Abstract
Long-term use of chemical fertilizers is affecting the environment, soil quality, and biodiversity. Organic agriculture is gaining global attention by using microbial-based biofertilizers. Carriers protect microbes by providing nutrition, energy, and suitable conditions for their survival while entering the natural environments. The purpose of this study was to evaluate the ability of different carrier materials to enhance the yield and the quality of spinach and to select the best carrier material for spinach biofertilizer. Three pre-isolated and characterized bacterial strains (AN-35, ZM-27, and ZM-63) were tested for their compatibility and used in this experiment through seed inoculation with organic carriers, i.e., compost, peat, press mud, biochar, and charcoal. A pot experiment and a field experiment were conducted to evaluate the efficacy of different organic carriers. The results of the pot study showed a significant increase in spinach growth, i.e., shoot length (25%), shoot fresh weight (24%), root length (25%), and root fresh weight (29%), spinach nutrition, i.e., nitrogen (18%), phosphorus (22%), potassium (15%), iron (17%), and zinc (14%), spinach physiology, i.e., relative water content (27%), chlorophyll content (9%), and the membrane stability index (28%) under peat coated treatments with 24% more soil microbial populations compared to the control. Similarly, in the field experiment, peat coating significantly enhanced spinach growth, i.e., shoot length (29%), shoot fresh weight (23%), root length (16%), and root fresh weight (24.7%), spinach nutrition, i.e., nitrogen (16%), phosphorus (19%), potassium (15%), iron (17%), and zinc (23%), spinach physiology, i.e., relative water content (28%), chlorophyll content (13%) and the membrane stability index (32%), and spinach yield per hectare (30%), as well as producing 20% higher soil microbial populations. From these results, it is concluded that peat is a good carrier material for biofertilizer production as it not only enhances crop production but also the microbial number, in addition to improving soil quality.
1. Introduction
By 2050, the world population is predicted to increase by 9 billion. To feed this burgeoning population, we need to double our crop production through the adaptation of new and eco-friendly technologies, focusing on the microenvironment of the rhizosphere [1]. The availability of suitable environmental conditions and fertile and healthy soils are the key factors involved in achieving this goal [2]. Chemical fertilizers have gained popularity due to their higher available nutrient contents, and because they meet the nutrient requirements of the high-yielding exhaustive crops. Approximately 53 billion tons of chemical fertilizers are applied every year for the provision of essential nutrients to plants, but a greater proportion of these minerals becomes fixed and unavailable for plant uptake [1]. The nutrient fixation by soil demands that fertilizers be used at higher rates, and this non-judicious use has a detrimental impact on the environment, as well as increasing input costs [3]. Moreover, the introduction of heavy metal residues and the rapid degradation of organic matter further lower the quality of the soil by reducing soil biodiversity [2,4].
Sustainable agricultural production demands the integration of different organic and inorganic nutrient sources. Among the organic sources, microbial-based biofertilizers have recently become more popular than chemical fertilizers [5]. Microbial biofertilizers contain living cells of beneficial microbes with crop-growth-enhancing traits, which work in the plant’s rhizosphere to enhance soil fertility, nutrient uptake, and crop productivity [6]. These tiny creatures perform key functions in the soil, such as organic matter degradation, improving nutrient availability and the synthesis and secretions of phytohormones, and inducing systemic resistance under biotic and abiotic stresses [7,8]. Organic amendments provide dual benefits to the farmers, being both economical and environment friendly [9]. Furthermore, the desired results from a product depend upon its formulation and application methods [10,11,12]. As such, biofertilizer formulation should mark the standards of biofertilizers’ shelf lives and their effectiveness [13,14].
Biofertilizers can be divided into two major categories, including solid- and liquid-based formulations [15,16]. The solution form contains microbial cultures, liquids such as water, oils, polymers, etc., and some adhesive materials [17]. Lee et al. [17] described solid formulations as a combination of plant-growth-promoting rhizobacteria (PGPR) culture and solid organic or inorganic carrier materials. Organic carriers may include peat moss [16,18], a mixture of fertile soil and biogas slurry [19,20], clay soil [21], and biochar material [22]. Carrier materials should be economically safe, non-toxic, chemically stable, and easily available from the market [23,24,25]. Biochar possesses various characteristics, such as high porosity, high water retention [26], and good sorption capacity [27], which favor prolonged microbial survival [28], stickiness, stabilization, and protection from harsh environmental conditions [22,29,30,31]. Press mud is rich in nutrients and provides energy to microbes. Press mud is a good carrier to nurture PGPR populations and provides nutrients when decomposed by these bacteria [32,33]. Hussain et al. [34] found that various organic materials improved growth attributes (shoot length, root length, and fresh and dry weights), physiological attributes (antioxidant status, chlorophyll content, the membrane stability index, and relative water content), and biological properties (microbial count) of the soil rhizosphere in wheat crops.
Similarly, compost is partially decomposed organic material, which provides a variety of nutrients and carbon to the soil and improves PGPR diversity to enhance crop growth and yields [2,35,36,37,38,39]. Sohaib and his colleagues [40] revealed that organic carriers such as compost and biogas slurry significantly improved the provision of suitable conditions for PGPR to flourish, ameliorating salinity stress and, ultimately, the growth traits, nutritional status, and physiology of wheat under saline conditions.
Peat is organic waste and an economical carrier material that is rich in nutrients due to the presence of a variety of organic materials in its composition; these not only aid the growth and survival of bacteria but also provide crevices and microcolony particle surfaces for them [23,24,41]. Charcoal is considered to be a reservoir of carbon for soil and forests, which supports the survival of saprotrophic microorganisms and increases nutrient availability in the soil [42,43,44,45]. Based on the qualities of different carrier materials, it is imperative to test which carrier material has the maximum number of microbial populations with a positive impact on crop growth and productivity. Moreover, the use of chemicals poses serious health concerns for humans via environmental degradation. No study has reported the role of different carrier materials in improving the soil microbial population and nutritional status of vegetables. The use of industrial by-products and organic amendments would not only reduce the environmental degradation caused by waste products, but also improve soil health and biodiversity. Based on this hypothesis, the present study aimed to determine the impact of different carrier materials in improving the quality and yield of spinach, along with the maximized bacterial population. The present research may help to minimize the utilization of chemical amendments and provide a better understanding of how to choose a better carrier material for the formulation of biofertilizers for leafy vegetables.
