Geographic Variation in the Species Composition of Parrotfish (Labridae: Scarini) in the South China Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Data Collection
2.3. Nestedness Analysis
2.4. Statistical Analyses
3. Results
3.1. Species Composition
3.2. Composition of Functional Groups
3.3. Nestedness of Parrotfish Assemblages
3.4. The Distribution Characteristics of Functional Groups
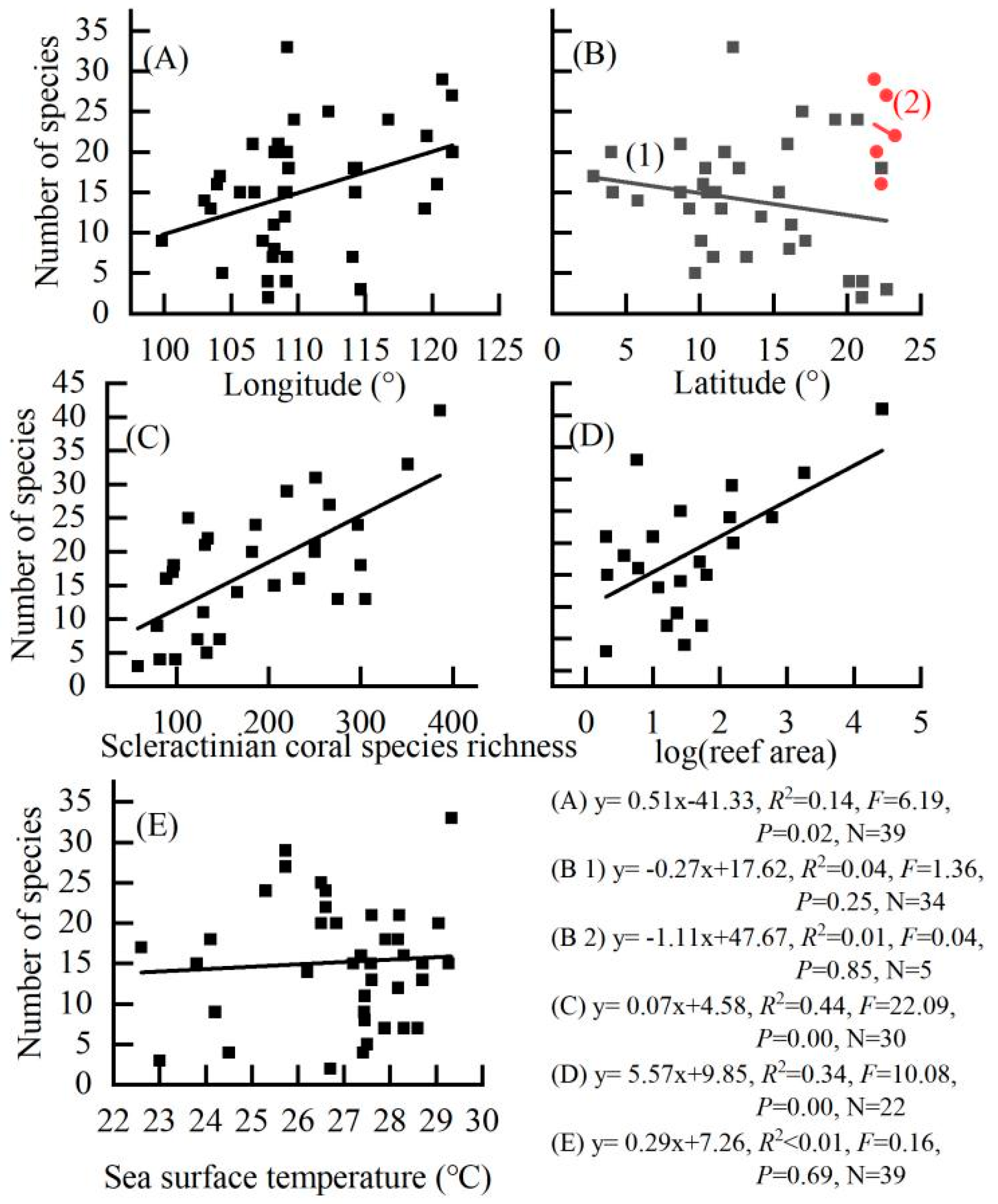
3.5. Patterns of Parrotfish Species Richness
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westneat, M.W.; Alfaro, M.E. Phylogenetic relationships and evolutionary history of the reef fish family Labridae. Mol. Phylogenet. Evol. 2005, 36, 370–390. [Google Scholar] [CrossRef]
- Sabetian, A. Parrotfish Fisheries and Population Dynamics: A Case-Study from Solomon Islands. Ph.D. Thesis, James Cook University, Townsville, Australia, 2010. [Google Scholar]
- Bonaldo, R.M.; Hoey, A.S.; Bellwood, D.R. The ecosystem roles of parrotfishes on tropical reefs. Oceanogr. Mar. Biol. 2014, 52, 81–132. [Google Scholar]
- Robertson, D.R.; Choat, J.H. Protogynous Hermaphroditism in Fishes of the Family Scaridae. In Intersexuality in the Animal Kingdom; Springer: Berlin/Heidelberg, Germany, 1975. [Google Scholar]
- Siebeck, U.E. Vision and Colour Diversity in Parrotfishes. In Biology of Parrotfishes; CRC Press: Boca Raton, FL, USA, 2018; pp. 99–118. [Google Scholar]
- Kulbicki, M.; Friedlander, A.M.; Mouillot, D.; Parravicini, V. Geographic Variation in the Composition and Function of Parrotfishes. In Biology of Parrotfishes; CRC Press: Boca Raton, FL, USA, 2018; pp. 215–244. [Google Scholar]
- Comeros-Raynal, M.; Choat, J.; Polidoro, B.; Clements, K.; Abesamis, R.; Craig, M.; Lazuardi, M.; McIlwain, J.; Muljadi, A.; Myers, R.; et al. The Likelihood of Extinction of Iconic and Dominant Herbivores and Detritivores of Coral Reefs: The Parrotfishes and Surgeonfishes. PLoS ONE 2012, 7, e39825. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fox, R.; Bellwood, D. Remote video bioassays reveal the potential feeding impact of the rabbitfish Siganus canaliculatus (f: Siganidae) on an inner-shelf reef of the Great Barrier Reef. Coral Reefs 2008, 27, 605–615. [Google Scholar] [CrossRef]
- Williams, I.; Polunin, N. Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs 2001, 19, 358–366. [Google Scholar] [CrossRef]
