Photoelectrochemical Water Splitting and H2 Generation Enhancement Using an Effective Surface Modification of W-Doped TiO2 Nanotubes (WT) with Co-Deposition of Transition Metal Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Situ Electrochemical Anodization Procedure
2.3. Photo-Deposition Procedure
2.4. Material Characterization
2.5. Photoelectrochemical (PEC) Characterization and PEC Water Splitting
3. Results and Discussion
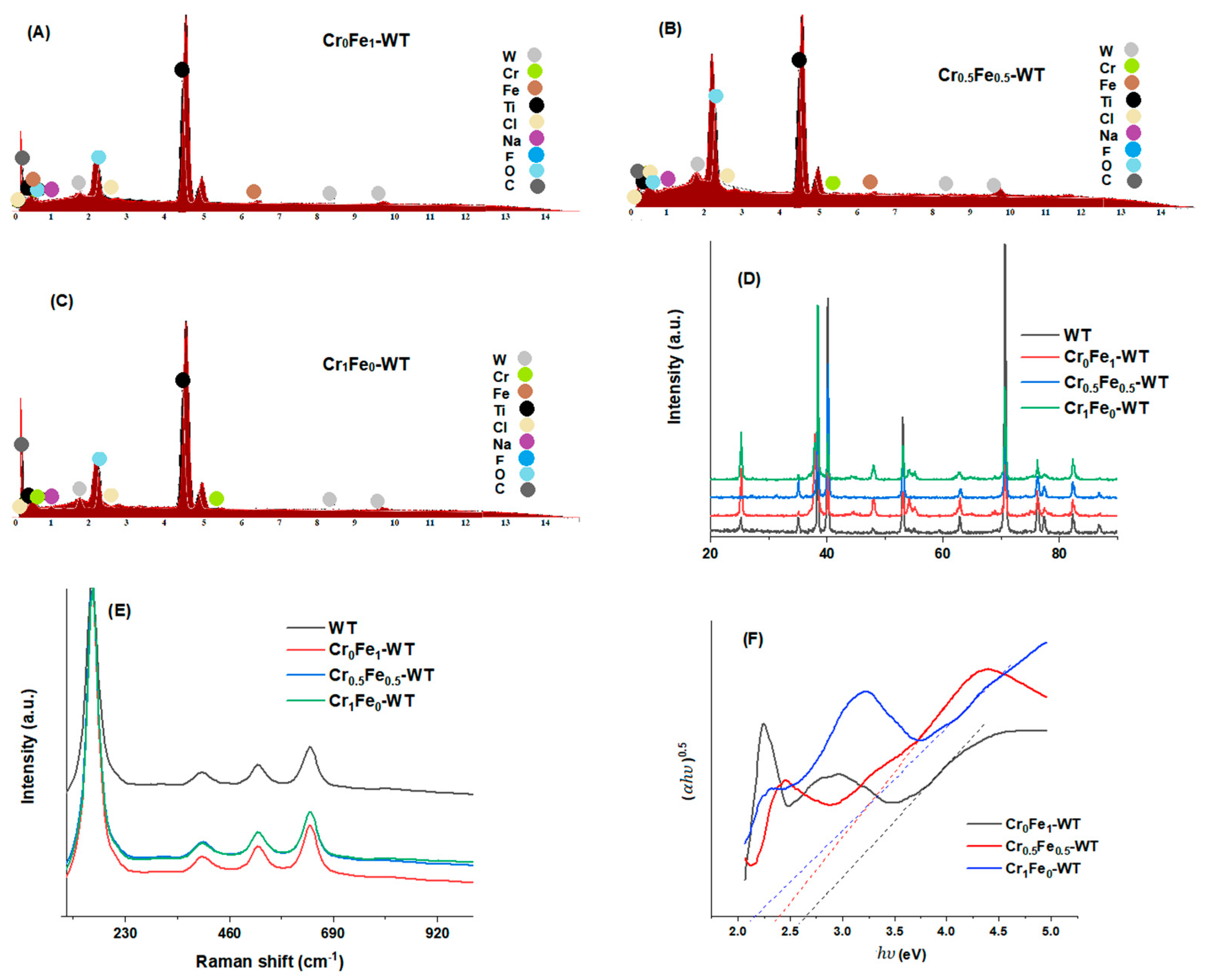
3.1. Material Characterization
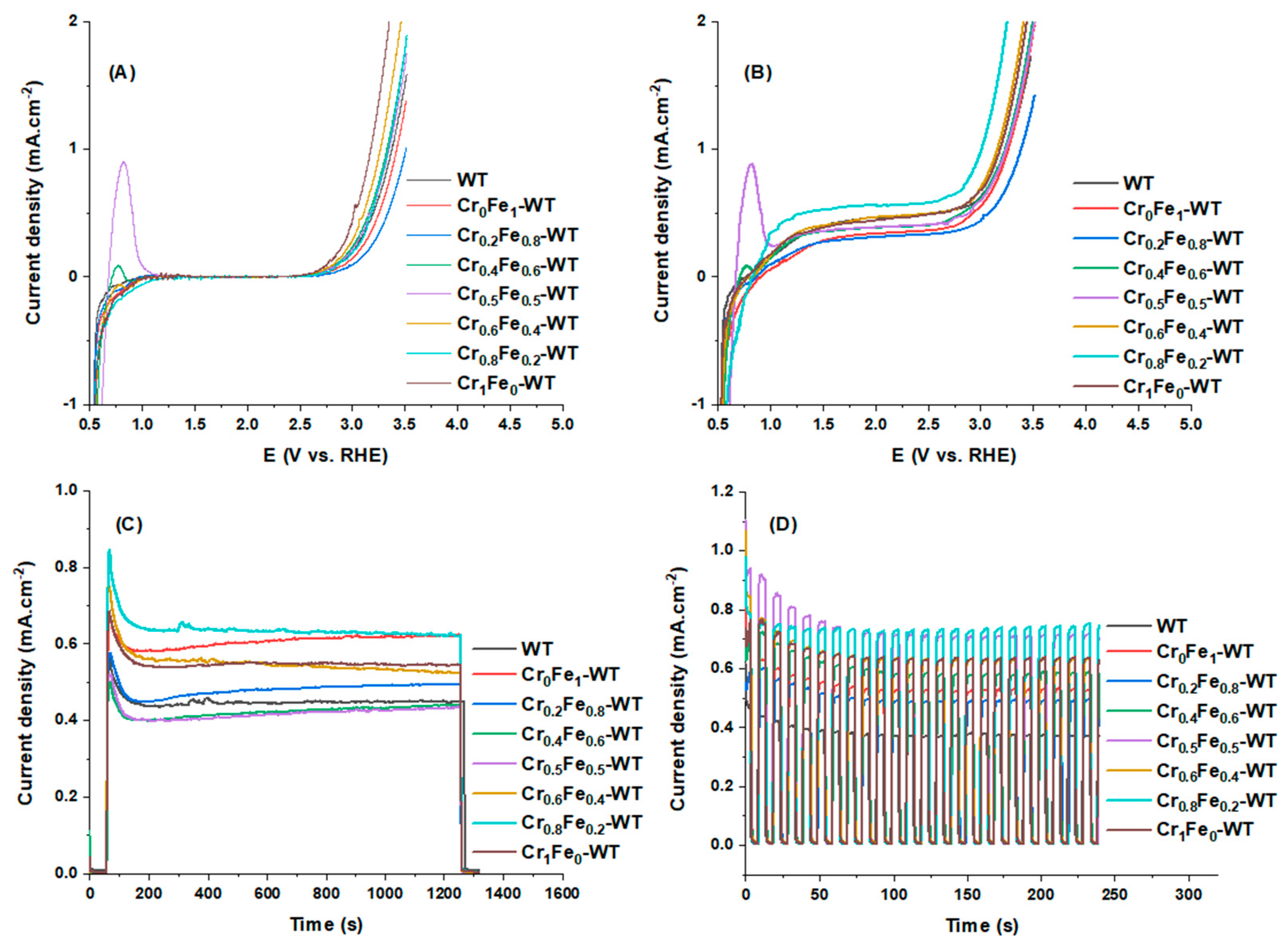
3.2. PEC Characterization
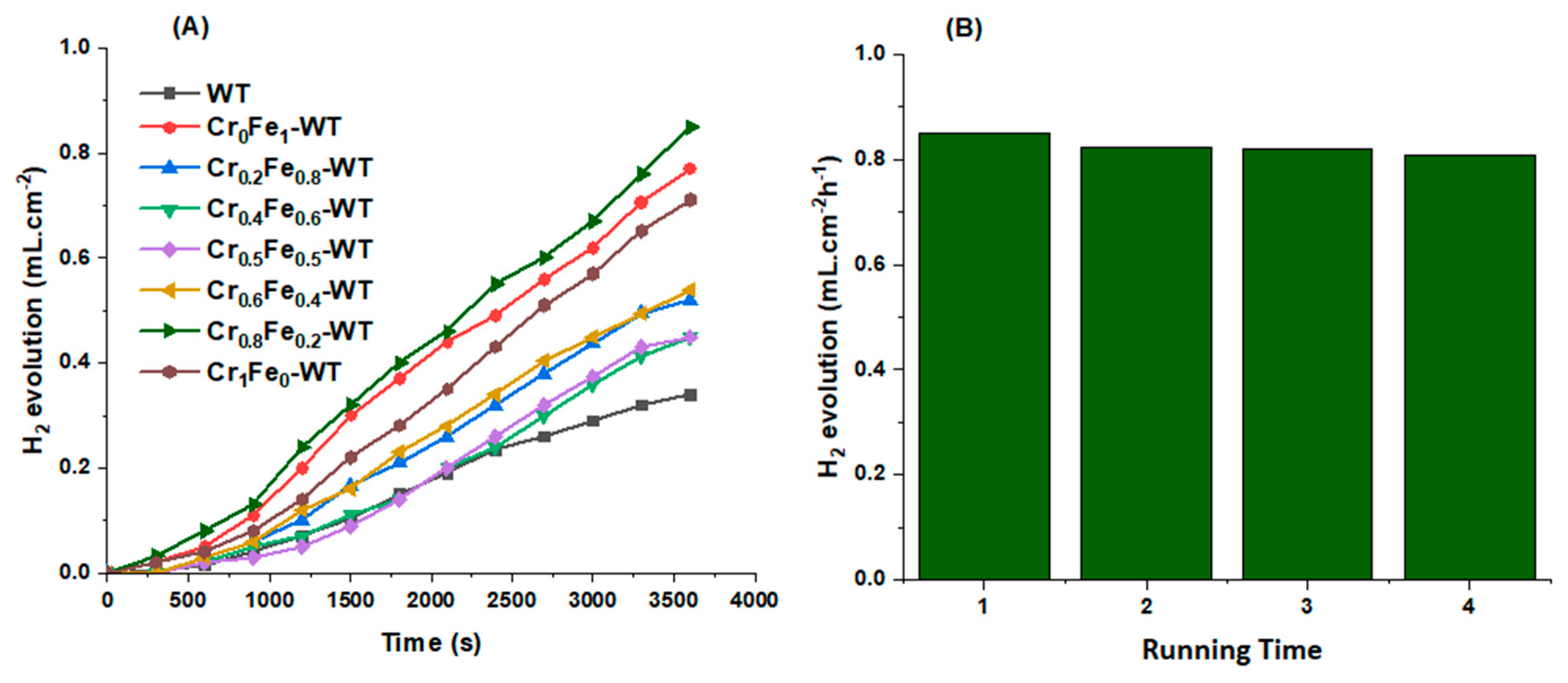
3.3. PEC Water Splitting and H2 Generation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nazir, H.; Muthuswamy, N.; Louis, C.; Jose, S.; Prakash, J.; Buan, M.E.; Flox, C.; Chavan, S.; Shi, X.; Kauranen, P. Is the H2 economy realizable in the foreseeable future? Part III: H2 usage technologies, applications, and challenges and opportunities. Int. J. Hydrogen Energy 2020, 45, 28217–28239. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, G.; Sun, Z.; Zhang, Y.; Xu, D. Review of renewable energy-based hydrogen production processes for sustainable energy innovation. Glob. Energy Interconnect. 2019, 2, 436–443. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Duy, L.T.; Seo, H. Recent Progress in Photoelectrochemical Water Splitting Activity of WO3 Photoanodes. Top. Catal. 2018, 61, 1043–1076. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar Water Splitting: Progress Using Hematite (α-Fe2O3) Photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef]
- dos Santos, W.S.; Rodriguez, M.; Khoury, J.M.O.; Nascimento, L.A.; Ribeiro, R.J.P.; Mesquita, J.P.; Silva, A.C.; Nogueira, F.G.E.; Pereira, M.C. Bismuth Vanadate Photoelectrodes with High Photovoltage as Photoanode and Photocathode in Photoelectrochemical Cells for Water Splitting. ChemSusChem 2018, 11, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, S.; Dewil, R.; Vanierschot, M.; Baeyens, J.; Deng, Y. Water Splitting by MnOx/Na2CO3 Reversible Redox Reactions. Sustainability 2022, 14, 7597. