1. Introduction
Humanity is significantly dependent on energy. There is a deep gap between consumers and fossil fuel accessibility due to the increase in economic growth and social development [
1]. Today, the energy crisis and environmental pollution caused by fossil fuels is a major problem [
2,
3]. Increasing human activities and the use of natural energy sources has caused a reduction in clean energy, and the methods currently in place to produce energy are not environmentally friendly [
4]. Worries about global climate warming require the development of new techniques of energy generation by natural sources of carbon and sustainable energy [
5,
6]. Different clean energy sources have been designed and utilized to solve this issue [
5]. For example, photovoltaic systems, wind turbines, and fuel cells (FCs) are some renowned sources in this area [
7]. Among them, fuel cells, as a new and environmentally friendly technology, have become more studied and utilized [
8]. Fuel cells are a technology with high efficiency for converting chemical energy into electrical energy [
9,
10]. Hydrogen is used as the cleanest energy as fuel in fuel cells [
11]. Hydrogen is one of the most abundant elements on Earth [
12]. This element does not exist in nature in its pure form, but it can be obtained in several different ways from other elements [
13]. Due to the depletion of oil reserves and pollution of fossil fuels, the use of fuel cells is necessary in the future which does not pollute the environment in cars and power plants. Fuel cells have several advantages, for example,
- -
They do not produce fuel, greenhouse gas, or pollution of air.
- -
They significantly cause environment enhancement [
14].
- -
They are more efficient than combustion engines.
- -
Unlike co-generation uses, these cells generate heat and electrical power with efficiency of about 80%.
- -
FCs generate water and heat without particles, GHGs, or toxins, i.e., these cells generate unpolluted air.
- -
They can be used in various sizes from mWs to MWs, such as in buildings, mobile phones, cars, etc.
- -
FC supplements are applicable in various energy techniques, such as wind turbines, batteries, solar panels, and super capacitors [
8].
There are several models of fuel cells that are divided based on their configurations. Each of these types also have their advantages and disadvantages [
15]. Recently, the utilization of solid oxide fuel cells (SOFCs) in high-temperature systems, such as power plants, has made them devices with high tendency from the industry society [
16]. Because of high temperature working points, designing and testing these models needs a lot of time and comes with a high cost. A proper method to prevent these problems is to first model and simulate these devices in the computer and after reaching to the best efficiency, they can be designed and constructed [
17]. Another problem is that the manufactured SOFCs after sending to the costumers, have some unknown parameters in their model which should be then selected optimally for the considered work. Due to the high cost of polymer electrolytes, it is better to predesign and simulate it. Some papers are published in this regard [
18]. However, literature showed that using metaheuristic-based techniques can be so useful for solving such complicated problems. Different metaheuristics are proposed in this direction. For example, Improved Red Fox Optimizer (IRFO) [
19], Marine Predator Optimizer (MPO) [
20], chaotic grey wolf metaheuristic algorithm [
21], Levenberg–Marquardt republishing optimization algorithm [
22], genetic algorithm, and radial movement optimization [
23] are some of these techniques. In the following, more details of the algorithms are explained.
Luo et al. [
19] used metaheuristic methods to detect solid oxide fuel cell (SOFC) parameters. In this research, an optimized metaheuristic method is used to detect the parameters of SOFC. The optimized metaheuristic method is called Improved Red Fox Optimizer (IRFO). For the evaluation of the effectiveness, an optimized metaheuristic model used the Sum of Squared Error (SSE). The finding displayed that the IRFO method had minimum error to generate power in various temperature conditions. It was obtained by about 0.0073 kW. Moreover, the optimized metaheuristic method had a minimum error value for output voltage by about 0.16 V. This finding was acceptable to detect SOFC parameters.
Yousri et al. [
20] detected unknown parameters of SOFCs dynamically simulating by comprehensive learning dynamic multiple-swarm MPO method. The most important step in presenting an energy storage system is to accurately identify the unknown parameters. In this research, with the help of the Marine Predator Optimizer (MPO), the proposed strategies detected the variables. The efficiency of the proposed optimization method was evaluated in different conditions of sudden load changes and dynamic voltage responses. Comparison of the results showed that the Marine Predator Optimizer (MPO), by the suggested strategies had the highest accuracy in identifying unknown parameters and presented the lowest variance between current and voltage.
Hao et al. [
21] utilized the improved chaotic grey wolf metaheuristic algorithm to detect solid oxide fuel cells variables. The purpose of their use of optimization methods is to increase the speed and accuracy of identifying the parameters of solid oxide fuel cells. The results of using the chaotic grey wolf optimization method showed that this optimization method had the least mean square error, the highest accuracy, and stability in the optimal identification of unknown parameters. It also had the highest convergence rate for resolving optimization problems. Moreover, it was able to provide the minimum variance between the current and voltage curves.
Yang et al. [
22] identified the unknown parameters of the SOFC using the metaheuristic method to identify SOFC parameters for modeling. For this objective in this study, the Levenberg–Marquardt republishing optimization algorithm was used. This algorithm was used to increase the efficiency of the artificial neural network in the training process to identify unknown parameters. The results suggested by the technique were compared with the electrochemical model (ECM) and steady-state model (SSM). The optimized artificial neural network had good simulation results in identifying the parameters so that this improved model was able to identify unknown parameters with high speed and stability and provide more accurate results.
Nassef et al. [
23] applied an optimal ANN method to identify the accuracy of solid oxide fuel cell parameters. The optimal ANN model was based on radial movement optimization. To confirm the optimum artificial neural network, it was compared to a genetic algorithm. For this objective, the genetic algorithm and radial movement optimization were compared in two different conditions. The investigation methods showed that the radial movement optimization method increased the power by about 17.28% compared to the genetic optimization algorithm. Furthermore, in the second condition, the radial movement metaheuristic method increased the power compared to the genetic method about 28.85%.
