Titania Thin Film Coated Glass for Simultaneous Ammonia Degradation and UV Light Blocking Layer in Photovoltaics
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
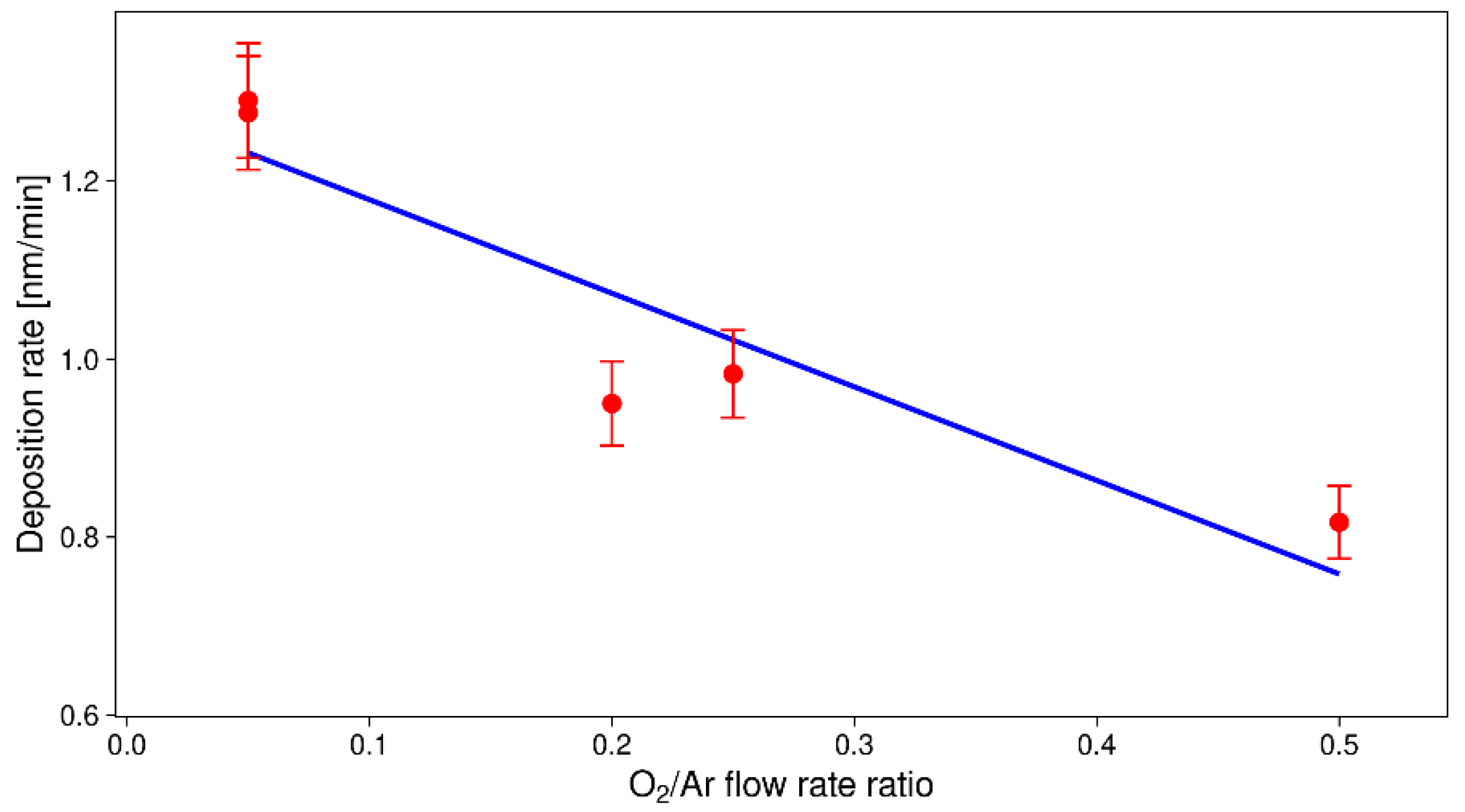
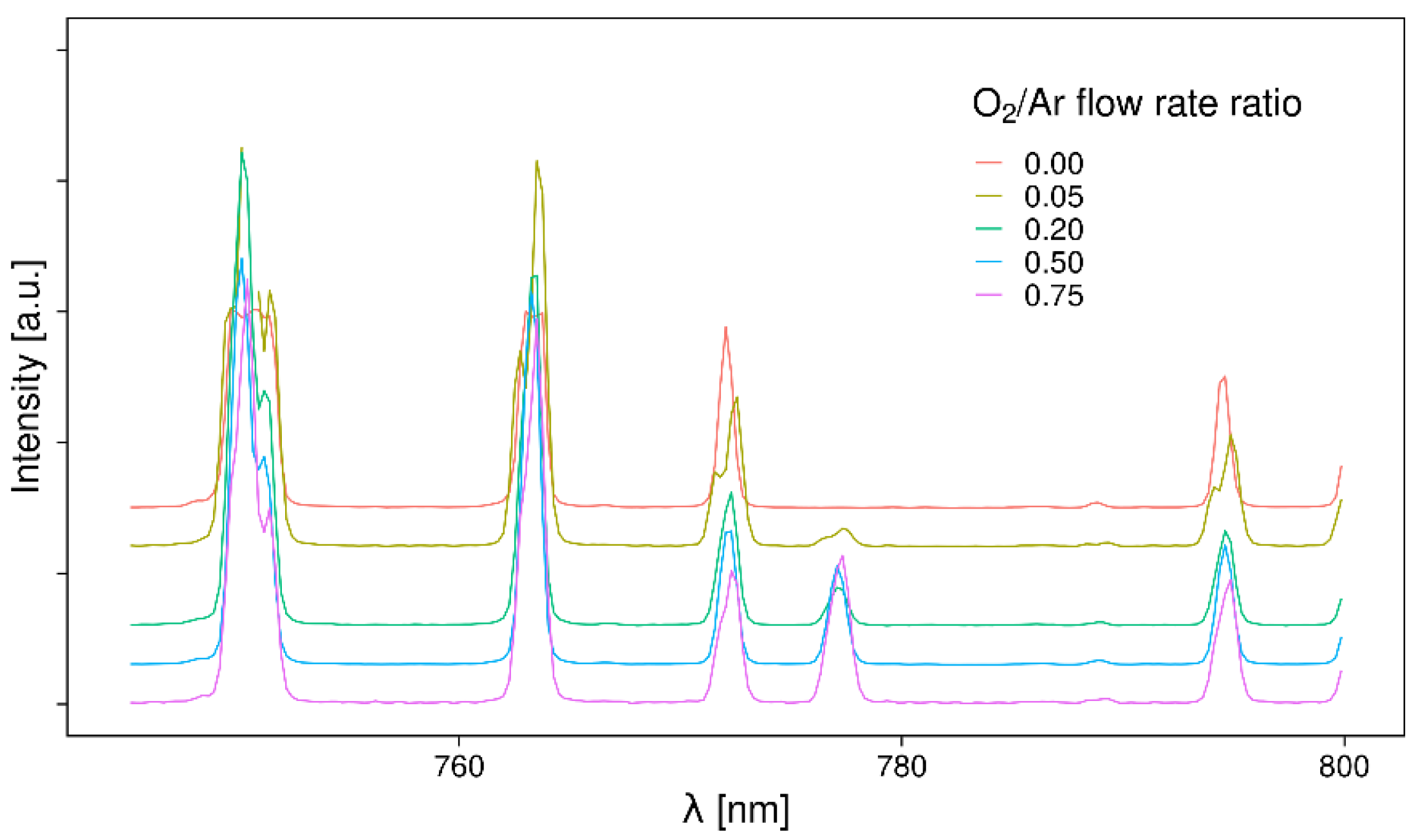
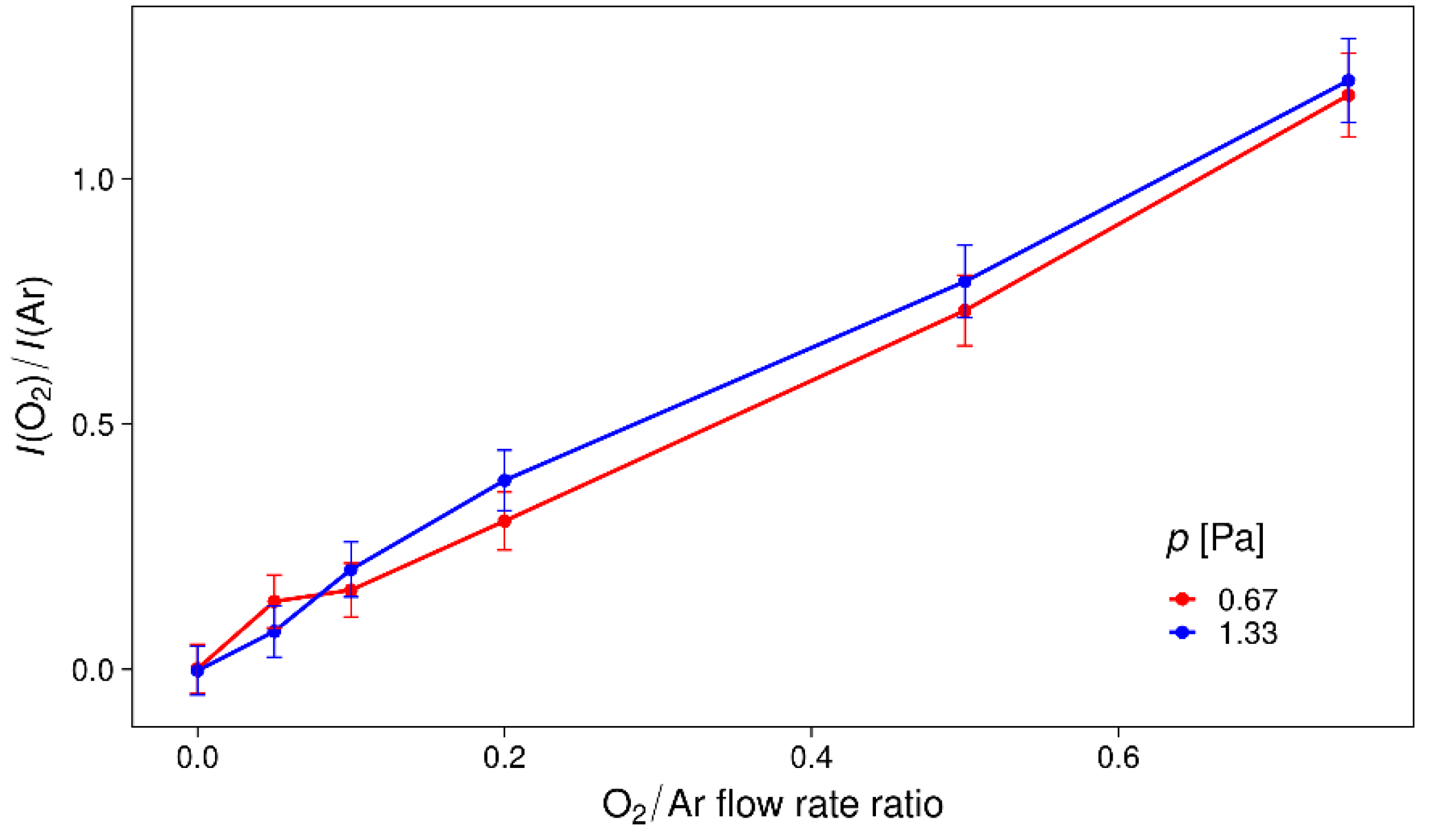
3.1. Optical Emission Spectroscopy (OES)
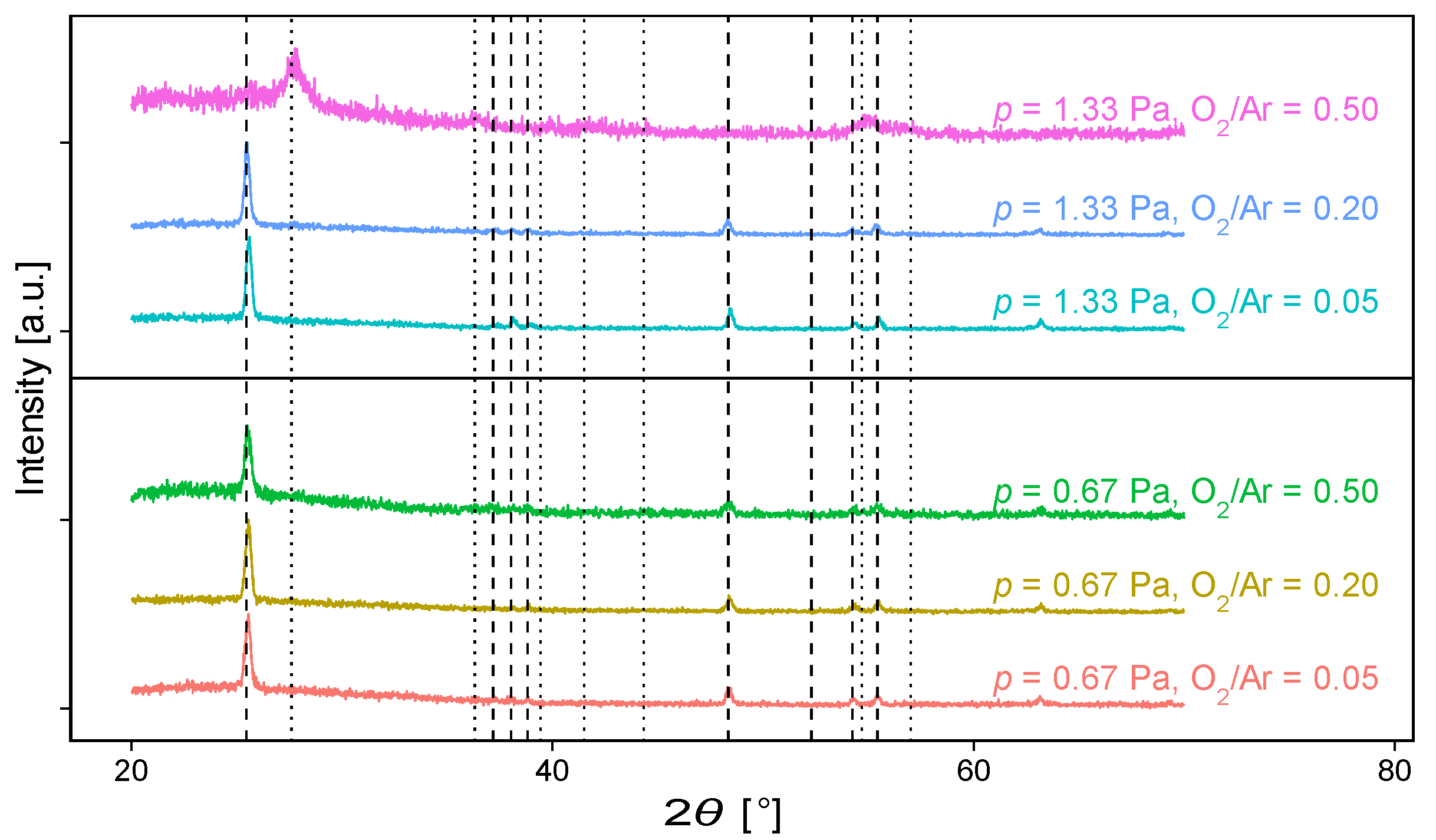
3.2. Structural Properties and Surface Morphology
3.3. Optical Properties
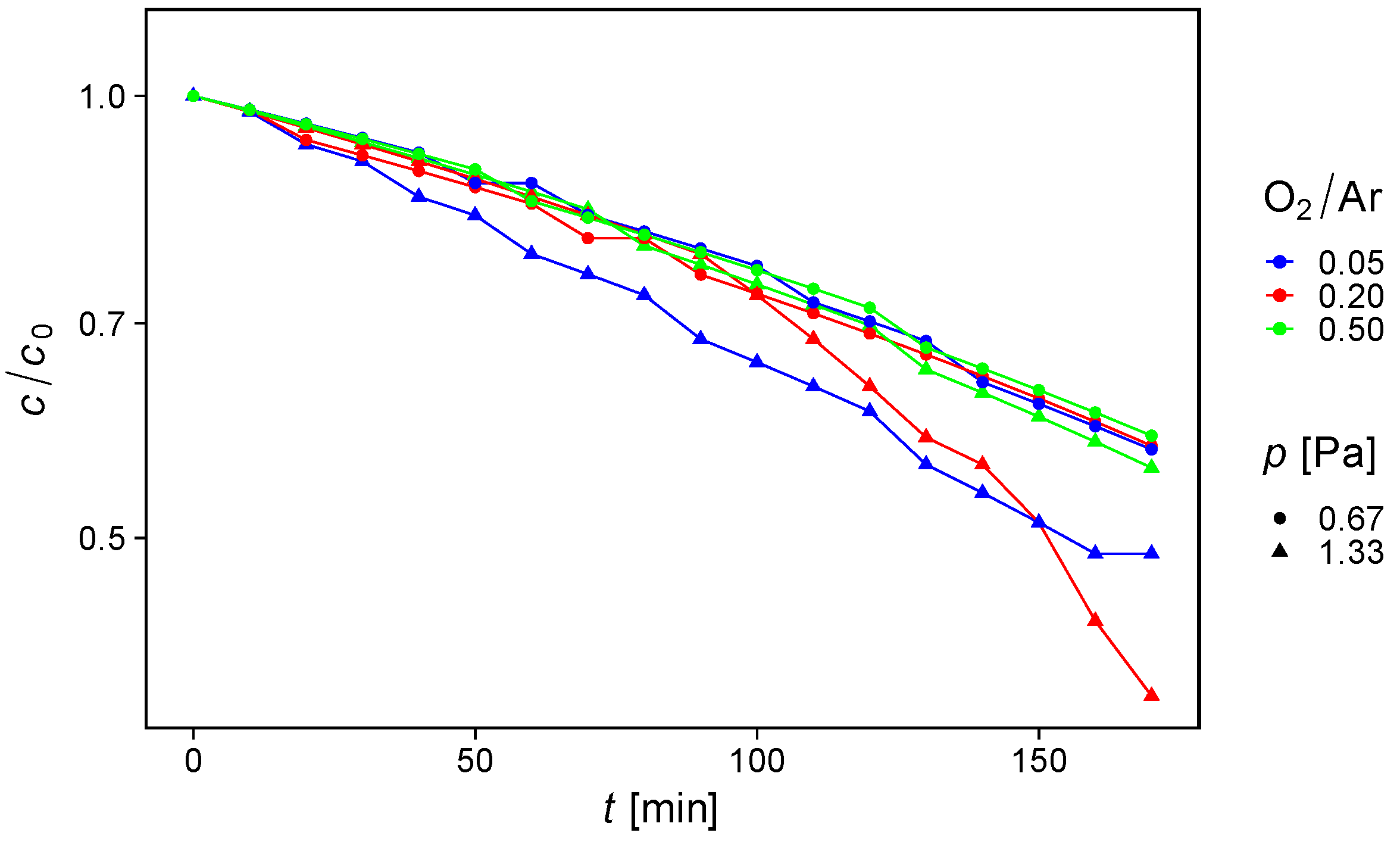
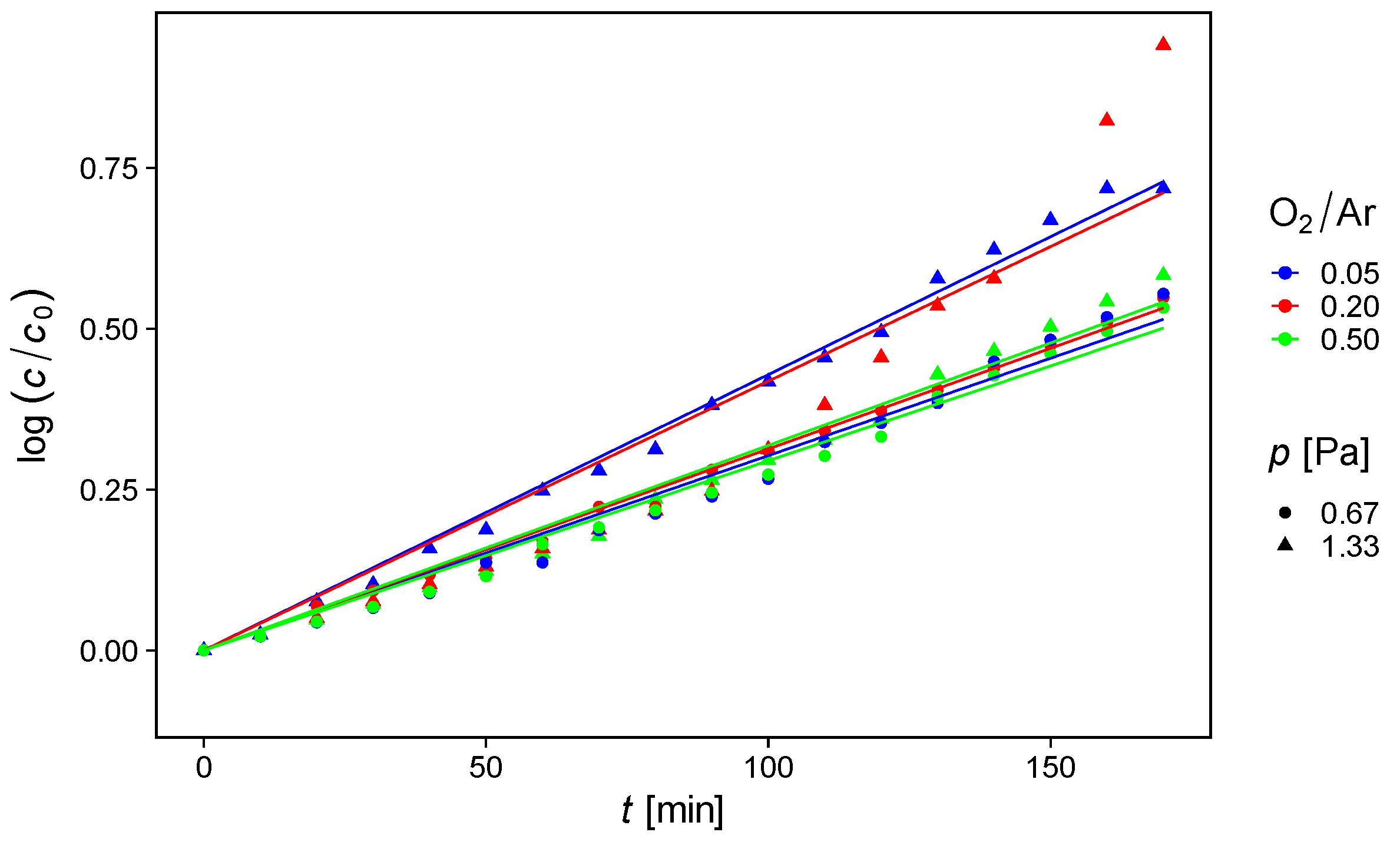
3.4. Photocatalysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghazaryan, L.; Handa, S.; Schmitt, P.; Beladiya, V.; Roddatis, V.; Tünnermann, A.; Szeghalmi, A. Structural, Optical, and Mechanical Properties of TiO2 Nanolaminates. Nanotechnology 2021, 32, 095709. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Henderson, M.A. A Surface Science Perspective on TiO2 Photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the Least Known TiO2 Photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Kim, T.; Lim, J.; Song, S. Recent Progress and Challenges of Electron Transport Layers in Organic–Inorganic Perovskite Solar Cells. Energies 2020, 13, 5572. [Google Scholar] [CrossRef]
