National Policies, Recent Research Hotspots, and Application of Sustainable Energy: Case of China, USA, and European Countries
Abstract
:1. Introduction
2. Theoretical Framework
2.1. Variation in National Energy Transition Politics
2.2. Technological Innovation of Sustainable Energy
3. Research Method
3.1. Process Tracing and Case Study
3.2. Data Source
4. National Policies
4.1. The United States: From Energy Neo-Realism to Conservative Neo-Liberalism
4.2. China: Authoritarian Environmentalism
4.3. European Countries: Integrated-Multinational Negotiation
5. Recent Research Hotspots
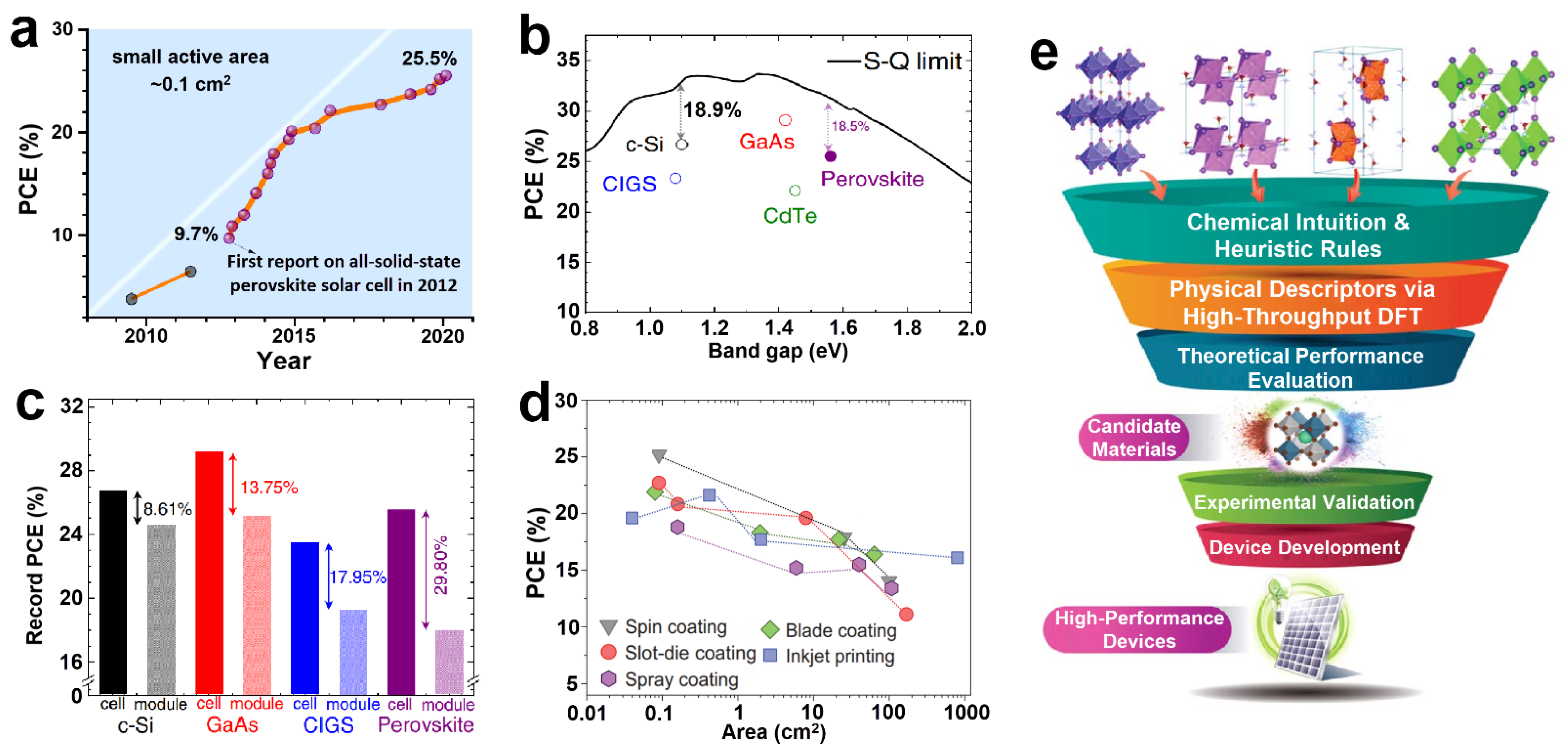
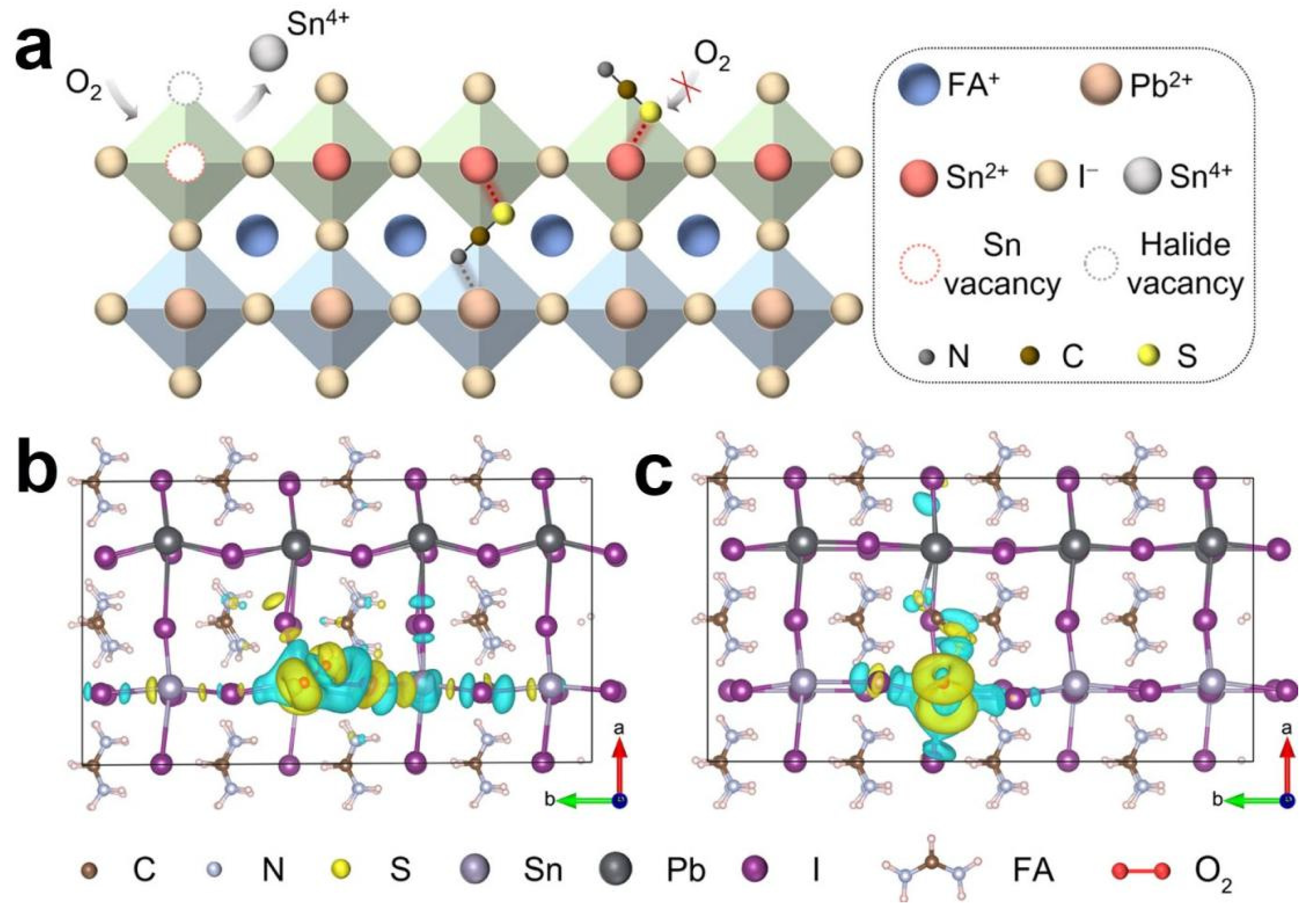
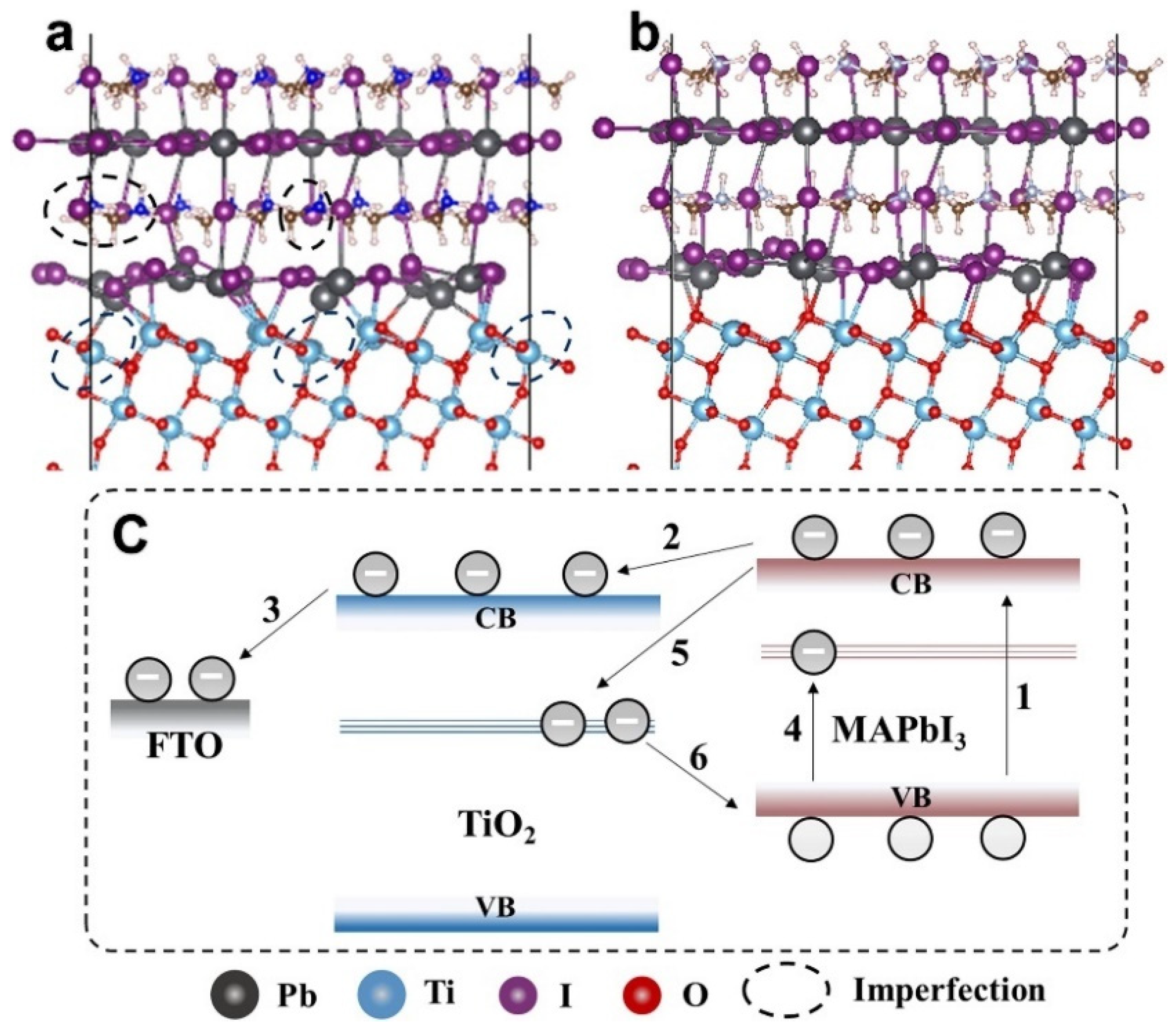
5.1. Solar Cells
5.2. Rechargeable Batteries
5.3. Hydrogen Production
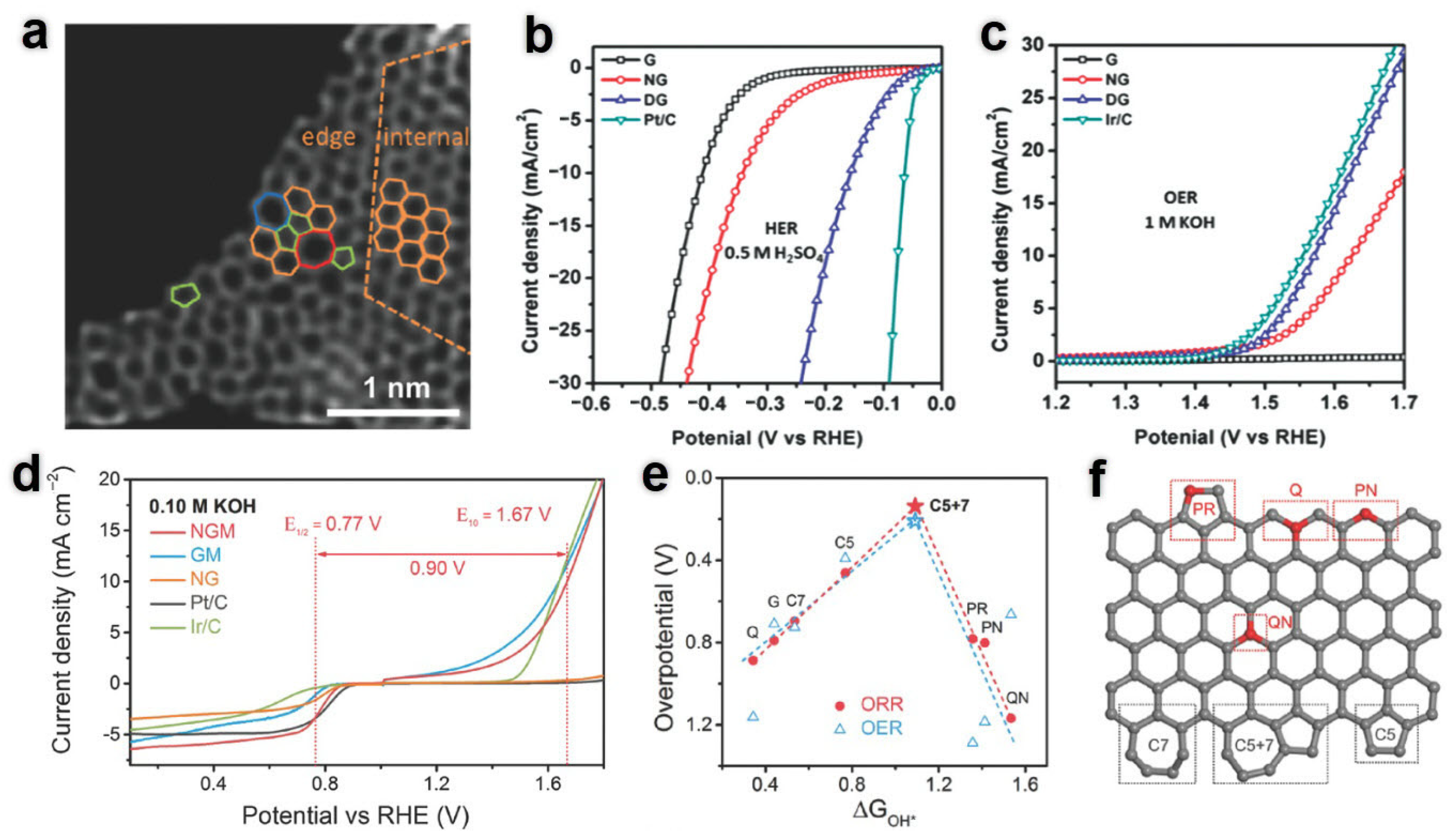
5.3.1. Noble Metal-Based Electrocatalysts
5.3.2. Transition Metal Electrocatalysts
5.3.3. Nonmetallic Electrocatalysts
6. Applications of the above Technologies
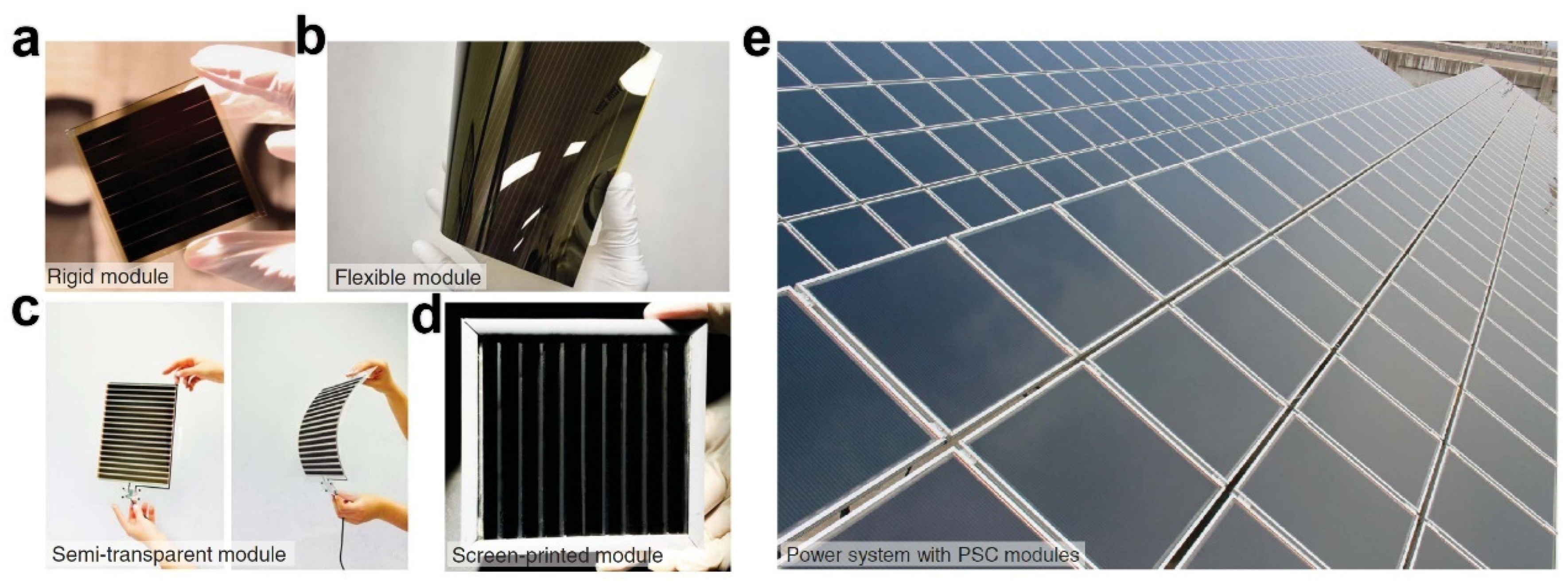
6.1. Solar Cells
6.2. Batteries
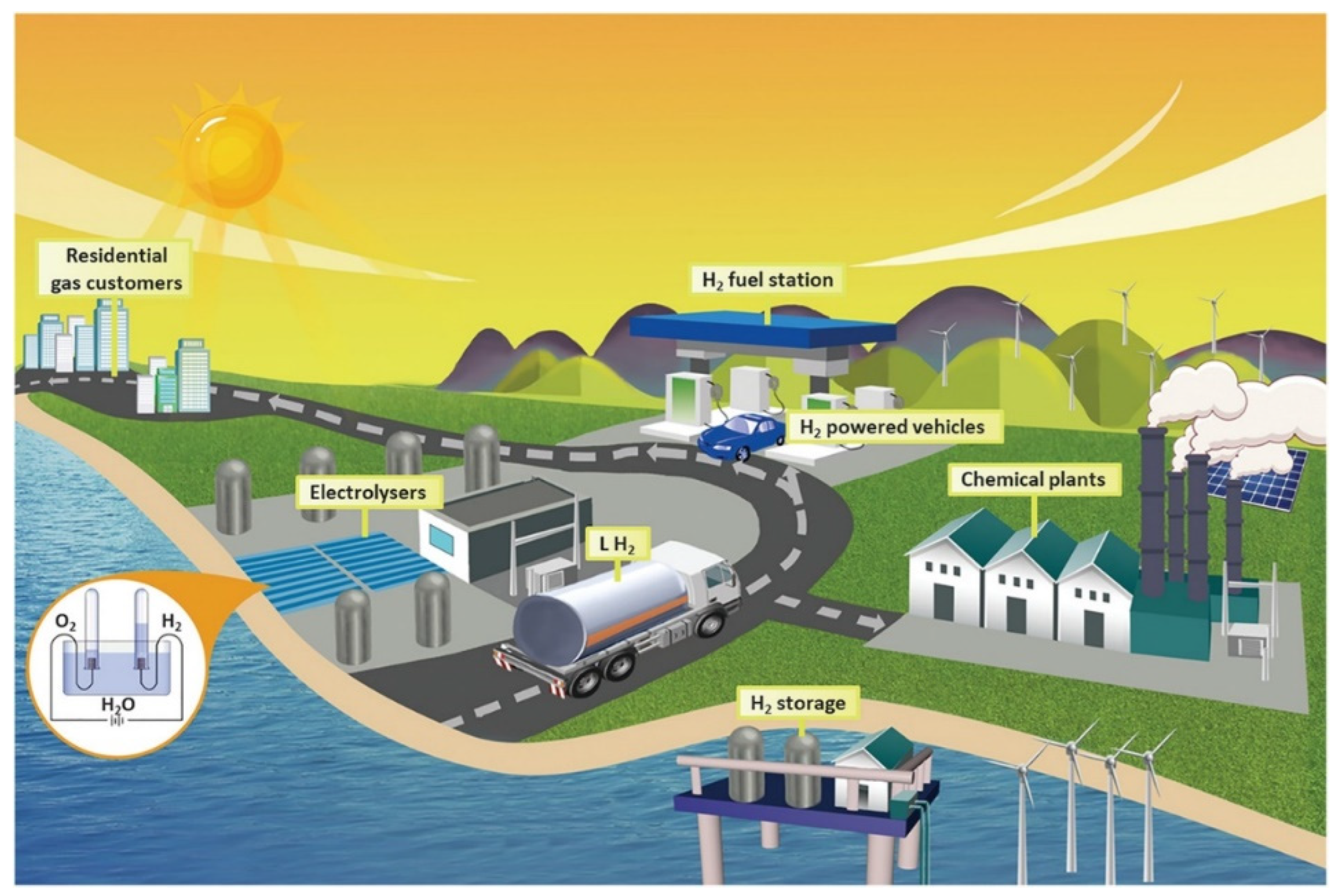
6.3. Hydrogen Production
7. Discussion and Limitation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Hosenuzzaman, M.; Rahim, N.A.; Selvaraj, J.; Hasanuzzaman, M.; Malek, A.B.M.A.; Nahar, A. Global prospects, progress, policies, and environmental impact of solar photovoltaic power generation. Renew. Sust. Energ. Rev. 2015, 41, 284–297. [Google Scholar] [CrossRef]
- Liu, C.F.; Yuan, J.F.; Masse, R.; Jia, X.X.; Bi, W.C.; Neale, Z.; Shen, T.; Xu, M.; Tian, M.; Zheng, J.Q.; et al. Interphases, Interfaces, and Surfaces of Active Materials in Rechargeable Batteries and Perovskite Solar Cells. Adv. Mater. 2020, 33, 1905245. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 2016, 1, 16070. [Google Scholar] [CrossRef]
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef]
- Hughes, L.; Lipscy, P.Y. The politics of energy. Annu. Rev. Political Sci. 2013, 16, 449–469. [Google Scholar] [CrossRef]
- Hughes, L.; Urpelainen, J. Interests, institutions, and climate policy: Explaining the choice of policy instruments for the energy sector. Environ. Sci. Policy 2015, 54, 52–63. [Google Scholar] [CrossRef]
- Lewis, N.S. Light work with water. Nature 2001, 414, 589–590. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Li, S.L.; Xu, Q. Metal-organic frameworks as platforms for clean energy. Energy Environ. Sci. 2013, 6, 1656–1683. [Google Scholar] [CrossRef]
- Jia, C.; Zhao, X.; Lai, Y.H.; Zhao, J.; Wang, P.C.; Liou, D.S.; Wang, P.; Liu, Z.; Zhang, W.; Chen, W.; et al. Highly flexible, robust, stable and high efficiency perovskite solar cells enabled by van der Waals epitaxy on mica substrate. Nano Energy 2019, 60, 476–484. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Priya, S.; Liu, S.F. Recent Advances in Flexible Perovskite Solar Cells: Fabrication and Applications. Angew. Chem. Int. Ed. Engl. 2019, 58, 4466–4483. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.J.; Pak, Y.C.; Kim, C.H.; Kim, J.S.; Ri, K.C.; Ri, K.H.; Choe, S.H.; Cottenier, S. Structural and electrochemical trends in mixed manganese oxides Nax(M0.44Mn0.56)O2 (M = Mn, Fe, Co, Ni) for sodium-ion battery cathode. J. Power Sources 2021, 511, 230395. [Google Scholar] [CrossRef]
- Li, M.; Zhu, K.; Zhao, H.; Meng, Z.; Wang, C.; Chu, P.K. Construction of α-MnO2 on Carbon Fibers Modified with Carbon Nanotubes for Ultrafast Flexible Supercapacitors in Ionic Liquid Electrolytes with Wide Voltage Windows. Nanomaterials 2022, 12, 2020. [Google Scholar] [CrossRef]
- Li, S.; Leng, D.; Li, W.; Long, Q.; Fan, Z. Recent Progress in Developing Li2S Cathodes for Li-S Batteries. Energy Storage Mater. 2020, 27, 279–296. [Google Scholar] [CrossRef]
