine Technosol: SEM/EDS Study to Investigate Pb and As Speciation. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Court, M.N.; Stephen, C.; Waidj, J.S. Toxicity as a Cause of the Inefficiency of Urea as a Fertilizer. J. Soil Sci. 1964, 15, 49–65. [Google Scholar] [CrossRef]
- Cantarella, H. Nitrogênio. In Fertilidade do Solo; Novais, R., Alvarez, V.H., Barros, N.F., Fontes, R.L., Cantarutti, R.B., Neves, J.C.L., Eds.; Sociedade Brasileira de Ciência do Solo: Viçosa, Brazil, 2007; pp. 375–470. [Google Scholar]
- Bayuelo-Jiménez, J.S.; Craig, R.; Lynch, J.P. Salinity Tolerance of Phaseolus Species during Germination and Early Seedling Growth. Crop Sci. 2002, 42, 1584–1594. [Google Scholar] [CrossRef]
- Santos, S.R.; Filho, J.F.L.; Vergütz, L.; Melo, L.C.A. Biochar Association with Phosphate Fertilizer and Its Influence on Phosphorus Use Efficiency by Maize. Cienc. Agrotecnologia 2019, 43. [Google Scholar] [CrossRef] [Green Version]
- De Freitas, I.F.; Novais, R.F.; de Villani, E.M.A.; Novais, S.V. Phosphorus Extracted by Ion Exchange Resins and Mehlich-1 from Oxisols (Latosols) Treated with Different Phosphorus Rates and Sources for Varied Soil-Source Contact Periods. Rev. Bras. Ciência Solo 2013, 37, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Lustosa Filho, J.F.; Carneiro, J.S.S.; Barbosa, C.F.; Lima, K.P.; Leite, A.A.; Melo, L.C.A. Aging of Biochar-Based Fertilizers in Soil: Effects on Phosphorus Pools and Availability to Urochloa Brizantha Grass. Sci. Total Environ. 2020, 709, 136028. [Google Scholar] [CrossRef] [PubMed]

| Properties | After Liming |
|---|---|
| pH water | 6.3 |
| Ca2+ (cmolc kg−1) | 2.96 |
| Al3+ (cmolc kg−1) | 0.06 |
| H+Al (cmolc kg−1) | 2.35 |
| Mg2+ (cmolc kg−1) | 1.10 |
| Resin P (mg kg−1) | 10.8 |
| P-rem (mg L−1) | 28.2 |
| K (mg kg−1) | 161 |
| SB (cmolc kg−1) | 4.45 |
| t (cmolc kg−1) | 4.51 |
| T (cmolc kg−1) | 6.80 |
| m (%) | 1.33 |
| V (%) | 65 |
| Organic matter (%) | 2.21 |
| Clay (%) | 47 |
| Silt (%) | 11 |
| Sand (%) | 42 |
| Property | PL | PLB |
|---|---|---|
| pH | 8.2 ± 0.02 a | 6.1 ± 0.1 |
| EC (dS m−1) | 4.03 ± 0.04 b | 0.46 ± 0.07 |
| Carbon (%) | 36.1 ± 0.2 a | 24. 9 ± 1.0 |
| Total content (g kg−1) | ||
| P | 15.6 ± 0.4 a | 164 ± 2 |
| Mg | 5.40 ± 0.20 a | 96.1 ± 3.1 |
| P solubility (g kg−1) | ||
| Water soluble | - | 6.13 ± 0.12 |
| Citric acid soluble | - | 72.8 ± 3.4 |
| NAC c + water-soluble | - | 142 ± 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, C.F.; Correa, D.A.; Carneiro, J.S.d.S.; Melo, L.C.A. Biochar Phosphate Fertilizer Loaded with Urea Preserves Available Nitrogen Longer than Conventional Urea. Sustainability 2022, 14, 686. https://doi.org/10.3390/su14020686
Barbosa CF, Correa DA, Carneiro JSdS, Melo LCA. Biochar Phosphate Fertilizer Loaded with Urea Preserves Available Nitrogen Longer than Conventional Urea. Sustainability. 2022; 14(2):686. https://doi.org/10.3390/su14020686
Chicago/Turabian StyleBarbosa, Cristiane Francisca, Dehon Aparecido Correa, Jefferson Santana da Silva Carneiro, and Leônidas Carrijo Azevedo Melo. 2022. "Biochar Phosphate Fertilizer Loaded with Urea Preserves Available Nitrogen Longer than Conventional Urea" Sustainability 14, no. 2: 686. https://doi.org/10.3390/su14020686
APA StyleBarbosa, C. F., Correa, D. A., Carneiro, J. S. d. S., & Melo, L. C. A. (2022). Biochar Phosphate Fertilizer Loaded with Urea Preserves Available Nitrogen Longer than Conventional Urea. Sustainability, 14(2), 686. https://doi.org/10.3390/su14020686






