Abstract
Pollution evaluation and health risk assessment are critical procedures for residents consuming black carp (Mylopharyngodon piceus) contaminated with non-essential hazardous trace elements in an artificial wetland also known as an aquaculture pond. Samples were collected, dissected and digested to analyze the pollution status and health risk associated with four heavy metals present in eleven tissues of black carp fish based on the metal pollution index (MPI) and target hazard quotient (THQ). The results indicated that the concentrations of Cd, Cr, Cu and Pb were 1.36 ± 0.04 mg/kg, 13.70 ± 0.50 mg/kg, 2.85 ± 0.10 mg/kg, and 4.98 ± 0.18 mg/kg in large black carp, while the concentrations of Cd, Cr, Cu and Pb were 4.27 ± 0.08 mg/kg, 50.84 ± 0.82 mg/kg, 9.33 ± 0.18 mg/kg, and 22.49 ± 0.42 mg/kg in small specimens. The MPI values showed that the heavy metal pollution detected in small fish was much more significant than in large fish. Notably, the polluted metals were more likely to accumulate in the viscera (e.g., brain and heart) rather than in the external tissues (e.g., muscle and epidermis). In addition, the estimated THQ and HI values for three edible tissues demonstrated that the health risk associated with muscle tissue intake of both small and large black carp was within the acceptable range, while the contaminants were likely to pose health risks associated with the consumption of fish head tissue. Small black carp are generally not fit for human consumption, thus both the epidermis and muscle of the fish are often cooked in China. However, the ingestion of large black carp is relatively safe. The contributions to THQ values of these four heavy metals decreased in the following sequence: Cr > Pb > Cd > Cu. Cr is the critical pollutant with its contribution to HI values measuring over 50%. We suggest that in artificial wetlands (e.g., aquaculture ponds) more attention should be paid to heavy metal pollution, the conservation of the aquaculture ecosystem, and effects on human health.
1. Introduction
In the natural world, wetland ecosystems are as important to birds as food, clothing and shelter are to humans. Additionally, wetland ecosystems play an irreplaceable role as the primary habitat of waterbirds. However, waterbird habitat loss is gradually increasing due to the conversion of natural wetlands to artificial wetlands or significant disturbances attributed to various other reasons [1]. Fortunately, aquaculture ponds are emerging as a type of environmentally friendly artificial wetland [2], and can provide important surrogate habitats for waterbirds [3]. Furthermore, when pond water levels fall, they offer more resources to attract higher densities of feeding birds and large numbers of species [4]. However, aquaculture ponds around the world are facing anthropogenic pressures that threaten ecosystem processes and have caused serious environmental problems [5,6]. The environmental problems associated with aquaculture ponds can be mainly manifested in the bioaccumulation of metals in the tissues of organisms [7,8,9,10]. Some researchers have considered that the metal pollution is caused by the unreasonable use of commercial feeds and the habitat of aquaculture organisms [11]. Therefore, the conservation of this type of farm pond requires collaborative efforts over scales ranging from within-pond to regional [12]. To protect both this type of wetland and human health, it is extremely important to investigate the risks of heavy metals and pollution for organisms in aquaculture ponds.
Black carp are cultured in freshwater aquaculture ponds in most areas of Huzhou city in Zhejiang Province [13] due to their rapid growth, high yield, delicious meat, broad physiological tolerances, and other attractive attributes [14]. Previous researchers only investigated the mercury concentration of this species from Jiaozhou Bay in 2014 [15]. However, fourteen trace elements (including Cu and Cr) are currently included as essential elements in the commercial feed of fish and shrimp [16,17]. The research of heavy metals in soil sampled from Huzhou has demonstrated that Cr, Pb, and Cd pollution showed a clear upward trend from 2002 to 2012 due to the excessive use of nitrogen and phosphorus fertilizers containing heavy metals, as well as surface irrigation and natural soil formation [18,19]. Therefore, these four heavy metals (Cd, Cr, Cu, and Pb) were selected as the focus of attention for this study.
According to the Project Management Body of Knowledge (PMBOK), risk is defined as the uncertainty associated with negative impacts on human health and ecological systems resulting from exposure to different types of stressors including specific chemical contaminants, disease-causing microbial agents and stressful conditions [20]. Researchers commonly use risk assessments to characterize the nature and magnitude of risks to human health and ecological receptors [21]. For precise and scientific decision making, researchers have developed many risk assessment methods as decision-support tools [22]. These methods include the Linear Assignment Method, the Multi-Attribute Decision Making (MADM) Method and MCDM Method, which can be employed in various fields including the mining industry, volcanic disasters, and various other arenas [23,24,25,26].
In this study, we individually used the Metal Pollution Index (MPI) and the Target Hazard Quotient (THQ) to evaluate the degree of pollution and health risks associated with four heavy metals (Cd, Cr, Cu, and Pb) in eleven tissue types of two sizes of black carp from aquaculture ponds. This evaluation is vital to help understand the bioaccumulation and health risks associated with this type of artificial wetland in Huzhou city, located in the south of the lower reaches of the Yangtze River.
2. Materials and Methods
2.1. Sampling and Sample Preparation
The study site is located in the area of primary aquaculture occupancy at the junction of the Wuxing and Nanxun regions of Huzhou City, northern Zhejiang (Figure 1). This area is situated in the north subtropical monsoon climate zone. Aquaculture represents the main agricultural industry in the region.
Figure 1.
The study location is in the aquaculture-rich region of Huzhou City, Zhejiang.
