Abstract
The process of melt quenching is utilized in the preparation of the PbO-TeO2-MgO-Na2O-B2O3 glasses. The effect of PbO and B2O3 on the physical, structural, and radiation shielding properties of present glasses have been presented in this study. As the lead concentration rises, both the density and the molecular weight rise, climbing from 3.283 to 3.923 g/cm3 and from 105.638 to 128.675 g, respectively. The utilization of PbO as an alternative to B2O3 contributes to an increase in the overall number of at-oms, which in turn contributes to an increase in the molar volume. The XRD spectra show that the samples are amorphous. The different bending and stretching vibrations of the bonds present in the samples are shown by the FTIR spectra. The mass attenuation coefficient (MAC), linear attenuation coefficients (LAC), half-value layers (HVL), and effective atomic number (Zeff) were calculated using Phy-X software within the energy range 0.284–2.506 MeV. These obtained verdicts advocate that pre-pared Pb4 glass containing the highest concentration of PbO showed supreme shielding ability comparing the rest of the pre-pared glasses. According to these results, it can be said that PbO and B2O3 are the weighty additive composites for glass composition in the interest of radiation shielding.
1. Introduction
Recently, there have been significant advances in materials science and nuclear science. A significant portion of the research, in particular, has been focused on experimental fields, such as the development of improvements in laser devices and radiation shielding.
Several glass systems are used in various applications such as solid-state lasers, optical switches, color displays, optical waveguides, nuclear diagnostics in the medical field, and radiation monitoring [1,2,3]. In addition, people are frequently exposed to hazardous radiation such as γ and X in various fields such as nuclear power plants, cancer centers, examination centers, and some examination laboratories. To avoid dangerous and harmful radiation, proper storage and protection are important and essential [4,5,6]. For a certain level of safety during the use and exposure to these rays, (ICRP) the International Commission on Radiological Protection has approved what is called these radiations.
Depending on the type and strength of radiation, different types of heavy concrete such as barite, magnetite, and serpentine were previously used to protect people from harmful radiation. Although concrete can be designed flexibly, it also has its disadvantages, such as requiring more space, high water content, opacity to visible light, and easy cracking [7,8]. The water content in concrete greatly affects its density and strength. The absorption of radiation by concrete makes it hotter when there is no water in it. Due to its many advantages, such as the low coefficient of thermal expansion, transparency to visible light, small footprint, non-toxicity, and low cost, glass can be used efficiently to overcome the disadvantages of concrete in shielding materials from harmful ionizing radiation [9,10,11,12,13]. For a material to serve as a shield, it should have an adequate and suitable thickness to attenuate the incident radiation from the source. To achieve an acceptable degree of luminescence in the electromagnetic spectrum, borate oxide glasses have been found to be very suitable hosts for rare earth ion doping. Other important properties such as refractive index, rare earth ion solubility, glass density, and glass transition temperature can also be increased [14].
Due to its higher atomic number (Z = 82), lead improves the ability to attenuate γ-radiation. Mechanical strength, thermal stability, and network strength are desirable requirements for radiation attenuators.
