A Review on Application of Biochar in the Removal of Pharmaceutical Pollutants through Adsorption and Persulfate-Based AOPs
Abstract
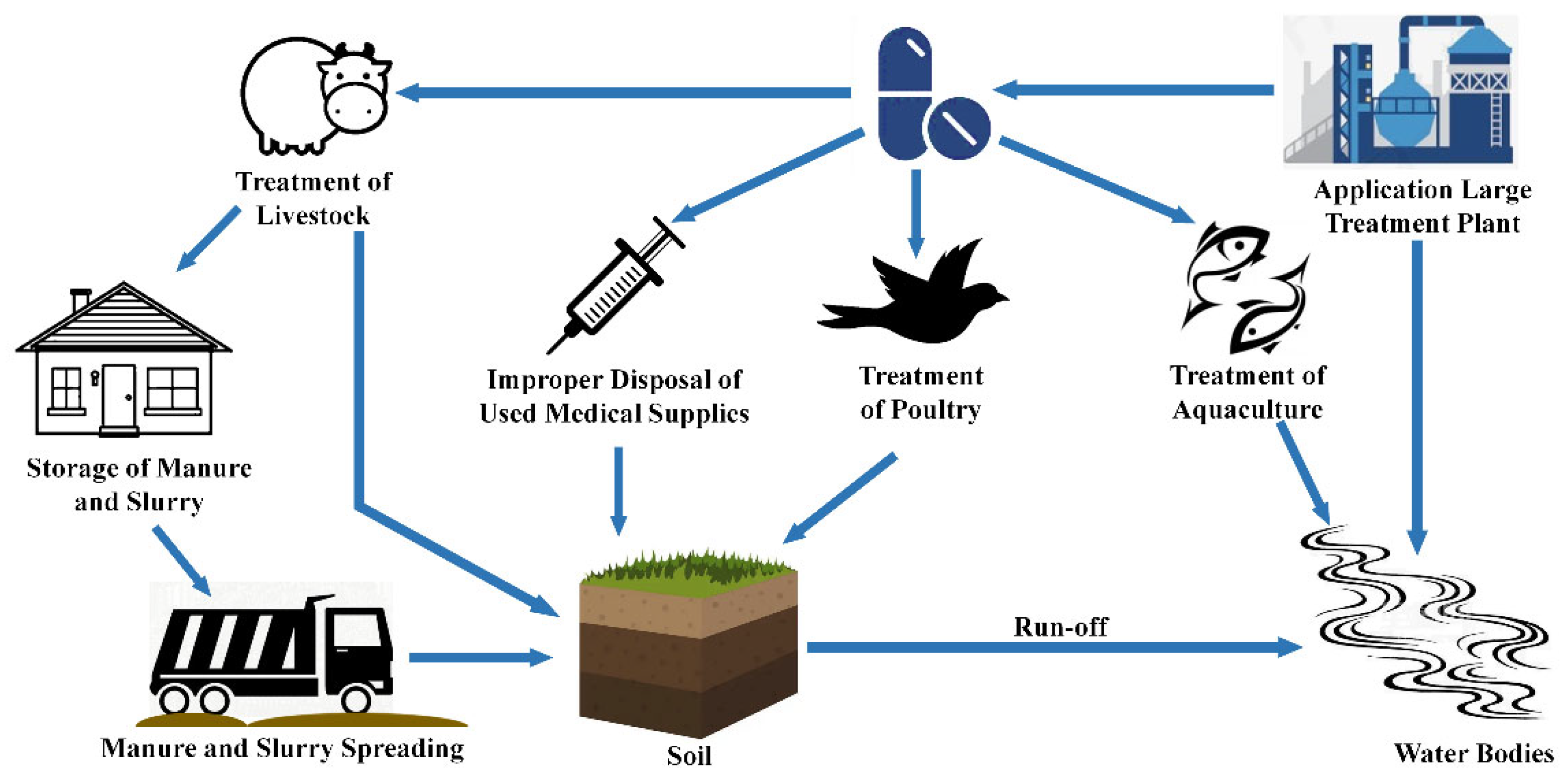
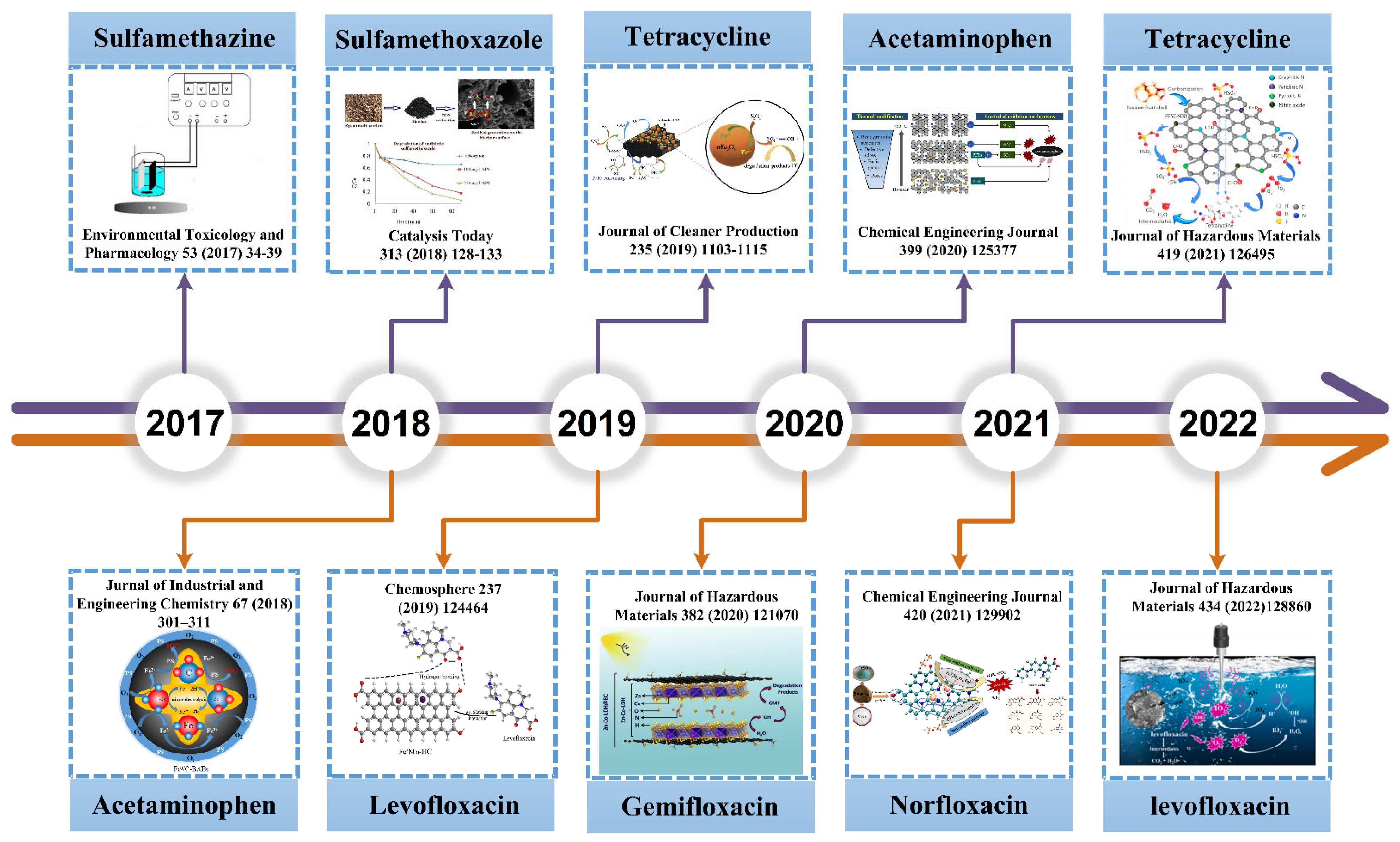
:1. Introduction
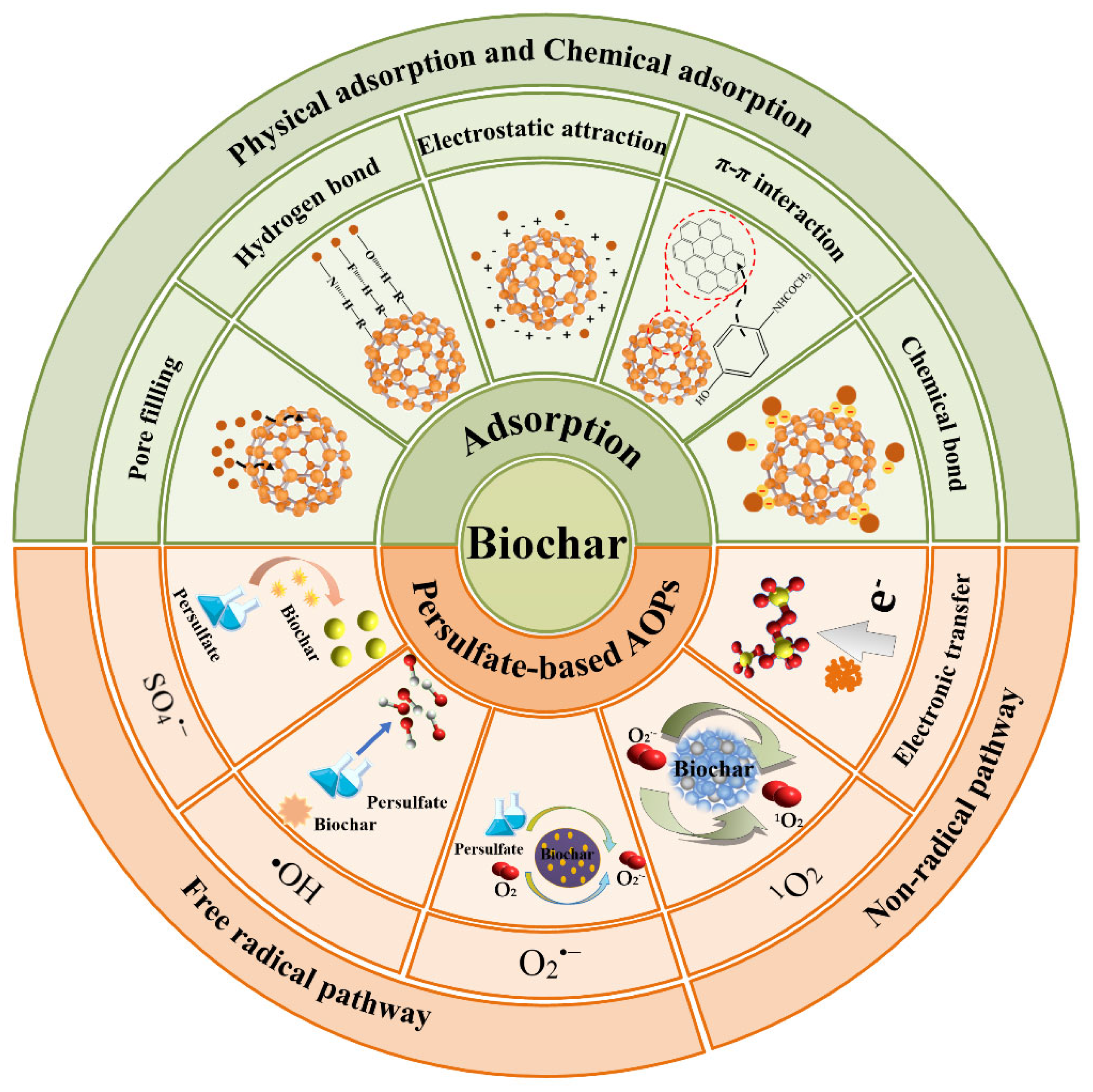
2. Removal Mechanism
2.1. Adsorption
2.2. Degradation via Persulfate-Based AOPs
2.2.1. Free Radical Pathway
2.2.2. Non-Radical Pathway
3. Adsorption and Degradation Behaviors of Various Drugs
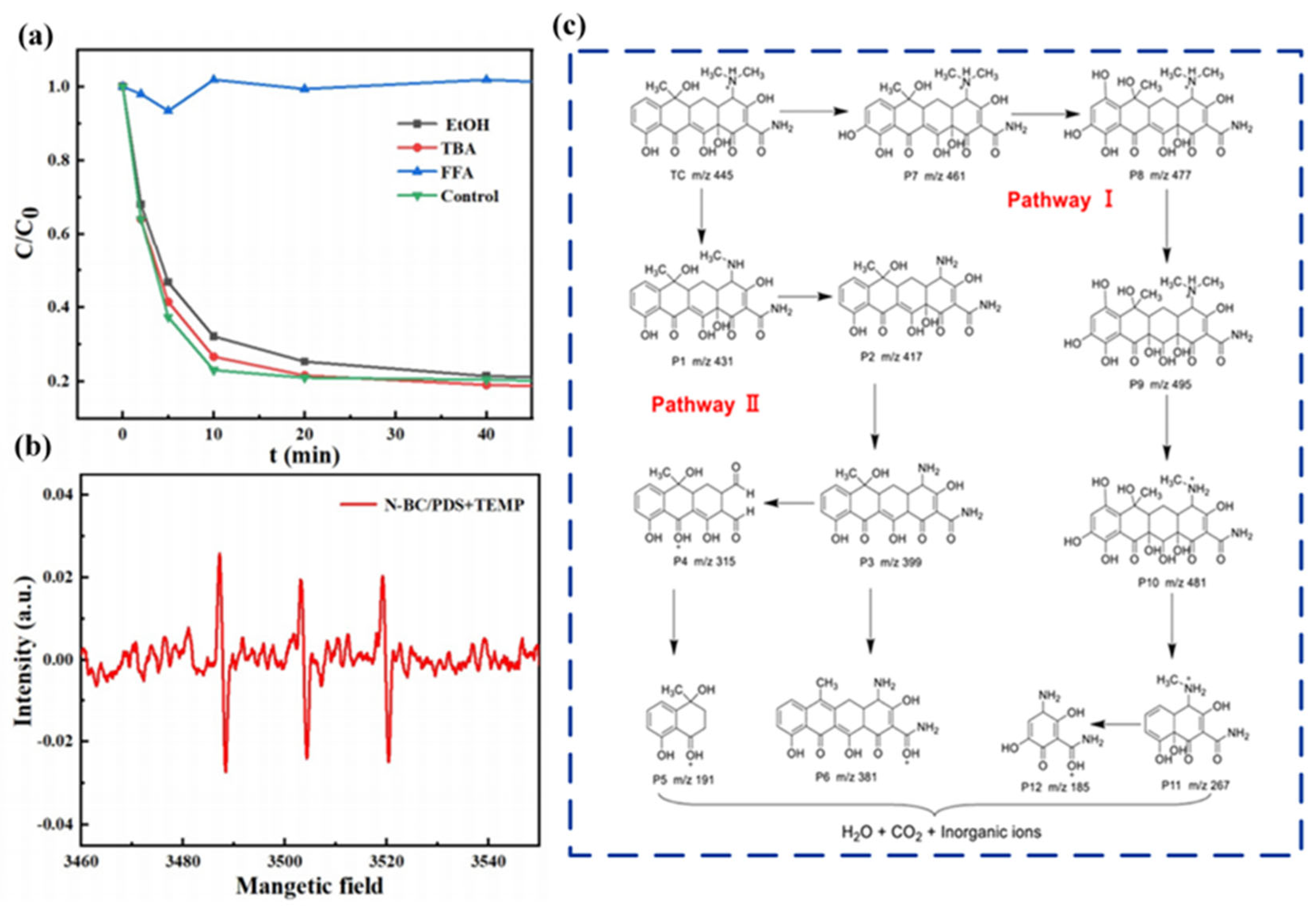
3.1. Tetracycline
3.1.1. Adsorption
3.1.2. Degradation via Persulfate-Based AOPs
3.2. Sulfamethoxazole
3.2.1. Adsorption
3.2.2. Degradation via Persulfate-Based AOPs
3.3. Acetaminophen
3.3.1. Adsorption
3.3.2. Degradation via Persulfate-Based AOPs
3.4. Cephalexin
3.4.1. Adsorption
3.4.2. Degradation via Persulfate-Based AOPs
3.5. Levofloxacin
3.5.1. Adsorption
3.5.2. Degradation via Persulfate-Based AOPs
3.6. Other Drugs
4. Effect of Parameters
4.1. Effect of Solution pH
4.2. Effect of Coexisting Ions
4.3. Effect of Solution Temperature
4.4. Effects of Biochar Dosage
4.5. Effects of Persulfate Dosage
5. Conclusions and Future Prospects
5.1. Conclusions
5.2. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tang, F.; He, T.; Zhang, H.; Wu, X.; Li, Y.; Long, F.; Xiang, Y.; Zhu, L.; Wu, J.; Wu, X. The MnO@N-doped carbon composite derived from electrospinning as cathode material for aqueous zinc ion battery. J. Electroanal. Chem. 2020, 873, 114368. [Google Scholar] [CrossRef]
- Li, B.; Xue, J.; Han, C.; Liu, N.; Ma, K.; Zhang, R.; Wu, X.; Dai, L.; Wang, L.; He, Z. A hafnium oxide-coated dendrite-free zinc anode for rechargeable aqueous zinc-ion batteries. J. Colloid Interface Sci. 2021, 599, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, Z.; Lv, Y.; Tang, A.; Dai, L.; Wang, L.; He, Z. Perovskite enables high performance vanadium redox flow battery. Chem. Eng. J. 2022, 443, 136341. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, M.; Jiang, Y.; Liu, S.; Li, J.; Wu, J.; Liu, Z.; Zhu, L.; Wu, X.; He, Z.; et al. Ionic liquid assisted hydrothermal synthesis of 0.5Li2MnO3·0.5LiNi0.5Mn0.5O2 for lithium ion batteries. J. Alloys Compd. 2021, 864, 158177. [Google Scholar] [CrossRef]
- Kong, P.; Zhu, L.; Li, F.; Xu, G. Self-Supporting Electrode Composed of SnSe Nanosheets, Thermally Treated Protein, and Reduced Graphene Oxide with Enhanced Pseudocapacitance for Advanced Sodium-Ion Batteries. ChemElectroChem 2019, 6, 5642–5650. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Y.; Han, C.; Huang, Z.; Wu, X.; He, Z.; Meng, W.; Dai, L.; Wang, L. Raising Lithium Storage Performances of NaTi2(PO4)3 by Nitrogen and Sulfur Dual-Doped Carbon Layer. J. Electrochem. Soc. 2020, 167, 20550. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Li, R.; Lu, X.; Yan, B.; Li, N.; Wang, S. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater. 2021, 408, 124461. [Google Scholar] [CrossRef]
- Ferrer-Polonio, E.; Fernandez-Navarro, J.; Iborra-Clar, M.I.; Alcaina-Miranda, M.I.; Mendoza-Roca, J.A. Removal of pharmaceutical compounds commonly-found in wastewater through a hybrid biological and adsorption process. J. Environ. Manag. 2020, 263, 110368. [Google Scholar] [CrossRef]
- Zhu, T.-T.; Su, Z.-X.; Lai, W.-X.; Zhang, Y.-B.; Liu, Y.-W. Insights into the fate and removal of antibiotics and antibiotic resistance genes using biological wastewater treatment technology. Sci. Total Environ. 2021, 776, 145906. [Google Scholar] [CrossRef]
- Igwegbe, C.A.; Aniagor, C.O.; Oba, S.N.; Yap, P.-S.; Iwuchukwu, F.U.; Liu, T.; de Souza, E.C.; Ighalo, J.O. Environmental protection by the adsorptive elimination of acetaminophen from water: A comprehensive review. J. Ind. Eng. Chem. 2021, 104, 117–135. [Google Scholar] [CrossRef]
- Yi, X.; Tran, N.H.; Yin, T.; He, Y.; Gin, K.Y. Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system. Water Res. 2017, 121, 46–60. [Google Scholar] [CrossRef]
- Szymańska, U.; Wiergowski, M.; Sołtyszewski, I.; Kuzemko, J.; Wiergowska, G.; Woźniak, M.K. Presence of antibiotics in the aquatic environment in Europe and their analytical monitoring: Recent trends and perspectives. Microchem. J. 2019, 147, 729–740. [Google Scholar] [CrossRef]
- Xu, W.; Zou, R.; Jin, B.; Zhang, G.; Su, Y.; Zhang, Y. The ins and outs of pharmaceutical wastewater treatment by microbial electrochemical technologies. Sustain. Horiz. 2022, 1, 100003. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Chen, X.; Huang, Y.; Peng, K.; Huang, Y.; Yan, Y.; Chen, Y. Pig manure-derived nitrogen-doped mesoporous carbon for adsorption and catalytic oxidation of tetracycline. Sci. Total Environ. 2020, 708, 135071. [Google Scholar] [CrossRef]
- Zungu, V.; Hadebe, L.; Mpungose, P.; Hamza, I.; Amaku, J.; Gumbi, B. Fabrication of Biochar Materials from Biowaste Coffee Grounds and Assessment of Its Adsorbent Efficiency for Remediation of Water-Soluble Pharmaceuticals. Sustainability 2022, 14, 2931. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R.; Yang, J.; Sillanpää, M. A review on persulfates activation by functional biochar for organic contaminants removal: Synthesis, characterizations, radical determination, and mechanism. J. Environ. Chem. Eng. 2021, 9, 106267. [Google Scholar] [CrossRef]
- Liang, J.; Xu, X.; Qamar Zaman, W.; Hu, X.; Zhao, L.; Qiu, H.; Cao, X. Different mechanisms between biochar and activated carbon for the persulfate catalytic degradation of sulfamethoxazole: Roles of radicals in solution or solid phase. Chem. Eng. J. 2019, 375, 121908. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, H.; Ji, J.; Yuan, X.; Li, X.; Jiang, L.; Wang, H. Resource utilization of luffa sponge to produce biochar for effective degradation of organic contaminants through persulfate activation. Sep. Purif. Technol. 2022, 288, 120650. [Google Scholar] [CrossRef]
