Potassium Determines Sugar Beets’ Yield and Sugar Content under Drip Irrigation Condition
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Materials
2.2. Experimental Design
2.3. Crop Sampling and Analysis
2.4. Statistical Analysis
3. Results
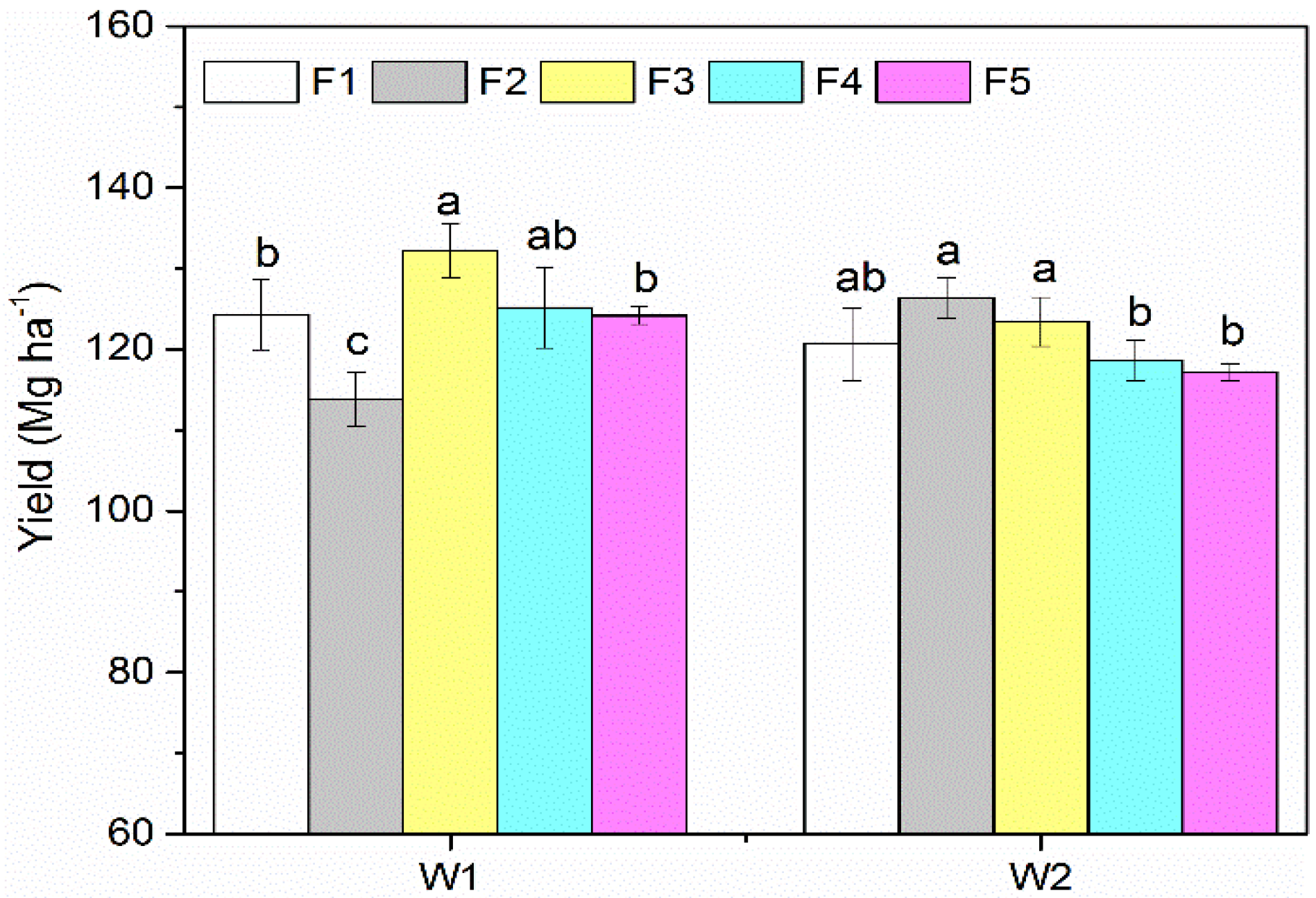
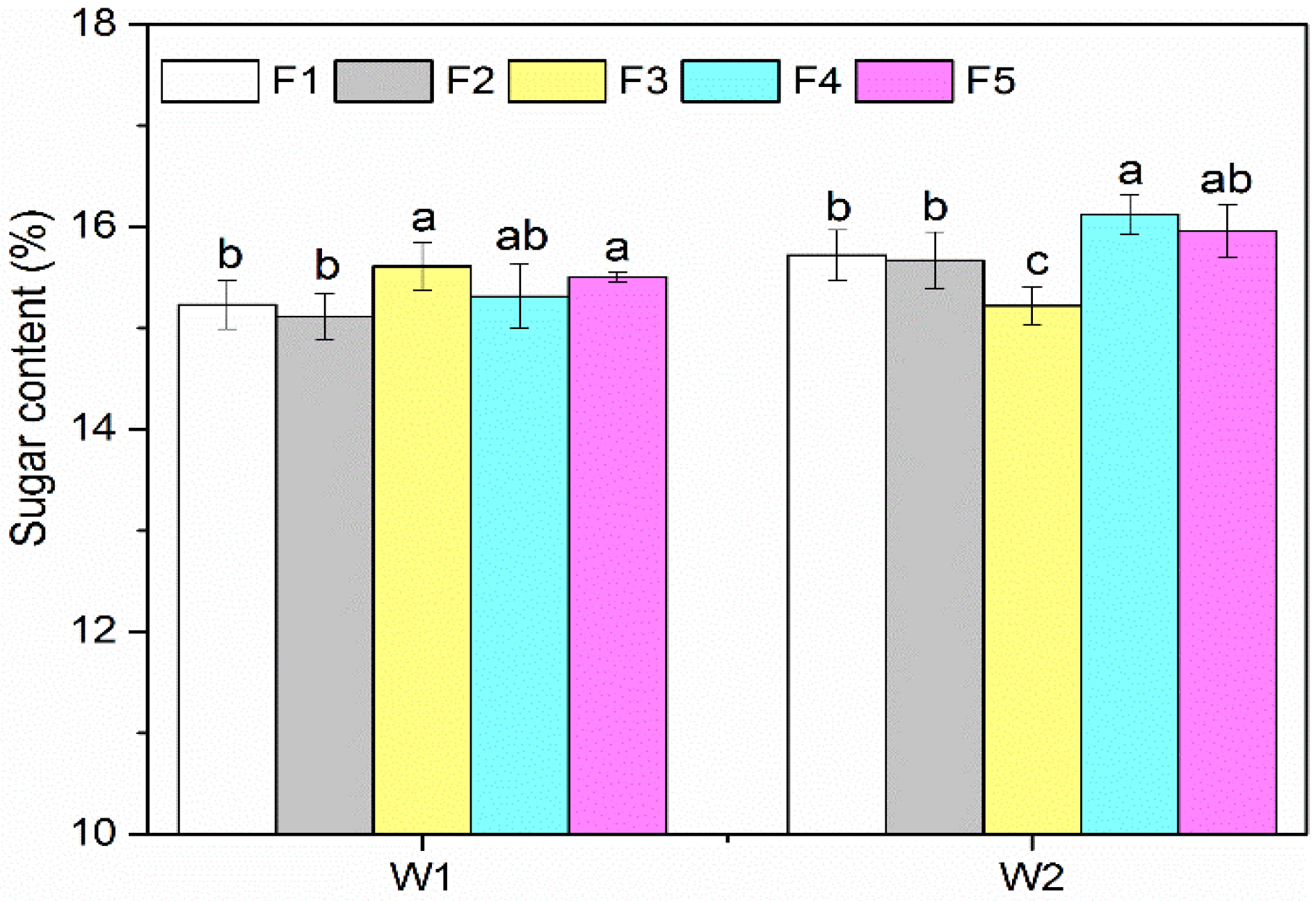
3.1. Yield and Sugar Content
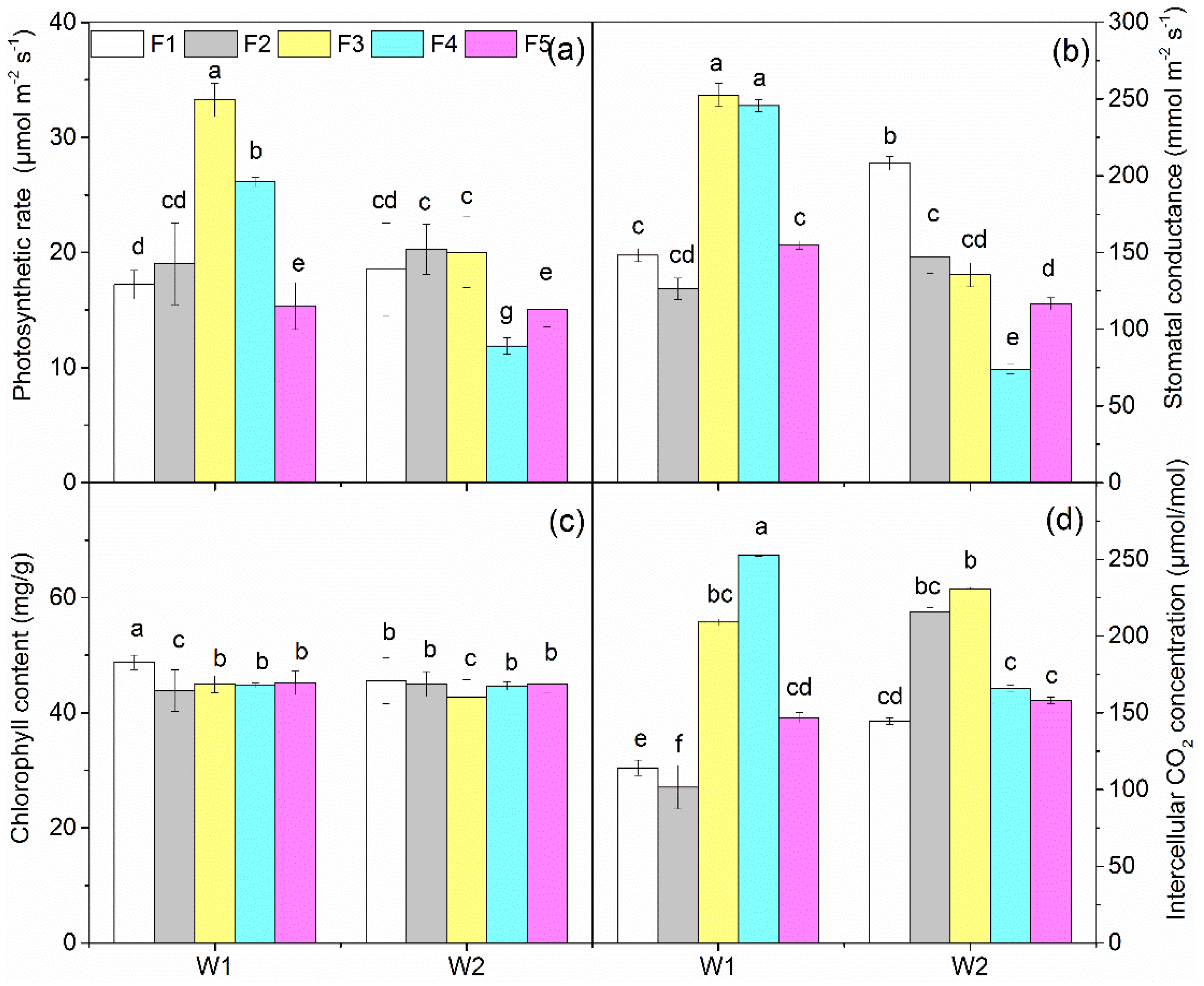
3.2. Crop Growth
3.3. Mineral Nitrogen-, Phosphorus-, and Potassium-Use Efficiency
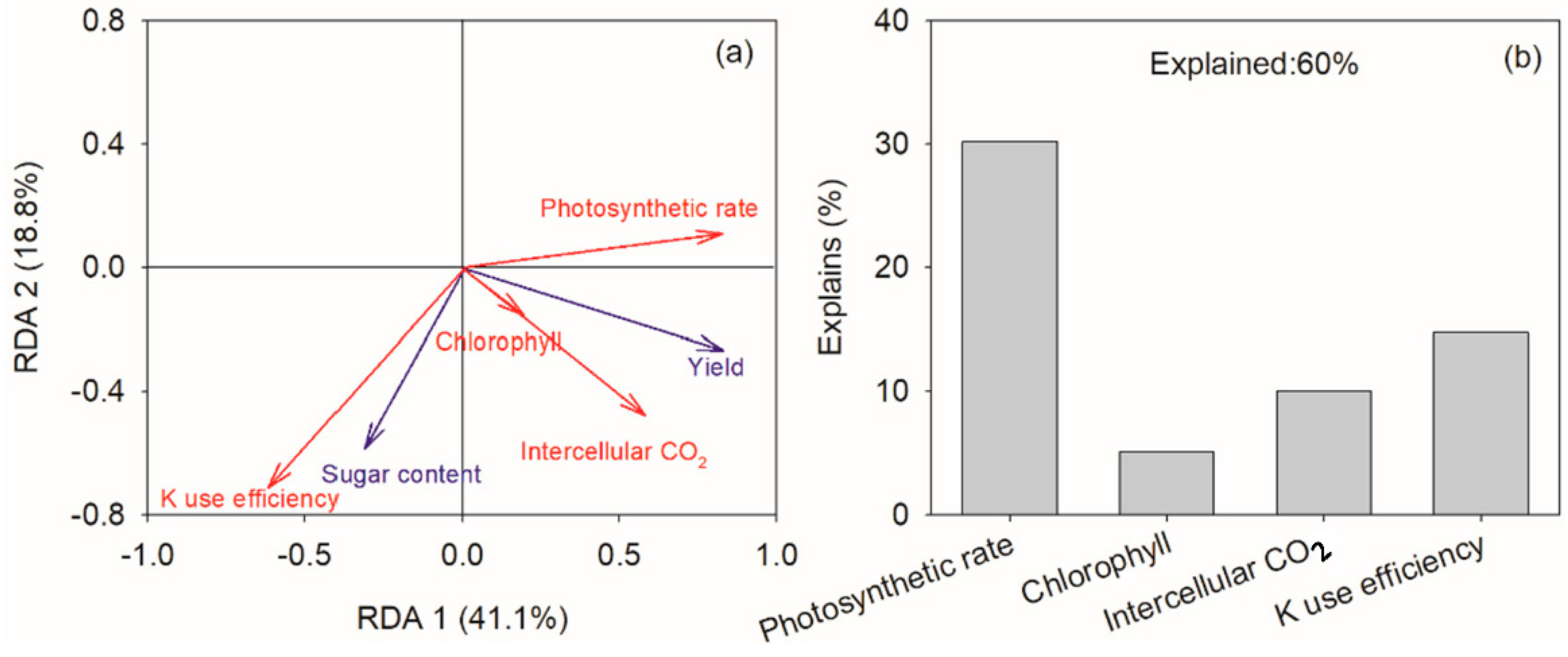
3.4. Driving Factors of Yield and Sugar Content
4. Discussion
4.1. Effects of Different Irrigation Levels and Fertilization Treatments on Yield, Sugar Content, and Growth Parameters
4.2. The Predominant Role of K in Sugar Beet Yield and Sugar Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hua, F.; Yangyang, L.; Cong, F.; Peishu, H.; Kaiyong, W. Energy-Use Efficiency and Economic Analysis of Sugar Beet Production in China: A Case Study in Xinjiang Province. Sugar Tech 2016, 18, 309–316. [Google Scholar] [CrossRef]
- Sagar, G.R.; Salter, P.J.; Goode, J.E. Crop Responses to Water at Different Stages of Growth. J. Ecol. 1967, 56, 626. [Google Scholar] [CrossRef]
- Ghamarnia, H.; Arji, I.; Sepehri, S.; Norozpour, S.; Khodaei, E. Evaluation and Comparison of Drip and Conventional Irrigation Methods on Sugar Beets in a Semiarid Region. J. Irrig. Drain. Eng. 2011, 138, 90–97. [Google Scholar] [CrossRef]
- Zhang, H.; Bai, C.; Wang, H.Z.; Zhang, H.Z.; Xiao, L.I.; Zeng, F.U. Research progress of proteomics in sugar beet. Sugar Crops China 2017, 39, 58–61+74. [Google Scholar]
- Cui, J. On the development and utilization of water resources and ecological environment in Xinjiang. West. Expl. Proj. 2018, 8, 129–140. [Google Scholar]
- Gençoğlan, C.; Altunbey, H.; Gençoğlan, S. Response of green bean (P. vulgaris L.) to subsurface drip irrigation and partial rootzone-drying irrigation. Agric. Water Manag. 2006, 84, 274–280. [Google Scholar] [CrossRef]
- Bhardwaj, S. Importance of drip irrigation in Indian agriculture. Kissan World 2001, 28, 32–33. [Google Scholar]
- Janat, M. Effect of drip fertigation on improvement of potato yield and water use efficiency. AECS-A/RRE 2003, 126, 1–38. [Google Scholar]
- Rahil, M.; Antonopoulos, V. Simulating soil water flow and nitrogen dynamics in a sunflower field irrigated with reclaimed wastewater. Agric. Water Manag. 2007, 92, 142–150. [Google Scholar] [CrossRef]
- Kuchaki, A.; Soltani, A. Sugar Beet Agronomy; Mashhad University Publisher: Mashhad, Iran, 1995. [Google Scholar]
- Allen, G.R.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration-Guidelines for computing crop water re-quirements-FAO Irrigation and drainage paper 56. Fao Rome 1998, 300, D05109. [Google Scholar]
