A Strategy for Conservation of Springsnails in Nevada and Utah, USA
Abstract
1. Introduction
2. Study Area
3. Study Organisms
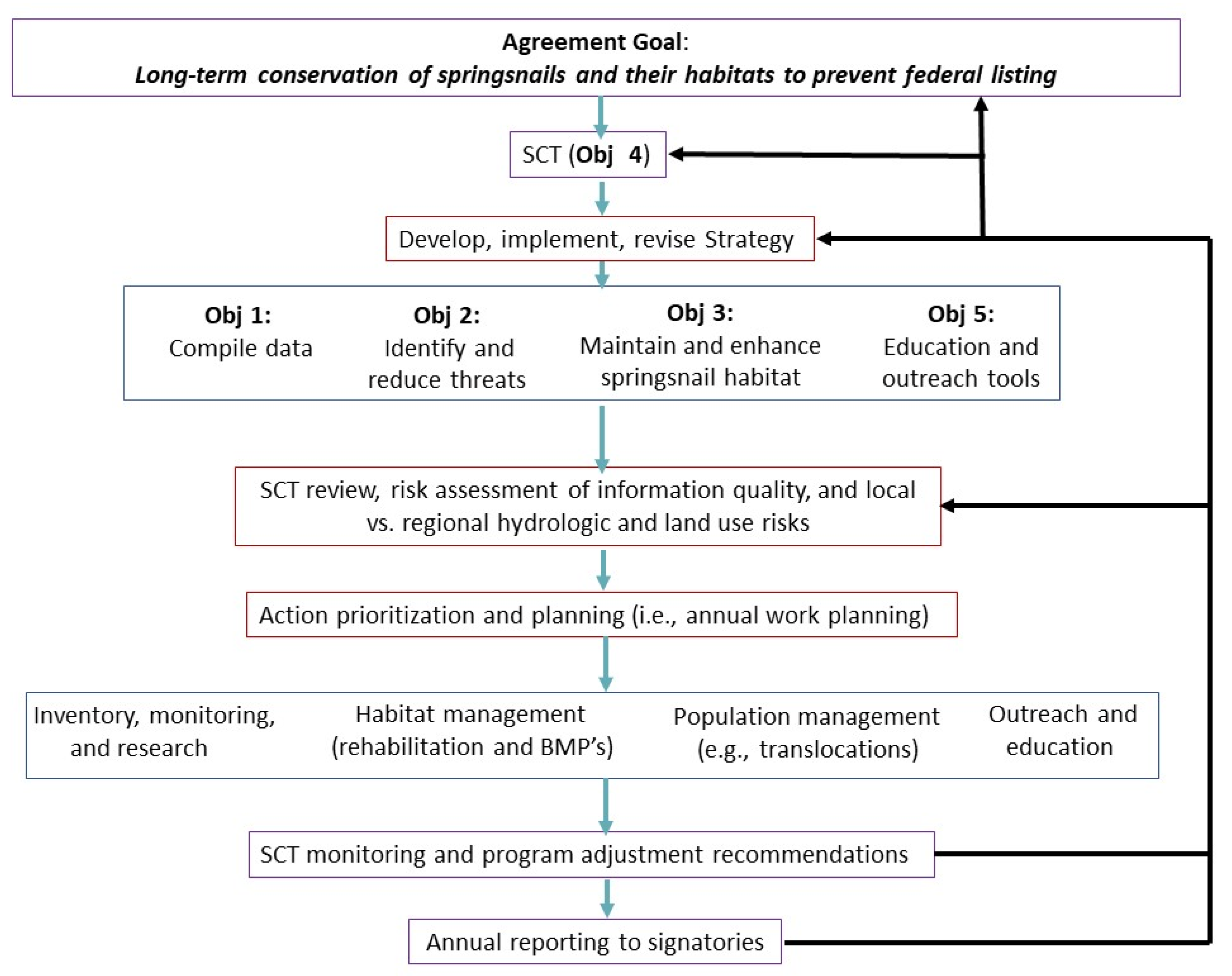
4. The Springsnail Conservation Program: Goals, Objectives, and Strategic Elements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hershler, R.; Liu, H.-P. Annotated Checklist of Freshwater Truncatelloidean Gastropods of the Western United States, with an Illustrated Key to the Genera; Technical Note; Information and Publishing Services Section, Bureau of Land Management National Operation Center: Denver, CO, USA, 2017; p. 449. [Google Scholar]
- Perez, K.E.; Leal, M.S.; Glover, H.; Chastain, R.T.; Hutchins, B.T.; Schwartz, B. Two new species of Pyrgulopsis Call & Pilsbry, 1886 (Mollusca: Caenogastropoda: Hydrobiidae) from springs in the Rio Grande watershed in Texas. Zootaxa 2021, 5071, 384–402. [Google Scholar] [PubMed]
- Ponder, W.F.; Hershler, R.; Jenkins, B. An endemic radiation of hydrobiid snails from artesian springs in northern South Australia: Their taxonomy, physiology, distribution, and anatomy. Malacologia 1989, 31, 1–140. [Google Scholar]
- Ponder, W.F.; Colgan, D.J.; Heraly, J.M.; Nützel, A.; Simone, L.R.L.; Strong, E.E. Caenogastropoda. In Phylogeny and Evolution of the Mollusca; Ponder, W.F., Lindberg, D.R., Eds.; University of California Press: Berkeley, CA, USA, 2008; pp. 331–383. [Google Scholar]
- Miller, J.P.; Ramos, M.A.; Hauffe, T.; Delicado, D. Global species richness of hydrobiid snails determined by climate and evolutionary history. Freshw. Biol. 2018, 63, 1225–1239. [Google Scholar] [CrossRef]
- Springs Conservation Plan Working Group. Nevada Springs Conservation Plan; Abele, S.L., Ed.; The Nature Conservancy: Reno, NV, USA, 2011. Available online: https://heritage.nv.gov//assets/documents/springcons_highres.pdf (accessed on 31 July 2022).
- Cantonati, M.; Fensham, R.J.; Stevens, L.E.; Gerecke, R.; Glazier, D.S.; Goldscheider, N.; Knight, R.L.; Richardson, J.S.; Springer, A.E.; Töckner, K. An urgent plea for global spring protection. Conserv. Biol. 2020, 35, 378–382. [Google Scholar] [CrossRef]
