Bacterial Isolates from Greek Sites and Their Efficacy in Degrading Petroleum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Media
2.2. Isolation of Oil Hydrocarbon Degraders
2.3. Phenotypical Characterization of Bacterial Isolates
2.4. Isolation of Genomic DNA and Detection of the 16S rDΝA Gene, Catechol 2,3-Dioxygenase Enzyme (nahH) Gene and Aliphatic Alcohol Dehydrogenase Enzyme (alkJ) Gene
2.5. Estimation of Biodegradation Activity in Batch Cultures
2.6. Analysis of Petroleum Hydrocarbons in Crude Oil and Liquid Naphthalene Cultures
3. Results
3.1. Physicochemical Parameters of the Sampling Sites
3.2. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) at the Sampling Sites
3.3. Bacterial Strains Displaying Petroleum-Degrading Ability
3.4. Presence of Petroleum-Hydrocarbon-Degrading Genes alkJ and nahH in the Specific Bacterial Strains
3.5. Growth of Isolated Bacterial Strains in Crude Oil or Naphthalene Batch Cultures
3.6. Patterns of Biodegradability of Specific Bacterial Strains
3.7. Development of Consortia for Crude Oil Degradation
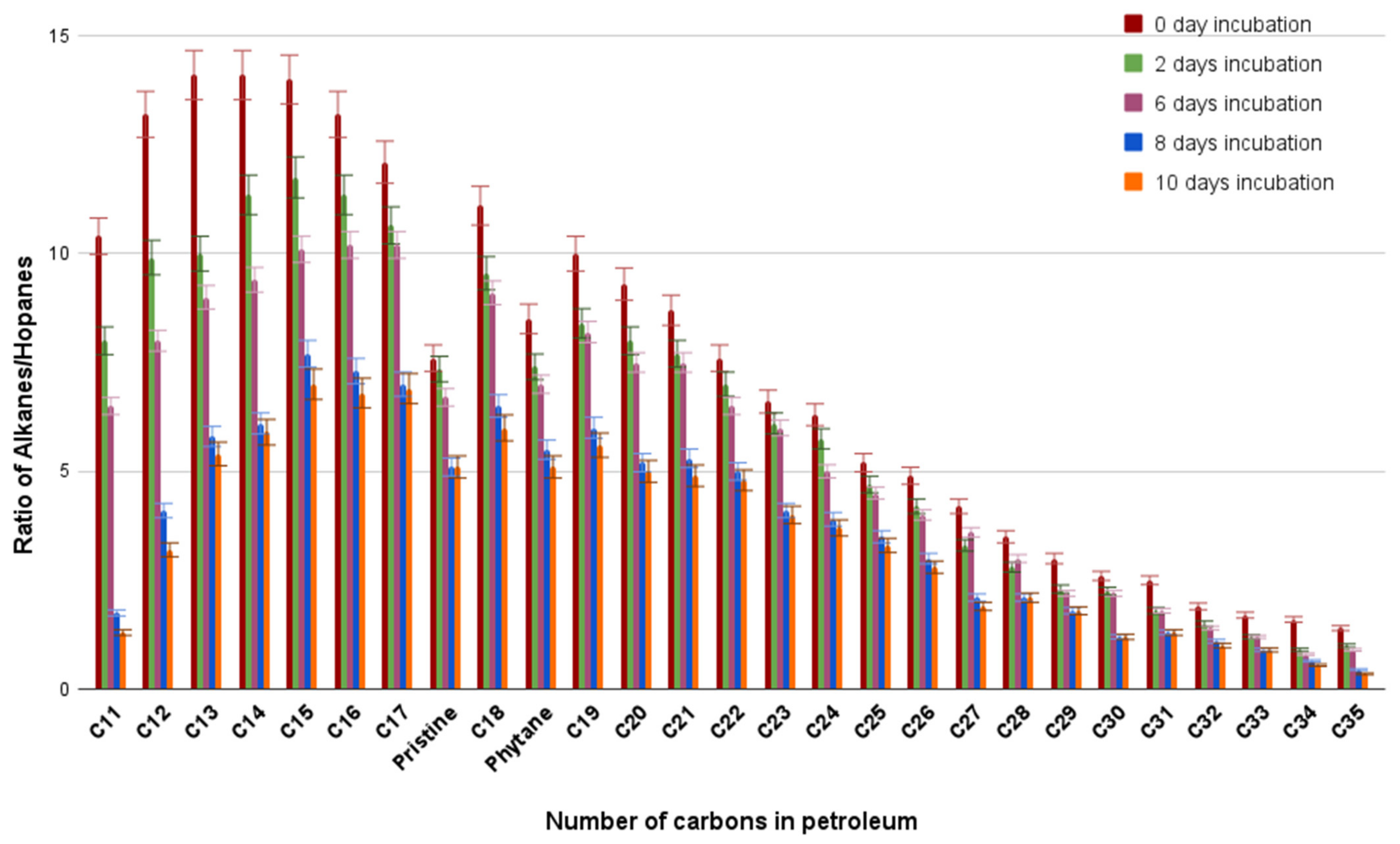
3.8. Alkanes/Hopanes Ratio during Crude Oil Degradation by Pseudomonas plecoglossicida
4. Discussion
4.1. Physicochemical Parameters of Sampling Sites and Microbial Diversity
4.2. Occurrence of alkJ and nahH Genes in Bacteria Populations Is Correlated with the Level of Contamination
4.3. Bacterial Population Diversity in Contaminated Sites
4.4. Cometabolism of Hydrocarbons by Microbial Community and Consortia Development
4.5. Selection of a Specific Strain and Its Potential Use in Autochthonous Bioaugmentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Xu, J.; Zhao, W.; Zhang, J. Effects and risk evaluation of oil spillage in the sea areas of Changxing Island. Int. J. Environ. Res. Public Health 2014, 11, 8491–8507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Wang, H.; Sheng, L.; Liu, X.; Zheng, X. Distribution characteristics and risk assessment of Polycyclic Aromatic Hydrocarbons in the Momoge Wetland, China. Int. J. Environ. Res. Public Health 2017, 14, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosal, D.; Ghosh, S.; Dutta, T.; Ahn, Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review. Front. Microbiol. 2016, 7, 1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrangelo, G.; Fadda, E.; Marzia, V. Polycyclic aromatic hydrocarbons and cancer in man. Environ. Health Perspect. 1996, 104, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Cerniglia, C.E. Biodegradation of polycyclic aromatic hydrocarbons. Curr. Opin. Biotechnol. 1992, 4, 331–338. [Google Scholar] [CrossRef]