We collected two sizes of black carp from aquaculture ponds in the regions of Huzhou city near Southern Taihu Lake. The number of individuals for each size category was 66. The large-sized black carp weighed 115.59 ± 4.29 g, while the small-sized sample individuals weighed 13.06 ± 0.31 g. The samples were placed in an oxygenated transport bag and stored in a refrigerator at a temperature of −20 °C. Next, the fish samples were weighed and dissected into 11 separate tissues, namely the fin, scale, epidermis, heart, liver, spleen, kidney, muscle, gill, brain and intestine, which were stored in centrifuge tubes.
2.2. Digestion of the Tissue Samples
Each tissue sample was placed in a digestion tube, to which 10 mL of nitric acid was added. Each tube was covered with a watch glass and placed in an electro-thermal digestion instrument overnight. The temperature of the digestion apparatus was set at 95 ± 5 ºC with a duration interval of 30 min. After the digestion period was completed, the watch glasses were removed, and the digestion solution was evaporated to about 3 mL. Then, 5 mL of concentrated nitric acid was added as the digestion process continued. Test samples that emanated brown smoke from the digestion tube had 5 mL of concentrated nitric acid added repeatedly until the bubbling and gas production ceased. A 1 mL volume of hydrogen peroxide was slowly added and the tubes were covered with the watch glasses. The digestion process was continued at the same temperature, until the bubbling stopped. If bubbles appeared, 1 mL of hydrogen peroxide was repeatedly added until the bubbling ceased. The watch glasses were removed, and the digestion solution was concentrated to about 3 mL. The digestion solution was then transferred to a 25 mL volumetric flask using 2% nitric acid.
2.3. Determination of the Four Heavy Metals
A standard series solution was prepared with a standard stock solution of 1000 μg/mL by using 2% nitric acid as a diluent. The standard series of solution concentrations for the four heavy metals were:
- For cadmium (Cd): 0.12, 0.24, 0.36, 0.48, 0.60 μg/mL
- For chromium (Cr): 0.50, 1.00, 1.50, 2.00, 2.50 μg/mL
- For copper (Cu): 0.30, 0.60, 0.90, 1.20, 1.50 μg/mL
- For lead (Pb): 1.00, 2.00, 3.00, 4.00, 5.00 μg/mL
The standards and digestion solutions were analyzed for cadmium, chromium, copper, and lead by FAAS (Agilent Varian AA240DUO). Three replicates were used per analyzed sample to control precision to below 10%. The lower limits of detection (LLOD) of Cd, Cr, Cu, and Pb were 0.05 μg/mL, 0.03 μg/mL, 0.05 μg/mL, and 0.20 μg/mL, respectively. A blank sample was processed to resolve any contamination by labware or chemicals. Verification of the method was conducted via analysis of standard reference materials (GSB 07-3186-2014) to confirm the accuracy of the data.
2.4. Data Manipulation and Statistical Methods
The concentrations of the four heavy metals are expressed as mg/kg wet weight, and the data are expressed as the arithmetic mean ± standard error (Mean ± SE). A scatter plot was developed using the standard series solution concentrations of cadmium, chromium, copper, and lead as abscissas and the absorbance as the ordinate. The scatter plot was fitted with the standard curve equations of cadmium, chromium, copper, and lead. The concentrations of the heavy metals in different tissues were calculated according to the volume of volumetric flask and the weight of each tissue. The experimental sample data were submitted to ANOVA in blocks on each different group (p < 0.05) to test their level of significance. When significant differences were identified in variance analysis, a multiple comparison Tukey Test was applied. SPSS software was used to administer these tests.
2.5. Pollution Evaluation
The concentrations of the four heavy metals were expressed as mg/kg wet weight and the data were expressed as the arithmetic mean ± standard error (Mean ± SE). The scatter plot was determined by using the standard series solution concentration of cadmium, chromium, copper, and lead as abscissas and the absorbance as the ordinate. The scatter plot was fitted with the standard curve equations of cadmium, chromium, copper and lead. Then, the concentrations of the heavy metals in different tissues were calculated according to the volume and the weight of each tissue. The experimental sample data were submitted to ANOVA in blocks on each different group (p < 0.05) to test their levels of significance. When significant differences were identified in variance analysis, a multiple comparison Tukey Test was applied. SPSS software was used to administer these tests.
The evaluation of each metal’s pollution level in various tissues of black carp was conducted by comparison between the limit values. The limit values were expressed as the minimum standard values derived from the Chinese National Standards (NY 5073-2006 and GB 2762-2012) (Table 1).

Table 1.
Limit values of four heavy metals in black carp.
The evaluation of total metal pollution in various tissues of black carp was conducted using the metal pollution index (MPI) [27], and was calculated according to the following formula (Equation (1)) [28]:
where is the concentration (mg/kg ww) of the metal n in the tissue sample of black carp [29].
2.6. Health Risk Assessment
The target hazard quotient (THQ) and hazard index (HI) were used to estimate the health risk of single and multiple metal intake via fish consumption, respectively [30]. The epidermis, muscle, and brain can be used for consumption in China when the whole fish is cooked. The estimated THQ and HI values for these three edible tissues were determined in our study. When THQ ≥ 1, there is a potential health risk to the exposed population, while the exposed population is unlikely to sustain conspicuous adverse effects when the THQ < 1 [31]. The THQ and HI values were calculated as follows:
where RfD is the reference dose of the toxicant (mg/kg/day). It is equivalent to the oral reference dose presented in Table 2. D is the intake dosage (mg/kg/day ww), as the exposure route of heavy metals in black carp for individual residents would occur via direct ingestion [32]. Thus, the ingestion intake dose (D) is described as:
where C is the heavy metal concentration in aquatic products (mg/kg ww), IR is the ingestion rate (g/day), EF is the exposure frequency (days/year), ED is the exposure duration (year), BW is the body weight (kg), and AT is the averaging time (days).