- Tang, J.; Durrant, J.R.; Klug, D.R. Mechanism of Photocatalytic Water Splitting in TiO2. Reaction of Water with Photoholes, Importance of Charge Carrier Dynamics, and Evidence for Four-Hole Chemistry. J. Am. Chem. Soc. 2008, 130, 13885–13891. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mazare, A.; Schmuki, P. One-Dimensional Titanium Dioxide Nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef]
- Smith, A.M.; Nie, S. Semiconductor Nanocrystals: Structure, Properties, and Band Gap Engineering. Acc. Chem. Res. 2010, 43, 190–200. [Google Scholar] [CrossRef]
- Selmi, W.; Hosni, N.; Ben Naceur, J.; Maghraoui-Meherzi, H.; Chtourou, R. Titanium Dioxide Thin Films for Environmental Applications. In Titanium Dioxide-Advances and Applications; Muhammad Ali, H., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-83969-475-2. [Google Scholar]
- Hadjoub, I.; Touam, T.; Chelouche, A.; Atoui, M.; Solard, J.; Chakaroun, M.; Fischer, A.; Boudrioua, A.; Peng, L.-H. Post-Deposition Annealing Effect on RF-Sputtered TiO2 Thin-Film Properties for Photonic Applications. Appl. Phys. A 2016, 122, 78. [Google Scholar] [CrossRef]
