Analysis of the Population Structure and Dynamic of Endemic Salvia ceratophylloides Ard. (Lamiaceae)
Abstract
1. Introduction
2. Materials and Methods
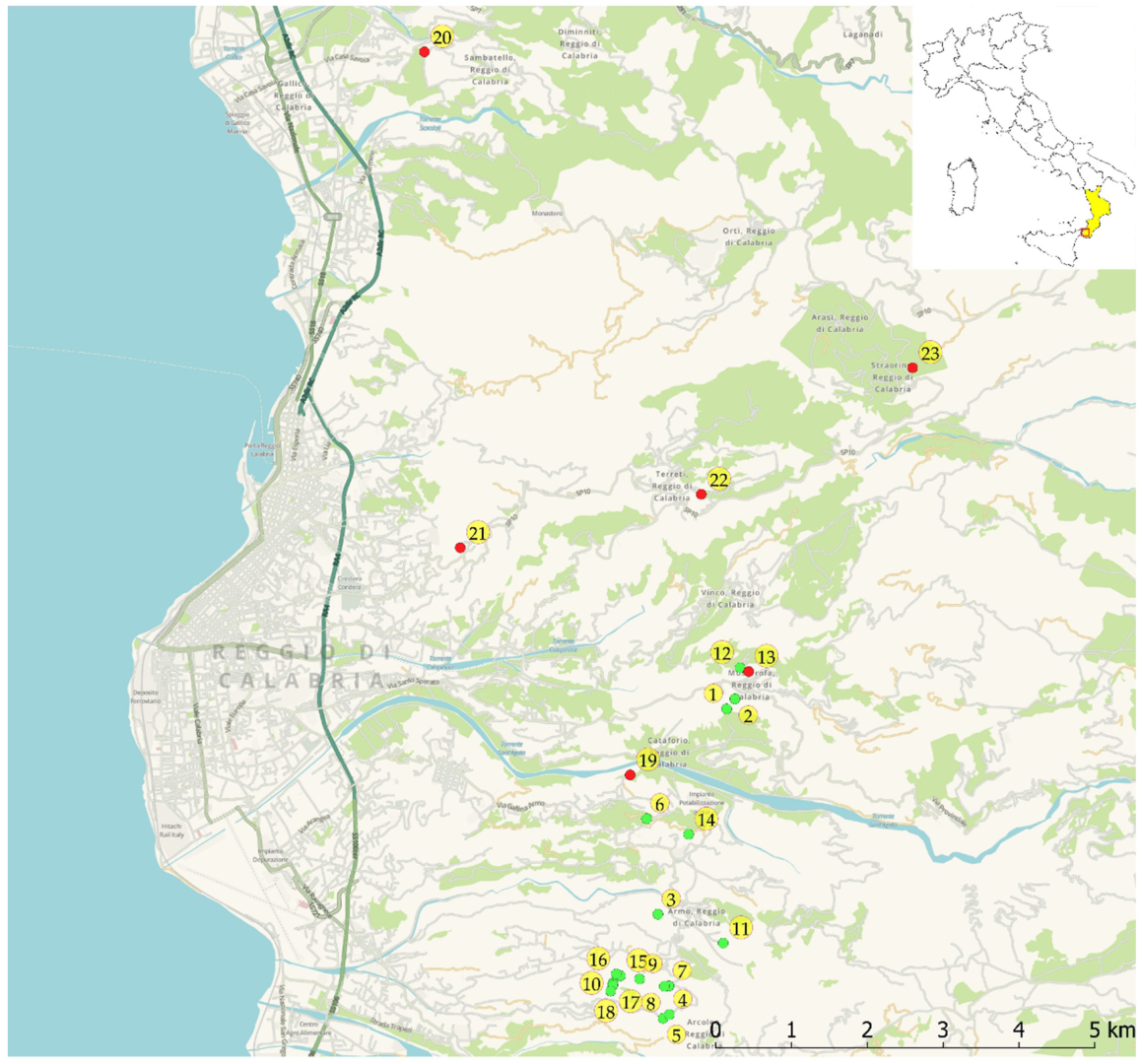
2.1. Study Area
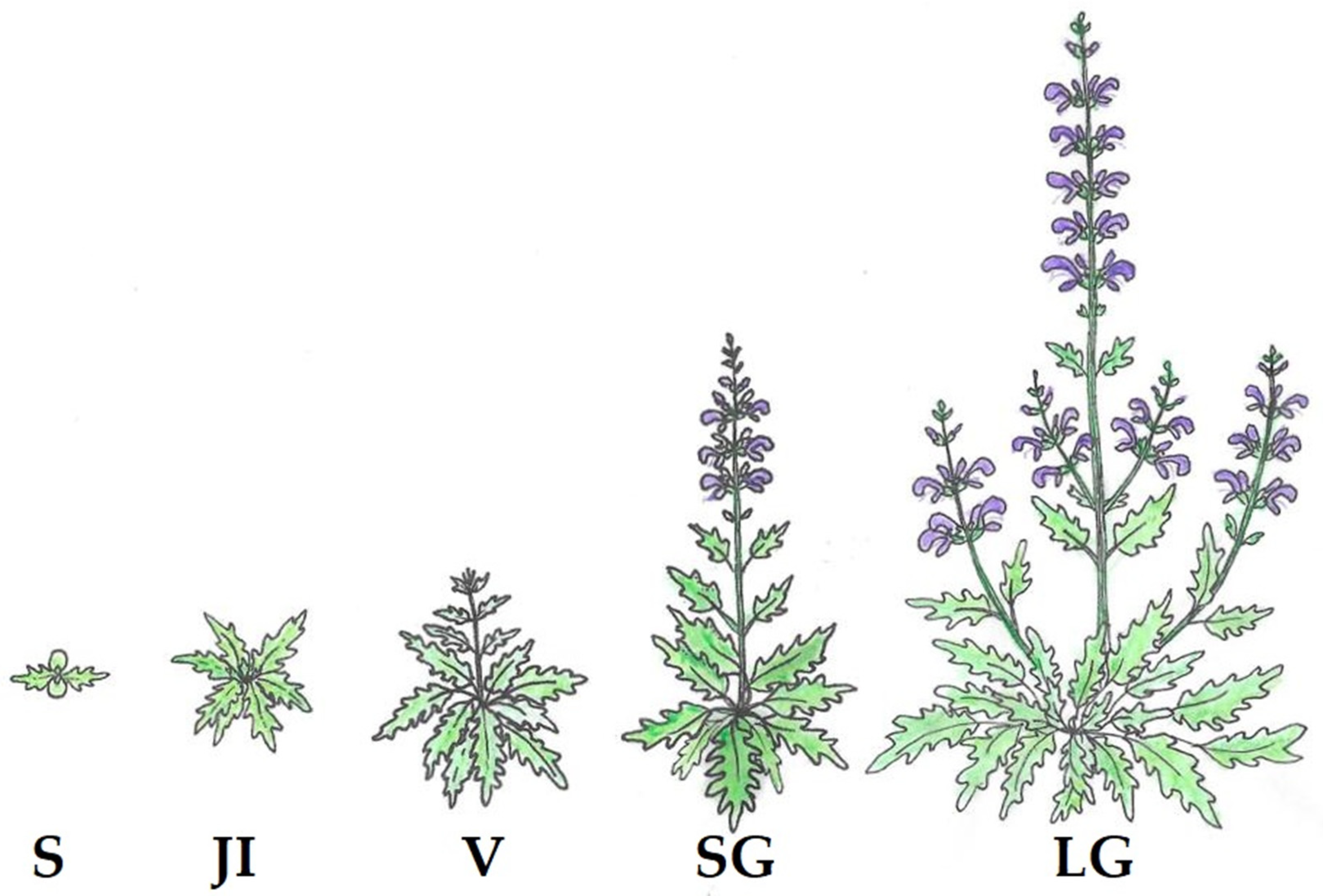
2.2. Population Analysis
- is the number of individuals at time zero (No. of individuals in 2019);
- is the number of individuals after a certain time interval (No. Individuals in 2021);
- is the Euler number;
- is the intrinsic population growth rate for each individual and for each unit of time considered (the difference between births and deaths in the unit of time);
- is the time from to the year considered.