- Li, J.; Liu, G.; Liu, B.; Min, Z.; Qian, D.; Jiang, J.; Li, J. Fe-doped CoSe2 nanoparticles encapsulated in N-doped bamboo-like carbon nanotubes as an efficient electrocatalyst for oxygen evolution reaction. Electrochim. Acta 2018, 265, 577–585. [Google Scholar] [CrossRef]
- Li, J.; Jiang, J.; Zhao, D.; Xu, Z.; Liu, M.; Liu, X.; Tong, H.; Qian, D. Novel hierarchical sea urchin-like Prussian blue@palladium core–shell heterostructures supported on nitrogen-doped reduced graphene oxide: Facile synthesis and excellent guanine sensing performance. Electrochim. Acta 2020, 330, 135196. [Google Scholar] [CrossRef]
- Wang, C.; Luo, D.; Zhang, X.; Huang, R.; Cao, Y.; Liu, G.; Zhang, Y.; Wang, H. Biochar-based slow-release of fertilizers for sustainable agriculture: A mini review. Environ. Sci. Ecotechnol. 2022, 10, 100167. [Google Scholar] [CrossRef]
- Selvarajoo, A.; Wong, Y.L.; Khoo, K.S.; Chen, W.H.; Show, P.L. Biochar production via pyrolysis of citrus peel fruit waste as a potential usage as solid biofuel. Chemosphere 2022, 294, 133671. [Google Scholar] [CrossRef]
- Irshad, M.K.; Ibrahim, M.; Noman, A.; Shang, J.; Mahmood, A.; Mubashir, M.; Khoo, K.S.; Ng, H.S.; Show, P.L. Elucidating the impact of goethite-modified biochar on arsenic mobility, bioaccumulation in paddy rice (Oryza sativa L.) along with soil enzyme activities. Process. Saf. Environ. Prot. 2022, 160, 958–967. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R. Highly dispersed iron-doped biochar derived from sawdust for Fenton-like degradation of toxic dyes. J. Clean. Prod. 2021, 297, 126681. [Google Scholar] [CrossRef]
- Zhang, X.; Gang, D.D.; Zhang, J.; Lei, X.; Lian, Q.; Holmes, W.E.; Zappi, M.E.; Yao, H. Insight into the activation mechanisms of biochar by boric acid and its application for the removal of sulfamethoxazole. J. Hazard. Mater. 2022, 424, 127333. [Google Scholar] [CrossRef]
- Fang, G.; Wu, W.; Liu, C.; Dionysiou, D.D.; Deng, Y.; Zhou, D. Activation of persulfate with vanadium species for PCBs degradation: A mechanistic study. Appl. Catal. B 2017, 202, 1–11. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Xu, Z.; Zhou, Y.; Wei, Y.; Long, X.; He, Y.; Zhi, D.; Yang, J.; Luo, L. A sustainable ferromanganese biochar adsorbent for effective levofloxacin removal from aqueous medium. Chemosphere 2019, 237, 124464. [Google Scholar] [CrossRef] [PubMed]
- Xi, M.; Cui, K.; Cui, M.; Ding, Y.; Guo, Z.; Chen, Y.; Li, C.; Li, X. Enhanced norfloxacin degradation by iron and nitrogen co-doped biochar: Revealing the radical and nonradical co-dominant mechanism of persulfate activation. Chem. Eng. J. 2021, 420, 129902. [Google Scholar] [CrossRef]
- Wang, B.-h.; Zhang, Q.; Hong, J.-m. Fe0/C-bentonite alginate beads and oyster shell fixed-bed column combined process to continuously remove N-acetyl-p-aminophenol in persulfate system. J. Ind. Eng. Chem. 2018, 67, 301–311. [Google Scholar] [CrossRef]
- Pi, Z.; Li, X.; Wang, D.; Xu, Q.; Tao, Z.; Huang, X.; Yao, F.; Wu, Y.; He, L.; Yang, Q. Persulfate activation by oxidation biochar supported magnetite particles for tetracycline removal: Performance and degradation pathway. J. Clean. Prod. 2019, 235, 1103–1115. [Google Scholar] [CrossRef]
- Ledjeri, A.; Yahiaoui, I.; Kadji, H.; Aissani-Benissad, F.; Amrane, A.; Fourcade, F. Combination of the Electro/Fe3+/peroxydisulfate (PDS) process with activated sludge culture for the degradation of sulfamethazine. Environ. Toxicol. Pharmacol. 2017, 53, 34–39. [Google Scholar] [CrossRef]
- Kim, D.-G.; Ko, S.-O. Effects of thermal modification of a biochar on persulfate activation and mechanisms of catalytic degradation of a pharmaceutical. Chem. Eng. J. 2020, 399, 125377. [Google Scholar] [CrossRef]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, D.; Zhang, R.; Ding, Y.; Ren, Z.; Fu, M.; Cao, X.; Zeng, G. Singlet oxygen-dominated activation of peroxymonosulfate by passion fruit shell derived biochar for catalytic degradation of tetracycline through a non-radical oxidation pathway. J. Hazard. Mater. 2021, 419, 126495. [Google Scholar] [CrossRef]
- He, L.; Yang, S.; Shen, S.; Ma, Y.; Chen, Y.; Xue, J.; Wang, J.; Zheng, L.; Wu, L.; Zhang, Z.; et al. Novel insights into the mechanism of periodate activation by heterogeneous ultrasonic-enhanced sludge biochar: Relevance for efficient degradation of levofloxacin. J. Hazard. Mater. 2022, 434, 128860. [Google Scholar] [CrossRef]
- Gholami, P.; Khataee, A.; Soltani, R.D.C.; Dinpazhoh, L.; Bhatnagar, A. Photocatalytic degradation of gemifloxacin antibiotic using Zn-Co-LDH@biochar nanocomposite. J. Hazard. Mater. 2020, 382, 121070. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Removal of emerging pharmaceutical contaminants by adsorption in a fixed-bed column: A review. Ecotoxicol. Environ. Saf. 2018, 149, 257–266. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Liang, J.; He, Z. Electrolysis-assisted recovery of reverse-fluxed solutes in forward osmosis. Desalination 2021, 520, 115346. [Google Scholar] [CrossRef]
- Xing, H.; Hu, P.; Li, S.; Zuo, Y.; Han, J.; Hua, X.; Wang, K.; Yang, F.; Feng, P.; Chang, T. Adsorption and diffusion of oxygen on metal surfaces studied by first-principle study: A review. J. Mater. Sci. Technol. 2021, 62, 180–194. [Google Scholar] [CrossRef]
- Sun, D.; Li, F.; Jin, J.; Khan, S.; Eltohamy, K.M.; He, M.; Liang, X. Qualitative and quantitative investigation on adsorption mechanisms of Cd(II) on modified biochar derived from co-pyrolysis of straw and sodium phytate. Sci. Total Environ. 2022, 829, 154599. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Braga, M.C.B. Adsorption of organic and inorganic pollutants onto biochars: Challenges, operating conditions, and mechanisms. Bioresour. Technol. Rep. 2021, 15, 100728. [Google Scholar] [CrossRef]