- Li, S.; Fan, Z. Encapsulation methods of sulfur particles for lithium-sulfur batteries: A review. Energy Storage Mater. 2021, 34, 107–127. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Y.; Zhang, S.; Yang, J.; Shao, Z.; Guo, Z. Recent progress on sodium ion batteries: Potential high-performance anodes. Energ. Environ. Sci. 2018, 11, 2310–2340. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, K.; Chen, J. Recent Advances and Prospects of Cathode Materials for Sodium-Ion Batteries. Adv. Mater. 2015, 27, 5343–5364. [Google Scholar] [CrossRef] [PubMed]
- Marbán, G.; Valdés-Solís, T. Towards the hydrogen economy? Int. J. Hydrogen Energy 2007, 3, 1625–1637. [Google Scholar] [CrossRef]
- Borgschulte, A.; Züttel, A.; Wittstadt, U. Hydrogen Production. In Hydrogen as a Future Energy Carrier; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 149–164. [Google Scholar]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Vayssieres, L. On Solar Hydrogen & Nanotechnology. J. Phys-Condens Mat. 2010, 20, 1005–1008. [Google Scholar]
- Gupta, R.B. Hydrogen Fuel: Production, Transport, and Storage; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Barreto, L.; Makihira, A.; Riahi, K. The hydrogen economy in the 21st century: A sustainable development scenario. Int. J. Hydrogen Energy 2003, 28, 267–284. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, Y.; Feng, X.U.; Yu, X.; Yu, S. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, 2007100. [Google Scholar] [CrossRef]
- Trampusch, C.; Palier, B. Between X and Y: How process tracing contributes to opening the black box of causality. New Polit. Econ. 2016, 2, 437–454. [Google Scholar] [CrossRef]
- Liu, H. The impact of conservative neo-liberalism on American climate policy. J. Fujian Norm. Univ. (Philos. Soc. Sci. Ed.) 2022, 2, 52–170. [Google Scholar]
- Spark, M. Matsunaga Hydrogen Research, Development, and Demonstration Act of 1990. COM/1990/1115; Congress: American, 1990. Available online: https://www.congress.gov/bill/101st-congress/senate-bill/639 (accessed on 31 July 2022).
- Hydrogen Future Act of 1996. COM/1996/1009; Congress: American, 1996. Available online: https://www.hydrogen.energy.gov/pdfs/hydrogen_future_act_1996.pdf (accessed on 31 July 2022).
- United States Department of Energy. A National Vision of America’s Transition to a Hydrogen Economy-to 2030 and Beyond; COM/2002/0212; United States Department of Energy: Washington, DC, USA, 2002. Available online: https://www.hydrogen.energy.gov/pdfs/vision_doc.pdf (accessed on 31 July 2022).
- United States Department of Energy. National Hydrogen Energy Roadmap; COM/2002/1031; United States Department of Energy: Washington, DC, USA, 2002. Available online: https://www.hydrogen.energy.gov/pdfs/national_h2_roadmap.pdf (accessed on 31 July 2022).
- The White House. The President’s Hydrogen Fuel Initiative; COM/2003/0128; The White House: Washington, DC, USA, 2003; Available online: https://georgewbush-whitehouse.archives.gov/news/releases/2003/01/20030130-20.html (accessed on 31 July 2022).
- The Council of Economic Advisers. The All-of-the-Above Energy Strategy; COM/2014/0529; The Council of Economic Advisers: Washington, DC, USA, 2014. Available online: https://obamawhitehouse.archives.gov/sites/default/files/docs/aota_report_updated_july_2014.pdf (accessed on 31 July 2022).
- United States Department of Energy. Hydrogen Energy Earth Shot Initiative; COM/2021/0607; United States Department of Energy: Washington, DC, USA, 2021. Available online: https://www.energy.gov/policy/energy-earthshots-initiative (accessed on 31 July 2022).