Based on the literature, efficient results can be provided by optimization algorithms for system identification of the SOFCs. Likewise, the application of metaheuristics as a famous part of optimizers is continuously being increased. It is worth noting that by the “No free lunch” theorems [
24], there is no metaheuristic algorithm with the capability of solving any type of problem. The major target here is to propose a developed design of a metaheuristic optimizer to deliver a SOFC model with higher efficiency. Therefore, the main contributions of the present study can be briefly highlighted as follows:
- -
New optimal parameters estimation of the solid oxide fuel cell system based on metaheuristics.
- -
The idea is to minimize the error between the model output and the empirical datapoints.
- -
A developed version of Battle Royale algorithm is utilized to minimize the error value.
- -
The method is performed on a 96-cell SOFC stack under different temperature and pressure values.
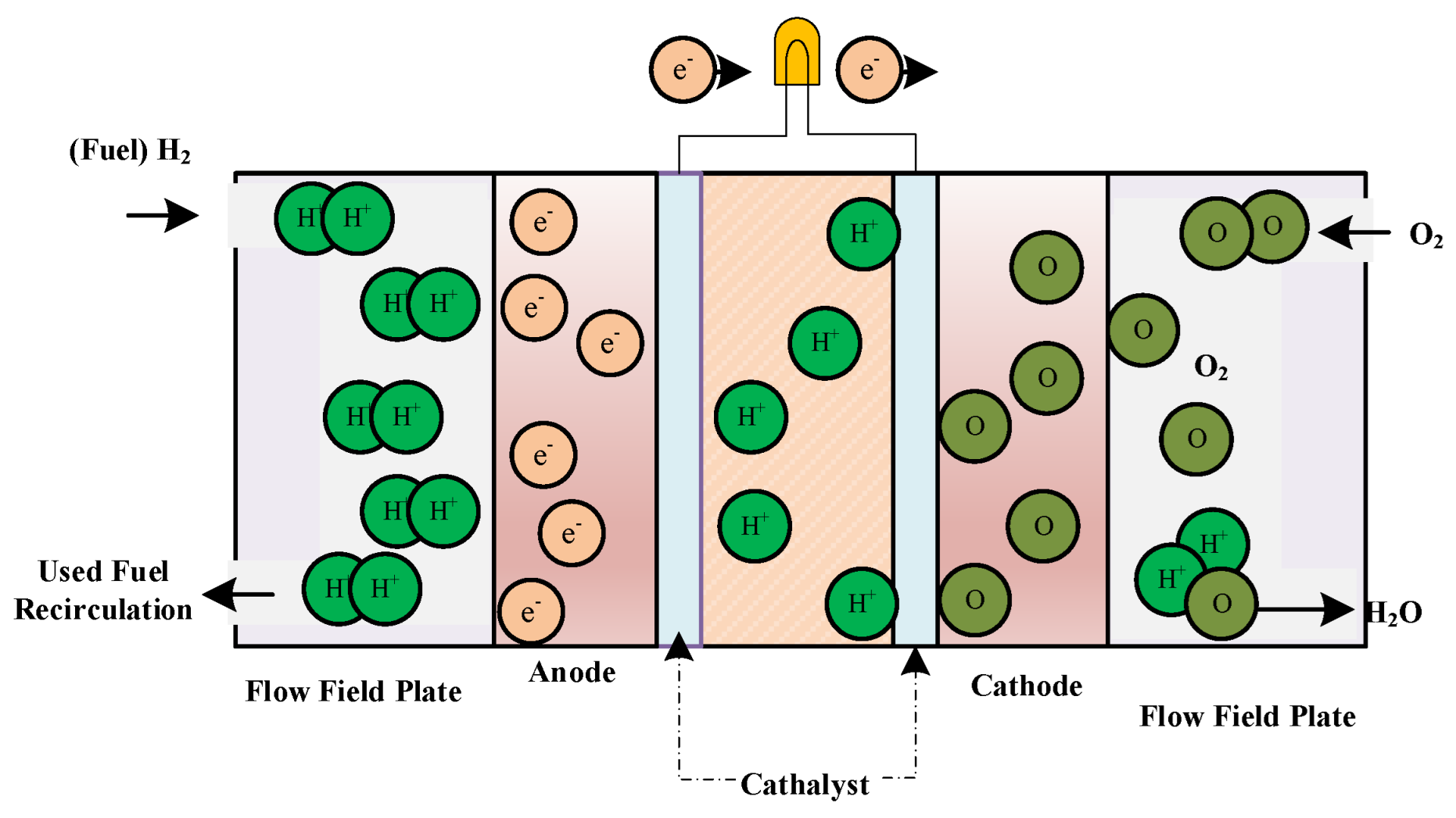
2. Modeling of a SOFC
The operational temperature of SOFC ranges between 600 °C and 1000 °C and therefore various kinds of fuels can be applied in it. This fuel cell has two plate and tube structures and a thin ceramic solid electrolyte is used instead of a liquid electrolyte. At high operating temperatures in a solid oxide fuel cell, oxygen ions (negatively charged) move over the crystal lattice [
25]. At the negative electrode, four electrons have been combined with
. While a hydrogen-containing gas fuel moves over the positive electrode,
ions with negative load moves through the electrolyte for fuel oxidization [
26]. The generated electrons at the positive electrode create an exterior circuit to produce electricity. SOFCs do not require an additional converter. Solid oxide combustion reactions are given below [
27]:
And the total reaction of the system is:
The efficiency of SOFC is almost 45–60% and the density of power generation in this type of fuel cell is 240–300. The lifespan of it is more than 40,000 h. The total efficiency is increased to 70% by combination of this cell with a turbine.