- Kiymaz, S.; Ertek, A. Yield and quality of sugar beet (Beta vulgaris L.) at different water and nitrogen levels under the climatic conditions of Kırsehir, Turkey. Agric. Water Manag. 2015, 158, 156–165. [Google Scholar] [CrossRef]
- Kirda, C. Deficit Irrigation Scheduling Based on Plant Growth Stages Showing Water Stress Tolerance; Deficit Irrigation Practices, Water Reports; Food and Agricultural Organization of the United Nations: Rome, Italy, 2002; Volume 22, 102p. [Google Scholar]
- Saif, U.; Maqsood, M.; Farooq, M.; Hussain, S.; Habib, A. Effect of planting patterns and different irrigation levels on yield and yield component of maize (Zea mays L.). Int. J. Agric. Biol. 2003, 1, 64–66. [Google Scholar]
- Li, Z.; Liu, M.; Wu, X.; Han, F.; Zhang, T. Effects of long-term chemical fertilization and organic amendments on dynamics of soil organic C and total N in paddy soil derived from barren land in subtropical China. Soil Tillage Res. 2010, 106, 268–274. [Google Scholar] [CrossRef]
- Zhu, Z.; Ge, T.; Liu, S.; Hu, Y.; Ye, R.; Xiao, M.; Tong, C.; Kuzyakov, Y.; Wu, J. Rice rhizodeposits affect organic matter priming in paddy soil: The role of N fertilization and plant growth for enzyme activities, CO2 and CH4 emissions. Soil Biol. Biochem. 2018, 116, 369–377. [Google Scholar] [CrossRef]
- Ouda, S.M. Effect of nitrogen and sulpher fertilizers levels on sugar beet in newly cultivated sandy soil. Zagazig J. Agric. Res. 2003, 29, 33–50. [Google Scholar]
- Abdel-Motagally, F.M.; Attia, K.K. Response of sugar beet plants to nitrogen and potassium fertilization in sandy calcareous soil. Int. J. Agric. Biol. 2009, 11, 695–700. [Google Scholar]
- Sharifi, H.; Hosseinpor, M.; Rahnama, A. Effect of irrigation termination before harvest and late nitrogen applica-tion on sugar beet, yield and quality and root rot in Dezful. Sugar Beet J. 2002, 17, 86–98. [Google Scholar]
- Vazifedoust, M.; van Dam, J.; Feddes, R.; Feizi, M. Increasing water productivity of irrigated crops under limited water supply at field scale. Agric. Water Manag. 2008, 95, 89–102. [Google Scholar] [CrossRef]
- Tsialtas, J.; Maslaris, N. Nitrogen effects on yield, quality and K/Na selectivity of sugar beets grown on clays under semi-arid, irrigated conditions. Int. J. Plant Prod. 2013, 7, 355–372. [Google Scholar] [CrossRef]
- Huang, S.; Peng, X.; Huang, Q.; Zhang, W. Soil aggregation and organic carbon fractions affected by long-term fer-tilization in a red soil of subtropical China. Geoderma 2010, 154, 364–369. [Google Scholar] [CrossRef]
- Aujla, M.; Thind, H.; Buttar, G. Fruit yield and water use efficiency of eggplant (Solanum melongema L.) as influ-enced by different quantities of nitrogen and water applied through drip and furrow irrigation. Sci. Hortic. 2007, 112, 142–148. [Google Scholar] [CrossRef]
