The Role of Digital Soil Information in Assisting Precision Soil Management
Abstract
1. Introduction
2. Materials and Methods
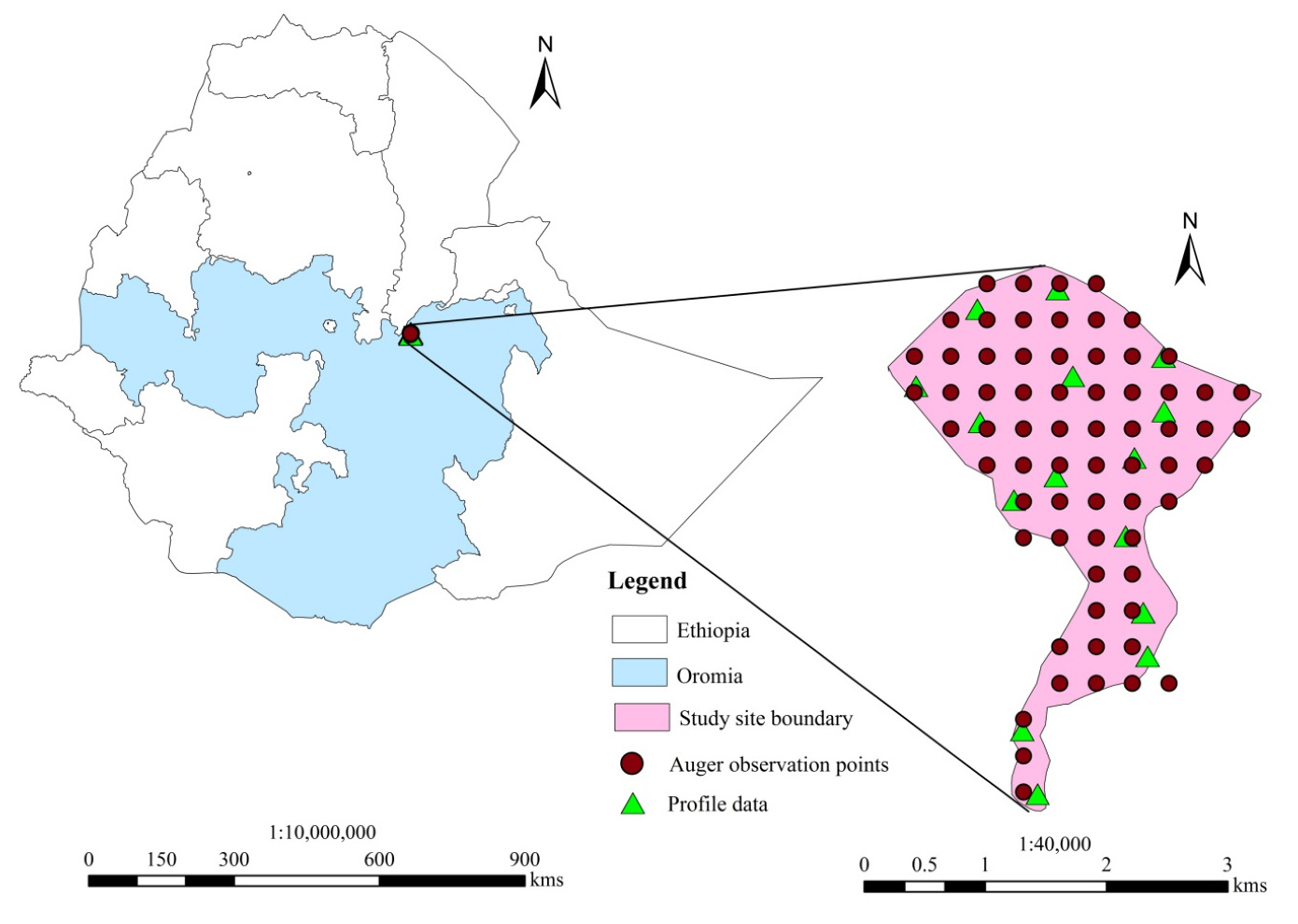
2.1. Study Site
2.2. Research Design and Procedures
2.2.1. Pre-Field Survey
2.2.2. Field Survey
2.2.3. Post-Field Survey
2.3. Sample Preparation and Analysis
2.4. Statistical Data Analysis
3. Results
3.1. Descriptive Statistics for the Entire Study Site
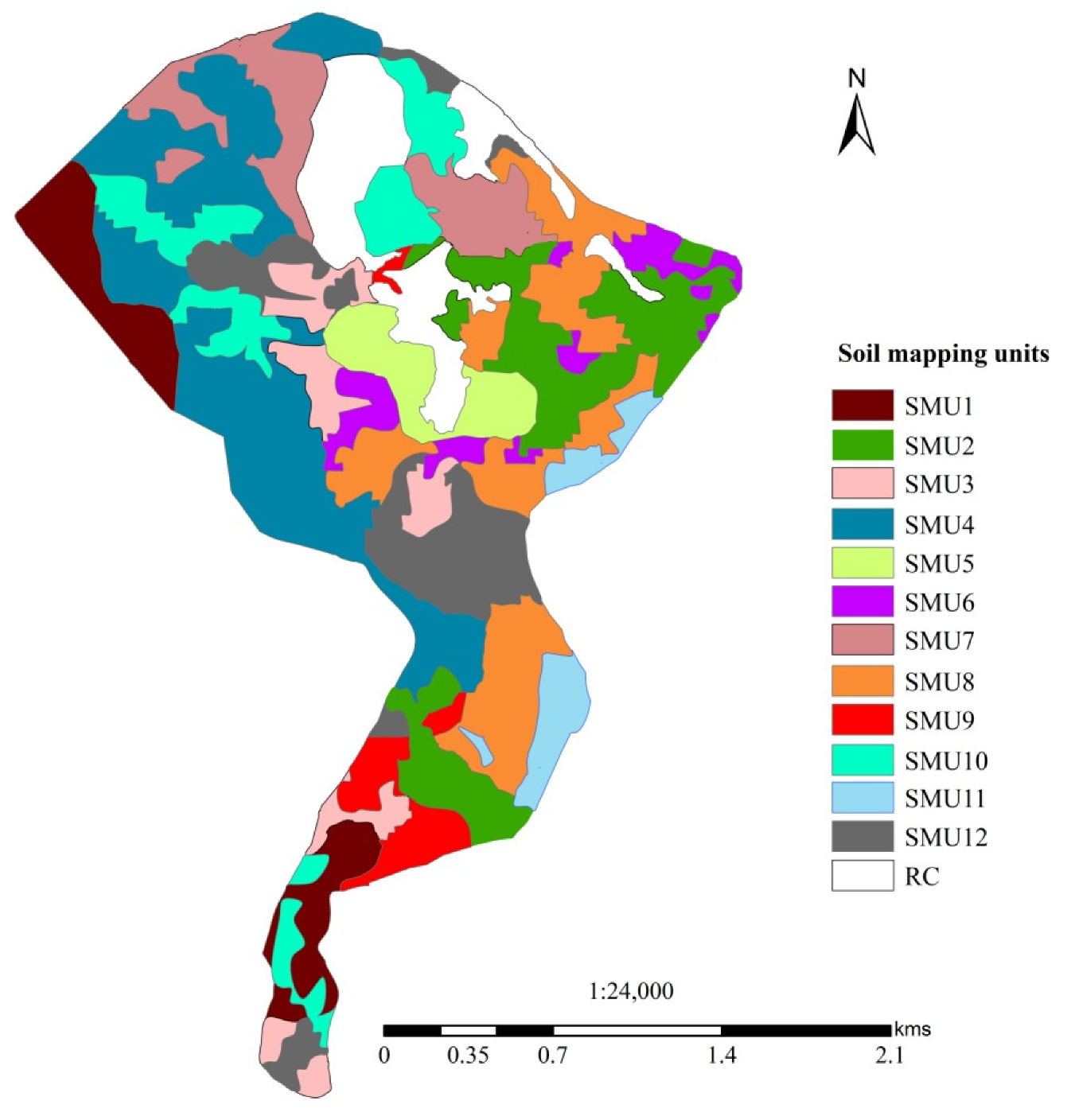
3.2. Extent and Distribution of Soil Mapping Units
3.3. Soil Nutrients and Salinity Problems across the Mapping Units
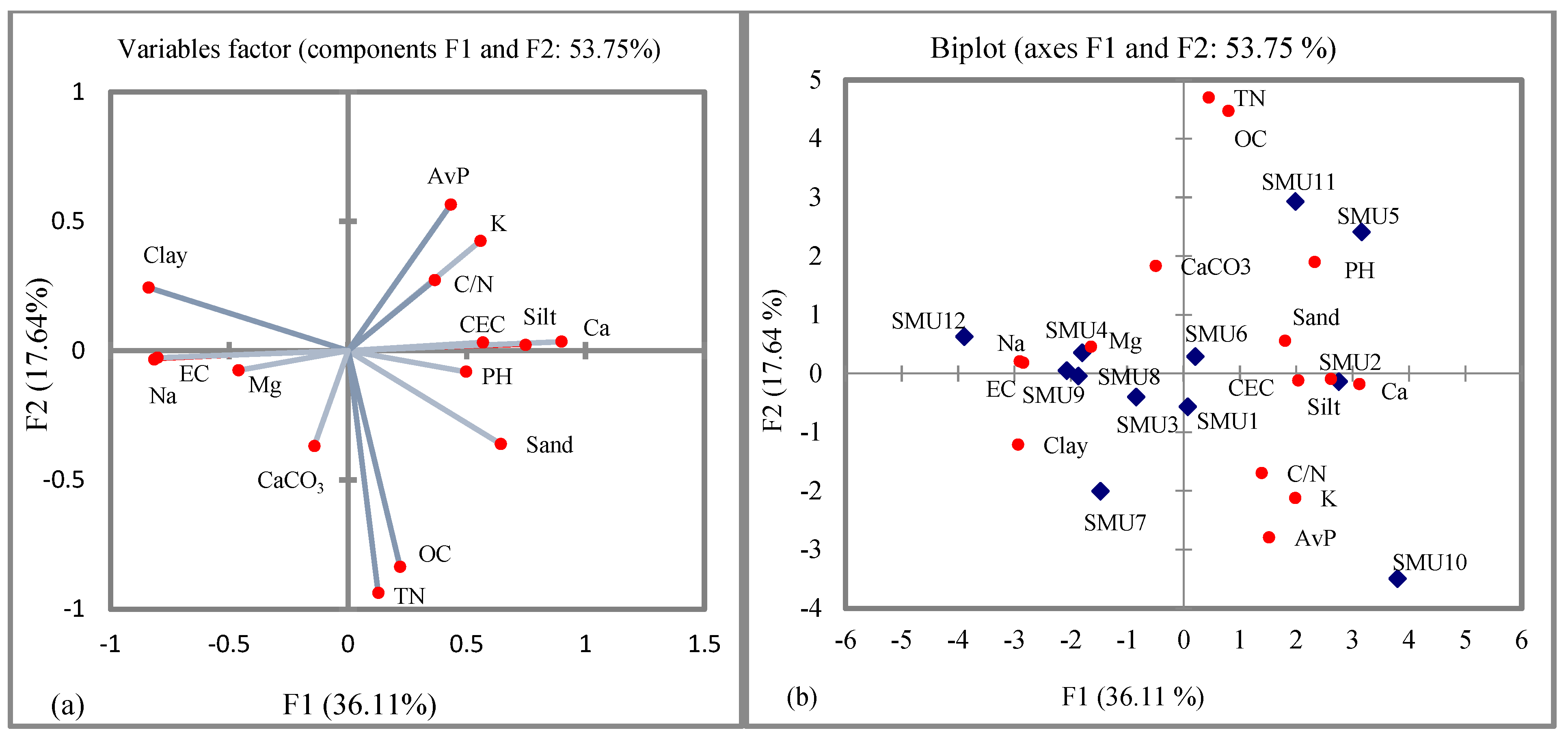
3.4. The Relationship between Soil Properties
4. Discussion
4.1. Management Zones for Spatially Targeted Nutrient Management
4.2. Site-Specific Management of Soil Chemical Constraints
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Economic and Social Development Department. World agriculture: Towards 2015/2030; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002. [Google Scholar]
- Hengl, T.; Heuvelink, G.B.M.; Kempen, B.; Leenaars, J.G.B.; Walsh, M.G.; Shepherd, K.D.; Sila, A.; MacMillan, R.A.; de Jesus, J.M.; Tamene, L.; et al. Mapping soil properties of Africa at 250 m resolution: Random forests significantly improve current predictions. PLoS ONE 2015, 10, e0125814. [Google Scholar] [CrossRef] [PubMed]
- Giller, K.E.; Witter, E.; Corbeels, M.; Tittonell, P. Conservation agriculture and smallholder farming in Africa: The heretics’ view. Field Crops Res. 2009, 114, 23–34. [Google Scholar] [CrossRef]
- Stewart, Z.P.; Pierzynski, G.M.; Middendorf, B.J.; Prasad, P.V.V. Approaches to improve soil fertility in sub-Saharan Africa. J. Exp. Bot. 2020, 71, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Gobezie, T.B.; Biswas, A. The need for streamlining precision agriculture data in Africa. Precis. Agric. 2022, 1–9. [Google Scholar] [CrossRef]