Attributed to these noteworthy features of glasses, researchers have invented a suitable glass system by adding numerous composites to improve the ionizing radiation shielding. A few noteworthy works have been abridged here. In 2021, Alotaibi et al. have examined an innovative glass system TeO2–CdO-PbO-B2O3. The addition of PbO instead of B2O3 on these studied glass systems enhanced the shielding ability as well as reduced the transmission factor. Moreover, the increase in the amount of PbO and the enhancement of sample thickness reduced the transmission factor (TF) [15]. During the next year, Fidan et al. studied the arch on the TeO2-BaO-B2O3-PbO-V2O5 glass systems. The enhancement of mol% of BaO decreased the intensity of the EPR spectra. Obtained results advocated that researched glass systems showed a capable possibility for the shielding of gamma, neutron, proton, and alpha radiation. BaO revealed the utmost reflective compound among the researched glasses, considering albedo parametric value [16]. In 2019, Sayyed et al. studied the glass systems with the composition of (50 + x) PbO–5 WO3–5 BaO–10 Na2O–(30-x) B2O3. Sample Pb50B30 showed more radiation attenuation ability due to gaining a greater effective atomic number by holding the higher amount of PbO [17]. In 2020, Singh et al. assessed Al2O3-PbO-B2O3 glass systems and obtained an outcome that the greater amount of WO3 is of utmost apt for shielding against gamma radiation as well as elastic constants and VHN [18]. In 2022, Aboud et al. calculated the γ-radiation shielding characteristics of (70-x) B2O3-10BaO-10Bi2O3-10CdO-xPbO glasses. Their obtained results showed that glass holding the supreme quantity of PbO revealed the best shielding efficiency [19]. In the same year, Showahy et al. researched on the radiological, mechanical, and structural traits of the (72.5-X) B2O3–20Bi2O3–7Al2O3–0.5CuO–x PbO (0 ≤ x ≤ 12 mol%) glass systems. Studied glass samples’ XRD spectra didn’t make clear crystalline peaks. The enlarged quantity of PbO on the worked glass systems lessened Young’s modulus, as well as the longitudinal ultrasonic velocity. The growing amount of PbO content on these worked glass systems increased the mass attenuation coefficient; moreover, lessened the half-value layer [20]. Furthermore, in that year, Geidam et al. explored the structural features of bismuth oxide containing lead-free silica boro tellurite glass systems (Bi2O3–SiO2–B2O3–TeO2). A greater amount of bismuth displayed terrific shielding properties against gamma radiation. The raises of mol% of Bi2O3 exposed nonlinear variation for the molar volume and density values [21]. In this work, the effect of PbO and B2O3 on the physical, structural, and radiation shielding properties have been studied of PbO-TeO2-MgO-Na2O-B2O3 glass systems.
2. Materials and Methods
The melt quenching method was used in the production of PbO-TeO2-MgO-Na2O-B2O3 glasses [22,23]. The AR-grade PbO, TeO2, MgO, NaOH and H3BO3 chemicals of Loba brand were procured from Laboratory Instruments and Chemicals at Ambala Cantt, Haryana, India. On an electronic scale with a resolution of 0.001 milligrams, high-purity chemicals were measured and recorded. In order to get a combination that was consistent throughout, it was first combined in an agate mortar and then heated in a muffle furnace for thirty minutes at 980 °C. In an annealing furnace, a graphite mold kept at 400 °C that had been filled with the molten mixture for two hours. After turning off the furnace, the samples were cooled to room temperature. The photographs of the samples that were made are shown in Figure 1. For the purpose of determining the samples’ densities, the Archimedes principle was used [24,25]. The amount of the chemical composition of the glass systems (PbO-TeO2-MgO-Na2O-B2O3) has been given in Table 1.
Figure 1.
Picture of PbO-TeO2-MgO-Na2O-B2O3 samples.

Table 1.
Compositional amount of the PbO-TeO2-MgO-Na2O-B2O3 glasses.
Using a copper source and a proportional detector, XRD spectra were obtained using the PAN analytical X-Pert Pro powder.
The spectra were collected using a Thermo Nicolet 380 FTIR spectrophotometer with a wavelength range of 4000 to 400 cm−1.
3. Results and Discussion
3.1. Physical Properties
It was observed that the density and molecular weight increased from 3.283–3.923 (g/cm3) and 105.638–128.675 (g) as lead concentration increased (Table 2). This increasing behavior may have been due to the replacement of boron atoms, which had a smaller atomic mass (10.811 u) with a higher atomic mass of lead (207.2 u) [26]. The substitution of PbO in place of B2O3 also increased the total number of atoms, which in turn increased the molar volume [27]. The ion concentration increased from 3.743–6.426 (×1021 ions cm−3) with lead content. Average boron separation decreased from 3.766–3.473 (×10−8 m) with lead oxide. The polaron radius and interionic distance decreased from 2.595–2.167 (×10−8 m) and 6.441–5.379 (×10−8 m) with the increase in ion concentration. Both these factors depended on the ion concentration. As the number of ions per unit volume increased, both these parameters decreased. Field strength increased from 4.455–6.387 (×1015 cm2). which was due to the decrease in Pb-O bond distance and increase in Pb-O bond strength [28].

Table 2.
Physical properties of the glasses.