- Musil, J.; Baroch, P.; Vlček, J.; Nam, K.H.; Han, J.G. Reactive Magnetron Sputtering of Thin Films: Present Status and Trends. Thin Solid Films 2005, 475, 208–218. [Google Scholar] [CrossRef]
- Depla, D.; Mahieu, S.; Depla, D. Reactive Sputter Deposition; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-76664-3. [Google Scholar]
- Liao, J.-Y.; He, J.-W.; Xu, H.; Kuang, D.-B.; Su, C.-Y. Effect of TiO2 Morphology on Photovoltaic Performance of Dye-Sensitized Solar Cells: Nanoparticles, Nanofibers, Hierarchical Spheres and Ellipsoid Spheres. J. Mater. Chem. 2012, 22, 7910. [Google Scholar] [CrossRef]
- Johansson, W.; Peralta, A.; Jonson, B.; Anand, S.; Österlund, L.; Karlsson, S. Transparent TiO2 and ZnO Thin Films on Glass for UV Protection of PV Modules. Front. Mater. 2019, 6, 259. [Google Scholar] [CrossRef]
- Verbruggen, S.W. TiO2 Photocatalysis for the Degradation of Pollutants in Gas Phase: From Morphological Design to Plasmonic Enhancement. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Gu, B.; Zhang, L.; Van Dingenen, R.; Vieno, M.; Van Grinsven, H.J.; Zhang, X.; Zhang, S.; Chen, Y.; Wang, S.; Ren, C.; et al. Abating Ammonia Is More Cost-Effective than Nitrogen Oxides for Mitigating PM2.5 Air Pollution. Science 2021, 374, 758–762. [Google Scholar] [CrossRef]
- Wu, H.; Ma, J.; Li, Y.; Zhang, C.; He, H. Photocatalytic Oxidation of Gaseous Ammonia over Fluorinated TiO2 with Exposed (001) Facets. Appl. Catal. B Environ. 2014, 152–153, 82–87. [Google Scholar] [CrossRef]
- Sopyan, I. Kinetic Analysis on Photocatalytic Degradation of Gaseous Acetaldehyde, Ammonia and Hydrogen Sulfide on Nanosized Porous TiO2 Films. Sci. Technol. Adv. Mater. 2007, 8, 33–39. [Google Scholar] [CrossRef]
- Čižmar, T.; Grčić, I.; Bohač, M.; Razum, M.; Pavić, L.; Gajović, A. Dual Use of Copper-Modified TiO2 Nanotube Arrays as Material for Photocatalytic NH3 Degradation and Relative Humidity Sensing. Coatings 2021, 11, 1500. [Google Scholar] [CrossRef]
- Yuzawa, H.; Mori, T.; Itoh, H.; Yoshida, H. Reaction Mechanism of Ammonia Decomposition to Nitrogen and Hydrogen over Metal Loaded Titanium Oxide Photocatalyst. J. Phys. Chem. C 2012, 116, 4126–4136. [Google Scholar] [CrossRef]