2. Materials and Methods
The current research was carried out at the Department of Soil Science, Faculty of Agriculture and Environment, The Islamia University of Bahawalpur, Pakistan. To evaluate the efficiency of organic carrier materials and plant-growth-promoting rhizosphere bacteria on spinach quality and production, pre-identified and pre-isolated rhizobacterial strains from the Bacillus genus, including Bacillus subtilis (ZM-63), Paenibacillus polymyxa (ZM-27) and Bacillus megaterium (AN-35), which have the accession numbers KX788861, KX788859, and MN005929, respectively, were selected from the culture bank of Soil Microbiology and Biotechnology Laboratory, Department of Soil Science, The Islamia University of Bahawalpur, Pakistan.
2.1. Testing Compatibility and Making Consortium
To test the compatibility of these bacterial strains with each other, fresh cultures of the strains were streaked on general-purpose medium (GPM) agar plates using the cross streak method. The petri dishes were placed in an incubator at 30 ± 2 °C for 48 h and observed for the inhibition zone. Afterward, equal proportions of the compatible strains were mixed in a media storage bottle and vortexed for 30 s to make a homogeneous consortium [46].
2.2. Experimental Materials
The Desi variety of spinach was taken from the grain market of Bahawalpur, Pakistan. Carrier materials were collected from different places; these materials included peat from a plant nursery at technical chowk Bahawalpur, Pakistan; press mud from Ashraf sugar mill at Khanka Sharif Road near Bahawalpur, Pakistan; and compost from the compost unit, the Islamia University of Bahawalpur, Pakistan. Charcoal and biochar were purchased from the market. Chemical characterization of the carrier materials was conducted before their use in the experiments (Table 1).

Table 1.
Physiochemical properties of carrier materials.
2.3. Soil Sampling and Analysis
The soil samples were taken from the research area of the Department of Soil Science, Faculty of Agriculture and Environment, the Islamia University of Bahawalpur; the physicochemical characteristics of the soil were determined before sowing. These pre-sowing samples were analyzed for different physicochemical analyses, following the method described in [47] (Table 2).

Table 2.
Physicochemical properties of the soil.
2.4. Seed Disinfection and Inoculation
Seeds of spinach were surface disinfected with sodium hypochlorite solution (5%) followed by ethanol (70%) and rinsed 6 times with the distilled autoclaved water. Surface disinfected seeds were coated using the slurry method; the slurry composition contained organic carrier-clay mixture (6:1), inoculum, and sugar solution (20%) in 5:4:1 ratios, respectively, for an effective slurry and seed coating [48]. The inoculated seeds were then spread under a shade for drying and then sown in the pot and field experiments.
2.5. Pot Experiment
The efficacy of the biofertilizer formulations was tested in pots at the wirehouse at the Department of Soil Science. For these purposes, the soil of a similar field was dried and sieved through a 2 mm mesh size sieve and filled into pots at 10 kg of soil per pot. The experiment consists of 7 treatments, named as follows: T0—control, T1—PGPR, T2—peat + PGPR, T3—press mud + PGPR, T4—compost + PGPR, T5—charcoal + PGPR, and T6—biochar + PGPR, arranged in a completely randomized design (CRD) with three repeats. In each pot, 10 inoculated seeds were sownafter germination, and a population of three seedlings was maintained throughout the experimental units. The recommended doses of nitrogen (N), phosphorus (P), and potassium (K) (120, 90, and 60 kg ha−1, respectively) were mixed in the pots while filling and before sowing through urea, diammonium phosphate, and sulfate of potash, respectively, while the N was applied in three splits with a gap of 25 days. All the intercultural operations were conducted as and when required, and pots were irrigated with high-quality tap water available at the wirehouse. The growth and physiological attributes (the membrane stability index, chlorophyll content, and relative water content) were measured at maturity, while yield and chemical analysis data were recorded after crop harvest.
The chlorophyll content was measured by lysing the fresh leaves of the plants (0.5 g) in acetone (80% pure) in a pestle and mortar until homogenized. The homogenate was then filtered and absorbance was read using a spectrophotometer; the readings were used to calculate the chlorophyll content following Arnon [49].
2.6. Field Experiment
A field experiment was conducted at the research area of the Department of Soil Science, Islamia University of Bahawalpur, Pakistan; the treatment used was similar to that used in the pot experiment, and was arranged in a randomized complete block design (RCBD) with three blocks. Recommended doses of P and K (90 and 60 kg ha−1) were applied as basal doses before sowing, while N (120 kg ha−1) was divided into 3 splits as basal after germination and 25 days after the second split. All of the intercultural operations were performed as and when required, and canal water was used for irrigation. At maturity, the growth and physiological attributes were measured, while measurements of the spinach yield and the chemical analysis were conducted after the crop harvest.
2.7. Membrane Stability Index
The membrane stability index was calculated using the method described by Sairam and Saxen [50], by measuring the electrical conductivity (EC) represented as C1 of a mixture of 100 mg fresh leaf samples and 10 mL distilled water in the test tube. The samples were then heated in a water bath at 100 °C for ten minutes and the second EC was taken as C2. The membrane stability index (MSI) was calculated using the following formula:
2.8. Relative Water Content
The relative water content of new leaves was estimated according to Lazcano-Ferrat and Lovatt [51]. Leaf samples were dipped in distilled water for 18 h, and extra water was removed with tissue paper before the turgid weight was measured. The dry weight was noted after placing the dipped samples at 65 °C in a hot air oven until they achieved a constant weight. Relative water content (RWC) was estimated using the given formula:
RWC (%) = (Fresh weight − Dry weight/Turgid Weight − Dry weight) × 100
2.9. Growth Parameters and Inorganic Nutrient Content
Plant samples from roots and shoots were taken at maturity for the roots’ fresh and dry weights. The shoot length was measured with a meter rod. The fresh samples were then kept in a hot air oven at 65 ± 2 °C for 48 h and the dry weights were taken with a digital analytical balance.
