Effect of Rising Temperature and Carbon Dioxide on the Growth, Photophysiology, and Elemental Ratios of Marine Synechococcus: A Multistressor Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Culturing Conditions
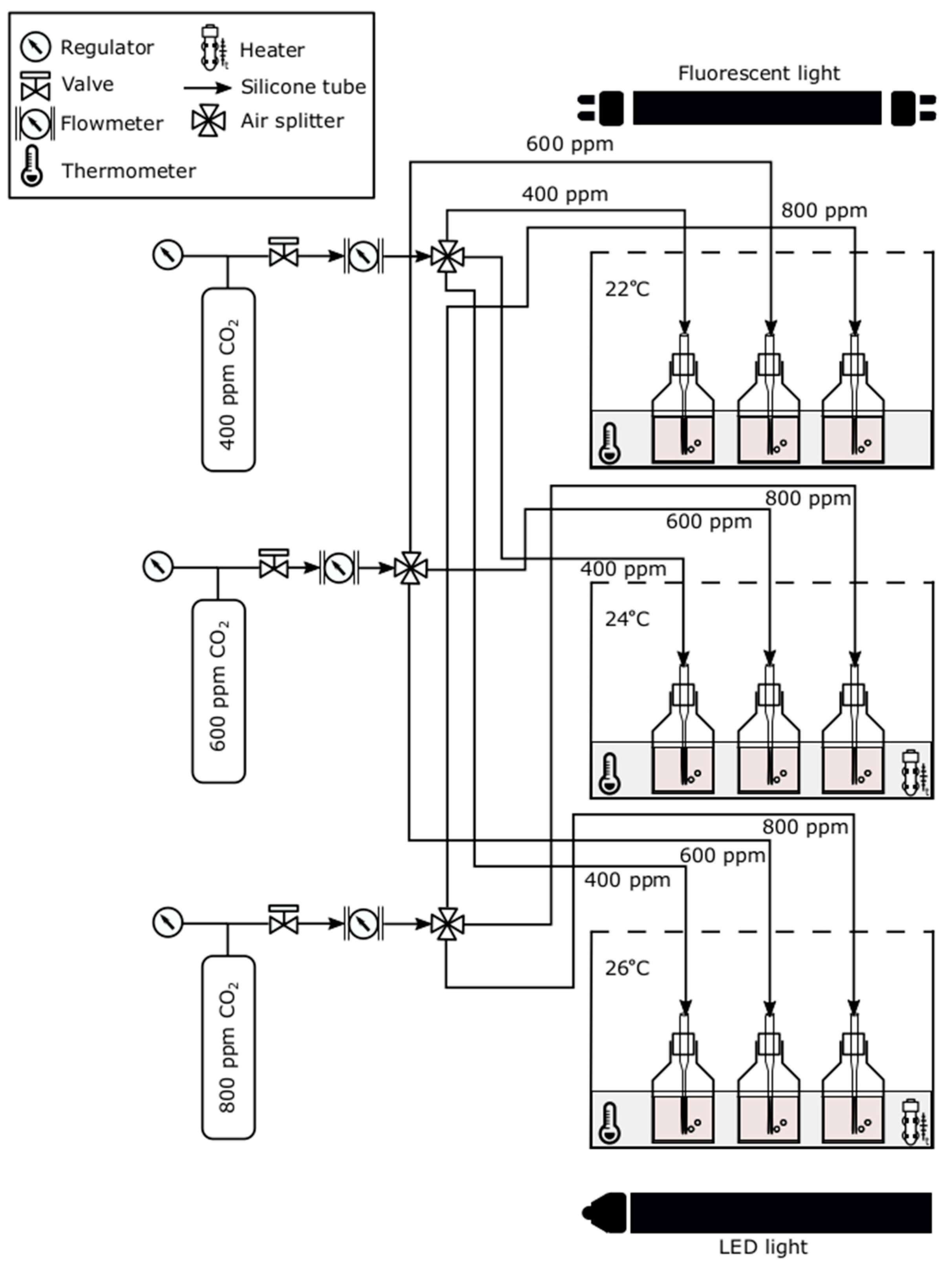
2.2. Experimental Set-Up and Procedure
