Application of Glass Waste on Red Ceramic to Improve Sintering
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
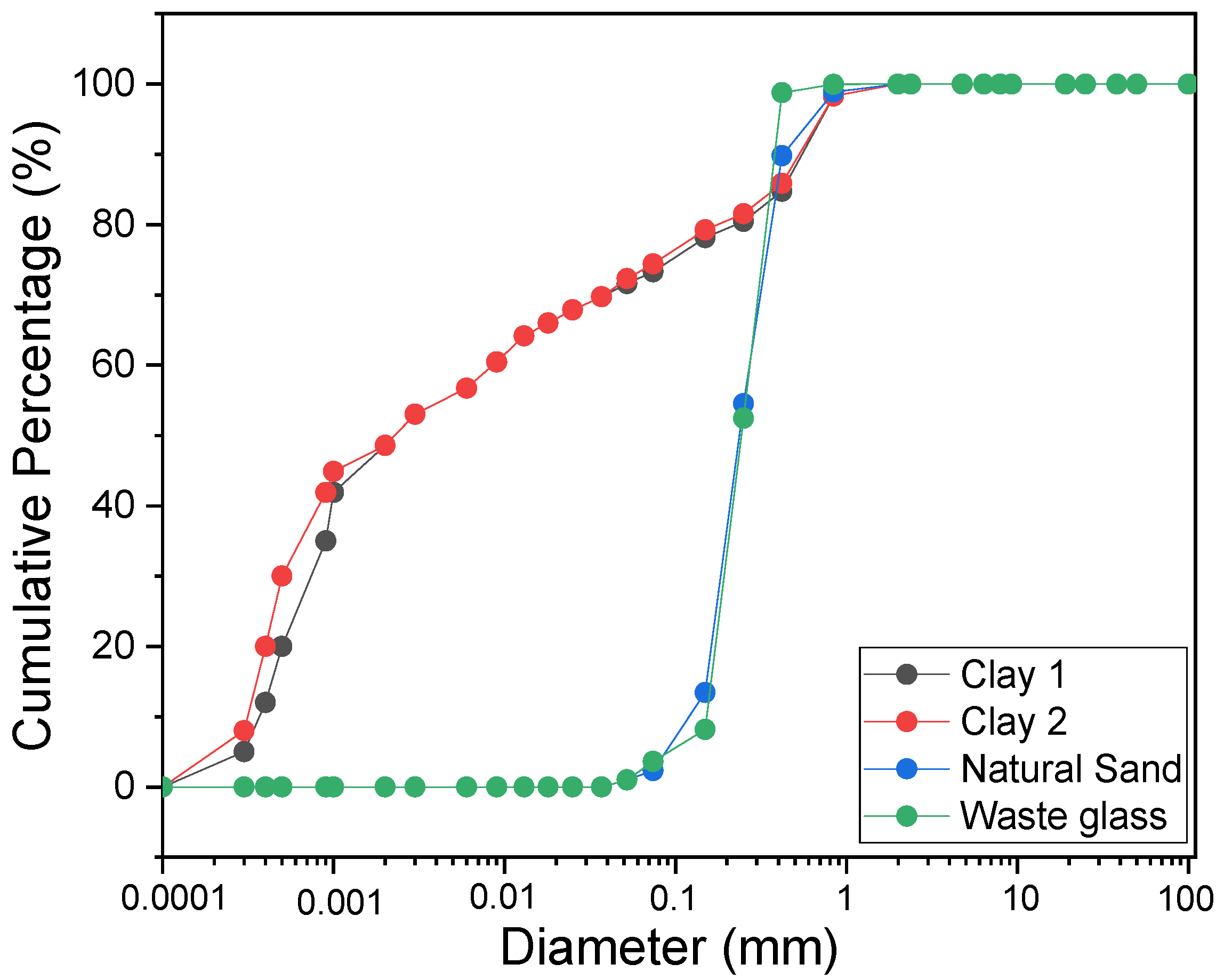
3.1. Characterization of Raw Materials
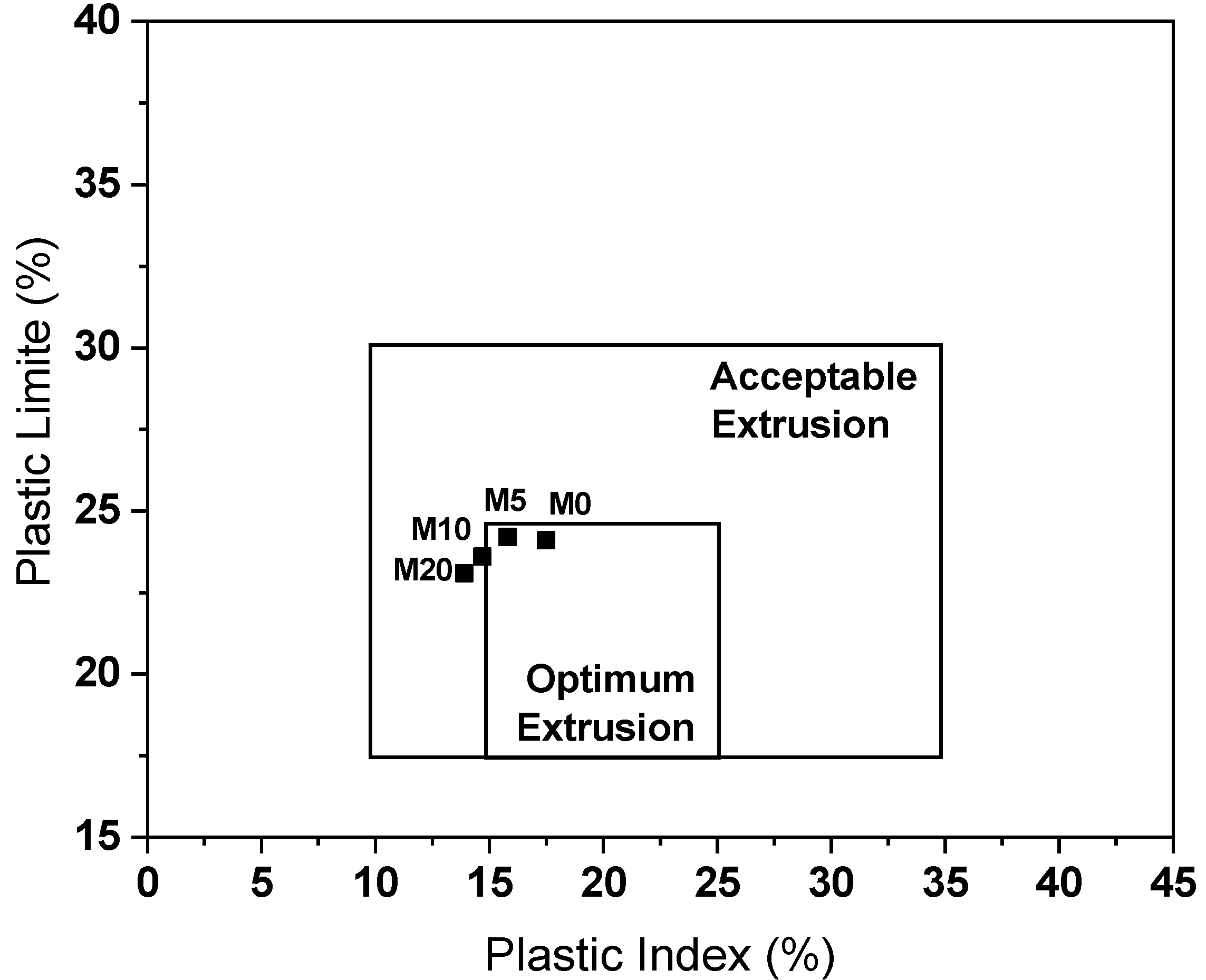