- Grčić, I.; Marčec, J.; Radetić, L.; Radovan, A.-M.; Melnjak, I.; Jajčinović, I.; Brnardić, I. Ammonia and Methane Oxidation on TiO2 Supported on Glass Fiber Mesh under Artificial Solar Irradiation. Environ. Sci. Pollut. Res. 2021, 28, 18354–18367. [Google Scholar] [CrossRef]
- Rebuffi, L.; Plaisier, J.R.; Abdellatief, M.; Lausi, A.; Scardi, P. MCX: A Synchrotron Radiation Beamline for X-Ray Diffraction Line Profile Analysis: MCX: A Synchrotron Radiation Beamline. Z. Anorg. Allg. Chem. 2014, 640, 3100–3106. [Google Scholar] [CrossRef]
- Gracin, D.; Sancho-Paramon, J.; Juraić, K.; Gajović, A.; Čeh, M. Analysis of Amorphous-Nano-Crystalline Multilayer Structures by Optical, Photo-Deflection and Photo-Current Spectroscopy. Micron 2009, 40, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.Y.; Cardona, M. Fundamentals of Semiconductors: Physics and Materials Properties, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1999; ISBN 978-3-540-65352-3. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Kramida, A.; Ralchenko, Y. NIST Standard Reference Database 78; NIST Atomic Spectra Database: Gaithersburg, MD, USA, 1999.