- Hershler, R.; Liu, H.-P.; Howard, J. Springsnails: A new conservation focus in western North America. Bioscience 2014, 64, 693–700. [Google Scholar] [CrossRef]
- Johannes, E.J.; Clark, S.A. Freshwater mollusc declines, local extinctions and introductions in five northern California streams. Tentacle 2016, 24, 22–25. [Google Scholar]
- Ledyeard, C.; Cowie, R.H.; Ponder, W.F.; Thompson, F.G. The global decline of nonmarine mollusks. BioScience 2004, 54, 321–330. [Google Scholar] [CrossRef]
- Noss, R.F. High-risk ecosystems as foci for considering biodiversity and ecological integrity in ecological risk assessment. Environ. Sci. Policy 2000, 3, 321–332. [Google Scholar] [CrossRef]
- Sada, D.W.; Lutz, A.D. Environmental Characteristics of Great Basin and Mojave Desert Springs; Unpublished Report to U.S. Fish and Wildlife Service; The Great Basin Landscape Conservation Cooperative: Reno, NV, USA, 2016. [Google Scholar]
- Stevens, L.E.; Jenness, J.; Ledbetter, J.D. Springs and springs-dependent taxa in the Colorado River Basin, southwestern North America: Geography, ecology, and human impacts. Water 2020, 12, 1501. [Google Scholar] [CrossRef]
- Utah Division of Wildlife Resources. Utah State Wildlife Action Plan: A plan for Managing Native Wildlife Species and Their Habitats to Help Prevent Listing under the Endangered Species Act; Publication Number 15–14; Utah Division of Wildlife Resources: Salt Lake City, UT, USA, 2015. [Google Scholar]
- United States. The Endangered Species Act as Amended by Public Law 97-304 (the Endangered Species Act Amendments of 1982); US Government Printing Office: Washington, DC, USA, 1983. [Google Scholar]
- Williams, J.E.; Sada, D.W. Ghosts of our making: Extinct aquatic species of North American deserts. In Standing between Life and Extinction; Williams, J.E., Probst, D., Eds.; University of Chicago Press: Chicago, IL, USA, 2020; pp. 89–108. [Google Scholar]
- Holcomb, K. Springsnail conservation in Nevada and Utah. Water Spot 2021, 1, 27. Available online: https://www.kelmanonline.com/httpdocs/files/NWEA/thewaterspot2021-issue1/index.html (accessed on 31 July 2022).