Figure 1 shows a general form of a SOFC.
As can be observed from
Figure 1, by considering the high operating temperature operation condition, the oxygen ions, which are charged negatively, move through membrane which is typically a blend of calcium oxide and zirconium oxide. The
molecules are then combined with 4 electrons at the cathode side. During the process, if a gassy fuel containing hydrogen molecules moves through the anode, there will be a movement by the negatively charged current including oxygen ions moving over the electrolyte for fuel oxidization. The electrons that are generated at the positive electrode side move over an exterior circuit to reach to the negative electrode side and produces electricity.
By considering the configuration explained before, the SOFC generate electricity with
voltage. This voltage is achieved by considering different voltage drops that have happened on the fuel cell. The main voltage losses in the SOFC include activation voltage drop, concentration voltage drops, and ohmic voltage drop. By considering these losses, the outputted voltage of the SOFC is achieved as follows [
28]:
where
describes the ohmic voltage drop,
defines the activation voltage, and
specifies the concentration voltage drop,
determines the quantity of the cells, and
is the open circuit voltage which is achieved by the following formula:
where
describes the reversible potentiality, and
specifies the operational temperature.
Table 1 reports the parameter value of the parameters.
,
, and
describe the partial pressure for
,
, and the
, respectively. The partial pressure for Oxygen and Hydrogen are achieved by the following [
29]:
where
and
represent the inlet pressure in anode and cathode, and
and
represent the relative humidity of vapor at the positive and negative electrodes sides.
The ohmic voltage loss of the SOFC can be calculated as follows:
And the concentration voltage loss for the cell is mathematically achieved as follows:
where
defines the partial pressure of the hydrogen,
partial pressure of oxygen, and
partial pressure of water.
The activation voltage drop is defined as given below:
where
represents the Tafel coefficient and
is the slope that can be formulated as follows:
Finally, the terminal voltage of a SOFC can be obtained as below:
where
signifies the resistance of the device area (kΩ cm
2),
specifies the constraint of current density (mA cm
2), and
and
refer to the exchanging flow’s current density of the cathode and anode, respectively. By considering the clarified equations, seven unknown parameters are defined for optimization. The parameters include
,
,
,
,
,
, and
.
5. Simulation Results
As can be observed from the previous sections, the major target of this paper is to provide an efficient method for system identification of the SOFCs. The major concept is to provide an optimum method by a developed design of Battle Royal Optimizer to minimize the sum of square error between the experimental data and the estimated outputted voltage from the designed system. The model is based on working on the mathematical conception of the SOFC with considering some undetermined variables on the model as decision values which should be optimally selected for minimizing the error value. The decision variables are , , ,, , , and . As mentioned before, due to using the stochastic algorithm here, we ran the algorithm 25 times independently and its mean value was considered as the final solution.
For ability validation of the presented Developed Battle Royal Optimizer in optimal parameter selection of the SOFC system, it was implemented to a studied case and its achievements were compared with some other related latest techniques by Chaotic Gray Wolf Optimization algorithm (CGWO) [
47], Satin Bowerbird Optimizer (SBO) [
48], Simplified Competitive Swarm Optimizer (SCSO) [
49], and Teaching-Learning Based Algorithm (TLBO) [
50]. To provide a fair comparison, like the Developed Battle Royal algorithm, all of the compared methods were run 25 times and the maximum iteration and the population size for all of them were similarly set to 200 and 50, respectively.
The case study in this research is a 96-cell SOFC stack where its information was collected from [
51]. The minimum and higher constraints of the major parameters for the studied case is given in
Table 5.
In the first step, the system efficiency was analyzed based on temperature variations under constant pressure. Therefore, this assessment shows how temperature changing can affect the identification system. Here, we used 160 datapoints for the analysis. In this study, five different temperatures, namely 550 °C, 600 °C, 650 °C, 700 °C, and 750 °C, under 3 atm constant pressure value were analyzed.
Table 6 reports the simulation achievements of the suggested technique under different temperatures in comparison with some of the latest algorithms.
As can be observed from
Table 6, the proposed DBRA with 9.41 × 10
−5 in lower temperature, i.e., 550 °C, provided the best confirmation with minimum error value than the other comparative methods. It is clear that by increasing the temperature value, the error value for all of the methods was increased. Because the above values were achieved after 25 runs as mean value of each algorithm, their standard deviation value should be also considered to show their consistency during different independent runs. The standard deviation results of the studied case under different temperature value and 3 atm constant pressure value are given in
Figure 3.
As can be inferred from the results of
Table 6 and
Figure 3, there is a minimum error value for all of the metaheuristic algorithms which shows their efficient ability in model parameter estimation of the SOFC. However, the proposed DBRA algorithm with 9.41 × 10
−5 error in 550 °C provided the minimum value toward the others which shows its higher accuracy than the others. It can also be inferred from
Figure 3 that there is an observable difference between the proposed DBRA and the other algorithms in their reliability, which shows the propose method’s higher consistency during 25 independent runs. After parameter estimation of the solid oxide fuel cell system, the value of the unknown variables can be achieved and are reported in
Table 7.
As can be inferred from
Table 7, the proposed method with 9.41 × 10
−5 SSE value in 550 °C provides the highest confirmation with the real value and its results get weaker by increasing the temperature value, where in the highest experimented temperature (750 °C), the maximum SSE value (3.97 × 10
−3) was achieved. The temperature variations provide a strong upshot on the estimator, i.e.,
ith incrementing of the value of temperature, the density amount of the exchange current for positive and negative is increased, though the voltage is decreased. Moreover,
Table 7 shows that
values for both training and testing data were extremely close to 1.00. As a result, we can infer that the proposed approach was flawlessly conducted and could precisely anticipate SOFC voltage with the exception of a few severe border situations. We can also prove the better efficiency of the proposed method from the accuracy (99.03%) results.
