Effects of Biochar and Plant Growth-Promoting Rhizobacteria on Plant Performance and Soil Environmental Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Field Sampling and Lab Measurements
2.3. Statistical Approach
3. Results and Discussion
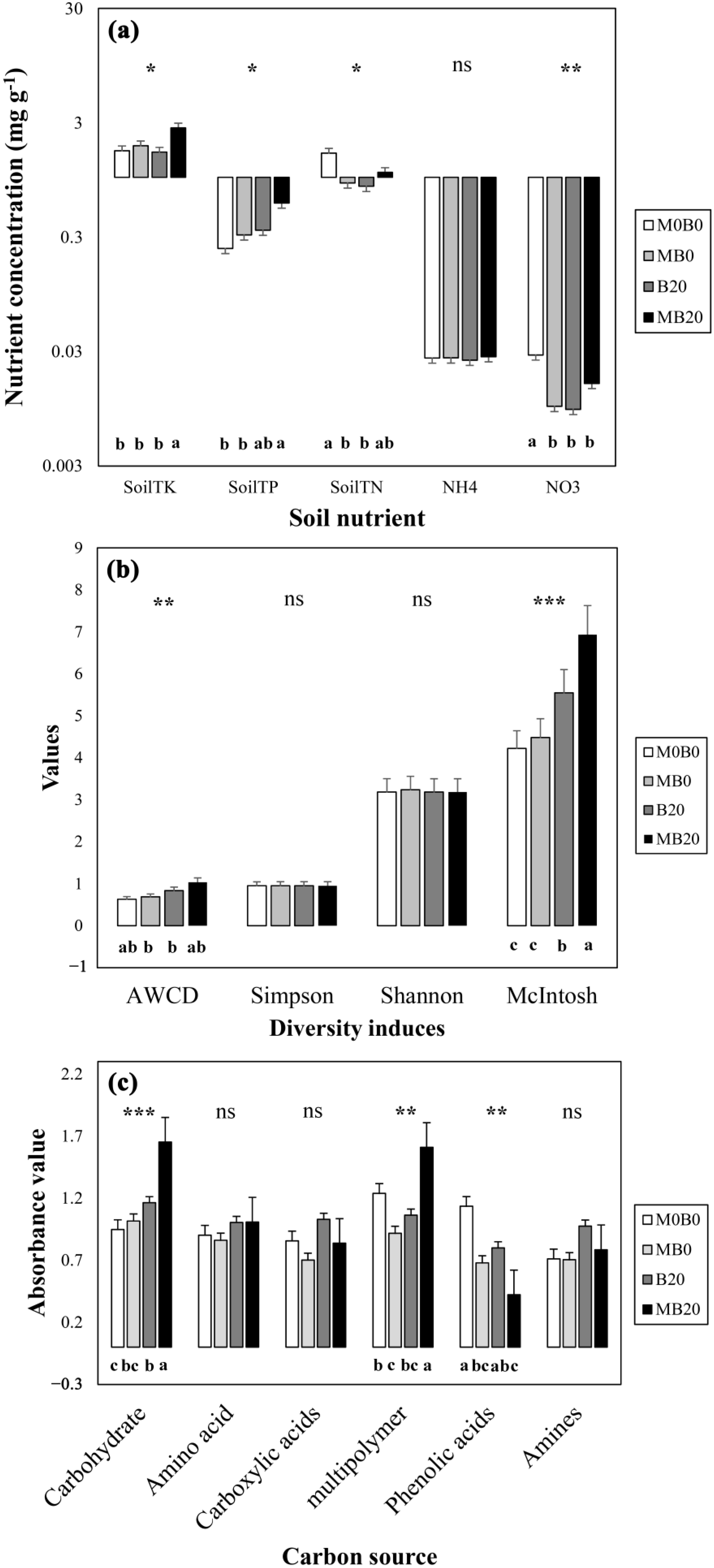
3.1. Soil Nutrient Contents
3.2. Diversity Indices of Soil Microbial Carbon Use
3.3. Differs in Soil Microbial Carbon Source Utilization
3.4. Specific Carbon Source Utilization
3.5. Plant Growth and Nutrient Status
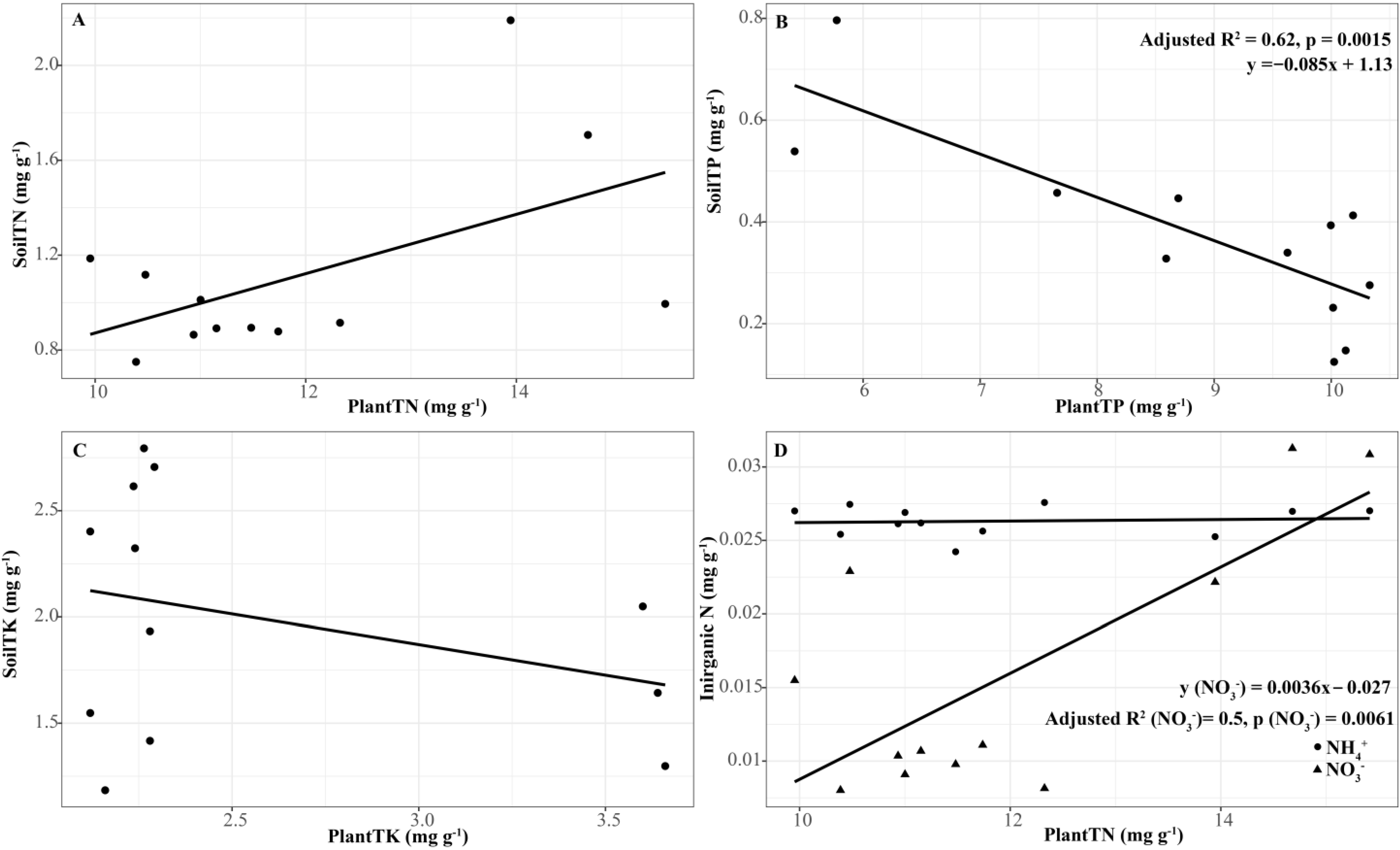
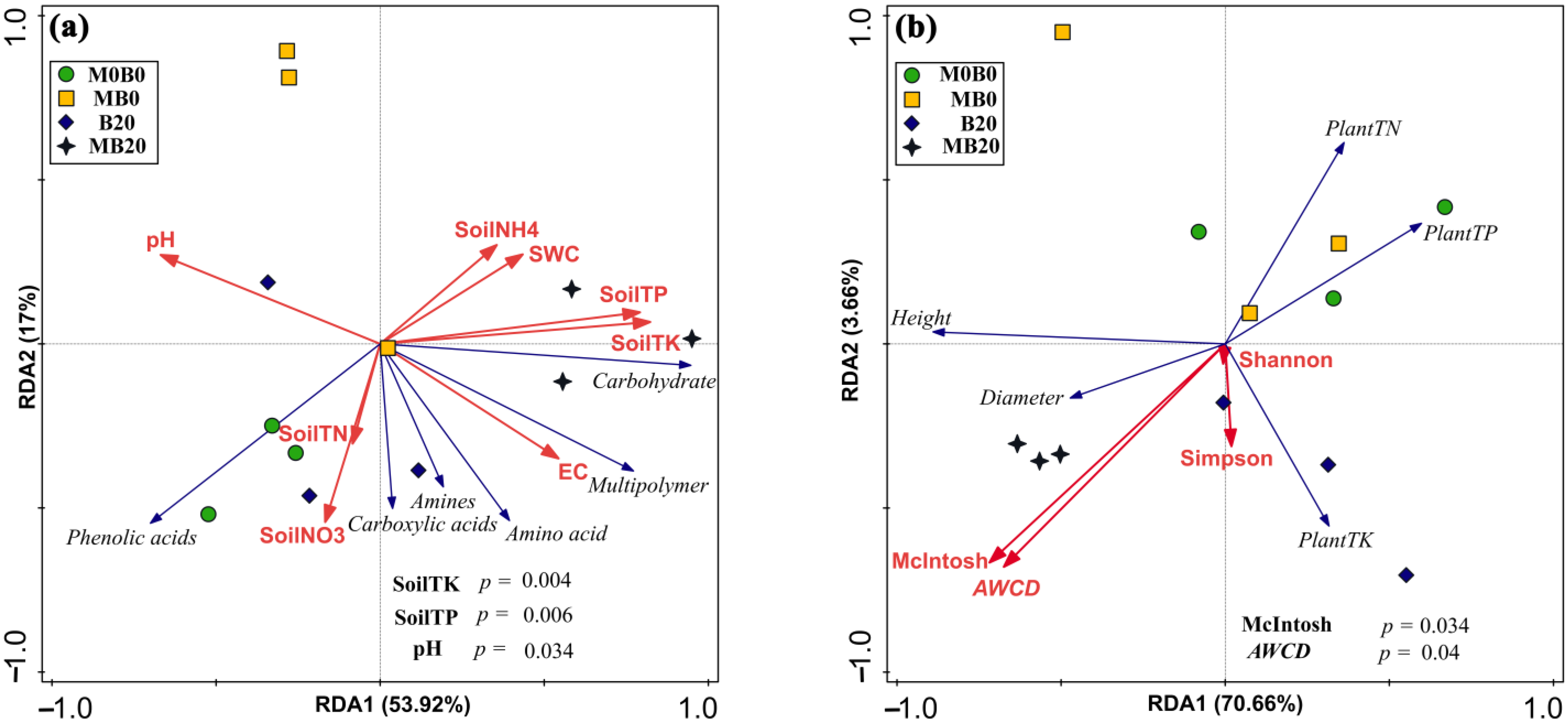
3.6. Relationship between Plant and Soil Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heidari, A.; Watkins, D.; Mayer, A.; Propato, T.; Verón, S.; de Abelleyra, D. Spatially Variable Hydrologic Impact and Biomass Production Tradeoffs Associated with Eucalyptus (E. grandis) Cultivation for Biofuel Production in Entre Rios, Argentina. GCB Bioenergy 2021, 13, 823–837. [Google Scholar] [CrossRef]
- Hua, L.; Yu, F.; Qiu, Q.; He, Q.; Su, Y.; Liu, X.; Li, J. Relationships between Diurnal and Seasonal Variation of Photosynthetic Characteristics of Eucalyptus Plantation and Environmental Factors under Dry-Season Irrigation with Fertilization. Agric. Water Manag. 2021, 248, 106737. [Google Scholar] [CrossRef]
- da Silva, L.P.; Heleno, R.H.; Costa, J.M.; Valente, M.; Mata, V.A.; Gonçalves, S.C.; da Silva, A.A.; Alves, J.; Ramos, J.A. Natural Woodlands Hold More Diverse, Abundant, and Unique Biota than Novel Anthropogenic Forests: A Multi-Group Assessment. Eur. J. For. Res. 2019, 138, 461–472. [Google Scholar] [CrossRef]
- Adekayode, F.; Olojugba, M. The Utilization of Wood Ash as Manure to Reduce the Use of Mineral Fertilizer for Improved Performance of Maize (Zea mays L.) as Measured in the Chlorophyll Content and Grain Yeild. J. Soil Sci. Environ. Manag. 2010, 1, 40–45. [Google Scholar]