After harvesting, the plant samples were dried, ground, and digested using Wolf’s method. The digested samples were diluted to 50 mL with distilled water and used for the nutrient analysis following standard protocols. The nitrogen content was determined using the Kjeldahl distillation method. The distilled sample was collected in a 4% boric acid solution and titrated with 0.01 N standard H2SO4 until the pink endpoint in the presence of mixed indicators (Bromo-cresol green and methylene red). The digested aliquot was directly run on the flame photometer to measure the potassium. The flame photometer reading was converted to K concentration by comparing it with the calibration curve of known concentration standards, using the formula:
where A = the final volume of the extract (mL) and w = the weight of the plant sample taken for digestion (g).
K (mg kg−1) = K (from the calibration curve) × A/w
Phosphorus was determined using a spectrophotometer through the yellow method. The 5 mL aliquot of the digested sample was mixed with 10 mL of color-developing reagent and incubated at room temperature for 15 min. After incubation, the absorbance of the samples was recorded at a wavelength of 410 nm. The P concentration was determined by comparing the sample readings with the calibration curve of known concentration standards [52].
The content of minerals such as Zn and Fe in the digested samples was determined using an atomic absorption spectrophotometer (AAS), according to the known concentration standards of those nutrients.
2.10. Microbial Population
The microbial population of the treatments was recorded at harvest. The samples were taken and transferred to the laboratory at 4 °C. Total microbial counts were determined by the serial dilution method described by Alexander and Zuberer [53]. Different dilutions of the samples were inoculated in agar plates with a general purpose medium (GPM) and incubated for 48–72 h in an incubator at 28 ± 2 °C. The microbial populations from each treatment were counted using the colony counter.
2.11. Statistical Analysis
The data were statistically analyzed through one-way analysis of variance (ANOVA) using CRD and RCBD for the pot and field experiments, respectively, using Statistix 8.1® [54]. The difference between the treatment means was compared using the Least Significant Difference (LSD) test at a probability level of 5%.
3. Results
3.1. Pot and Field Trial
Carrier-based bio-inoculated amendments were tested for their effects on different parameters of the spinach in a pot and field experiment. The consortium of the bacterial isolates ZM-27, ZM-63, and AN-35 was tested with different carrier materials, and significant results were obtained in terms of growth parameters, physiological parameters, mineral content, and bacterial population in the rhizosphere of spinach. All applied treatments had a statistically significant impact, but the application of peat along with PGPR showed maximum results as compared to the control, followed by compost + PGPR and press mud + PGPR, charcoal + PGPR, biochar + PGPR, and sole PGPR.
3.2. Growth Parameters
Data presented in Table 3 and Table 4 show that all of the inoculated treatments resulted in a significant increase in the growth attributes of spinach, as compared to the un-inoculated treatment, under pot and field trials. However, the maximum increase in shoot fresh weight (24%), shoot dry weight (25%), shoot height (25%), root fresh weight (29%), root dry weight (33%), and total root length (25%) was observed when the PGPR consortium was applied with peat as the carrier material in the pot trial, as compared to the un-inoculated control treatment (T0). Similarly, the maximum increase in shoot fresh weight (23%) shoot dry weight (35%), shoot height (29%), root fresh weight (24.7%), root dry weight (27%), and total root length (16%) was observed when the PGPR consortium was applied with peat as the carrier material in the field trial, as compared to the un-inoculated control treatment.

Table 3.
Effects of different carrier materials, along with the PGPR consortium, on the growth parameters of spinach in a pot trial.

Table 4.
Effect of different carrier materials, along with the PGPR consortium, on the growth parameters of spinach in field trial.
3.3. Spinach Physiology
Physiological characteristics were significantly enhanced by the application of the PGPR consortium with different types of carrier materials, as compared to the un-inoculated control (Table 5 and Table 6). As compared to the un-inoculated control, the combined application of peat with the PGPR consortium showed the maximum improvement in spinach physiological attributes, i.e., the membrane stability index, chlorophyll content, and relative water content, by 28, 9, and 27%, respectively, followed by compost+ PGPR, press mud+ PGPR, charcoal+ PGPR, and biochar+ PGPR.

Table 5.
Effects of different carrier materials, along with the PGPR consortium, on the physiological parameters of spinach in a pot trial.

Table 6.
Effects of different carrier materials, along with the PGPR consortium, on the physiological parameters of spinach in the field trial.
Similar results were found in the field experiment, as the application of peat along with the PGPR consortium (T2) showed the maximum improvement in membrane stability index, chlorophyll content, and relative water content, increasing their values by 32, 13, and 28%, respectively, as compared to the un-inoculated control (T0); the next most substantial improvements were produced by compost + PGPR (T4), press mud+ PGPR (T3), charcoal + PGPR (T5), and biochar + PGPR (T6).
3.4. Mineral Content of Spinach
The inoculation of the PGPR consortium with organic carriers significantly enhanced the nutritional value of spinach, as compared with the un-inoculated treatment (T0) in both the pot and field studies. The data presented in Table 7 show that the macronutrient (N, P, K) content of spinach was significantly improved by the application of the consortium with peat in the pot trial. The maximum increases in nitrogen, phosphorus, and potassium content were 18, 22, and 15%, respectively, when the PGPR consortium was applied with peat, as compared to the un-inoculated control treatment (T0). Moreover, the application of the PGPR consortium with peat also enhanced the iron and zinc fortification in spinach under pot conditions. The maximum increases in iron (17%) and zinc (14%), as compared to the un-inoculated control, were recorded under the T2 treatment (Figure 1). Similarly, the data presented in Table 8 show that the macronutrient contents of spinach were significantly improved by the application of the PGPR consortium with peat in the field trial, with a maximum increase in nitrogen (16%), phosphorus (19%), and potassium (15%), as compared to the un-inoculated control (T0). Meanwhile, the same treatment also caused the maximum biofortification of Fe and Zn, by 17 and 23%, respectively, as compared to the un-inoculated control treatment (Figure 2).

Table 7.
Effects of different carrier materials along with the PGPR consortium on the mineral content of spinach in a pot trial.
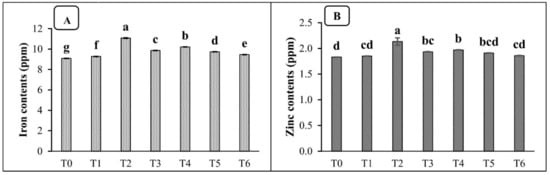
Figure 1.