- Ohsaka, T.; Izumi, F.; Fujiki, Y. Raman Spectrum of Anatase, TiO2. J. Raman Spectrosc. 1978, 7, 321–324. [Google Scholar] [CrossRef]
- Ottermann, C.; Otto, J.; Jeschkowski, U.; Anderson, O.; Heming, M.; Bange, K. Stress of TiO2 Thin Films Produced by Different Deposition Techniques. MRS Proc. 1993, 308, 69. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001; ISBN 978-0-201-61091-8. [Google Scholar]
- Dehghani, Z.; Shadrokh, Z.; Nadafan, M. The Effect of Magnetic Metal Doping on the Structural and the Third-Order Nonlinear Optical Properties of ZnS Nanoparticles. Optik 2017, 131, 925–931. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, R. Effect of Different UV Cut off Wavelength of EVA Encapsulant on Cr-Si PV Module’s Performance & Reliability. In Proceedings of the 32nd European Photovoltaic Solar Energy Conference and Exhibition, Munich, Germany, 20–24 June 2016; pp. 1823–1825. [Google Scholar] [CrossRef]
- Coto, M.; Troughton, S.C.; Knight, P.; Joshi, R.; Francis, R.; Kumar, R.V.; Clyne, T.W. Optimization of the Microstructure of TiO2 Photocatalytic Surfaces Created by Plasma Electrolytic Oxidation of Titanium Substrates. Surf. Coat. Technol. 2021, 411, 127000. [Google Scholar] [CrossRef]