3.2. Structural Properties
To further understand their amorphous character, the XRD plots of the glasses are shown in Figure 2. The lack of prominent peaks implied that all the samples were amorphous.
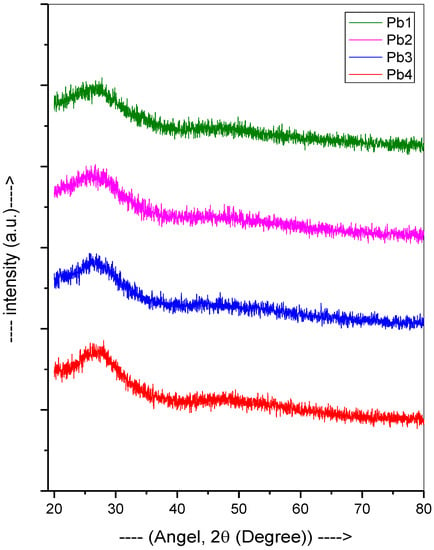
Figure 2.
XRD plot of the PbO-TeO2-MgO-Na2O-B2O3 samples.
The recorded FTIR spectra are shown in Figure 3. The major band at ~670 cm−1 in the lower wavenumber region was observed, which attributed to the bending vibrations of O-B-O bond and stretching vibrations of Te-O bonds in TeO4 units [29]. The other intense band was observed in mid wavenumber region at ~1000 cm−1, which clearly indicated the presence of stretching vibrations of B-O bonds in BO4 tetrahedra [30]. The two strong bands were observed at ~1235 cm−1 and 1365 cm−1 due to asymmetric stretching vibration of B-O bonds from the ortho borate group and vibration of BO3 units [31,32]. The low wavenumber region band 600–800 cm−1 was due to the bending vibrations of B-O-B groups [33]. The mid-wavenumber region band 800–1200 cm−1 was attributed to the B-O stretching vibration of BO4 units in tri, tetra, and penta borate groups. The high wavenumber region band 1200–1600 cm−1 confirmed the presence of B-O stretching vibration of polymerized BO3 unit [34].
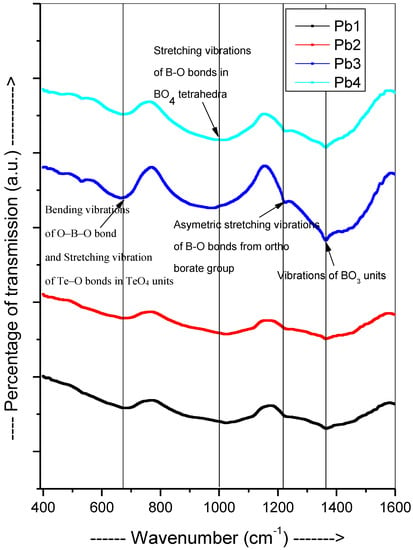
Figure 3.
FTIR plot of the PbO-TeO2-MgO-Na2O-B2O3 samples.
3.3. Radiation Shielding Study
Figure 4 plots the MAC of the investigated glass systems against increasing energy. The MAC of the tested samples was mainly affected by two factors: the energy of the incoming radiation and the concentration of PbO and B2O3. Focusing on the first factor, the maximum MAC values occurred at 0.284 MeV, and were equal to 0.252, 0.274, 0.293, and 0.310 cm2/g for Pb1 to Pb4, respectively. The MAC values then rapidly decreased to 0.183, 0.196, 0207, and 0.217 cm2/g for the same respective glasses as energy increased to 0.347 MeV. From this, we concluded that an increase in the energy of the incoming photons led to a decrease in the MAC values of the glasses. This trend was important as it indicated that the glasses were effective radiation shields against low-energy photons, with a decrease in performance when exposed to higher energy radiation. Regarding the second aforementioned factor, the Pb4 sample had the greatest MAC values at all energies, followed by Pb3, and so on, with Pb1 having the smallest MAC values. Since the Pb4 glass, which had the highest concentration of PbO, also has the highest MAC values, this correlation led to the conclusion that the glass with high amounts of PbO had a high MAC value. In other words, increasing the amount of PbO in the glass systems led to more interactions between the incoming photons and the atoms of the material, so more photons were absorbed or scattered by the glass being blocked from penetrating through the material.