Effects of different carrier materials along with the PGPR consortium on the iron content (A) and zinc content (B) of spinach in a pot trial. T0 = control, T1 = consortium, T2 = peat + PGPR, T3 = press mud + PGPR, T4 = compost + PGPR, T5 = charcoal + PGPR, and T6 = biochar + PGPR. The bars with different letters are significantly different at p ≤ 0.05.

Table 8.
Effects of different carrier materials, along with the PGPR consortium, on the mineral content of spinach in the field trial.
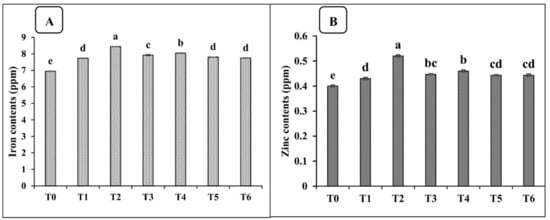
Figure 2.
Effects of different carrier materials along with the PGPR consortium on the iron contents (A) and zinc contents (B) of spinach in the field trial. T0 = control, T1 = consortium, T2 = peat + PGPR, T3 = press mud + PGPR, T4 = compost + PGPR, T5 = charcoal + PGPR, and T6 = biochar + PGPR. The bars with different letters are significantly different at p ≤ 0.05.
3.5. Microbial Count
The viability of the sole consortium and the combined application of the consortium with different carrier materials on the bacterial colonies in the rhizosphere of spinach is shown in Figure 3.
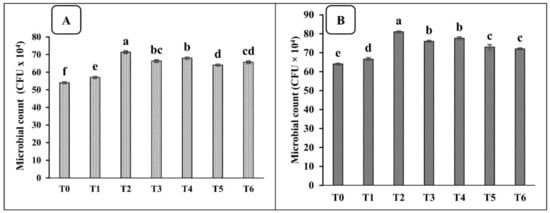
Figure 3.
Effects of different carrier materials, along with the PGPR consortium, on the bacterial population in the rhizosphere of spinach in pot (A) and field (B) trials. T0 = control, T1 = consortium, T2 = peat + PGPR, T3 = press mud + PGPR, T4 = compost + PGPR, T5 = charcoal + PGPR, and T6 = biochar + PGPR. The bars with different letters are significantly different at p ≤ 0.05.
The application of the PGPR consortium significantly enhanced the microbial population in the spinach rhizosphere, as compared to the un-inoculated control treatment. The maximum increase in the microbial count in terms of colony-forming units (CFU g−1 dry soil) was observed at 24% under the application of the PGPR consortium with peat (T2), followed by compost (T4), which showed a 20.6% increase as compared to the control (Figure 3A).
Similar findings were obtained from field experimentation as the maximum CFU g−1 dry soil was obtained by the application of the PGPR consortium with peat, which was 20% more than the un-inoculated control treatment, followed by compost + PGPR (T4), press mud+ PGPR (T3), charcoal + PGPR (T5), biochar + PGPR (T6), and the sole consortium (T1) which significantly improved the CFU g−1 dry soil by 17.6, 15.8, 12.3, 11.1 and 4.0%, respectively (Figure 3B).
3.6. Spinach Yield
The application of the PGPR consortium along with different carriers significantly improved the yield of spinach as compared to the un-inoculated control (Figure 4). The data presented in Figure 4 show that application of the PGPR consortium with peat (T2) caused the maximum increase in the per hectare yield of spinach, improving it by 30% as compared to the un-inoculated control; this was followed by the compost T4, T3, T5, and T6 treatments, which produced a 22, 14, 6.9, and 6.8% increase in the spinach yield, respectively, as compared to the un-inoculated control. However, the sole application of the PGPR consortium (T1) caused the minimum increase in spinach yield: 2.5%, as compared to the un-inoculated control.
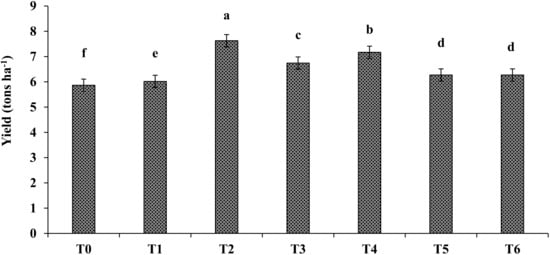
Figure 4.
Effects of different carrier materials, along with the PGPR consortium, on the yield of spinach under a field trial. T0 = control, T1 = consortium, T2 = peat + PGPR, T3 = press mud + PGPR, T4 = compos t+ PGPR, T5 = charcoal + PGPR, and T6 = biochar + PGPR. The bars with different letters are significantly different at p ≤ 0.05.
4. Discussion
The soil is a complex and enriched medium that aids in plant production. However, the soil environment is affected by various factors (weeds, pathogens, drought, salinity, heavy metals, floods, temperature, etc.) that impede plant growth [55,56,57]. Several chemical-based products have been used to suppress the effects of these harmful factors and boost plant productivity, but these chemicals are dangerous to the environment [55]. Advanced research is required to identify natural substitutes for chemical fertilizers; one area for investigation concerns the benefits of PGPR in biofertilizers in terms of the improved growth, quality, and yield of vegetable crops.
The PGPRs present in biofertilizers have several beneficial properties, including the biological fixation of nitrogen, the solubilization of fixed nutrients, and various enzymatic activities that make the soil environment suitable for plant growth and help the plants to combat stressful ecological situations [58]. In our current research, the physiological, growth, and mineral parameters of plants and the microbial population in the plant rhizosphere were increased by applying a sole PGPR consortium and a PGPR consortium grouped with various organic carrier materials. Our findings are similar to the findings of Hussain et al. [59], who stated that the combination of different organic carrier materials with a consortium of PGPR improve the quality, growth, and output of maize grains. Moreover, this growth enhancement might be due to the PGPR strains’ possession of growth-promoting traits, which, when applied with organic carriers as a consortium, might synergize for a growth-promoting impact on spinach, as described for maize in Dar et al. [60].
