Elicitation Promoability with Gamma Irradiation, Chitosan and Yeast to Perform Sustainable and Inclusive Development for Marjoram under Organic Agriculture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Practical Field Experiment
2.2. Biomass Yield Production
2.3. Bioactive Secondary Metabolites Production (BSMs)
2.3.1. Phenolics (TPC)
2.3.2. Flavonoids (TFC)
2.3.3. Flavonols (TFL)
2.3.4. Tannins (TAE)
2.3.5. Essential Oils (EO)
3. Statistical Analysis
4. Results and Discussion
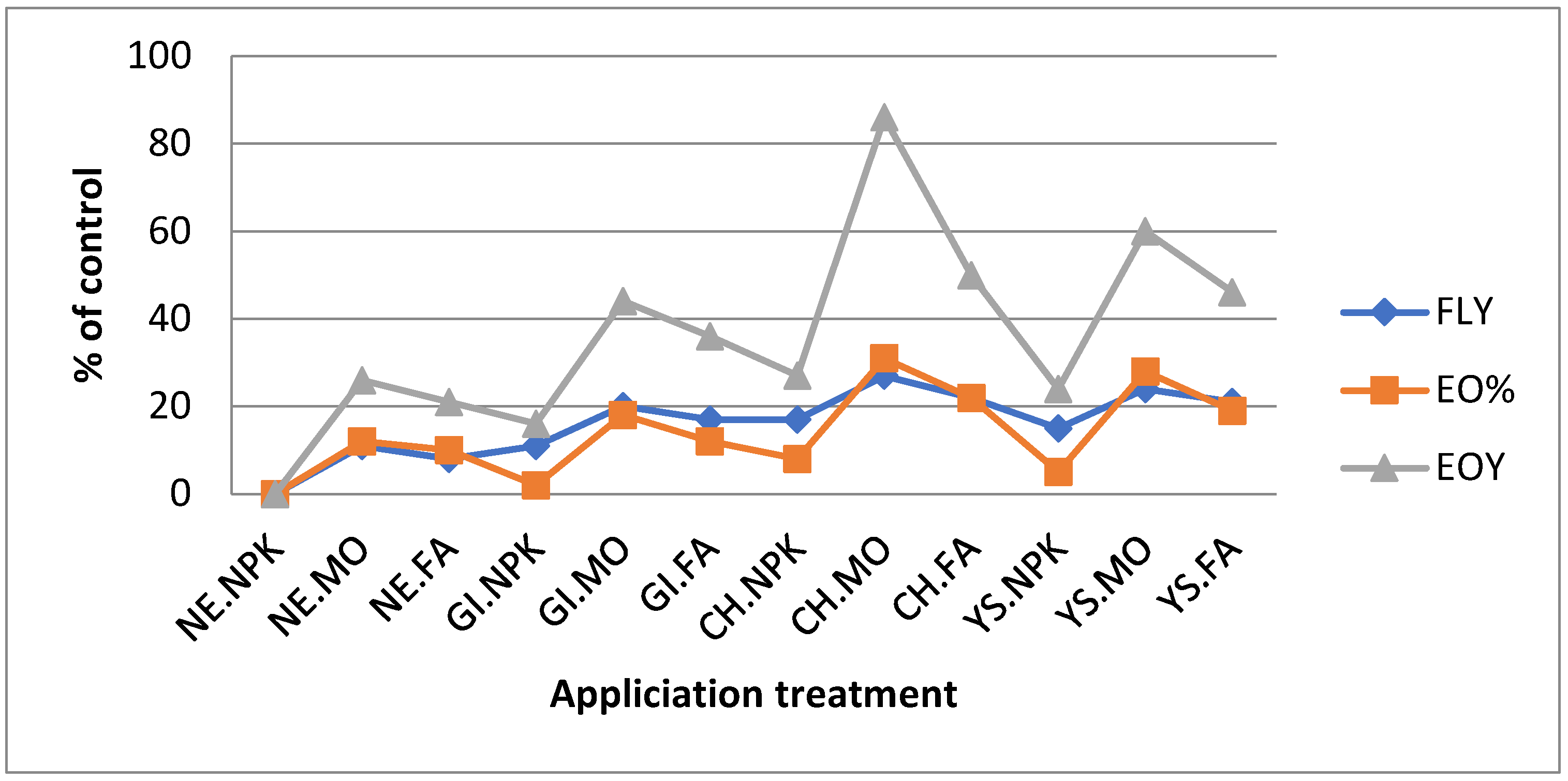
4.1. Biomass Yield Production
4.2. Essential oils % (EO%)
4.3. Essential Oils Yield (EOY, Kg/m2)
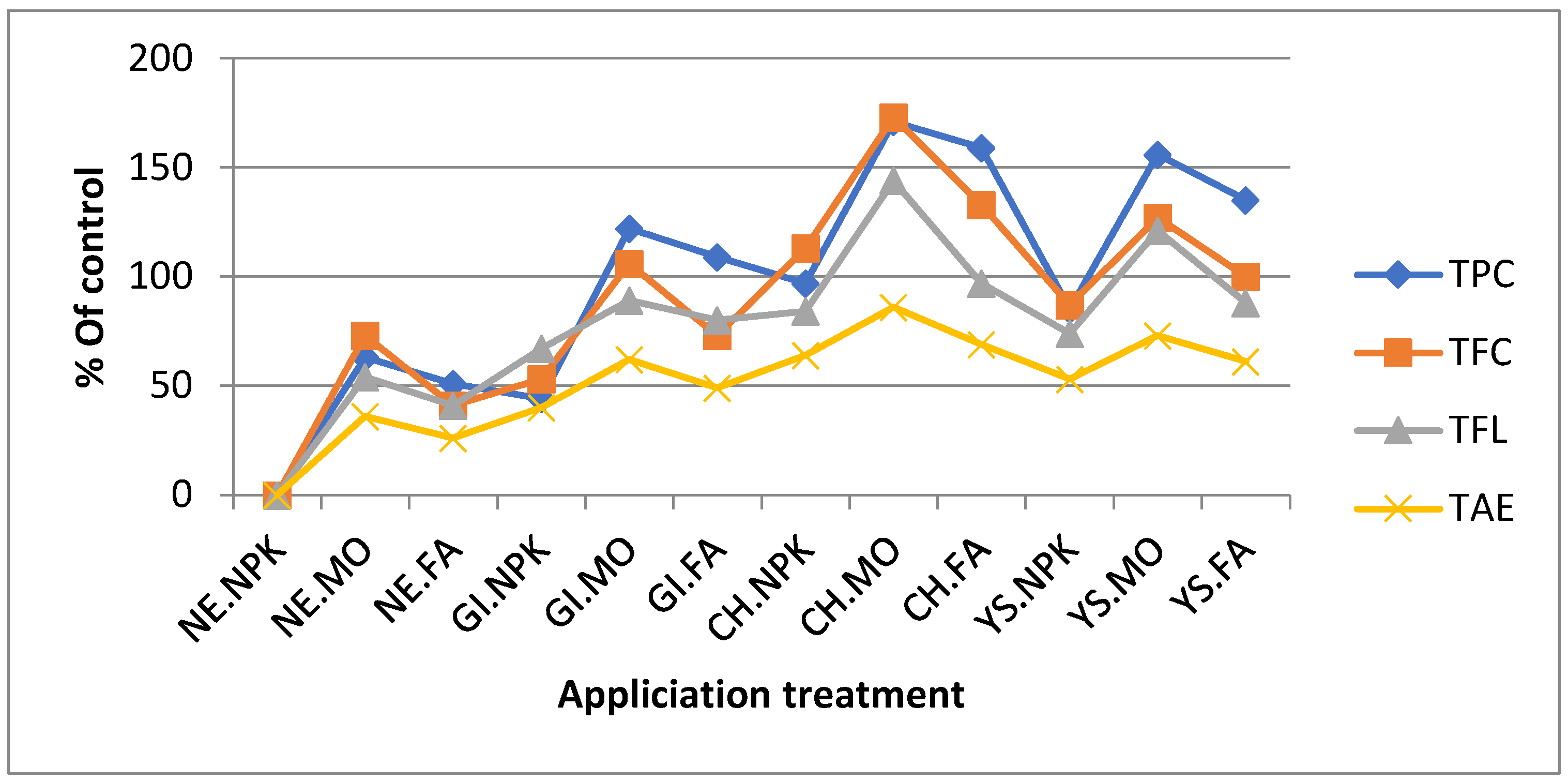
4.4. Quali–Quantitative Bioactive Secondary Metabolites (BSMs)
4.4.1. Essential Oil Contents (EOC)
4.4.2. Total Phenolic (TPC)
4.4.3. Total Flavonoids Content (TFC)
4.4.4. Total Flavonols (TFL)
4.4.5. Tannin (TAN):
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleh, H.M.; Mahmoud, H.H.; Abdou, M.I.; Eskander, S.B. Health Risk Assessment Based on Metal Analysis of Soil and Crops in Al-Dakhla Oasis. Arab. J. Geosci. 2021, 14, 260. [Google Scholar] [CrossRef]
- Saleh, H.M.; Eskander, S.B.; Mahmoud, H.H.; Abdou, M.I. Groundwater Quality and Health Assessments Based on Heavy Metals and Trace Elementscontent in DakhlaOasis, NewValley Governorate, Egypt. Water Sci. 2022, 36, 1–12. [Google Scholar] [CrossRef]
- Saleh, H.M.; Mahmoud, H.H.; Aglan, R.F.; Bayoumi, T.A. Biological Treatment of Wastewater Contaminated with Cu(II), Fe(II) and Mn(II) Using Ludwigia Stolonifera Aquatic Plant. Environ. Eng. Manag. J. 2019, 18, 1327–1336. [Google Scholar] [CrossRef]
- Saleh, H.M.; Moussa, H.R.; El-Saied, F.A.; Dawoud, M.; Nouh, E.S.A.; Abdel Wahed, R.S. Adsorption of Cesium and Cobalt onto Dried Myriophyllum Spicatum L. from Radio-Contaminated Water: Experimental and Theoretical Study. Prog. Nucl. Energy 2020, 125, 103393. [Google Scholar] [CrossRef]
- Saleh, H.M.; Aglan, R.F.; Mahmoud, H.H. Qualification of Corroborated Real Phytoremediated Radioactive Wastes under Leaching and Other Weathering Parameters. Prog. Nucl. Energy 2020, 119, 103178. [Google Scholar] [CrossRef]