- Fox, R.J.; Bellwood, D.R. Quantifying herbivory across a coral reef depth gradient. Mar. Ecol. Prog. Ser. 2007, 339, 49–59. [Google Scholar] [CrossRef]
- Afeworki, Y.; Videler, J.J.; Bruggemann, J.H. Seasonally changing habitat use patterns among roving herbivorous fishes in the southern Red Sea: The role of temperature and algal community structure. Coral Reefs 2013, 32, 475–485. [Google Scholar] [CrossRef]
- Cinner, J.E.; McClanahan, T.R. Socioeconomic factors that lead to overfishing in small-scale coral reef fisheries of Papua New Guinea. Environ. Conserv. 2006, 33, 73–80. [Google Scholar] [CrossRef]
- Edwards, C.B.; Friedlander, A.M.; Green, A.G.; Hardt, M.J.; Sala, E.; Sweatman, H.P.; Williams, I.D.; Zgliczynski, B.; Sandin, S.A.; Smith, J.E. Global assessment of the status of coral reef herbivorous fishes: Evidence for fishing effects. Proc. R. Soc. B Biol. Sci. 2013, 281, 20131835. [Google Scholar] [CrossRef] [PubMed]
- McClanahan, T.; Maina, J.; Muthiga, N. Associations between climate stress and coral reef diversity in the Western Indian Ocean. Glob. Chang. Biol. 2011, 17, 2023–2032. [Google Scholar] [CrossRef]
- Pratchett, M.; Bay, L.; Gehrke, P.; Koehn, J.; Osborne, K.; Pressey, R.; Sweatman, H.; Wachenfeld, D. Contribution of climate change to degradation and loss of critical fish habitats in Australian marine and freshwater environments. Mar. Freshw. Res. 2011, 62, 1062–1081. [Google Scholar] [CrossRef]
- Hughes, T.P.; Rodrigues, M.J.; Bellwood, D.R.; Ceccarelli, D.; Hoegh-Guldberg, O.; McCook, L.; Moltschaniwskyj, N.; Pratchett, M.S.; Steneck, R.S.; Willis, B. Phase Shifts, Herbivory, and the Resilience of Coral Reefs to Climate Change. Curr. Biol. 2007, 17, 360–365. [Google Scholar] [CrossRef]
- Adam, T.C.; Burkepile, D.E.; Ruttenberg, B.I.; Paddack, M.J. Herbivory and the Resilience of Caribbean Coral Reefs: Knowledge Gaps and Implications for Management. Mar. Ecol. Prog. 2015, 520, 1–20. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hoey, A.S.; Hughes, T.P. Human activity selectively impacts the ecosystem roles of parrotfishes on coral reefs. Proc. R. Soc. B Biol. Sci. 2012, 279, 1621–1629. [Google Scholar] [CrossRef]
- Thurber, R.V.; Burkepile, D.; Correa, A.; Thurber, A.; Shantz, A.; Welsh, R.; Pritchard, C.; Rosales, S. Macroalgae Decrease Growth and Alter Microbial Community Structure of the Reef-Building Coral, Porites astreoides. PLoS ONE 2012, 7, e44246. [Google Scholar] [CrossRef] [PubMed]