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, H.; Zhu, M.; Li, Y.; Li, W. Interfacial Charge Transport in 1D TiO2 Based Photoelectrodes for Photoelectrochemical Water Splitting. Small 2021, 17, 1903378. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, T.; Ghayeb, Y.; Mohammadi, T.; Momeni, M.M.; Bagheri, R.; Song, Z. Surface treatment of titanium by in-situ anodizination and NiO photodeposition: Enhancement of photoelectrochemical properties for water splitting and photocathodic protection of stainless steel. Appl. Phys. A 2021, 127, 72. [Google Scholar] [CrossRef]
- Zhao, Y.; Hoivik, N.; Wang, K. Recent advance on engineering titanium dioxide nanotubes for photochemical and photoelectrochemical water splitting. Nano Energy 2016, 30, 728–744. [Google Scholar] [CrossRef]
- Ge, M.; Li, Q.; Cao, C.; Huang, J.; Li, S.; Zhang, S.; Chen, Z.; Zhang, K.; Al-Deyab, S.S.; Lai, Y. One-dimensional TiO2 nanotube photocatalysts for solar water splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Enhanced photocatalytic activity of bismuth-doped TiO2 nanotubes under direct sunlight irradiation for degradation of Rhodamine B dye. J. Nanoparticle Res. 2013, 15, 1669. [Google Scholar] [CrossRef]
- Mohammadi, T.; Ghayeb, Y.; Sharifi, T.; Momeni, M.M. RuO2 photodeposited on W-doped and Cr-doped TiO2 nanotubes with enhanced photoelectrochemical water splitting and capacitor properties. New J. Chem. 2020, 44, 2339–2349. [Google Scholar] [CrossRef]
- Huo, K.; Gao, B.; Fu, J.; Zhao, L.; Chu, P.K. Fabrication, modification, and biomedical applications of anodized TiO2 nanotube arrays. Rsc Adv. 2014, 4, 17300–17324. [Google Scholar] [CrossRef]
- Sharifi, T.; Mohammadi, T.; Momeni, M.M.; Kusic, H.; Kraljic Rokovic, M.; Loncaric Bozic, A.; Ghayeb, Y. Influence of Photo-Deposited Pt and Pd onto Chromium Doped TiO2 Nanotubes in Photo-Electrochemical Water Splitting for Hydrogen Generation. Catalysts 2021, 11, 212. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Kočí, K.; Edelmannová, M.; Lajaunie, L.; Figueiredo, B.; Calvino, J.J.; Seabra, M.P.; Labrincha, J.A. CuxO and carbon–modified TiO2–based hybrid materials for photocatalytically assisted H2 generation. Mater. Today Energy 2021, 19, 100607. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Chávez-Angulo, G.; Carrasco-Jaim, O.A.; Torres-Martínez, L.M.; Garza-Navarro, M.A. Enhanced photoelectrochemical water splitting on heterostructured α-Fe2O3-TiO2:X (X = Co, Cu, Bi) photoanodes: Role of metal doping on charge carrier dynamics improvement. J. Photochem. Photobiol. A Chem. 2021, 410, 113077. [Google Scholar] [CrossRef]
- Draz, M.A.; El-Maghrabi, H.H.; Soliman, F.S.; Selim, H.; Razik, A.A.; Amin, A.E.-s.; Moustafa, Y.M.; Hamdy, A.; Nada, A.A. Large scale hybrid magnetic ZnFe2O4/TiO2 nanocomposite with highly photocatalytic activity for water splitting. J. Nanopart. Res. 2021, 23, 10. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, D.; Xu, S.; Fang, Z.; Wang, L.; Yang, W.; Hou, H. Synergistic promotion of photoelectrochemical water splitting efficiency of TiO2 nanorod arrays by doping and surface modification. J. Mater. Chem. C 2021, 9, 12263–12272. [Google Scholar] [CrossRef]
- Montoya, A.T.; Gillan, E.G. Enhanced Photocatalytic Hydrogen Evolution from Transition-Metal Surface-Modified TiO2. ACS Omega 2018, 3, 2947–2955. [Google Scholar] [CrossRef]
- Šuligoj, A.; Arčon, I.; Mazaj, M.; Dražić, G.; Arčon, D.; Cool, P.; Štangar, U.L.; Tušar, N.N. Surface modified titanium dioxide using transition metals: Nickel as a winning transition metal for solar light photocatalysis. J. Mater. Chem. A 2018, 6, 9882–9892. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Jin, Q.; Iwaszuk, A.; Nolan, M. Molecular-scale transition metal oxide nanocluster surface-modified titanium dioxide as solar-activated environmental catalysts. J. Phys. Chem. C 2014, 118, 12077–12086. [Google Scholar] [CrossRef]
- Shopova-Gospodinova, D.; Jeurgens, L.P.; Welzel, U.; Bauermann, L.P.; Hoffmann, R.C.; Bill, J. Synthesis of V-doped TiO2 films by chemical bath deposition and the effect of post-annealing on their properties. Thin Solid Films 2012, 520, 5928–5935. [Google Scholar] [CrossRef]