- Saleh, H.M.; Moussa, H.R.; El-Saied, F.A.; Dawod, M.; Bayoumi, T.A.; Abdel Wahed, R.S. Mechanical and Physicochemical Evaluation of Solidifed Dried Submerged Plants Subjected to Extreme Climatic Conditions to Achieve an Optimum Waste Containment. Prog. Nucl. Energy 2020, 122, 103285. [Google Scholar] [CrossRef]
- Jagetia, G.C. Radioprotective Potential of Plants and Herbs against the Effects of Ionizing Radiation. J. Clin. Biochem. Nutr. 2007, 40, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Khosravian, P.; Heidari-Soureshjani, S.; Yang, Q. Effects of Medicinal Plants on Radiolabeling and Biodistribution of Diagnostic Radiopharmaceuticals: A Systematic Review. Plant Sci. Today 2019, 6, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Shala, A.Y. Effect of Different Doses of Gamma Irradiation on Vegetative Growth and Oil Yield of Ocimum basilicum L. J. Plant Prod. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef] [Green Version]
- Shylaja, M.R.; Peter, K.V. The Functional Role of Herbal Spices. Handb. Herbs Spices 2004, 2, 26–45. [Google Scholar]
- Peter, K.V.; Shylaja, M.R. Introduction to Herbs and Spices: Definitions, Trade and Applications. In Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–24. [Google Scholar]
- Khan, I.A.; Abourashed, E.A. Leung’s Encyclopedia of Common Natural Ingredients: Used in Food, Drugs and Cosmetics; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 1118213068. [Google Scholar]
- Hossain, M.B.; Brunton, N.P.; Barry-Ryan, C.; Martin-Diana, A.B.; Wilkinson, M. Antioxidant Activity of Spice Extracts and Phenolics in Comparison to Synthetic Antioxidants. Rasayan J. Chem. 2008, 1, 751–756. [Google Scholar]
- Namlı, S.; Işıkalan, Ç.; Akbaş, F.; Toker, Z.; Tilkat, E.A. Effects of UV-B Radiation on Total Phenolic, Flavonoid and Hypericin Contents in Hypericum Retusum Aucher Grown under in Vitro Conditions. Nat. Prod. Res. 2014, 28, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