- Cheng, G.; Tan, B.; Zhang, Z.; Fu, S.; Haiyan, W.; Wang, F. Characteristics of coal-oxygen chemisorption at the low-temperature oxidation stage: DFT and experimental study. Fuel 2022, 315, 123120. [Google Scholar] [CrossRef]
- Liu, N.; Yu, F.; Wang, Y.; Ma, J. Effects of environmental aging on the adsorption behavior of antibiotics from aqueous solutions in microplastic-graphene coexisting systems. Sci. Total Environ. 2022, 806, 150956. [Google Scholar] [CrossRef]
- Guo, R.; Xi, B.; Guo, C.; Cheng, X.; Lv, N.; Liu, W.; Borthwick, A.G.L.; Xu, J. Persulfate-based advanced oxidation processes: The new hope brought by nanocatalyst immobilization. Environ. Funct. Mater. 2022, 1, 67–91. [Google Scholar] [CrossRef]
- Tian, K.; Hu, L.; Li, L.; Zheng, Q.; Xin, Y.; Zhang, G. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, J.; Wu, D.; Zhou, Z.; Deng, Y.; Zhang, T.; Shih, K. Efficient degradation of sulfamethazine with CuCo2O4 spinel nanocatalysts for peroxymonosulfate activation. Chem. Eng. J. 2015, 280, 514–524. [Google Scholar] [CrossRef]
- Luo, R.; Li, M.; Wang, C.; Zhang, M.; Nasir Khan, M.A.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Li, J. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition. Water Res. 2019, 148, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.-f.; An, L.; Wu, D.-d. The use of carbon materials in persulfate-based advanced oxidation processes: A review. New Carbon Mater. 2020, 35, 667–683. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. Activation of peroxymonosulfate by cobalt-impregnated biochar for atrazine degradation: The pivotal roles of persistent free radicals and ecotoxicity assessment. J. Hazard. Mater. 2020, 398, 122768. [Google Scholar] [CrossRef]
- Huang, R.; Yang, J.; Cao, Y.; Dionysiou, D.D.; Wang, C. Peroxymonosulfate catalytic degradation of persistent organic pollutants by engineered catalyst of self-doped iron/carbon nanocomposite derived from waste toner powder. Sep. Purif. Technol. 2022, 291, 120963. [Google Scholar] [CrossRef]
- Ioannidi, A.; Oulego, P.; Collado, S.; Petala, A.; Arniella, V.; Frontistis, Z.; Angelopoulos, G.N.; Diaz, M.; Mantzavinos, D. Persulfate activation by modified red mud for the oxidation of antibiotic sulfamethoxazole in water. J. Environ. Manag. 2020, 270, 110820. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; Fang, L.; Nie, Y.; Tian, N.; Tian, X.; Lu, L.; Zhou, Z.; Yang, C.; Li, Y. Enhanced peroxymonosulfate activation by supported microporous carbon for degradation of tetracycline via non-radical mechanism. Sep. Purif. Technol. 2020, 240, 116617. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, P.; Xu, S.; Cheng, G.; Li, Z.; Li, Y.; Li, K.; Ma, S. Fabrication of Fe3O4 and graphitized porous biochar composites for activating peroxymonosulfate to degrade p-hydroxybenzoic acid: Insights on the mechanism. Chem. Eng. J. 2019, 375, 121980. [Google Scholar] [CrossRef]
- Al-Othman, Z.A.; Ali, R.; Naushad, M. Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: Adsorption kinetics, equilibrium and thermodynamic studies. Chem. Eng. J. 2012, 184, 238–247. [Google Scholar] [CrossRef]
- Fu, Y.; Shen, Y.; Zhang, Z.; Ge, X.; Chen, M. Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption. Sci. Total Environ. 2019, 646, 1567–1577. [Google Scholar] [CrossRef]
- Xia, D.; Tan, F.; Zhang, C.; Jiang, X.; Chen, Z.; Li, H.; Zheng, Y.; Li, Q.; Wang, Y. ZnCl2-activated biochar from biogas residue facilitates aqueous As(III) removal. Appl. Surf. Sci. 2016, 377, 361–369. [Google Scholar] [CrossRef]
- Ye, S.; Xiong, W.; Liang, J.; Yang, H.; Wu, H.; Zhou, C.; Du, L.; Guo, J.; Wang, W.; Xiang, L.; et al. Refined regulation and nitrogen doping of biochar derived from ramie fiber by deep eutectic solvents (DESs) for catalytic persulfate activation toward non-radical organics degradation and disinfection. J. Colloid Interface Sci. 2021, 601, 544–555. [Google Scholar] [CrossRef]
- Sun, F.; Chen, T.; Liu, H.; Zou, X.; Zhai, P.; Chu, Z.; Shu, D.; Wang, H.; Chen, D. The pH-dependent degradation of sulfadiazine using natural siderite activating PDS: The role of singlet oxygen. Sci. Total Environ. 2021, 784, 147117. [Google Scholar] [CrossRef]
- Sun, C.; Chen, T.; Huang, Q.; Duan, X.; Zhan, M.; Ji, L.; Li, X.; Yan, J. Selective production of singlet oxygen from zinc-etching hierarchically porous biochar for sulfamethoxazole degradation. Environ. Pollut. 2021, 290, 117991. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Wu, Q.; Chang, J.-S.; Ren, N. Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: Performance and mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Moreno-Pérez, J.; Pauletto, P.S.; Cunha, A.M.; Bonilla-Petriciolet, Á.; Salau, N.P.G.; Dotto, G.L. Three-dimensional mass transport modeling of pharmaceuticals adsorption inside ZnAl/biochar composite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126170. [Google Scholar] [CrossRef]