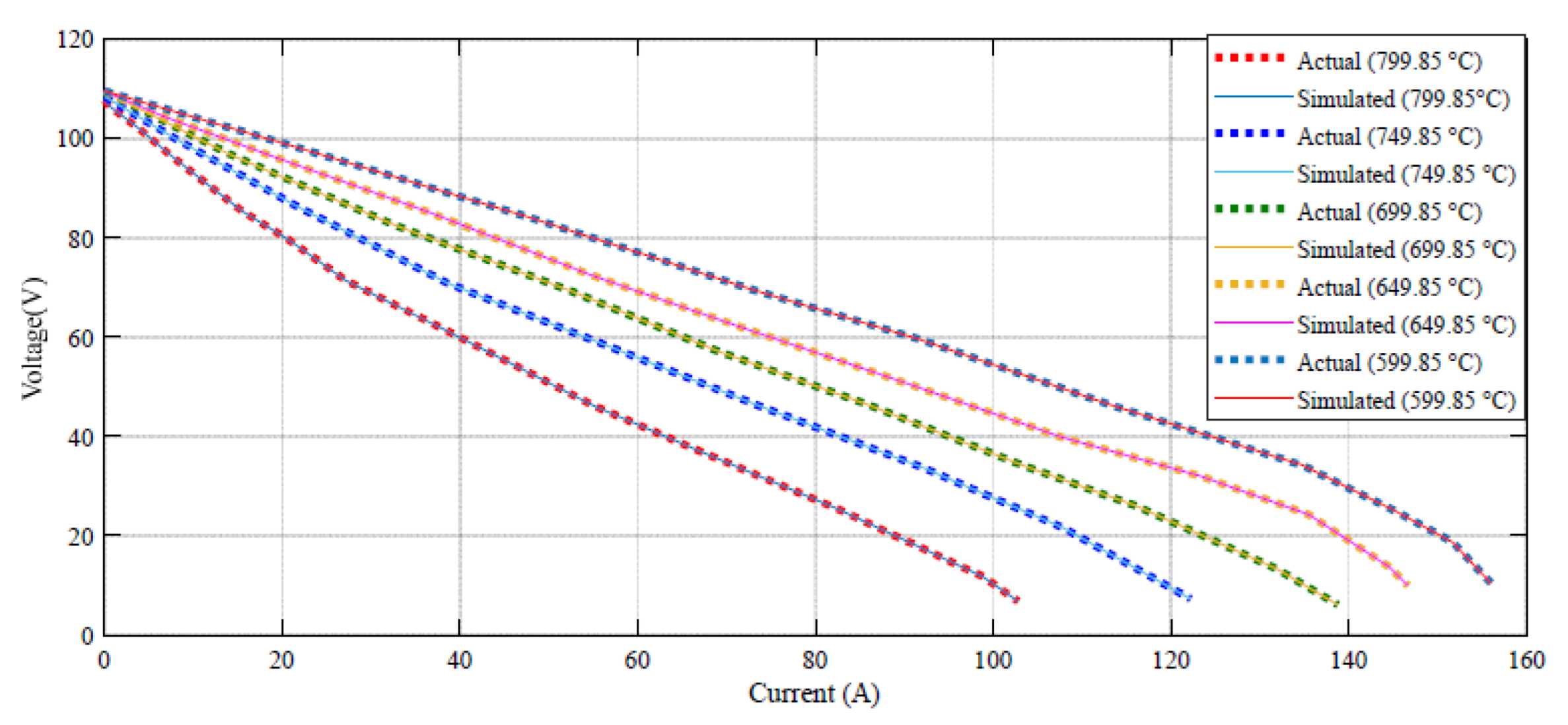
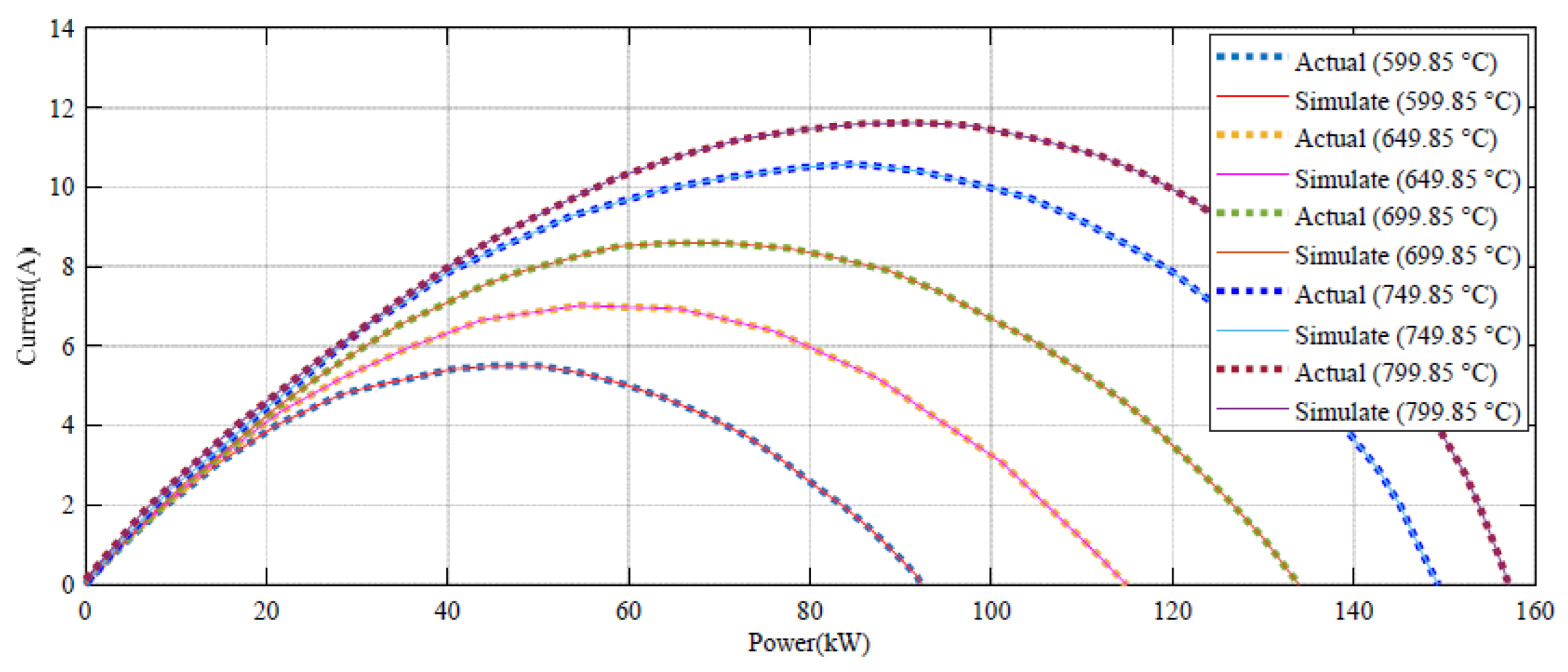
Figure 4 indicates how the suggested technique gives a promising confirmation with the empirical data under different temperatures.