The selection of organic carriers for the formulation of biofertilizers is very important, as the physical and chemical properties of the carriers vary. Selected carriers for biofertilizers may influence the life of the PGPRs present in it. The carrier materials should be able to maintain the bacterial population and different strains of bacteria in one formulation, as noted by Malusá et al. [23]. In short, the goal of these experiments was to evaluate the suitable carrier material for the inoculation of microbes. Pre-isolated AN-35, ZM-27, and ZM-63 significantly enhanced the physiology of spinach in terms of chlorophyll content, RWC, and MSI when applied with peat, followed by compost and press mud; these constitute superior carrier materials for biofertilizers in comparison to biochar and charcoal. Similar findings were observed by Hammad et al. [61], who used organic waste for the application of the PGPR consortium and found that the growth-promoting traits of the PGPR, such as miners solubilization, indole acetic acid (IAA) production, and siderophore production, not only enhanced the chlorophyll, MSI, RWC, and mineral nutrients, but also biofortify okra with Zn and Fe. The reason for this might be that the use of organic carriers with entrapped nutrients and microorganisms solubilizes mineral nutrients by secreting organic acids in the rhizosphere of spinach [62].
In the current study, growth parameters including shoot length, root length, shoot fresh weight, root fresh weight, shoot dry weight, and root dry weight were recorded as being improved by the use of peat, which demonstrated its significant crop-improvement abilities. However, its efficiency was statistically close to that of compost and press mud containing biofertilizers. Similar results were also demonstrated by Janssen [63]. As carbon is a basic component of organic compounds, it is found in huge quantities in peat because it is an organic layer of soil that contains 50 to 89% carbon. As rhizobacteria flourish on carbon [64], a large amount of carbon in peat also supports the bacterial population under unfavorable soil conditions. Similar results were observed by Priyanka and Koshy [65], who found that organic waste materials such as peat resulted in a remarkable uplift in growth parameters such as plant height, fresh and dry weight, and germination percentages, due to the production of phytohormones.
An increase in the accumulation of NPK, iron, and zinc in shoots was observed, due to improvements in the root architecture resulting from the enhanced activity of bacterial cells via the production of IAA, which is a phytohormone responsible for root development [63,64,65]. These instant effects might be due to the availability of nutrients for plants [22,66]. Peat, compost, and press mud act as good carriers, containing higher N and K concentrations that could be more effective for the improved growth and life of the bacterial population in an unfavorable environment. As potassium (K) is key to the activation of enzymes, its accessibility could be amplified by the application of PGPR. Nitrogen (N) is the basic component of chlorophyll molecules and is necessary for its production in plants. As such, these minerals help the bacteria to live and the plant to grow sustainably [67,68,69].
The application of biofertilizers with suitable carriers significantly increases the activity and population of rhizospheric microorganisms [34,70,71]. All of the carrier materials significantly enhanced the microbial population, but the treatment with peat had the maximum microbial count as compared to the other organic carriers. The reason for this might be the provision of a large surface area for microbial attachment to peat, their compatibility, and the ease of degrading the peat to attain energy and nutrients for their population growth, even under harsh climatic conditions [62]. The improved bacterial population is responsible for the enhanced-growth promoting activities, i.e., mineral solubilization, nutrient uptake, root proliferation, and water for better crop growth, as well as physiological trait improvements and, ultimately, yield improvement [70,71]. Moreover, the enhancement of moisture content and the availability of carbon and nitrogen to the microbes and plants is responsible for higher yields and the higher fortification of micronutrients [72,73].
5. Conclusions
From the results of the pot and field experiments, it is concluded that the application of carrier-containing biofertilizers enhances the life of bacterial cells, the growth, physiology, and quality of spinach, and microbial numbers in the soil, as compared to the sole consortium and the control. The applied carrier materials, i.e., peat, compost, and press mud, resulted in significantly better performance in comparison with the control without any carrier material. Among all the tested carrier materials, peat caused the maximum improvement in growth and physiological attributes, nutritional status, soil microbial population, and biomass production. In the future, we would strongly recommend that an extensive evaluation of these carriers, especially peat, is carried out in the field in various ecological zones; this will enable the formulation of biofertilizers for the biofortification of nutrients (Fe and Zn) and the sustainable production of vegetables, fulfilling the UN’s sustainable development goal of zero hunger.
Author Contributions