In this study, IR originated from the 2017 edition of China’s health and family planning statistical yearbook, Section 9-8-3, which covers the food intake per person per day for urban and rural residents. The EF duration for adult residents in Huzhou is 350 days/year, as this is the primary area of aquaculture production, and thus aquatic products represent the primary source of food for residents. ED is equal to the life expectancy in Section 9-2-2 from the 2017 edition of China’s health and family planning statistical yearbook based on the conception from the exposure factors handbook (2011 Edition) [33]. BW was calculated by interpolation based on the relationship between age and body weight from Section 8-7 in the 2008 edition of China’s health statistical yearbook. The detailed values for all these parameters are presented in Table 2. AT is equal to ED multiplied by 365 days/year.

Table 2.
The selected values of parameters in pollution evaluation and health risk assessment.
Table 2.
The selected values of parameters in pollution evaluation and health risk assessment.
| Parameters | Unit | Values | References |
|---|---|---|---|
| RfD (Cd) | mg/kg/day | 0.001 | [34] |
| RfD (Cr) | mg/kg/day | 0.003 | [34] |
| RfD (Cu) | mg/kg/day | 0.04 | [34] |
| RfD (Pb) | mg/kg/day | 0.004 | [35] |
| IR | g/day | 23.7 | [36] (p. 245) |
| EF | day/year | 350 | [37] |
| ED | year | 77.73 | [36] (p. 233) |
| BW | kg | 52.79 | [38] |
3. Results
3.1. Concentration of Heavy Metals in Large and Small Fish
The concentrations of Cd, Cr, Cu, and Pb in both large and small black carp are given in Figure 2. In total, the concentrations of Cd, Cr, Cu, and Pb in black carp were 3.31 ± 0.04 mg/kg, 38.53 ± 0.47 mg/kg, 7.18 ± 0.10 mg/kg, and 16.69 ± 0.24 mg/kg, respectively. In large black carp, the concentrations of Cd, Cr, Cu, and Pb were 1.36 ± 0.04 mg/kg, 13.70 ± 0.50 mg/kg, 2.85 ± 0.10 mg/kg, and 4.98 ± 0.18 mg/kg, while in small carp the concentrations of Cd, Cr, Cu, and Pb were 4.27 ± 0.08 mg/kg, 50.84 ± 0.82 mg/kg, 9.33 ± 0.18 mg/kg, and 22.49 ± 0.42 mg/kg, respectively. In comparison to health concern limit values, the concentrations of Cd, Cr, and Pb concentrations were above the permissible level for human consumption, while the Cu concentration was lower than the limit value. Notably, the concentrations of each metal in large fish were lower than in small ones. This indicates that black carp may be easily contaminated by Cr, Pb, and Cd pollution. The degree of pollution and bioaccumulation appears to be directly related to the size of the fish.
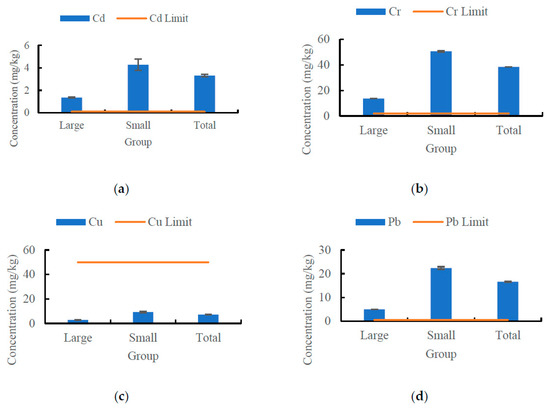
Figure 2.
The concentration of four heavy metals in two size categories of black carp. (a) Cd. (b) Cr. (c) Cu. (d) Pb.
3.2. Concentrations of Heavy Metals in Different Tissues
3.2.1. Concentration Levels of the Four Heavy Metals in Large Black Carp
The concentration levels of Cd, Cr, Cu, and Pb in 11 different tissues of large black carp are presented in Figure 3, ranging from:
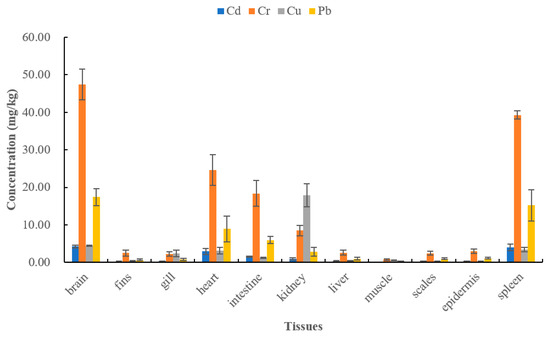
Figure 3.
The concentration levels of four heavy metals in different tissues of large black carp.
- 0.10 (muscle) to 4.26 (brain) mg/kg
- 0.77 (muscle) to 47.49 (brain) mg/kg
- 0.27 (epidermis) to 17.97 (kidney) mg/kg
- 0.24 (muscle) to 17.45 (brain) mg/kg
The pattern of heavy metal occurrences in all 11 tissues:
The Cd concentration levels listed in descending order was measured in the brain, spleen, heart, intestine, kidney, liver, gill, epidermis, scale, fin, and muscle.
The Cr concentration levels decreased in the sequence of brain, spleen, heart, intestine, kidney, epidermis, liver, fin, scale, gill, and muscle.