Based on
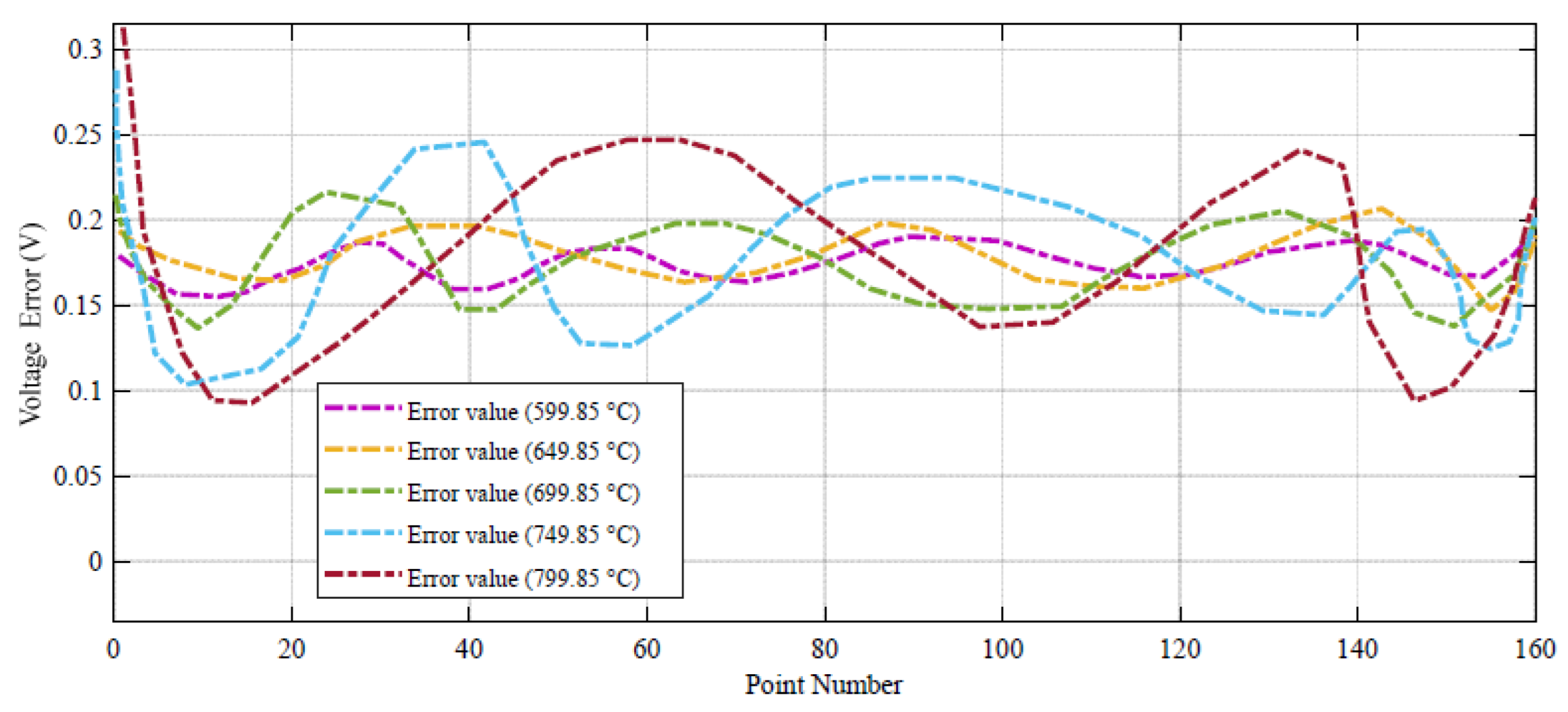
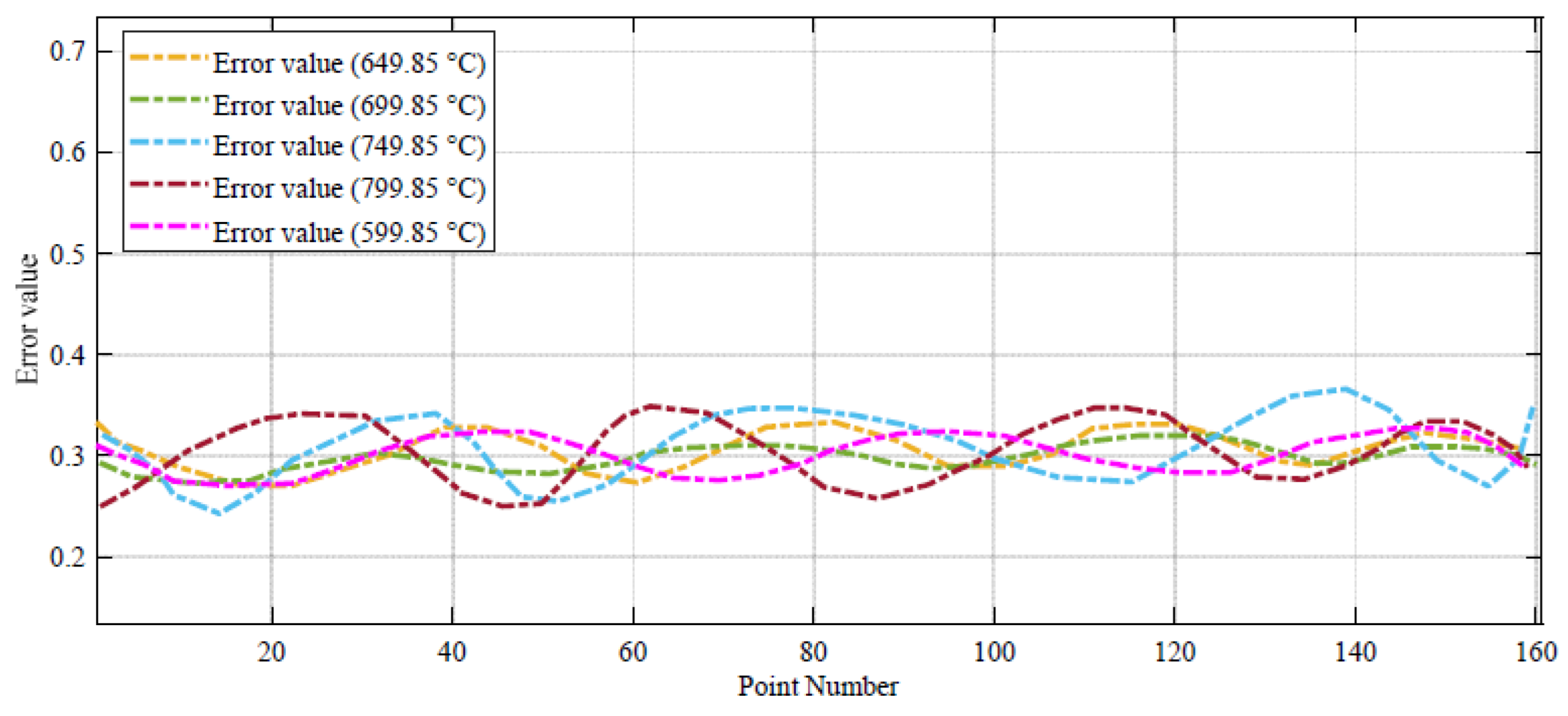
Figure 4, the error between the DBRA and experimental data is negligible in high values of voltage, such that it cannot be detected in the profile. The error profile of the voltage–current profile during 160 different datapoints are shown in
Figure 5.
As can be seen from
Figure 5, the voltage error value in 599.85 °C has the minimum range. For more clarification, the power–current profile of the system during 160 different datapoints are shown in
Figure 6.
Based on
Figure 6, the power–current error between the DBRA and empirical data is negligible in high values of power, such that it cannot be detected in the profile. The error profile of the power–current profile during 160 different datapoints is shown in
Figure 7.
It can be observed from
Figure 7 that the presented DBRA has a high validation with the experimental data based on power–current datapoints. In the second step, the system efficiency was analyzed based on pressure variations under constant temperature. Therefore, this calculation indicates how pressure variations affect the estimator. Like the previous analysis, 160 datapoints were also utilized for the assessment. Here, five various pressure values which were 1–5 atm under 750 °C constant temperature value were studied.