- Mohammadparast, B.; Rasouli, M.; Rustaiee, A.R.; Zardari, S.; Agrawal, V. Quantification of Asiatic Acid from Plant Parts of Centella Asiatica L. and Enhancement of Its Synthesis through Organic Elicitors in in Vitro. Hortic. Environ. Biotechnol. 2014, 55, 578–582. [Google Scholar] [CrossRef]
- Mejdoub-Trabelsi, B.; Touihri, S.; Ammar, N.; Riahi, A.; Daami-Remadi, M. Effect of Chitosan for the Control of Potato Diseases Caused by Fusarium Species. J. Phytopathol. 2020, 168, 18–27. [Google Scholar] [CrossRef]
- Andrys, D.; Adaszyńska-Skwirzyńska, M.; Kulpa, D. Jasmonic Acid Changes the Composition of Essential Oil Isolated from Narrow-Leaved Lavender Propagated in in Vitro Cultures. Nat. Prod. Res. 2018, 32, 834–839. [Google Scholar] [CrossRef]
- Ahamed, T.E.S.; Ahamed, E.S.S. Synergy Prospect Low Gamma Irradiation Doses Incorporating Elicitation with Iron Nanoparticles to Hyper Production Biomass Yield and Bioactive Secondary Metabolites for Cress, Medicinal Plant. J. Plant Sci. 2018, 6, 157–163. [Google Scholar]
- Ahamed, T.E.S. Bioprospecting Elicitation with Gamma Irradiation Combine with Chitosan to Enhance, Yield Production, Bioactive Secondary Metabolites and Antioxidant Activity for Saffron. J. Plant Sci. 2019, 7, 137–143. [Google Scholar]
- Sheshbahreh, M.J.; Dehnavi, M.M.; Salehi, A.; Bahreininejad, B. Effect of Irrigation Regimes and Nitrogen Sources on Biomass Production, Water and Nitrogen Use Efficiency and Nutrients Uptake in Coneflower (Echinacea purpurea L.). Agric. Water Manag. 2019, 213, 358–367. [Google Scholar] [CrossRef]
- Yadav, K.K.; Sarkar, S. Biofertilizers, Impact on Soil Fertility and Crop Productivity under Sustainable Agriculture. Environ. Ecol. 2019, 37, 89–93. [Google Scholar]
- Bombardi, L.M. Geografia Do Uso de Agrotóxicos No Brasil e Conexões Com a União Europeia; FFLCH- USP: São Paulo, Brazil, 2017; ISBN 8575063103. [Google Scholar]
- Pignati, W.A.; de Souza e Lima, F.A.N.; de Lara, S.S.; Correa, M.L.M.; Barbosa, J.R.; da Costa Leão, L.H.; Pignatti, M.G. Distribuição Espacial Do Uso de Agrotóxicos No Brasil: Uma Ferramenta Para a Vigilância Em Saúde. Cienc. Saúde Colet. 2017, 22, 3281–3293. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, P.H.B.; Alonzo, H.G.A. Rural Work and Health Risks: A Review into de”Safe Use” of Pesticides in Brazil. Cienc. Saúde Colet. 2014, 19, 4197. [Google Scholar]