2.3. Habitat Characterisation and Georeferencing
2.4. Pressures and Threats
2.5. Statistical Analysis
3. Results
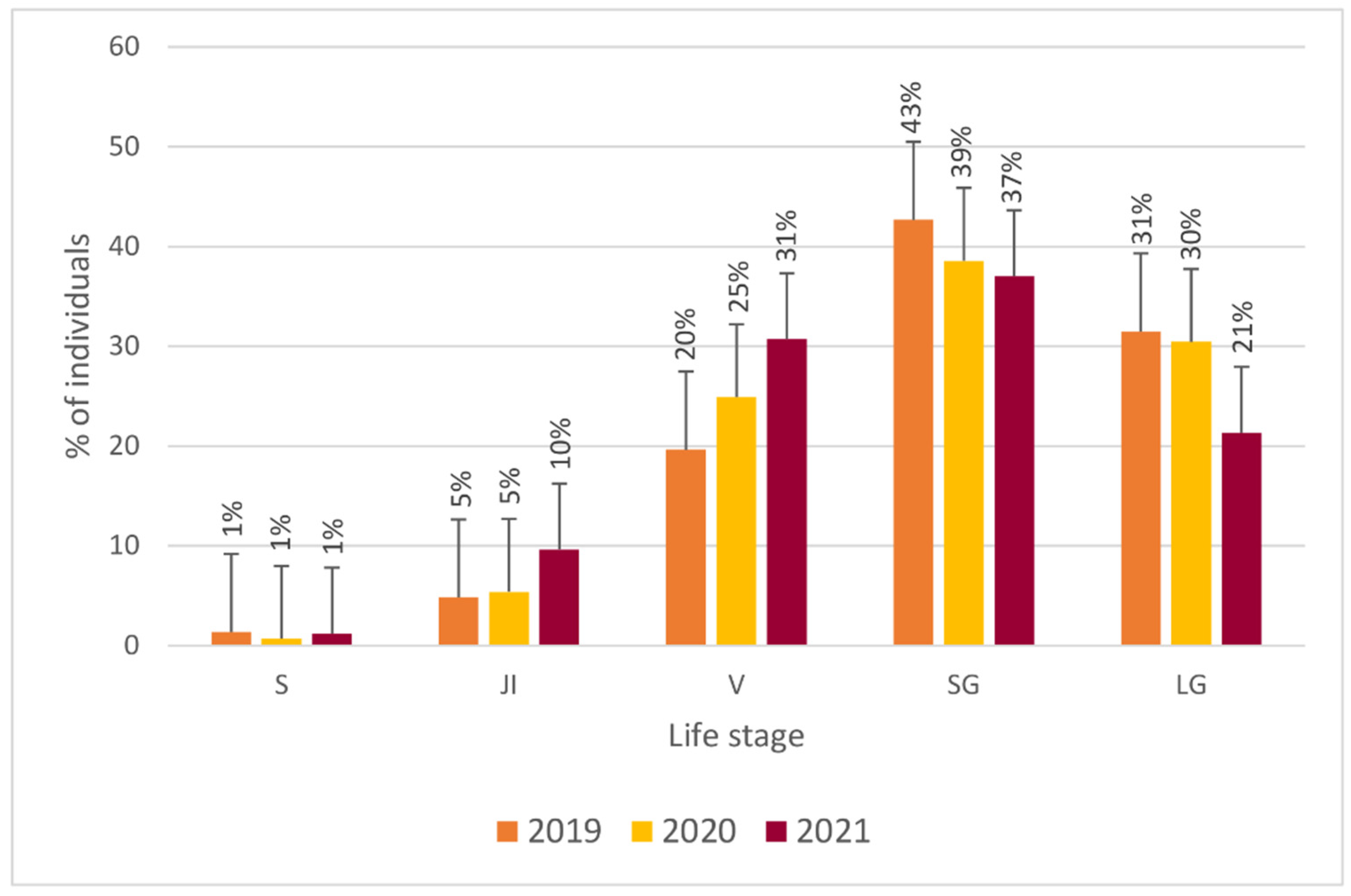
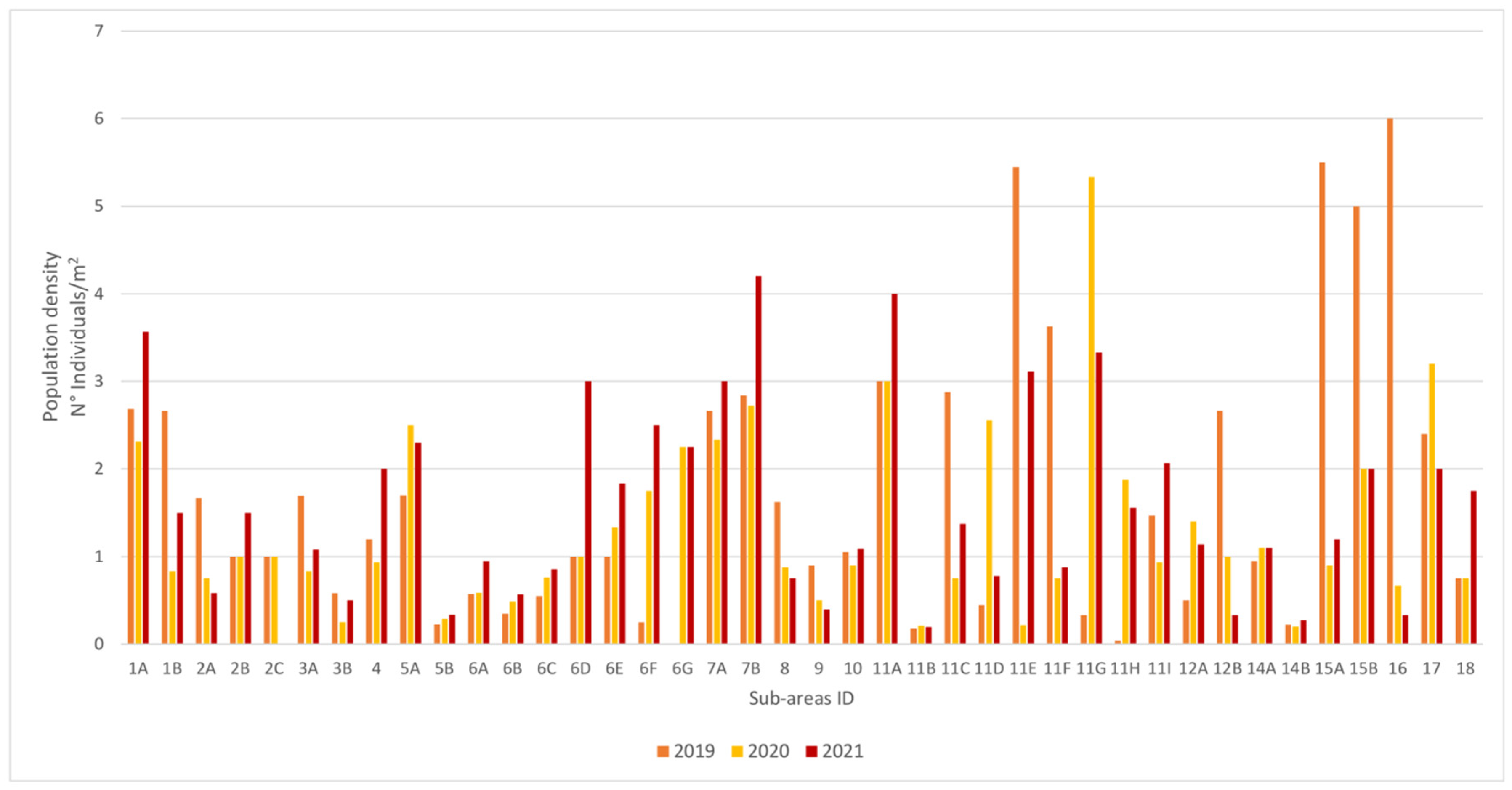
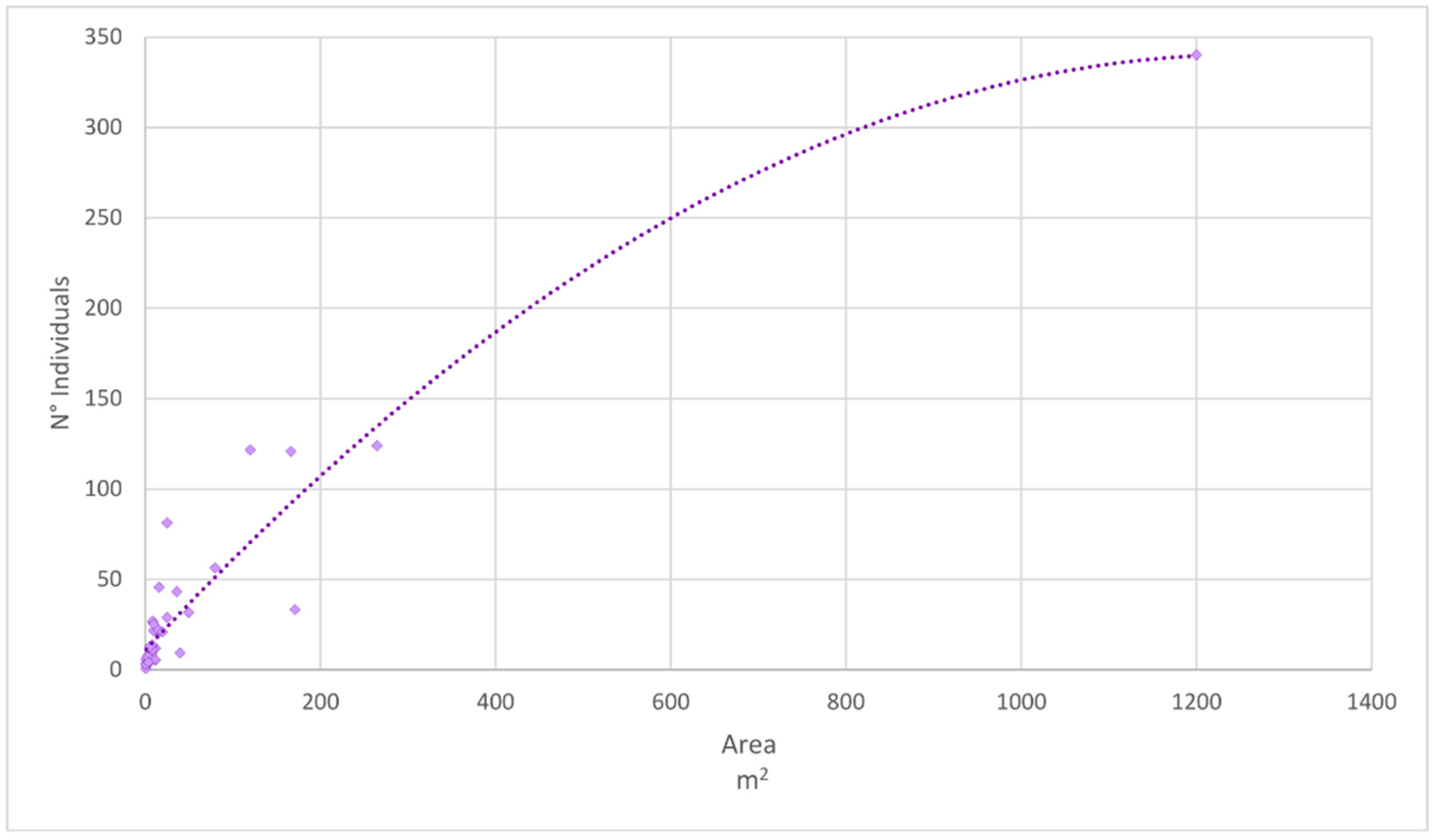
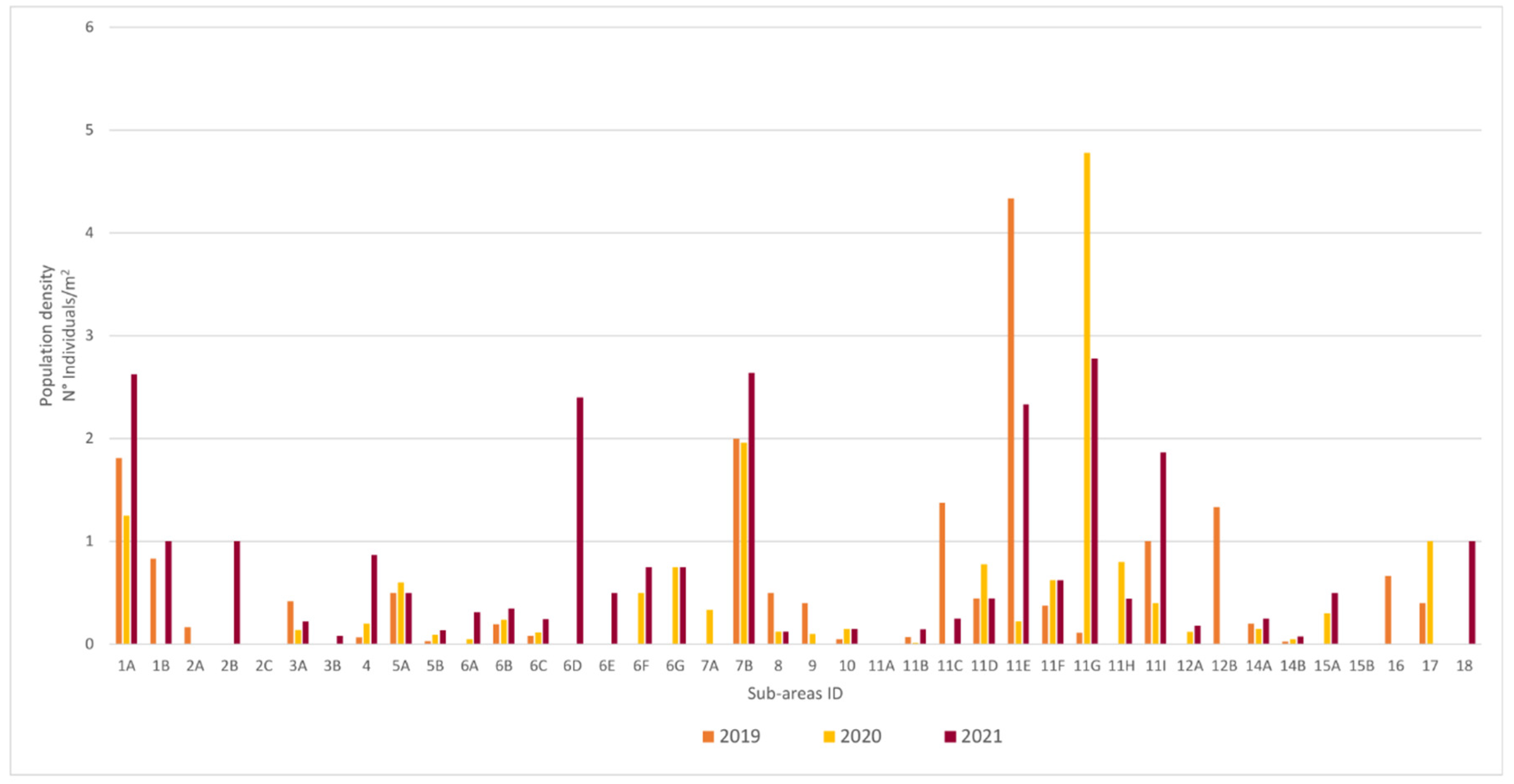
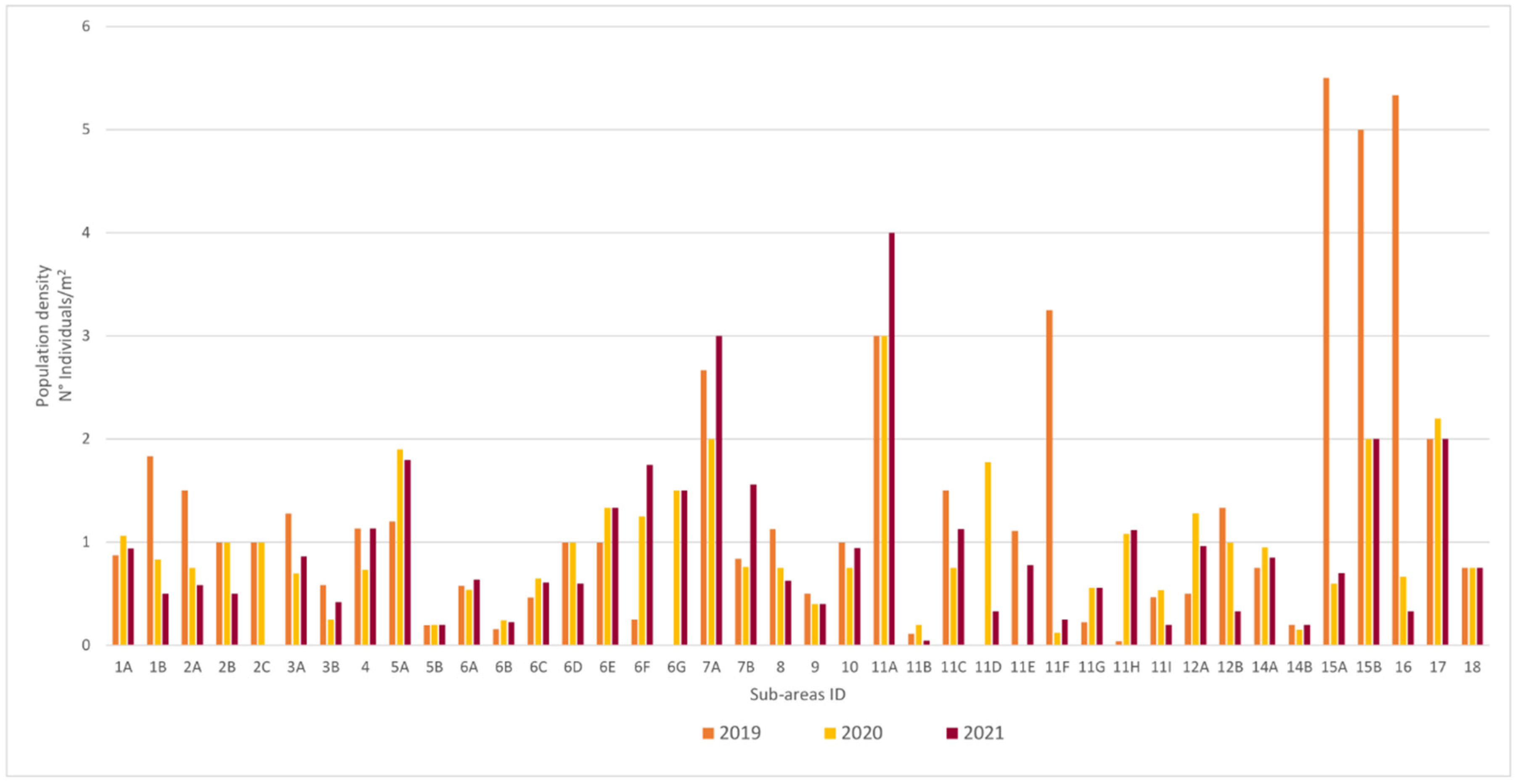
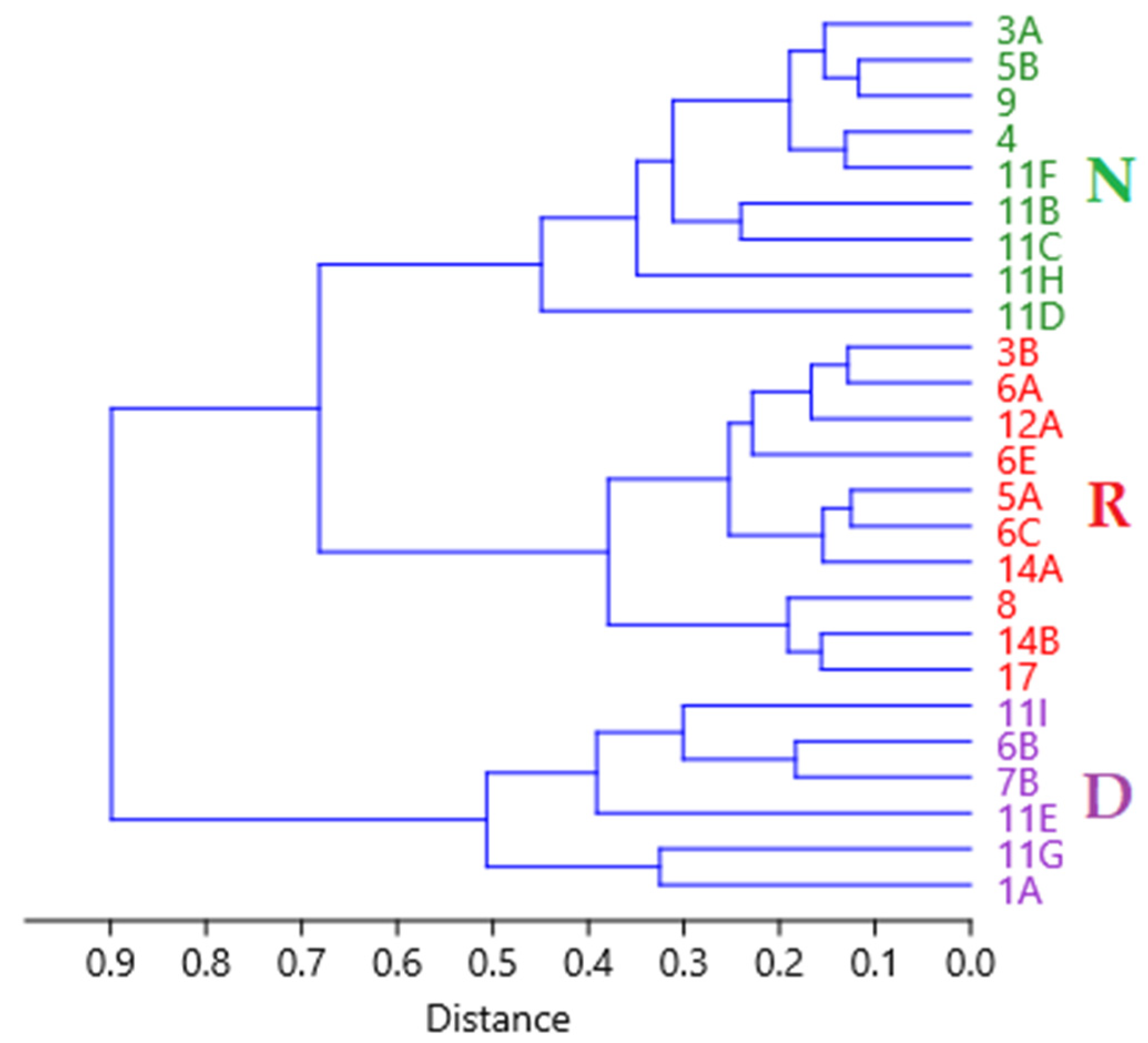
3.1. Population Structure
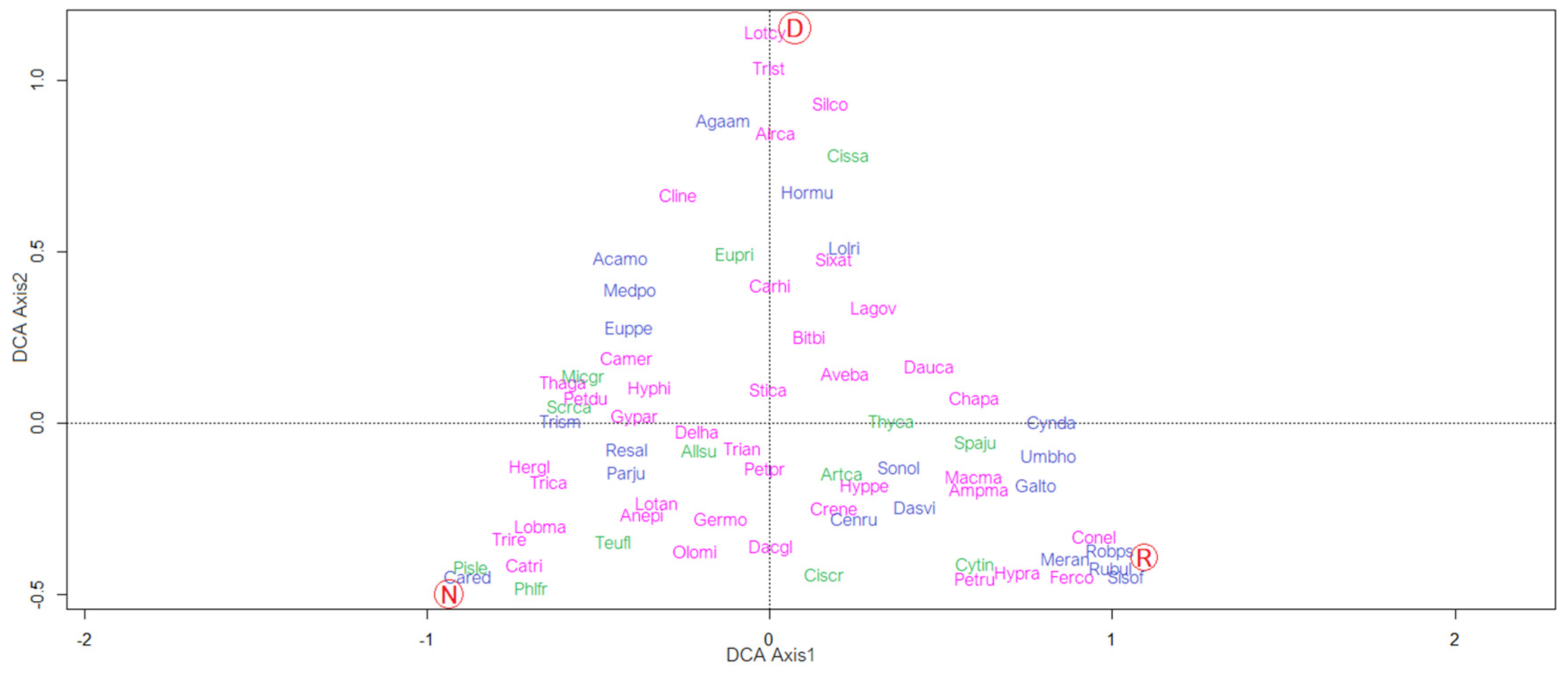
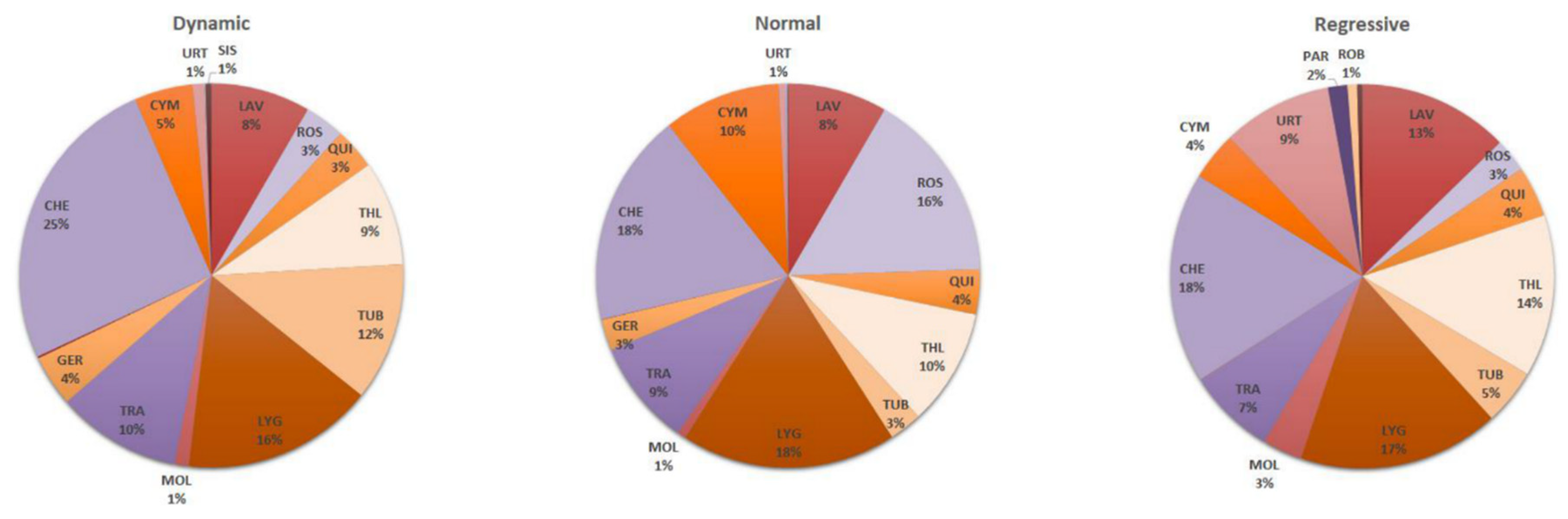
3.2. Relationships between “Life Stages” and Pressures
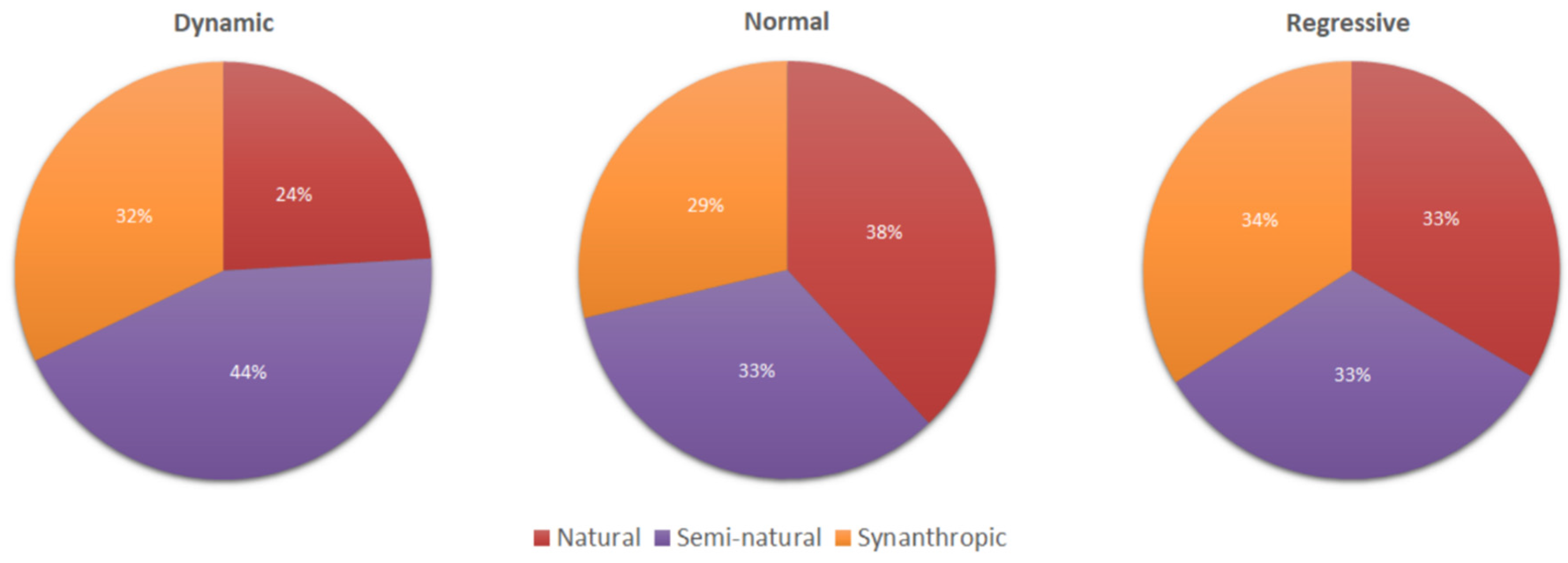
3.3. Relationships between “Life Stage” and Vegetation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchings, M.J. Monitoring plant populations: Census as an aid to conservation. In Monitoring for Conservation and Ecology; Goldsmith, F.B., Ed.; Chapman & Hall: London, UK, 1991; pp. 61–76. [Google Scholar]
- Oostermeijer, J.G.B.; Brugman, M.L.; de Boer, E.R.; den Nijs, J.C.M. Temporal and spatial variation in the demography of Gentiana pneumonanthe, a rare perennial herb. J. Ecol. 1996, 84, 153–166. [Google Scholar] [CrossRef]
- Menges, E.S. Population viability analyses in plants: Challenges and opportunities. Trends Ecol. Evol. 2000, 15, 51–56. [Google Scholar] [CrossRef]
- Aikens, M.L.; Roach, D.A. Population dynamics in central and edge populations of a narrowly endemic plant. Ecology 2014, 95, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Shaltout, K.H.; Ahmed, D.A.; Hatem, A. Shabana, Population structure and dynamics of the endemic species Phlomis aurea Decne in different habitats in southern Sinai Peninsula, Egypt. Glob. Ecol. Conserv. 2015, 4, 505–515. [Google Scholar] [CrossRef][Green Version]
- Shomurodov, H.F.; Khuzhanazarov, U.E.; Beshko, N.Y.; Akhmadalieva, B.; Sharipova, V.K.A. Demographic structure of populations of Salvia lilacinocoerulea Nevski, a rare species endemic to the Western Pamir-Alay (Uzbekistan, Turkmenistan). Am. J. Plant Sci. 2017, 8, 1411. [Google Scholar]