- Nevada Department of Wildlife and Utah Division of Wildlife Resources. Nevada-Utah Springsnail Conservation Agreement; Utah State Government Office: Salt Lake City, UT, USA, 2018. [Google Scholar]
- Nevada and Utah Springsnail Conservation Team. 2020. Available online: https://springstewardshipinstitute.org/springsnail-conservation-strategy (accessed on 31 July 2022).
- Forest Guardians. A Petition to List 206 Critically Imperiled or Imperiled Species in the Mountain-Prairie Region of the United States as Threatened or Endangered under the Endangered Species Act, 16 U.S.C. §§ 1531 Et Seq.; Forest Guardians: Santa Fe, NM, USA, 2007. [Google Scholar]
- The Center for Biological Diversity; Curry, T.; Greenwald, N.; Deacon, J.; Duff, D.; The Freshwater Mollusk Conservation Society Hereby Formally Petition the U.S.; Fish and Wildlife Service (FWS). Petition to List 42 Species of GREAT Basin Springsnails from Nevada, Utah, and California as Threatened or Endangered under the Endangered Species Act; Department of the Interior: Washington, DC, USA, 2009. [Google Scholar]
- USFWS. Endangered and Threatened Wildlife and Plants; 90-Day Finding on a Petition to list 42 Great Basin and Mojave Desert Springsnails as Threatened or Endangered with Critical Habitat; Proposed Rule. Federal Register 76:56608-56630. 2011. Available online: https://www.fws.gov/species-publication-action/90-day-finding-petition-list-42-great-basin-and-mojave-desert-18 (accessed on 31 July 2022).
- USFWS. Species Status Assessment Report for 14 Springsnails in Nevada and Utah; U.S. Fish and Wildlife Service, Pacific Southwest Region: Sacramento, CA, USA, 2017. [Google Scholar]
- USFWS. Endangered and threatened wildlife and plants; 17 species not warranted for Listing as Endangered or Threatened Species. Fed. Regist. 2021, 86, 53255–53261. [Google Scholar]
- Nevada Wildlife Management Plan Team. Nevada Wildlife Action Plan; Nevada Department of Wildlife: Reno, NV, USA, 2012. [Google Scholar]
- Benson, A.J.; Kipp, R.M.; Larson, J.; Fusaro, A. Potamopyrgus antipodarum (J.E. Gray, 1853): U.S. Geological Survey; Nonindigenous Aquatic Species Database: Gainesville, FL, USA, 2020. Available online: https://nas.er.usgs.gov/queries/factsheet.aspx?SpeciesID=1008 (accessed on 1 June 2020).
- Hershler, R. Springsnails (Gastropoda: Hydrobiidae) of Owens and Amargosa River (exclusive of Ash Meadows) drainages, Death Valley system, California-Nevada. Proc. Biol. Soc. Wash. 1989, 102, 176–248. [Google Scholar]
- Hershler, R.; Sada, D.W. Biogeography of Great Basin Freshwater Snails of the Genus Pyrgulopsis; Smithsonian Institution Press: Washington, DC, USA, 2002; pp. 255–276. [Google Scholar]
- Hershler, R.; Liu, H.-P. Ancient vicariance and recent dispersal of springsnails (Hydrobiidae: Pyrgulopsis) in the Death Valley system, California-Nevada. In Late Cenozoic Drainage History of the Southwestern Great Basin and lower Colorado River Region: Geologic and Biotic Perspectives; Reheis, M.C., Hershler, R., Miller, D.M., Eds.; Special Paper 439; Geological Society of America: Denver, CO, USA, 2008. [Google Scholar]
- Heilweil, V.M.; Brooks, L.E. (Eds.) Conceptual Model of the Great Basin Carbonate and Alluvial Aquifer System; US Geological Survey Investigations Report 2010-5193; US Geological Survey: Washington, DC, USA, 2011. [Google Scholar]