- Pereira, M.M.A.; Morais, L.C.; Marques, E.A.; Martins, A.D.; Cavalcanti, V.P.; Rodrigues, F.A.; Gonçalves, W.M.; Blank, A.F.; Pasqual, M.; Dória, J. Humic Substances and Efficient Microorganisms: Elicitation of Medicinal Plants—A Review. J. Agric. Sci. 2019, 11, 268–280. [Google Scholar] [CrossRef]
- Arabshahi-Delouee, S.; Urooj, A. Antioxidant Properties of Various Solvent Extracts of Mulberry (Morus indica L.) Leaves. Food Chem. 2007, 102, 1233–1240. [Google Scholar] [CrossRef]
- Ordonez, A.A.L.; Gomez, J.D.; Vattuone, M.A. Antioxidant Activities of Sechium Edule (Jacq.) Swartz Extracts. Food Chem. 2006, 97, 452–458. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Jimoh, F.O.; Koduru, S.; Afolayan, A.J.; Masika, P.J. Antibacterial and Antioxidant Properties of the Methanol Extracts of the Leaves and Stems of Calpurnia Aurea. BMC Complement. Altern. Med. 2008, 8, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagerman, A.E.; Butler, L.G. Protein Precipitation Method for the Quantitative Determination of Tannins. J. Agric. Food Chem. 1978, 26, 809–812. [Google Scholar] [CrossRef]
- Towler, M.J.; Weathers, P.J. Variations in Key Artemisinic and Other Metabolites throughout Plant Development in Artemisia Annua L. for Potential Therapeutic Use. Ind. Crops Prod. 2015, 67, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Boghozian, A.; Amjad, L.; Shahanipour, K. Chemical Constituents and Identification of the Essential Oil of Artemisia Aucheri Boiss. in Iran. Adv. Environ. Biol. 2014, 8, 2339–2344. [Google Scholar]
- Adekiya, A.O.; Agbede, T.M.; Aboyeji, C.M.; Dunsin, O.; Ugbe, J.O. Green Manures and NPK Fertilizer Effects on Soil Properties, Growth, Yield, Mineral and Vitamin C Composition of Okra (Abelmoschus esculentus (L.) Moench). J. Saudi Soc. Agric. Sci. 2019, 18, 218–223. [Google Scholar] [CrossRef]