- de Graaff, J.; Kessler, A.; Nibbering, J.W. Agriculture and food security in selected countries in Sub-Saharan Africa: Diversity in trends and opportunities. Food Secur. 2011, 3, 195–213. [Google Scholar] [CrossRef]
- Iticha, B.; Takele, C. Soil–landscape variability: Mapping and building detail information for soil management. Soil Use Manag. 2018, 34, 111–123. [Google Scholar] [CrossRef]
- Panday, R.; Babu, O.R.; Chalise, D.; Das, S.; Twanabasu, B. Spatial variability of soil properties under different land use in the Dang district of Nepal. Cogent Food Agric. 2019, 5, 1. [Google Scholar] [CrossRef]
- Dewitte, O.; Jones, A.; Spaargaren, O.; Breuning-Madsen, H.; Brossard, M.; Dampha, A.; Deckers, J.; Gallali, T.; Hallett, S.; Jones, R.; et al. Harmonisation of the soil map of Africa at the continental scale. Geoderma 2013, 211–212, 138–153. [Google Scholar] [CrossRef]
- McBratney, A.B.; Mendonca Santos, M.L.; Minasny, B. On digital soil mapping. Geoderma 2003, 117, 3−52. [Google Scholar] [CrossRef]
- Oliver, M.A.; Webster, R. Basic Steps in Geostatistics: The Variogram and Kriging; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Taghizadeh-Mehrjardi, R.; Nabiollahi, K.; Kerry, R. Digital mapping of soil organic carbon at multiple depths using different data mining techniques in baneh region, iran. Geoderma 2016, 266, 98–110. [Google Scholar] [CrossRef]
- Zeraatpisheh, M.; Jafari, A.; Bodaghabadi, M.B.; Ayoubi, S.; Taghizadeh-Mehrjardi, R.; Toomanian, N.; Kerry, R.; Xu, M. Conventional and digital soil mapping in Iran: Past, present, and future. Catena 2020, 188, 104424. [Google Scholar] [CrossRef]