- Kyeremateng, N.A.; Lebouin, C.; Knauth, P.; Djenizian, T. The electrochemical behaviour of TiO2 nanotubes with Co3O4 or NiO submicron particles: Composite anode materials for Li-ion micro batteries. Electrochim. Acta 2013, 88, 814–820. [Google Scholar] [CrossRef]
- Sharifi, T.; Ghayeb, Y.; Mohammadi, T.; Momeni, M.M. Enhanced photoelectrochemical water splitting of CrTiO2 nanotube photoanodes by the decoration of their surface via the photodeposition of Ag and Au. Dalton Trans. 2018, 47, 11593–11604. [Google Scholar] [CrossRef]
- Hariri, A.; Gilani, N.; Pasikhani, J.V. Promoting the photo-induced charge separation and photoelectrocatalytic hydrogen generation: Z-scheme configuration of WO3 quantum nanodots-decorated immobilized Ti/TiO2 nanorods. J. Alloys Compd. 2021, 871, 159528. [Google Scholar] [CrossRef]
- Yang, B.; Chai, Y.; Yang, F.; Zhang, Q.; Liu, H.; Wang, N. Hydrogen generation by aluminum-water reaction in acidic and alkaline media and its reaction dynamics. Int. J. Energy Res. 2018, 42, 1594–1602. [Google Scholar] [CrossRef]
- Mishra, P.; Shukla, P.; Srivastava, O. Study of modular PEC solar cells for photoelectrochemical splitting of water employing nanostructured TiO2 photoelectrodes. Int. J. Hydrogen Energy 2007, 32, 1680–1685. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y.; Davarzadeh, M. Single-step electrochemical anodization for synthesis of hierarchical WO3–TiO2 nanotube arrays on titanium foil as a good photoanode for water splitting with visible light. J. Electroanal. Chem. 2015, 739, 149–155. [Google Scholar] [CrossRef]
- Momeni, M.M.; Akbarnia, M.; Ghayeb, Y. Preparation of S–W-codoped TiO2 nanotubes and effect of various hole scavengers on their photoelectrochemical activity: Alcohol series. Int. J. Hydrogen Energy 2020, 45, 33552–33562. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; Gu, G.; Li, Q.; Kang, S.; Zhang, Y.; Ng, D.H.; Zhao, H. One pot microwave-assisted synthesis of Ag decorated yolk@ shell structured TiO2 microspheres. Rsc Adv. 2015, 5, 11349–11357. [Google Scholar] [CrossRef]