- O’Connor, J.E.; Costa, J.E. The world’s largest floods, past and present—Their causes and magnitudes. US Geol. Surv. Circ. 2004, 1254, 1–13. [Google Scholar]
- Prudic, D.E.; Sweetkind, D.S.; Jackson, T.R.; Dotson, K.E.; Plume, R.W.; Hatch, C.E.; Halford, K.J. Evaluating Connection of Aquifers to Springs and Streams, GREAT Basin National Park and Vicinity, Nevada; Professional Paper 1819; US Geological Survey: Reston, VA, USA, 2015. [Google Scholar]
- Winograd, I.J.; Coplen, T.B.; Landwehr, J.M.; Riggs, A.C.; Ludwig, K.R.; Szabo, B.J.; Kolesar, P.R.; Revesz, K.M. Continuous 500,000-year climate record from vein calcite in Devils Hole, Nevada. Science 1992, 258, 255–260. [Google Scholar] [CrossRef]
- Thomas, J.M.; Mihevc, T.M. Evaluation of Groundwater Origins, Flow Paths, and Ages in East-Central and Southeastern Nevada; Desert Research Institute Publication N: Reno, NV, USA, 2011. [Google Scholar]
- Harrill, J.R.; Prudic, D.E. Aquifer Systems in the Great Basin Region of Nevada, Utah, and Adjacent States: Summary Report; Professional Paper 1409-A; US Geological Survey: Washington, DC, USA, 1998. [Google Scholar]
- Wittmeyer, G. Summary of Groundwater Studies of the Great Basin, Death Valley Regional Flow System, and Yucca Mountain Area. US Nuclear Regulatory Commission Contract NRC-HQ-12-C-0089. 2017. Available online: https://www.nrc.gov/docs/ML1717/ML17172A193.pdf (accessed on 31 July 2022).
- Masbruch, M.D.; Heilweil, V.M.; Buto, S.G.; Brooks, L.E.; Susong, D.D.; Flint, A.L.; Flint, L.E.; Gardner, P.M. Chapter D: Estimated Groundwater Budgets; Scientific Investigations Report 2010–5193; US Geological Survey: Washington, DC, USA, 2011. [Google Scholar]
- Hershler, R.; Liu, H.-P.; Stevens, L.E. A new springsnail (Hydrobiidae: Pyrgulopsis) from the lower Colorado River basin, northwestern Arizona. West. N. Am. Nat. 2016, 76, 72–81. [Google Scholar] [CrossRef]
- Criscione, F.; Ponder, W.F. A phylogenetic analysis of rissooidean and cingulopsoidean families (Gastropoda: Caenogastropoda). Mol. Phylogenetics Evol. 2013, 11, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, P.; Rocroi, J.-P. Classification and nomenclature of gastropod families. Malacologia 2005, 47, 1–397. [Google Scholar]
- Ledyeard, C.; Cummings, K.S. (Eds.) Freshwater Mollusks of the World: A Distribution Atlas; John Hopkins University Press: Baltimore, MD, USA, 2019. [Google Scholar]
- Hershler, R.; Liu, H.-P.; Babbitt, C.; Kellogg, M.G.; Howard, J.K. Three new species of western California springsnails previously confused with Pyrgulopsis stearnsiana (Caenogastropoda, Hydrobiidae). ZooKeys 2016, 601, 1–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mladenka, G.C. The Ecological Life History of the Bruneau Hot Springs Snail (Pyrgulopsis bruneauensis); Final Report; Stream Ecology Center, Department of Biological Sciences, Idaho State University: Pocatello, ID, USA, 1992. [Google Scholar]
- Mladenka, G.C.; Minshall, G.W. Variation in the life history and abundance of three populations of Bruneau Hot Springs snails (Pyrgulopsis bruneauensis). West. N. Am. Nat. 2001, 61, 204–212. [Google Scholar]
- Sada, D.W.; Herbst, D.B. Ecology of Aquatic Macroinvertebrates in Travertine and Nevares Springs, Death Valley National Park, California, with an Examination of Water Diversion Effects on Their Abundance and Community Structure; Desert Research Institute: Reno, NV, USA, 2006. [Google Scholar]