- Hengl, T.; Heuvelink, G.B.M.; Rossiter, D.G. About Regression-Kriging: From Equations to Case Studies. Comput. Geoscie. 2007, 33, 1301–1315. [Google Scholar] [CrossRef]
- Vasu, D.; Singh, S.K.; Sahu, N.; Tiwary, P.; Chandran, P.; Durisami, V.P.; Ramamurthy, V.; Lalitha, M.; Kalaiselvi, B. Assessment of spatial variability of soil properties using geospatial techniques for farm level nutrient management. Soil Till. Res. 2017, 169, 25−34. [Google Scholar] [CrossRef]
- Ichami, S.M.; Shepherd, K.D.; Hoffland, E.; Karuku, G.N.; Stoorvogel, J.J. Soil spatial variation to guide the development of fertilizer use recommendations for smallholder farms in western Kenya. Geoderma Reg. 2020, 22, e00300. [Google Scholar] [CrossRef]
- Shepherd, K.D. Soil spectral diagnostics-infrared, X-ray and laser diffraction spectroscopy for rapid soil characterization in the Africa Soil Information Service. In Proceedings of the 19th World Congress of Soil Science, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Kamau, M.N.; Shepherd, K.D. Mineralogy of Africa’s soils as a predictor of soil fertility. In Proceedings of the RUFORUM Third Biennial Conference, Entebbe, Uganda, 24–28 September 2012. [Google Scholar]
- Song, F.; Xu, M.; Duan, Y.; Cai, Z.; Wen, S.; Chen, X.; Shi, W.; Colinet, G. Spatial variability of soil properties in red soil and its implications for site-specific fertilizer management. J. Integr. Agric. 2020, 19, 2313–2325. [Google Scholar] [CrossRef]
- Yao, R.J.; Yang, J.S.; Zhang, T.J.; Gao, P.; Wang, X.P.; Hong, L.Z.; Wang, M.W. Determination of site-specific management zones using soil physico-chemical properties and crop yields in coastal reclaimed farmland. Geoderma 2014, 232–234, 381–393. [Google Scholar] [CrossRef]