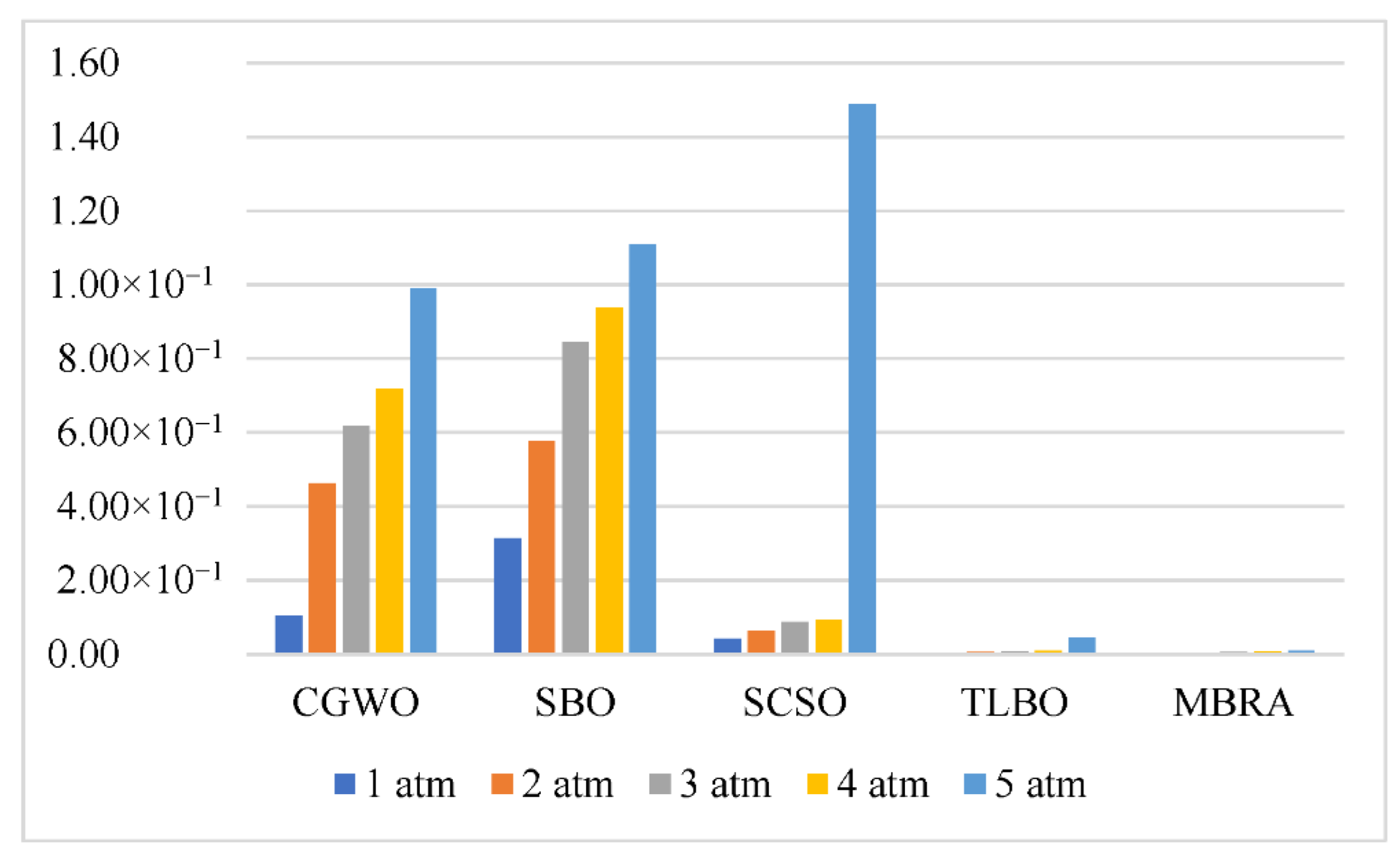
Table 7 reports the simulation achievements of the presented method under different pressures in comparison with some of the latest optimizers.
Based on
Table 8, the error value by the suggested DBRA was minimal in comparison with other newest optimizers, which shows the suggested technique’s higher efficiency toward the others. The error curve of the current–voltage of the system under different pressure during 160 different datapoints are shown in
Figure 8.
According to
Table 8, the lowest error amount was achieved for the proposed DBRA. However, TLBO also had a satisfying error value as the second rank. Furthermore, based on
Figure 8, the suggested technique with minimum standard deviation value provided the best confirmation by the experimental data.
Table 9 indicates the achieved optimum amount of the undetermined parameters.