- Congress. Energy Tax Act; COM/1977/0321; Congress of the United States: Washington, DC, USA, 1977. Available online: https://www.congress.gov/bill/95th-congress/house-bill/5263 (accessed on 31 July 2022).
- United States Department of Energy. The Energy Policy Act of 1992; COM/1992/1024; United States Department of Energy: Washington, DC, USA, 1992. Available online: https://www.energy.gov/gc/epact-1992-usc (accessed on 31 July 2022).
- United States Department of Energy. The Energy Policy Act of 2005; COM/2005/0808; United States Department of Energy: Washington, DC, USA, 2005. Available online: https://www.energy.gov/gc/epact-2005-usc (accessed on 31 July 2022).
- Energy Independence and Security Act of 2007; COM/2007/1219; Congress of the United States: Washington, DC, USA,, 2007. Available online: https://www.epa.gov/greeningepa/energy-independence-and-security-act-2007 (accessed on 31 July 2022).
- Young, O.R.; Guttman, D.; Qi, Y.; Bachus, K.; Belis, D.; Cheng, H.; Zhu, X. Institutionalized governance processes: Comparing environmental problem-solving in China and the United States. Global Environ. Chang 2015, 31, 163–173. [Google Scholar] [CrossRef]
- Gilley, B. Authoritarian environmentalism and China’s response to climate change. Environ. Polit. 2012, 21, 287–307. [Google Scholar] [CrossRef]
- Richard, L.B. An Inquiry into the Human Prospect. J. Econ. Issues 1984, 18, 941–994. [Google Scholar]
- Beeson, M. The coming of environmental authoritarianism. Environ. Polit. 2010, 19, 276–294. [Google Scholar] [CrossRef]
- The State Council of the People’s Republic of China. Outline of National Medium and Long-Term Science and Technology Development Plan (2006–2020); COM/2006/0209; The State Council of the People’s Republic of China: Beijing, China, 2006. Available online: http://www.gov.cn/gongbao/content/2006/content_240244.htm (accessed on 31 July 2022).
- The State Council of the People’s Republic of China. Energy Conservation and New Energy Vehicle Industry Development Plan (2012–2020); COM/2012/0709; The State Council of the People’s Republic of China: Beijing, China, 2012. Available online: http://www.gov.cn/zwgk/2012-07/09/content_2179032.htm (accessed on 31 July 2022).
- The National Development and Reform Commission and the National Energy Administration. Energy Technology Revolution Innovation Action Plan (from 2016 to 2030); COM/2016/0601; The National Development and Reform Commission and the National Energy Administration: Beijing, China, 2016. Available online: http://www.gov.cn/xinwen/2016-06/01/content_5078628.htm (accessed on 31 July 2022).
- The National Development and Reform Commission and the National Energy Administration. Energy Law of the People’s Republic of China (Draft for Comment); COM/2020/0410; The National Development and Reform Commission and the National Energy Administration: Beijing, China, 2020. Available online: http://www.nea.gov.cn/2020-04/10/c_138963212.htm (accessed on 31 July 2022).
- The National People’s Congress. The 14th Five-Year Plan for National Economic and Social Development of the People’s Republic of China and the Outline of Long-Term Goals for 2035; COM/2021/0312; The National People’s Congress: Beijing, China, 2021. Available online: http://www.gov.cn/xinwen/2021-03/13/content_5592681.htm (accessed on 31 July 2022).
- Urban-Rural Development of the People’s Republic of China. The Opinions on Accelerating the Implementation and Applications of Solar Photovoltaic Buildings; COM/2009/0329; Urban-Rural Development of the People’s Republic of China: Beijing, China, 2009. Available online: http://www.gov.cn/zwgk/2009-03/26/content_1269282.htm (accessed on 31 July 2022).