- Prince, R.C. The Microbiology of Marine Oil Spill Bioremediation. In Petroleum Microbiology, 1st ed.; Ollivier, B., Magot, M., Eds.; American Society for Microbiology Press: Washington, DC, USA, 2005; pp. 317–335. [Google Scholar]
- Wilkes, H.; Rabus, R.; Fischer, T.; Armstroff, A.; Behrends, A.; Widdel, F. Anaerobic degradation of n-hexane in a denitrifying bacterium: Further degradation of the initial intermediate (1-methylpentyl) succinate via C-skeleton rearrangement. Arch. Microbiol. 2002, 177, 235–243. [Google Scholar] [CrossRef]
- Mallick, S.; Chakraborty, J.; Dutta, T. Role of oxygenases in guiding diverse metabolic pathways in the bacterial degradation of low-molecular-weight polycyclic aromatic hydrocarbons: A review. Crit. Rev. Microbiol. 2010, 37, 64–90. [Google Scholar] [CrossRef]
- Arvanitis, N.; Katsifas, E.A.; Chalkou, K.I.; Meintanis, C.; Karagouni, A.D. A refinery sludge deposition site: Presence of nahH and alkJ genes and crude oil biodegradation ability of bacterial isolates. Biotechnol. Lett. 2008, 30, 2105–2110. [Google Scholar] [CrossRef]
- Kirmair, L.; Skerra, A. Biochemical analysis of recombinant alkJ from Pseudomonas putida reveals a membrane-associated, flavin adenine dinucleotide-dependent dehydrogenase suitable for the biosynthetic production of aliphatic aldehydes. Appl. Environ. Microbiol. 2014, 80, 2468–2477. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Tang, J.; Bai, Z.; Hecker, M.; Giesy, J.P. Distribution of petroleum degrading genes and factor analysis of petroleum contaminated soil from the Dagang Oilfield, China. Sci. Rep. 2015, 5, 11068. [Google Scholar] [CrossRef]
- Paisse, S.; Duran, R.; Coulon, F.; Goñi-Urriza, M. Are alkane hydroxylase genes (alkB) relevant to assess petroleum bioremediation processes in chronically polluted coastal sediments? Appl. Microbiol. Biot. 2011, 92, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Kloos, K.; Munch, J.C.; Schloter, M. A new method for the detection of alkane-monooxygenase homologous genes (alkB) in soils based on PCR-hybridization. J. Microbiol. Meth. 2006, 66, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Panicker, G.; Mojib, N.; Aislabie, J.; Bej, A.K. Detection, expression and quantitation of the biodegradative genes in Antarctic microorganisms using PCR. Antonie Van Leeuwenhoek 2009, 97, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.M.; Ferguson, S.H.; Bowman, J.P.; Snape, I. Using Real-Time PCR to assess changes in the hydrocarbon-degrading microbial community in antarctic soil during bioremediation. Microbial. Ecol. 2006, 52, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zheng, L.; Yin, X.; Gao, W.; Han, B.; Li, Q.; Zhu, A.; Chen, H.; Yang, H. Reconstruction and evaluation of oil-degrading consortia isolated from sediments of hydrothermal vents in the South Mid-Atlantic Ridge. Sci. Rep. 2021, 11, 1456. [Google Scholar] [CrossRef]