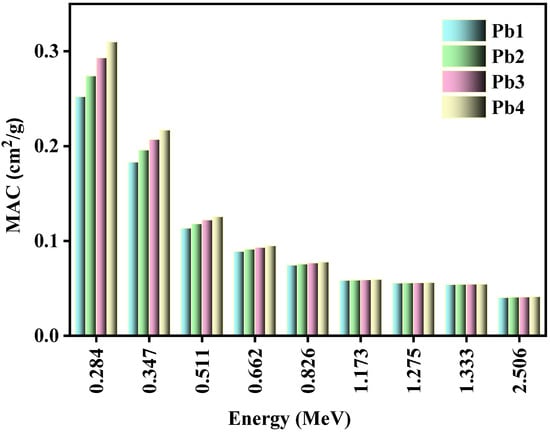
Figure 4.
The relation between the MAC and the energy for the Pb1, Pb2, Pb3, and Pb4 glasses.
The linear attenuation coefficients (LAC) of two glasses, Pb1 and Pb4, were calculated (Figure 5). These two samples were chosen since they had the lowest and highest PbO concentration, which allowed us to investigate the impact of changing the PbO concentration of the glasses on their LAC values. The figure illustrated that Pb4′s LAC was greater than Pb1′s LAC at all tested energies. These results reinforced the idea that adding PbO to the glasses led to the photons interacting with the glasses with a higher probability, enhancing their attenuation capabilities. From these results, it could be reasoned that high-density glass had a desirable attenuation performance, and thus it was recommended to use a high PbO concentration when preparing a glass system for radiation shields. Additionally, the figure also showed the impact of energy on the LAC values of glasses with varying PbO content. More specifically, at 0.284 MeV, in the low energy range, Pb4′s LAC was almost double Pb1′s. At 0.511 MeV, in the middle energy range, Pb4′s LAC was 1.3 times Pb1′s, and at 1.173 MeV, in the high energy range, Pb4′s LAC was 1.2 times Pb1′s. These results indicated that the advantage of a higher PbO concentration was more pronounced at lower energies, and as energy increased, this advantage became less recognizable.
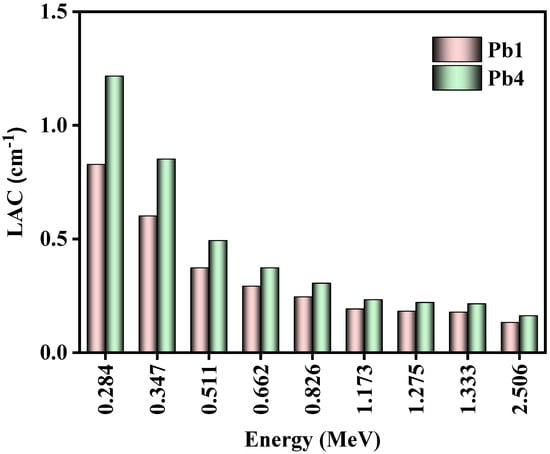
Figure 5.
The LAC for Pb1 and Pb4 glasses.
Figure 6 illustrated the relationship between the Zeff and the energy of the incoming photons. As the PbO concentration increased, the Zeff values increase, as B (Z = 5) was replaced with Pb (Z = 82). More specifically, the respective Zeff values of Pb1 and Pb4 were equal to 26.21 and 37.72 at 0.284 MeV, 22.14 and 32.22 at 0.347 MeV, and 13.78 and 19.00 at 1.333 MeV. Furthermore, at low energies, the probability of the incoming photons interacting with the glasses was higher than at higher energies, since Zeff was high at low energies, and this probability decreased as energy increased, leading to a smaller Zeff at higher energies (0.826–2.506 MeV).