- Hoekstra, J.M.; Boucher, T.M.; Ricketts, T.H.; Roberts, C. Confronting a biome crisis: Global disparities of habitat loss and protection. Ecol. Lett. 2005, 8, 23–29. [Google Scholar] [CrossRef]
- Schemske, D.W.; Husband, B.C.; Ruckelshaus, M.H.; Goodwillie, C.; Parker, I.M.; Bishop, J.G. Evaluating Approaches to the Conservation of Rare and Endangered Plants. Ecology 1994, 75, 585–606. [Google Scholar] [CrossRef]
- Menges, E.S. Evaluating extinction risks in plant populations. In Conservation Biology for the Coming Decade; Fiedler, P.L., Jain, S.K., Eds.; Chapman & Hall: New York, NY, USA, 1998; pp. 49–65. [Google Scholar]
- Harvey, H.J. Population biology and the conservation of rare species. In Studies on Plant Demography: A Festschrift for John L. Harper; White, J., Ed.; Academic Press: London, UK, 1985; pp. 111–123. [Google Scholar]
- Hegland, S.J.; Van Leeuwen, M.; Oostermeijer, J.G.B. Population structure of Salvia pratensis in relation to vegetation and management of Dutch dry floodplain grasslands. J. Appl. Ecol. 2001, 38, 1277–1289. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389. [Google Scholar] [CrossRef]
- Jump, A.S.; Penuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Del Río, S.; Canas, R.; Cano, E.; Cano-Ortiz, A.; Musarella, C.; Pinto-Gomes, C.; Penas, A. Modelling the impacts of climate change on habitat suitability and vulnerability in deciduous forests in Spain. Ecol. Indic. 2021, 131, 108202. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2022: Mitigation of Climate Change. In Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Shukla, P.R., Skea, J., Slade, R., al Khourdajie, A., van Diemen, R., McCollum, D., Pathak, M., Some, S., Vyas, P., Fradera, R., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022. [Google Scholar]