- Johns, N.I.; Blazejewski, T.; Gomes, A.L.; Wang, H.H. Principles for designing synthetic microbial communities. Curr. Opin. Microbiol. 2016, 31, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, R.; Nagai, M.; Morikawa, M.; Okuyama, H. Autochthonous bioaugmentation and its possible application to oil spills. World J. Microbiol. Biotechnol. 2009, 25, 1519–1528. [Google Scholar] [CrossRef] [Green Version]
- Avramidis, P.; Geraga, M.; Lazarova, M.; Kontopoulos, N. Holocene record of environmental changes and palaeoclimatic implications in Alykes Lagoon, Zakynthos Island, western Greece, Mediterranean Sea. Quat. Int. 2013, 293, 184–195. [Google Scholar] [CrossRef]
- Chandankere, R.; Jun, Y.; Choi, M.M.F.; Masakorala, K.; Chan, Y. An efficient biosurfactant-producing and crude-oil emulsifying bacterium Bacillus methylotrophicus USTBa isolated from petroleum reservoir. Biochem. Eng. J. 2013, 74, 46–53. [Google Scholar] [CrossRef]
- Kanini, S.G.; Katsifas, E.A.; Savvides, A.L.; Karagouni, A.D. Streptomyces rochei ACTA1551, an indigenous Greek isolate studied as a potential biocontrol agent against Fusarium oxysporum f.sp. lycopersici. Biomed. Res. Int. 2013, 2013, 387230. [Google Scholar]
- Atlas, R.M. Handbook of Microbiological Media, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Kai, S.; Matsuo, Y.; Nakagawa, S.; Kryukov, K.; Matsukawa, S.; Tanaka, H.; Iwai, T.; Imanishi, T.; Hirota, K. Rapid bacterial identification by direct PCR amplification of 16S rRNA genes using the MinIONTM nanopore sequencer. FEBS Open Bio. 2019, 9, 548–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, K.; Ogawa, M.; Tanigughi, H.; Saito, M. Molecular approaches to studying microbial communities: Targeting the 16S ribosomal RNA gene. J. UOEH 2016, 3, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meintanis, C.; Chalkou, K.; Kormas, K.; Karagouni, A.D. Biodegradation of crude oil by thermophilic bacteria isolated from a volcano island. Biodegradation 2006, 17, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Costopoulou, D.; Vassiliadou, I.; Chrysafidis, D.; Bergele, K.; Tzavara, E.; Tzamtzis, V.; Leondiadis, L. Determination of PCDD/F, dioxin-like PCB and PAH levels in olive and olive oil samples from areas affected by the fires in summer 2007 in Greece. Chemosphere 2010, 79, 285–291. [Google Scholar] [CrossRef]
- Tang, X.; Xie, G.; Shao, K.; Tian, W.; Gao, G.; Qin, B. Aquatic bacterial diversity, community composition and assembly in the semi-arid inner Mongolia plateau: Combined effects of salinity and nutrient Levels. Microorganisms 2021, 9, 208. [Google Scholar] [CrossRef]