The rank of Cu concentration levels in various tissues: kidney, brain, spleen, heart, gill, intestine, muscle, liver, fin, scale, and epidermis.
The Pb concentration value measured in the tissues decreased in the following order, the brain, spleen, heart, intestine, kidney, epidermis, liver, scale, gill, fin, and muscle.
In comparison to the health concern limit values, the metal concentrations showed:
The Cu concentration value in all tissues measured below the permissible level.
The Cd, Cr, and Pb concentration values in various tissues exceeded the permissible levels in the order of brain, fin, gill, heart, intestine, kidney, liver, scale, epidermis, and spleen.
The Cd, Cr, and Pb concentration values in muscle tissue were below the permissible level.
These results revealed that Cu was not more likely to be above levels of concern in three edible tissues (muscle, epidermis, and brain). However, Cd, Cr, and Pb could concentrate in the epidermis and brain, while they were not more likely to exceed levels of concern in muscle tissue. Thus, the concentration levels of these four metals in the muscle tissue of large black carp were within the safety limits
3.2.2. Concentration Levels of Four Heavy Metals in Different Tissues of Small Black Carp
In small black carp, the concentrations of Cd, Cr, Cu, and Pb in the 11 tissues were within the following range of values:
- 0.15 (muscle) to 14.58 (heart) mg/kg
- 1.77 (muscle) to 158.73 (heart) mg/kg
- 0.98 (muscle) to 26.07 (heart) mg/kg
- 0.53 (muscle) to 87.65 (heart) mg/kg, as listed in Figure 4.
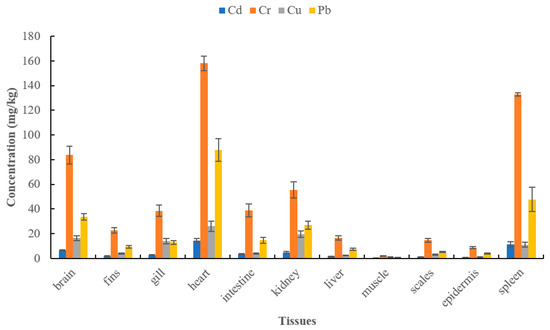 Figure 4. Concentration levels of four heavy metals in different tissues of small black carp.
Figure 4. Concentration levels of four heavy metals in different tissues of small black carp.
From the 11 tissues:
The order of Cd concentration from largest to smallest was heart, spleen, brain, kidney, intestine, gill, fin, liver, scale, epidermis, and muscle. The Cd concentrations in the epidermis and muscle were below the permissible level.
The Cr concentration levels decreased in the following order, the heart, spleen, brain, kidney, intestine, gill, fin, liver, scale, epidermis, and muscle. Only the muscle tissue had Cr concentrations below the level of health concern.
The Cu concentration levels decreased in the following order, the heart, kidney, brain, gill, spleen, intestine, fin, scale, liver, epidermis, and muscle. The Cu concentration in all tissues of small fish were below the level of health concern.
Listed in decreasing order of Pb concentration was the heart, spleen, brain, kidney, intestine, gill, fin, liver, scale, epidermis, and muscle, and the heavy metal concentrations of all tissues exceeded health safety concern levels.
These results revealed that the concentrations of Cu and Cr in three edible tissues were the same as those in large fish. In small fish, Pb concentration was high in muscle tissue, while Cd was only concentrated in the brain. This finding indicates that Pb contamination should be taken into consideration when handling the muscle tissue of small black carp for human consumption.
3.3. Degree of Concentration of Heavy Metals in Various Tissues of Black Carp
The levels of heavy metal contamination in various tissues of black carp are presented in Figure 5. The MPI values for the 11 tissues decreased in the following order:
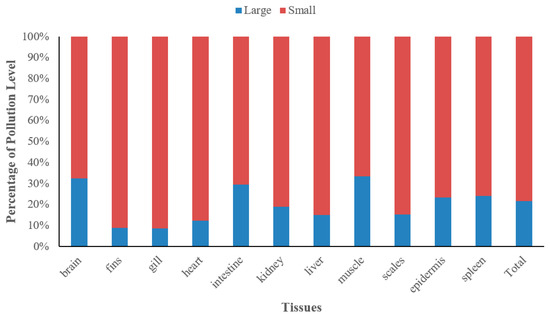
Figure 5.
Relative degree of heavy metal concentrations in various tissues of black carp.
- In large fish: brain, spleen, heart, kidney, intestine, gill, liver, scale, epidermis, fin, muscle.
- In small fish: heart, spleen, brain, kidney, gill, intestine, fin, liver, scale, epidermis, muscle.
The findings showed that the four heavy metals were more likely to be of concern in the viscera (e.g., brain, heart, kidney, spleen, and so forth) rather than in the external tissues (e.g., muscle, epidermis, scale, etc.). The MPI values for various tissues of small black carp were higher than those found in large black carp.
3.4. Health Risk Assessment of Heavy Metals in Black Carp
The THQ and HI values for each heavy metal in three edible tissues (epidermis, muscle and brain) of two sizes of black carp for adults are listed in Table 3. The HI values for large fish were less than those found in small fish. The HI values for muscle tissue of both large and small fish were well below the hazard quotient threshold of 1, while those for the brain were greater than 1. The HI value for the epidermis of large fish was lower than 1, while that for small fish was higher than 1. In addition, the contributions to THQ values of these four heavy metals decreased in the following sequence: Cr > Pb > Cd > Cu, where the contributions of Cr to HI values were over 50%. The THQ values of Cu for all three edible tissues were well below the hazard threshold of 1. Furthermore, the THQ values for the muscle tissue of both large and small fish were below 1, while the THQ values of Cd, Cr, and Pb for the brain were greater than 1. Unlike the other two edible tissues, the THQ value for the epidermis of large fish was also well below than 1, but that of small fish was higher than 1. These results indicate that the risks associated with the consumption of small black carp warrant consideration. Individuals consuming these fish should select muscle as the edible tissue instead of the brain and epidermis. Environmental managers should focus primarily on the effect of Cr on human health, and address the effect of Pb secondarily.