- Song, H.; Li, Q.; Ye, Y.; Pan, F.; Zhang, D.; Xia, D. Degradation of cephalexin by persulfate activated with magnetic loofah biochar: Performance and mechanism. Sep. Purif. Technol. 2021, 272, 118971. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Wang, M.; Kang, J.; Zhang, J.; Liu, S.; Tang, Y.; Li, S. Peroxymonosulfate activation by cobalt particles embedded into biochar for levofloxacin degradation: Efficiency, stability, and mechanism. Sep. Purif. Technol. 2022, 294, 121082. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, B.; Zhang, H.; Ma, J.; Mu, B.; Zhang, W. A novel Biochar modified by Chitosan-Fe/S for tetracycline adsorption and studies on site energy distribution. Bioresour. Technol. 2019, 294, 122152. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.; Shi, J.; Luo, X. Rapid and efficient adsorption of tetracycline from aqueous solution in a wide pH range by using iron and aminoacetic acid sequentially modified hierarchical porous biochar. Bioresour. Technol. 2022, 346, 126672. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, J.; Cui, Q.; Zhang, W.; Zhu, X. A feasible biochar derived from biogas residue and its application in the efficient adsorption of tetracycline from an aqueous solution. Environ. Res. 2022, 207, 112175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Wu, M.; Pang, Y.; Hao, Z.; Hu, M.; Qiu, R.; Chen, Z. Enhanced adsorption of tetracycline by an iron and manganese oxides loaded biochar: Kinetics, mechanism and column adsorption. Bioresour. Technol. 2021, 320, 124264. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; He, W.; Ma, H. Enhanced adsorption of tetracycline by the modified tea-based biochar with the developed mesoporous and surface alkalinity. Bioresour. Technol. 2021, 342, 126001. [Google Scholar] [CrossRef]
- Liu, Q.; Li, D.; Cheng, H.; Cheng, J.; Du, K.; Hu, Y.; Chen, Y. High mesoporosity phosphorus-containing biochar fabricated from Camellia oleifera shells: Impressive tetracycline adsorption performance and promotion of pyrophosphate-like surface functional groups (C-O-P bond). Bioresour. Technol. 2021, 329, 124922. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R.; Wang, H. Superior fenton-like degradation of tetracycline by iron loaded graphitic carbon derived from microplastics: Synthesis, catalytic performance, and mechanism. Sep. Purif. Technol. 2021, 270, 118773. [Google Scholar] [CrossRef]
- Kang, X.; Jia, X.; Kang, Z.; Zhang, Y.; Zhang, D.; Wei, J.; Guo, A.; Ge, M.; He, Z. Activation of peroxydisulfate by black fungus-derived N-doped biochar for tetracycline degradation via non-radical dominated oxidation pathway. Surf. Interfaces 2022, 31, 102007. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 132387. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Zheng, S.; Xu, Z.; Zhu, D. Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes. Langmuir 2009, 25, 11608–11613. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K. Single and competitive sorption properties and mechanism of functionalized biochar for removing sulfonamide antibiotics from water. Chem. Eng. J. 2017, 311, 348–358. [Google Scholar] [CrossRef]
- Yang, W.; Lu, Y.; Zheng, F.; Xue, X.; Li, N.; Liu, D. Adsorption behavior and mechanisms of norfloxacin onto porous resins and carbon nanotube. Chem. Eng. J. 2012, 179, 112–118. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K.; Gao, W. Synthesis of magnetic biochar from pine sawdust via oxidative hydrolysis of FeCl2 for the removal sulfamethoxazole from aqueous solution. J. Hazard. Mater. 2017, 321, 868–878. [Google Scholar] [CrossRef]
- Reguyal, F.; Sarmah, A.K. Adsorption of sulfamethoxazole by magnetic biochar: Effects of pH, ionic strength, natural organic matter and 17alpha-ethinylestradiol. Sci. Total Environ. 2018, 628–629, 722–730. [Google Scholar] [CrossRef]
- Du, L.; Xu, W.; Liu, S.; Li, X.; Huang, D.; Tan, X.; Liu, Y. Activation of persulfate by graphitized biochar for sulfamethoxazole removal: The roles of graphitic carbon structure and carbonyl group. J. Colloid Interface Sci. 2020, 577, 419–430. [Google Scholar] [CrossRef]
- Qi, Y.; Ge, B.; Zhang, Y.; Jiang, B.; Wang, C.; Akram, M.; Xu, X. Three-dimensional porous graphene-like biochar derived from Enteromorpha as a persulfate activator for sulfamethoxazole degradation: Role of graphitic N and radicals transformation. J. Hazard. Mater. 2020, 399, 123039. [Google Scholar] [CrossRef]
- Ji, Y.; Fan, Y.; Liu, K.; Kong, D.; Lu, J. Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds. Water Res. 2015, 87, 1–9. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, Z.; Xu, Y.; Tian, J.; Qi, H.; Lin, W.; Cui, F. Oxidation of sulfamethoxazole (SMX) by chlorine, ozone and permanganate—A comparative study. J. Hazard. Mater. 2014, 274, 258–269. [Google Scholar] [CrossRef]
- Liu, L.; Lin, S.; Zhang, W.; Farooq, U.; Shen, G.; Hu, S. Kinetic and mechanistic investigations of the degradation of sulfachloropyridazine in heat-activated persulfate oxidation process. Chem. Eng. J. 2018, 346, 515–524. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.H.; Park, H.; Kwak, C.; Yang, J.; Kim, J.; Ryu, S.Y.; Lee, J. Role of electrostatic interactions in the adsorption of dye molecules by Ti3C2-MXenes. RSC Adv. 2021, 11, 6201–6211. [Google Scholar] [CrossRef] [PubMed]
- Boudrahem, N.; Delpeux-Ouldriane, S.; Khenniche, L.; Boudrahem, F.; Aissani-Benissad, F.; Gineys, M. Single and mixture adsorption of clofibric acid, tetracycline and paracetamol onto Activated carbon developed from cotton cloth residue. Process. Saf. Environ. Prot. 2017, 111, 544–559. [Google Scholar] [CrossRef]
- Zhou, X.; Lai, C.; Liu, S.; Li, B.; Qin, L.; Liu, X.; Yi, H.; Fu, Y.; Li, L.; Zhang, M.; et al. Activation of persulfate by swine bone derived biochar: Insight into the specific role of different active sites and the toxicity of acetaminophen degradation pathways. Sci. Total Environ. 2022, 807, 151059. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ye, Q.; Gan, J. Degradation and transformation products of acetaminophen in soil. Water Res. 2014, 49, 44–52. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Villota, N.; Lomas, J.M.; Camarero, L.M. Study of the paracetamol degradation pathway that generates color and turbidity in oxidized wastewaters by photo-Fenton technology. J. Photochem. Photobiol. A 2016, 329, 113–119. [Google Scholar] [CrossRef]