- Vié, J.C.; Hilton-Taylor, C.; Stuart, S.N. (Eds.) Wildite in a Changing Word—An Analysis of the 2008 /UN Red List of Threatened Species; IUCN: Gland, Switzerland, 2009; p. 180. [Google Scholar]
- Içik, K. Rare and endemic species: Why are they prone to extinction? Turk. J. Bot. 2011, 35, 411–417. [Google Scholar]
- Spampinato, G.; Laface, V.L.A.; Ortiz, A.C.; Canas, R.Q.; Musarella, C.M. Salvia ceratophylloides Ard. (Lamiaceae): A rare endemic species of Calabria (Southern Italy). In Endemic Species; Cano Carmona, E., Musarella, C.M., Cano Ortiz, A., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Fenu, G.; Ferretti, G.; Gennai, M.; Lahora, A.; Mendoza-Fernández, A.J.; Mota, J.; Robles, J.; Serra, L.; Schwarzer, H.; Sánchez-Gómez, P.; et al. Global and Regional IUCN Red List Assessments: 4. Ital. Bot. 2017, 4, 61–71. [Google Scholar] [CrossRef]
- Thuiller, W.; Richardson, D.M.; Midgley, G.F. Will Climate Change Promote Alien Plant Invasions? Springer: Berlin/Heidelberg, Germany, 2008; pp. 197–211. [Google Scholar]
- Laface, V.L.A.; Musarella, C.M.; Cano Ortiz, A.; Quinto Canas, R.; Cannavò, S.; Spampinato, G. Three new alien taxa for Europe and a chorological update on the alien vascular flora of Calabria (Southern Italy). Plants 2020, 9, 1181. [Google Scholar] [CrossRef]
- Musarella, C.M.; Stinca, A.; Cano-Ortíz, A.; Laface, V.L.A.; Petrilli, R.; Esposito, A.; Spampinato, G. New data on the alien vascular flora of Calabria (Southern Italy). Ann. Bot. 2020, 10, 55–66. [Google Scholar] [CrossRef]
- Spampinato, G.; Laface, V.L.A.; Posillipo, G.; Cano Ortiz, A.; Quinto Canas, R.; Musarella, C.M. Alien flora in Calabria (Southern Italy): An updated checklist. Biol. Invasions 2022, 24, 2323–2334. [Google Scholar] [CrossRef]