- Kaleri, A.A.; Baloch, A.W.; Baloch, M.; Wahocho, N.A.; Abro, T.F.; Jogi, Q.; Soomro, A.A.; Marri, A.; Bhutto, L.A. 30. Heritability and Correlation Analysis in Bt and Non-Bt Cotton (Gossypium hirsutum L.) Genotypes. Pure Appl. Biol. 2021, 5, 906–912. [Google Scholar]
- El-Mohamedy, R.S.R.; Mohamed, S.K. Effect of Moringa Oleifera Seed Oil, Root and Leave Extracts on Growth of Major Pathogenic Fungi of Tomato, Green Bean and Potato in Vitro. Int. J. Agric. Technol. 2018, 14, 505–520. [Google Scholar]
- Tarek Elsayed, S.A.; El Sayed, S.A. The Potentiality Biotic-Elicitation with Chitosan or Vitamin C to Achieve Integrated and Sustainable Development for Sage Salvia Officiealis Under Sustainable Agriculture Systems. J. Plant Sci. 2021, 9, 151–162. [Google Scholar]
- Valletta, A.; De Angelis, G.; Badiali, C.; Brasili, E.; Miccheli, A.; Di Cocco, M.E.; Pasqua, G. Acetic Acid Acts as an Elicitor Exerting a Chitosan-like Effect on Xanthone Biosynthesis in Hypericum perforatum L. Root Cultures. Plant Cell Rep. 2016, 35, 1009–1020. [Google Scholar] [CrossRef]
- Gabaston, J.; El Khawand, T.; Waffo-Teguo, P.; Decendit, A.; Richard, T.; Mérillon, J.-M.; Pavela, R. Stilbenes from Grapevine Root: A Promising Natural Insecticide against Leptinotarsa Decemlineata. J. Pest Sci. 2018, 91, 897–906. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Akila, G.; Charles, R.E. Chitosan-Induced Defence Responses in Tomato Plants against Early Blight Disease Caused by Alternaria Solani (Ellis and Martin) Sorauer. Arch. Phytopathol. Plant Prot. 2014, 47, 1963–1973. [Google Scholar] [CrossRef]
- Bondok, A.M. Response of Tomato Plants to Salicyli c Acid and Chitosan under Infection with Tomato Mosaic Virus. Am. J. Agric. Environ. Sci. 2015, 15, 1520–1529. [Google Scholar] [CrossRef]
- Firmansyah, D.; Widodo; Hidayat, S.H. Chitosan and Plant Growth Promoting Rhizobacteria Application to Control Squash Mosaic Virus on Cucumber Plants. Asian J. Plant Pathol. 2017, 11, 148–155. [Google Scholar] [CrossRef]
- Li, Y.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Li, K.; Li, P. Preparation, Characterization, and Insecticidal Activity of Avermectin-Grafted-Carboxymethyl Chitosan. BioMed Res. Int. 2016, 2016, 9805675. [Google Scholar] [CrossRef] [Green Version]