- Yang, M.T.; Du, Y.; Tong, W.C.; Yip, A.C.K.; Lin, K.A. Cobalt-impregnated biochar produced from CO2-mediated pyrolysis of Co/lignin as an enhanced catalyst for activating peroxymonosulfate to degrade acetaminophen. Chemosphere 2019, 226, 924–933. [Google Scholar] [CrossRef]
- Zhuo, S.-N.; Ren, H.-Y.; Cao, G.-L.; Xie, G.-J.; Xing, D.-F.; Ren, N.-Q.; Liu, B.-F. Highly efficient activation of persulfate by encapsulated nano-Fe0 biochar for acetaminophen degradation: Rich electron environment and dominant effect of superoxide radical. Chem. Eng. J. 2022, 440, 135947. [Google Scholar] [CrossRef]
- Shirani, Z.; Song, H.; Bhatnagar, A. Efficient removal of diclofenac and cephalexin from aqueous solution using Anthriscus sylvestris-derived activated biochar. Sci. Total Environ. 2020, 745, 140789. [Google Scholar] [CrossRef]
- Al-Gheethi, A.; Noman, E.; Saphira Radin Mohamed, R.M.; Talip, B.; Vo, D.N.; Algaifi, H.A. Cephalexin removal by a novel Cu-Zn bionanocomposite biosynthesized in secondary metabolic products of Aspergillus arenarioides EAN603 with pumpkin peels medium: Optimization, kinetic and artificial neural network models. J. Hazard. Mater. 2021, 419, 126500. [Google Scholar] [CrossRef]
- Naghipour, D.; Amouei, A.; Estaji, M.; Taghavi, K.; Allahabadi, A. Cephalexin adsorption from aqueous solutions by biochar prepared from plantain wood: Equilibrium and kinetics studies. Desalin. Water Treat. 2019, 143, 374–381. [Google Scholar] [CrossRef]
- Ali Noman, E.; Al-Gheethi, A.; Saphira Radin Mohamed, R.M.; Talip, B.A.; Hossain, M.S.; Ali Hamood Altowayti, W.; Ismail, N. Sustainable approaches for removal of cephalexin antibiotic from non-clinical environments: A critical review. J. Hazard. Mater. 2021, 417, 126040. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, A.; Ren, S.; Wen, Z.; Tian, X.; Li, D.; Li, J. Effect of surface properties of activated carbon fiber cathode on mineralization of antibiotic cefalexin by electro-Fenton and photoelectro-Fenton treatments: Mineralization, kinetics and oxidation products. Chemosphere 2019, 221, 423–432. [Google Scholar] [CrossRef]
- Droguett, C.; Salazar, R.; Brillas, E.; Sires, I.; Carlesi, C.; Marco, J.F.; Thiam, A. Treatment of antibiotic cephalexin by heterogeneous electrochemical Fenton-based processes using chalcopyrite as sustainable catalyst. Sci. Total Environ. 2020, 740, 140154. [Google Scholar] [CrossRef]
- Wang, Z.; Jang, H.M. Comparative study on characteristics and mechanism of levofloxacin adsorption on swine manure biochar. Bioresour. Technol. 2022, 351, 127025. [Google Scholar] [CrossRef]
- Xu, Z.; Xiang, Y.; Zhou, H.; Yang, J.; He, Y.; Zhu, Z.; Zhou, Y. Manganese ferrite modified biochar from vinasse for enhanced adsorption of levofloxacin: Effects and mechanisms. Environ. Pollut. 2021, 272, 115968. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, X.; Xu, Z.; Hu, W.; Zhou, Y.; Wan, Z.; Yang, Y.; Wei, Y.; Yang, J.; Tsang, D.C.W. Fabrication of sustainable manganese ferrite modified biochar from vinasse for enhanced adsorption of fluoroquinolone antibiotics: Effects and mechanisms. Sci. Total Environ. 2020, 709, 136079. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Luo, Z.; Du, S.; Yang, J.; Zhi, D.; Zhou, Y. Sustainable biochar/MgFe2O4 adsorbent for levofloxacin removal: Adsorption performances and mechanisms. Bioresour. Technol. 2021, 340, 125698. [Google Scholar] [CrossRef] [PubMed]
- Abukhadra, M.R.; Fathallah, W.; El Kashief, F.A.; El-Sherbeeny, A.M.; El-Meligy, M.A.; Awwad, E.M.; Luqman, M. Insight into the antimicrobial and photocatalytic properties of NiO impregnated MCM-48 for effective removal of pathogenic bacteria and toxic levofloxacin residuals. Microporous Mesoporous Mater. 2021, 312, 110769. [Google Scholar] [CrossRef]
- Hu, Z.; Ge, M.; Guo, C. Efficient removal of levofloxacin from different water matrices via simultaneous adsorption and photocatalysis using a magnetic Ag3PO4/rGO/CoFe2O4 catalyst. Chemosphere 2021, 268, 128834. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Lu, S.; Wang, Z.; Wang, Y.; Zhang, G.; Guo, X.; Guo, W.; Zhang, T.; Xi, B. Degradation difference of ofloxacin and levofloxacin by UV/H2O2 and UV/PS (persulfate): Efficiency, factors and mechanism. Chem. Eng. J. 2020, 385, 123987. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Guo, H.; Zhang, L.; Liang, C.; Zeng, G.-M. Photocatalytic degradation of levofloxacin by ternary Ag2CO3/CeO2/AgBr photocatalyst under visible-light irradiation: Degradation pathways, mineralization ability, and an accelerated interfacial charge transfer process study. J. Catal. 2018, 358, 211–223. [Google Scholar] [CrossRef]
- Luo, J.; Yi, Y.; Ying, G.; Fang, Z.; Zhang, Y. Activation of persulfate for highly efficient degradation of metronidazole using Fe(II)-rich potassium doped magnetic biochar. Sci. Total Environ. 2022, 819, 152089. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.N.; Wang, L. Metal-free activation of persulfates by corn stalk biochar for the degradation of antibiotic norfloxacin: Activation factors and degradation mechanism. Chemosphere 2019, 237, 124454. [Google Scholar] [CrossRef]
- Dong, F.-X.; Yan, L.; Huang, S.-T.; Liang, J.-Y.; Zhang, W.-X.; Yao, X.-W.; Chen, X.; Qian, W.; Guo, P.-R.; Kong, L.-J.; et al. Removal of antibiotics sulfadiazine by a biochar based material activated persulfate oxidation system: Performance, products and mechanism. Process. Saf. Environ. Prot. 2022, 157, 411–419. [Google Scholar] [CrossRef]