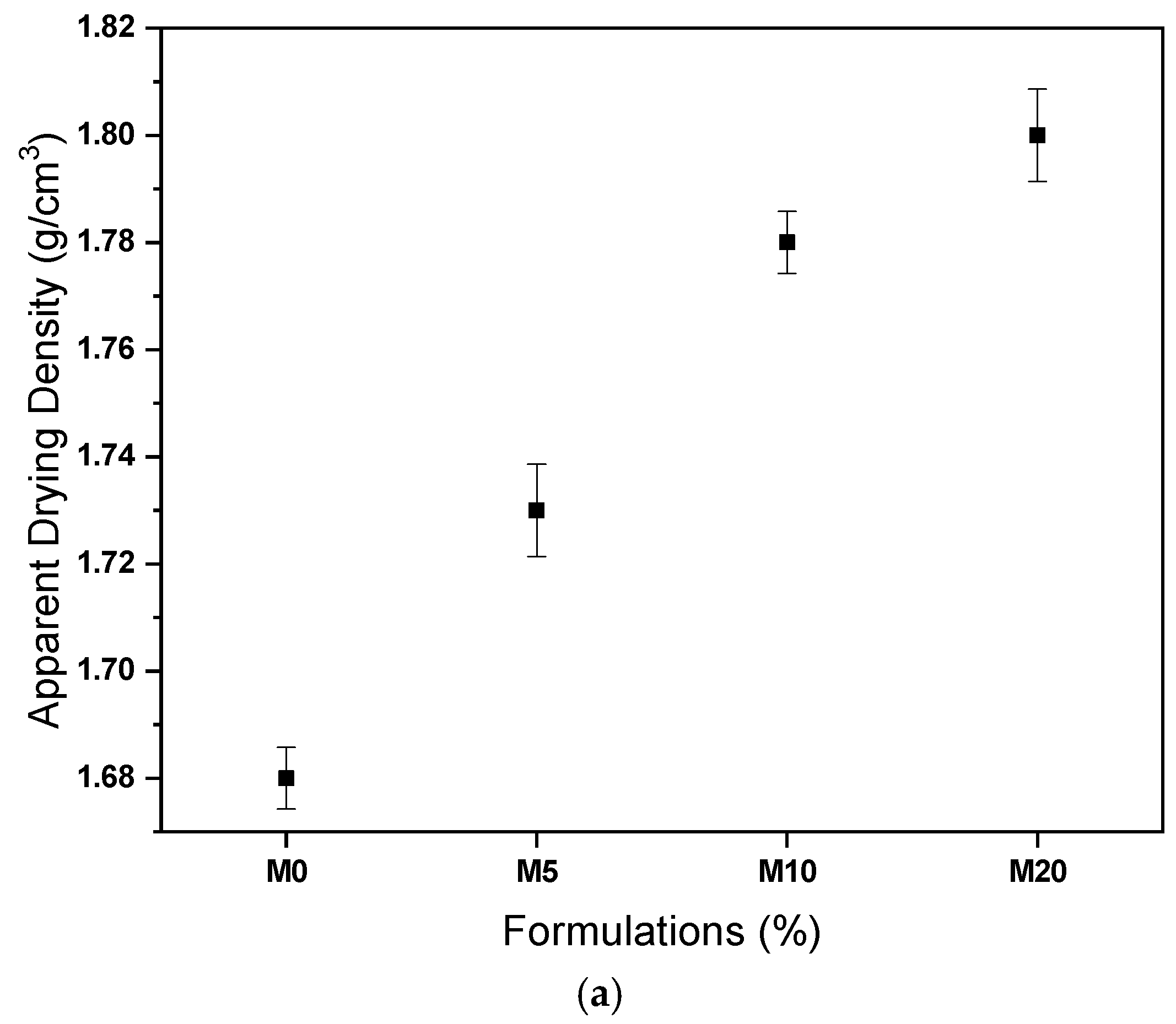
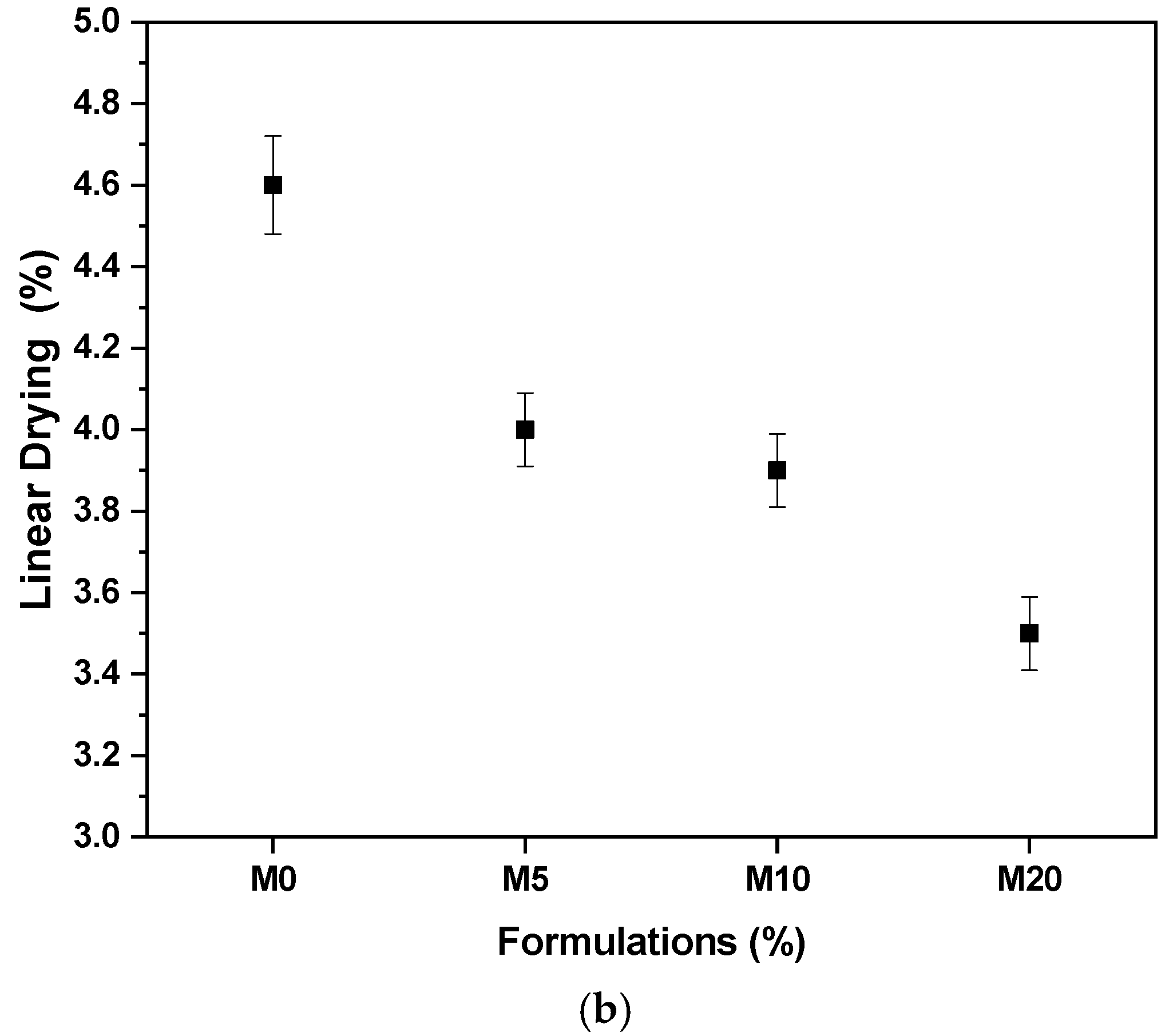
3.2. Characterization of Ceramic Masses before Firing
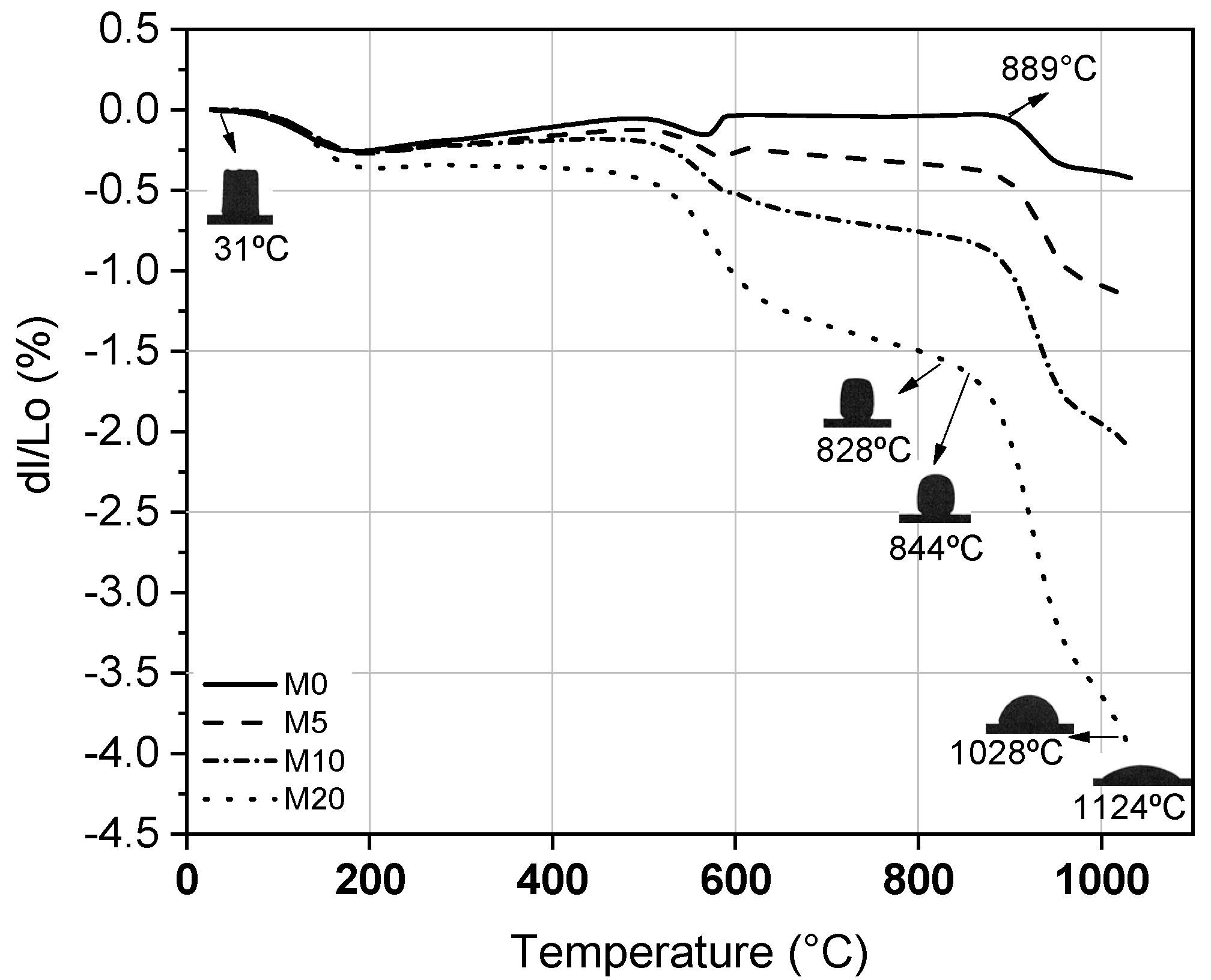
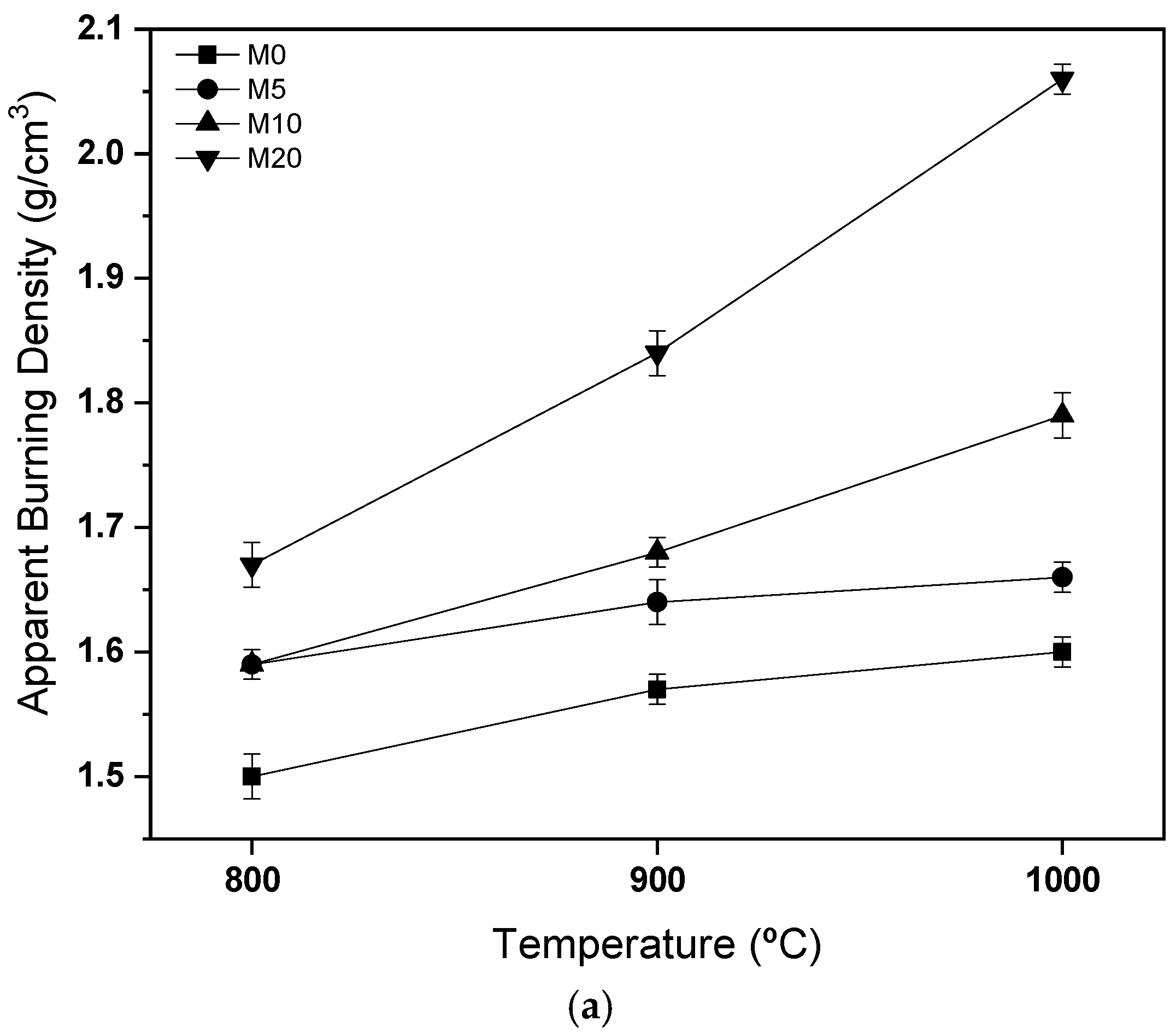
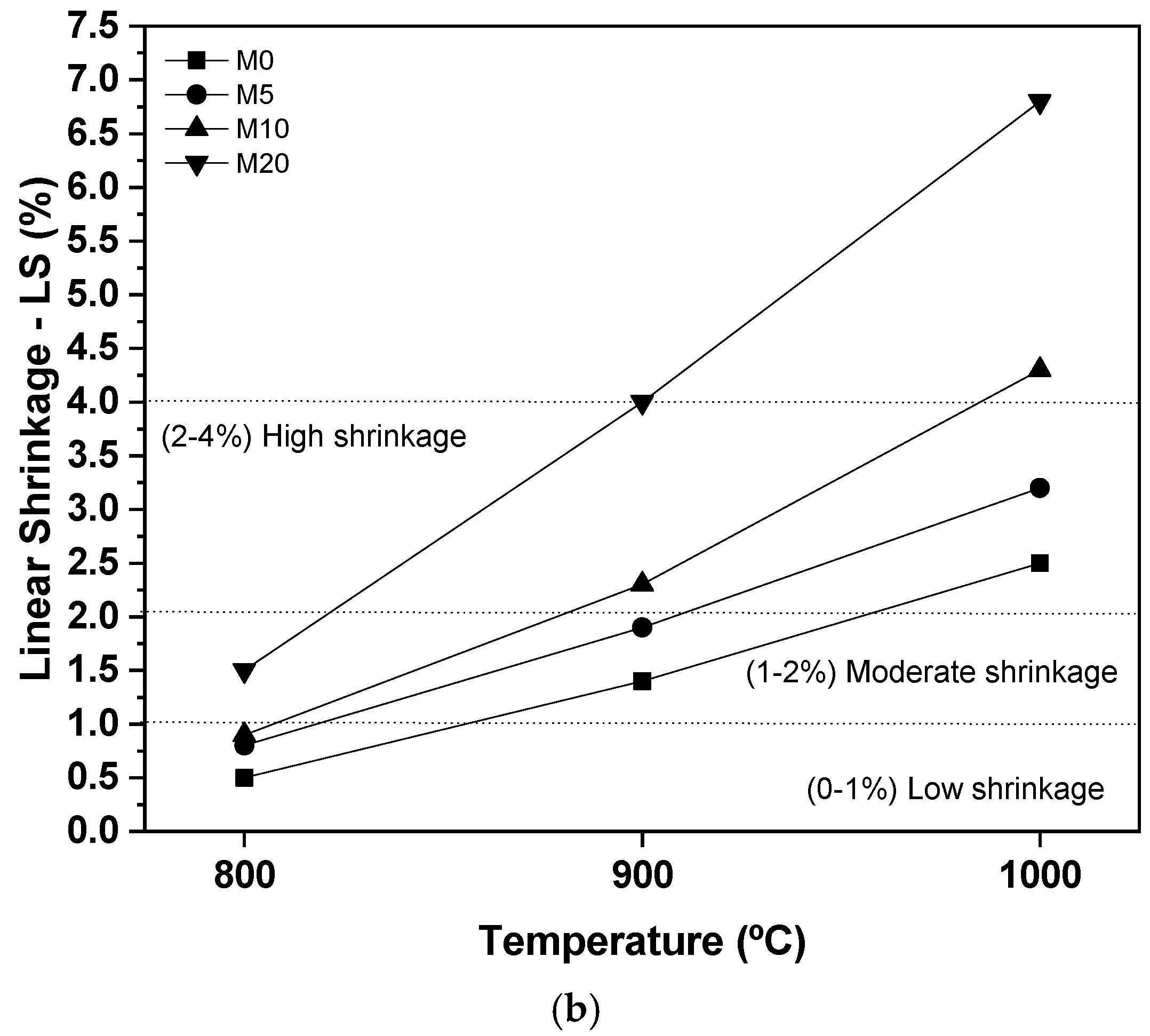