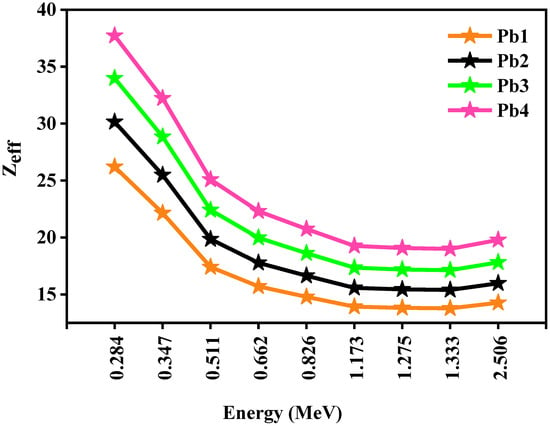
Figure 6.
The Zeff for the Pb1, Pb2, Pb3, and Pb4 glasses.
Figure 7 graphs the HVL of the glasses as a function of the PbO content. At 0.284 MeV, adding 15 mol% PbO to the glass system (from 20 to 35 mol%) reduced the HVL from 0.837 cm to 0.570 cm. In other words, adding 15 mol% PbO cut the thickness of the glass needed in half. The same trend could be observed at 0.347 MeV, where the glass with 20 mol% PbO had an HVL of 1.153 cm, while the glass with 35 mol% PbO had an HVL of 0.814 cm, a reduction by a factor of 0.7.
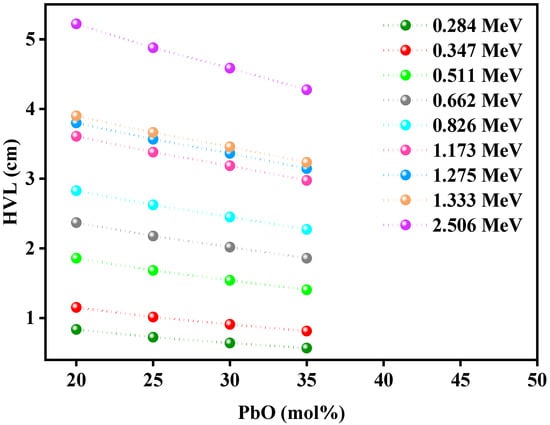
Figure 7.
The HVL for the Pb1, Pb2, Pb3, and Pb4 glasses as a function of PbO.
Figure 8 demonstrated the effect of PbO content on the TVL of the glasses at 0.284 MeV. Similar trends could be observed at other energies, which was why only this specific energy was chosen. At this energy, the TVL for Pb1 was equal to 2.780 cm, while the TVL for Pb4 was equal to 1.893 cm. This result confirmed the conclusion from the previous figure that increasing the PbO led to the TVL values decreasing by a factor of 0.68 at 0.284 MeV.
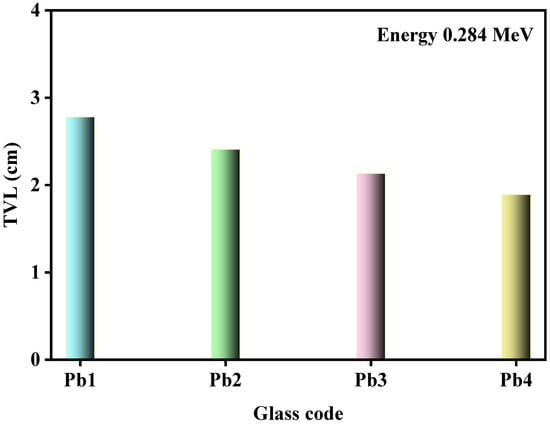
Figure 8.
The TVL of the Pb1, Pb2, Pb3, and Pb4 glasses at 0.284 MeV.