Conceptualization, H.S., M.J., A.H., A.D. (Allah Ditta), A.D. (Abubakar Dar), A.A., H.T.A., Q.N. and M.A.; data curation, H.S., A.D. (Abubakar Dar), A.A., H.T.A., Q.N. and M.A.; formal analysis, H.S., M.J., A.H., B.F.A.A., A.D. (Allah Ditta), A.D. (Abubakar Dar), A.A., H.T.A., Q.N. and M.A.; funding acquisition, M.J., A.H. and B.F.A.A.; investigation, H.S.; methodology, A.H. and A.D. (Allah Ditta); project administration, M.J., A.H., B.F.A.A. and M.A.; resources, M.J., A.H., B.F.A.A., A.D. (Allah Ditta), A.A., H.T.A., Q.N. and M.A.; software, B.F.A.A., A.D. (Allah Ditta), A.D. (Abubakar Dar), A.A., H.T.A. and Q.N.; supervision, A.H. and M.A.; validation, M.J., A.H., B.F.A.A., A.D. (Allah Ditta), A.D. (Abubakar Dar), A.A., H.T.A., Q.N. and M.A.; visualization, H.S., B.F.A.A., A.D. (Allah Ditta), A.D. (Abubakar Dar), A.A., H.T.A. and Q.N.; writing—original draft, H.S.; writing—review and editing, H.S., M.J., A.H., B.F.A.A., A.D. (Allah Ditta), A.D. (Abubakar Dar), A.A., H.T.A., Q.N. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ORIC-funded research project No. 3911/ORIC/IUB/2021 entitled “Optimization of carrier materi-al for bio-inoculant to enhance vegetable crops growth and yield”, which is run by Azhar Hussain, Department of Soil Science, the Islamia University of Bahawalpur.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the financial assistance of the Islamia University of Bahawalpur under the ORIC-funded research project No. 3911/ORIC/IUB/2021 entitled “Optimization of carrier material for bio-inoculant to enhance vegetable crops growth and yield”, which is run by Azhar Hussain, Department of Soil Science, the Islamia University of Bahawalpur.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. The State of Food Security and Nutrition in the World 2018: Building Climatic Resilience for Food Security and Nutrition; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Ullah, N.; Ditta, A.; Imtiaz, M.; Li, X.; Jan, A.U.; Mehmood, S.; Rizwan, M.S.; Rizwan, M. Appraisal for organic amendments and plant growth-promoting rhizobacteria to enhance crop productivity under drought stress: A review. J. Agron. Crop Sci. 2021, 207, 783–802. [Google Scholar] [CrossRef]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and soil microbial community: A review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Yadav, K.K.; Sarkar, S. Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture. Environ. Ecol. 2019, 37, 89–93. [Google Scholar]
- Okur, N. A review-bio-fertilizers-power of beneficial microorganisms in soils. Biomed. J. Scient. Tech. Res. 2018, 4, 4028–4029. [Google Scholar] [CrossRef]
- Sehrawat, A.; Sindhu, S.S. Potential of biocontrol agents in plants disease control for improving food safety. Life Sci. 2019, 4, 220–225. [Google Scholar] [CrossRef]
- Zhang, J.; Cook, J.; Nearing, J.T.; Zhang, J.; Raudonis, R.; Glick, B.R.; Langille, M.G.; Cheng, Z. Harnessing the plant microbiome to promote the growth of agricultural crops. Microbiol. Res. 2021, 245, 126690. [Google Scholar] [CrossRef]
- Glick, B.R. Introduction to plant growth-promoting bacteria. In Beneficial Plant-Bacterial Interactions; Springer: Cham, Switzerland, 2020; pp. 1–37. [Google Scholar]
- Etesami, H.; Jeong, B.R. Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria, and silicon to P uptake by plant: A review. Front. Plant Sci. 2021, 12, 1355. [Google Scholar] [CrossRef]
- Patel, S.H.; Viradiya, M.B.; Prajapati, B.J. Effect of potassium and potassium mobilizing bacteria (KMB) with and without FYM on yield of wheat (Triticum aestivum L.). J. Pharmacog. Phytochem. 2021, 10, 1615–1620. [Google Scholar]
- Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.D.L.; Orozco-Mosqueda, M.D.C.; Glick, B.R. Plant growth stimulation by microbial consortia. Agronomy 2021, 11, 219. [Google Scholar] [CrossRef]
- Soumare, A.; Boubekri, K.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. From isolation of phosphate solubilizing microbes to their formulation and use as biofertilizers: Status and needs. Front. Bioeng. Biotechnol. 2020, 7, 425. [Google Scholar] [CrossRef] [PubMed]
- Amenaghawon, A.N.; Anyalewechi, C.L.; Kusuma, H.S. Fabrication approaches for biofertilizers. Biofertil. Study Impact 2021, 75, 491–515. [Google Scholar]
- Berninger, T.; Mitter, B.; Preininger, C. Zeolite-based, dry formulations for conservation and practical application of Paraburkholderia phytofirmans Ps JN. J. Appl. Microbiol. 2017, 122, 974–986. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Santos, O.J.; Marcelino, P.R.; Milani, K.M.; Zuluaga, M.Y.; Zucareli, C.; Gonçalves, L.S. Maize inoculation with Azospirillum brasilense Ab-V5 cells enriched with exopolysaccharides and polyhydroxybutyrate results in high productivity under low N fertilizer input. Front. Microbiol. 2017, 8, 1873. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Lur, H.S.; Lo, K.J.; Cheng, K.C.; Chuang, C.C.; Tang, S.J.; Yang, Z.W.; Liu, C.T. Evaluation of the effects of different liquid inoculant formulations on the survival and plant-growth-promoting efficiency of Rhodopseudomonas palustris strain PS3. Appl. Microbiol. Biotechnol. 2016, 100, 7977–7987. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Deng, B.; Zhang, Y.; Cobb, A.B.; Zhang, Z. Molybdate in rhizobial seed-coat formulations improves the production and nodulation of alfalfa. PLoS ONE 2017, 12, e0170179. [Google Scholar] [CrossRef]
- Mukhtar, S.; Shahid, I.; Mehnaz, S.; Malik, K.A. Assessment of two carrier materials for phosphate solubilizing biofertilizers and their effect on growth of wheat (Triticum aestivum L.). Microbiol. Res. 2017, 205, 107–117. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, L.M.; Sharma, S.; Singh, N. Overview on agricultural potentials of biogas slurry (BGS): Applications, challenges, and solutions. Biomass Convers. Biorefin. 2022, 1–41. [Google Scholar] [CrossRef]
- Schoebitz, M.; Mengual, C.; Roldán, A. Combined effects of clay immobilized Azospirillum brasilense and Pantoea dispersa and organic olive residue on plant performance and soil properties in the revegetation of a semiarid area. Sci. Total Environ. 2014, 466, 67–73. [Google Scholar] [CrossRef]