- Kuscu, H.; Turhan, A.; Ozmen, N.; Aydinol, P.; Demir, A.O. Optimizing levels of water and nitrogen applied through drip irrigation for yield, quality, and water productivity of processing tomato (Lycopersicon esculentum Mill.). Hortic. Environ. Biotechnol. 2014, 55, 103–114. [Google Scholar] [CrossRef]
- Radin, J.W.; Matthews, M.A. Water transport properties of cortical cells in roots of nitrogen-and phospho-rus-deficient cotton seedlings. Plant Physiol. 1989, 89, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Füllgrabe, H.; Claassen, N.; Hilmer, R.; Koch, H.; Dittert, K.; Kreszies, T. Potassium deficiency reduces sugar yield in sugar beet through decreased growth of young plants. J. Plant Nutr. Soil Sci. 2022. [Google Scholar] [CrossRef]
- Hu, W.; Coomer, T.D.; Loka, D.A.; Oosterhuis, D.M.; Zhou, Z. Potassium deficiency affects the carbon-nitrogen balance in cotton leaves. Plant Physiol. Biochem. 2017, 115, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Humble, G.; Raschke, K. Stomatal opening quantitatively related to potassium transport: Evidence from electron probe analysis. Plant Physiol. 1971, 48, 447–453. [Google Scholar] [CrossRef]
- Kaiser, W.M. Correlation between changes in photosynthetic activity and changes in total protoplast volume in leaf tissue from hygro-, meso- and xerophytes under osmotic stress. Planta 1982, 154, 538–545. [Google Scholar] [CrossRef]
- DU, Y.-D.; Cao, H.-X.; Liu, S.-Q.; Gu, X.; Cao, Y.-X. Response of yield, quality, water and nitrogen use efficiency of tomato to different levels of water and nitrogen under drip irrigation in Northwestern China. J. Integr. Agric. 2017, 16, 1153–1161. [Google Scholar] [CrossRef]
- El-Mageed, T.A.A.; Mekdad, A.A.A.; Rady, M.O.A.; Abdelbaky, A.S.; Saudy, H.S.; Shaaban, A. Physio-biochemical and Agronomic Changes of Two Sugar Beet Cultivars Grown in Saline Soil as Influenced by Potassium Fertilizer. J. Soil Sci. Plant Nutr. 2022, 1–19. [Google Scholar] [CrossRef]
- Hadir, S.; Gaiser, T.; Hüging, H.; Athmann, M.; Pfarr, D.; Kemper, R.; Ewert, F.; Seidel, S. Sugar beet shoot and root pheno-typic plasticity to nitrogen, phosphorus, potassium and lime omission. Agriculture 2020, 11, 21. [Google Scholar] [CrossRef]
- Campbell, L.G.; Kern, J.J. Relationships among components of yield and quality of sugarbeets. J. Am. Soc. Sugar Beet Technol. 1983. Available online: https://bsdf-assbt.org/wp-content/uploads/2017/04/JSBRVol22No2P135to145RelationshipsAmongComponentsofYieldandQualityofSugarbeets.pdf (accessed on 1 August 2022). [CrossRef]