- The State Electricity Regulatory Commission. The Notice on Renewable Energy Electricity Price Subsidies and Quota Trading Schemes from July to December of 2008; COM/2009/0701; The State Electricity Regulatory Commission: Beijing, China, 2009. Available online: http://www.gov.cn/zwgk/2009-07/01/content_1354370.htm (accessed on 31 July 2022).
- National Energy Administration. The Notice on Declaring a Demonstration Zone for the Large-Scale Application of Distributed Photovoltaic Power Generation; COM/2012/0928; National Energy Administration: Beijing, China, 2012. Available online: http://www.gov.cn/zwgk/2012-09/28/content_2235051.htm (accessed on 31 July 2022).
- The State Council of the People’s Republic of China. The Several Opinions on Promoting the Healthy Development of the Photovoltaic Industry; COM/2013/0715; The State Council of the People’s Republic of China: Beijing, China, 2013. Available online: http://www.gov.cn/zwgk/2013-07/15/content_2447814.htm (accessed on 31 July 2022).
- National Energy Administration. The Notice on Further Implementing the Policies Related to Distributed Photovoltaic Power Generation; COM/2014/0902; National Energy Administration: Beijing, China, 2014. Available online: http://zfxxgk.nea.gov.cn/auto87/201409/t20140904_1837.htm (accessed on 31 July 2022).
- National Energy Administration. The Notice on the Price Policy of Photovoltaic Power Generation Project of 2018; COM/2018/0531; National Energy Administration: Beijing, China, 2018. Available online: http://www.gov.cn/zhengce/zhengceku/2018-12/31/content_5433580.htm (accessed on 31 July 2022).
- National Energy Administration. The Notice on Actively Promoting the Work Related to the Subsidy-free Grid Parity of Wind Power and Photovoltaic Power Generation; COM/2019/0110; National Energy Administration: Beijing, China, 2019. Available online: http://www.nea.gov.cn/2019-01/10/c_137731320.htm (accessed on 31 July 2022).
- National Development and Reform Commission. The Notice on Problems Related to Improve the Feed-in Tariff Mechanism for Photovoltaic Power Generation; COM/2019/0428; National Development and Reform Commission: Beijing, China, 2019. Available online: https://www.ndrc.gov.cn/xxgk/zcfb/tz/201904/t20190430_962433.html?code=&state=123 (accessed on 31 July 2022).
- National Energy Administration. The Notice on Matters Related to the Development and Construction of Wind Power and Photovoltaic Power Generation of 2021; COM/2021/0511; National Energy Administration: Beijing, China, 2021. Available online: http://zfxxgk.nea.gov.cn/2021-05/11/c_139958210.htm (accessed on 31 July 2022).
- The State Council of the People’s Republic of China. Implementation Plan on Promoting the High-Quality Development of New Energy in the New Era; COM/2022/0514; The State Council of the People’s Republic of China: Beijing, China, 2022. Available online: http://www.gov.cn/zhengce/content/2022-05/30/content_5693013.htm (accessed on 31 July 2022).
- Li, X.; Yang, X.; Wei, Q.; Zhang, B. Authoritarian environmentalism and environmental policy implementation in China. Resources. Resour. Conserv. Recy. 2019, 145, 86–93. [Google Scholar] [CrossRef]
- Van Baal, P.A.; Finger, M. The Effect of European Integration on Swiss Energy Policy and Governance. Politics Gov. 2019, 7, 6–16. [Google Scholar] [CrossRef]
- Commission of the European Communities. Energy in Europe; COM/1986/0905; Commission of the European Communities: Luxembourg, 1986. Available online: http://aei.pitt.edu/79850/1/5._Sept_1986.pdf (accessed on 31 July 2022).
- European Commission. 2020 Climate and Energy Package; COM (2007); European Commission: Brussels, Belgium, 2007. Available online: https://ec.europa.eu/clima/eu-action/climate-strategies-targets/2020-climate-energy-package_en (accessed on 31 July 2022).
- European Commission. Energy Roadmap 2050; COM (2011); European Commission: Luxembourg, 2011. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/2012_energy_roadmap_2050_en_0.pdf (accessed on 31 July 2022).
- European Commission. The Roadmap for Transforming the EU into a Competitive, Low-Carbon Economy by 2050; COM/2011/0308; European Commission: Brussels, Belgium, 2011. Available online: https://ec.europa.eu/clima/system/files/2016-12/2050_roadmap_en.pdf (accessed on 31 July 2022).
- European Commission. The 2030 Climate and Energy Framework; COM/2014/0122; European Commission: Brussels, Belgium, 2014. Available online: https://ec.europa.eu/clima/eu-action/climate-strategies-targets/2030-climate-energy-framework_sl (accessed on 31 July 2022).
- Fuel Cells and Hydrogen Joint Undertaking. European Hydrogen Roadmap: A Sustainable Pathway for the European Energy Transition; COM/2019/0212; Fuel Cells and Hydrogen Joint Undertaking: Brussels, Belgium, 2019. Available online: https://www.fch.europa.eu/sites/default/files/Hydrogen%20Roadmap%20Europe_Report.pdf (accessed on 31 July 2022).
- European Commission. A European Green Deal; COM/2019/1211; European Commission: Brussels, Belgium, 2019. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 31 July 2022).