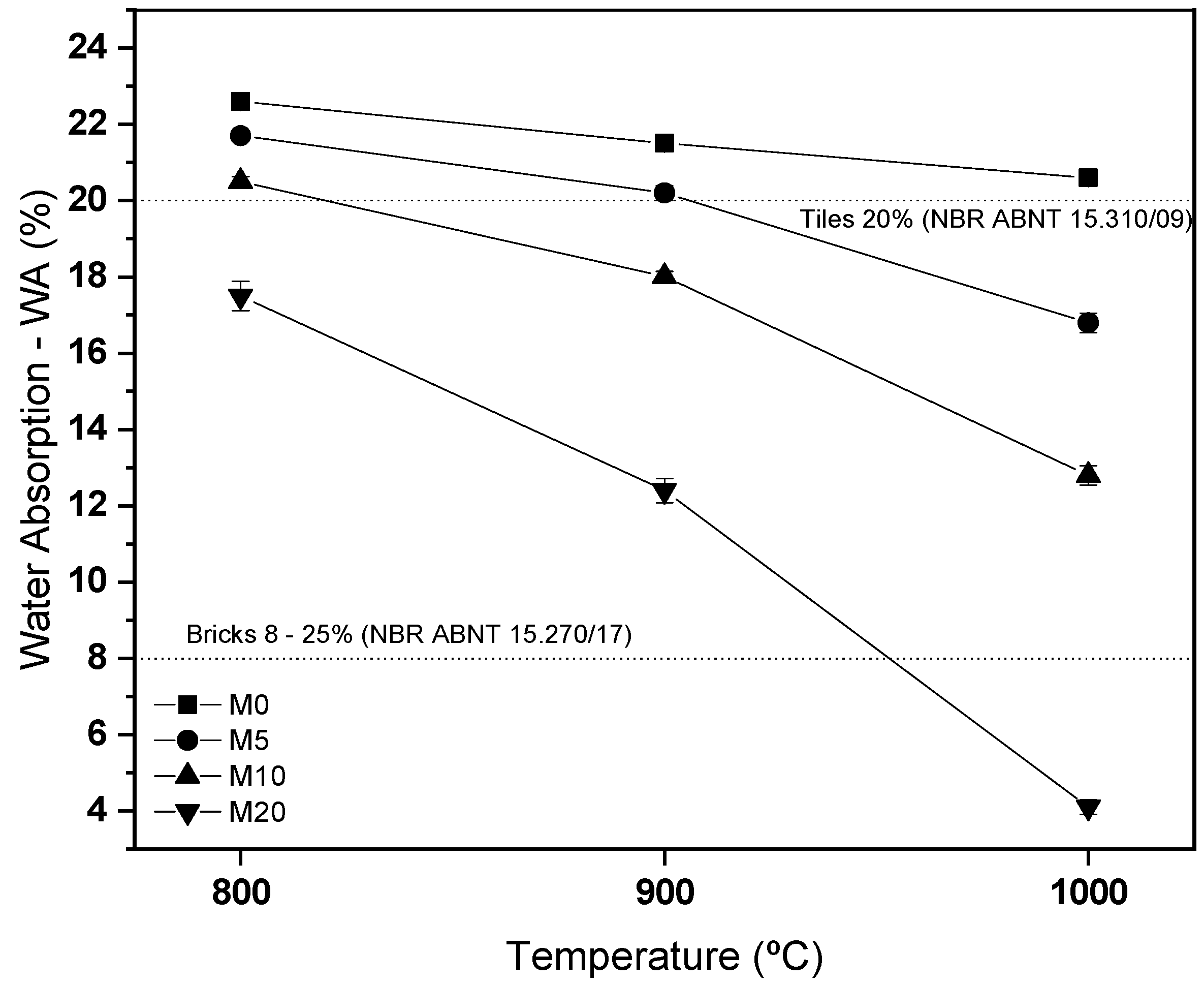
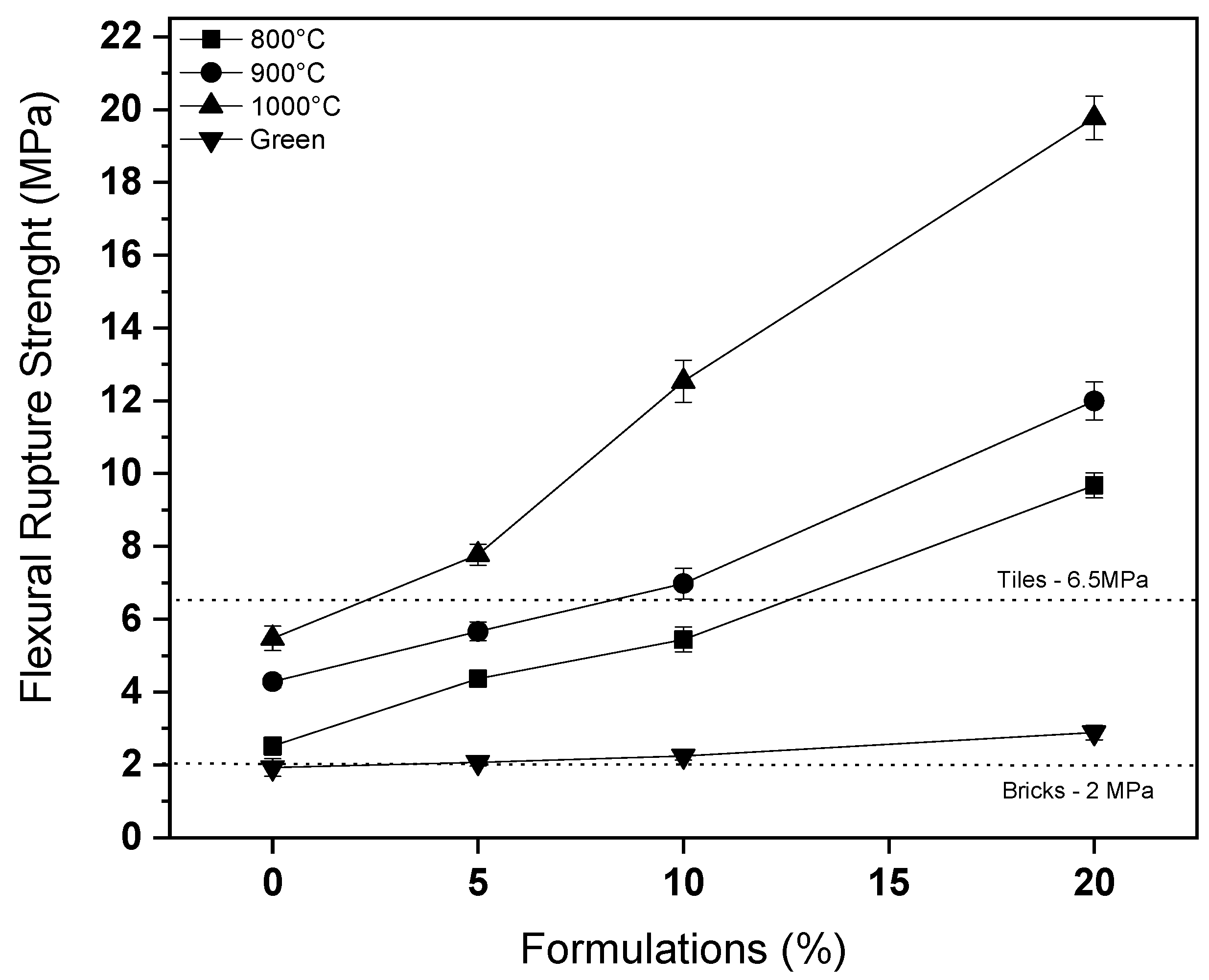
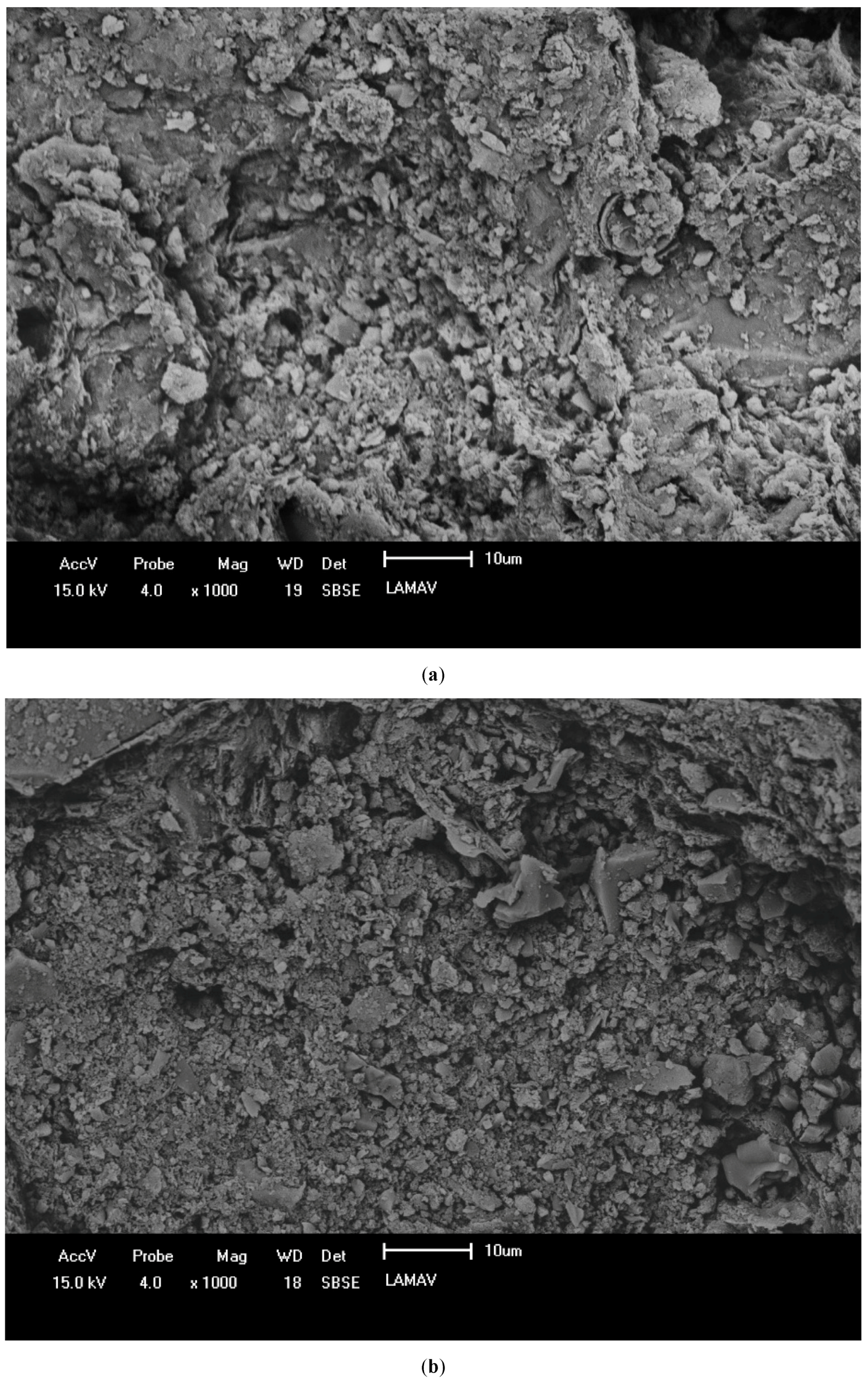
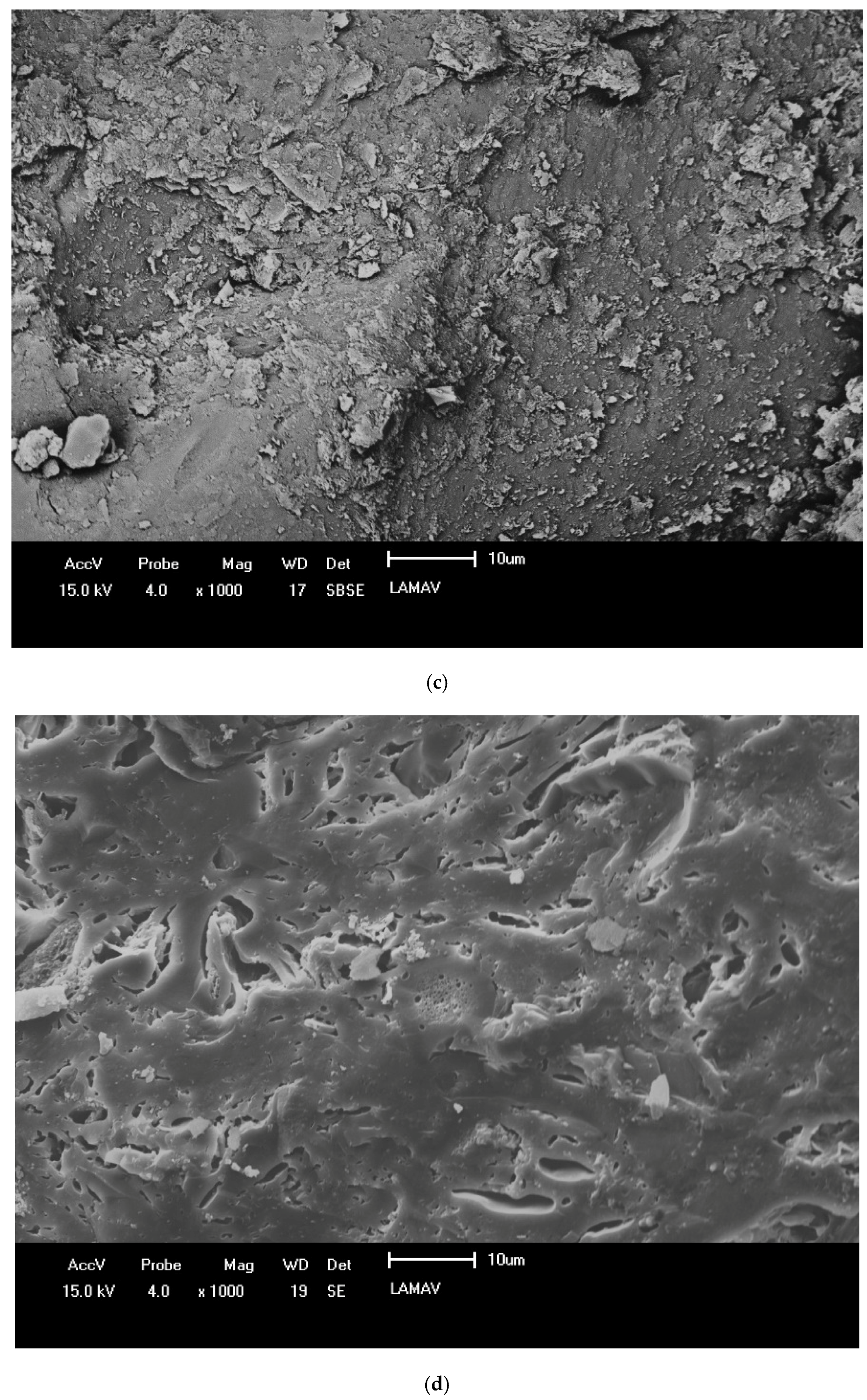
3.3. Characterization of Ceramic Masses after Firing
4. Conclusions
- The characterization of the glass waste shows the material’s compatibility with natural sand, enabling its use, in addition to indicating a potential for improving flexural rupture strength, due to the alkali levels, which increases the sintering of the ceramic.
- The masses containing glass waste did not present problems in the extrusion prognosis, in addition to improving the densification of the ceramic material after drying and after firing.
- After firing, the use of 20% glass waste promoted the sintering of the ceramic mass, due to the formation of a liquid phase, reducing porosity and improving the properties of water absorption and flexural strength. There were problems with burn retraction, but within tolerated limits.