- Sada, D.W. Demography and habitat use of the Badwater snail (Assiminea infima), with observations on its conservation status, Death Valley National Park, California, USA. Hydrobiologia 2001, 466, 255–265. Available online: https://musnaz-my.sharepoint.com/:b:/g/personal/amendoza_musnaz_org/EVDezW5S0ZJPr5-9lQ5cfdYBnlKI-WwEWQDRAUsYSlRi8Q?e=yuNvSi (accessed on 31 July 2022). [CrossRef]
- Sada, D.W. Great Basin Springsnail Natural History; Desert Research Institute: Reno, NV, USA, 2008. [Google Scholar]
- Martinez, M.A.; Thome, D.M. Habitat usage by the Page Springsnail, Pyrgulopsis morrisoni (Gastropoda: Hydrobiidae), from central Arizona. Veliger 2006, 18, 8–16. [Google Scholar]
- Martinez, M.A.; Myers, T.L. Associations between aquatic habitat variables and Pyrgulopsis trivialis presence/absence. J. Freshw. Ecol. 2008, 23, 189–194. [Google Scholar] [CrossRef]
- Sada, D.W.; Rosamond, C. Abundance, Distribution, and Habitat Use of the Elongate Mud Meadows Springsnail (Pyrgulopsis notidicola); Desert Research Institute Report to U.S. Fish and Wildlife Service; Soldier Meadow: Reno, NV, USA, 2013. [Google Scholar]
- Tsai, Y.J.; Maloney, K.; Arnold, A.E. Biotic and abiotic factors influencing the distribution of the Huachuca springsnail (Pyrgulopsis thompsoni). J. Freshw. Ecol. 2007, 22, 213–218. [Google Scholar] [CrossRef]
- Southern Nevada Water Authority. Spring Valley stipulation Biological Monitoring Plan 2010 Annual Report; Southern Nevada Water Authority: Las Vegas, NV, USA, 2011. [Google Scholar]
- Sada, D.W. Environmental and Biological Factors Affecting Great Basin and Surrounding Areas Springsnail (Superfamily rissooidea) Abundance and Distribution; Unpublished Report; U.S. Fish and Wildlife Service: Reno, NV, USA, 2016. [Google Scholar]
- Ledbetter, J.D.; Sevens, L.E.; Springer, A.E.; Brandt, B. Springs Inventory Database. Online Database. Springs and Springs-Dependent Species Database. Vers. 1.0. Springs Stewardship Institute, Flagstaff. 2022. Available online: https://SpringsData.org (accessed on 31 July 2022).
- USFWS. Species Status Assessment Framework: An Integrated Analytical Framework for Conservation; Version 3.4; USFWS: Washington, DC, USA, 2016. [Google Scholar]
- Kilburn, S. Impacts of Introduced Crayfish on Ash Meadows Aquatic Communities: Ash Meadows National Wildlife Refuge. Master’s Thesis, University of Illinois at Urbana-Champaign, Urbana, IL, USA, 2012. [Google Scholar]
- Smithsonian Environmental Research Center. Mollusks—Gastropods: Melanoides tuberculate, Red-Rimmed Melania. 2022. Available online: https://invasions.si.edu/nemesis/species_summary/71533 (accessed on 31 July 2022).
- Benson, L.; Thompson, R. Lake-level variation in the Lahontan basin for the past 50,000 years. Quat. Res. 1987, 28, 69–85. [Google Scholar] [CrossRef]
- Courtenay, W.R.; Deacon, J.E. Fish introductions in the American southwest: A case history of Rogers Spring, Nevada. Southwest. Nat. 1983, 28, 221–224. [Google Scholar] [CrossRef]
- Williams, J.E.; Probst, D. (Eds.) Standing between Life and Extinction; University of Chicago Press: Chicago, IL, USA, 2021. [Google Scholar]
- Utah Division of Wildlife Resources. Don’t Ditch a Fish. Salt Lake City. 2021. Available online: https://wildlife.utah.gov/dont-ditch.html (accessed on 31 July 2022).