- Sabir, A.; Naveed, M.; Bashir, M.A.; Hussain, A.; Mustafa, A.; Zahir, Z.A.; Kamran, M.; Ditta, A.; Núñez-Delgado, A.; Saeed, Q.; et al. Cadmium mediated phytotoxic impacts in Brassica napus: Managing growth, physiological and oxidative disturbances through the combined use of biochar and Enterobacter sp. MN17. J. Environ. Manag. 2020, 265, 110522. [Google Scholar] [CrossRef]
- Malusá, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for beneficial microorganisms inocula used as biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Aloo, B.N.; Mbega, E.R.; Makumba, B.A.; Tumuhairwe, J.B. Effects of Carrier Materials and Storage Temperatures on the Viability and Stability of Three Biofertilizer Inoculants Obtained from Potato (Solanum tuberosum L.) Rhizosphere. Agriculture 2022, 12, 140. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.A.; Gonnermann, H.M. Biochar particle size, shape, and porosity act together to influence soil water properties. PLoS ONE 2017, 12, e0179079. [Google Scholar] [CrossRef]
- Batista, E.M.; Shultz, J.; Matos, T.T.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; de Freitas, R.A.; Mangrich, A.S. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci. Rep. 2018, 8, 10677. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management; Routledge: Oxfordshire, UK, 2012; pp. 117–138. [Google Scholar]
- Sun, T.; Levin, B.D.; Guzman, J.J.; Enders, A.; Muller, D.A.; Angenent, L.T.; Lehmann, J. Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nat. Comm. 2017, 8, 14873. [Google Scholar] [CrossRef]
- Ajema, L. Effects of biochar application on beneficial soil organisms. Int. J. Res. Stud. Sci. Eng. Technol. 2018, 5, 9–18. [Google Scholar]
- Tripti; Kumar, A.; Kumar, V.; Anshumali; Bruno, L.B.; Rajkumar, M. Synergism of Industrial and Agricultural Waste as a Suitable Carrier Material for Developing Potential Biofertilizer for Sustainable Agricultural Production of Eggplant. Horticulturae 2022, 8, 444. [Google Scholar] [CrossRef]
- Zaman, M.; Di, H.J.; Sakamoto, K.; Goto, S.; Hayashi, H.; Inubushi, K. Effects of sewage sludge compost and chemical fertilizer application on microbial biomass and N mineralization rates. Soil Sci. Plant Nutr. 2002, 48, 195–201. [Google Scholar] [CrossRef]
- Mushtaq, Z.; Asghar, H.N.; Zahir, Z.A. Comparative growth analysis of okra (Abelmoschus esculentus) in the presence of PGPR and press mud in chromium-contaminated soil. Chemosphere 2021, 262, 127865. [Google Scholar] [CrossRef]
- Hussain, A.; Iqbal, Z.; Aurangzaib, M.; Naeem, M.; Mustafa, S.; Shoaib, M.; Khalid, M. Comparative effectiveness of plant growth promoting rhizobacteria and various organic carriers on wheat growth, physiology, antioxidative activities, and rhizosphere properties. Pak. J. Bot. 2022, 54, 1–8. [Google Scholar] [CrossRef]
- Ditta, A.; Muhammad, J.; Imtiaz, M.; Mehmood, S.; Qian, Z.; Tu, S. Application of rock phosphate enriched composts increases nodulation, growth, and yield of chickpea. Int. J. Recycl. Org. Waste Agri. 2018, 7, 33–40. [Google Scholar] [CrossRef]
- Ditta, A.; Imtiaz, M.; Mehmood, S.; Rizwan, M.S.; Mubeen, F.; Aziz, O.; Tu, S. Rock phosphate-enriched organic fertilizer with phosphate-solubilizing microorganisms improves nodulation, growth, and yield of legumes. Comm. Soil Sci. Plant Anal. 2018, 49, 2715–2725. [Google Scholar] [CrossRef]
- Singh, D.P.; Prabha, R.; Renu, S.; Sahu, P.k.; Singh, V. Agro waste bioconversion and microbial fortification have prospects for soil health, crop productivity, and eco-enterprising. Int. J. Recycl. Org. Waste Agric. 2019, 8, 457–472. [Google Scholar] [CrossRef]
- Tahiri, A.I.; Meddich, A.; Raklami, A.; Alahmad, A.; Bechtaoui, N.; Anli, M.; Göttfert, M.; Heulin, T.; Achouak, W.; Oufdou, K. Assessing the potential role of compost, PGPR, and AMF in improving tomato plant growth, yield, fruit quality, and water stress tolerance. J. Soil Sci. Plant Nutr. 2022, 22, 743–764. [Google Scholar] [CrossRef]
- Yasir, T.A.; Aslam, S.; Rizwan, M.S.; Wasaya, A.; Ateeq, M.; Khan, M.N.; Tanveer, S.K.; Soufan, W.; Ali, B.; Ditta, A.; et al. Role of Organic Amendments to Mitigate Cd Toxicity and Its Assimilation in Triticum aestivum L. Phyton Int. J. Exp. Bot. 2022, 91, 2491–2504. [Google Scholar]
- Sohaib, M.; Zahir, Z.A.; Khan, M.Y.; Ans, M.; Asghar, H.N.; Yasin, S.; Al-Barakah, F.N. Comparative evaluation of different carrier-based multi-strain bacterial formulations to mitigate the salt stress in wheat. Saudi J. Biol. Sci. 2020, 27, 777–787. [Google Scholar] [CrossRef]
- Kaljeet, S.; Keyeo, F.; Amir, H.G. Influence of carrier materials and storage temperature on the survivability of rhizobial inoculant. Asian J. Plant Sci. 2011, 10, 331–337. [Google Scholar] [CrossRef]
- Makoto, K.; Kamata, N.; Kamibayashi, N.; Koike, T.; Tani, H. Bark-beetle-attacked trees produced more charcoal than unattacked trees during a forest fire on the Kenai Peninsula, Southern Alaska. Scand. J. For. Res. 2012, 27, 30–35. [Google Scholar] [CrossRef]
- Javeed, H.M.R.; Ali, M.; Ahmed, I.; Wang, X.; Al-ashkar, I.; Qamar, R.; Ibrahim, A.; Habib-Ur-Rahman, M.; Ditta, A.; Sabagh, A.E. Biochar enriched with buffalo slurry improved soil nitrogen and carbon dynamics, nutrient uptake, and growth attributes of wheat by reducing leaching losses of nutrients. Land 2021, 10, 1392. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R. Valorizing biomass to engineered biochar and its impact on soil, plant, water, and microbial dynamics: A review. Biomass Convers. Biorefin. 2022, 12, 4183–4199. [Google Scholar] [CrossRef]
- Mehmood, S.; Ahmed, W.; Alatalo, J.M.; Mahmood, M.; Imtiaz, M.; Ditta, A.; Ali, E.F.; Abdelrahman, H.; Slaný, M.; Antoniadis, V.; et al. Herbal plants- and rice straw-derived biochars reduced metal mobilization in fishpond sediments and improved their potential as fertilizers. Sci. Total Environ. 2022, 826, 154043. [Google Scholar] [CrossRef]