- D’Elia, D.; Beauger, C.; Hochepied, J.-F.; Rigacci, A.; Berger, M.-H.; Keller, N.; Keller-Spitzer, V.; Suzuki, Y.; Valmalette, J.-C.; Benabdesselam, M.; et al. Impact of three different TiO2 morphologies on hydrogen evolution by methanol assisted water splitting: Nanoparticles, nanotubes and aerogels. Int. J. Hydrogen Energy 2011, 36, 14360–14373. [Google Scholar] [CrossRef]
- Spanu, D.; Recchia, S.; Mohajernia, S.; Schmuki, P.; Altomare, M. Site-selective Pt dewetting on WO3-coated TiO2 nanotube arrays: An electron transfer cascade-based H2 evolution photocatalyst. Appl. Catal. B Environ. 2018, 237, 198–205. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y. Photoelectrochemical water splitting on chromium-doped titanium dioxide nanotube photoanodes prepared by single-step anodizing. J. Alloys Compd. 2015, 637, 393–400. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Ozkan, S.; Hejazi, S.; Denisov, N.; Tomanec, O.; Zboril, R.; Schmuki, P. Providing significantly enhanced photocatalytic H2 generation using porous PtPdAg alloy nanoparticles on spaced TiO2 nanotubes. Int. J. Hydrogen Energy 2019, 44, 22962–22971. [Google Scholar] [CrossRef]
- Li, F.; Huang, H.; Li, G.; Leung, D.Y. TiO2 nanotube arrays modified with nanoparticles of platinum group metals (Pt, Pd, Ru): Enhancement on photoelectrochemical performance. J. Nanopart. Res. 2019, 21, 29. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y. Fabrication, characterization and photoelectrochemical behavior of Fe–TiO2 nanotubes composite photoanodes for solar water splitting. J. Electroanal. Chem. 2015, 751, 43–48. [Google Scholar] [CrossRef]




| Photoelectrode | Rct (kΩcm−2) | CPE | ||
|---|---|---|---|---|
| n | Q (μFcm−2) | C (μFcm−2) | ||
| WT | 7.247 | 0.97 | 71.9 | 70.3 |
| Cr0Fe1–WT | 7.541 | 0.90 | 106 | 104 |
| Cr0.2Fe0.8–WT | 9.670 | 0.92 | 86.0 | 84.7 |
| Cr0.4Fe0.6–WT | 5.716 | 0.95 | 73.8 | 70.4 |
| Cr0.5Fe0.5–WT | 6.511 | 0.94 | 81.3 | 78.3 |
| Cr0.6Fe0.4–WT | 4.253 | 0.95 | 81.9 | 77.8 |