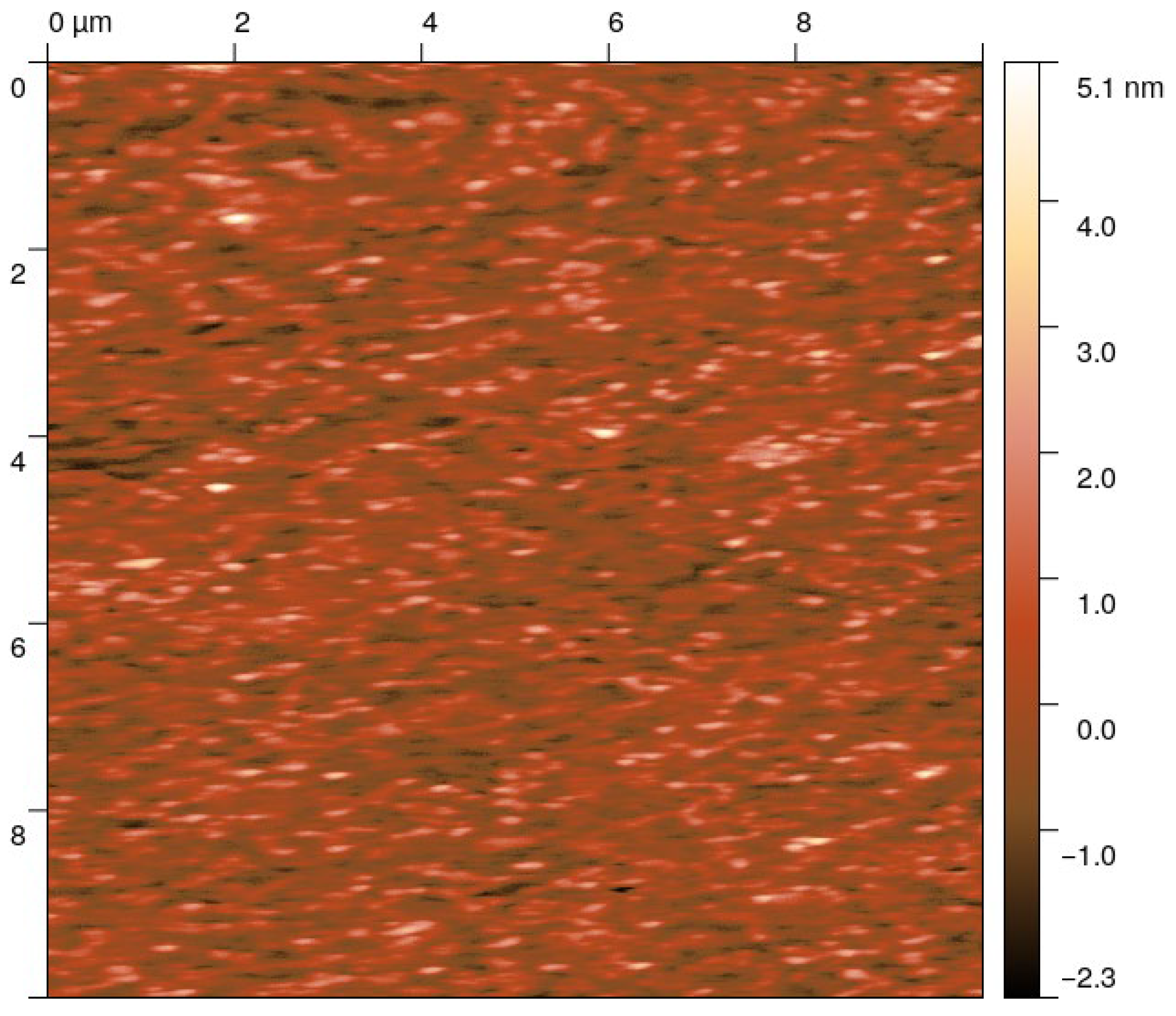
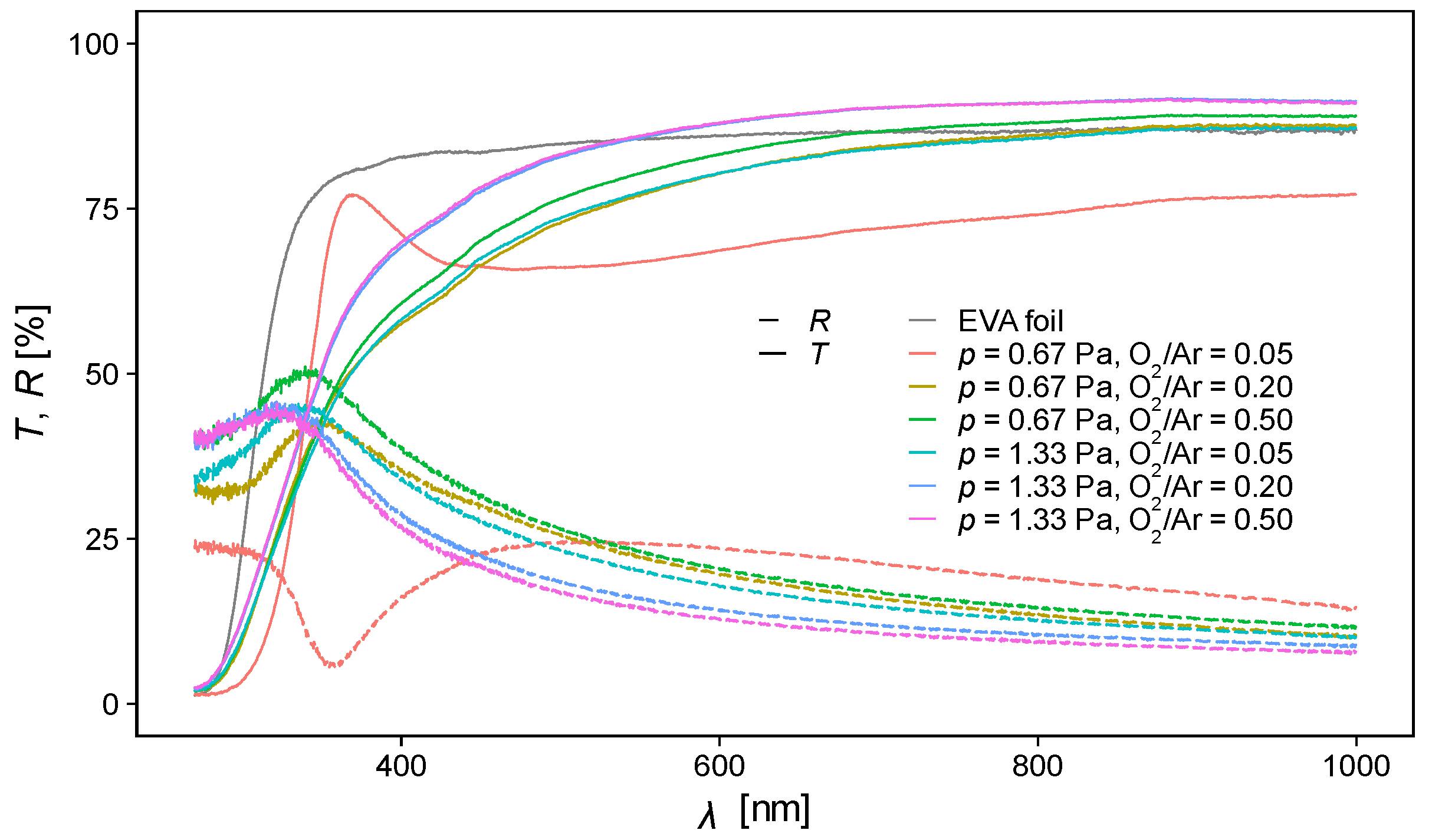
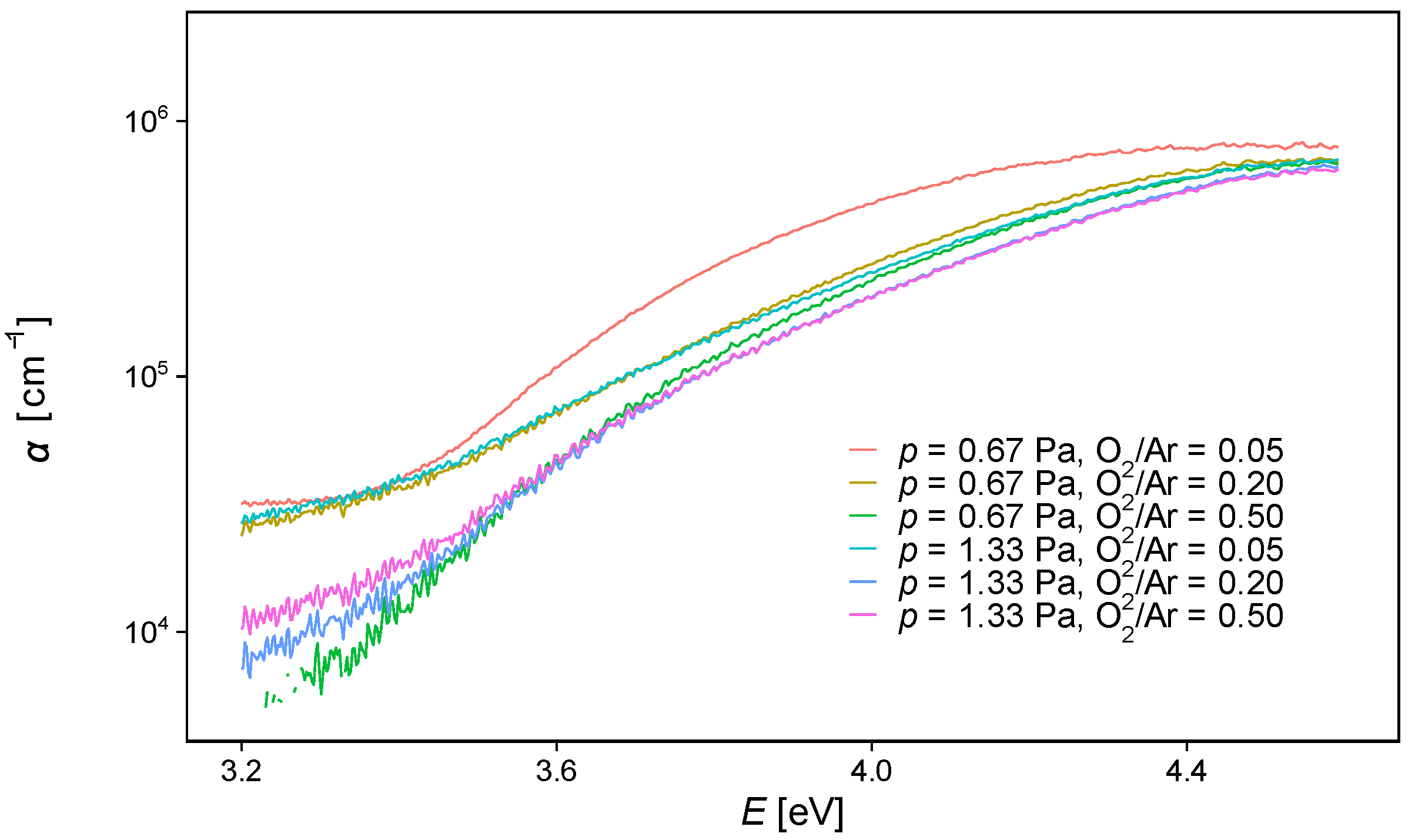
| O2/Ar Flow Rate Ratio | O2/Ar Pressure (Pa) | Raman Eg Band Position (cm−1) | GIXRD DScherrer (nm) | AFM RMS Roughness (nm) | UV-Vis Optical Gap Eg (eV) |
|---|---|---|---|---|---|
| 0.05 | 0.67 | 144.25 ± 0.05 | 4.32 ± 0.05 | 3.96 | 3.27 ± 0.01 |
| 0.20 | 0.67 | 142.9 ± 0.1 | 4.44 ± 0.05 | 1.70 | 3.34 ± 0.01 |
| 0.50 | 0.67 | 142.99 ± 0.09 | 3.87 ± 0.05 | 2.12 | 3.35 ± 0.02 |
| 0.05 | 1.33 | 144.1 ± 0.1 | 4.71 ± 0.05 | 4.66 | 3.32 ± 0.02 |
| 0.20 | 1.33 | 142.5 ± 0.1 | 4.60 ± 0.05 | 4.36 | 3.36 ± 0.02 |
| 0.50 | 1.33 | 142.5 ± 0.1 | - | 2.80 | 3.36 ± 0.02 |
| O2/Ar Ratio | p (Pa) | kz (ppm s−1) | αUVB (105 cm−1) | αUVA (105 cm−1) | kintrinsic 10−9 ppm cm2.5 mW−0.5 s−1 |
|---|---|---|---|---|---|
| 0.05 | 0.67 | 0.00220 | 6.333 | 1.124 | 3.63 |
| 0.20 | 0.67 | 0.00225 | 4.316 | 0.705 | 5.51 |
| 0.50 | 0.67 | 0.00210 | 3.878 | 0.457 | 5.94 |
| 0.05 | 1.33 | 0.00281 | 3.993 | 0.706 | 7.35 |
| 0.20 | 1.33 | 0.00277 | 3.408 | 0.436 | 8.83 |