- Roos, N.C.; Pennino, M.G.; de Macedo Lopes, P.F.; Carvalho, A.R. Multiple management strategies to control selectivity on parrotfishes harvesting. Ocean. Coast. Manag. 2016, 134, 20–29. [Google Scholar] [CrossRef]
- Choat, J.H.; Klanten, O.S.; Lynne, V.H.; Ross, R.D.; Clements, K.D. Patterns and processes in the evolutionary history of parrotfishes (Family Labridae). Biol. J. Linn. Soc. 2012, 107, 529–557. [Google Scholar] [CrossRef]
- Heena, A.; Hoey, A.S.; Williams, G.J.; Williams, I.D. Natural bounds on herbivorous coral reef fishes. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161716. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Roberts, C. Effects of fishing on sex-changing Caribbean parrot fishes. Biol. Conserv. 2004, 115, 213–226. [Google Scholar] [CrossRef]
- Hamilton, R.J.; Almany, G.R.; Brown, C.J.; Pita, J.; Peterson, N.A.; Howard Choat, J. Logging degrades nursery habitat for an iconic coral reef fish. Biol. Conserv. 2017, 210, 273–280. [Google Scholar] [CrossRef]
- Azhar, M.A.; Ulkhaq, M.F.; Kenconojati, H. Inventorization of reef fish on Tabuhan Island, Banyuwangi, East Java, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 236, 012041. [Google Scholar]
- Bellwood, D.R.; Hoey, A.S.; Ackerman, J.L.; Depczynski, M. Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Glob. Chang. Biol. 2006, 12, 1587–1594. [Google Scholar] [CrossRef]
- Emslie, M.J.; Cheal, A.J.; Johns, K.A.; Geraldine, C.M. Retention of Habitat Complexity Minimizes Disassembly of Reef Fish Communities following Disturbance: A Large-Scale Natural Experiment. PLoS ONE 2014, 9, e105384. [Google Scholar] [CrossRef] [PubMed]
- Andrew, H.; Emily, H.; Jacob, J.; Jean-Paul, H.; Vanessa, M.; Dominique, M.C.; Shaun, W.; Morgan, P. Recent Advances in Understanding the Effects of Climate Change on Coral Reefs. Diversity 2016, 8, 12. [Google Scholar]
- Maraun, M. The Theory of Island Biogeography Revisited. Basic Appl. Ecol. 2011, 12, 275. [Google Scholar] [CrossRef]
- Mora, C.; Chittaro, P.M.; Sale, P.F.; Kritzer, J.P.; Ludsin, S.A. Patterns and processes in reef fish diversity. Nature 2003, 421, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Bellwood, D.R.; Hughes, T.P. Regional-scale assembly rules and biodiversity of coral reefs. Science 2001, 292, 1532–1534. [Google Scholar] [CrossRef] [PubMed]