- Butphu, S.; Rasche, F.; Cadisch, G.; Kaewpradit, W. Eucalyptus Biochar Application Enhances Ca Uptake of Upland Rice, Soil Available P, Exchangeable K, Yield, and N Use Efficiency of Sugarcane in a Crop Rotation System. J. Plant Nutr. Soil Sci. 2020, 183, 58–68. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant Growth Promoting Rhizobacteria as Biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Barbosa, C.F.; Correa, D.A.; Carneiro, J.S.; Melo, L.C. Biochar Phosphate Fertilizer Loaded with Urea Preserves Available Nitrogen Longer than Conventional Urea. Sustainability 2022, 14, 686. [Google Scholar] [CrossRef]
- Marzeddu, S.; Cappelli, A.; Ambrosio, A.; Décima, M.A.; Viotti, P.; Boni, M.R. A Life Cycle Assessment of an Energy-Biochar Chain Involving a Gasification Plant in Italy. Land 2021, 10, 1256. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Piater, L.A.; Dubery, I.A.; Tugizimana, F.; Mhlongo, M.I. Rhizosphere Tripartite Interactions and PGPR-Mediated Metabolic A Metabolomics Review. Biology 2022, 11, 346. [Google Scholar]
- Adoko, M.Y.; Sina, H.; Amogou, O.; Agbodjato, N.A.; Noumavo, P.A.; Aguégué, R.M.; Assogba, S.A.; Adjovi, N.A.; Dagbénonbakin, G.; Adjanohoun, A.; et al. Potential of Biostimulants Based on PGPR Rhizobacteria Native to Benin’s Soils on the Growth and Yield of Maize (Zea mays L.) under Greenhouse Conditions. Open J. Soil Sci. 2021, 11, 177–196. [Google Scholar] [CrossRef]
- Hungria, M.; Rondina, A.B.L.; Nunes, A.L.P.; Araujo, R.S.; Nogueira, M.A. Seed and Leaf-Spray Inoculation of PGPR in Brachiarias (Urochloa spp.) as an Economic and Environmental Opportunity to Improve Plant Growth, Forage Yield and Nutrient Status. Plant Soil 2021, 463, 171–186. [Google Scholar] [CrossRef]
- Daryabeigi Zand, A.; Tabrizi, A.M.; Heir, A.V. The Influence of Association of Plant Growth-Promoting Rhizobacteria and Zero-Valent Iron Nanoparticles on Removal of Antimony from Soil by Trifolium Repens. Environ. Sci. Pollut. Res. 2020, 27, 42815–42829. [Google Scholar] [CrossRef] [PubMed]