- Shi, B.; Duan, L.; Zhao, Y.; Luo, J.; Zhang, X. Semitransparent Perovskite Solar Cells: From Materials and Devices to Applications. Adv. Mater. 2020, 32, 1806474.1–1806474.12. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Li, G.; Chen, P.; Gao, X. Two-Terminal Perovskite-Based Tandem Solar Cells for Energy Conversion and Storage. Small 2021, 17, 2006145. [Google Scholar] [CrossRef]
- Badawy, W.A. A review on solar cells from Si-single crystals to porous materials and quantum dots. J. Adv. Res. 2015, 6, 123–132. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, J.W.; Jung, H.S.; Shin, H.; Park, N.G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, C.; Lv, P.; Ku, Z.; Lu, J.; Huang, F.; Cheng, Y.B. Printing strategies for scaling-up perovskite solar cells. Natl. Sci. Rev. 2021, 8, 24. [Google Scholar] [CrossRef]
- Huang, Y.T.; Kavanagh, S.R.; Scanlon, D.O.; Walsh, A.; Hoye, R. Perovskite-inspired materials for photovoltaics and beyond-from design to devices. Nanotechnology 2021, 32, 132004. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Yun, J.H.; Chen, P.; Hao, M.; Wang, L. Addressing Toxicity of Lead: Progress and Applications of Low-Toxic Metal Halide Perovskites and Their Derivatives. Adv. Energy Mater. 2017, 7, 1602512. [Google Scholar] [CrossRef]
- Park, B.W.; Philippe, B.; Zhang, X.; Rensmo, H.; Boschloo, G.; Johansson, E. Bismuth Based Hybrid Perovskites A3Bi2I9 (A: Methylammonium or Cesium) for Solar Cell Application. Adv. Mater. 2016, 27, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hao, F.; Stoumpos, C.C.; Phelan, B.T.; Wasielewski, M.R.; Kanatzidis, M.G. Carrier Diffusion Lengths of over 500 nm in Lead-Free Perovskite CH3NH3SnI3 Films. J. Am. Chem. Soc. 2016, 138, 14750–14755. [Google Scholar] [CrossRef]
- Chakrabarti, T.; Saha, M.; Khanda, A.; Sarkar, S.K. Modeling of Lead-Free CH3NH3SnI3-Based Perovskite Solar Cell Using ZnO as ETL. In Advances in Communication, Devices and Networking; Bera, R., Sarkar, S., Chakraborty, S., Eds.; Lecture Notes in Electrical Engineering; Springer: Singapore, 2018; Volume 462, pp. 125–131. [Google Scholar]
- Wang, L.; Wang, Z.X.; Li, H.; Chang, B.; Pan, L.; Xie, Z.; Yin, L. Pseudohalide anions to suppress oxidative degradation for efficient formamidinium-based Sn−Pb halide perovskite solar cells. ACS Appl. Mater. Interfaces 2022, 14, 18302–18312. [Google Scholar] [CrossRef]
- Lin, R.; Xiao, K.; Qin, Z.; Han, Q.; Zhang, C.; Wei, M.; Saidaminov, M.I.; Gao, Y.; Xu, J.; Xiao, M.; et al. Monolithic all-perovskite tandem solar cells with 24.8% efficiency exploiting comproportionation to suppress Sn(ii) oxidation in precursor ink. Nat. Energy 2019, 4, 864–873. [Google Scholar]
- Giuri, A.; Munir, R.; Listorti, A.; Esposito Corcione, C.; Gigli, G.; Rizzo, A.; Amassian, A.; Colella, S. Implication of polymeric template agent on the formation process of hybrid halide perovskite films. Nanotechnology 2021, 32, 265707. [Google Scholar] [CrossRef]
- Han, Q.; Yang, S.; Wang, L.; Yu, F.; Zhang, C.; Wu, M.; Ma, T. The sulfur-rich small molecule boosts the efficiency of carbon-based CsPbI2Br perovskite solar cells to approaching 14%. Sol. Energy 2021, 216, 351–357. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, J.; Li, H.; Yan, Y.; Zhou, W.; Yu, D.; Zhao, Q. A polymer scaffold for self-healing perovskite solar cells. Nat. Commun. 2016, 7, 10228. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Heumueller, T.; Zhang, J.Y.; Luo, J.S.; Kasian, O.; Langner, S.; Kupfer, C.; Liu, B.; Zhong, Y.; Elia, J.; et al. A bilayer conducting polymer structure for planar perovskite solar cells with over 1400 h operational stability at elevated temperatures. Nat. Energy 2022, 7, 144–152. [Google Scholar] [CrossRef]
- Enomoto, J.; Sato, R.; Yokoyama, M.; Kimura, T.; Oshita, N.; Umemoto, K.; Asakura, S.; Masuhara, A. Highly luminescent MAPbI3 perovskite quantum dots with a simple purification process via ultrasound-assisted bead milling. RSC Adv. 2022, 12, 5571–5576. [Google Scholar] [CrossRef]
- Fan, R.; Zhou, N.; Zhang, L.; Yang, R.; Meng, Y.; Li, L.; Guo, T.; Chen, Y.; Xu, Z.; Zheng, G.; et al. Toward Full Solution Processed Perovskite/Si Monolithic Tandem Solar Device With PCE Exceeding 20%. Solar RRL 2017, 1, 1700149. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, H.; Hong, Z.; Luo, S.; Duan, H.S.; Wang, H.H.; Liu, Y.; Li, G.; Yang, Y. Planar Heterojunction Perovskite Solar Cells via Vapor-Assisted Solution Process. J. Am. Chem. Soc. 2014, 136, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.P.; Chen, Q.; Li, G.; Luo, S.; Song, T.B.; Duan, H.S.; Hong, Z.R.; You, J.B.; Liu, Y.S.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yan, H.; Duan, M.; Ji, J.; Liu, X.; Jiang, H.; Liu, B.; Sajid, S.; Cui, P.; Li, Y.; et al. TiO2 surface oxygen vacancy passivation towards mitigated interfacial lattice distortion and efficient perovskite solar cell. Appl. Surf. Sci. 2021, 544, 148583. [Google Scholar] [CrossRef]
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef]
- Yin, X.; Sarkar, S.; Shi, S.; Huang, Q.-A.; Zhao, H.; Yan, L.; Zhao, Y.; Zhang, J. Recent Progress in Advanced Organic Electrode Materials for Sodium-Ion Batteries: Synthesis, Mechanisms, Challenges and Perspectives. Adv. Funct. Mater. 2020, 30, 1908445. [Google Scholar] [CrossRef]