- Wolf, S.; Hartl, B.; Carroll, C.; Neel, M.C.; Greenwald, D.N. Beyond PVA: Wy recover under the Endangered Species Act is more than population viability. BioScience 2015, 65, 200–207. [Google Scholar] [CrossRef]
- Gurrieri, J.T. Rangeland Water Developments at Springs: Best Practices for Design, Rehabilitation, and Restoration; General Technical Report RMRS-GTR-405; Rocky Mountain Research Station, U.S. Department of Agriculture, Forest Service: Fort Collins, CO, USA, 2020. [Google Scholar]
- Kodrick-Brown, A.; Brown, J.H. Native fishes, exotic mammals, and the conservation of desert springs. Front. Ecol. Environ. 2007, 5, 549–553. [Google Scholar] [CrossRef]
- Holling, C.S. Adaptive Environmental Assessment and Management; John Wiley & Sons: Hoboken, NJ, USA, 1978. [Google Scholar]
- Walters, C. Adaptive Management of Renewable Resources; Blackburn Press: Caldwell, NJ, USA, 1986. [Google Scholar]
- Bormann, B.T.; Martin, J.R.; Wagner, F.H.; Wood, G.W.; Alegria, J.; Cunningham, P.G.; Brookes, M.H.; Friesema, P.; Berg, J.; Henshaw, J.R. Adaptive Management. In Ecological Stewardship: A Common Reference for Ecosystem Management; Johnson, N.C., Malk, A.J., Sexton, W.T., Szaro, R., Eds.; Elsevier Science Ltd: Oxford, UK, 1999; Volume 3, pp. 505–534. [Google Scholar]
- Paulich, N. Increasing private conservation through incentive mechanisms. Stanf. J. Anim. Law Policy 2010, 3, 106–158. Available online: https://www-cdn.law.stanford.edu/wp-content/uploads/2018/05/paulich.pdf (accessed on 31 July 2022).
- Paffett, K.; Stevens, L.E.; Springer, A.E. Ecological assessment and rehabilitation prioritization for improving springs ecosystem stewardship. In Wetland and Stream Rapid Assessments: Development, Validation, and Application; Dorney, J., Tiner, R.W., Savage, R., Adamus, P., Eds.; Elsevier: London, UK, 2018; pp. 475–487. [Google Scholar]
- Stevens, L.E.; Aly, A.A.; Arpin, S.M.; Apostolova, I.; Ashley, G.M.; Barba, P.Q.; Barquín, J.; Beauger, A.; Benaabidate, L.; Bhat, S.U.; et al. Springs of the world: Distribution, ecology, and conservation status. In Imperiled: The Encyclopedia of Conservation; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