- Liang, J.; Xu, X.; Zhong, Q.; Xu, Z.; Zhao, L.; Qiu, H.; Cao, X. Roles of the mineral constituents in sludge-derived biochar in persulfate activation for phenol degradation. J. Hazard. Mater. 2020, 398, 122861. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Gan, S.; He, H.; Cai, N.; Xu, J.; Guo, P.; Chen, B.; Pan, X. Effective degradation of COVID-19 related drugs by biochar-supported red mud catalyst activated persulfate process: Mechanism and pathway. J. Clean. Prod. 2022, 340, 130753. [Google Scholar] [CrossRef]
- Tian, W.; Lin, J.; Zhang, H.; Duan, X.; Sun, H.; Wang, H.; Wang, S. Enhanced removals of micropollutants in binary organic systems by biomass derived porous carbon/peroxymonosulfate. J. Hazard. Mater. 2021, 408, 124459. [Google Scholar] [CrossRef]
- Naima, A.; Ammar, F.; Abdelkader, O.; Rachid, C.; Lynda, H.; Syafiuddin, A.; Boopathy, R. Development of a novel and efficient biochar produced from pepper stem for effective ibuprofen removal. Bioresour. Technol. 2022, 347, 126685. [Google Scholar] [CrossRef]
- Zheng, X.; He, X.; Peng, H.; Wen, J.; Lv, S. Efficient adsorption of ciprofloxacin using Ga2S3/S-modified biochar via the high-temperature sulfurization. Bioresour. Technol. 2021, 334, 125238. [Google Scholar] [CrossRef]
- He, X.; Li, J.; Meng, Q.; Guo, Z.; Zhang, H.; Liu, Y. Enhanced adsorption capacity of sulfadiazine on tea waste biochar from aqueous solutions by the two-step sintering method without corrosive activator. J. Environ. Chem. Eng. 2021, 9, 104898. [Google Scholar] [CrossRef]
- Tomul, F.; Arslan, Y.; Kabak, B.; Trak, D.; Kenduzler, E.; Lima, E.C.; Tran, H.N. Peanut shells-derived biochars prepared from different carbonization processes: Comparison of characterization and mechanism of naproxen adsorption in water. Sci. Total Environ. 2020, 726, 137828. [Google Scholar] [CrossRef]
- Escudero-Curiel, S.; Penelas, U.; Sanroman, M.A.; Pazos, M. An approach towards Zero-Waste wastewater technology: Fluoxetine adsorption on biochar and removal by the sulfate radical. Chemosphere 2021, 268, 129318. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Z.; Xu, G.; Li, G. Pyrolysis of penicillin fermentation residue and sludge to produce biochar: Antibiotic resistance genes destruction and biochar application in the adsorption of penicillin in water. J. Hazard. Mater. 2021, 413, 125385. [Google Scholar] [CrossRef]
- Chayid, M.A.; Ahmed, M.J. Amoxicillin adsorption on microwave prepared activated carbon from Arundo donax Linn: Isotherms, kinetics, and thermodynamics studies. J. Environ. Chem. Eng. 2015, 3, 1592–1601. [Google Scholar] [CrossRef]
- Ushani, U.; Lu, X.; Wang, J.; Zhang, Z.; Dai, J.; Tan, Y.; Wang, S.; Li, W.; Niu, C.; Cai, T.; et al. Sulfate radicals-based advanced oxidation technology in various environmental remediation: A state-of-the–art review. Chem. Eng. J. 2020, 402, 126232. [Google Scholar] [CrossRef]
- Fan, X.; Li, S.; Sun, M.; Song, C.; Xiao, J.; Du, J.; Tao, P.; Sun, T.; Shao, M.; Wang, T. Degradation of phenol by coal-based carbon membrane integrating sulfate radicals-based advanced oxidation processes. Ecotoxicol. Environ. Saf. 2019, 185, 109662. [Google Scholar] [CrossRef]
- Ersan, M.; Dogan, H. Development of new adsorbents via microwave treatment magnetic PET synthesis from waste PET and investigation of TC removal. Colloid Interface Sci. Commun. 2021, 42, 100416. [Google Scholar] [CrossRef]
- Lemaire, J.; Buès, M.; Kabeche, T.; Hanna, K.; Simonnot, M.-O. Oxidant selection to treat an aged PAH contaminated soil by in situ chemical oxidation. J. Environ. Chem. Eng. 2013, 1, 1261–1268. [Google Scholar] [CrossRef]
- Sun, Y.; Bian, J.; Zhu, Q. Sulfamethoxazole removal of adsorption by carbon—Doped boron nitride in water. J. Mol. Liq. 2022, 349, 118216. [Google Scholar] [CrossRef]
- Yang, D.; Li, J.; Luo, L.; Deng, R.; He, Q.; Chen, Y. Exceptional levofloxacin removal using biochar-derived porous carbon sheets: Mechanisms and density-functional-theory calculation. Chem. Eng. J. 2020, 387, 124103. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Huang, G.; Yang, C.; Chen, C.; Zhou, T.; Zhao, Y.; Ma, J. Adsorption behavior of the antibiotic levofloxacin on microplastics in the presence of different heavy metals in an aqueous solution. Chemosphere 2020, 260, 127650. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Ghanam, A.M.; Mohamed, R.H.A.; Saad, S.R. Enhanced adsorption of Levofloxacin and Ceftriaxone antibiotics from water by assembled composite of nanotitanium oxide/chitosan/nano-bentonite. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110199. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Bai, X.; Li, Y.; Yang, B.; Yang, P.; Yu, F.; Ma, J. HKUST-1 derived carbon adsorbents for tetracycline removal with excellent adsorption performance. Environ. Res. 2022, 205, 112425. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Sharma, N.; Mann, A. Removal of ofloxacin hydrochloride and paracetamol from aqueous solutions: Binary mixtures and competitive adsorption. Mater. Today Proc. 2020, 28, 1514–1519. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Zhu, Z.; Cui, F.; Zhu, Y.-A.; Duan, X.; Wang, S. Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: Integrated experimental and theoretical investigations for mechanism and application. Chem. Eng. J. 2018, 354, 507–516. [Google Scholar] [CrossRef]
- Yang, S.; Wang, P.; Yang, X.; Shan, L.; Zhang, W.; Shao, X.; Niu, R. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide. J. Hazard. Mater. 2010, 179, 552–558. [Google Scholar] [CrossRef]
- Geng, G.; Gao, Y.; Zhang, Z.; Gao, K.; Zhang, W.; Song, J. Renewable and robust biomass waste-derived Co-doped carbon aerogels for PMS activation: Catalytic mechanisms and phytotoxicity assessment. Ecotoxicol. Environ. Saf. 2021, 220, 112381. [Google Scholar] [CrossRef]