Table 3.
Health risk assessment for edible tissues in two size categories of black carp.
4. Discussion
Our results revealed that the concentrations of these four metals in the muscle tissue of both large and small black carp are at safe levels for human consumption. We compared the results derived from large black carp with those from muscle tissues of grass carp (Ctenopharyngodon idella), silver carp (Hypophthalmichthy smolitrix), and bighead carp (Hypophthalmichthys nobilis), respectively (Table 4). The Cu concentration level was only lower than that of grass carp in Guangdong, while the Cd concentration was below the level of grass carp in both Guangdong and the Pearl River Delta. The Cr concentration level was less that of grass carp in both Tianjing and Beijing, and lower than that of silver carp in Tianjing. The Pb concentration level was less than that of grass carp in both Guangdong and the Pearl River Delta, and below that of both silver carp and bighead carp in Meizhou. The Pb concentration levels in the muscle tissue of black carp from the Huzhou pond study were less than that of fish from Caohu Lake. In summary, the concentrations of these four heavy metals in black carp were at relatively high levels. Li et al. (2022) found that aquaculture ponds contribute significantly to heavy metal pollution [39]. Therefore, our determination that the pollution in the muscle tissue of black carp in a constructed environment was more serious than that in the natural environment was unsurprising.

Table 4.
Comparison of heavy metal concentrations in the muscle tissue of four major Chinese carp from ponds in different areas (mg/kg).
Previous studies proved that the highest metal concentrations for fish from aquaculture ponds are located in the brain, kidney, liver and gills, and the lowest are found in the muscle tissue [7,48]. These results are consistent with the findings of this study. The bioaccumulation of four heavy metals was greater in the viscera (e.g., brain, heart, kidney, spleen, etc.) than in the external tissues (e.g., muscle, epidermis, etc.). Yin et al. (2018) stated that the consumption of black carp from Caohu Lake posed no risk to human health [40]. This differs from our findings, which showed that the risk associated with epidermis and muscle tissue consumption of black carp is acceptable, while the brain of black carp is not safe to consume. Similar findings were also observed in other fish. In addition, a similar pattern was found for other fish cultivated in aquaculture ponds or in the wild environment, such as common, grass, silver, and bighead carp [41,45,46,47,48,49].
The biological dilution of trace metals is ubiquitous in the predatory and omnivorous food chains [50,51,52,53]. The degree of pollution found in our study suggests that the metal bioaccumulation in black carp is negatively correlated with fish size. Thus, with the increasing of the fish’s weight, this biodilution pattern in relation to size effect could play a dominant role in the growth of black carp [54,55]. To promote changes in cholesterol metabolism, Cr is used as a nutritional element and supplemented in the diets of the fish [56,57]. Spatiotemporal analysis suggests that Cr pollution levels in the fish’s habitat have increased in recent years [58]. Therefore, we recommend that the Cr pollution in this area should be further researched and given more attention, as Cr has a higher mobility and is more available in these pond ecosystems [59]. Moreover, it bears a significant positive correlation with fat concentration in the tissues of fish [60].
5. Conclusions
In this study, we conducted a comprehensive survey of four metals (Cd, Cr, Cu, and Pb) in 11 tissue categories from 4 fish species grown in a type of artificial wetlands in Huzhou city, located in the south of the lower reaches of the Yangtze River. Although the four heavy metals were found to be widely present in various black carp tissues from the Huzhou aquaculture ponds in the region of Southern Taihu Lake, they have only been polluted by Cd, Cr, and Pb. The heavy metal pollution of small fish in these regions was much more serious than in the large fish. A biodilution effect related to body size could play a role in protecting the black carp, although an indoor simulation experiment is required for verification. Compared to the other three major Chinese carp species grown in ponds in other regions of China, the heavy metal pollution in the aquaculture ponds in Huzhou contained relatively high levels of the studied pollutants. Thus, the pollution levels of the black carp in our study area are more serious than pollution levels found in fish from elsewhere. Through analysis of the MPI values, we found that there are four heavy metals that are more likely to accumulate in the viscera (e.g., brain, heart, kidney, spleen, and so forth) than in the external tissues (e.g., muscle, epidermis, scale, etc.). The estimated THQ and HI values indicate that the health risks regarding human consumption of epidermis and muscle tissue are within the acceptable range, but three heavy metals (Cr, Cd, Pb) pose a significant health risk concerning consumption of the fish head. When assessing the health risks posed by consuming black carp grown in aquaculture ponds, we should not focus solely upon the risk of chromium pollution on human health, but should also be aware of heavy metal pollution present in the brain tissue.
Author Contributions
Conceptualization, R.Z. and J.Z.; methodology, R.Z. and J.Z.; software, Q.S.; validation, Q.S., Y.Z. and J.Y.; data curation, R.Z. and J.Z.; writing, R.Z.; visualization, Q.S.; project administration, Y.Z and J.Y.; funding acquisition, J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Social Science Foundation of China (16@ZH005), the Post Scientist Task of the National Technology System for Conventional Freshwater Fish Industries (CARS-45-11), the Key Research and Development Project of Zhejiang Province (2015C03018) and the Huzhou City Science and Technology Bureau (2016GY01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the reported results are available from the corresponding author upon reasonable request.