It is observed from
Table 9, with pressure value increasing, the Nernst voltage value of the mode increased. This indicates the direct relation between pressure value and Nernst voltage value. However, there is no clear elation between the pressure value and the ohmic resistance. Furthermore,
Table 9 shows that
values for both training and testing data were extremely close to 1.00. As a result, we can infer that the proposed approach was flawlessly conducted and can precisely anticipate SOFC voltage with the exception of a few severe border situations. We can also prove the better efficiency of the proposed method from the accuracy (99.18%) results.
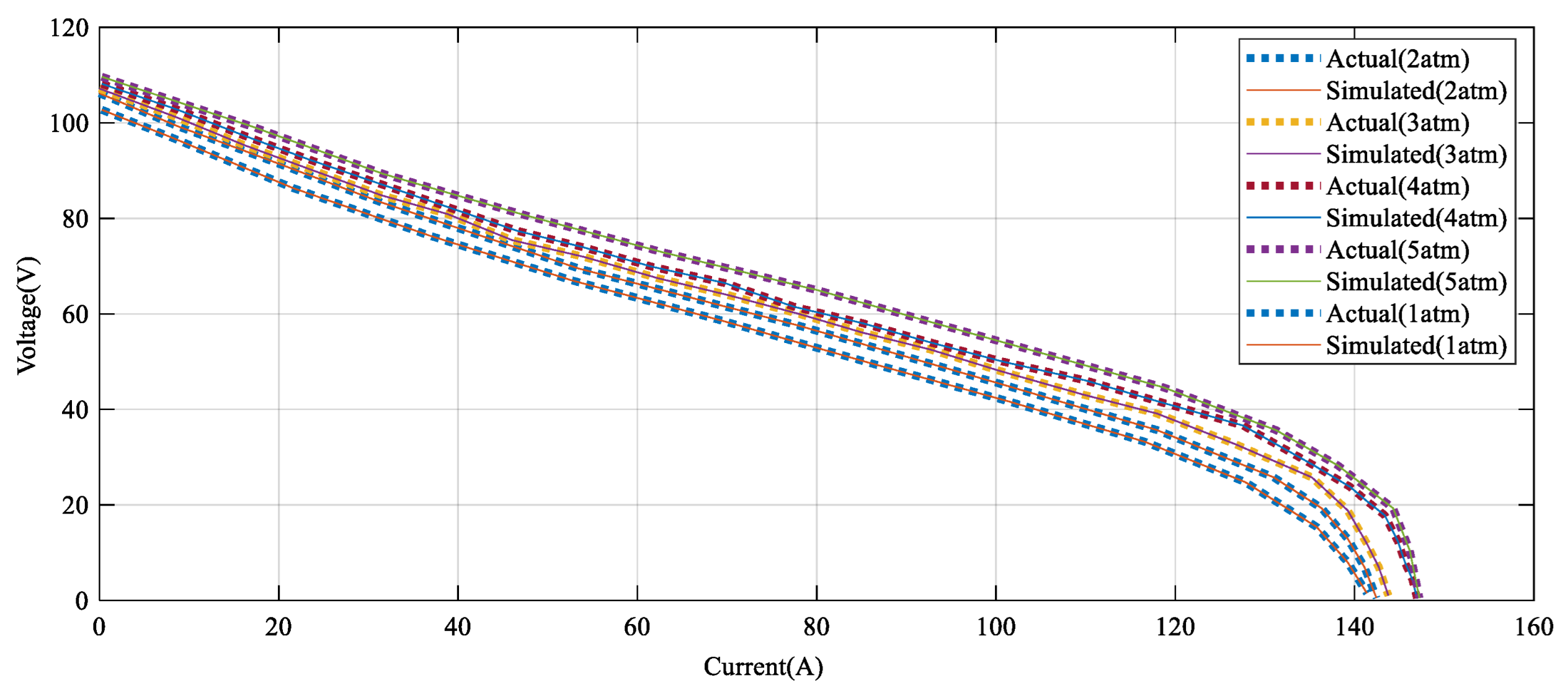
Figure 9 indicates how the suggested technique gives a promising confirmation with the empirical data under different pressure.