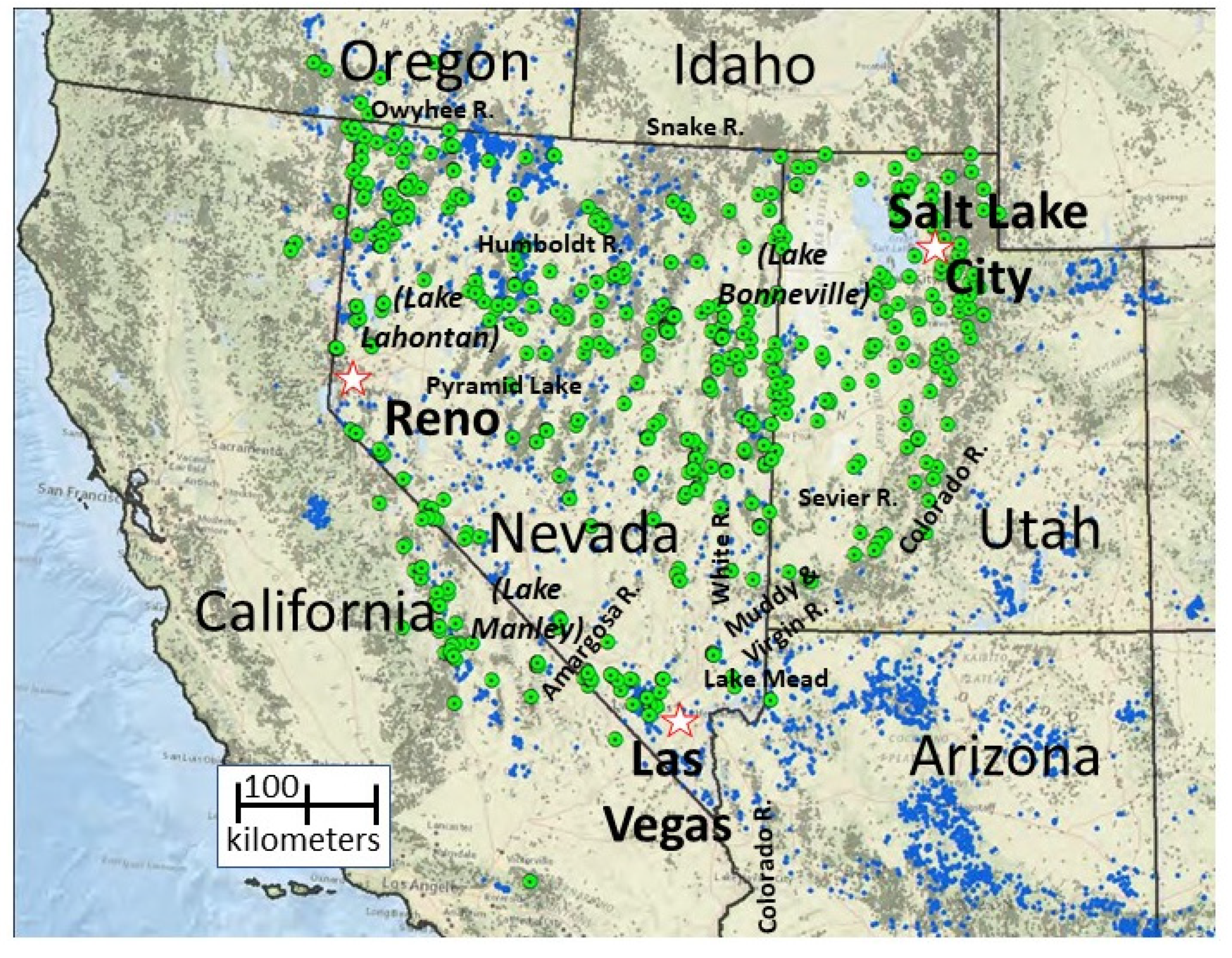
| Variable | Nevada | Utah | Total |
|---|---|---|---|
| No. Reported Springs on Federal Land | 17,835 | 6129 | 23,964 |
| No. Inventoried Springs on Federal Land | 2610 | 832 | 3442 |
| No. Springs on State or City Land | 280 | 603 | 883 |
| No. Inventoried Springs on State or City Land | 12 | 43 | 55 |
| No. Springs Tribal Land | 133 | 354 | 487 |
| No. Springs Inventoried on Tribal Land | 20 | 2 | 22 |
| No. Springs on Private Land | 7221 | 4559 | 11,780 |
| No. Springs Inventoried on Private Land | 761 | 186 | 947 |
| Total No. Reported Springs | 25,469 | 11,645 | 37,114 * |
| Total No. Inventoried Springs | 3403 | 1063 | 4466 |
| No. Federally-owned Springs with Springsnails | 399 | 116 | 515 |
| No. State- or City-owned Springs with Springsnails | 5 | 32 | 37 |
| No. Tribally-owned Springs with Springsnails | 11 | 1 | 12 |
| No. Privately-owned Springs with Springsnails | 365 | 164 | 529 |
| Total No. Springs with Springsnails | 780 | 313 | 1093 |
| No. Species of Springsnails | 86 | 21 | 103 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevens, L.E.; Holcomb, K.; Crookshanks, C.; Sada, D.W.; Jenness, J.; Szabo, K. A Strategy for Conservation of Springsnails in Nevada and Utah, USA. Sustainability 2022, 14, 9546. https://doi.org/10.3390/su14159546
Stevens LE, Holcomb K, Crookshanks C, Sada DW, Jenness J, Szabo K. A Strategy for Conservation of Springsnails in Nevada and Utah, USA. Sustainability. 2022; 14(15):9546. https://doi.org/10.3390/su14159546
Chicago/Turabian StyleStevens, Lawrence E., Kathryn Holcomb, Chris Crookshanks, Donald W. Sada, Jeff Jenness, and Kristin Szabo. 2022. "A Strategy for Conservation of Springsnails in Nevada and Utah, USA" Sustainability 14, no. 15: 9546. https://doi.org/10.3390/su14159546
APA StyleStevens, L. E., Holcomb, K., Crookshanks, C., Sada, D. W., Jenness, J., & Szabo, K. (2022). A Strategy for Conservation of Springsnails in Nevada and Utah, USA. Sustainability, 14(15), 9546. https://doi.org/10.3390/su14159546