2.3. Fluorescence Measurements
2.4. Determination of Specific Growth Rate
2.5. Determination of Elemental Carbon and Nitrogen Content and Nitrogen Stable Isotopic Composition
2.6. Construction of Phylogenetic Trees
2.7. Statistical Analysis
3. Results
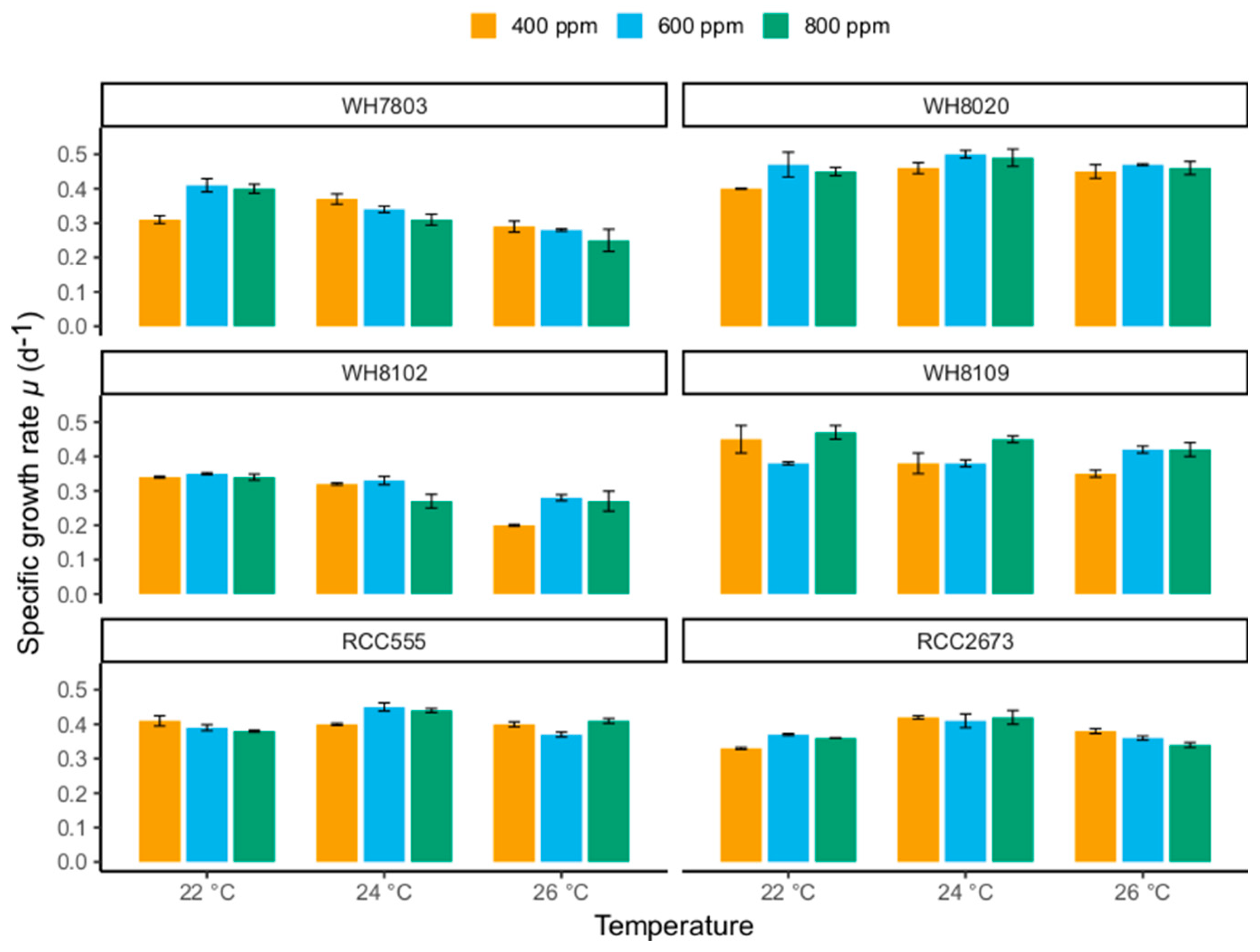
3.1. Specific Growth Rates