- Dar, A.; Zahir, Z.A.; Asghar, H.N.; Ahmad, R. Preliminary screening of rhizobacteria for biocontrol of little seed canary grass (Phalaris minor Retz.) and wild oat (Avena fatua L.) in wheat. Can. J. Microbiol. 2020, 66, 368–376. [Google Scholar] [CrossRef]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant Analysis Laboratory Manual, 2nd ed.; International Center for Agriculture in Dry Areas (ICARDA): Aleppo, Syria, 2001. [Google Scholar]
- Zahir, Z.A.; Ahmad, M.; Hilger, T.H.; Dar, A.; Malik, S.R.; Abbas, G.; Rasche, F. Field evaluation of multistrain biofertilizer for improving the productivity of different mungbean genotypes. Soil Environ. 2018, 37, 45–52. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Sairam, R.K.; Saxena, D.C. Oxidative stress and antioxidants in wheat genotypes: Possible mechanism of water stress tolerance. J. Agron. Crop Sci. 2000, 184, 55–61. [Google Scholar] [CrossRef]
- Lazcano-Ferrat, I.; Lovatt, C.J. Relationship between relative water content, nitrogen pools, and growth of Phaseolus vulgaris L. and P. acutifolius A. Gray during water deficit. Crop Sci. 1999, 39, 467–475. [Google Scholar] [CrossRef]
- Champman, H.D.; Pratt, P.F. Methods of Analysis for Soil Plants and Water; University of California Division of Agricultural Sciences, Office of Agriculre Publication: Berkeley, CA, USA, 1978. [Google Scholar]
- Alexander, D.B.; Zuberer, D.A. Use of chrome Azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol. Fert. Soil 1991, 12, 39–45. [Google Scholar] [CrossRef]
- Steel, D. Bayesian statistics in radiocarbon calibration. Philos. Sci. 2001, 68, S153–S164. [Google Scholar] [CrossRef]
- Mustafa, A.; Naveed, M.; Saeed, Q.; Ashraf, M.N.; Hussain, A.; Abbas, T.; Minggang, X. Application potentials of plant growth promoting rhizobacteria and fungi as an alternative to conventional weed control methods. In Sustainable Crop Production; IntechOpen: London, UK, 2019. [Google Scholar]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. 2007. Promotion of plant growth by ACC deaminase-producing soil bacteria. In New Perspectives and Approaches in Plant Growth-Promoting Rhizobacteria Research; Springer: Berlin/Heidelberg, Germany, 2007; pp. 329–339. [Google Scholar]
- Ahmad, M.; Naseer, I.; Hussain, A.; Zahid Mumtaz, M.; Mustafa, A.; HHilger, T.; Ahmad Zahir, Z.; Xu, M. Appraising endophyte–plant symbiosis for improved growth, nodulation, nitrogen fixation, and abiotic stress tolerance: An experimental investigation with chickpea (Cicer arietinum L.). Agronomy 2019, 9, 621. [Google Scholar] [CrossRef]
- Hussain, A.; Ahmad, M.; Mumtaz, M.Z.; Nazli, F.; Farooqi, M.A.; Khalid, I.; Iqbal, Z.; Arshad, H. Impact of integrated use of enriched compost, biochar, humic acid, and Alcaligenes sp. AZ9 on maize productivity and soil biological attributes in natural field conditions. Italian J. Agron. 2019, 14, 101–107. [Google Scholar] [CrossRef]
- Dar, A.; Zahir, Z.A.; Iqbal, M.; Mehmood, A.; Javed, A.; Hussain, A.; Ahmad, M. Efficacy of rhizobacterial exopolysaccharides in improving plant growth, physiology, and soil properties. Environ. Monit. Assess. 2021, 193, 1–15. [Google Scholar] [CrossRef]
- Anwar, H.; Wang, X.; Hussain, A.; Rafay, M.; Ahmad, M.; Latif, M.; Jamshaid, M.U.; Khalid, I.; Dar, A.; Mustafa, A. Comparative Effects of Bio-Wastes in Combination with Plant Growth-Promoting Bacteria on Growth and Productivity of Okra. Agronomy 2021, 11, e2065. [Google Scholar] [CrossRef]
- Iqbal, Z.; Hussain, A.; Dar, A.; Ahmad, M.; Wang, X.; Brtnicky, M.; Mustafa, A. Combined Use of Novel Endophytic and Rhizobacterial Strains Upregulates Antioxidant Enzyme Systems and Mineral Accumulation in Wheat. Agronomy 2022, 12, e551. [Google Scholar] [CrossRef]
- Janssen, B.H. Nitrogen mineralization in relation to the C: N ratio and decomposability of organic materials. In Progress in Nitrogen Cycling Studies; Springer: Dordrecht, The Netherlands, 1996; pp. 69–75. [Google Scholar]
- Vance, E.D.; Chapin, F.S. Substrate limitations to microbial activity in taiga forest floors. Soil Biol. Biochem. 2001, 33, 173–188. [Google Scholar] [CrossRef]
- Priyanka, M.; Koshy, E.P. Effect of vegetable and fruit waste on seed germination and growth of Solanum lycopersicum. Asian J. BioSci. 2016, 11, 1–5. [Google Scholar]
- Ditta, A.; Arshad, M.; Zahir, Z.A.; Jamil, A. Comparative efficacy of rock phosphate enriched organic fertilizer vs. mineral phosphatic fertilizer for nodulation, growth, and yield of lentil. Int. J. Agri. Biol. 2015, 17, 589–595. [Google Scholar]
- Hussain, A.; Zahir, Z.A.; Ditta, A.; Tahir, M.U.; Ahmad, M.; Mumtaz, M.Z.; Hayat, K.; Hussain, S. Production and implications of bio-activated organic fertilizer enriched with zinc-solubilizing bacteria to boost up maize (Zea mays L.) production and biofortification under two cropping seasons. Agronomy 2019, 10, 39. [Google Scholar] [CrossRef]
- Nazir, Q.; Wang, X.; Hussain, A.; Ditta, A.; Aimen, A.; Saleem, I.; Naveed, M.; Aziz, T.; Mustafa, A.; Panpluem, N. Variation in growth, physiology, yield, and quality of wheat under the application of different zinc-coated formulations. Appl. Sci. 2021, 11, 4797. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Iqbal, M.; Ashraf, M. Gibberellic acid-mediated induction of salt tolerance in wheat plants: Growth, ionic partitioning, photosynthesis, yield, and hormonal homeostasis. Environ. Exp. Bot. 2013, 86, 76–85. [Google Scholar] [CrossRef]
- Manivannan, N.; Thilagavathy, D. Use of vermicompost as carrier material for microbial inoculants for enhanced crop production. J. Pure Appl. Microbiol. 2009, 3, 255–260. [Google Scholar]
- Azeem, M.; Hassan, T.U.; Tahir, M.I.; Ali, A.; Jeyasundar, P.G.S.A.; Hussain, Q.; Zhang, Z. Tea leaves biochar as a carrier of Bacillus cereus improves the soil function and crop productivity. Appl. Soil Ecol. 2021, 157, 103732. [Google Scholar] [CrossRef]
- Pérez-Piqueres, A.; Edel-Hermann, V.; Alabouvette, C.; Steinberg, C. Response of soil microbial communities to compost amendments. Soil Biol. Biochem. 2006, 38, 460–470. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).