- The masses containing 20% of glass waste obtained parameters compatible with industrial applications at a calcination temperature of 800 °C, while the composition containing only natural sand did not present the same behavior even at temperatures of 1000 °C. This indicates the feasibility of using glass waste.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parthan, S.R.; Milke, M.W.; Wilson, D.C.; Cocks, J.H. Cost Estimation for Solid Waste Management in Industrialising Regions—Precedents, Problems and Prospects. Waste Manag. 2012, 32, 584–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrayyan, B.; Hamdi, M.R. Management Approaches to Integrated Solid Waste in Industrialized Zones in Jordan: A Case of Zarqa City. Waste Manag. 2006, 26, 195–205. [Google Scholar] [CrossRef]
- Hu, D.; Wang, R.; Yan, J.; Xu, C.; Wang, Y. A Pilot Ecological Engineering Project for Municipal Solid Waste Reduction, Disinfection, Regeneration and Industrialization in Guanghan City, China. Ecol. Eng. 1998, 11, 129–138. [Google Scholar] [CrossRef]
- Dondi, M.; Raimondo, M.; Zanelli, C. Clays and Bodies for Ceramic Tiles: Reappraisal and Technological Classification. Appl. Clay Sci. 2014, 96, 91–109. [Google Scholar] [CrossRef]
- Hossain, S.; Roy, K. Cerâmica sustentável derivada de resíduos sólidos: Uma revisão. J. Asian Ceram. Soc. 2020, 8, 984–1009. [Google Scholar] [CrossRef]
- Mota, L.; Toledo, R.; Machado, F.; Holanda, J.N.F.; Vargas, H.; Fariajr, R. Thermal characterisation of red clay from the Northern Region of Rio de Janeiro State, Brazil using an open photoacoustic cell, in relation to structural changes on firing. Appl. Clay Sci. 2008, 42, 168–174. [Google Scholar] [CrossRef]
- Moreira, J.; Manhães, J.P.; Holanda, J.N. Processing of red ceramic using ornamental rock powder waste. J. Mater. Process. Technol. 2008, 196, 88–93. [Google Scholar] [CrossRef]
- Amaral, L.F.; Carvalho, J.P.R.G.d.; da Silva, B.M.; Delaqua, G.C.G.; Monteiro, S.N.; Vieira, C.M.F. Development of Ceramic Paver with Ornamental Rock Waste. J. Mater. Res. Technol. 2019, 8, 599–608. [Google Scholar] [CrossRef]
- Girondi, G.D.; Marvila, M.M.; de Azevedo, A.R.G.; de Souza, C.C.; Souza, D.; de Brito, J.; Vieira, C.M.F. Recycling Potential of Powdered Cigarette Waste in the Development of Ceramic Materials. J. Mater. Cycles Waste Manag. 2020, 22, 1672–1681. [Google Scholar] [CrossRef]
- Girondi Delaqua, G.C.; Marvila, M.T.; Souza, D.; Sanchez Rodriguez, R.J.; Colorado, H.A.; Fontes Vieira, C.M. Evaluation of the Application of Macrophyte Biomass Salvinia Auriculata Aublet in Red Ceramics. J. Environ. Manag. 2020, 275, 111253. [Google Scholar] [CrossRef]
- Vieira, C.M.F.; Morais, A.S.C.; Monteiro, S.N.; Delaqua, G.C.G. Teste Industrial de Cerâmica Vermelha Incorporada Com Resíduo de Vidro de Lâmpada Fluorescente. Cerâmica 2016, 62, 376–385. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, B.; Zhang, S. A Review of Glass Ceramic Foams Prepared from Solid Wastes: Processing, Heavy-Metal Solidification and Volatilization, Applications. Sci. Total Environ. 2021, 781, 146727. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, Y.; Fang, J. Effect of MgO Addition on Crystallization, Microstructure and Properties of Glass-Ceramics Prepared from Solid Wastes. J. Alloy. Compd. 2021, 881, 159821. [Google Scholar] [CrossRef]
- Silva, R.V.; de Brito, J.; Lye, C.Q.; Dhir, R.K. The Role of Glass Waste in the Production of Ceramic-Based Products and Other Applications: A Review. J. Clean. Prod. 2017, 167, 346–364. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.; Ma, S.; Liu, C.; Wang, X. Ceramic Tiles Derived from Coal Fly Ash: Preparation and Mechanical Characterization. Ceram. Int. 2017, 43, 11953–11966. [Google Scholar] [CrossRef]
- Menezes, R.R.; Neto, H.G.M.; Santana, L.N.L.; Lira, H.L.; Ferreira, H.S.; Neves, G.A. Optimization of Wastes Content in Ceramic Tiles Using Statistical Design of Mixture Experiments. J. Eur. Ceram. Soc. 2008, 28, 3027–3039. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, J.; Wu, Y.; Li, X.; Chu, P.K.; Qi, T. Substitution of Quartz and Clay with Fly Ash in the Production of Architectural Ceramics: A Mechanistic Study. Ceram. Int. 2021, 47, 12514–12525. [Google Scholar] [CrossRef]
- Sasui, S.; Kim, G.; Nam, J.; van Riessen, A.; Hadzima-Nyarko, M. Effects of Waste Glass as a Sand Replacement on the Strength and Durability of Fly Ash/GGBS Based Alkali Activated Mortar. Ceram. Int. 2021, 47, 21175–21196. [Google Scholar] [CrossRef]
- Yang, H.; Chen, C.; Pan, L.; Lu, H.; Sun, H.; Hu, X. Preparation of Double-Layer Glass-Ceramic/Ceramic Tile from Bauxite Tailings and Red Mud. J. Eur. Ceram. Soc. 2009, 29, 1887–1894. [Google Scholar] [CrossRef]
- NBR 7181; Solo-Análise Granulométrica. Associação Brasileira de Normas Técnicas-ABNT: São Paulo, Brasil, 2016.
- de Strieder, M.C.; Ratusznei, F.; Pereira, M.; Montedo, O.R.K. Laser-Assisted Glass-Based Sealing of Polished Porcelain Stoneware Tile Surface to Increase Stain Resistance. J. Eur. Ceram. Soc. 2020, 40, 3478–3488. [Google Scholar] [CrossRef]
- Girondi Delaqua, G.C.; Ferreira, M.d.N.; Amaral, L.F.; Sánchez Rodríguez, R.J.; Atem de Carvalho, E.; Fontes Vieira, C.M. Incorporation of Sludge from Effluent Treatment Plant of an Industrial Laundry into Heavy Clay Ceramics. J. Build. Eng. 2022, 47, 103451. [Google Scholar] [CrossRef]
- NBR 6459:2016; Solo—Determinação Do Limite de Liquidez. Associação Brasileira de Normas Técnicas-ABNT: São Paulo, Brasil, 2016.
- NBR 7180:2016; Solo—Determinação Do Limite de Plasticidade. Associação Brasileira de Normas Técnicas-ABNT: São Paulo, Brasil, 2016.
- NBR 15310; Componentes Cerâmicos—Telhas—Terminologia, Requisitos e Métodos de Ensaio. ABNT: São Paulo, Brasil, 2005.