- Hafez, E.M.; Alsohim, A.S.; Farig, M.; Omara, A.E.D.; Rashwan, E.; Kamara, M.M. Synergistic Effect of Biochar and Plant Growth Promoting Rhizobacteria on Alleviation of Water Deficit in Rice Plants under Salt-Affected Soil. Agronomy 2019, 9, 847. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Bhutta, T.S.; Shaaban, M.; Hussain, S.; Qayyum, M.F.; Aslam, U.; Zahir, Z.A. Influence of Plant Growth Promoting Rhizobacterial Inoculation on Wheat Productivity under Soil Salinity Stress. Phyton 2019, 88, 119–129. [Google Scholar] [CrossRef]
- Welbaum, G.E. Managing Soil Microorganisms to Improve Productivity of Agro-Ecosystems of Agro-Ecosystems. CRC. Crit. Rev. Plant Sci. 2004, 23, 175–193. [Google Scholar] [CrossRef]
- Dinnage, R.; Simonsen, A.K.; Barrett, L.G.; Cardillo, M.; Raisbeck-Brown, N.; Thrall, P.H.; Prober, S.M. Larger Plants Promote a Greater Diversity of Symbiotic Nitrogen-Fixing Soil Bacteria Associated with an Australian Endemic Legume. J. Ecol. 2019, 107, 977–991. [Google Scholar] [CrossRef]
- Thomas, J.; Kim, H.R.; Rahmatallah, Y.; Wiggins, G.; Yang, Q.; Singh, R.; Glazko, G.; Mukherjee, A. RNA-Seq Reveals Differentially Expressed Genes in Rice (Oryza Sativa) Roots during Interactions with Plant-Growth Promoting Bacteria, Azospirillum Brasilense. PLoS ONE 2019, 14, e0217309. [Google Scholar] [CrossRef]
- Pham, V.T.K.; Rediers, H.; Ghequire, M.G.K.; Nguyen, H.H.; De Mot, R.; Vanderleyden, J.; Spaepen, S. The Plant Growth-Promoting Effect of the Nitrogen-Fixing Endophyte Pseudomonas Stutzeri A15. Arch. Microbiol. 2017, 199, 513–517. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Yang, Y.; Xia, X.; Li, F.; Yang, Z.; Xing, B. Biochar’s Stability and Effect on the Content, Composition and Turnover of Soil Organic Carbon. Geoderma 2020, 364, 114184. [Google Scholar] [CrossRef]
- Ren, H.; Huang, B.; Fernández-garcía, V.; Miesel, J.; Yan, L. Biochar and Rhizobacteria Amendments Improve Several Soil Properties. Microorganisms 2020, 8, 502. [Google Scholar] [CrossRef]
- Boni, M.R.; Chiavola, A.; Antonucci, A.; Di Mattia, E.; Marzeddu, S. A Novel Treatment for Cd-Contaminated Solution through Adsorption on Beech Charcoal: The Effect of Bioactivation. Desalin. Water Treat. 2018, 127, 104–110. [Google Scholar] [CrossRef]