- Parravicini, V.; Kulbicki, M.; Bellwood, D.; Friedlander, A.; Arias, E.; Chabanet, P.; Floeter, S.; Myers, R.; Vigliola, L.; D’Agata, S.; et al. Global patterns and predictors of tropical reef fish species richness. Ecography 2013, 36, 1254–1262. [Google Scholar] [CrossRef]
- Allen, G. Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 541–556. [Google Scholar] [CrossRef]
- Choat, J.H. The Biology of Herbivorous Fishes on Coral Reefs. In The Ecology of Fishes on Coral Reefs; Academic Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Taylor, B.M.; Lindfield, S.J.; Choat, J.H. Hierarchical and scale-dependent effects of fishing pressure and environment on the structure and size distribution of parrotfish communities. Ecography 2014, 38, 520–530. [Google Scholar] [CrossRef]
- Wang, Z.-D.; Guo, Y.-S.; Liu, X.-M.; Fan, Y.-B.; Liu, C.-W. DNA barcoding South China Sea fishes. Mitochondrial DNA 2012, 23, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.; Tan, K.S. The state of marine biodiversity in the South China Sea. Raffles Bull. Zool. 2000, 48, 3–7. [Google Scholar]
- Randall, J.E.; Lim, K. A checklist of the fishes of the South China Sea. Raffles Bull. Zool. 2000, 8, 569–667. [Google Scholar]
- Gao, Y. The species diversity and trophic structure of reef fishes in the waters of the Xisha Archipelago. Biodivers. Sci. 2014, 22, 618–623. [Google Scholar]
- UNEP. Reversing Environmental Degradation Trends in the South China Sea and Gulf of Thailand. 2004. Available online: www.reefbase.org/gefll/pdf/Reversing%20Environmental%20Degradation%20Trends%20in%20the%20South%20China%20Sea%20and%20Gulf%20of%20Thailand.pdf (accessed on 1 March 2020).
- Emmers, R. Resource Management and Contested Territories in East Asia; Palgrave Pivot: London, UK, 2013. [Google Scholar] [CrossRef]
- Bellwood, D.; Howardchoat, J.; Bellwood, D.; Howardchoat, J.; Bellwood, D.; Howardchoat, J. A Functional Analysis of Grazing in Parrotfishes (family Scaridae): The Ecological Implications; Springer: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Ong, L.; Holland, K.N. Bioerosion of coral reefs by two Hawaiian parrotfishes: Species, size differences and fishery implications. Mar. Biol. 2010, 157, 1313–1323. [Google Scholar] [CrossRef]
- Shuihua, C.; Yujun, W. Nestedness pattern of insular community assemblages and its applications. Chin. J. Ecol. 2004, 23, 81–87. [Google Scholar]