- WRB, I.W.G. World reference base for soil resources 2014: International soil classification system for naming soils and creating legends for soil maps. World Soil Resour. Rep. 2014, 106, 12–21. [Google Scholar]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis: Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 1982. [Google Scholar]
- Klute, A.; Dirksen, C. Hydraulic conductivity and diffusivity: Laboratory methods. Methods Soil Anal. Part 1 Phys. Mineral. Methods 1986, 5, 687–734. [Google Scholar]
- Afshar, R.K.; Nilahyane, A.; Chen, C.; He, H.; Stevens, W.B.; Iversen, W.M. Impact of conservation tillage and ni-trogen on sugarbeet yield and quality. Soil Tillage Res. 2019, 191, 216–223. [Google Scholar] [CrossRef]
- Afshar, R.K.; Chen, C.; Eckhoff, J.; Flynn, C. Impact of a living mulch cover crop on sugarbeet establishment, root yield and sucrose purity. Field Crops Res. 2018, 223, 150–154. [Google Scholar] [CrossRef]
- Watson, D.J. Comparative Physiological Studies on the Growth of Field Crops: I. Variation in Net Assimilation Rate and Leaf Area between Species and Varieties, and within and between Years. Ann. Bot. 1947, 11, 41–76. [Google Scholar] [CrossRef]
- López-Bellido, R.; López-Bellido, L. Efficiency of nitrogen in wheat under Mediterranean conditions: Effect of tillage, crop rotation and N fertilization. Field Crops Res. 2001, 71, 31–46. [Google Scholar] [CrossRef]
- Yavuz, D.; Seymen, M.; Yavuz, N.; Türkmen, Ö. Effects of irrigation interval and quantity on the yield and quality of confectionary pumpkin grown under field conditions. Agric. Water Manag. 2015, 159, 290–298. [Google Scholar] [CrossRef]
- Chen, J.; Kang, S.; Du, T.; Qiu, R.; Guo, P.; Chen, R. Quantitative response of greenhouse tomato yield and quality to water deficit at different growth stages. Agric. Water Manag. 2013, 129, 152–162. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, K.L.; Fan, Z.B.; Wei, Y.P.; Lin, S.; Wang, J.G. Simulating the fate of nitrogen and optimizing water and nitrogen management of greenhouse tomato in North China using the EU-Rotate_N model. Agric. Water Manag. 2013, 128, 72–84. [Google Scholar] [CrossRef]
- Mitchell, J.; Shennan, C.; Grattan, S.; May, D. Tomato fruit yields and quality under water deficit and salinity. J. Am. Soc. Hortic. Sci. 1991, 116, 215–221. [Google Scholar] [CrossRef]
- Guichard, S.; Gary, C.; Longuenesse, J.; Leonardi, C. Water fluxes and growth of greenhouse tomato fruits under summer conditions. In III International Workshop on Models for Plant Growth and Control of the Shoot and Root Environments in Greenhouses; ISHS: Leuven, Belgium, 1999; Volume 507, pp. 223–230. [Google Scholar]
- Hamzei, J.; Soltani, J. Deficit irrigation of rapeseed for water-saving: Effects on biomass accumulation, light interception and radiation use efficiency under different N rates. Agric. Ecosyst. Environ. 2012, 155, 153–160. [Google Scholar] [CrossRef]