- Luo, S.; Wang, S.; Tian, L.; Li, S.; Li, X.; Shen, Y.; Tian, C. Long-Term Biochar Application Influences Soil Microbial Community and Its Potential Roles in Semiarid Farmland. Appl. Soil Ecol. 2017, 117–118, 10–15. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and Mechanisms of Biochar-Microbe Interactions in Soil Improvement and Pollution Remediation: A Review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- He, M.; Xiong, X.; Wang, L.; Hou, D.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Tsang, D.C.W. A Critical Review on Performance Indicators for Evaluating Soil Biota and Soil Health of Biochar-Amended Soils. J. Hazard. Mater. 2021, 414, 125378. [Google Scholar] [CrossRef] [PubMed]
- Halmi, M.F.A.; Simarani, K. Effect of Two Contrasting Biochars on Soil Microbiota in the Humid Tropics of Peninsular Malaysia. Geoderma 2021, 395, 115088. [Google Scholar] [CrossRef]
- Oguntunde, P.G.; Fosu, M.; Ajayi, A.E.; Van De Giesen, N. De Effects of Charcoal Production on Maize Yield, Chemical Properties and Texture of Soil. Biol. Fertil. Soils 2004, 39, 295–299. [Google Scholar] [CrossRef]
- Kant, S.; Kant, R.; Yang, Y. An Overview of Microdiesel—A Sustainable Future Source of Renewable Energy. Renew. Sustain. Energy Rev. 2017, 79, 1078–1090. [Google Scholar]
- Singh, B.; MacDonald, L.M.; Kookana, R.S.; Van Zwieten, L.; Butler, G.; Joseph, S.; Weatherley, A.; Kaudal, B.B.; Regan, A.; Cattle, J.; et al. Opportunities and Constraints for Biochar Technology in Australian Agriculture: Looking beyond Carbon Sequestration. Soil Res. 2014, 52, 739–750. [Google Scholar] [CrossRef]
- Ren, H.; Lv, C.; Fernández-García, V.; Huang, B.; Yao, J.; Wei, D. Biochar and PGPR Amendments Influence Soil Enzyme Activities and Nutrient Concentrations in a Eucalyptus Seedling Plantation. Biomass Convers. Biorefinery 2021, 11, 1865–1874. [Google Scholar] [CrossRef]
- Gałazka, A.; Jończyk, K.; Gawryjołek, K.; Ciepiel, J. The Impact of Biochar Doses on Soil Quality and Microbial Functional Diversity. BioResources 2019, 14, 7852–7868. [Google Scholar]