- Meyer, S.; Moser, R.; Neef, A.; Stahl, U.; Kämpfer, P. Differential detection of key enzymes of polyaromatic-hydrocarbon-degrading bacteria using PCR and gene probes. Microbiology 1999, 145, 1731–1741. [Google Scholar] [CrossRef] [Green Version]
- Milcic-Terzic, J.; Lopez-Vidal, Y.; Vrvic, M.; Saval, S. Detection of catabolic genes in indigenous microbial consortia isolated from a diesel-contaminated soil. Bioresour. Technol. 2001, 78, 47–54. [Google Scholar] [CrossRef]
- Sei, K.; Sugimoto, Y.; Mori, K.; Maki, H.; Kohno, T. Monitoring of alkane-degrading bacteria in a sea-water microcosm during crude oil degradation by polymerase chain reaction based on alkane-catabolic genes. Environ. Microbiol. 2003, 5, 517–522. [Google Scholar] [CrossRef]
- van Beilen, J.B.; Panke, S.; Lucchini, S.; Franchini, A.G.; Rothlisberger, M.; Witholt, B. Analysis of Pseudomonas putida alkane degradation gene clusters and flanking insertion sequences: Evolution and regulation of the alk genes. Microbiology 2001, 147, 1621–1630. [Google Scholar] [CrossRef] [Green Version]
- Throne-Holst, M.; Markussen, S.; Winnberg, A.; Ellingsen, T.E.; Kotlar, H.K.; Zotchev, S.B. Utilization of n-alkanes by a newly isolated strain of Acinetobacter venetianus: The role of two AlkB-type alkane hydroxylases. Appl. Microbiol. Biot. 2006, 72, 353–360. [Google Scholar] [CrossRef]
- Whyte, L.G.; Slagman, S.J.; Pietrantonio, F.; Bourbonnière, L.; Koval, S.F.; Lawrence, J.R.; Inniss, W.E.; Greer, C.W. Physiological adaptations involved in alkane assimilation at a low temperature by Rhodococcus sp. strain Q15. Appl. Environ. Microbiol. 1999, 65, 2961–2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavrodi, D.V.; Kovalenko, N.P.; Sokolov, S.L.; Parfeniuk, V.G.; Kosheleva, I.A.; Boronin, A.M. Identification of the key genes of naphthalene catabolism in soil DNA. Mikrobiologiia 2003, 72, 672–680. [Google Scholar] [PubMed]
- Kahng, H.Y.; Malinverni, J.C.; Majko, M.M.; Kukor, J.J. Genetic and functional analysis of the tbc operons for catabolism of alkyl- and chloroaromatic compounds in Burkholderia sp. strain JS150. Appl. Environ. Microb. 2001, 67, 4805–4816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldwin, B.R.; Nakatsu, C.H.; Nebe, J.; Wickham, G.S.; Parks, C.; Nies, L. Enumeration of aromatic oxygenase genes to evaluate biodegradation during multi-phase extraction at a gasoline-contaminated site. J. Hazard. Mater. 2009, 163, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Bakaeva, M.; Kuzina, E.; Vysotskaya, L.; Kudoyarova, G.; Arkhipova, T.; Rafikova, G.; Chetverikov, S.; Korshunova, T.; Chetverikova, D.; Loginov, O. Capacity of Pseudomonas strains to degrade hydrocarbons, produce auxins and maintain plant growth under normal conditions and in the presence of petroleum contaminants. Plants 2020, 9, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.; Jyot, J.; Kuhad, R.C.; Lal, B. In situ bioremediation potential of an oily sludge degrading bacterial consortium. Curr. Microbiol. 2001, 43, 328–335. [Google Scholar] [CrossRef]