| Cr0.8Fe0.2–WT | 3.420 | 0.94 | 88.3 | 81.8 |
| Cr1Fe0–WT | 10.016 | 0.91 | 117 | 119 |
| Photoelectrode | Electrolyte | Light Source | Rate of H2 Evolution | Ref. |
|---|---|---|---|---|
| TiO2 NP | MeOH + H2O | 150 W halide | 0.270 µmol g−1min−1 | [33] |
| 1Pt-3WO3 -TiO2 NTs | H2O +20 vol% EtOH | UV | 5 µL h−1 | [34] |
| Cr-doped TiO2 NTs | NaOH | Vis | 9.70 μL cm−2 h−1 | [35] |
| Pd-TiO2 NTs | H2O +50 vol% MeOH | UV | 70 μL cm−2 h−1 | [36] |
| Ru-TiO2 NTs | Na2SO4 (2 M) | Vis | 29 μmol cm−2 h−1 | [37] |
| Fe-TiO2 NTs | NaOH | Vis | 10 μL cm−2 h−1 | [38] |
| Cu-TiO2 | H2O +50 vol% EtOH | UV | 81.1 µmol g−1h−1 | [20] |
| Cr0.8Fe0.2–WT | KOH + 10 vol% EG | Vis | 0.85 mL cm−2 h−1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, T.; Sharifi, S.; Ghayeb, Y.; Sharifi, T.; Momeni, M.M. Photoelectrochemical Water Splitting and H2 Generation Enhancement Using an Effective Surface Modification of W-Doped TiO2 Nanotubes (WT) with Co-Deposition of Transition Metal Ions. Sustainability 2022, 14, 13251. https://doi.org/10.3390/su142013251
Mohammadi T, Sharifi S, Ghayeb Y, Sharifi T, Momeni MM. Photoelectrochemical Water Splitting and H2 Generation Enhancement Using an Effective Surface Modification of W-Doped TiO2 Nanotubes (WT) with Co-Deposition of Transition Metal Ions. Sustainability. 2022; 14(20):13251. https://doi.org/10.3390/su142013251
Chicago/Turabian StyleMohammadi, Tecush, Somayeh Sharifi, Yousef Ghayeb, Tayebeh Sharifi, and Mohamad Mohsen Momeni. 2022. "Photoelectrochemical Water Splitting and H2 Generation Enhancement Using an Effective Surface Modification of W-Doped TiO2 Nanotubes (WT) with Co-Deposition of Transition Metal Ions" Sustainability 14, no. 20: 13251. https://doi.org/10.3390/su142013251
APA StyleMohammadi, T., Sharifi, S., Ghayeb, Y., Sharifi, T., & Momeni, M. M. (2022). Photoelectrochemical Water Splitting and H2 Generation Enhancement Using an Effective Surface Modification of W-Doped TiO2 Nanotubes (WT) with Co-Deposition of Transition Metal Ions. Sustainability, 14(20), 13251. https://doi.org/10.3390/su142013251