3.1.1. Specific Growth Rate Responses in WH7803 and WH8102
3.1.2. Specific Growth Rate Responses in RCC555 and RCC2673
3.1.3. Specific Growth Rate Responses in WH8020 and WH8109
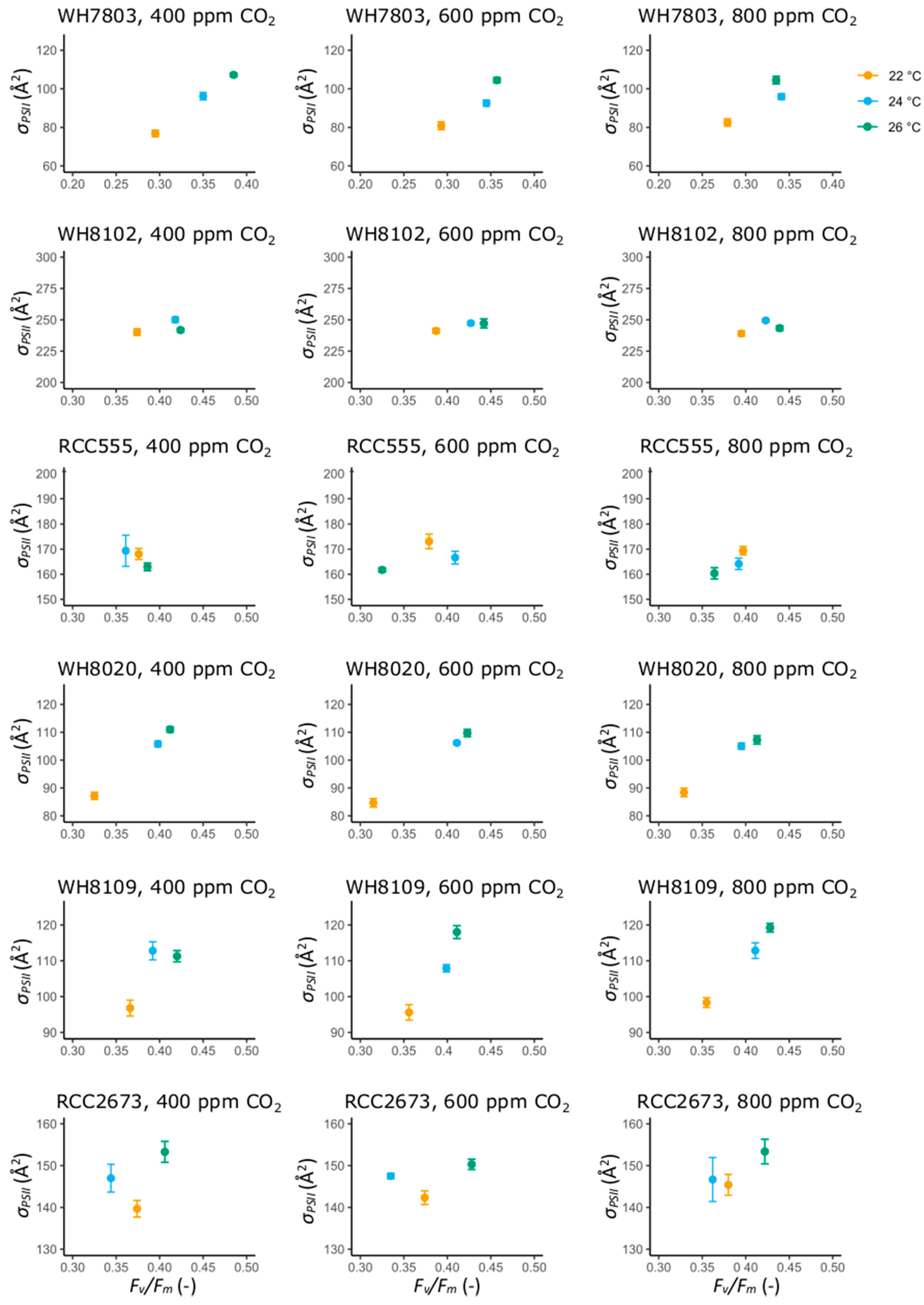
3.2. Photosynthetic Performance Parameters