- Vinas, M.; Griffol, M.; Sabate, J.; Solanas, A.M. Biodegradation of a crude oil by three microbial consortia of different origins and metabolic capabilities. J. Ind. Microbiol. Biotechnol. 2002, 28, 252–260. [Google Scholar] [CrossRef]
- Korshunova, T.Y.; Chetverikov, S.P.; Loginov, O.; Ramírez-Bahena, M.-H.; Igual, J.M.; Peix, Á. Pseudomonas turukhanskensis sp. nov., isolated from oil-contaminated soils. Int. J. Syst. Evol. Microbiol. 2016, 6, 4657–4664. [Google Scholar] [CrossRef]
- Vignesh, R.; Arularasan, A.; Gandhiraj, V.; Deepika, R.C. Isolation identification and characterization of potential oil degrading bacteria from oil contaminated sites. Int. Res. J. Eng. Technol. 2016, 3, 2503–2508. [Google Scholar]
- Koshlaf, E.; Ball, A.S. Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol. 2017, 3, 25–49. [Google Scholar] [CrossRef]
- Katsifas, E.A.; Giannoutsou, E.P.; Karagouni, A.D. Diversity of streptomycetes among specific Greek terrestrial ecosystems. Lett. Appl. Microbiol. 1999, 29, 48–51. [Google Scholar] [CrossRef] [Green Version]
- Giannoutsou, E.P.; Meintanis, C.; Karagouni, A.D. Identification of yeast strains isolated from a two-phase decanter system olive oil waste and investigation of their ability for its fermentation. Bioresour. Technol. 2004, 93, 301–306. [Google Scholar] [CrossRef] [PubMed]

| Physicochemical Parameter | Keri Lake | Glyfada Beach |
|---|---|---|
| pH | 8.5 | 8.0 |
| Temperature | 19.0 °C | 22.0 °C |
| Salinity | 6.9 ppt * | 37.8 ppt * |
| Dissolved Oxygen | 10.1 mg/L | 8.5 mg/L |
| Sampling Site | Naphthalene (NAPH) | Anthracene (ANTH) | Fluoranthene (FLUO) | Benzo(b) Fluoranthene (B[b]F) | Benzo(k) Fluoranthene (B[k]F) | Benzo(a) Pyrene (B[a]P) | Benzo(ghi) Perylene (B[ghi]P) | Indeno (1,2,3-cd) Pyrene (IP) |
|---|---|---|---|---|---|---|---|---|
| Sampling Site 1 Glyfada Beach A | 8.63 | 0.55 | 7.01 | 46 | 7.36 | 22.4 | 7.16 | 5.69 |
| Sampling Site 2 Glyfada Beach B | 3.3 | 32.2 | 79.7 | 122 | 17 | 60 | 19.2 | 7.27 |
| Sampling Site 3 Glyfada Beach C | NT * | NT * | NT * | <LOQ ** | NT * | NT * | NT * | NT * |
| Sampling Site 4 Keri Lake D | 9 | 35.7 | 75.5 | 131 | 17 | 62 | 17.8 | 7.51 |
| Sampling Site | Total Isolated Bacterial Strains | Strains with Ability to Degrade Crude Oil | Strains with alkJ Gene | Strains with nahH Gene | Strains with Both Genes Present |
|---|---|---|---|---|---|
| Sampling Site 1 Glyfada Beach A | 19 | 16 (84.21%) | 7 (46.7%) | 9 (60.0%) | 2(12.5%) |