- D’Elia, C.F.; Steudler, P.A.; Corwin, N. Determination of Total Nitrogen in Aqueous Samples Using Persulfate Digestion. Limnology 1977, 22, 760–764. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Development Core Team: Vienna, Austria, 2007. [Google Scholar]
- Mia, S.; van Groenigen, J.W.; van de Voorde, T.F.J.; Oram, N.J.; Bezemer, T.M.; Mommer, L.; Jeffery, S. Biochar Application Rate Affects Biological Nitrogen Fixation in Red Clover Conditional on Potassium Availability. Agric. Ecosyst. Environ. 2014, 191, 83–91. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Salazar, P.; Barrón, V.; Torrent, J.; Del Campillo, M.D.C.; Gallardo, A.; Villar, R. Enhanced Wheat Yield by Biochar Addition under Different Mineral Fertilization Levels. Agron. Sustain. Dev. 2013, 33, 475–484. [Google Scholar] [CrossRef]
- Chintala, R.; Schumacher, T.E.; McDonald, L.M.; Clay, D.E.; Malo, D.D.; Papiernik, S.K.; Clay, S.A.; Julson, J. Phosphorus Sorption and Availability from Biochars and Soil/Biochar Mixtures. J. Endourol. 2009, 23, A21–A22. [Google Scholar] [CrossRef]
- Ogawa, M.; Okimori, Y. Pioneering Works in Biochar Research, Japan. Aust. J. Soil Res. 2010, 48, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, N.; Dai, Z.; Xiao, K.; Meng, J.; Brookes, P.C.; Liu, X.; Wang, H.; Wu, J.; Xu, J. Changes in Microbial Community Structure Due to Biochars Generated from Different Feedstocks and Their Relationships with Soil Chemical Properties. Geoderma 2014, 226–227, 270–278. [Google Scholar] [CrossRef]
- Ren, H.; Qin, X.; Huang, B.; Fernández-García, V.; Lv, C. Responses of Soil Enzyme Activities and Plant Growth in a Eucalyptus Seedling Plantation Amended with Bacterial Fertilizers. Arch. Microbiol. 2020, 202, 1381–1396. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn Growth and Nitrogen Nutrition after Additions of Biochars with Varying Properties to a Temperate Soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Deslippe, J.R.; Hartmann, M.; Simard, S.W.; Mohn, W.W. Long-Term Warming Alters the Composition of Arctic Soil Microbial Communities. FEMS Microbiol. Ecol. 2012, 82, 303–315. [Google Scholar] [CrossRef]