- Zaker, M. Natural Plant Products as Eco-Friendly Fungicides for Plant Diseases Control—A Review. Agriculturists 2016, 14, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Phiri, C. Influence of Moringa Oleifera Leaf Extracts on Germination and Early Seedling Development of Major Cereals. Agric. Biol. J. N. Am. 2010, 1, 774–777. [Google Scholar] [CrossRef]
- Ho, T.-T.; Lee, J.-D.; Jeong, C.-S.; Paek, K.-Y.; Park, S.-Y. Improvement of Biosynthesis and Accumulation of Bioactive Compounds by Elicitation in Adventitious Root Cultures of Polygonum Multiflorum. Appl. Microbiol. Biotechnol. 2018, 102, 199–209. [Google Scholar] [CrossRef] [PubMed]
| Application Treatment | Fresh Leaves Yield, Kg/m2 | Essential Oil % | Essential Oil Yield, Kg/m2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | PM | 2019 | 2020 | PM | 2019 | 2020 | PM | |
| NE.NPK | 5.920 | 6.200 | 6.060(0) | 0.522 | 0.524 | 0.532(0) | 3.090 | 3.294 | 3.192(0) |
| NE.MO | 6.630 | 6.820 | 6.725(11) | 0.600 | 0.592 | 0.596(12) | 3.979 | 4.038 | 4.009(26) |
| NE.FA | 6.453 | 6.696 | 6.574(8) | 0.590 | 0.582 | 0.586(10) | 3.807 | 3.897 | 3.852(21) |
| GI.NPK | 6.690 | 6.882 | 6.786(11) | 0.548 | 0.540 | 0.544(2) | 3.666 | 3.716 | 3.691(16) |
| GI.MO | 7.163 | 7.440 | 7.301(20) | 0.632 | 0.629 | 0.630(18) | 4.527 | 4.680 | 4.604(44) |
| GI.FA | 7.045 | 7.254 | 7.149(17) | 0.616 | 0.603 | 0.609(12) | 4.340 | 4.374 | 4.357(36) |
| CH.NPK | 6.926 | 7. 228 | 7.228(19) | 0.580 | 0.571 | 0.575(8) | 4.017 | 4.071 | 4.044(27) |
| CH.MO | 7.637 | 7.750 | 7.693(27) | 0.705 | 0.692 | 0.699(31) | 5.385 | 5.363 | 5.374(68) |
| CH.FA | 7.282 | 7.502 | 7.392(22) | 0.653 | 0.645 | 0.649(22) | 4.744 | 4.839 | 4.792(50) |
| YS.NPK | 6.808 | 7.316 | 7.062(17) | 0.564 | 0.556 | 0.560(5) | 3.840 | 4.068 | 3.954(24) |
| YS.MO | 7.518 | 7.564 | 7.541(24) | 0.679 | 0.676 | 0.678(28) | 5.105 | 5.113 | 5.109(60) |
| YS.FA | 7.400 | 7.378 | 7.389(21) | 0.642 | 0. 624 | 0.633(19) | 4.751 | 4.604 | 4.678(46) |
| LSD1% | 0.035 | 0.031 | 0.027 | 0.003 | 0.005 | 0.004 | 0.022 | 0.025 | 0.026 |