- Perrino, E.V.; Valerio, F.; Jallali, S.; Trani, A.; Mezzapesa, G.N. Ecological and biological properties of Satureja cuneifolia Ten. and Thymus spinulosus Ten.: Two wild officinal species of conservation concern in Apulia (Italy). A preliminary survey. Plants 2021, 10, 1952. [Google Scholar] [CrossRef]
- Valerio, F.; Mezzapesa, G.N.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.F.; Perrino, E.V. Characterization and antimicrobial properties of essential oils from four wild taxa of Lamiaceae family growing in Apulia. Agronomy 2021, 11, 1431. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, Y.P.; Salmaki, Y.; Drew, B.T.; Wilson, T.C.; Scheen, A.C.; Celep, F.; Bräuchler, C.; Bendiksby, M.; Wang, Q.; et al. An updated tribal classification of Lamiaceae based on plastome phylogenomics. BMC Biol. 2021, 19, 2. [Google Scholar] [CrossRef]
- Walker, J.B.; Sytsma, K.J.; Treutlein, J.; Wink, M. Salvia (Lamiaceae) is not monophyletic: Implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae. Am. J. Bot. 2004, 91, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Kintzios, S.E. Sage—The Genus Salvia; CRC Press: Boca Raton, FL, USA, 2003; p. 318. [Google Scholar]
- Askari, S.F.; Avan, R.; Tayarani-Najaran, Z.; Sahebkar, A.; Eghbali, S. Iranian Salvia species: A phytochemical and pharmacological update. Phytochemistry 2021, 183, 112619. [Google Scholar] [CrossRef] [PubMed]
- Hedge, I.C. Salvia L. Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1972; Volume 3, pp. 188–192. [Google Scholar]
- Nikolova, M.; Aneva, I.Y. European Species of Genus Salvia: Distribution, Chemodiversity and Biological Activity. Salvia Biotechnol. 2017, 1–30. [Google Scholar] [CrossRef]
- Portal to the Flora of Italy. Available online: http:/dryades.units.it/floritaly (accessed on 21 June 2022).
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982; Volume 2, pp. 502–507. [Google Scholar]
- Pignatti, S. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2018; Volume 3, pp. 301–310. [Google Scholar]