- Shah, G.; Jan, M.; Afreen, M.; Anees, M.; Rehman, S.; Daud, M.K.; Malook, I.; Jamil, M. Halophilic Bacteria Mediated Phytoremediation of Salt-Affected Soils Cultivated with Rice. J. Geochem. Explor. 2015, 174, 59–65. [Google Scholar] [CrossRef]
- Lu, W.; Ding, W.; Zhang, J.; Li, Y.; Luo, J.; Bolan, N.; Xie, Z. Biochar Suppressed the Decomposition of Organic Carbon in a Cultivated Sandy Loam Soil: A Negative Priming Effect. Soil Biol. Biochem. 2014, 76, 12–21. [Google Scholar] [CrossRef]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar Addition Rate Influences Soil Microbial Abundance and Activity in Temperate Soils. Eur. J. Soil Sci. 2014, 65, 28–39. [Google Scholar] [CrossRef]
- Song, Q.; Qin, F.; He, H.; Wang, H.; Yu, S. Allelopathic Potential of Rain Leachates from Eucalyptus Urophylla on Four Tree Species. Agrofor. Syst. 2019, 93, 1307–1318. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Biochar Mitigates Salinity Stress in Potato. J. Agron. Crop Sci. 2015, 201, 368–378. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Z.; Li, Z.; Wu, H. Impacts of Aeration and Biochar Addition on Extracellular Polymeric Substances and Microbial Communities in Constructed Wetlands for Low C/N Wastewater Treatment: Implications for Clogging. Chem. Eng. J. 2020, 396, 125349. [Google Scholar] [CrossRef]
- Nuccio, E.E.; Starr, E.; Karaoz, U.; Brodie, E.L.; Zhou, J.; Tringe, S.G.; Malmstrom, R.R.; Woyke, T.; Banfield, J.F.; Firestone, M.K.; et al. Niche Differentiation Is Spatially and Temporally Regulated in the Rhizosphere. ISME J. 2020, 14, 999–1014. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential Mechanisms for Achieving Agricultural Benefits from Biochar Application to Temperate Soils: A Review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Shen, X.; Hu, H.; Peng, H.; Wang, W.; Zhang, X. Comparative Genomic Analysis of Four Representative Plant Growth-Promoting Rhizobacteria in Pseudomonas. BMC Genom. 2013, 14, 271. [Google Scholar] [CrossRef]