- FAO. Guidelines for Soil Description, 4th ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Bouyocous, G.J. Hydrometer method improved for making particle size analysis of soil. Agron. J. 1962, 54, 3. [Google Scholar]
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 7th ed.; Technical Report 9; ISRIC—World Soil Information: Wageningen, The Netherlands, 2006. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining organic carbon in soils: Effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci. 1934, 63, 251–263. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Total nitrogen. In Methods of Soil Analysis; Black, C.A., Ed.; Part 2; American Society of Agronomy Inc.: Madison, WI, USA, 1982; pp. 1149–1178. [Google Scholar]
- Fanuel, L.; Kibebew, K. Explaining soil fertility heterogeneity in smallholder farms of Southern Ethiopia. Appl. Environ. Soil Sci. 2020, 2020, 6161059. [Google Scholar]
- Tittonell, P.; Muriuki, A.; Klapwijk, C.J.; Shepherd, K.D.; Coe, R.; Vanlauwe, B. Soil heterogeneity and soil fertility gradients in smallholder farms of the East African highlands. Soil Sci. Soc. Am. J. 2013, 77, 525–538. [Google Scholar] [CrossRef]
- Chivenge, P.; Zingore, S.; Ezui, S.; Njoroge, S.; Bunquin, M.A.; Dobermann, A.; Saito, K. Progress in research on site-specific nutrient management for smallholder farmers in sub-Saharan Africa. Field Crops Res. 2022, 281, 108503. [Google Scholar] [CrossRef] [PubMed]
- Karltun, E.; Lemenih, M.; Tolera, M. Comparing farmers’ perception of soil fertility change with soil properties and crop performance in Beseku, Ethiopia. Land Degrad. Dev. 2013, 24, 228−235. [Google Scholar] [CrossRef]
- Bruce, R.C.; Rayment, G.E. Soil Testing and Some Soil Test Interpretations Used by the Queensland Department of Primary Industries; Bulletin QB8 (2004); Queensland Department of Primary Industries: Brisbane, Australia, 1982.