- Del Carratore, F.; Garbari, F. Indagini Biosistematiche Sul Genere Salvia L. Sect. Plethiosphace Benyham (Labiatae). Inf. Bot. Ital. 1997, 29, 297–299. [Google Scholar]
- Tenore, M. Sylloge Plantarum Vascularium Florae Neapolitanae Hucusque Detectarum; Ex Typographia Fibreni: Neapoli, Italy, 1831; Volume 1. [Google Scholar]
- Macchiati, C. Catalogo delle piante raccolte nei dintorni di Reggio Calabria dal settembre 1881 al febbraio 1883. Nuovo G. Bot. Ital. 1884, 16, 59–100. [Google Scholar]
- Lacaita, C. Addenda et emendanda ad floram italicam. Bull. Della Soc. Bot. Ital. 1921, 18–19. [Google Scholar]
- Lacaita, C. Piante italiane critiche o rare: 67. Salvia ceratophylloides Arduino. Nuovo G. Bot. Ital. 1921, 28, 144–147. [Google Scholar]
- Conti, F.; Manzi, A.; Pedrotti, F. Liste Rosse Regionali delle Piante d’Italia; Assoc. Ital. WWF, Società Botanica Italiana: Camerino, Italy, 1997. [Google Scholar]
- Del Carratore, F.; Garbari, F. Il Gen. Salvia Sect. Plethiosphace (Lamiaceae) in Italia. Arch. Geobot. 2001, 7, 41–62. [Google Scholar]
- Scoppola, A.; Spampinato, G. Atlante delle specie a rischio di estinzione. Versione 1.0. CD-Rom enclosed to the volume. In Stato Delle Conoscenze Sulla Flora Vascolare d’Italia; Scoppola, A., Blasi, C., Eds.; Palombi Editori: Roma, Italy, 2005. [Google Scholar]
- Spampinato, G.; Crisafulli, A. Struttura delle popolazioni e sinecologia di Salvia ceratophylloides (Lamiaceae) specie endemica minacciata di estinzione, 56. In Book of Abstracts of 103° S.B.I. Congress; Università Mediterranea di: Reggio Calabria, Italy, 2008; pp. 17–19. [Google Scholar]
- Prediction of Worldwide Energy Resource (POWER) Project Funded through the National Aeronautics and Space Administration (NASA) Earth Science/Applied Science Program. Available online: https://power.larc.nasa.gov/data-access-viewer/ (accessed on 26 July 2022).