- Pan, H.L.; Yong-Sheng, H.U.; Hong, L.I.; Chen, L.Q. Recent progress in structure study of electrode materials for room-temperature sodium-ion stationary batteries. Sci. Sin. Chim. 2014, 44, 1269. [Google Scholar]
- Li, M.; Zhu, K.L.; Meng, Z.Y.; Hu, R.H.; Wang, J.L.; Wang, C.R.; Chu, P.K. Efficient coupling of MnO2/TiN on carbon cloth positive electrode and Fe2O3/TiN on carbon cloth negative electrode for flexible ultra-fast hybrid supercapacitors. RSC Adv. 2021, 11, 35726–35736. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lu, Y.; Chen, L.; Hu, Y.S. Flexible Na batteries. InfoMat 2020, 2, 126–138. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Han, N.; Huang, W.; Li, J.; Ye, H.; Chen, F.; Li, Y. Nanostructured CuP2/C composites as high-performance anode materials for sodium ion batteries. J. Mater. Chem. A 2015, 3, 21754–21759. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Wang, L.; Huang, W.; Zheng, S.; Hu, P.; Du, Y. High-capacity organic sodium ion batteries using a sustainable C4Q/CMK-3/SWCNT electrode. Inorg. Chem. Front. 2019, 6, 1977–1985. [Google Scholar] [CrossRef]
- Senthilkumar, B.; Murugesan, C.; Sharma, L.; Lochab, S.; Barpanda, P. An Overview of Mixed Polyanionic Cathode Materials for Sodium-Ion Batteries. Small Methods 2019, 3, 1800253. [Google Scholar] [CrossRef]
- Wheeler, S.; Capone, I.; Day, S.; Tang, C.; Pasta, M. Low-Potential Prussian Blue Analogues for Sodium-Ion Batteries: Manganese Hexacyanochromate. Chem. Mater. 2019, 31, 2619–2626. [Google Scholar] [CrossRef]
- Amin, K.; Mao, L.; Wei, Z. Recent Progress in Polymeric Carbonyl-Based Electrode Materials for Lithium and Sodium Ion Batteries. Macromol. Rapid Comm. 2019, 40, 1800565. [Google Scholar] [CrossRef]
- Liu, R.; Liang, Z.; Gong, Z.; Yang, Y. Research Progress in Multielectron Reactions in Polyanionic Materials for Sodium-Ion Batteries. Small Methods 2019, 3, 1800221. [Google Scholar] [CrossRef]
- Desai, A.V.; Morris, R.E.; Armstrong, A.R. Advances in Organic Anode Materials for Na-/K-Ion Rechargeable Batteries. ChemSusChem 2020, 13, 4866–4884. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Meng, Z.; Feng, R.; Zhu, K.; Zhao, F.; Wang, C.; Wang, J.; Wang, L.; Chu, P.K. Fabrication of Bimetallic Oxides (MCo2O4: M=Cu, Mn) on Ordered Microchannel Electro-Conductive Plate for High-Performance Hybrid Supercapacitors. Sustainability 2021, 13, 9896. [Google Scholar] [CrossRef]
- Feng, R.C.; Li, M.; Wang, Y.; Lin, J.; Zhu, K.L.; Wang, J.L.; Wang, C.R.; Chu, P.K. High-performance multi-dimensional nitrogen-doped N+MnO2@TiC/C electrodes for supercapacitors. Electrochim. Acta 2021, 370, 137716. [Google Scholar] [CrossRef]
- Deng, J.; Luo, W.B.; Chou, S.L.; Liu, H.K.; Dou, S.X. Sodium-Ion Batteries: From Academic Research to Practical Commercialization. Adv. Energy Mater. 2018, 8, 1701428. [Google Scholar] [CrossRef]
- Tang, W.; Liang, R.; Li, D.; Yu, Q.H.; Hu, J.H.; Cao, B.; Fan, C. Highly Stable and High Rate-Performance Na-Ion Batteries Using Polyanionic Anthraquinone as the Organic Cathode. Chemsuschem 2019, 12, 2181–2185. [Google Scholar] [CrossRef]
- Lee, J.E.; Jeon, K.-J.; Show, P.L.; Lee, I.H.; Jung, S.-C.; Choi, Y.J.; Rhee, G.H.; Lin, K.-Y.A.; Park, Y.-K. Mini review on H2 production from electrochemical water splitting according to special nanostructured morphology of electrocatalysts. Fuel 2022, 308, 122048. [Google Scholar] [CrossRef]
- Schneider, S.; Bajohr, S.; Graf, F.; Kolb, T. State of the Art of Hydrogen Production via Pyrolysis of Natural Gas. ChemBioEng Rev. 2020, 7, 150–158. [Google Scholar] [CrossRef]
- Yang, H.; Xu, G.; Wei, X.; Cao, J.; Yang, L.; Chu, P.K. Ultrafast hetero-assembly of monolithic interwoven V2O5 nanobelts/carbon nanotubes architectures for high-energy alkali-ion batteries. J. Power Sources 2018, 395, 295–304. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Q.; Feng, X. Support and Interface Effects in Water-Splitting Electrocatalysts. Adv. Mater. 2019, 31, 1808167. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Ling, T.; Davey, K.; Zheng, Y.; Qiao, S.Z. Transition-Metal-Doped RuIr Bifunctional Nanocrystals for Overall Water Splitting in Acidic Environments. Adv. Mater. 2019, 31, 1900510.1–1900510.7. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, H.; Li, J.; Yue, X.; Cui, Y. Porous MoO2 Nanosheets as Non-noble Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Mater. 2016, 28, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ali, R.; Ma, J.; Jiao, W.; Jian, X. Graphene-Decorated Boron–Carbon–Nitride-Based Metal-Free Catalysts for an Enhanced Hydrogen Evolution Reaction. ACS Appl. Energ. Mater. 2021, 4, 3861–3868. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Wang, J.L.; Wang, C.R.; Li, W.; Chu, P.K. NiFeP nanoflakes composite with CoP on carbon cloth as flexible and durable electrocatalyst for efficient overall water splitting. Nanotechnology 2019, 30, 485402. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Feng, R.C.; Li, M.; Zhao, X.; Zhang, B.H.; Liang, Y.; Ning, H.P.; Wang, J.L.; Wang, C.R.; Chu, P.K. Needle-like CoO nanowire composites with NiO nanosheets on carbon cloth for hybrid flexible supercapacitors and overall water splitting electrodes. RSC Adv. 2020, 10, 37489. [Google Scholar] [CrossRef]