Acknowledgments
This experiment was completed with the technical support of Xiao He and He Zhou. This paper has been professionally checked and corrected by Ting Wang.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study.
References
- Xu, P.; Zhang, X.; Zhang, F.; Bempah, G.; Lu, C.; Lv, S.; Zhang, W.; Cui, P. Use of aquaculture ponds by globally endangered red-crowned crane (Grus japonensis) during the wintering period in the Yancheng National Nature Reserve, a Ramsar wetland. Glob. Ecol. Conserv. 2020, 23, e1123. [Google Scholar] [CrossRef]
- Deguchi, S.; Katayama, N.; Tomioka, Y.; Miguchi, H. Ponds support higher bird diversity than rice paddies in a hilly agricultural area in Japan. Biodivers. Conserv. 2020, 29, 3265–3285. [Google Scholar] [CrossRef]
- Feaga, J.S.; Vilella, F.J.; Kaminski, R.M.; Davis, J.B. Waterbird use of catfish ponds and migratory bird habitat initiative wetlands in Mississippi. Waterbirds 2015, 38, 269–281. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Huang, K.; Tsai, C. Can aquaculture ponds be managed as foraging habitats for overwintering water birds? An experimental approach. Sustainability 2020, 12, 10335. [Google Scholar] [CrossRef]
- Ren, C.; Wang, Z.; Zhang, B.; Li, L.; Chen, L.; Song, K.; Jia, M. Remote monitoring of expansion of aquaculture ponds along coastal region of the Yellow River Delta from 1983 to 2015. Chin. Geogra. Sci. 2018, 28, 430–442. [Google Scholar] [CrossRef]
- Hardwick, L.J.; Fryirs, K.A.; Hose, G.C. Spatial and temporal variation in macrophyte litter decomposition in a rare chain-of-ponds, an intermittent stream and wetland system. Wetlands 2022, 42, 33. [Google Scholar] [CrossRef]
- Maurya, P.K.; Malik, D.S. Bioaccumulation of heavy metals in tissues of selected fish species from Ganga river, India, and risk assessment for human health. Hum. Ecol. Risk Assess. 2019, 25, 905–923. [Google Scholar] [CrossRef]
- Subotic, S.; Spasic, S.; Visnjic-Jeftic, Z.; Hegedis, A.; Krpo-Cetkovic, J.; Mickovic, B.; Skoric, S.; Lenhardt, M. Heavy metal and trace element bioaccumulation in target tissues of four edible fish species from the Danube River (Serbia). Ecotox. Environ. Saf. 2013, 98, 196–202. [Google Scholar] [CrossRef]
- Kumar, B.; Mukherjee, D.P.; Kumar, S.; Mishra, M.; Prakash, D.; Singh, S.K.; Sharma, C.S. Bioaccumulation of heavy metals in muscle tissue of fishes from aquaculture ponds in east Kolkata wetlands. Ann. Biol. Res. 2011, 2, 125–134. [Google Scholar]
- Hao, W.; Jinling, L.; Xiangyang, B.; Guanghui, L.; Feng, C.C.; Zhengjie, L.; Fei, Q.; Tianling, Z.; Liqi, X. Trace metals in sediments and benthic animals from aquaculture ponds near a mangrove wetland in southern China. Mar. Pollut. Bull. 2017, 117, 486–491. [Google Scholar] [CrossRef]
- Gu, Y.-G.; Wang, X.-N.; Wang, Z.-H.; Huang, H.-H.; Gong, X.-Y. Metal biological enrichment capacities, distribution patterns, and health risk implications in Sea Bass (Lateolabrax japonicus). Biol. Trace. Elem. Res. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; He, B.; Nover, D.; Lu, H.; Liu, J.; Sun, W.; Chen, W. Farm ponds in southern China: Challenges and solutions for conserving a neglected wetland ecosystem. Sci. Total Environ. 2019, 659, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- FABMOARPC. The 2021 Edition of China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2021; p. 30. (In Chinese) [Google Scholar]
- Dong, L.; Sifa, L.; Wenqiao, T. Invasion status worldwide of the four major culture fishes and their adaptive features. Chin. J. Zool. 2012, 47, 143–152. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, L. A preliminary study on mercury pollution in fish and shellfish from Jiaozhou Bay in North of China. Adv. Mater. Res. 2014, 864–867, 234–238. [Google Scholar] [CrossRef]
- Prabhu, P.A.J.; Schrama, J.W.; Kaushik, S.J. Mineral requirements of fish: A systematic review. Rev. Aquac. 2016, 8, 172–219. [Google Scholar] [CrossRef]
- Lall, S.P.; Kaushik, S.J. Nutrition and metabolism of minerals in fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef]
- Hu, B.; Zhou, Y.; Jiang, Y.; Ji, W.; Fu, Z.; Shao, S.; Li, S.; Huang, M.; Zhou, L.; Shi, Z. Spatio-temporal variation and source changes of potentially toxic elements in soil on a typical plain of the Yangtze River Delta, China (2002–2012). J. Environ. Manag. 2020, 271, 110943. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Jun, Z.; Gao, L. Sources and ecological risk assessment of heavy metal(loid)s in agricultural soils of Huzhou, China. Soil Sediment Contam. 2015, 24, 437–453. [Google Scholar] [CrossRef]
- Richardson, G.L. Project management body of knowledge. In Project Management Theory and Practice; Auerbach Publications: New York, NY, USA, 2021; pp. 49–60. [Google Scholar] [CrossRef]