According to
Figure 9, the error amount between the DBRA and experimental data is negligible in high values of voltage, such that it cannot be detected in the profile. Finally,
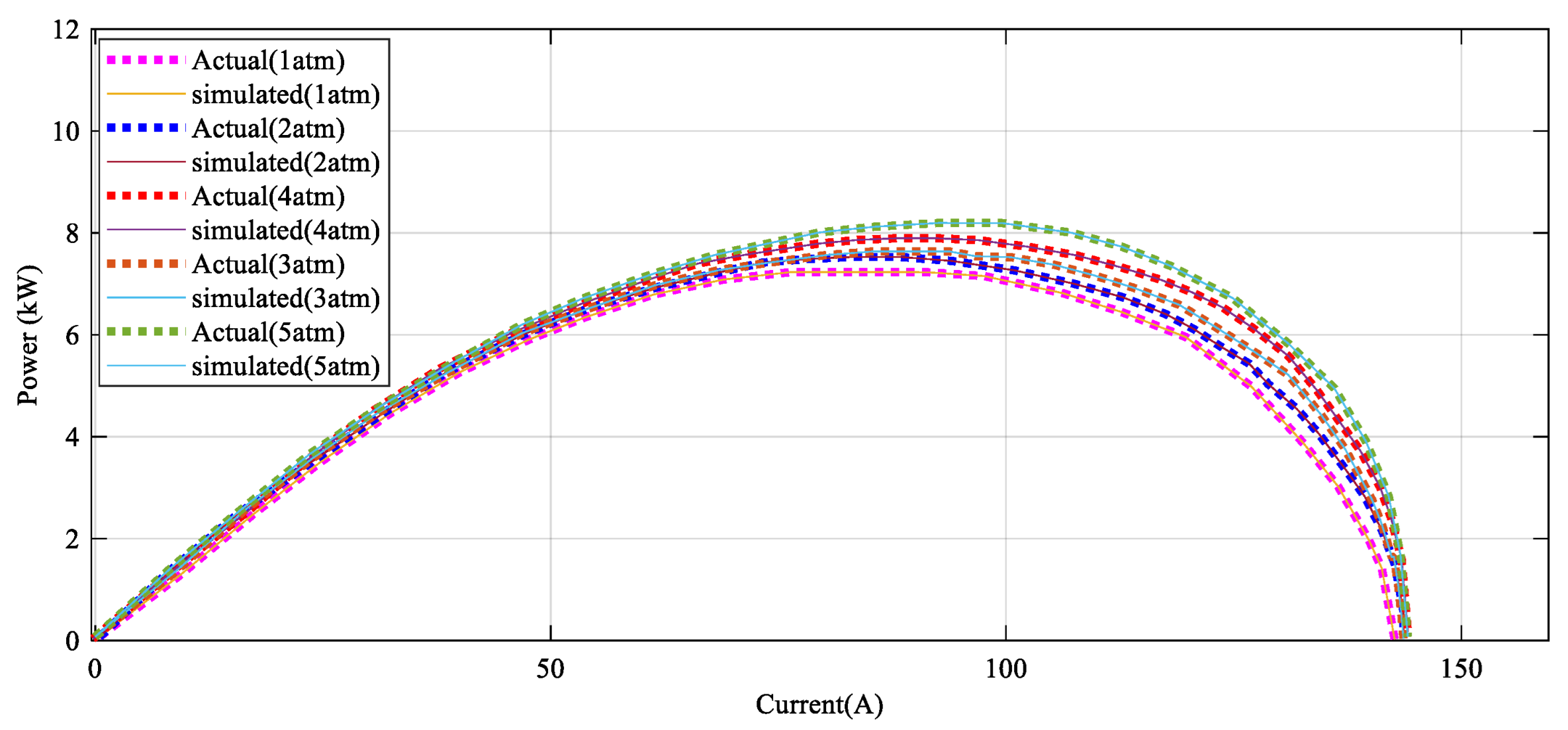
Figure 10 illustrates how the power–current profile provides a satisfying confirmation with the experimental data by various pressure.
As seen in
Figure 10, the power–current error between the DBRA and experimental data under rent pressure values is negligible in high values of power, such that it cannot be detected in the profile.