- Jiang, M.; Lu, J.; Ji, Y.; Kong, D. Bicarbonate-activated persulfate oxidation of acetaminophen. Water Res. 2017, 116, 324–331. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Chen, D.; Dai, G.; Wei, D.; Shu, Y. Activation of persulfate with biochar for degradation of bisphenol A in soil. Chem. Eng. J. 2020, 381, 122637. [Google Scholar] [CrossRef]
- Peiris, C.; Gunatilake, S.R.; Mlsna, T.E.; Mohan, D.; Vithanage, M. Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: A critical review. Bioresour. Technol. 2017, 246, 150–159. [Google Scholar] [CrossRef]
- Reza Samarghandi, M.; Tari, K.; Shabanloo, A.; Salari, M.; Zolghadr Nasab, H. Synergistic degradation of acid blue 113 dye in a thermally activated persulfate (TAP)/ZnO-GAC oxidation system: Degradation pathway and application for real textile wastewater. Sep. Purif. Technol. 2020, 247, 116931. [Google Scholar] [CrossRef]
- Mei, Y.; Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H. Effect of Fe-N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour. Technol. 2021, 325, 124732. [Google Scholar] [CrossRef]
- Xiong, S.; Deng, Y.; Gong, D.; Tang, R.; Zheng, J.; Li, L.; Zhou, Z.; Su, L.; Liao, C.; Yang, L. Magnetically modified in-situ N-doped Enteromorpha prolifera derived biochar for peroxydisulfate activation: Electron transfer induced singlet oxygen non-radical pathway. Chemosphere 2021, 284, 131404. [Google Scholar] [CrossRef]
- Miserli, K.; Kogola, D.; Paraschoudi, I.; Konstantinou, I. Activation of persulfate by biochar for the degradation of phenolic compounds in aqueous systems. Chem. Eng. J. Adv. 2022, 9, 100201. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Yin, R.; Du, J.; Wu, Q.; Luo, H.; Liu, B.; Sseguya, F.; Ren, N. Biochar-induced Fe(III) reduction for persulfate activation in sulfamethoxazole degradation: Insight into the electron transfer, radical oxidation and degradation pathways. Chem. Eng. J. 2019, 362, 561–569. [Google Scholar] [CrossRef]
- Li, Q.; Tang, Y.; Zhou, B.; Zhou, J.; Shi, B. Resource utilization of tannery sludge to prepare biochar as persulfate activators for highly efficient degradation of tetracycline. Bioresour. Technol. 2022, 358, 127417. [Google Scholar] [CrossRef]
- Zhong, Q.; Lin, Q.; He, W.; Fu, H.; Huang, Z.; Wang, Y.; Wu, L. Study on the nonradical pathways of nitrogen-doped biochar activating persulfate for tetracycline degradation. Sep. Purif. Technol. 2021, 276, 119354. [Google Scholar] [CrossRef]
- Shen, B.; Liu, Y.; Liu, S.; Tan, X.; Zhang, P.; Du, L.; Wen, J. Catalytic degradation of sulfamethoxazole by persulfate activated with magnetic graphitized biochar: Multiple mechanisms and variables effects. Process. Saf. Environ. Prot. 2020, 144, 143–157. [Google Scholar] [CrossRef]
- Wang, W.; Chen, M. Catalytic degradation of sulfamethoxazole by peroxymonosulfate activation system composed of nitrogen-doped biochar from pomelo peel: Important roles of defects and nitrogen, and detoxification of intermediates. J. Colloid Interface Sci. 2022, 613, 57–70. [Google Scholar] [CrossRef]
- Yao, B.; Luo, Z.; Du, S.; Yang, J.; Zhi, D.; Zhou, Y. Magnetic MgFe2O4/biochar derived from pomelo peel as a persulfate activator for levofloxacin degradation: Effects and mechanistic consideration. Bioresour. Technol. 2022, 346, 126547. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, B.; Guo, Y.; Guo, Y.; Zheng, Z.; Pak, T.; Li, G. Effect of hydrothermal pretreatment on pyrolyzed sludge biochars for tetracycline adsorption. J. Environ. Chem. Eng. 2021, 9, 106557. [Google Scholar] [CrossRef]
- Zeng, S.; Choi, Y.K.; Kan, E. Iron-activated bermudagrass-derived biochar for adsorption of aqueous sulfamethoxazole: Effects of iron impregnation ratio on biochar properties, adsorption, and regeneration. Sci. Total Environ. 2021, 750, 141691. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, X.; Chen, B.; Ma, J.; Niu, X.; Zhang, D.; Lin, Z.; Fu, M.; Zhou, S. Enhanced adsorption of sulfamethoxazole from aqueous solution by Fe-impregnated graphited biochar. J. Clean. Prod. 2020, 256, 120662. [Google Scholar] [CrossRef]
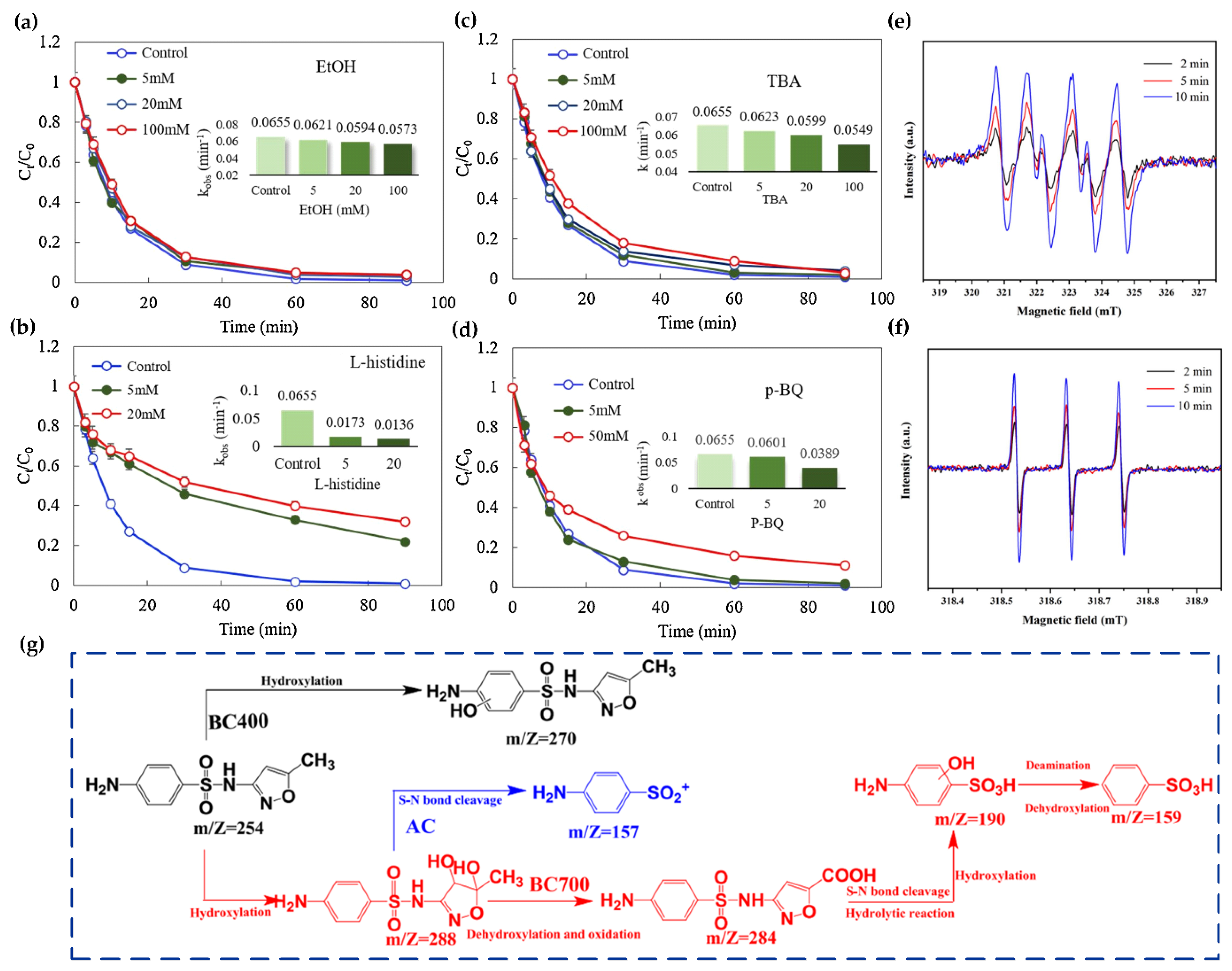
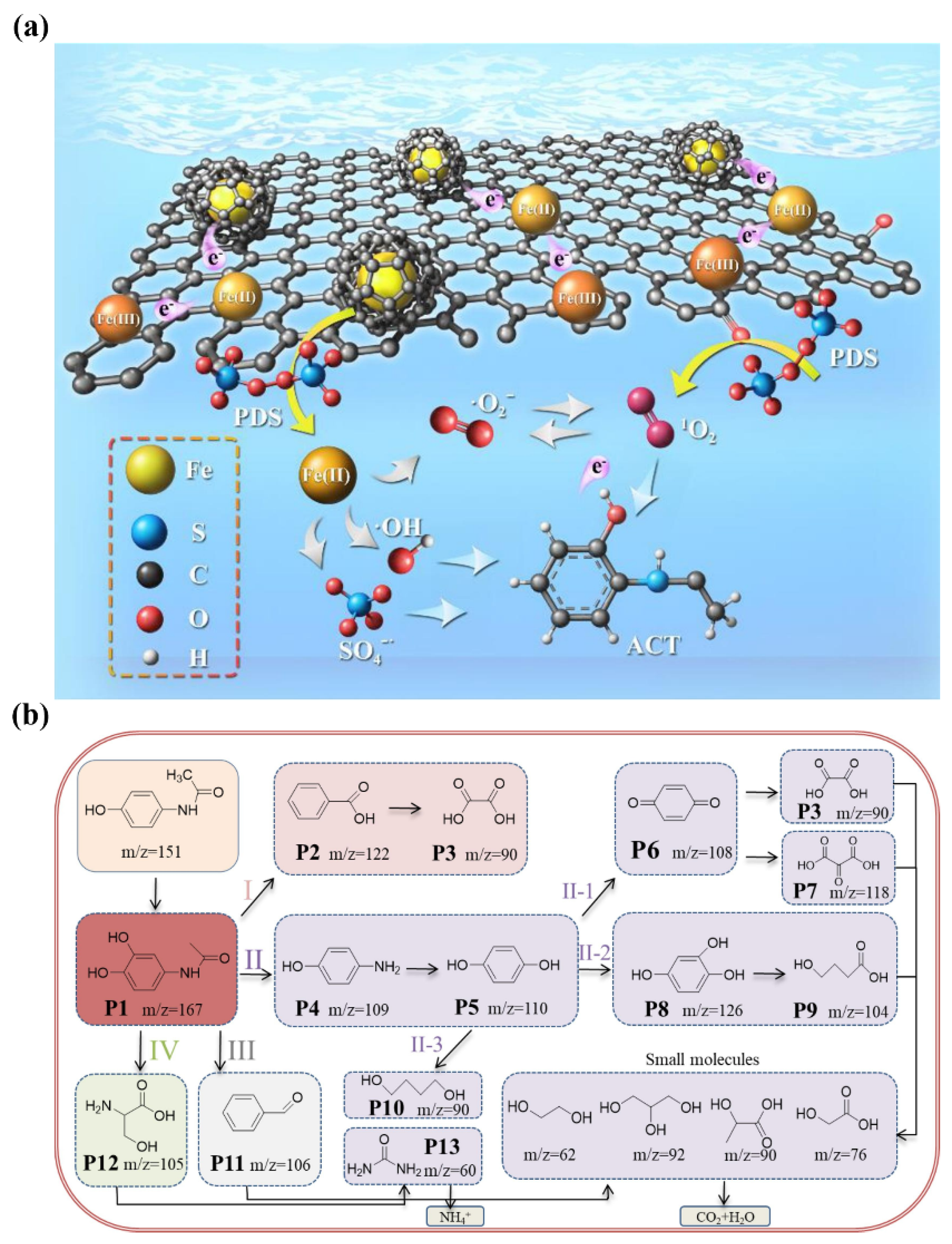
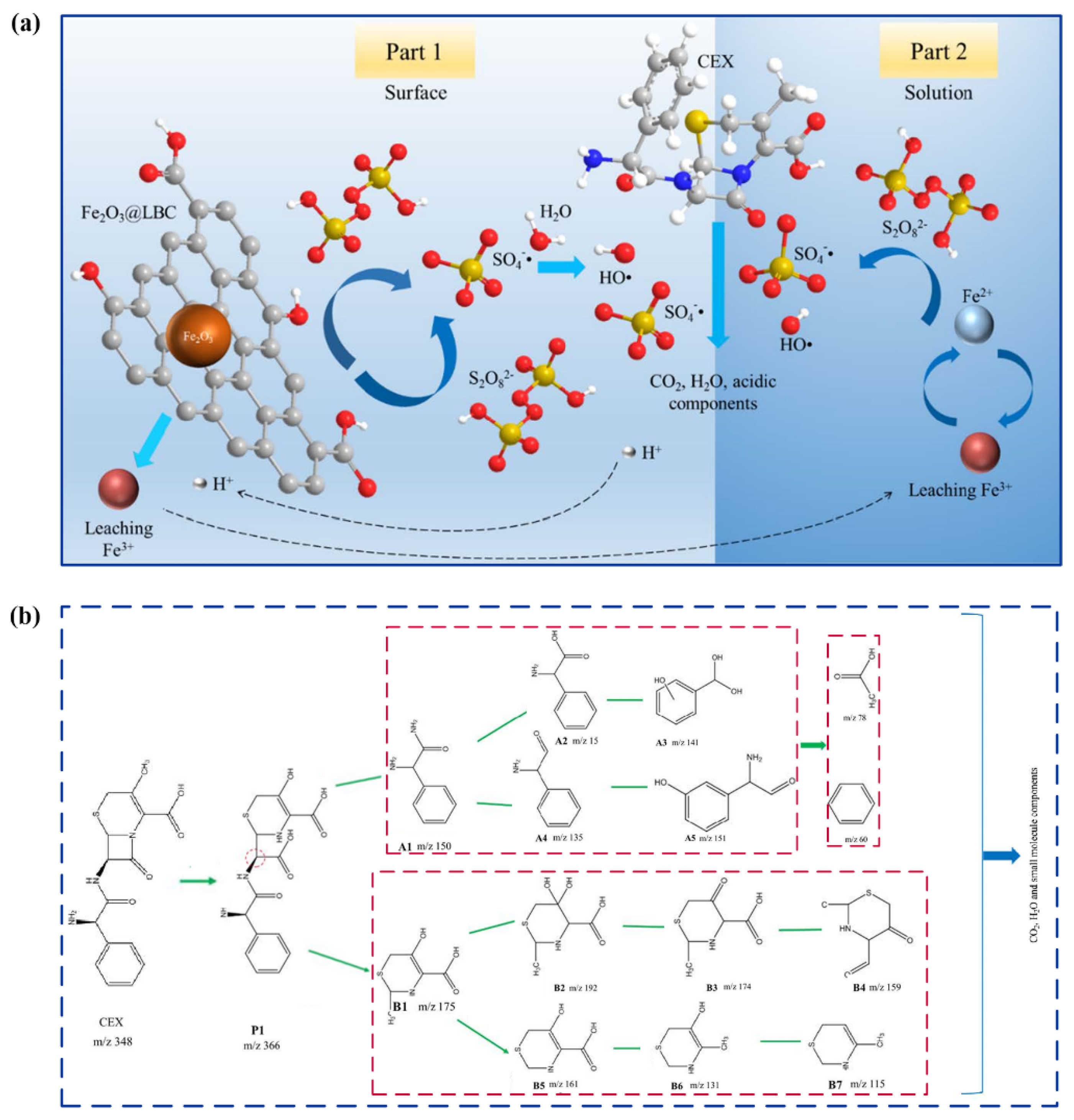
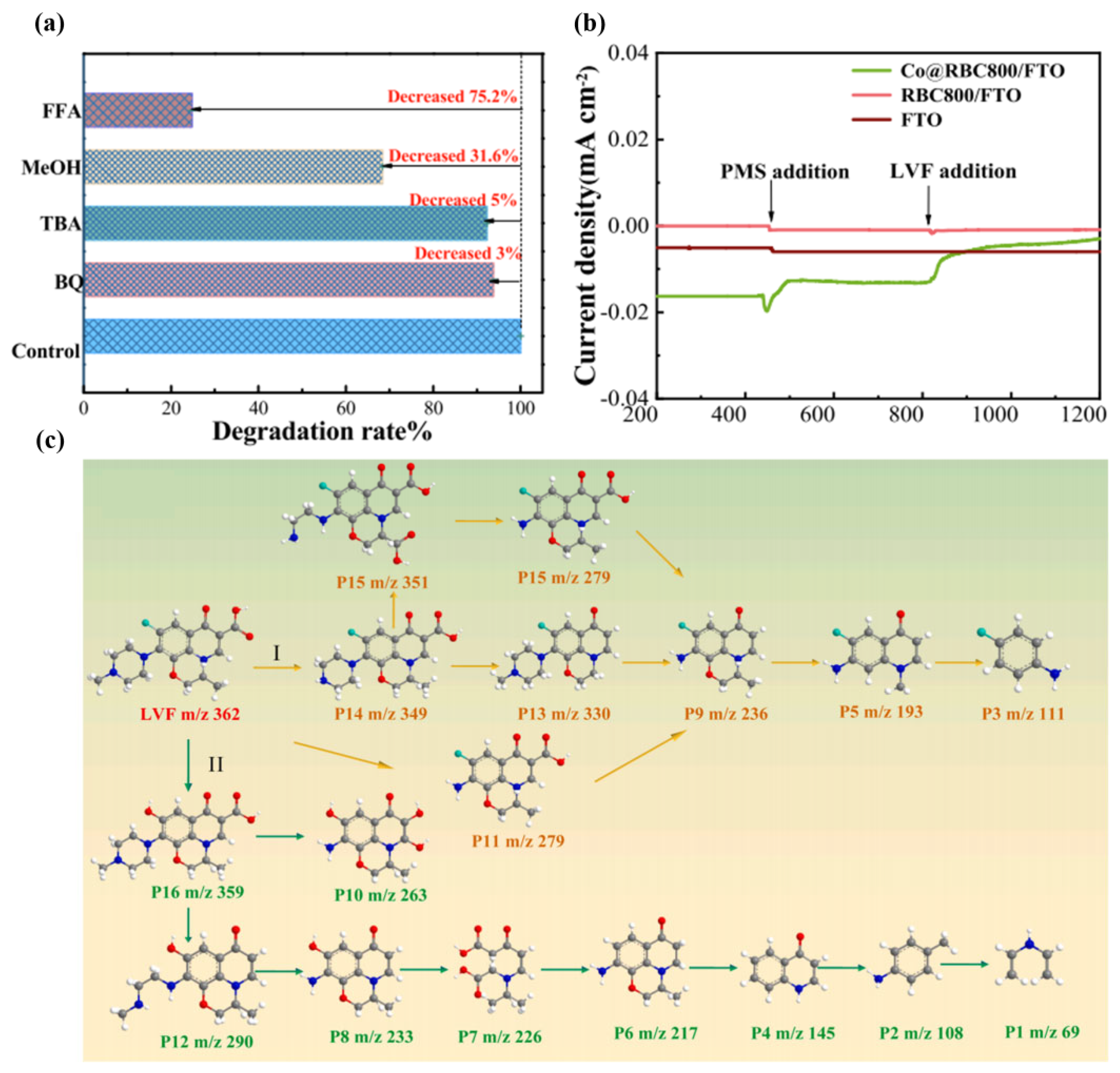
| Target Pollutants | Formula | Molecular Weight (g/mol) | Molar Volume | Solubility (mg/mL) | Application | Ref. |
|---|---|---|---|---|---|---|
| TC | C22H24N2O8 | 444.43 | 270.3 m3/mol | 1.7 | For rickettsial disease, mycoplasma infection, chlamydia infection, etc., caused by sensitive microorganisms | [36] |
| SMX | C10H11N3O3S | 253.28 | 173.1 cm3/mol | Almost insoluble in water | For respiratory system infection, intestinal infection, and wound infection caused by sensitive bacteria, etc. | [18] |
| ACT | C8H9NO2 | 151.16 | 120.9 cm3/mol | 14 | Used to relieve both fever and pain of various causes | [63] |
| CPX | C16H17N3O4S·H2O | 365.4 | 231.3 m3/mol | 13.5 | Used to treat multiple site infections caused by sensitive bacteria | [64] |
| LEV | C18H20FN3O4 | 361.37 | 243.9 cm3/mol | 50 | For the treatment of respiratory tract infections caused by bacteria, mycoplasma, etc. | [65] |
| Target Pollutants | Catalyst | Catalyst Dosage (g/L) | Pollutant Concentration (mg/L) | Persulfate Dosage (mM) | pH | Reaction Temperature (°C) | Reaction Time (min) | Vulnerable Areas | Degradation Efficiency. (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Metronidazole (MNZ) | Potassium-doped magnetic biochar (KMBC) | 0.5 | 20.0 | 1.0 | 6.5 | 25 | 180 | C–N, –NO2 | 98.40 | [107] |
| Norfloxacin (NOR) | Iron and nitrogen co-doped biochar material (Fe@N co-doped biochar) | 0.1 | 10.0 | 10 | 7 | 25 | 20 | the nalidixic and piperazine rings | 95 | [108] |