- Yifru, A.; Taye, B. Effects of land use on soil organic carbon and nitrogen in soils of Bale, southeastern Ethiopia. Trop. Subtrop. Agroecosyst. 2011, 14, 229–235. [Google Scholar]
- Iticha, B.; Takele, C. Digital soil mapping for site-specific management of soils. Geoderma 2019, 351, 85–91. [Google Scholar] [CrossRef]
- Dawit, S.; Fritzsche, F.; Tekalign, M.; Lehmann, J.; Zech, W. Phosphorus forms and dynamics as influenced by land use changes in the sub-humid Ethiopian highlands. Geoderma 2002, 105, 21–48. [Google Scholar]
- Von Wandruszka, R. Phosphorus retention in calcareous soils and the effect of organic matter on its mobility. Geochem Trans. 2006, 7, 6. [Google Scholar] [CrossRef]
- Laekemariam, F.; Kibret, K.; Shiferaw, H. Potassium (K)-to-magnesium (Mg) ratio, its spatial variability and implications to potential Mg-induced K deficiency in Nitisols of Southern Ethiopia. Agric. Food. Secur. 2018, 7, 13. [Google Scholar] [CrossRef]
- Sori, G.; Iticha, B.; Takele, C. Spatial prediction of soil acidity and nutrients for site-specific soil management in Bedele district, Southwestern Ethiopia. Agric. Food Secur. 2021, 10, 59. [Google Scholar] [CrossRef]
- Ethiopian Soils Information System (EthioSIS). Soil fertility status and fertilizer recommendation atlas of Amhara National Regional State, Ethiopia; Ethiopian Soils Information System (EthioSIS): Addis Ababa, Ethiopia, 2016. [Google Scholar]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant–soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Xu, Q.; Fu, H.; Zhu, B.; Hussain, H.A.; Zhang, K.; Tian, X.; Duan, M.; Xie, X.; Wang, L. Potassium improves drought stress tolerance in plants by affecting root morphology, root exudates and microbial diversity. Metabolites 2021, 11, 131. [Google Scholar] [CrossRef]
- Osemwota, I.O.; Omueti, J.A.I.; Ogboghodo, A.I. Effect of calcium/magnesium ratio in soil on magnesium availability, yield, and yield components of maize. Commun. Soil Sci. Plant Anal. 2007, 38, 19–20. [Google Scholar] [CrossRef]
- Eckert, D.J. Soil test interpretations: Basic cation saturation ratios and sufficiency levels. In Soil Testing: Sampling, Correlation, Calibration, and Interpretation; Brown, J.R., Ed.; SSSA: Madison, WI, USA, 1987; pp. 53–64. [Google Scholar]
- Loide, V. About the effect of the contents and ratios of soil’s available calcium, potassium and magnesium in liming of acid soils. Agron. Res. 2004, 2, 71–82. [Google Scholar]
- Hillette, H.; Tekalign, M.; Riikka, K.; Erik, K.; Heluf, G.; Taye, B. Soil fertility status and wheat nutrient content in Vertisol cropping systems of central highlands of Ethiopia. Agric Food Secur. 2015, 4, 19. [Google Scholar]
- Chianu, J.N.; Chianu, J.N.; Mairura, F. Mineral fertilizers in the farming systems of sub-Saharan Africa. A review. Agron. Sustain. Dev. 2012, 32, 545–566. [Google Scholar] [CrossRef]
- Chikowo, R.; Zingore, S.; Snapp, S.; Johnston, A. Farm typologies, soil fertility variability and nutrient management in smallholder farming in Sub-Saharan Africa. Nutr Cycling Agroecosyst. 2014, 100, 1–18. [Google Scholar] [CrossRef]
- Amacher, M.C.; O’Neil, K.P.; Perry, C.H. Soil Vital Signs: A New Soil Quality Index (sqi) for Assessing Forest Soil Health; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2007.