- Pesaresi, S.; Galdenzi, D.; Biondi, E.; Casavecchia, S. Bioclimate of Italy: Application of the worldwide bioclimatic classification system. J. Maps 2014, 10, 538–553. [Google Scholar] [CrossRef]
- Cassa per il Mezzogiorno—Cassa per Opere Straordinarie di Pubblico Interesse nell’Italia Meridionale, Carta Geologica della Calabria; Cassa per il Mezzogiorno: Rome, Italy, 1967–1972.
- Laface, V.L.A.; Musarella, C.M.; Spampinato, G. Conservation status of the Aspromontana flora: Monitoring and new stations of Salvia ceratophylloides Ard. (Lamiaceae) endemic species in Reggio Calabria (southern Italy). In Abstracts Book of 113° Congresso della Società Botanica Italiana (V International Plant Science Conference (IPSC); Società Botanica Italiana: Fisciano, Italy, 2018; pp. 12–15. ISBN 978-88-85915-22-0. [Google Scholar]
- Malthus, T.R. An Essay on the Principle of Population; Printed for J. Johnson in St. Paul’s Church-Yard: London, UK, 1798. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge der Vegetationskunde, 3rd ed.; Springer: Berlin, Germany, 1964; Volume 631. [Google Scholar]
- Ighbareyeh, J.M.H.; Suliemieh, A.A.R.A.; Sheqwarah, M.; Cano-Ortiz, A.; Carmona, E.C. Flora and Phytosociological of Plant in Al-Dawaimah of Palestine. Res. J. Ecol. Environ. Sci. 2022, 2, 58–91. [Google Scholar] [CrossRef]
- Raposo, M.; Pinto-Gomes, C.; Quinto-Canas, R. Priority tree and shrubs for use in Landscape Architecture based on the dynamic states of native vegetation with the highest ecological value in mainland Portugal. Res. J. Ecol. Environ. Sci. 2022, 2, 46–57. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; Gavilán García, R.; et al. Vegetation of Europe: Hierarchical floristic classification of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Biondi, E.; Blasi, C. Prodromo alla vegetazione d’Italia. Available online: https://www.prodromo-vegetazione-italia.org/ (accessed on 21 June 2022).
- Salafsky, N.; Salzer, D.; Stattersfield, A.J.; Hilton-Taylor, C.; Neugarten, R.; Butchart, S.H.M.; Collen, B.; Cox, N.; Master, L.L.; O’Connor, S.; et al. A Standard Lexicon for Biodiversity Conservation: Unified Classifications of Threats and Actions. Conserv. Biol. 2007, 22, 4. [Google Scholar] [CrossRef]
- EIONET (European Environment Information and Observation Network). Available online: http://biodiversity.eionet.europa.eu/activities/Reporting/Article_17/Reports_2019/Files_2019/Pressures_Threats_Final_20180507.xls (accessed on 12 April 2022).
- Hartigan, J.A. Clustering Algorithms. Wiley and Sons Inc.: New York, NY, USA, 1975. [Google Scholar]
- Hammer, Ø. Past Software. Natural History Museum; University of Oslo: Oslo, Norway, 2022. [Google Scholar]
- Oostermeijer, J.G.B.; van’t Veer, R.; den Nijs, J.C.M. Population structure of the rare long-lived perennial Gentiana pneumonanthe in relation to vegetation and management in the Netherlands. J. Appl. Ecol. 1994, 31, 428–438. [Google Scholar] [CrossRef]
- Hill, M.O. DECORANA—A FORTRAN Program for Detrended Correspondence Analysis and Reciprocal Averaging; Cornell University: New York, NY, USA, 1979. [Google Scholar]
- Jongman, R.H.G.; Ter Braak, C.J.F.; van Tongeren, O.F.R. Data Analysis in Community and Landscape Ecology; Pudoc: Wageningen, The Netherlands, 1987. [Google Scholar]
- De Luca, G.; Modica, G. Canopy fire effects estimation using Sentinel-2 imagery and deep learning approach. A case study on the Aspromonte National Park. In Communications in Computer and Information Science series (CCIS)—AII. International Conference on Applied Intelligence and Informatics; Springer: Berlin/Heidelberg, Germany, 2022; accepted. [Google Scholar]
- European Forest Fire Informationon System (EFFIS). Available online: https://effis.jrc.ec.europa.eu/ (accessed on 30 June 2022).
- Bonsignore, C.P.; Laface, V.L.A.; Vono, G.; Marullo, R.; Musarella, C.M.; Spampinato, G. Threats Posed to the Rediscovered and Rare Salvia ceratophylloides Ard. (Lamiaceae) by Borer and Seed Feeder Insect Species. Diversity 2021, 13, 33. [Google Scholar] [CrossRef]
- Vescio, R.; Abenavoli, M.R.; Araniti, F.; Musarella, C.M.; Sofo, A.; Laface, V.L.A.; Spampinato, G.; Sorgonà, A. The assessment and the within-plant variation of the morpho-physiological traits and vocs profile in endemic and rare Salvia ceratophylloides Ard. (Lamiaceae). Plants 2021, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Crawley, M.J. The relative importance of vertebrate and invertebrate herbivores in plant population dynamics. In Insect-Plant Interactions; CRC Press: Boca Raton, FL, USA, 2019; pp. 45–71. [Google Scholar]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Hahs, A.K.; McDonnell, M.J.; McCarthy, M.A.; Vesk, P.A.; Corlett, R.T.; Norton, B.A.; Clemants, S.E.; Duncan, R.P.; Thompson, K.; Schwartz, M.W.; et al. A global synthesis of plant extinction rates in urban areas. Ecol. Lett. 2009, 12, 1165–1173. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Hui, C.; Castillo, M.L.; Iriondo, J.M.; Keet, J.H.; Khapugin, A.A.; Médail, F.; Rejmánek, M.; Theron, G.; Yannelli, F.A.; et al. Recent anthropogenic plant extinctions differ in biodiversity hotspots and coldspots. Curr. Biol. 2019, 29, 2912–2918. [Google Scholar] [CrossRef]