- NBR 15270-1; Componentes Cerâmicos—Blocos e Tijolos Para Alvenaria Parte 1: Requisitos. ABNT: São Paulo, Brasil, 2017.
- Gencel, O.; Sutcu, M.; Erdogmus, E.; Koc, V.; Cay, V.V.; Gok, M.S. Properties of Bricks with Waste Ferrochromium Slag and Zeolite. J. Clean. Prod. 2013, 59, 111–119. [Google Scholar] [CrossRef]
- Gülen, Ş.M.; Çöpoğlu, N.; Yılmaz, Y.B.; Karaahmet, O.; Cengiz, T.; Gökdemir, H.; Çiçek, B. TiO2-Based Glass-Ceramic Coatings: An Innovative Approach to Architectural Panel Applications. Case Stud. Constr. Mater. 2022, 16, e00805. [Google Scholar] [CrossRef]
- Özcan, M.; Birol, B.; Kaya, F. Investigation of Photocatalytic Properties of TiO2 Nanoparticle Coating on Fly Ash and Red Mud Based Porous Ceramic Substrate. Ceram. Int. 2021, 47, 24270–24280. [Google Scholar] [CrossRef]
- Conconi, M.S.; Morosi, M.; Maggi, J.; Zalba, P.E.; Cravero, F.; Rendtorff, N.M. Thermal Behavior (TG-DTA-TMA), Sintering and Properties of a Kaolinitic Clay from Buenos Aires Province, Argentina. Cerâmica 2019, 65, 227–235. [Google Scholar] [CrossRef]
- Pardo, F.; Jordan, M.M.; Montero, M.A. Ceramic behaviour of clays in Central Chile. Appl. Clay Sci. 2018, 157, 158–164. [Google Scholar] [CrossRef]
- Dondi, M.; Guarini, G.; Raimondo, M.; Zanelli, C. Recycling PC and TV Waste Glass in Clay Bricks and Roof Tiles. Waste Manag. 2009, 29, 1945–1951. [Google Scholar] [CrossRef] [Green Version]
- Karamanov, A.; Dzhantov, B.; Paganelli, M.; Sighinolfi, D. Glass Transition Temperature and Activation Energy of Sintering by Optical Dilatometry. Thermochim. Acta 2013, 553, 1–7. [Google Scholar] [CrossRef]
- Khoeini, M.; Hesaraki, S.; Kolahi, A. Effect of BaO Substitution for CaO on the Structural and Thermal Properties of SiO2–B2O3–Al2O3–CaO–Na2O–P2O5 Bioactive Glass System Used for Implant Coating Applications. Ceram. Int. 2021, 47, 31666–31680. [Google Scholar] [CrossRef]
- Fontes Vieira, C.M.; Monteiro, S.N. Firing Behavior of the Clay Fraction of a Natural Kaolinitic Clay: Are They Different? Mater. Res. 2019. [Google Scholar] [CrossRef]
- Assías, S.G.; Pabón, F.; Cala, N.; Delvasto, P. Incorporation of Fluorescent Lamp Waste into Red-Clay Bricks: Defect Formation, Physical and Mechanical. Properties. Waste Biomass Valorization 2021, 12, 1621–1632. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Martínez-Martínez, S.; Pérez-Villarejo, L.; Iglesias-Godino, F.J.; Martínez-García, C.; Corpas-Iglesias, F.A. Valorization of Biodiesel Production Residues in Making Porous Clay Brick. Fuel Proces. Technol. 2012, 103, 166–173. [Google Scholar] [CrossRef]
- Oummadi, S.; Nait-Ali, B.; Alzina, A.; Paya, M.-C.; Gaillard, J.-M.; Smith, D.S. Optical Method for Evaluation of Shrinkage in Two Dimensions during Drying of Ceramic Green Bodies. Open Ceram. 2020, 2, 100016. [Google Scholar] [CrossRef]
- Kagonbé, B.P.; Tsozué, D.; Nzeukou, A.N.; Ngos, S. Mineralogical, physico-chemical and ceramic properties of clay materials from Sekandé and Gashiga (North, Cameroon) and their suitability in earthenware production. Heliyon 2021, 7, e07608. [Google Scholar] [CrossRef]
- Izzo, F.; Guarino, V.; Ciotola, A.; Verde, M.; de Bonis, A.; Capaldi, C.; Morra, V. An Archaeometric Investigation in a Consumption Context: Exotic, Imitation and Traditional Ceramic Productions from the Forum of Cumae (Southern Italy). J. Archaeol. Sci. Rep. 2021, 35, 102768. [Google Scholar] [CrossRef]

| Formulation | Clay 1 (%) | Clay 2 (%) | Natural Sand (%) | Glass Waste (%) |
|---|---|---|---|---|
| 0% | 60 | 20 | 20 | 0 |
| 5% | 60 | 20 | 15 | 5 |
| 10% | 60 | 20 | 10 | 10 |
| 20% | 60 | 20 | 0 | 20 |
| Composition | Clay 1 (wt%) | Clay 2 (wt%) | Natural Sand (wt%) | Glass Waste (wt%) |
|---|---|---|---|---|
| SiO2 | 47.04 | 49.34 | 81.10 | 72.58 |
| Al2O3 | 32.56 | 30.71 | 11.90 | 1.82 |
| Fe2O3 | 3.48 | 3.66 | 1.20 | 0.55 |
| TiO2 | 1.29 | 1.21 | 0.47 | 0.01 |
| K2O | 1.01 | 0.99 | 1.50 | 0.36 |
| MgO | 0.55 | 0.61 | 0.62 | 2.53 |
| Na2O | 0.34 | 0.24 | 0.84 | 6.86 |
| CaO | 0.24 | 0.22 | 0.51 | 15.54 |
| Others | 0.10 | 0.11 | 0.16 | 0.02 |
| L.O.I. | 13.39 | 12.91 | 1.70 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delaqua, G.; Magalhães, J.; Marvila, M.; Vernilli, F., Jr.; Monteiro, S.; Colorado, H.; Vieira, C. Application of Glass Waste on Red Ceramic to Improve Sintering. Sustainability 2022, 14, 10454. https://doi.org/10.3390/su141610454
Delaqua G, Magalhães J, Marvila M, Vernilli F Jr., Monteiro S, Colorado H, Vieira C. Application of Glass Waste on Red Ceramic to Improve Sintering. Sustainability. 2022; 14(16):10454. https://doi.org/10.3390/su141610454
Chicago/Turabian StyleDelaqua, Geovana, Juan Magalhães, Markssuel Marvila, Fernando Vernilli, Jr., Sérgio Monteiro, Henry Colorado, and Carlos Vieira. 2022. "Application of Glass Waste on Red Ceramic to Improve Sintering" Sustainability 14, no. 16: 10454. https://doi.org/10.3390/su141610454
APA StyleDelaqua, G., Magalhães, J., Marvila, M., Vernilli, F., Jr., Monteiro, S., Colorado, H., & Vieira, C. (2022). Application of Glass Waste on Red Ceramic to Improve Sintering. Sustainability, 14(16), 10454. https://doi.org/10.3390/su141610454