- US Salinity Laboratory Staff. Diagnosis and Improvement of Saline and Alkali Soils; US Department of Agriculture Handbook 60; US Salinity Laboratory Staff: Washington, DC, USA, 1954. [Google Scholar]
- Bauder, T.A.; Waskom, R.M.; Southerland, P.L.; Davis, J.G. Irrigation Water Quality Criteria; Colorado State University: Fort Collins, CO, USA, 2011. [Google Scholar]
- Burger, F.; Celková, A. Salinity and sodicity hazard in water flow processes in the soil. Plant Soil Environ. 2003, 49, 314–320. [Google Scholar] [CrossRef]
- Rietz, D.N.; Haynes, R.J. Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 2003, 35, 845–854. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef]
- Minhas, P.S.; Qadir, M.; Yadav, R.K. Groundwater irrigation induced soil sodification and response options. Agric. Water Manag. 2019, 215, 74–85. [Google Scholar] [CrossRef]
- Mohanavelu, A.; Naganna, S.R.; Al-Ansari, N. Irrigation induced salinity and sodicity hazards on soil and groundwater: An overview of its causes, impacts and mitigation strategies. Agriculture 2021, 11, 983. [Google Scholar] [CrossRef]
- Zaman, M.; Shahid, S.A.; Heng, L. Irrigation Water Quality. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Ali, Y.; Aslam, Z. Use of environmental friendly fertilizers in saline and saline sodic soils. Int. J. Environ. Sci. Technol. 2005, 1, 97–98. [Google Scholar] [CrossRef]
- Selim, M.M. Introduction to the Integrated Nutrient Management Strategies and Their Contribution to Yield and Soil Properties. Int. J. Agron. 2020, 2020, 2821678. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential Benefits and Risks for Soil Health Derived From the Use of Organic Amendments in Agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sagasti, M.T.; Hernández, A.; Artetxe, U.; Garbisu, C.; Becerril, J.M. How Valuable Are Organic Amendments as Tools for the Phytomanagement of Degraded Soils? The Knowns, Known Unknowns, and Unknowns. Front. Sustain. Food Syst. 2018, 2, 68. [Google Scholar] [CrossRef]
- Bonanomi, G.; De Filippis, F.; Zotti, M.; Idbella, M.; Cesarano, G.; Al-Rowaily, S.; Abd-ElGawad, A. Repeated applications of organic amendments promote beneficial microbiota, improve soil fertility and increase crop yield. Appl. Soil Ecol. 2020, 156, 103714. [Google Scholar] [CrossRef]
- Bello, S.K.; Alayafi, A.H.; AL-Solaimani, S.G.; Abo-Elyousr, K.A.M. Mitigating soil salinity stress with gypsum and bio-organic amendments: A review. Agronomy 2021, 11, 1735. [Google Scholar] [CrossRef]
- Munns, R.; Husain, S.; Rivelli, A.R. Avenues for increasing salt tolerance of crops, and the role of physiologically based selection traits. Plant Soil 2002, 247, 93–105. [Google Scholar] [CrossRef]
- Nicholas, P. Soil, Irrigation and Nutrition; Grape Production Series No. 2; South Australian Research and Development Institute: Adelaide, Australia, 2004.
- Horneck, D.A.; Ellsworth, J.W.; Hopkins, B.G.; Sullivan, D.M.; Stevens, R.G. Managing Salt-Affected Soils for Crop Production; PNW 601-E; Oregon State University: Corvallis, OR, USA, 2007. [Google Scholar]
- Kumar, P.; Sharma, P.K. Soil Salinity and Food Security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar]
- Yadav, S.K.; Soni, R. Integrated soil fertility management. In Soil Fertility Management for Sustainable Development; Springer: Singapore, 2019; pp. 71–80. [Google Scholar]