| Sampling Site 2 Glyfada Beach B | 15 | 11 (73.33%) | 7 (63.6%) | 7 (63.6%) | 5 (45.5%) |
| Sampling Site 3 Glyfada Beach C | 31 | 4 (12.9%) | 1 (25.0%) | 3 (75.0%) | 1 (25.0%) |
| Sampling Site 4 Keri Lake D | 8 | 7 (87.5%) | 5 (71.4%) | 4 (54.1%) | 2 (28.6%) |
| ATHUBA Culture Collection | - | 10 | 4 (40.0%) | 4 (40.0%) | 2 (20.0%) |
| Sampling Site | Strain Name and Classification | Biodegradability Percentage of Crude Oil (%) | Biomass (g/L) of Crude Oil | Biomass (g/L) of Naphthalene |
|---|---|---|---|---|
| Sampling Site 1 | Streptomyces flavoviridis, ATHUBA 682 | 25.80 ± 0.69 | 0,11 ± 0,03 | 0.10 ± 0.02 |
| Streptomyces aridus, ATHUBA 683 | 27.80 ± 1.01 | 0.44 ± 0.05 | 0.21 ± 0.01 | |
| Staphylococcus sp., ATHUBA 684 | 30.40 ± 0.99 | 0.38 ± 0.04 | 0.27 ± 0.02 | |
| Isoptericola chiayiensis, ATHUBA 685 | 23.40 ± 0.65 | 0.19 ± 0.02 | 0.11 ± 0.01 | |
| Isoptericola chiayiensis, ATHUBA 686 | 38.70 ± 1.21 | 0.34 ± 0.03 | 0.28 ± 0.01 | |
| Bacillus wiedmannii sp., ATHUBA 687 | 57.70 ± 0.36 | 0.61 ± 0.04 | 0.41 ± 0.02 | |
| Staphylococcus warneri, ATHUBA 688 | 32.10 ± 0.51 | 0.19 ± 0.01 | 0.17 ± 0.01 | |
| Streptomyces sp., ATHUBA 689 | 66.10 ± 0.56 | 0.49 ± 0.04 | 0.42 ± 0.02 | |
| Paenibacillus polymyxa, ATHUBA 690 | 40.00 ± 2.30 | 0.12 ± 0.01 | 0.09 ± 0.01 | |
| Bacillus flexus, ATHUBA 691 | 60.80 ± 1.14 | 0.67± 0.05 | 0.47 ± 0.02 | |
| Isoptericola chiayiensis, ATHUBA 692 | 33.00 ± 0.39 | 0.13 ± 0.01 | 0.06 ± 0.01 | |
| Bacillus sp., ATHUBA 693 | 35.50 ± 0.69 | 0.11 ± 0.02 | 0.05 ± 0.01 | |
| Isoptericola sp., ATHUBA 694 | 45.50 ± 1.00 | 0.22 ± 0.02 | 0.10 ± 0.01 | |
| Bacillus sp., ATHUBA 695 | 40.20 ± 1.27 | 0.16 ± 0.02 | 0.12 ± 0.01 | |
| Brevundimonas sp., ATHUBA 696 | 38.70 ± 1.91 | 0.21 ± 0.01 | 0.13 ± 0,01 | |
| Isoptericola chiayiensis, ATHUBA 697 | 52.00 ± 1.36 | 0.66 ± 0.04 | 0.25 ± 0.01 | |
| Sampling Site 2 | Streptomyces sp.,ATHUBA 698 | 45.60 ± 0.98 | 0.64 ± 0.04 | 0.41 ± 0.02 |
| Halomonas lionensis, ATHUBA 699 | 27.00 ± 0.87 | 0.14 ± 0.01 | 0.14 ± 0.01 | |
| Halomonas quamarina, ATHUBA 700 | 50.00 ± 1.03 | 0.41 ± 0.03 | 0.39 ± 0.02 | |
| Isoptericola chiayiensis, ATHUBA 701 | 37.50 ± 1.63 | 0.27 ± 0.01 | 0.38 ± 0.02 | |
| Isoptericola halotolerans, ATHUBA 702 | 14.50 ± 0.62 | 0.19 ± 0.02 | 0.21 ± 0.01 | |
| Isoptericola rhizophila, ATHUBA 703 | 43.20 ± 0.28 | 0.10 ± 0.01 | 0.12 ± 0.01 | |
| Isoptericola chiayiensis, ATHUBA 704 | 28.80 ± 1.35 | 0.15 ± 0.01 | 0.12 ± 0.01 | |
| Bacillus sp., ATHUBA 705 | 21.00 ± 1.28 | 0.37 ± 0.02 | 0.30 ± 0.02 | |
| Streptomyces flavoviridis, ATHUBA 706 | 51.10 ± 1.87 | 0.49 ± 0.02 | 0.41 ± 0.02 | |
| Pseudomonas plecoglossicida, ATHUBA 707 | 76.70 ± 1.23 | 0.73 ± 0.04 | 0.66 ± 0.04 | |
| Paenibacillus abyssi, ATHUBA 708 | 69.30 ± 0.99 | 0.53 ± 0.03 | 0.48 ± 0.03 | |