| Sulfadiazine (SDZ) | Biochar-based iron material (MBC) | 1.0 | 40.0 | 1.5 | 5.16 | 25 | 60 | N–H, S–N, C–S | 91.97 | [109] |
| Phenol | Demineralized biochar (DSS700) | 0.5 | 200.0 | 10 | 7 | 25 | 800 | benzene-OH | 57.80 | [110] |
| Arbidol (ARB) | Biochar-supported red mud (RM-BC) | 0.2 | 20 | 0.6 | 7 | 25 | 15 | Sulfur atom, 4-(dimethylamino) methyle group | 100 | [111] |
| Gemifloxacin (GMF) | Biochar nanocomposite (Zn-Co-LDH) | 0.75 | 15 | 20 | 5.5 | 35 | 130 | C=C, –OH, C–O, C–N | 92.7 | [38] |
| P-hydroxybenzoic acid (HBA) | Biomass-derived N-doped porous carbon (Y-PC) | 0.05 | 20 | 5.7 | 4.5 | 25 | 120 | –OH | 97.6 | [112] |
| Target Pollutants | Adsorbent | Adsorbent Dosage (g/L) | Pollutant Concentration (mg/L) | pH | Time | Isotherms/Kinetics | Adsorption Capacity (mg/g) | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Ibuprofen (IBP) | Biochar from pepper stems (PS-biochar) | 1.0 | 40 | 7 | 240 min | Langmuir/ Pseudo-second-order | 596.6 | π–π interaction, pore filling, H-bonding | [113] |
| Ciprofloxacin | Ga2S3 and sulfur co-modified biochar (Ga/S-BC) | 0.25 | 140 | 5 | 60 min | Langmuir/ Pseudo-second-order | 330.21 | Electrostatic interaction, hydrogen bonding, π–π interactions. | [114] |
| Sulfadiazine | Tea waste biochar | 0.5 | 50 | 10.97 | 720 min | Langmuir/ Pseudo-second-order | 99.47 | π–π interactions. | [115] |
| Naproxen | Peanutshells-derivedbiochars | 5.0 | 1000 | 7 | 1440 min | Langmuir/ Pseudo-second-order | 81.6 | π–π interactions. | [116] |
| Fluoxetine | Biochar | 2.3 | 50 | 7.1 | 60 min | Freundlich/Pseudo-second-order | 70 | Electrostatic attractions | [117] |
| Antibiotic fermentation residues (AFRB) | Sludge (AFSB) | 0.01 | 40 | 7 | 12 h | Pseudo-first-order | 82.6 | Aromatic structures, the chemisorption, active sites | [118] |
| Amoxicillin antibiotic (AMX). | Giant reed (AMX) | 0.5 | 250 | 7 | 400 min | Sips isotherm/ Pseudo-second-order | 345.4 | Hydrogen bonding, aromatic structures, the chemisorption, active sites | [119] |
| Target Pollutants | Catalyst | Catalyst Dosage (g/L) | Pollutant Concentration (mg/L) | Persulfate Dosage (mM) | pH | Reaction Time (min) | Degradation Efficiency. (%) | Cycle Times | Last Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| TC | biochart from tannery sludge (TSBC) | 0.3 | 50 | 3 | 7 | 60 | 99.1 | Four times | 79.8 | [141] |
| nitrogen-doped biochar (N-BCX) | 0.2 | 20 | 2 | 7 | 120 | 100 | Three times | 55 | [142] | |
| SMX | magnetic graphitized biochar (GMBC) | 0.06 | 10 | 3 | 5.04 | 60 | 99.4 | Four times | 23.1 | [143] |
| nitrogen-doped biochar from pomelo peel | 0.1 | 10 | 0.5 | 7 | 30 | 95 | Four times | 80 | [144] | |
| ACT | nanoscale zero-valent iron biochar (BC-Fe0) derived from waste lignocellulose rice | 0.5 | 10 | 1.8 | 7 | 20 | 100 | Five times | 93.8 | [92] |
| cobalt-impregnated biochar produced from CO2-mediated pyrolysis of Co/lignin | 0.05 | 5 | 0.3 | 7 | 30 | 90 | Four times | 90 | [91] | |
| CPX | the magnetic biochar (Fe2O3@LBC) derived from loofah | 0.4 | 10 | 0.4 | 7 | 200 | 73.9 | Four times | 50 | [64] |
| LEV | innovative magnetic MgFe2O4/BC (MMB) derived from pomelo peel | 0.4 | 10 | 4.2 | 5 | 240 | 87.87 | Three times | 67.9 | [145] |
| Co@RBC prepared from cobalt nanoparticles embedded in biochar | 0.2 | 10 | 0.5 | 4.5 | 10 | 100 | Five times | 100 | [65] |
| Target Pollutants | Adsorbent | Adsorbent Dosage (g/L) | Pollutant Concentration (mg/L) | pH | Time | Adsorption Capacity (mg/g) | Regeneration | Cycle Times | Last Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| TC | pyrolyzed sludge biochar | 0.2 | 20 | 7 | 24 h | 54.8 | NaOH, ethanol, and thermal treatment | Four times | 35.4 (NaOH treatment) 49.8 (thermal treatment) | [146] |
| tea residue-based biochar | 1 | 100 | 2 | 360 min | 70.8 | Ethanol treatment | Five times | 42.6 | [70] | |
| SMX | FeCl3-activated bermudagrass (BG)-derived biochar (IA-BCs) | 10 | 100 | 3 | 48 h | 280 | NaOH desorption, thermal oxidation, and Fenton oxidation | Four times | 151.2 (NaOH treatment) 128.8 (thermal treatment) | [147] |
| Fe-impregnated graphited biochar | 0.2 | 50 | 5 | 240 min | 187.31 | NaOH treatment | Three times | 34.85 | [148] | |
| ACT | ZnAl/biochar | 0.1 | 125 | 5 | 180 min | 1108.43 | NaOH treatment | Three times | 332.5 | [63] |
| CPX | anthriscus sylvestris-derived activated biochar | 0.1 | 30 | 4 | 24 h | 384.31 | NaOH treatment | Three times | 115.3 | [93] |
| LEV | biochar-supported MgFe2O4 (BMF) | 0.3 | 100 | 5 | 1400 min | 44.9 | NaOH treatment | Four times | 33.3 | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Z.; Jia, X.; Zhang, Y.; Kang, X.; Ge, M.; Liu, D.; Wang, C.; He, Z. A Review on Application of Biochar in the Removal of Pharmaceutical Pollutants through Adsorption and Persulfate-Based AOPs. Sustainability 2022, 14, 10128. https://doi.org/10.3390/su141610128
Kang Z, Jia X, Zhang Y, Kang X, Ge M, Liu D, Wang C, He Z. A Review on Application of Biochar in the Removal of Pharmaceutical Pollutants through Adsorption and Persulfate-Based AOPs. Sustainability. 2022; 14(16):10128. https://doi.org/10.3390/su141610128
Chicago/Turabian StyleKang, Ziyang, Xigai Jia, Yuchen Zhang, Xiaoxuan Kang, Ming Ge, Dong Liu, Chongqing Wang, and Zhangxing He. 2022. "A Review on Application of Biochar in the Removal of Pharmaceutical Pollutants through Adsorption and Persulfate-Based AOPs" Sustainability 14, no. 16: 10128. https://doi.org/10.3390/su141610128
APA StyleKang, Z., Jia, X., Zhang, Y., Kang, X., Ge, M., Liu, D., Wang, C., & He, Z. (2022). A Review on Application of Biochar in the Removal of Pharmaceutical Pollutants through Adsorption and Persulfate-Based AOPs. Sustainability, 14(16), 10128. https://doi.org/10.3390/su141610128