- Arrouays, D.; Mulder, V.L.; Richer-de-Forges, A.C. Soil mapping, digital soil mapping and soil monitoring over large areas and the dimensions of soil security—A review. Soil Secur. 2021, 5, 100018. [Google Scholar] [CrossRef]
- Sistani, N.; Moeinaddini, M.; Ali-Taleshi, M.S.; Khorasani, N.; Hamidian, A.H.; Yancheshmeh, R.A. Source identification of heavy metal pollution nearby Kerman steel industries. J. Nat. Environ. 2017, 70, 627–641. [Google Scholar]
| Statistic | pH (H2O) | EC (mS cm) | Exchangeable Bases (cmol+ kg−1 Soil) | T.N (%) | O.C (%) | Av.P (ppm) | Ca:Mg | K:Mg | PBS (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | K | Ca | Mg | CEC | |||||||||
| Minimum | 7.3 | 0.11 | 0.04 | 0.04 | 29.74 | 4.27 | 42.40 | 0.08 | 0.98 | 1.14 | 3.39 | 0.01 | 73.22 |
| Maximum | 8.7 | 0.28 | 1.16 | 1.13 | 42.22 | 8.93 | 56.52 | 0.24 | 2.43 | 5.34 | 8.66 | 0.16 | 94.16 |
| Median | 7.7 | 0.22 | 0.24 | 0.44 | 37.23 | 6.38 | 52.40 | 0.15 | 1.55 | 2.54 | 5.84 | 0.06 | 87.12 |
| Mean | 7.9 | 0.21 | 0.42 | 0.49 | 37.09 | 6.44 | 51.52 | 0.16 | 1.69 | 2.69 | 6.04 | 0.08 | 86.30 |
| SD | 0.40 | 0.05 | 0.39 | 0.28 | 4.05 | 1.49 | 3.29 | 0.04 | 0.42 | 1.27 | 1.45 | 0.04 | 6.43 |
| Skewness | 0.60 | −0.58 | 0.76 | 0.64 | −0.62 | 0.29 | −1.35 | 0.21 | 0.48 | 0.80 | −0.07 | 0.32 | −0.50 |
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | |
|---|---|---|---|---|---|---|---|---|
| Eigenvalue | 5.29 | 2.52 | 1.91 | 1.83 | 1.24 | 0.51 | 0.15 | 0.12 |
| Variability (%) | 35.29 | 16.83 | 12.75 | 12.18 | 8.27 | 3.37 | 1.02 | 0.78 |
| Cumulative (%) | 35.29 | 52.11 | 64.86 | 77.04 | 85.31 | 88.68 | 89.70 | 90.48 |
| SMU | Slope (%) | Soil Depth (cm) | Soil Particle Distribution (%) | Soil Texture | Area (%) | ||
|---|---|---|---|---|---|---|---|
| Sand | Silt | Clay | |||||
| SMU1 | 0–2 | >150 | 23 | 30 | 47 | C | 7.47 |
| SMU2 | 0–2 | 50–100 | 42 | 34 | 24 | L | 12.16 |
| SMU3 | 0–2 | 50–100 | 27 | 27 | 46 | C | 5.47 |
| SMU4 | 2–4 | 100–150 | 26 | 28 | 46 | C | 20.75 |
| SMU5 | 4–6 | 50–100 | 47 | 27 | 26 | SCL | 4.45 |
| SMU6 | 4–6 | 50–100 | 39 | 26 | 35 | CL | 3.79 |
| SMU7 | 4–6 | >150 | 27 | 29 | 44 | C | 7.88 |
| SMU8 | 6–8 | 100–150 | 37 | 29 | 34 | CL | 13.17 |
| SMU9 | 6–8 | 100–150 | 35 | 12 | 53 | C | 3.44 |
| SMU10 | 6–8 | >150 | 27 | 39 | 34 | CL | 8.66 |
| SMU11 | 8–15 | <50 | 35 | 41 | 24 | L | 3.35 |
| SMU12 | 4–6 | 100–150 | 35 | 23 | 42 | C | 9.42 |
| SMUs | n | Mean, SD | Exchangeable Bases-Mean (cmol+ kg−1 Soil), SD | Mean, SD | Mean, SD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH (H2O) | EC (mS cm−1) | Ex. Na | Ex. K | Ex. Ca | Ex. Mg | CEC | Ca:Mg | K:Mg | AvP (ppm) | PBS (%) | OC (%) | TN (%) | ||
| SMU1 | 5 | 7.9, 0.28 | 0.62, 0.61 | 0.28, 0.20 | 0.48, 0.22 | 34.48, 2.10 | 6.65, 0.40 | 47.31, 3.65 | 5.21, 0.49 | 0.07, 0.03 | 2.21, 1.74 | 88.69, 3.11 | 1.53, 0.52 | 0.13, 0.04 |
| SMU2 | 6 | 7.3, 0.06 | 0.20, 0.01 | 0.25, 0.05 | 0.35, 0.05 | 38.47, 1.76 | 5.41, 0.03 | 50.90, 2.12 | 7.11, 0.29 | 0.06, 0.01 | 2.79, 1.43 | 87.39, 0.11 | 1.45, 0.01 | 0.12, 0.09 |
| SMU3 | 5 | 7.7, 0.02 | 0.23, 0.08 | 1.43, 0.60 | 0.13, 0.14 | 33.48, 5.29 | 5.64, 0.58 | 50.90, 3.54 | 6.02, 1.56 | 0.02, 0.03 | 1.44, 0.51 | 79.82, 2.81 | 1.30, 0.14 | 0.12, 0.03 |
| SMU4 | 9 | 7.6, 0.40 | 1.36, 1.67 | 1.67, 1.43 | 0.53, 0.47 | 30.92, 2.75 | 9.07, 2.54 | 51.82, 2.65 | 3.67, 1.15 | 0.07, 0.07 | 1.66, 1.16 | 81.46, 2.93 | 1.43, 0.56 | 0.14, 0.06 |