4. Conclusions
The effect of PbO and B2O3 on the physical and structural properties of PbO-TeO2-MgO-Na2O-B2O3 glasses have been presented in this study. As the lead concentration rises, both the density and the molecular weight rise, going from 3.283 to 3.923 g/cm3 and from 105.638 to 128.675 g, respectively. Increased lead content results in an increase in ion concentration from 3.743 × 1021–6.426 × 1021 ions/cm3 per unit volume. The utilization of PbO as an alternative to B2O3 contributes to an increase in the overall number of atoms, which in turn contributes to an increase in the molar volume. According to the XRD spectra, the materials have an amorphous structure in their natural state. The FTIR spectra provides the information on the numerous bending and stretching vibrations that are exhibited by the bonds present in the samples. the substances being analyzed. The MAC, LAC, TVL, and Zeff were calculated. The maximum MAC values occurred at 0.284 MeV, and are equal to 0.252, 0.274, 0.293, and 0.310 cm2/g for Pb1 to Pb4, respectively. The MAC values then rapidly decreased to 0.183, 0.196, 0207, and 0.217 cm2/g for the same respective glasses as energy increased to 0.347 MeV. The MAC results demonstrated that increasing the amount of PbO led to more interactions between the incoming photons and the atoms of the material, so more photons were absorbed or scattered by the glass, being blocked from penetrating through the material. From the HVL results, we found that adding 15 mol% PbO to the glass system reduces the HVL at 0.284 MeV from 0.837 cm to 0.570 cm. The shielding parameters showed that prepared Pb4 glass, which had the highest concentration of PbO showed supreme shielding ability comparing the rest of the prepared glasses.
Author Contributions
Data curation, M.I.S.; Formal analysis, A.K.; Funding acquisition, H.A.-G. and A.H.A.; Investigation, S.Y.; Project administration, A.H.A.; Supervision, H.A.-G.; Writing—original draft, M.I.S., S.Y. and B.O.E.; Writing—review & editing, A.K., S.Y. and B.O.E. All authors have read and agreed to the published version of the manuscript.
Funding
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R28), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R28), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abouhaswa, A.S.; Kavaz, E. Bi2O3 effect on physical, optical, structural and radiation safety characteristics of B2O3-Na2O-ZnO-CaO glass system. J. Non-Cryst. Solids 2020, 535, 119993. [Google Scholar] [CrossRef]
- Al-Yousef, H.A.; Alotiby, M.; Hanfi, M.Y.; Alotaibi, B.M.; Mahmoud, K.A.; Sayyed, M.I.; Al-Hadeethi, Y. Effect of the Fe2O3 addition on the elastic and gammaray shielding features of bismuth sodium-borate glass system. J. Mater. Sci. Mater. Electron. 2021, 32, 6942–6954. [Google Scholar] [CrossRef]
- Rajesh, M.; Kavaz, B.E.; Raju, D.P. Photoluminescence, radiative shielding properties of Sm3+ ions doped fluoroborosilicate glasses for visible (reddish-orange) display and radiation shielding applications. Mater. Res. Bull. 2021, 142, 111383. [Google Scholar] [CrossRef]
- Abouhaswa, A.S.; Kavaz, E. A novel B2O3-Na2O-BaO-HgO glass system: Synthesis, physical, optical and nuclear shielding features. Ceram. Int. 2020, 46, 16166–16177. [Google Scholar] [CrossRef]
- Kavaz, E.; Ghanim, E.H.; Abouhaswa, A.S. Optical, structural and nuclear radiation security properties of newly fabricated V2O5-SrO-PbO glass system. J. Non-Cryst. Solids 2020, 538, 120045. [Google Scholar] [CrossRef]