- Cook, R.R.; Angermeier, P.L.; Finn, D.S.; Krueger, P.K.L. Geographic variation in patterns of nestedness among local stream fish assemblages in Virginia. Oecologia 2004, 140, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gironés, M.A.; Santamaría, L. A new algorithm to calculate the nestedness temperature of presence–absence matrices. J. Biogeogr. 2006, 33, 924–935. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Jiang, P.; Peng, L.; Ping, D. Nested analysis of passeriform bird assemblages in the Thousand Island Lake region. Biodivers. Sci. 2008, 16, 321–331. [Google Scholar]
- Boecklen, W.J. Nestedness, biogeographic theory, and the design of nature reserves. Oecologia 1997, 112, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.H.; Patterson, B.D.; Mikkelson, G.M.; Atmar, C.W. A Comparative Analysis of Nested Subset Patterns of Species Composition. Oecologia 1998, 113, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; Swihart, R.K. Toward ecologically explicit null models of nestedness. Oecologia 2007, 152, 763–777. [Google Scholar] [CrossRef] [PubMed]
- Schouten, M.; Verweij, P.A.; Barendregt, A.; Kleukers, R.; Ruiter, P. Nested assemblages of Orthoptera species in the Netherlands: The importance of habitat features and life-history traits. J. Biogeogr. 2007, 34, 1938–1946. [Google Scholar] [CrossRef]
- Azeria, E.; Kolasa, J. Nestedness, niche metrics and temporal dynamics of a metacommunity in a dynamic natural model system. Oikos 2008, 117, 1006–1019. [Google Scholar] [CrossRef]
- Frick, W.; Hayes, J.; Heady, P. Nestedness of desert bat assemblages: Species composition patterns in insular and terrestrial landscapes. Oecologia 2008, 158, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Alabdeh, D.; Omidvar, B.; Karbassi, A.; Sarang, A. Study of speciation and spatial variation of pollutants in Anzali Wetland (Iran) using linear regression, Kriging and multivariate analysis. Environ. Sci. Pollut. Res. 2020, 27, 16827–16840. [Google Scholar] [CrossRef]
- Parravicini, V.; Villéger, S.; Mcclanahan, T.R.; Arias-González, J.E.; Bellwood, D.R.; Belmaker, J.; Chabanet, P.; Floeter, S.R.; Friedlander, A.M.; Guilhaumon, F. Global mismatch between species richness and vulnerability of reef fish assemblages. Ecol. Lett. 2014, 17, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Cheal, A.; Emslie, M.J.; Miller, I.; Sweatman, H. The distribution of herbivorous fishes on the Great Barrier Reef. Mar. Biol. 2012, 159, 1143–1154. [Google Scholar] [CrossRef]