- Su, J.; Yang, Y.; Xia, G.; Chen, J.; Jiang, P.; Chen, Q. Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media. Nat. Commun. 2017, 8, 14969. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, Z.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y.M.; et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef]
- Yang, H.; Shang, L.; Zhang, Q.; Shi, R.; Waterhouse, G.I.N.; Gu, L.; Zhang, T. A universal ligand mediated method for large scale synthesis of transition metal single atom catalysts. Nat. Commun. 2019, 10, 4585. [Google Scholar] [CrossRef]
- Li, P.; Wang, M.; Duan, X.; Zheng, L.; Cheng, X.; Zhang, Y.; Kuang, Y.; Li, Y.; Ma, Q.; Feng, Z.; et al. Boosting oxygen evolution of single-atomic ruthenium through electronic coupling with cobalt-iron layered double hydroxides. Nat. Commun. 2019, 10, 1711. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, Z.; Yu, P.; Cai, Y.; Du, Y.; Wu, D.; Gao, S.; Tan, C.; Li, Z.; Ren, M.; et al. Lithiation-induced amorphization of Pd3P2S8 for highly efficient hydrogen evolution. Nat. Catal. 2018, 1, 460–468. [Google Scholar] [CrossRef]
- Feng, T.; Yu, G.; Tao, S.; Zhu, S.; Ku, R.; Zhang, R.; Zeng, Q.; Yang, M.; Chen, Y.; Chen, W. A highly efficient overall water splitting ruthenium-cobalt alloy electrocatalyst across a wide pH range via electronic coupling with carbon dots. J. Mater. Chem. A 2020, 8, 9638–9645. [Google Scholar] [CrossRef]
- Xu, H.; Liu, T.; Bai, S.; Li, L.; Zhu, Y.; Wang, J.; Yang, S.; Li, Y.; Shao, Q.; Huang, X. Cation Exchange Strategy to Single-Atom Noble-Metal Doped CuO Nanowire Arrays with Ultralow Overpotential for H2O Splitting. Nano Lett. 2020, 20, 5482–5489. [Google Scholar] [CrossRef] [PubMed]
- Anxolabehere-Mallart, E.; Costentin, C.; Fournier, M.; Nowak, S.; Robert, M.; Saveant, J.M. Boron-capped tris (glyoximato) cobalt clathrochelate as a precursor for the electrodeposition of nanoparticles catalyzing H2 evolution in water. J. Am. Chem. Soc. 2012, 134, 6104–6107. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.H.; Zhang, H.J.; Ma, J.L.; Huang, Z.Y.; Li, J.; Wang, Y. Transition-Metal Carbides as Hydrogen Evolution Reduction Electrocatalysts: Synthetic Methods and Optimization Strategies. Chem.—Eur. J. 2021, 27, 5074–5090. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Li, K.; Mu, X.; Zou, J.; Xie, S.; Xiong, X.; Zeng, X. Nanoporous NiFeMoP alloy as a bifunctional catalyst for overall water splitting—ScienceDirect. Int. J. Hydrogen Energ. 2020, 45, 16447–16457. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water. Angew. Chem. Int. Ed. 2015, 126, 9731–9735. [Google Scholar] [CrossRef]
- Dai, L.; Chen, Z.; Li, L.; Yin, P.; Zhang, H. Ultrathin Ni(0)-Embedded Ni(OH)2 Heterostructured Nanosheets with Enhanced Electrochemical Overall Water Splitting. Adv. Mater. 2020, 32, 1906915. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, X.; Sun, Y.; Qi, K.; Jin, Z.; Wei, S.; Li, W.; Zhang, L.; Zheng, W. Nanoporous Sulfur-Doped Copper Oxide (Cu2OxS1−x) for Overall Water Splitting. ACS Appl. Energ. Mater. 2018, 10, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A.; Sahar, S.; Qazi, U.Y.; Odda, A.H.; Ullah, N.; Liu, Y.N.; Qazi, I.A.; Xu, A.W. Intrinsic peroxidase-like activity and enhanced photo-Fenton reactivity of iron-substituted polyoxometallate nanostructures. Dalton T. 2018, 47, 7344–7352. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Du, A.; Gao, G.; Chen, J.; Yan, X.; Brown, C.L.; Yao, X. Defect Graphene as a Trifunctional Catalyst for Electrochemical Reactions. Adv. Mater. 2016, 28, 9532–9538. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.F.; Chen, X.; Li, B.-Q.; Hou, T.Z.; Zhang, B.; Zhang, Q.; Titirici, M.-M.; Wei, F. Topological Defects in Metal-Free Nanocarbon for Oxygen Electrocatalysis. Adv. Mater. 2016, 28, 6845–6851. [Google Scholar] [CrossRef] [PubMed]
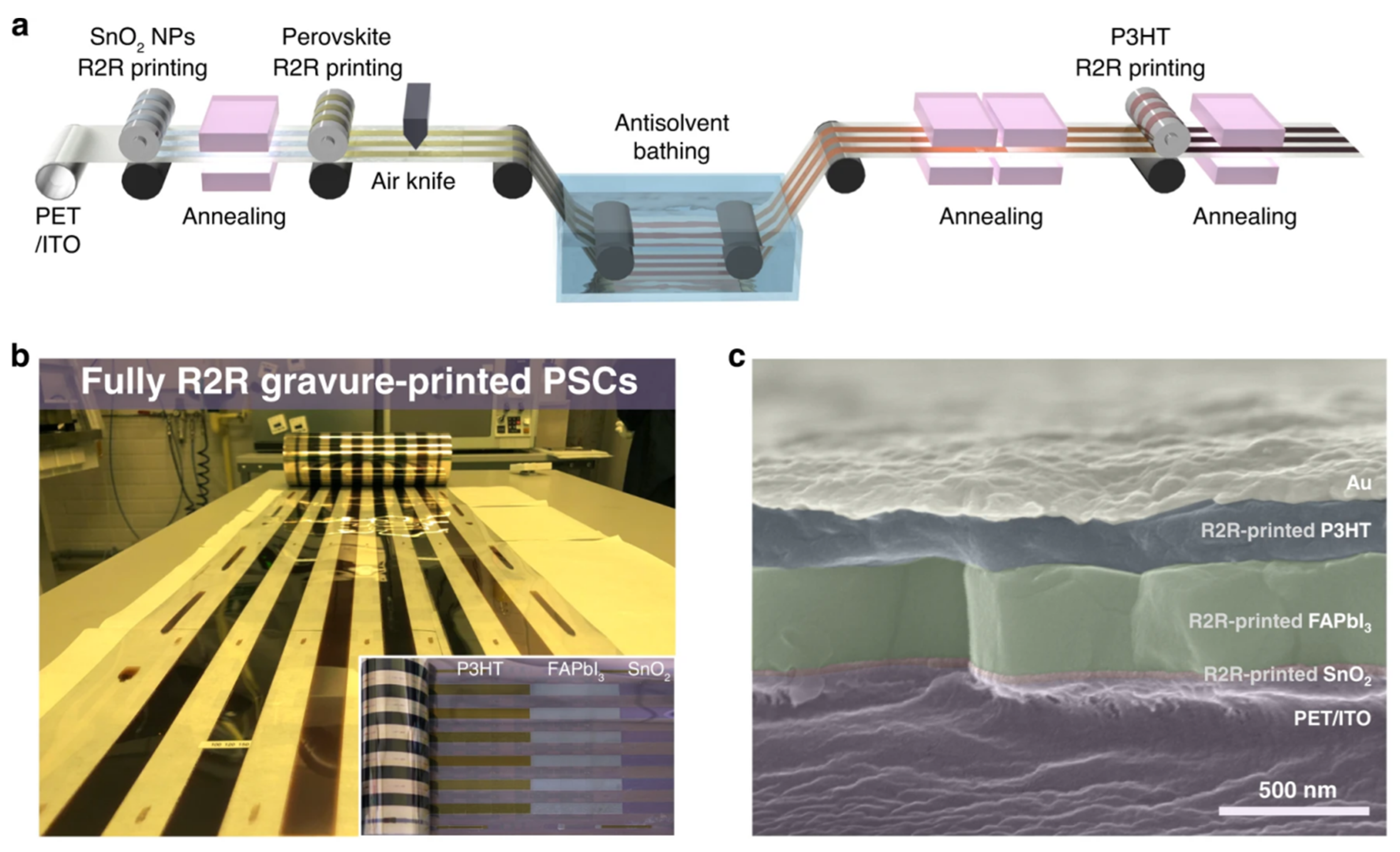
- Kim, Y.Y.; Yang, T.Y.; Suhonen, R.; Kemppainen, A.; Hwang, K.; Jeon, N.J.; Seo, J. Roll-to-roll gravure-printed flexible perovskite solar cells using eco-friendly antisolvent bathing with wide processing window. Nat. Commun. 2020, 11, 5146. [Google Scholar] [CrossRef] [PubMed]
- Giacomo, F.D.; Shanmugam, S.; Fledderus, H.; Bruijnaers, B.J.; Verhees, W.; Dorenkamper, M.S.; Veenstra, S.C.; Qiu, W.; Gehlhaar, R.; Merckx, T. Up-scalable sheet-to-sheet production of high efficiency perovskite module and solar cells on 6-in. substrate using slot die coating. Sol. Energ. Mat. Sol. C. 2017, 181, 53–59. [Google Scholar] [CrossRef]
- Hu, Y.; Si, S.; Mei, A.; Rong, Y.; Liu, H.; Li, X.; Han, H. Stable large-area (10 × 10 cm2) printable mesoscopic perovskite module exceeding 10% efficiency. Solar RRL 2017, 1, 1600019. [Google Scholar] [CrossRef]
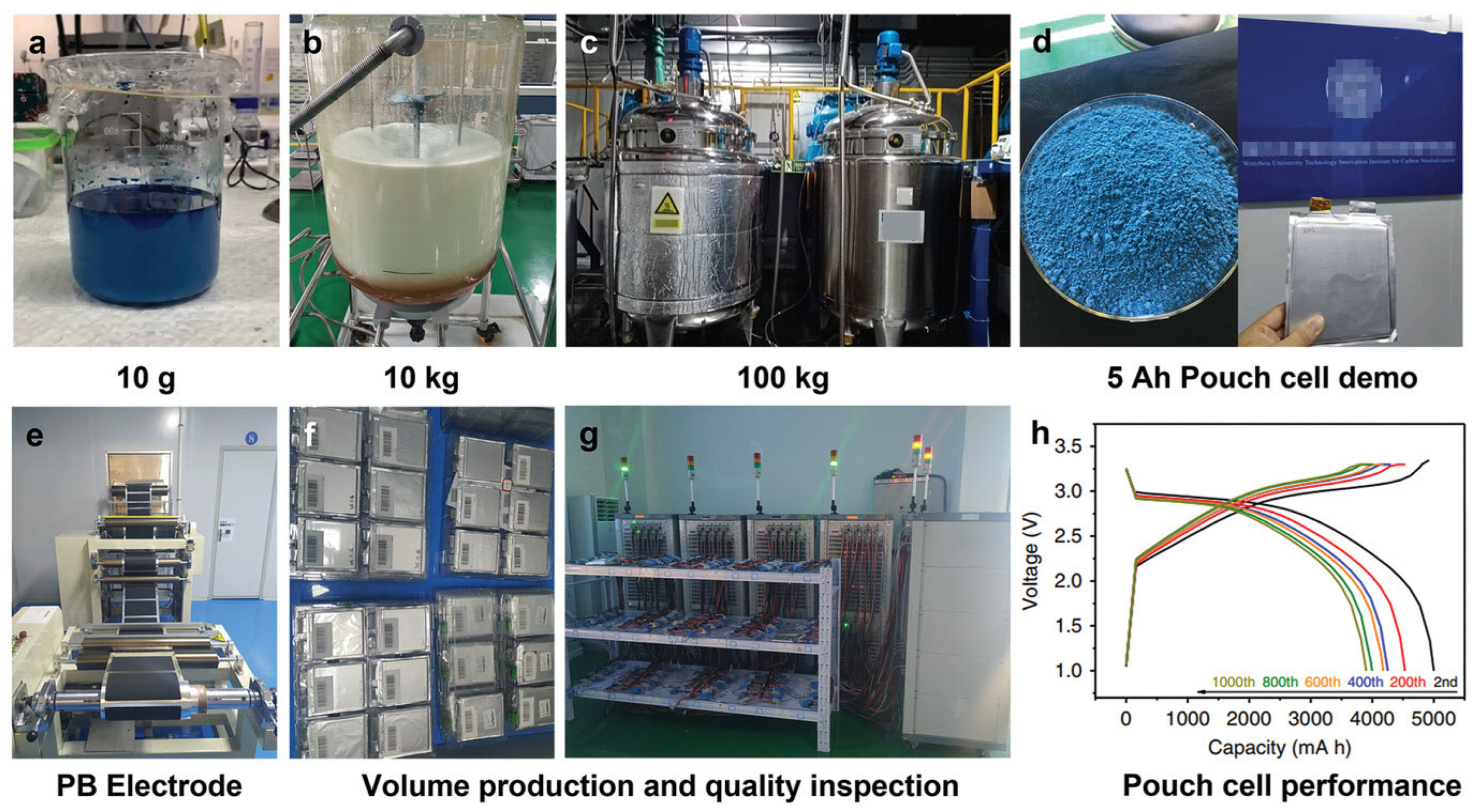