- Council, N.R. The nature of risk assessment. In Risk Assessment in the Federal Government: Managing the Process; National Academies Press: Washington, DC, USA, 1983; pp. 17–48. [Google Scholar] [CrossRef]
- Wild, A.; Lindsay, J.M.; Bebbington, M.; Clive, M.; Wilson, T. Suitability of 1uantitative volcanic hazard and risk assessment methods and tools for crisis management in Auckland, New Zealand. Lower Hutt. NZ GNS Sci. 2020, 16, 35. [Google Scholar] [CrossRef]
- Yari, M.; Bagherpour, R.; Jamali, S.; Asadi, F. Selection of most proper blasting pattern in mines using linear assignment method: Sungun copper mine. Arch. Min. Sci. 2015, 60, 375–386. [Google Scholar] [CrossRef]
- Yari, M.; Bagherpour, R.; Almasi, N. An approach to the evaluation and classification of dimensional stone quarries with an emphasis on safety parameters. Rud.-Geol.-Naft. Zb. 2016, 31, 15–26. [Google Scholar] [CrossRef]
- Yari, M.; Bagherpour, R.; Khoshouei, M.; Pedram, H. Investigating a comprehensive model for evaluating occupational and environmental risks of dimensional stone mining. Rud.-Geol.-Naft. Zb. 2020, 35, 101–109. [Google Scholar] [CrossRef]
- Tonini, R.; Sandri, L.; Thompson, M.A. PyBetVH: A python tool for probabilistic volcanic hazard assessment and for generation of bayesian hazard curves and maps. Comput. Geosci. 2015, 79, 38–46. [Google Scholar] [CrossRef]
- Vaseem, H.; Banerjee, T.K. Metal bioaccumulation in fish Labeo rohita exposed to effluent generated during metals extraction from polymetallic sea nodules. Int. J. Environ. Sci. Technol. 2015, 12, 53–60. [Google Scholar] [CrossRef]
- Usero, J.; González-Regalado, E.; Gracia, I. Trace metals in the bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic coast of Southern Spain. Environ. Int. 1997, 23, 291–298. [Google Scholar] [CrossRef]
- Zhuang, P.; Li, Z.; Mcbride, M.B.; Wang, G.; Zou, B. Concentrations of heavy metals in fish from a mine-affected area and potential health risk. Fresen. Environ. Bull. 2013, 22, 2402–2408. [Google Scholar]
- Miri, M.; Akbari, E.; Amrane, A.; Jafari, S.J.; Eslami, H.; Hoseinzadeh, E.; Zarrabi, M.; Salimi, J.; Sayyad-Arbabi, M.; Taghavi, M. Health risk assessment of heavy metal intake due to fish consumption in the Sistan Region, Iran. Environ. Monit. Assess. 2017, 189, 583. [Google Scholar] [CrossRef]
- Kwaansa-Ansah, E.E.; Nti, S.O.; Opoku, F. Heavy metals concentration and human health risk assessment in seven commercial fish species from Asafo Market, Ghana. Food Sci. Biotechnol. 2019, 28, 569–579. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, W.; Shi, X.; Yu, X.; Li, M.; Xiao, L.; Cui, Y. Health risk of semi-volatile organic pollutants in Wujin river inflow into Taihu Lake. Ecotoxicology 2011, 20, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Exposure Factors Handbook, 2011st ed.; National Center for Environmental Assessment, Office of Research and Development: Washington, DC, USA, 2011; pp. 11–18.
- USEPA. Regional Screening Levels (RSLs) Summary Table. Available online: https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables (accessed on 17 November 2017).
- Varol, M.; Kaya, G.K.; Alp, A. Heavy metal and arsenic concentrations in rainbow trout (Oncorhynchus mykiss) farmed in a dam reservoir on the Firat (Euphrates) River: Risk-based consumption advisories. Sci. Total Environ. 2017, 599–600, 1288–1296. [Google Scholar] [CrossRef]
- NHFPC. The 2017 Edition of China’s Health and Family Planning Statistical Yearbook; Peking Union Medical College Press: Beijing, China, 2017; pp. 233–245. (In Chinese)
- USEPA. Human health evaluation manual, supplemental guidance: Standard default exposure factors. In OSWER Directive 9285.6-03; USEPA: Washington, DC, USA, 1991. [Google Scholar]
- MOHPRC. The 2008 Edition of China’s Health Statistical Yearbook; Peking Union Medical College Press: Beijing, China, 2008; p. 191. (In Chinese) [Google Scholar]
- Li, P.; Li, X.; Bai, J.; Meng, Y.; Diao, X.; Pan, K.; Zhu, X.; Lin, G. Effects of land use on the heavy metal pollution in mangrove sediments: Study on a whole island scale in Hainan, China. Sci. Total Environ. 2022, 824, 153856. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liu, Q.; Wang, L.; Li, J.; Li, S.; Zhang, X. The distribution and risk assessment of heavy metals in water, sediments, and fish of Chaohu Lake, China. Environ. Earth Sci. 2018, 77, 97. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, Y.; Wu, Z.; Yang, R.; Chen, X.; Yang, J.; Zhu, L. Health risk assessment of heavy metals in freshwater fish in the central and eastern North China. Ecotox. Environ. Safe 2018, 157, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Hu, Z.; Liu, F.; Zhang, R.; Duo, B.; Fu, J.; Cui, Y.; Li, M. Heavy metals levels in fish from aquaculture farms and risk assessment in Lhasa, Tibetan Autonomous Region of China. Ecotoxicology 2014, 23, 577–583. [Google Scholar] [CrossRef]