| Sampling Site 3 | Bacillus cereus, ATHUBA 709 | 37.80 ± 0.68 | 0.10 ± 0.01 | 0.05 ± 0.01 |
| Bacillus sp., ATHUBA 710 | 21.10 ± 2.01 | 0.15 ± 0.01 | 0.09 ± 0.01 | |
| Halomonas sp., ATHUBA 711 | 10.80 ± 1.27 | 0.15 ± 0.01 | 0.12 ± 0.01 | |
| Bacillus sp., ATHUBA 712 | 10.00 ± 0.36 | 0.19 ± 0.02 | 0.13 ± 0.01 | |
| Sampling Site 4 | Pseudomonas wadenswilerensis, ATHUBA 713 | 46.70 ± 0.87 | 0.47 ± 0.04 | 0.48 ± 0.03 |
| Pseudomonas sp., ATHUBA 714 | 29.10 ± 0.61 | 0.23 ± 0.01 | 0.31 ± 0.02 | |
| Halomonas sp., ATHUBA 715 | 27.80 ± 0.53 | 0.25 ± 0.01 | 0.19 ± 0.01 | |
| Kocuria arsenatis, ATHUBA 716 | 61.30 ± 1.41 | 0.88 ± 0.05 | 0.75 ± 0.5 | |
| Brevundimonas vesiculari, ATHUBA 717 | 59.20 ± 1.18 | 0.71 ± 0.05 | 0.79 ± 0.06 | |
| Pseudomonas flavescens, ATHUBA 718 | 52.10 ± 1.39 | 0.68 ± 0.04 | 0.56 ± 0.04 | |
| Bacillus sp., ATHUBA 719 | 38.70 ± 0.97 | 0.27 ± 0.01 | 0.31 ± 0.2 | |
| ATHUBA | Streptomyces griseus, ATHUBA 720 | 25.20 ± 0.67 | 0.32 ± 0.02 | 0.13 ± 0.01 |
| Culture | Streptomyces chromofuscus, ATHUBA 721 | 18.20 ±0.57 | 0.41 ± 0.03 | 0.32 ± 0.4 |
| Collection | Streptomyces lividans, ATHUBA 722 | 21.90 ± 0.61 | 0.47 ± 0.02 | 0.45 ± 0.04 |
| Streptomyces rochei, ATHUBA 723 | 24.00 ± 0.32 | 0.60 ± 0.04 | 0.35 ± 0.02 | |
| Streptomyces griseus, ATHUBA 724 | 25.20 ± 0.32 | 0.83 ± 0.06 | 0.66 ± 0.05 | |
| Nitratireductor aquamarina, ATHUBA 725 | 50.80 ± 1.25 | 0.27 ± 0.01 | 0.15 ± 0.01 | |
| Thalassospira sp., ATHUBA 726 | 47.00 ± 1.10 | 0.50 ± 0.04 | 0.47 ± 0.03 | |
| Pseudomonas plecoglossicida, ATHUBA 727 | 60.10 ± 1.38 | 0.73 ± 0.05 | 0.27 ± 0.01 | |
| Nitratireductor sp., ATHUBA 728 | 41.50 ± 0.99 | 0.28 ± 0.01 | 0.28 ± 0.02 | |
| Pseudomonas sp., ATHUBA 729 | 40.00 ± 0.87 | 0.80 ± 0.06 | 0.45 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntroumpogianni, G.C.; Giannoutsou, E.; Karagouni, A.D.; Savvides, A.L. Bacterial Isolates from Greek Sites and Their Efficacy in Degrading Petroleum. Sustainability 2022, 14, 9562. https://doi.org/10.3390/su14159562
Ntroumpogianni GC, Giannoutsou E, Karagouni AD, Savvides AL. Bacterial Isolates from Greek Sites and Their Efficacy in Degrading Petroleum. Sustainability. 2022; 14(15):9562. https://doi.org/10.3390/su14159562
Chicago/Turabian StyleNtroumpogianni, Georgia C., Eleni Giannoutsou, Amalia D. Karagouni, and Alexandros L. Savvides. 2022. "Bacterial Isolates from Greek Sites and Their Efficacy in Degrading Petroleum" Sustainability 14, no. 15: 9562. https://doi.org/10.3390/su14159562
APA StyleNtroumpogianni, G. C., Giannoutsou, E., Karagouni, A. D., & Savvides, A. L. (2022). Bacterial Isolates from Greek Sites and Their Efficacy in Degrading Petroleum. Sustainability, 14(15), 9562. https://doi.org/10.3390/su14159562