- Ishihara, T.; Tachihara, K. Pelagic Larval Duration and Settlement Size of Apogonidae, Labridae, Scaridae, and Tripterygiidae Species in a Coral Lagoon of Okinawa Island, Southern Japan. Pac. Sci. 2011, 65, 87–93. [Google Scholar] [CrossRef][Green Version]
- Cook, R.R. The relationship between nested subsets, habitat subdivision, and species diversity. Oecologia 1995, 101, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Fleishman, E.; Betrus, C.; Blair, R.; Mac Nally, R.; Murphy, D. Nestedness analysis and conservation planning: The importance of place, environment, and life history across taxonomic groups. Oecologia 2002, 133, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.; Leprieur, F.; Mouillot, D.; Kulbicki, M.; Parravicini, V.; Pie, M.; Barneche, D.; Oliveira-Santos, L.; Floeter, S. Isolation drives taxonomic and functional nestedness in tropical reef fish faunas. Ecography 2016, 40, 425–435. [Google Scholar] [CrossRef]
- Wang, T.; Sun, T.; Xiao, Y.Y.; Liu, Y.; Li, C.H.; Chen, D.F.; Li, H. Nested Distribution Patterns of Fish Assemblages in Daya Bay. Chin. J. Zool. 2019, 54, 327–338. [Google Scholar] [CrossRef]
- Luiz, O.J.; Madin, J.S.; Robertson, D.R.; Rocha, L.A.; Wirtz, P.; Floeter, S.R. Ecological traits influencing range expansion across large oceanic dispersal barriers: Insights from tropical Atlantic reef fishes. Proc. Biol. Sci. 2012, 279, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Luiz, O.J.; Allen, A.P.; Robertson, D.R.; Floeter, S.R.; Kulbicki, M.; Vigliola, L.; Becheler, R.; Madin, J.S. Adult and larval traits as determinants of geographic range size among tropical reef fishes. Proc. Natl. Acad. Sci. USA 2013, 110, 16498–16502. [Google Scholar] [CrossRef] [PubMed]
- Kulbicki, M.; Parravicini, V.; Mouillot, D. Patterns and Processes in Reef Fish Body Size. In Ecology of Fishes on Coral Reefs; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Siqueira, A.C.; Morais, R.A.; Bellwood, D.R.; Cowman, P.F. Planktivores as trophic drivers of global coral reef fish diversity patterns. Proc. Natl. Acad. Sci. USA 2021, 118, e2019404118. [Google Scholar] [CrossRef]
- Floeter, S.; Behrens, M.; Ferreira, C.; Paddack, M.; Horn, M. Geographical gradients of marine herbivorous fishes: Patterns and processes. Mar. Biol. 2005, 147, 1435–1447. [Google Scholar] [CrossRef]
- Wu, F.-Q.; Ni, Y.; Zhong, X.-M.; Tang, J.-H.; Wu, L.; Gao, Y.-S. Three new records on the fish fauna of Jiangsu. Mar. Fish. 2015, 37, 87–92. [Google Scholar] [CrossRef]