| SMU5 | 6 | 8.6, 0.03 | 0.22, 0.04 | 0.49, 0.63 | 0.47, 0.36 | 40.64, 1.02 | 6.69, 2.94 | 54.59, 3.66 | 6.69, 2.79 | 0.09, 0.09 | 1.81, 0.61 | 88.41, 1.82 | 1.66, 0.38 | 0.16, 0.06 |
| SMU6 | 4 | 7.4, 0.23 | 0.49, 0.54 | 1.05, 1.02 | 0.17, 0.20 | 38.47, 5.29 | 8.27, 2.79 | 52.39, 1.43 | 5.05, 2.34 | 0.03, 0.03 | 1.35, 0.49 | 91.53, 0.71 | 1.37, 0.54 | 0.11, 0.06 |
| SMU7 | 6 | 7.5, 0.12 | 0.67, 0.25 | 1.34, 0.12 | 0.12, 0.03 | 31.40, 1.44 | 4.54, 0.63 | 44.07, 2.08 | 7.01, 0.99 | 0.03, 0.01 | 2.22, 0.62 | 84.91, 3.22 | 1.10, 0.05 | 0.10, 0.02 |
| SMU8 | 7 | 7.5, 0.22 | 1.62, 1.85 | 1.23, 1.21 | 0.16, 0.18 | 33.57, 3.75 | 7.78, 2.01 | 47.07, 1.53 | 4.56, 1.47 | 0.02, 0.03 | 1.80, 0.79 | 90.80, 2.36 | 1.40, 0.14 | 0.13, 0.01 |
| SMU9 | 4 | 7.7, 0.14 | 1.01, 0.88 | 1.34, 1.20 | 0.19, 0.27 | 36.06, 4.30 | 9.53, 0.89 | 51.47, 1.90 | 3.83, 0.83 | 0.02, 0.03 | 1.39, 0.60 | 91.53, 2.30 | 1.48, 0.31 | 0.13, 0.02 |
| SMU10 | 6 | 8.4, 0.13 | 0.23, 0.03 | 0.37, 0.27 | 0.95, 0.34 | 42.01, 2.39 | 7.46, 1.71 | 54.94, 2.64 | 5.89, 1.46 | 0.13, 0.05 | 4.48, 2.48 | 92.60, 5.62 | 1.15, 0.26 | 0.10, 0.03 |
| SMU11 | 4 | 8.0, 0.53 | 0.24, 0.04 | 0.31, 0.28 | 0.16, 0.17 | 37.33, 3.67 | 5.83, 1.47 | 47.50, 7.21 | 3.72, 4.99 | 0.61, 0.85 | 2.07, 0.66 | 92.00, 2.20 | 1.89, 0.77 | 0.17, 0.06 |
| SMU12 | 6 | 7.8, 0.14 | 2.05, 2.08 | 3.95, 2.75 | 0.20, 0.15 | 24.75, 4.99 | 9.51, 2.27 | 46.07, 3.79 | 2.75, 0.97 | 0.02, 0.02 | 2.83, 1.62 | 83.58, 5.02 | 1.41, 0.24 | 0.13, 0.03 |
| SMUs | Potential Management Measures | References |
|---|---|---|
| SMU1 | Variable rate ammonium containing N and P fertilizers, K-fertilizer, good-quality irrigation water, integrated soil nutrient management, organic amendments such as biochar, compost, animal manures, crop residues, digestate, and biosolids. | [54,55,56,57] |
| SMU2 | ||
| SMU3 | ||
| SMU4 | ||
| SMU5 | ||
| SMU6 | ||
| SMU7 | ||
| SMU8 | ||
| SMU9 | ||
| SMU10 | Ammonium containing N and P fertilizers, good-quality irrigation water, organic amendments. | [58,59,60] |
| SMU11 | Ammonium containing P fertilizers, K-fertilizer, good-quality irrigation water, organic amendments. | [55,56,57,60] |
| SMU12 | Gypsum, ammonium containing N and P fertilizers, K fertilizer, good-quality irrigation water, salt tolerant crops, organic amendments. | [58,61,62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iticha, B.; Kamran, M.; Yan, R.; Siuta, D.; Al-Hashimi, A.; Takele, C.; Olana, F.; Kukfisz, B.; Iqbal, S.; Elshikh, M.S. The Role of Digital Soil Information in Assisting Precision Soil Management. Sustainability 2022, 14, 11710. https://doi.org/10.3390/su141811710
Iticha B, Kamran M, Yan R, Siuta D, Al-Hashimi A, Takele C, Olana F, Kukfisz B, Iqbal S, Elshikh MS. The Role of Digital Soil Information in Assisting Precision Soil Management. Sustainability. 2022; 14(18):11710. https://doi.org/10.3390/su141811710
Chicago/Turabian StyleIticha, Birhanu, Muhammad Kamran, Rui Yan, Dorota Siuta, Abdulrahman Al-Hashimi, Chalsissa Takele, Fayisa Olana, Bożena Kukfisz, Shehzad Iqbal, and Mohamed S. Elshikh. 2022. "The Role of Digital Soil Information in Assisting Precision Soil Management" Sustainability 14, no. 18: 11710. https://doi.org/10.3390/su141811710
APA StyleIticha, B., Kamran, M., Yan, R., Siuta, D., Al-Hashimi, A., Takele, C., Olana, F., Kukfisz, B., Iqbal, S., & Elshikh, M. S. (2022). The Role of Digital Soil Information in Assisting Precision Soil Management. Sustainability, 14(18), 11710. https://doi.org/10.3390/su141811710