- Kaewjang, S.; Maghanemi, U.; Kothan, S.; Kim, H.; Limkitjaroenporn, P.; Kaewkhao, J. New gadolinium based glasses for gamma-rays shielding materials. Nucl. Eng. Des. 2014, 280, 21–26. [Google Scholar] [CrossRef]
- Gökç, H.S.; Canbaz-Öztürk, B.; Çam, N.F.; Andiç-Çakır, Ö. Gamma-ray attenuation coefficients and transmission thickness of high consistency heavyweight concrete containing mineral admixture. Cem. Concr. Compos. 2018, 92, 56–69. [Google Scholar] [CrossRef]
- Gökçe, H.S.; Yalçınkaya, Ç.; Tuyan, M. Optimization of reactive powder concrete by means of barite aggregate for neutrons and gamma rays. Constr. Build. Mater. 2018, 189, 470–477. [Google Scholar] [CrossRef]
- Albarzan, B.; Hanfi, M.Y.; Almuqrin, A.H.; Sayyed, M.I.; Alsafi, H.M.; Mahmoud, K.A. The Influence of Titanium Dioxide on Silicate-Based Glasses: An Evaluation of the Mechanical and Radiation Shielding Properties. Materials 2021, 14, 3414. [Google Scholar] [CrossRef]
- Chanthima, N.; Kaewkhao, J.; Limkitjaroenporn, P.; Tuscharoen, S.; Kothan, S.; Tungjai, M.; Kaewjaeng, S.; Sarachai, S.; Limsuwan, P. Development of BaO–ZnO–B2O3 glasses as a radiation shielding material. Radiat. Phys. Chem. 2017, 137, 72–77. [Google Scholar] [CrossRef]
- Kaewjaeng, S.; Chanthima, N.; Thongdang, J.; Reungsri, S.; Kothan, S.; Kaewkhao, J. Synthesis and radiation properties of Li2O-BaO-Bi2O3-P2O5 glasses. Mater. Today Proc. 2021, 43, 2544–2553. [Google Scholar] [CrossRef]
- Almuqrin, A.; Hanfi, M.; Mahmoud, K.; Sayyed, M.; Al-Ghamdi, H.; Aloraini, D. The Role of La2O3 in Enhancement the Radiation Shielding Efficiency of the Tellurite Glasses: Monte-Carlo Simulation and Theoretical Study. Materials 2021, 14, 3913. [Google Scholar] [CrossRef]
- Akyildirim, H.; Kavaz, E.; El-Agawany, F.; Yousef, E.; Rammah, Y. Radiation shielding features of zirconolite silicate glasses using XCOM and FLUKA simulation code. J. Non-Crystalline Solids 2020, 545, 120245. [Google Scholar] [CrossRef]
- Mariselvam, K.; Liu, J. Concentration effect of Tm3+ ions doped B2O3-Li2CO3-BaCO3-CaF2-ZnO glasses: Blue laser and radiation shielding investigations. Opt. Laser Technol. 2022, 154, 108262. [Google Scholar] [CrossRef]
- Alotaibi, B.M.; Alotiby, M.; Kumar, A.; Mahmoud, K.A.; Sayyed, M.I.; Al-Yousef, H.A.; Al-Hadeethi, Y. Gamma-ray shielding, physical, and structural characteristics of TeO2–CdO–PbO–B2O3 glasses. Opt. Mater. 2021, 119, 111333. [Google Scholar] [CrossRef]
- Fidan, M.; Acikgoz, A.; Demircan, G.; Yilmaz, D.; Aktas, B. Optical, structural, physical, and nuclear shielding properties, and albedo parameters of TeO2–BaO–B2O3–PbO–V2O5 glasses. J. Phys. Chem. Solids 2022, 163, 110543. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Aşkın, A.; Ali, A.M.; Kumar, A.; Rashad, M.; Alshehri, A.M.; Singh, M. Extensive study of newly developed highly dense transparent PbO-WO3-BaO-Na2O-B2O3 glasses for radiation shielding applications. J. Non-Cryst. Solids 2019, 521, 119521. [Google Scholar] [CrossRef]
- Singh, G.P.; Singh, J.; Kaur, P.; Kaur, S.; Arora, D.; Kaur, R.; Singh, D.P. Analysis of enhancement in gamma-ray shielding proficiency by adding WO3 in Al2O3-PbO-B2O3 glasses using Phy-X/PSD. J. Mater. Res. Technol. 2020, 9, 14425–14442. [Google Scholar] [CrossRef]
- Aboud, H.; Aldhuhaibat, M.J.; Alajermi, Y. Gamma radiation shielding traits of B2O3–Bi2O3–CdO–BaO–PbO glasses. Radiat. Phys. Chem. 2022, 191, 109836. [Google Scholar] [CrossRef]
- Showahy, A.A.; Elsaman, R.; Issa, S.A.; El-Denglawey, A.; Saddeek, Y.B. Effect of PbO on the elastic and radiation shielding properties of B2O3–Bi2O3–Al2O3–CuO glasses. Radiat. Phys. Chem. 2022, 196, 110129. [Google Scholar] [CrossRef]