- Han, F.; Hu, W.; Zheng, J.; Du, F.; Zhang, X. Estimating Soil Organic Carbon Storage and Distribution in a Catchment of Loess Plateau, China. Geoderma 2010, 154, 261–266. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; Mackowiak, C.L.; Comerford, N.B.; da Veiga Moline, E.F.; Shirley, J.P.; Guimaraes, D.V. Pyrolysis Methods Impact Biosolids-Derived Biochar Composition, Maize Growth and Nutrition. Soil Tillage Res. 2017, 165, 59–65. [Google Scholar] [CrossRef]
- Ren, H.; Warnock, D.D.; Tiemann, L.K.; Quigley, K.; Miesel, J.R. Evaluating Foliar Characteristics as Early Indicators of Plant Response to Biochar Amendments. For. Ecol. Manag. 2021, 489, 119047. [Google Scholar] [CrossRef]
- Liqun, X.; Weiming, Z.; Di, W.; Yanyan, S.; Honggui, Z.; Wenqi, G.; Jun, M.; Chen, W. Heat Storage Capacity and Temporal-Spatial Response in the Soil Temperature of Albic Soil Amended with Maize-Derived Biochar for 2 Years. Soil Tillage Res. 2021, 205, 104762. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Ali, S.; Ali, B.; Hameed, S.; Zhou, W. Plant Growth Promoting Bacteria Confer Salt Tolerance in Vigna Radiata by Up-Regulating Antioxidant Defense and Biological Soil Fertility. Plant Growth Regul. 2016, 80, 23–36. [Google Scholar] [CrossRef]

| Fixed C (mg g−1) | Bulk Density (g cm−3) | pH | EC (mS cm−1) | CEC (cmol kg−1) |
|---|---|---|---|---|
| 650 | 0.19 | 10.24 | 4.68 | 60.80 |
| Carbon Type | Order | Carbon Source | PC1 | PC2 |
|---|---|---|---|---|
| Carbohydrate | C6 | D-cellose | −0.11 | 0.033 |
| C7 | a-D-lactose | −0.26 | −0.072 | |
| C8 | ß-methyl D-glycoside | −0.25 | 0.056 | |
| C9 | D-xylose | −0.24 | −0.15 | |
| C10 | L-erythritol | −0.059 | −0.079 | |
| C11 | D-mannitol | −0.30 | −0.12 | |
| C12 | N-acetyl-D-gluosamine | −0.18 | 0.061 | |
| C14 | Glucose−1-phosphate | −0.13 | −0.24 | |
| C15 | D, L-a-glycerol | −0.29 | −0.071 | |
| C16 | D-glactonicacid γ lactone | −0.29 | 0.025 | |
| Amino acid | C24 | L-arginine | −0.14 | 0.25 |
| C25 | L-asparagine | 0.055 | 0.29 | |
| C26 | L-phenylalanine | 0.084 | −0.30 | |
| C27 | L-serine | −0.22 | 0.25 | |
| C28 | L-threonine | 0.10 | −0.27 | |
| C29 | Glycyl-L-glutamate | −0.26 | −0.22 | |
| Carbonxylic acid | C1 | Methyl pyruvate | 0.0025 | 0.16 |
| C20 | r-hydroxybutyric acid | −0.14 | 0.30 | |