| Application Treatmen | Essential Oil Content from Terpinoids | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ∞-Ter-Pinene | Cineol | Y-Ter Pinene | P–Cy–Mene | Terp-Ineolene | D-Lin-Alool | Terpin-Eol 4ol | Bcar-Yophe Llene | ∞-Ter-Pineol | Tymol | Carvacol | Total% | |
| NE.NPK | 7.15 | 1.44 | 12.51 | 2.62 | 2.24 | 1.23 | 23.41 | 1.92 | 3.64 | 9.95 | 9.32 | 67.43 |
| NE.MO | 7.75 | 1.62 | 12.81 | 2.75 | 2.62 | 1.71 | 23.99 | 1.97 | 3.92 | 9.89 | 1.52 | 69.03 |
| NE.FA | 7.53 | 1.48 | 12.60 | 2.68 | 2.42 | 1.53 | 23.66 | 1.95 | 3.71 | 9.75 | 1.44 | 68.75 |
| GI.NPK | 7.75 | 1.10 | 12.79 | 2.72 | 2.64 | 1.52 | 23.89 | 1.85 | 3.75 | 9.87 | 1.70 | 69.58 |
| GI.MO | 7.92 | 1.18 | 12.91 | 2.82 | 2.86 | 1.76 | 23.93 | 1.92 | 3.89 | 9.91 | 1.87 | 71.60 |
| GI.FA | 7.89 | 1.77 | 12.99 | 2.83 | 2.71 | 1.60 | 23.95 | 1.90 | 3.81 | 9.97 | 1.62 | 71.04 |
| CH.NPK | 7.69 | 1.52 | 12.75 | 2.66 | 2.63 | 1.05 | 23.76 | 1.78 | 3.72 | 9.75 | 1.75 | 69.66 |
| CH.MO | 8.77 | 2.72 | 13.80 | 3.51 | 3.45 | 2.32 | 24.81 | 2.75 | 3.63 | 10.80 | 2.25 | 79.81 |
| CH.FA | 8.42 | 2.35 | 13.31 | 3.25 | 3.31 | 2.25 | 24.35 | 2.27 | 4.35 | 10.42 | 2.20 | 76.48 |
| YS.NPK | 7.80 | 1.72 | 12.82 | 2.71 | 2.60 | 2.55 | 23.85 | 1.88 | 3.81 | 9.86 | 1.72 | 71.32 |
| YS.MO | 8.35 | 2.18 | 13.32 | 3.15 | 3.21 | 3.32 | 24.35 | 2.27 | 4.19 | 10.25 | 2.35 | 70.94 |
| YS.FA | 8.25 | 2.11 | 13.25 | 3.12 | 3.18 | 3.27 | 24.11 | 2.15 | 4.12 | 10.17 | 2.23 | 75.86 |
| LSD1% | 0.25 | |||||||||||
| Application Treatment | TPC, mg GAE, g/DW | TFC, mg QE/DW | TFL, mg QE/gDW | TAN, mg TAE/gDW | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | PM | 2019 | 2020 | PM | 2019 | 2020 | PM | 2019 | 2020 | PM | |
| NE.NPK | 7.85 | 7.81 | 7.83(0) | 0.16 | 0.14 | 0.15(0) | 2.58 | 2.53 | 2.55(0) | 1.26 | 1.23 | 1.25(0) |
| NE.MO | 12.64 | 12.89 | 12.77(63) | 0.29 | 0.24 | 0.26(73) | 3.90 | 3.97 | 3.93(54) | 1.70 | 1.69 | 1.70(36) |
| NE.FA | 11.78 | 11.87 | 11.83(51) | 0.22 | 0.20 | 0.21(41) | 3.61 | 3.59 | 3.60(41) | 1.59 | 1.58 | 1.58(26) |
| GI.NPK | 11.15 | 11.32 | 11.24(44) | 0.24 | 0.22 | 0.23(53) | 4.26 | 4.28 | 4.27(67) | 1.74 | 1.75 | 1.75(40) |