- Li, X.; FLiu GLi, Q.L.i.n.; Jensen, C.R. Soil microbial response, water and nitrogen use by tomato under different irrigation regimes. Agric. Water Manag. 2010, 98, 414–418. [Google Scholar] [CrossRef]
- Masri, M.I.; Ramadan, B.S.B.; El-Shafai, A.M.A.; El-Kady, M.S. Effect of water stress and fertilization on yield and quality of sugar beet under drip and sprinkler irrigation systems in sandy soil. Int. J. Agric. Sci. 2015, 5, 414–425. [Google Scholar]
- Schmehl, W.R.; Finkner, R.; Swink, J. Effect of Nitrogen Fertilization on Yield and Quality of Sugar beets. J. Sugar Beet Res. 1963, 12, 538–544. [Google Scholar] [CrossRef]
- Jahedi, A.; Noorozi, A.; Hasani, M.; Hamdi, F. Effect of irrigation methods and nitrogen application on sugar beet yield and quality. J. Sugar Beet 2012, 28, 23–28. [Google Scholar]
- Mofokeng, M.; Steyn, J.; Plooy, C.D.; Prinsloo, G.; Araya, H. Growth of Pelargonium sidoides DC. in response to water and nitrogen level. S. Afr. J. Bot. 2015, 100, 183–189. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, J. Controlled alternate partial root-zone irrigation: Its physiological consequences and impact on water use efficiency. J. Exp. Bot. 2004, 55, 2437–2446. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y. Study on effects of fertilizing on crop yield and its mechanism to raise water use efficiency. Res. Soil Water Conserv. 1996, 3, 185–191. [Google Scholar]
- Yang, Q.; Zhang, F.; Li, F. Effect of different drip irrigation methods and fertilization on growth, physiology and water use of young apple tree. Sci. Hortic. 2011, 129, 119–126. [Google Scholar] [CrossRef]
- Song, X.-Z.; Zhao, C.-X.; Wang, X.-L.; Li, J. Study of nitrate leaching and nitrogen fate under intensive vegetable production pattern in northern China. Comptes Rendus. Biol. 2009, 332, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Xu, Z.; Wei-Ming, S.; Guang-Xi, X.; Zhao-Liang, Z. Nitrogen balance and loss in a greenhouse vegetable system in southeastern China. Pedosphere 2011, 21, 464–472. [Google Scholar]
- Scholberg, J.; Parsons, L.; Wheaton, T.; McNeal, B.; Morgan, K. Soil temperature, nitrogen concentration, and resi-dence time affect nitrogen uptake efficiency in citrus. J. Environ. Qual. 2002, 31, 759–768. [Google Scholar] [CrossRef] [PubMed]
- El-Hadidi, E.; Aziz, H.A.; Enab, M. Efficient Utilization of Nitrogen by Sugar Beet Irrigated with Saline Water under Fertilizer Rates and Water Regime Using 15N Tracer Technique. J. Soil Sci. Agric. Eng. 2017, 8, 655–660. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.; Lovelock, C.; Plaxton, W.; Price, C.A.; Scheible, W.-R.; Shane, M.W.; White, P.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Massonneau, A.; Martinoia, E.; Römheld, V. Physiological adaptations to phosphorus deficiency during proteoid root development in white lupin. Planta 1999, 208, 373–382. [Google Scholar] [CrossRef]