| C21 | Itaconic acid | −0.022 | 0.15 | |
| C22 | a-ketobutyric acid | 0.022 | 0.17 | |
| C23 | D-malic acid | 0.034 | 0.15 | |
| C13 | D-glucosaminicacid | −0.26 | −0.11 | |
| C17 | D-galactose | −0.27 | 0.16 | |
| Multipolymer | C2 | Tween 40 | −0.16 | −0.029 |
| C3 | Tween 80 | −0.15 | −0.087 | |
| C4 | a-cyclodextrin | −0.12 | −0.048 | |
| C5 | Glycogenin | −0.18 | −0.10 | |
| Phenoliacids | C18 | 2-hydroxy-benzoic acid | 0.19 | −0.12 |
| C19 | 4-hydroxy-benzoic acid | 0.16 | 0.22 | |
| Amines | C30 | Phenylethylamine | −0.10 | 0.27 |
| C31 | Putrescine | −0.068 | 0.27 |
| Plant Variable | Unit | M0B0 | MB0 | B20 | MB20 |
|---|---|---|---|---|---|
| Height | cm | 91 ± 6.24 b | 96.67 ± 6.03 ab | 90.33 ± 3.79 b | 102 ± 2.65 a |
| Diameter | mm | 10.14 ± 0.58 | 9.87 ± 1.14 | 9.17 ± 0.17 | 11.09 ± 0.98 |
| TN | mg g−1 | 14.68 ± 0.73 a | 11.74 ± 0.59 b | 10.94 ± 0.55 bc | 10.48 ± 0.52 c |
| TP | mg g−1 | 6.91 ± 0.8 a | 9.52 ± 0.87 a | 10.07 ± 0.1 a | 6.28 ± 1.2 b |
| TK | mg g−1 | 2.13± 0.023 c | 2.27 ± 0.023 b | 3.63 ± 0.031 a | 2.26 ± 0.028 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Li, Z.; Chen, H.; Zhou, J.; Lv, C. Effects of Biochar and Plant Growth-Promoting Rhizobacteria on Plant Performance and Soil Environmental Stability. Sustainability 2022, 14, 10922. https://doi.org/10.3390/su141710922
Ren H, Li Z, Chen H, Zhou J, Lv C. Effects of Biochar and Plant Growth-Promoting Rhizobacteria on Plant Performance and Soil Environmental Stability. Sustainability. 2022; 14(17):10922. https://doi.org/10.3390/su141710922
Chicago/Turabian StyleRen, Han, Zilu Li, Hualin Chen, Jiangmin Zhou, and Chengqun Lv. 2022. "Effects of Biochar and Plant Growth-Promoting Rhizobacteria on Plant Performance and Soil Environmental Stability" Sustainability 14, no. 17: 10922. https://doi.org/10.3390/su141710922
APA StyleRen, H., Li, Z., Chen, H., Zhou, J., & Lv, C. (2022). Effects of Biochar and Plant Growth-Promoting Rhizobacteria on Plant Performance and Soil Environmental Stability. Sustainability, 14(17), 10922. https://doi.org/10.3390/su141710922