| GI.MO | 17.27 | 17.57 | 17.42(122) | 0.33 | 0.29 | 0.31(106) | 4.82 | 4.83 | 4.82(89) | 2.03 | 2.03 | 2.03(62) |
| GI.FA | 16.25 | 16.48 | 16.37(109) | 0.28 | 0.25 | 0.26(73) | 4.59 | 4.60 | 4.59(80) | 1.85 | 1.86 | 1.86(49) |
| CH.NPK | 15.31 | 15.46 | 15.39(97) | 0.34 | 0.30 | 0.32(113) | 4.67 | 4.73 | 4.70(84) | 2.03 | 2.07 | 2.05(64) |
| CH.MO | 21.20 | 21.24 | 21.22(71) | 0.43 | 0.38 | 0.41(173) | 5.94 | 6.55 | 6.25(144) | 2.33 | 2.32 | 2.33(86) |
| CH.FA | 20.18 | 20.38 | 20.28(59) | 0.38 | 0.33 | 0.35(133) | 5.03 | 5.01 | 5.02(97) | 2.16 | 2.09 | 2.12(69) |
| YS.NPK | 14.37 | 14.60 | 14.49(85) | 0.29 | 0.26 | 0.28(87) | 4.44 | 4.43 | 4.44(74) | 1.92 | 1.89 | 1.91(53) |
| YS.MO | 19.70 | 20.15 | 19.93(125) | 0.35 | 0.32 | 0.34(127) | 5.39 | 5.87 | 5.63(121) | 2.17 | 2.14 | 2.16(73) |
| YS.FA | 18.13 | 18.67 | 18.40(135) | 0.32 | 0.29 | 0.30(100) | 4.82 | 4.78 | 4.80(88) | 2.03 | 1.99 | 2.01(61) |
| LSD1% | 0.07 | 0.08 | - | 0.04 | 0.03 | - | 0.05 | 0.06 | - | 0.06 | 0.07 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, T.E.; Ahmed, E.-S.S. Elicitation Promoability with Gamma Irradiation, Chitosan and Yeast to Perform Sustainable and Inclusive Development for Marjoram under Organic Agriculture. Sustainability 2022, 14, 9608. https://doi.org/10.3390/su14159608
Sayed TE, Ahmed E-SS. Elicitation Promoability with Gamma Irradiation, Chitosan and Yeast to Perform Sustainable and Inclusive Development for Marjoram under Organic Agriculture. Sustainability. 2022; 14(15):9608. https://doi.org/10.3390/su14159608
Chicago/Turabian StyleSayed, Tarek E., and El-Sayed S. Ahmed. 2022. "Elicitation Promoability with Gamma Irradiation, Chitosan and Yeast to Perform Sustainable and Inclusive Development for Marjoram under Organic Agriculture" Sustainability 14, no. 15: 9608. https://doi.org/10.3390/su14159608
APA StyleSayed, T. E., & Ahmed, E.-S. S. (2022). Elicitation Promoability with Gamma Irradiation, Chitosan and Yeast to Perform Sustainable and Inclusive Development for Marjoram under Organic Agriculture. Sustainability, 14(15), 9608. https://doi.org/10.3390/su14159608