- Shenoy, V.; Kalagudi, G. Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnol. Adv. 2005, 23, 501–513. [Google Scholar] [CrossRef]
- White, P.J. Improving potassium acquisition and utilisation by crop plants. J. Plant Nutr. Soil Sci. 2013, 176, 305–316. [Google Scholar] [CrossRef]
- Marschner, H. Mineral nutrition of higher plants. J. Ecol. 1995, 76, 1250. [Google Scholar]
- Arquero, O.; Barranco, D.; Benlloch, M. Potassium Starvation Increases Stomatal Conductance in Olive Trees. HortScience 2006, 41, 433–436. [Google Scholar] [CrossRef]
- Yang, X.E.; Liu, J.X.; Wang, W.M.; Ye, Z.Q.; Luo, A.C. Potassium Internal Use Efficiency Relative to Growth Vigor, Potassium Distribution, and Carbohydrate Allocation in Rice Genotypes. J. Plant Nutr. 2004, 27, 837–852. [Google Scholar] [CrossRef]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y.; Saifullah; Ahmad, M. Improving agricultural water use efficiency by nu-trient management in crop plants. Acta Agric. Scand. Sect. B-Soil Plant Sci. 2011, 61, 291–304. [Google Scholar]
- Chatterjee, A.; Subedi, K.; Franzen, D.; Mickelson, H.; Cattanach, N. Nitrogen Fertilizer Optimization for Sugarbeet in the Red River Valley of North Dakota and Minnesota. Agron. J. 2018, 110, 1554–1560. [Google Scholar] [CrossRef]
- Kaiser, W.M. Mineral Nutrition of Plants: Principles and Perspectives; Epstein, E., Bloom, A., Eds.; Sinauer Associates, Inc. Publishers: Sunderland, MA, USA, 2004; 400p, ISBN 0-87893-172-4. [Google Scholar]
- Topak, R.; Acar, B.; Uyanöz, R.; Ceyhan, E. Performance of partial root-zone drip irrigation for sugar beet produc-tion in a semi-arid area. Agric. Water Manag. 2016, 176, 180–190. [Google Scholar] [CrossRef]
- Saykhul, A.; Chatzistathis, T.; Chatzissavvidis, C.; Koundouras, S.; Therios, I.; Dimassi, K. Potassium utilization effi-ciency of three olive cultivars grown in a hydroponic system. Sci. Hortic. 2013, 162, 55–62. [Google Scholar] [CrossRef]
- George, M.S.; Lu, G.; Zhou, W. Genotypic variation for potassium uptake and utilization efficiency in sweet potato (Ipomoea batatas L.). Field Crops Res. 2002, 77, 7–15. [Google Scholar] [CrossRef]
- Wang, X.; Mohamed, I.; Xia, Y.; Chen, F. Effects of water and potassium stresses on potassium utilization efficiency of two cotton genotypes. J. Soil Sci. Plant Nutr. 2014, 14, 833–844. [Google Scholar] [CrossRef]
- Naumann, J.C.; Young, D.R.; Anderson, J.E. Linking leaf chlorophyll fluorescence properties to physiological responses for detection of salt and drought stress in coastal plant species. Physiol. Plant. 2007, 131, 422–433. [Google Scholar] [CrossRef]