- Spampinato, G.; Crisarà, R.; Cameriere, P.; Cano-Ortiz, A.; Musarella, C.M. Analysis of the Forest Landscape and Its Transformations through Phytotoponyms: A Case Study in Calabria (Southern Italy). Land 2022, 11, 518. [Google Scholar] [CrossRef]
- Spampinato, G.; Crisarà, R.; Cannavò, S.; Musarella, C.M. Phytotoponims of southern Calabria: A tool for the analysis of the landscape and its transformations. Atti Della Soc. Toscana Di Sci. Nat. Mem. Ser. B 2017, 124, 61–72. [Google Scholar] [CrossRef]
- Spampinato, G.; Cameriere, P.; Caridi, D.; Crisafulli, A. Carta della biodiversità vegetale del Parco Nazionale dell’Aspromonte (Italia meridionale). Quad. Bot. Amb. Appl. 2008, 19, 3–36. [Google Scholar]
- Mercurio, R. Restauro Della Foresta Mediterranea; Clueb, Ed.; Cooperativa Libraria Universitaria Editrice Bologna: Bologna, Italy, 2008; ISBN 978-88-491-3399-8. [Google Scholar]
- Médail, F.; Diadema, K.; Pouget, M.; Baumel, A. Identification of plant micro-reserves using conservation units and population vulnerability: The case of an endangered endemic Snowflake (Acis nicaeensis) in the Mediterranean Basin hotspot. J. Nat. Conserv. 2021, 61, 125980. [Google Scholar] [CrossRef]
- Laguna, E.; Deltoro, V.I.; Pérez-Botella, J.; Pérez-Rovira, P.; Serra, L.; Olivares, A.; Fabregat, C. The role of small reserves in plant conservation in a region of high diversity in eastern Spain. Biol. Conserv. 2004, 119, 421–426. [Google Scholar] [CrossRef]
- Laguna, E.; Fosa, S.; Jim´enez, J.; Volis, S. Role of micro-reserves in conservation of endemic, rare and endangered plants of the Valencian region (Eastern Spain). Isr. J. Plant Sci. 2016, 63, 320–332. [Google Scholar] [CrossRef]
- Kadis, C.; Thanos, C.A.; Laguna Lumbreras, E. (Eds.) Plant micro-reserves: From theory to practice. Experiences Gained FROM EU LIFE and other Related Projects; Utopia Publishing: Athens, Greece, 2013. [Google Scholar]



| Parameter | 1991/2021 | 2019 | 2020 | 2021 |
|---|---|---|---|---|
| Specific Humidity at 2 Meters (g/kg) | 9.59 | 9.7 | 9.58 | 9.64 |
| Relative Humidity at 2 Meters (%) | 71.52 | 70.88 | 69.56 | 70.06 |
| Temperature at 2 Meters Maximum | 35.43 | 35.19 | 34.13 | 38.08 |
| Temperature at 2 Meters Minimum | 4.64 | 4.43 | 6.55 | 4.44 |
| Temperature at 2 Meters Range | 30.79 | 30.76 | 27.59 | 33.64 |
| Precipitation Corrected (mm/day) | 1.86 | 1.85 | 1.54 | 1.94 |
| Precipitation Corrected Sum (mm) | 609.68 | 606.45 | 511.52 | 706.65 |
| Sub Aree | 1A | 6B | 7B | 11E | 11G | 11I | 3A | 4 | 5B | 9 | 11B | 11C | 11D | 11F | 11H | 3B | 5A | 6A | 6C | 6E | 8 | 12A | 14A | 14B | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population Tipe | Dynamic | Normal | Regressive | |||||||||||||||||||||||
| Threats Code | Threats Name | |||||||||||||||||||||||||
| A08 | Mowing or cutting of grasslands | L | L | L | L | L | L | L | L | L | L | L | ||||||||||||||
| A10 | Extensive grazing or undergrazing by livestock | L | L | L | L | M | M | |||||||||||||||||||
| A11 | Burning for agriculture | H | L | L | ||||||||||||||||||||||
| A15 | Tillage practices in agriculture | H | L | M | L | L | M | |||||||||||||||||||
| F07 | Sports, tourism and leisure activities | H | H | H | H | H | H | H | H | |||||||||||||||||
| I01-I02 | Invasive alien species of Union/not Union concern | M | M | L | L | L | L | L | L | M | L | H | L | M | L | |||||||||||
| L06 | Interspecific faunal and floral relations (competition, predation, parasitism, pathogens) | H | H | H | M | M | M | M | M | H | H | H | H | M | M | L | ||||||||||
| M05 | Collapse of terrain, landslide | L | ||||||||||||||||||||||||
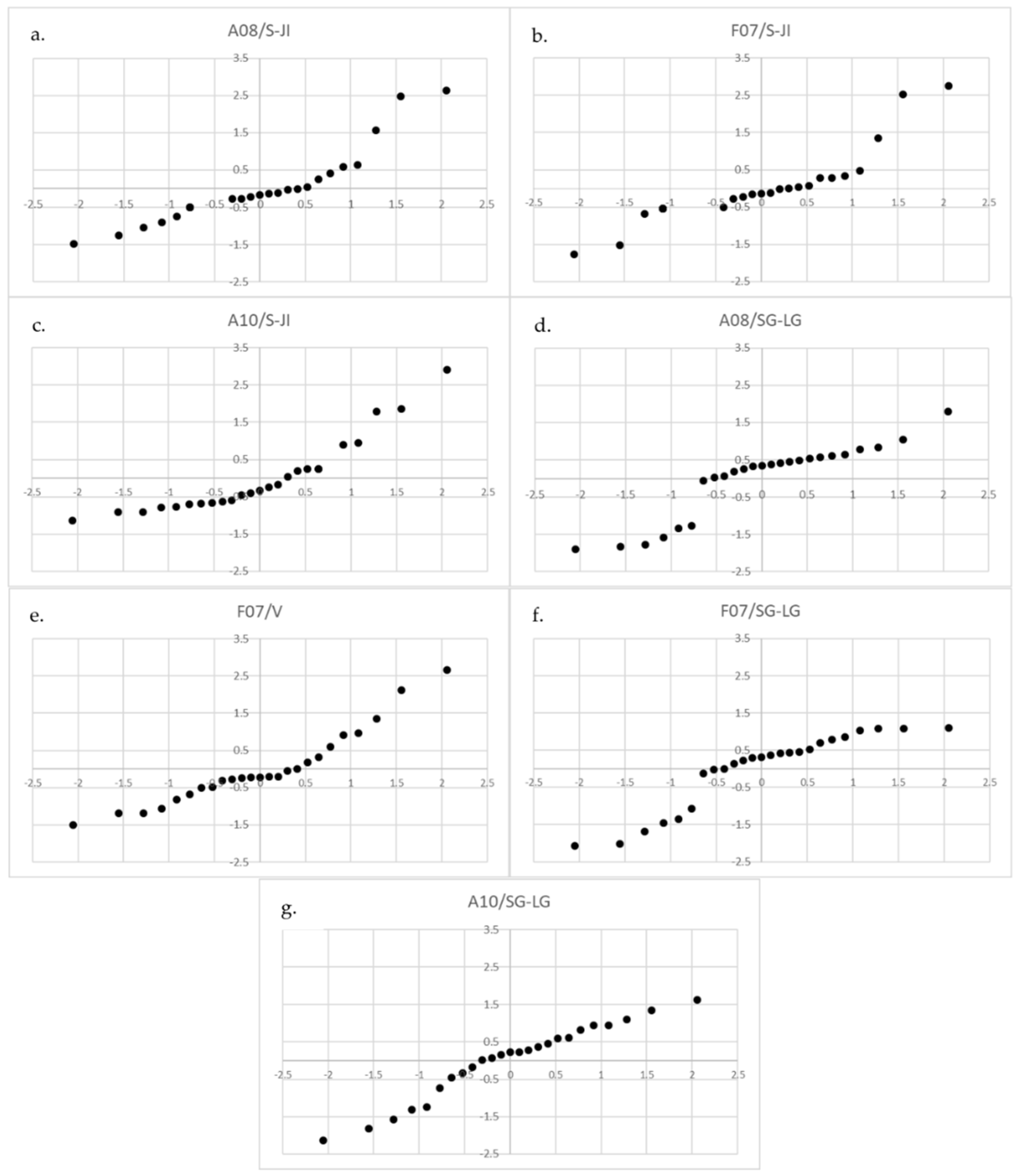
| Life Stage | Threats | R2 | p | Sign. |
|---|---|---|---|---|
| S-JI | A08 | 0.434 | 0.03 | ** |
| F07 | 0.576 | 0.003 | ** | |
| A10 | −0.403 | 0.046 | ** | |
| V | F07 | 0.352 | 0.08 | * |
| SG-LG | A08 | −0.343 | 0.09 | * |
| F07 | −0.523 | 0.01 | ** | |
| A10 | 0.395 | 0.05 | * |
| Population Typology | Min. Species | Max. Species | Mean |
|---|---|---|---|
| Normal | 17 | 70 | 43 |
| Dynamic | 15 | 60 | 42.5 |
| Regressive | 18 | 34 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laface, V.L.A.; Musarella, C.M.; Sorgonà, A.; Spampinato, G. Analysis of the Population Structure and Dynamic of Endemic Salvia ceratophylloides Ard. (Lamiaceae). Sustainability 2022, 14, 10295. https://doi.org/10.3390/su141610295
Laface VLA, Musarella CM, Sorgonà A, Spampinato G. Analysis of the Population Structure and Dynamic of Endemic Salvia ceratophylloides Ard. (Lamiaceae). Sustainability. 2022; 14(16):10295. https://doi.org/10.3390/su141610295
Chicago/Turabian StyleLaface, Valentina Lucia Astrid, Carmelo Maria Musarella, Agostino Sorgonà, and Giovanni Spampinato. 2022. "Analysis of the Population Structure and Dynamic of Endemic Salvia ceratophylloides Ard. (Lamiaceae)" Sustainability 14, no. 16: 10295. https://doi.org/10.3390/su141610295
APA StyleLaface, V. L. A., Musarella, C. M., Sorgonà, A., & Spampinato, G. (2022). Analysis of the Population Structure and Dynamic of Endemic Salvia ceratophylloides Ard. (Lamiaceae). Sustainability, 14(16), 10295. https://doi.org/10.3390/su141610295