- Wang, J.F.; Si, J.C.; Yu, F. Progress in studies of the characteristics and mechanisms of variations in the Taiwan Warm Current. Mar. Sci. 2020, 44, 141–148. [Google Scholar]
- Floeter, S.R.; Guimaraes, R.; Rocha, L.A.; Ferreira, C.; Gasparini, R. Geographic Variation in Reef-Fish Assemblages along the Brazilian Coast. Glob. Ecol. Biogeogr. 2001, 10, 423–431. [Google Scholar] [CrossRef]
- Musburger, C.A. The Biogeography of Central Pacific Coral Reef Fishes. Ph.D. Thesis, University of Hawaii at Manoa, Honolulu, HI, USA, 2012. [Google Scholar]
- Hoey, A.S.; Feary, D.A.; Burt, J.A.; Vaughan, G.; Pratchett, M.S.; Berumen, M.L. Regional variation in the structure and function of parrotfishes on Arabian reefs. Mar. Pollut. Bull. 2016, 105, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Licuanan, W.Y.; Gomez, E.D. Philippine coral reefs: Status and the role of the academe to improve their management. In Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000. [Google Scholar]
- Johnson, M.K.; Holbrook, S.J.; Schmitt, R.J.; Brooks, A.J. Fish communities on staghorn coral: Effects of habitat characteristics and resident farmerfishes. Environ. Biol. Fishes 2011, 91, 429–448. [Google Scholar] [CrossRef]
- Ivan, N.; Grol, M.; Mumby, P.J.; Simon, T. Effects of Marine Reserves versus Nursery Habitat Availability on Structure of Reef Fish Communities. PLoS ONE 2012, 7, e36906. [Google Scholar]
- Yu, K.F. Introduction to the Science of Coral Reefs; Science Press: Beijing, China, 2018; p. 24. [Google Scholar]
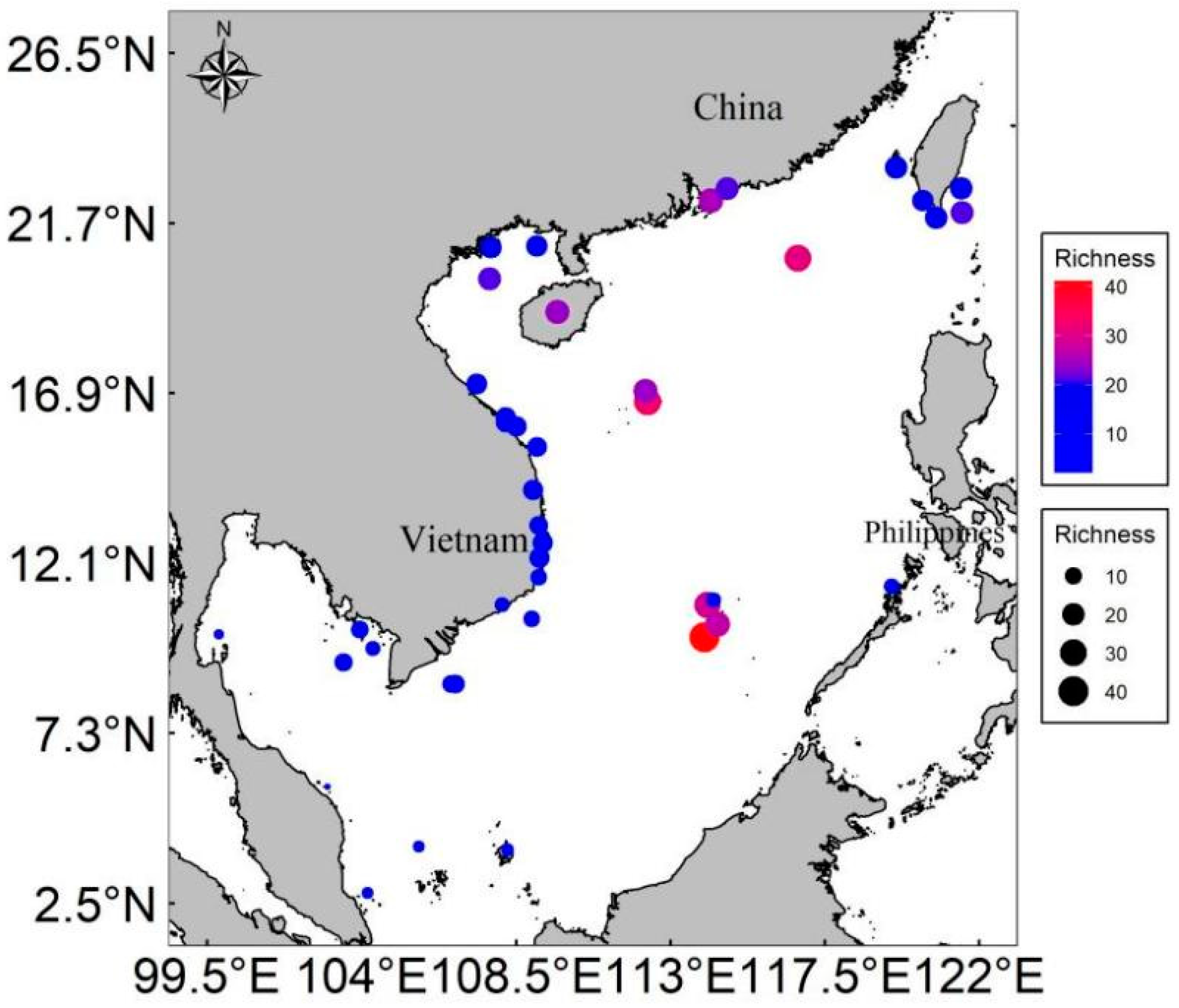
| Site | Scrapers | Excavators | Browsers | Site | Scrapers | Excavators | Browsers |
|---|---|---|---|---|---|---|---|
| Nansha Islands | 26 | 12 | 3 | Nui Chua | 16 | 4 | 0 |
| Xisha Islands | 20 | 7 | 4 | Hon Cau | 12 | 3 | 0 |
| Dongsha Islands | 16 | 5 | 3 | Phu Quy | 12 | 3 | 0 |
| Subi Reef | 3 | 3 | 1 | Con Dao | 16 | 5 | 0 |
| Qilianyu | 18 | 6 | 1 | Phu Quoc | 12 | 4 | 0 |
| Taiping Island | 12 | 6 | 0 | Nam Du | 4 | 1 | 0 |
| Hainan Island | 16 | 5 | 3 | Tho Chu | 13 | 0 | 0 |
| Hong Kong | 11 | 5 | 2 | Cu Lao Cau Bay | 6 | 1 | 0 |
| Daya Bay | 2 | 1 | 0 | Zhongye Island 1 | 10 | 2 | 1 |
| Weizhou Island | 3 | 1 | 0 | EI Nido 2 | 8 | 3 | 0 |
| Kenting National Park | 19 | 7 | 3 | Natuna Islands | 12 | 8 | 0 |
| Green Island | 18 | 6 | 3 | Anambas Islands | 9 | 6 | 0 |