- Chaves, M.M.; Pereira, J.S.; Marôco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How Plants Cope with Water Stress in the Field? Photosynthesis and Growth. Ann. Bot. 2002, 89, 907–916. [Google Scholar] [CrossRef]
- Centritto, M.; Loreto, F.; Chartzoulakis, K. The use of low [CO2] to estimate diffusional and non-diffusional limita-tions of photosynthetic capacity of salt-stressed olive saplings. Plant Cell Environ. 2003, 26, 585–594. [Google Scholar] [CrossRef]
- Xia, Y.; Jiang, C.; Wang, X.; Chen, F. Studies on potassium uptake and use efficiency of different cotton (Gossypium hirsutum L.) genotypes by grafting. J. Food Agric. Environ. 2013, 11, 472–476. [Google Scholar]
- Cassman, K.G.; Kerby, T.A.; Roberts, B.A.; Bryant, D.C.; Brouder, S.M. Differential Response of Two Cotton Cultivars to Fertilizer and Soil Potassium. Agron. J. 1989, 81, 870–876. [Google Scholar] [CrossRef]
- Rengel, Z.; Damon, P.M. Crops and genotypes differ in efficiency of potassium uptake and use. Physiol. Plant. 2008, 133, 624–636. [Google Scholar] [CrossRef]
- Gerendás, J.; Abbadi, J.; Sattelmacher, B. Potassium efficiency of safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.). J. Plant Nutr. Soil Sci. 2008, 171, 431–439. [Google Scholar] [CrossRef]
| Fertilization | Inorganic N (kg ha−1) | Inorganic P (kg ha−1) | Inorganic K (kg ha−1) | Hydroquinone (kg ha−1) | Microbial Fertilizer (kg ha−1) |
|---|---|---|---|---|---|
| F1 | 300 | 270 | 150 | 0 | 0 |
| F2 | 270 | 180 | 202.5 | 0 | 0 |
| F3 | 229.5 | 180 | 202.5 | 0.2295 | 0 |
| F4 | 229.5 | 180 | 202.5 | 0 | 300 |
| F5 | 67.5 | 180 | 202.5 | 0 | 0 |
| Irrigation | Fertilization | Seedling Stage | Luxuriant Foliage Stage | Expanding Stage | Sugar Accumulation |
|---|---|---|---|---|---|
| W1 | F1 | 39.45 ± 5.61 bcd | 29.42 ± 4.48 cd | 42.28 ± 3.25 abcd | 35.95 ± 11.22 bcd |
| F2 | 42.90 ± 8.09 abcd | 34.47 ± 7.15 bcd | 41.94 ± 3.98 abcd | 41.54 ± 0.84 abcd | |
| F3 | 36.04 ± 15.56 bcd | 31.46 ± 2.54 cd | 57.83 ± 8.29 ab | 65.49 ± 37.44 a | |
| F4 | 28.65 ± 8.21 d | 33.62 ± 13.34 bcd | 45.16 ± 5.99 abcd | 53.96 ± 14.37 abc | |
| F5 | / | / | / | / | |
| W2 | F1 | 38.78 ± 7.11 abc | 28.08 ± 9.61 c | 39.18 ± 5.30 abc | 43.98 ± 20.06 abc |
| F2 | 49.75 ± 20.07 abc | 41.63 ± 11.18 abc | 58.45 ± 3.43 abc | 62.13 ± 12.04 bc | |
| F3 | 33.35 ± 29.03 bc | 27.74 ± 11.05 c | 64.47 ± 6.31 a | 47.34 ± 11.04 abc | |
| F4 | 46.28 ± 27.73 abc | 27.77 ± 15.74 c | 55.11 ± 16.93 abc | 56.54 ± 15.17 abc | |
| F5 | / | / | / | / |
| Irrigation | Fertilization | Seedling Stage | Luxuriant Foliage Stage | Expanding Stage | Sugar Accumulation |
|---|---|---|---|---|---|