3.2.1. Photosynthetic Performance Parameters in the Open Ocean Isolates WH7803 and WH8102
3.2.2. Photosynthetic Performance Parameters in RCC555
3.2.3. Photosynthetic Performance Parameters in the Coastal Isolates WH8020, WH8109 and RCC2673
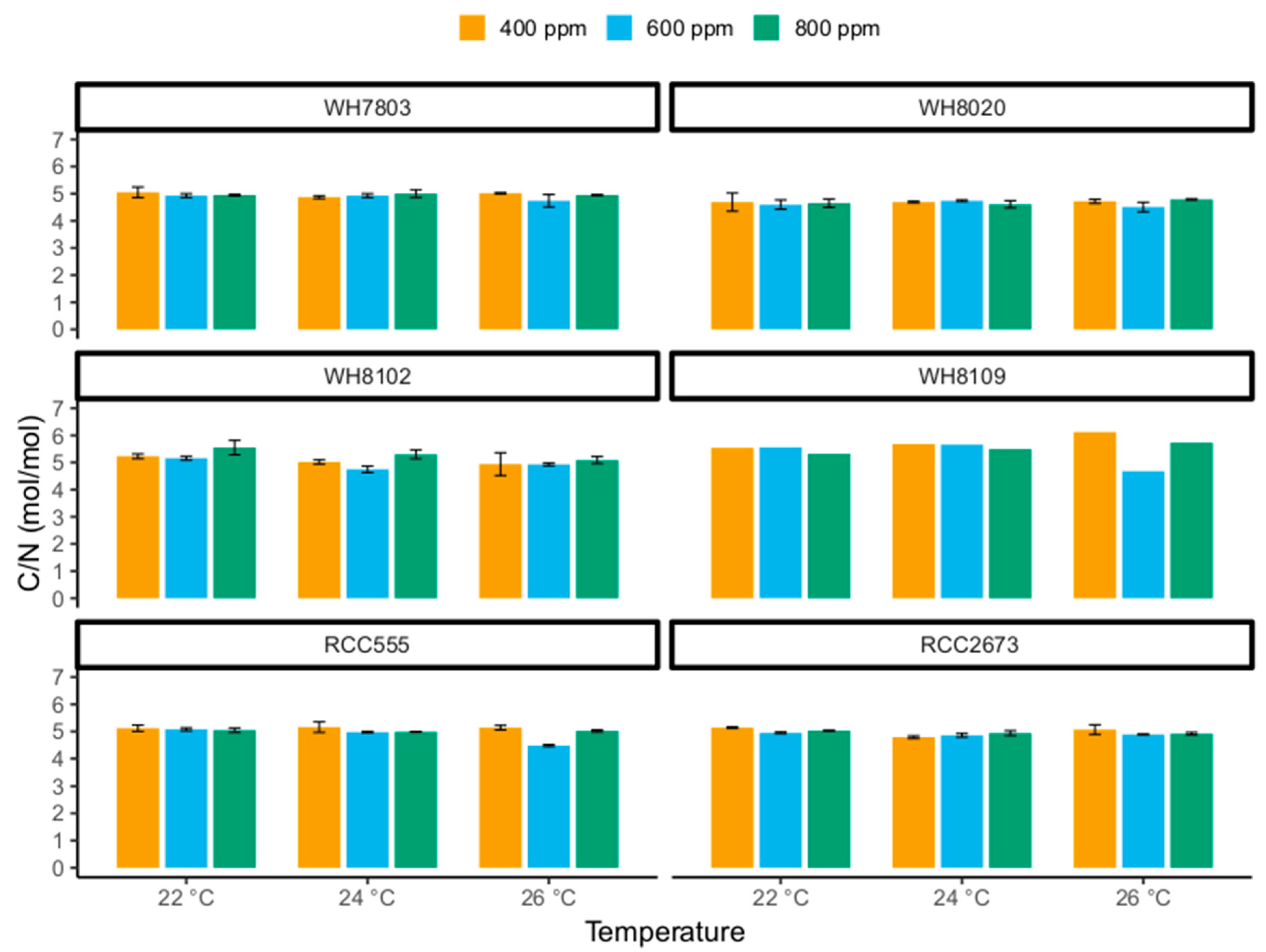
3.3. Carbon-to-Nitrogen Ratios