| Lanyu | 12 | 6 | 2 | Timon Island | 11 | 6 | 0 |
| Ryukyu | 10 | 3 | 3 | Redang Island | 10 | 4 | 0 |
| South Penghu National Park 3 | 15 | 5 | 1 | Koh Tao | 8 | 1 | 0 |
| Co To | 1 | 1 | 0 | Pearl River estuary | 7 | 2 | 2 |
| Bach Long Vi | 2 | 2 | 0 | Minjiang River estuary | 1 | 0 | 1 |
| Con Co | 6 | 3 | 0 | Jiulong River estuary | 3 | 1 | 1 |
| Hai Van-Son Cha | 8 | 3 | 0 | Taiwan | 24 | 10 | 4 |
| Da Nang | 7 | 1 | 0 | Eastern Taiwan | 11 | 5 | 1 |
| Cu Lao Cham | 16 | 5 | 0 | Southern Taiwan | 22 | 10 | 4 |
| Ly Son | 12 | 3 | 0 | Western Taiwan | 4 | 3 | 0 |
| Binh Dinh | 10 | 2 | 0 | Northern Taiwan | 13 | 2 | 2 |
| Phu Yen | 6 | 1 | 0 | Brunei Darussalam | 1 | 3 | 1 |
| Van Phong | 13 | 5 | 0 | Cambodia | 3 | 0 | 0 |
| Nha Trang | 21 | 9 | 3 |
| Nested Rank for Species 1 | Nested Rank for Sites 2 | |||
|---|---|---|---|---|
| R | p | r | p | |
| Maximum body length 1 (mm) | −0.024 | 0.869 | ||
| Longitude 2 (°) | −0.371 * | 0.020 | ||
| Latitude 2 (°) | −0.070 | 0.673 | ||
| Scleractinian coral species richness 2 | −0.569 ** | 0.001 | ||
| log(reef area) 2 (km²) | −0.453 * | 0.034 | ||
| Sea surface temperature 2 (°C) | −0.117 | 0.498 | ||
| t | Sig. (2-Sided) | |
|---|---|---|
| Scraper | 6.817 | 0.000 ** |
| Browser | 4.346 | 0.000 ** |
| Excavator | 7.142 | 0.000 ** |
| Scraper/Total species of parrotfish | −1.609 | 0.120 |
| Browser/Total species of parrotfish | 3.407 | 0.002 ** |
| Excavator/Total species of parrotfish | 0.475 | 0.640 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, Q.; Liu, Y.; Wang, T.; Li, C. Geographic Variation in the Species Composition of Parrotfish (Labridae: Scarini) in the South China Sea. Sustainability 2022, 14, 11524. https://doi.org/10.3390/su141811524
Quan Q, Liu Y, Wang T, Li C. Geographic Variation in the Species Composition of Parrotfish (Labridae: Scarini) in the South China Sea. Sustainability. 2022; 14(18):11524. https://doi.org/10.3390/su141811524
Chicago/Turabian StyleQuan, Qiumei, Yong Liu, Teng Wang, and Chunhou Li. 2022. "Geographic Variation in the Species Composition of Parrotfish (Labridae: Scarini) in the South China Sea" Sustainability 14, no. 18: 11524. https://doi.org/10.3390/su141811524
APA StyleQuan, Q., Liu, Y., Wang, T., & Li, C. (2022). Geographic Variation in the Species Composition of Parrotfish (Labridae: Scarini) in the South China Sea. Sustainability, 14(18), 11524. https://doi.org/10.3390/su141811524