| W1 | F1 | 5.02 ± 0.7 cd | 4.92 ± 0.21 cd | 6.71 ± 0.94 abc | 3.78 ± 1.13 d |
| F2 | 7.82 ± 2.49 ab | 5.33 ± 0.51 cd | 5.56 ± 0.58 bcd | 5.12 ± 1.09 cd | |
| F3 | 4.77 ± 1.77 cd | 5.10 ± 0.7 cd | 6.49 ± 0.62 abc | 7.07 ± 1.57 bcd | |
| F4 | 3.77 ± 1.74 d | 5.17 ± 1.41 cd | 6.18 ± 0.93 abcd | 5.84 ± 1.57 abcd | |
| F5 | 6.20 ± 1.74 abcd | 6.05 ± 0.65 abcd | 8.35 ± 0.78 a | 5.89 ± 1.21 abcd | |
| W2 | F1 | 5.84 ± 1.46 abcd | 4.68 ± 1.10 bcd | 5.83 ± 0.91 abcd | 5.42 ± 2.00 abcd |
| F2 | 6.52 ± 2.46 abcd | 7.18 ± 1.84 abcd | 8.36 ± 1.31 a | 7.45 ± 1.40 ab | |
| F3 | 4.13 ± 3.04 d | 4.28 ± 1.16 bcd | 7.21 ± 0.45 abcd | 6.02 ± 0.79 abcd | |
| F4 | 6.06 ± 2.80 abcd | 4.13 ± 1.79 d | 5.67 ± 1.62 abcd | 6.99 ± 0.41 abcd | |
| F5 | 4.20 ± 1.20 cd | 4.46 ± 1.81 bcd | 7.42 ± 1.15 abc | 6.11 ± 0.62 abcd |
| Irrigation | Fertilization | Seedling Stage | Luxuriant Foliage Stage | Expanding Stage | Sugar Accumulation |
|---|---|---|---|---|---|
| W1 | F1 | 58.96 ± 11.9 defg | 50.93 ± 3.54 efg | 101 ± 16.47 abc | 84.93 ± 23 bcde |
| F2 | 57.17 ± 15 defg | 46.19 ± 9.54 fg | 66.06 ± 12 cdefg | 76.88 ± 18 bcde | |
| F3 | 44.45 ± 23.70 fg | 35.87 ± 2.46 g | 98.24 ± 22.68 abc | 128.10 ± 40.92 a | |
| F4 | 32.62 ± 11.67 g | 35.31 ± 8.80 g | 74.55 ± 19 bcdef | 104.8 ± 37.35 ab | |
| F5 | 55.18 ± 14.8 defg | 43.01 ± 7.22 fg | 84.16 ± 3.32 bcde | 88.95 ± 18 bcd | |
| W2 | F1 | 61.38 ± 4.31 bcde | 47.50 ± 13.20 de | 94.02 ± 12.64 ab | 93.29 ± 34.63 ab |
| F2 | 64.24 ± 23 bcde | 52.74 ± 13 cde | 94.83 ± 16.85 ab | 121.77 ± 27.95 a | |
| F3 | 43.95 ± 39.65 e | 32.16 ± 12.76 e | 107.47 ± 14.75 a | 99.63 ± 22.39 ab | |
| F4 | 62.11 ± 31 bcde | 31.12 ± 14.80 e | 89.47 ± 21.90 abc | 116.98 ± 12.93 a | |
| F5 | 40.11 ± 15.07 e | 32.78 ± 16.24 e | 90.57 ± 9.02 abc | 84.69 ± 4.8 abcd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Zhu, Q.; Xu, Y.; Ma, X.; Ding, F.; Li, G. Potassium Determines Sugar Beets’ Yield and Sugar Content under Drip Irrigation Condition. Sustainability 2022, 14, 12520. https://doi.org/10.3390/su141912520
Xie X, Zhu Q, Xu Y, Ma X, Ding F, Li G. Potassium Determines Sugar Beets’ Yield and Sugar Content under Drip Irrigation Condition. Sustainability. 2022; 14(19):12520. https://doi.org/10.3390/su141912520
Chicago/Turabian StyleXie, Xiangwen, Qianqian Zhu, Yongmei Xu, Xiaopeng Ma, Feng Ding, and Guangyong Li. 2022. "Potassium Determines Sugar Beets’ Yield and Sugar Content under Drip Irrigation Condition" Sustainability 14, no. 19: 12520. https://doi.org/10.3390/su141912520
APA StyleXie, X., Zhu, Q., Xu, Y., Ma, X., Ding, F., & Li, G. (2022). Potassium Determines Sugar Beets’ Yield and Sugar Content under Drip Irrigation Condition. Sustainability, 14(19), 12520. https://doi.org/10.3390/su141912520