- Peng, J.; Zhang, W.; Liu, Q.; Wang, J.; Chou, S.; Liu, H.; Dou, S. Prussian blue analogues for sodium-ion batteries: Past, present and future. Adv. Mater. 2022, 34, 2108384. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Han, C.; Chou, S.L.; Wang, J.Z.; Li, Z.; Kang, Y.M.; Liu, H.K.; Dou, S.X. Graphite-Nanoplate-Coated Bi2S3 Composite with High-Volume Energy Density and Excellent Cycle Life for Room-Temperature Sodium–Sulfide Batteries. Chem. Eur. J. 2016, 22, 590. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Gang, Y.; Peng, J.; Hu, Z.; Yan, Z.; Lai, W.; Zhu, Y.; Appadoo, D.; Ye, M.; Cao, Y.; et al. Effect of Eliminating Water in Prussian Blue Cathode for Sodium-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2111727. [Google Scholar] [CrossRef]
- Zhang, X.; Weng, W.; Gu, H.; Hong, Z.; Xiao, W.; Wang, F.; Li, W.; Gu, D. Versatile Preparation of Mesoporous Single-Layered Transition-Metal Sulfide/Carbon Composites for Enhanced Sodium Storage. Adv. Mater. 2022, 34, 2104427. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, M.; Zhao, L.; Wang, L.; Gong, Y. Ru doped bimetallic phosphide derived from 2D metal-organic framework as active and robust electrocatalyst for water splitting. Appl. Surf. Sci. 2021, 536, 147952. [Google Scholar] [CrossRef]
- Ye, L.K.; Rouhani, F.; Kaviani, H.; Miao, Q.; Cai, X.Q.; Morsali, A.; Hu, M.L. Effect of Proton Conduction on the Charge Storage Mechanism of a MOF as a Supercapacitor Electrode. J. Phys. Chem. C 2021, 125, 22951–22959. [Google Scholar] [CrossRef]
- Lin, R.; Xu, J.; Wei, M.; Wang, Y.R.; Qin, Z.Y.; Liu, Z.; Wu, J.L.; Xiao, K.; Chen, B.; Park, S.M.; et al. All-perovskite tandem solar cells with improved grain surface passivation. Nature 2022, 603, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ma, F.; Qu, Z.H.; Yu, S.Q.; Shen, T.; Deng, H.X.; Chu, X.B.; Peng, X.X.; Yuan, Y.B.; Zhang, X.W.; et al. Inactive (PbI2)2RbCl stabilizes perovskite films for efficient solar cells. Science 2022, 377, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.K.; Zhang, X.; Li, C.; Wang, K.; Sun, X.Z.; Ma, Y.W. Recent advances in prelithiation materials and approaches for lithium-ion batteries and capacitors. Energy Stor. Mater. 2020, 32, 497–516. [Google Scholar] [CrossRef]
- Wang, Y.R.; Chen, R.P.; Chen, T.; Lv, H.L.; Zhu, G.Y.; Ma, L.B.; Wang, C.X.; Jin, Z.; Liu, J. Emerging non-lithium ion batteries. Energy Stor. Mater. 2016, 4, 103–129. [Google Scholar] [CrossRef]
- Liang, Y.R.; Lai, W.H.; Miao, Z.C.; Chou, S.L. Nanocomposite Materials for the Sodium–Ion Battery: A Review. Small 2017, 14, 1702514. [Google Scholar] [CrossRef]
- Bossche, P.V.D.; Vergels, F.; Mierlo, J.V.; Matheys, J.; Autenboer, W.V. SUBAT: An assessment of sustainable battery technology. J. Power Sources 2006, 162, 913–919. [Google Scholar] [CrossRef]
- Joshi, B.V.; Vipin, B.; Ramkumar, J.; Amit, R.K. Impact of policy instruments on lead-acid battery recycling: A system dynamics approach. Resour. Conserv. Recy. 2021, 169, 105528. [Google Scholar] [CrossRef]
- Chen, Y.N.; Xu, S.M.; Li, Y.C.; Jaco, R.J.; Kuang, Y.; Liu, B.; Wang, Y.; Pastel, G.; Salamanca-Riba, L.; Zachariah, M.; et al. FeS2 Nanoparticles Embedded in Reduced Graphene Oxide toward Robust, High-Performance Electrocatalysts. Adv. Energy Mater. 2017, 7, 1700482. [Google Scholar] [CrossRef]
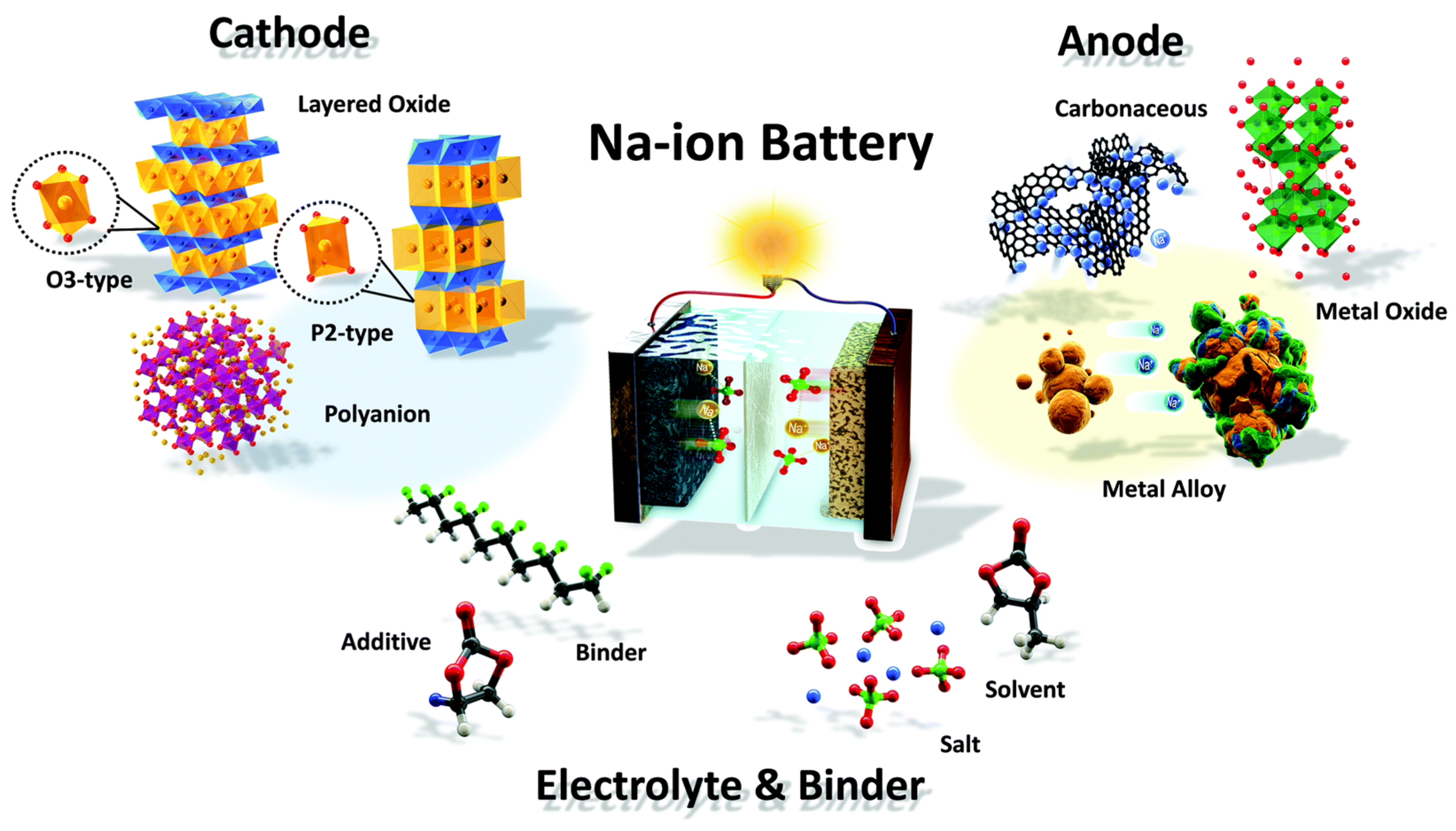
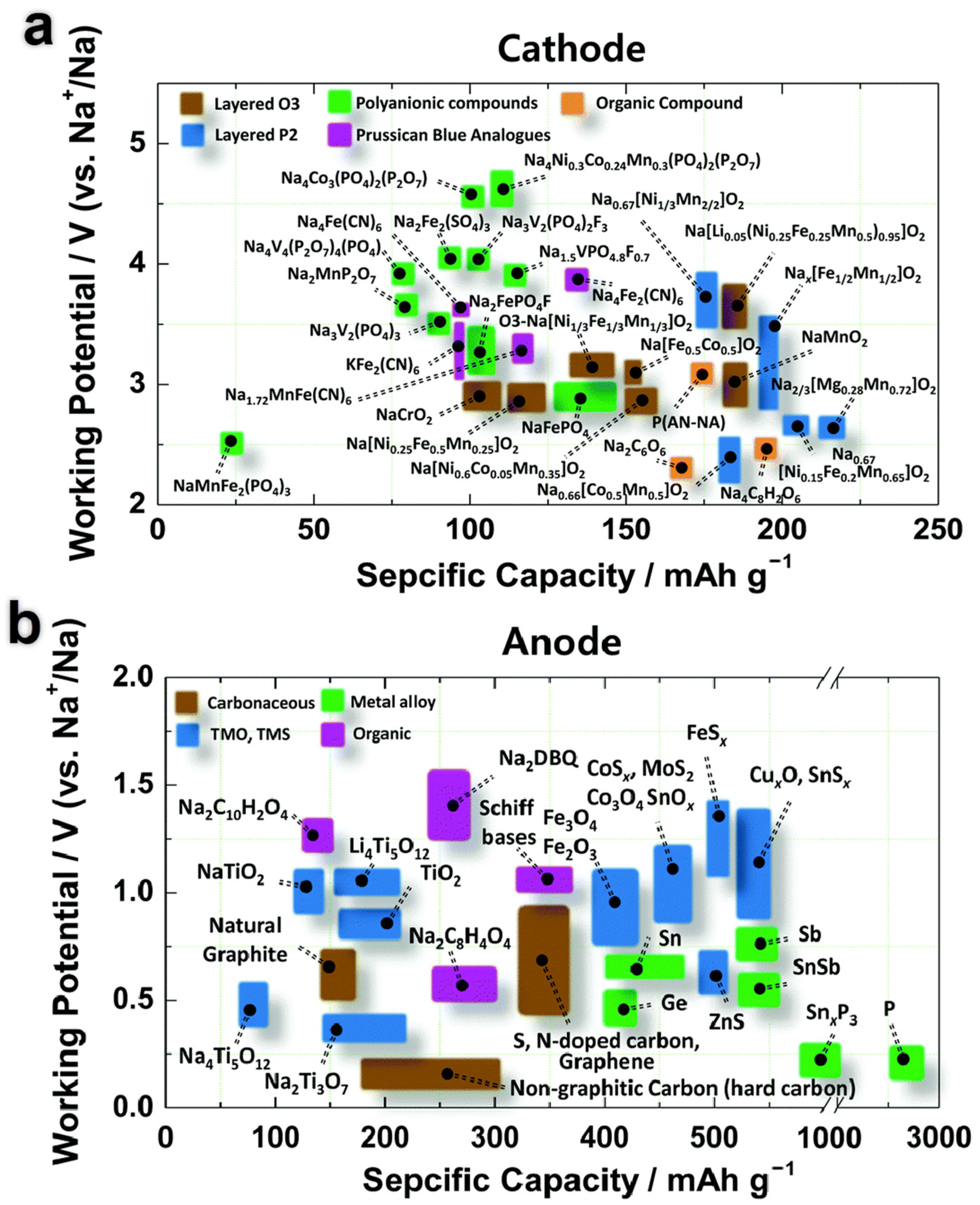

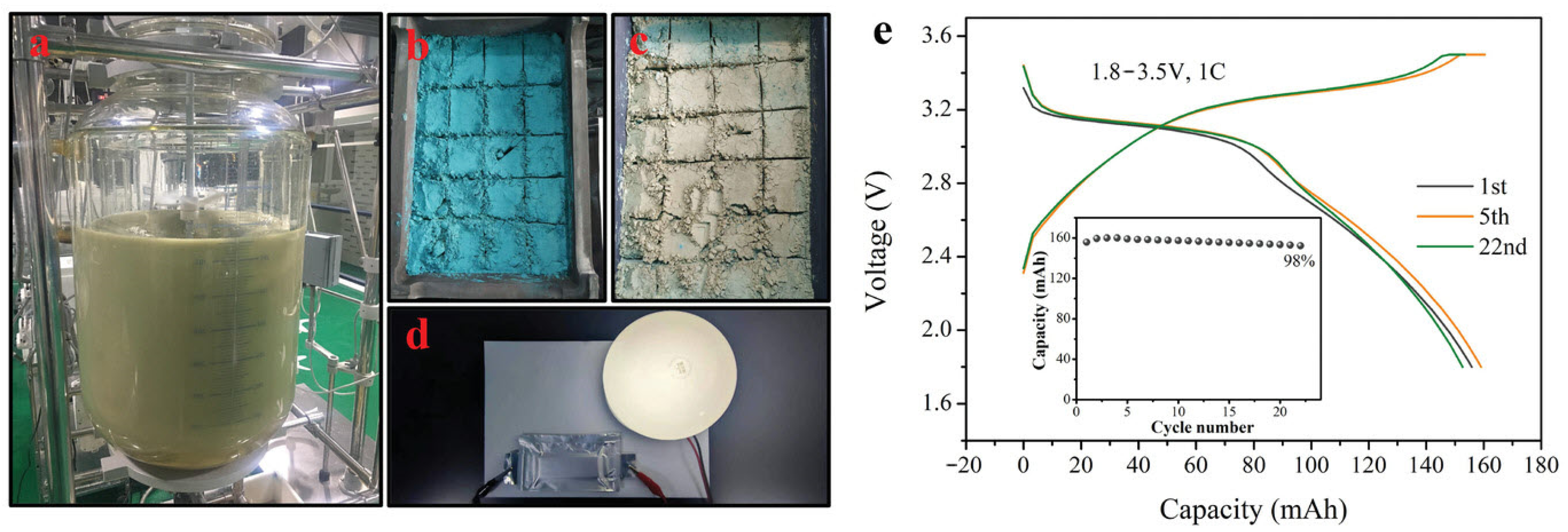
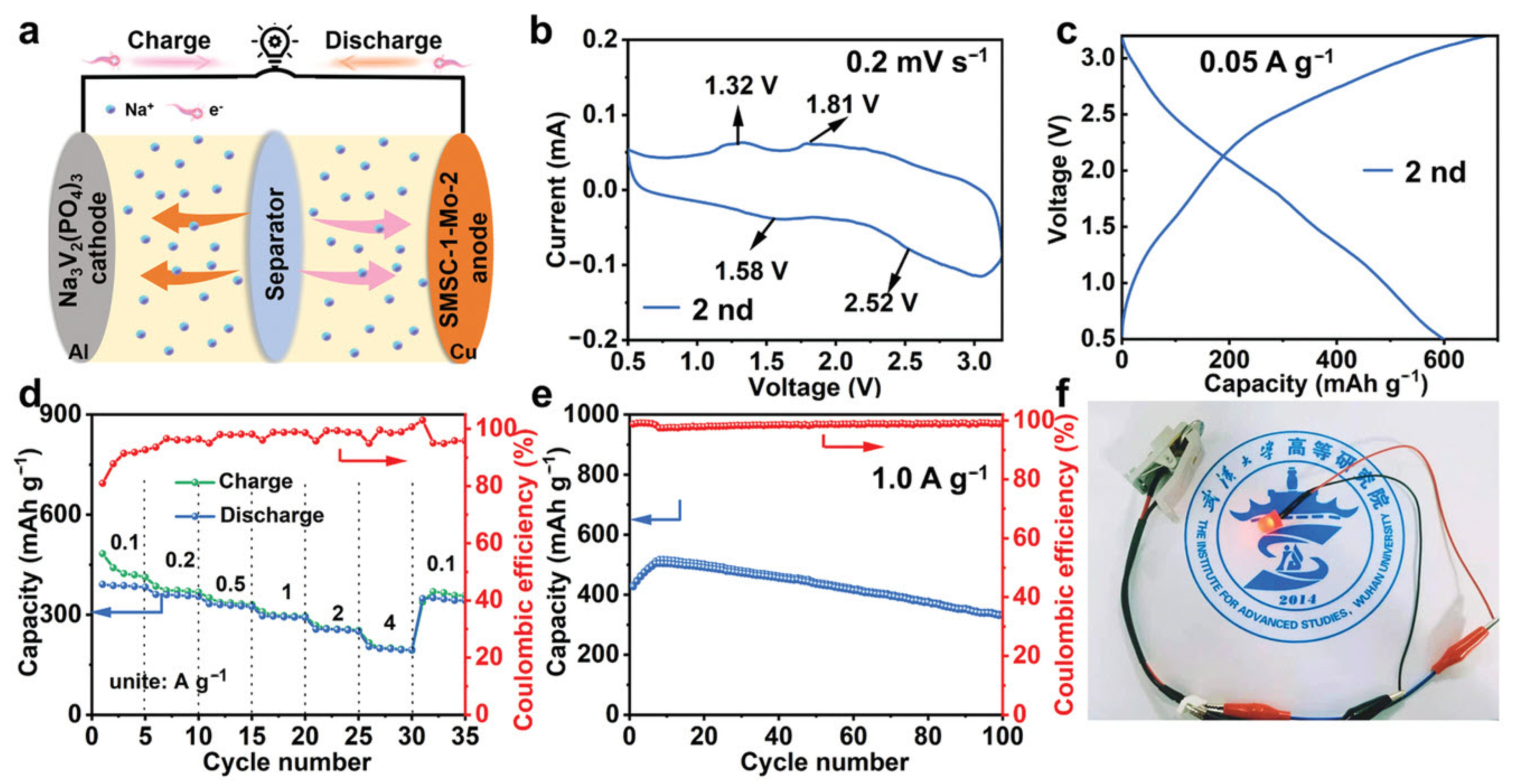
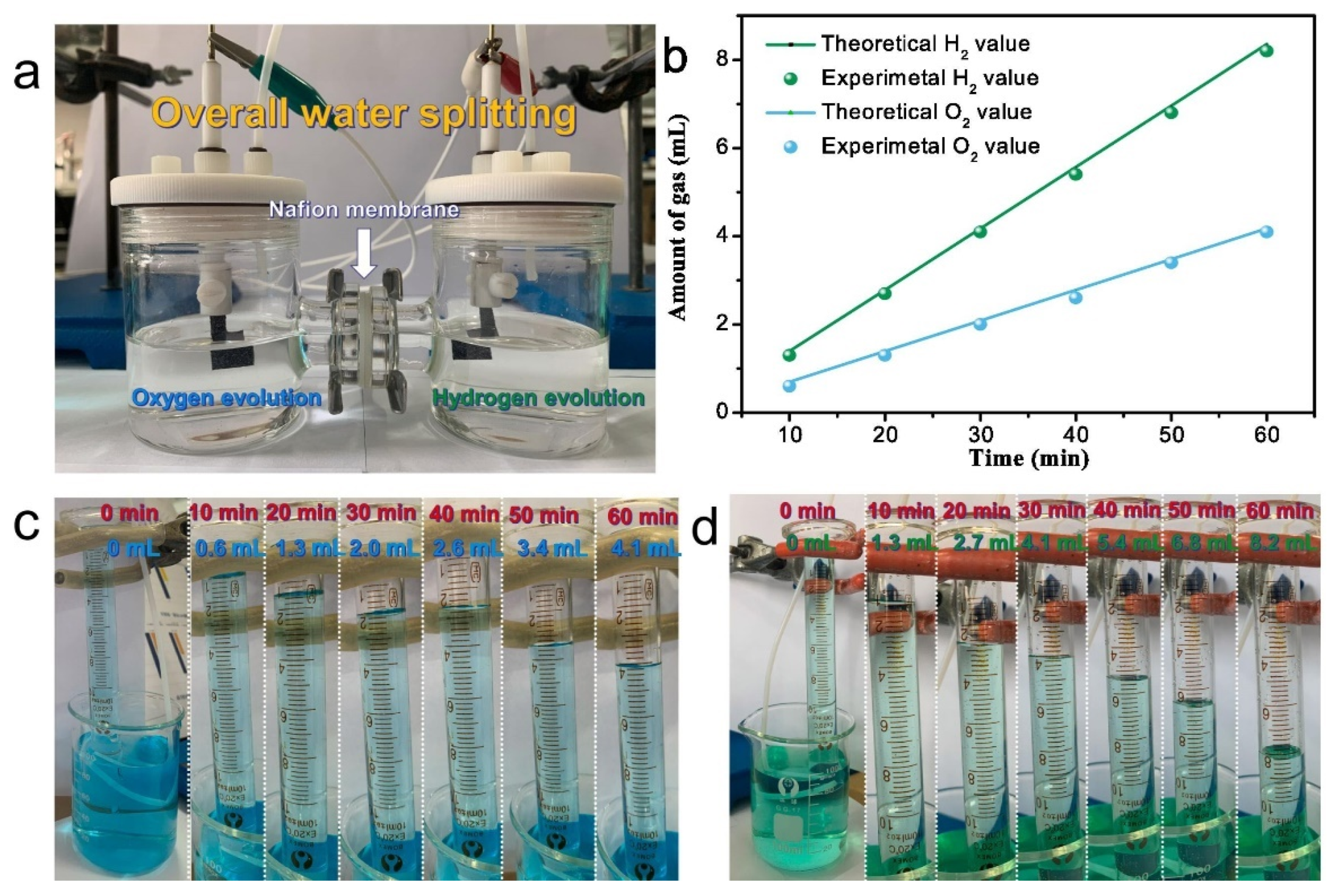
| Transition Metal Compounds | Organic Compounds | Prussian Blue Derivatives | Polyanionic Compounds | |
|---|---|---|---|---|
| Structure | 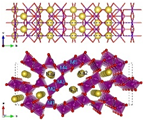 | 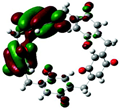 | 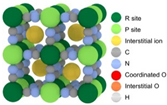 | 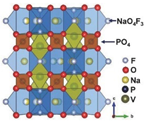 |
| Advantage | High reversible specific capacity High energy density High odds performance Easy technology transformation | High theoretical capacity Low cost Environmentally benign Structural versatility | The working voltage is adjustable High reversible specific capacity High energy density Low synthesis temperature | High working voltage Good thermal stability Good circulation Good air stability |
| Shortcoming | Easy to absorb moisture The cycle performance is slightly poor | Poor conductivity Low voltage platform | Poor conductivity Low coulomb efficiency | Low reversible capacity ratio Some contain toxic elements |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Qu, J.; Li, M. National Policies, Recent Research Hotspots, and Application of Sustainable Energy: Case of China, USA, and European Countries. Sustainability 2022, 14, 10014. https://doi.org/10.3390/su141610014
Xu M, Qu J, Li M. National Policies, Recent Research Hotspots, and Application of Sustainable Energy: Case of China, USA, and European Countries. Sustainability. 2022; 14(16):10014. https://doi.org/10.3390/su141610014
Chicago/Turabian StyleXu, Min, Jinjun Qu, and Mai Li. 2022. "National Policies, Recent Research Hotspots, and Application of Sustainable Energy: Case of China, USA, and European Countries" Sustainability 14, no. 16: 10014. https://doi.org/10.3390/su141610014
APA StyleXu, M., Qu, J., & Li, M. (2022). National Policies, Recent Research Hotspots, and Application of Sustainable Energy: Case of China, USA, and European Countries. Sustainability, 14(16), 10014. https://doi.org/10.3390/su141610014