- Tian, Q.B.; Ren, H.L.; Hou, S.M.; Yang, Y.H. Investigation and evaluation of heavy metal content of aquaculture products in Shanxi. Freshw. Fish. 2017, 47, 57–62. (In Chinese) [Google Scholar] [CrossRef]
- Qin, D.; Jiang, H.; Bai, S.; Tang, S.; Mou, Z. Determination of 28 trace elements in three farmed cyprinid fish species from Northeast China. Food Control 2015, 50, 1–8. [Google Scholar] [CrossRef]
- PuYang, X.; Gao, C.; Han, L.H.C. Risk assessment of heavy metals in water and two fish species from golf course ponds in Beijing, China. Bull. Environ. Contam. Tox. 2015, 94, 437–443. [Google Scholar] [CrossRef]
- Cheung, K.C.; Leung, H.M.; Wong, M.H. Metal concentrations of common freshwater and marine fish from the Pearl River Delta, south China. Arch. Environ. Con. Tox. 2008, 54, 705–715. [Google Scholar] [CrossRef]
- Zheng, Q.M.; Han, C.Y.; Zhong, Y.M.; Wen, R.S.; Zhang, J.L. The determination and evaluation of heavy metal content in Polyodon Spathula, Aristichthys Nobilis and Hypophthalmichthys Molitrix from Meizhou Areas. J. Jiaying Univ. 2014, 32, 52–57. (In Chinese) [Google Scholar]
- Adhikari, S.; Ghosh, L.; Rai, S.P.; Ayyappan, S. Metal concentrations in water, sediment, and fish from sewage-fed aquaculture ponds of Kolkata, India. Environ. Monit. Assess. 2009, 159, 217–230. [Google Scholar] [CrossRef]
- Damodharan, U.; Reddy, M.V. Heavy metal bioaccumulation in edible fish species from an industrially polluted river and human health risk assessment. Arch. Pol. Fish. 2013, 21, 19–27. [Google Scholar] [CrossRef]
- Cheng, Z.; Man, Y.B.; Nie, X.P.; Wong, M.H. Trophic relationships and health risk assessments of trace metals in the aquaculture pond ecosystem of Pearl River Delta, China. Chemosphere 2013, 90, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Yohannes, Y.B.; Ikenaka, Y.; Nakayama, S.M.M.; Saengtienchai, A.; Watanabe, K.; Ishizuka, M. Organochlorine pesticides and heavy metals in fish from Lake Awassa, Ethiopia: Insights from stable isotope analysis. Chemosphere 2013, 91, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.M.; Duzgoren-Aydin, N.S.; Au, C.K.; Krupanidhi, S.; Fung, K.Y.; Cheung, K.C.; Wong, Y.K.; Peng, X.L.; Ye, Z.H.; Yung, K.K.L.; et al. Monitoring and assessment of heavy metal contamination in a constructed wetland in Shaoguan (Guangdong Province, China): Bioaccumulation of Pb, Zn, Cu and Cd in aquatic and terrestrial components. Environ. Sci. Pollut. R. 2017, 24, 9079–9088. [Google Scholar] [CrossRef]
- Shinn, C.; Dauba, F.; Grenouillet, G.; Guenard, G.; Lek, S. Temporal variation of heavy metal contamination in fish of the river lot in southern France. Ecotox. Environ. Saf. 2009, 72, 1957. [Google Scholar] [CrossRef]
- Aoyama, I.; Inoue, Y.; Inoue, Y. Simulation analysis of the concentration process of trace heavy metals by aquatic organisms from the viewpoint of nutrition ecology. Water Res. 1978, 12, 837–842. [Google Scholar] [CrossRef]
- Sun, T.; Wu, H.; Wang, X.; Ji, C.; Shan, X.; Li, F. Evaluation on the biomagnification or biodilution of trace metals in global marine food webs by meta-analysis. Environ. Pollut. 2020, 264, 113856. [Google Scholar] [CrossRef]
- Haq, Z.; Jain, R.K.; Khan, N.; Dar, M.Y.; Ali, S.; Gupta, M.; Varun, T.K. Recent advances in role of chromium and its antioxidant combinations in poultry nutrition: A review. Vet. World 2016, 9, 1392–1399. [Google Scholar] [CrossRef]
- Pires, K.A.; Santos, D.C.C.D.; Graça, D.S.; Melo, M.M.; Barbosa, F.A.; Sotoblanco, B. Effects of two sources of chromium on performance, blood and liver lipid levels in Nile tilapia (Oreochromis niloticus). Acta Sci. Vet. 2015, 43, 1302. [Google Scholar]
- Li, H.; Li, Y.; Lee, M.-K.; Liu, Z.; Miao, C. Spatiotemporal analysis of heavy metal water pollution in transitional China. Sustainability 2015, 7, 9067–9087. [Google Scholar] [CrossRef]
- Mukherjee, P.; Das, P.K.; Ghosh, P. The extent of heavy metal pollution by chemical partitioning and risk assessment code of sediments of sewage-fed fishery ponds at east Kolkata Wetland, a Ramsar Site, India. Bull. Environ. Contam. Toxicol. 2022, 108, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, L.; Chen, Y.; Zhang, D.; Hegazy, A.M.; Zhang, X. A comparison of accumulation and depuration effect of dissolved hexavalent chromium (Cr6+) in head and muscle of Bighead Carp (Aristichthys nobilis) and assessment of the potential health risk for consumers. Food Chem. 2019, 286, 388–394. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).