| 0.50 | 1.33 | 0.00221 | 3.364 | 0.460 | 7.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juraić, K.; Bohač, M.; Plaisier, J.R.; Hodzic, A.; Dubček, P.; Gracin, D.; Grčić, I.; Marčec, J.; Čižmar, T.; Gajović, A. Titania Thin Film Coated Glass for Simultaneous Ammonia Degradation and UV Light Blocking Layer in Photovoltaics. Sustainability 2022, 14, 10970. https://doi.org/10.3390/su141710970
Juraić K, Bohač M, Plaisier JR, Hodzic A, Dubček P, Gracin D, Grčić I, Marčec J, Čižmar T, Gajović A. Titania Thin Film Coated Glass for Simultaneous Ammonia Degradation and UV Light Blocking Layer in Photovoltaics. Sustainability. 2022; 14(17):10970. https://doi.org/10.3390/su141710970
Chicago/Turabian StyleJuraić, Krunoslav, Mario Bohač, Jasper Rikkert Plaisier, Aden Hodzic, Pavo Dubček, Davor Gracin, Ivana Grčić, Jan Marčec, Tihana Čižmar, and Andreja Gajović. 2022. "Titania Thin Film Coated Glass for Simultaneous Ammonia Degradation and UV Light Blocking Layer in Photovoltaics" Sustainability 14, no. 17: 10970. https://doi.org/10.3390/su141710970
APA StyleJuraić, K., Bohač, M., Plaisier, J. R., Hodzic, A., Dubček, P., Gracin, D., Grčić, I., Marčec, J., Čižmar, T., & Gajović, A. (2022). Titania Thin Film Coated Glass for Simultaneous Ammonia Degradation and UV Light Blocking Layer in Photovoltaics. Sustainability, 14(17), 10970. https://doi.org/10.3390/su141710970