- Geidam, I.; Matori, K.; Halimah, M.; Chan, K.; Muhammad, F.; Ishak, M.; Umar, S. Oxide ion polarizabilities and gamma radiation shielding features of TeO2–B2O3–SiO2 glasses containing Bi2O3 using Phy-X/PSD software. Mater. Today Commun. 2022, 31, 103472. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Sayyed, M.I.; Kumar, A.; Mahmoud, K.A.; Olarinoye, O.I.; Alhuthali, A.; Al-Hadeethi, Y. A comprehensive investigation on the role of PbO in the structural and radiation shielding attribute of P2O5–CaO–Na2O–K2O–PbO glass system. J. Mater. Sci. Mater. Elec. 2021, 32, 12371–12382. [Google Scholar] [CrossRef]
- Almuqrin, A.; Albarzan, B.; Olarinoye, O.; Kumar, A.; Alwadai, N.; Sayyed, M. Mechanical and Gamma Ray Absorption Behavior of PbO-WO3-Na2O-MgO-B2O3 Glasses in the Low Energy Range. Materials 2021, 14, 3466. [Google Scholar] [CrossRef]
- Al-Harbi, F.F.; Prabhu, N.S.; Sayyed, M.I.; Almuqrin, A.H.; Kumar, A.; Kamath, S.D. Evaluation of structural and gamma ray shielding competence of Li2O-K2O-B2O3-HMO (HMO = SrO/TeO2/PbO/Bi2O3) glass system. Optik 2021, 248, 168074. [Google Scholar] [CrossRef]
- Aloraini, D.A.; Almuqrin, A.H.; Sayyed, M.I.; Al-Ghamdi, H.; Kumar, A.; Elsafi, M. Experimental Investigation of Radiation Shielding Competence of Bi2O3-CaO-K2O-Na2O-P2O5 Glass Systems. Materials 2021, 14, 5061. [Google Scholar] [CrossRef]
- Al-Buriahi, M.S.; Alajerami, Y.S.M.; Abouhaswa, A.S.; Alalawi, A.; Nutaro, T.; Tonguc, B. Effect of chromium oxide on the physical, optical, and radiation shielding properties of lead sodium borate glasses. J. Non-Cryst. Solids 2020, 544, 120171. [Google Scholar] [CrossRef]
- Subhadra, M.; Sulochana, S.; Kistaiah, P. Effect of V2O5 content on physical and optical properties of lithium bismuth borate glasses. Mater. Today Proc. 2018, 5, 26417–26423. [Google Scholar] [CrossRef]
- Naseer, K.A.; Marimuthu, K.; Al-Buriahi, M.S.; Alalawi, A.; Tekin, H.O. Influence of Bi2O3 concentration on barium-telluro-borate glasses: Physical, structural and radiation-shielding properties. Ceram. Int. 2021, 47, 329–340. [Google Scholar] [CrossRef]
- Devaraja, C.; Gowda, G.J.; Keshavamurthy, K.; Eraiah, B.; Devarajulu, G.; Jagannath, G. Physical, structural and photo luminescence properties of lead boro-tellurite glasses doped with Eu3+ ions. Vacuum 2020, 177, 109426. [Google Scholar] [CrossRef]
- Gaafar, M.; Marzouk, S. Mechanical and structural studies on sodium borosilicate glasses doped with Er2O3 using ultrasonic velocity and FTIR spectroscopy. Phys. B Condens. Matter 2007, 388, 294–302. [Google Scholar] [CrossRef]
- Pawar, P.P.; Munishwar, S.R.; Gautam, S.; Gedam, R.S. Physical, Thermal, Structural and Optical Properties of Dy 3+ doped Lithium Alumino-borate Glasses for Bright W-LED. J. Lumin. 2017, 183, 79–88. [Google Scholar] [CrossRef]
- Singh, S.; Kalia, G.; Singh, K. Effect of intermediate oxide (Y2O3) on thermal, structural and optical properties of lithium borosilicate glasses. J. Mol. Struct. 2015, 1086, 239–245. [Google Scholar] [CrossRef]
- Khan, S.; Kaur, G.; Singh, K. Effect of ZrO2 on dielectric, optical and structural properties of yttrium calcium borosilicate glasses. Ceram. Int. 2017, 43, 722–727. [Google Scholar] [CrossRef]
- Kaur, R.; Singh, S.; Pandey, O.P. Influence of CdO and gamma irradiation on the infrared absorption spectra of borosilicate glass. J. Mol. Struct. 2013, 1049, 409–413. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).