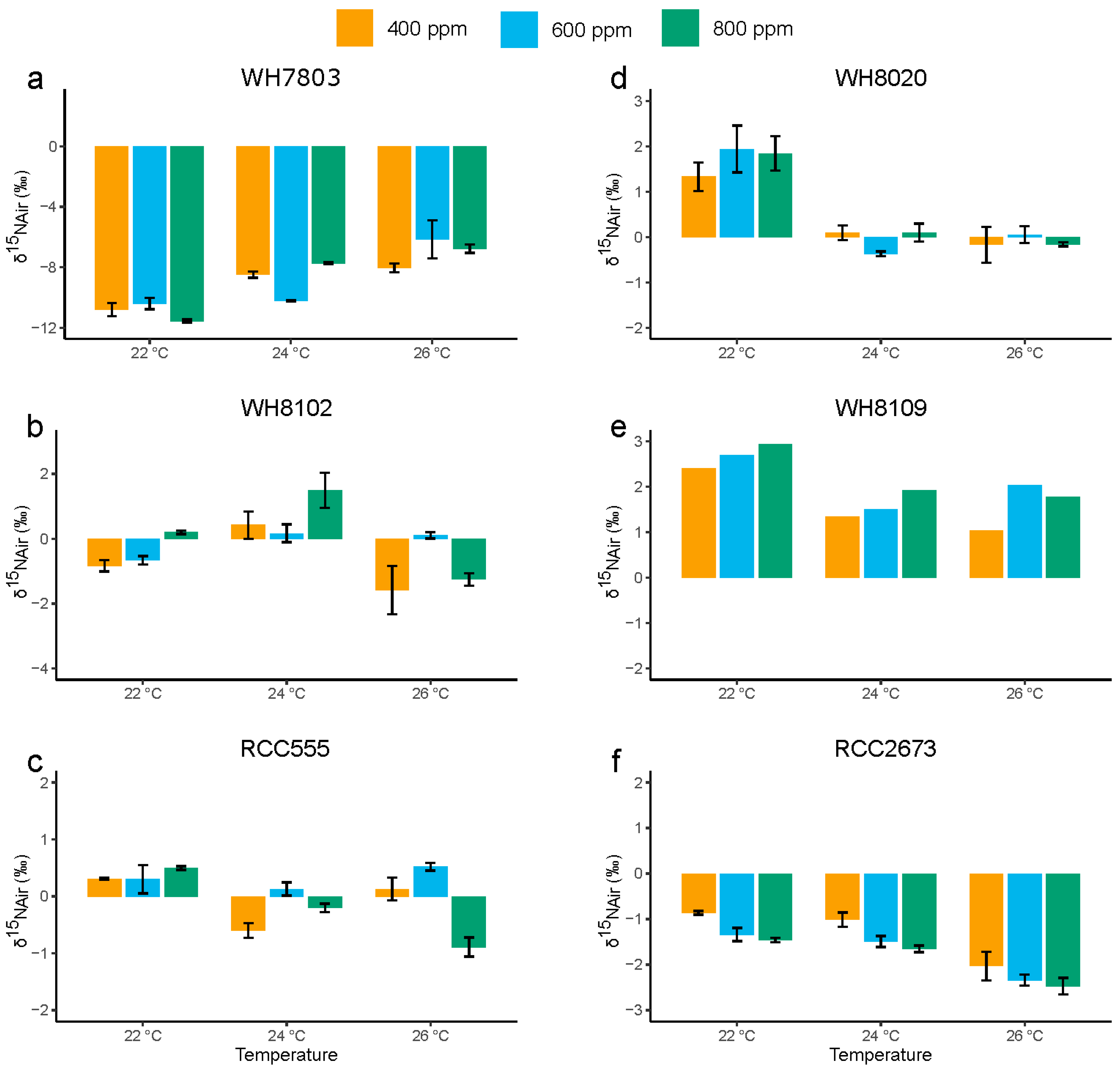
3.4. Nitrogen Stable Isotopic Composition
3.4.1. Nitrogen Stable Isotopic Composition in the Open Ocean Isolates WH7803 and WH8102
3.4.2. Nitrogen Stable Isotopic Composition in RCC555
3.4.3. Nitrogen Stable Isotopic Composition in the Coastal Isolates WH8020, WH8109 and RCC2673
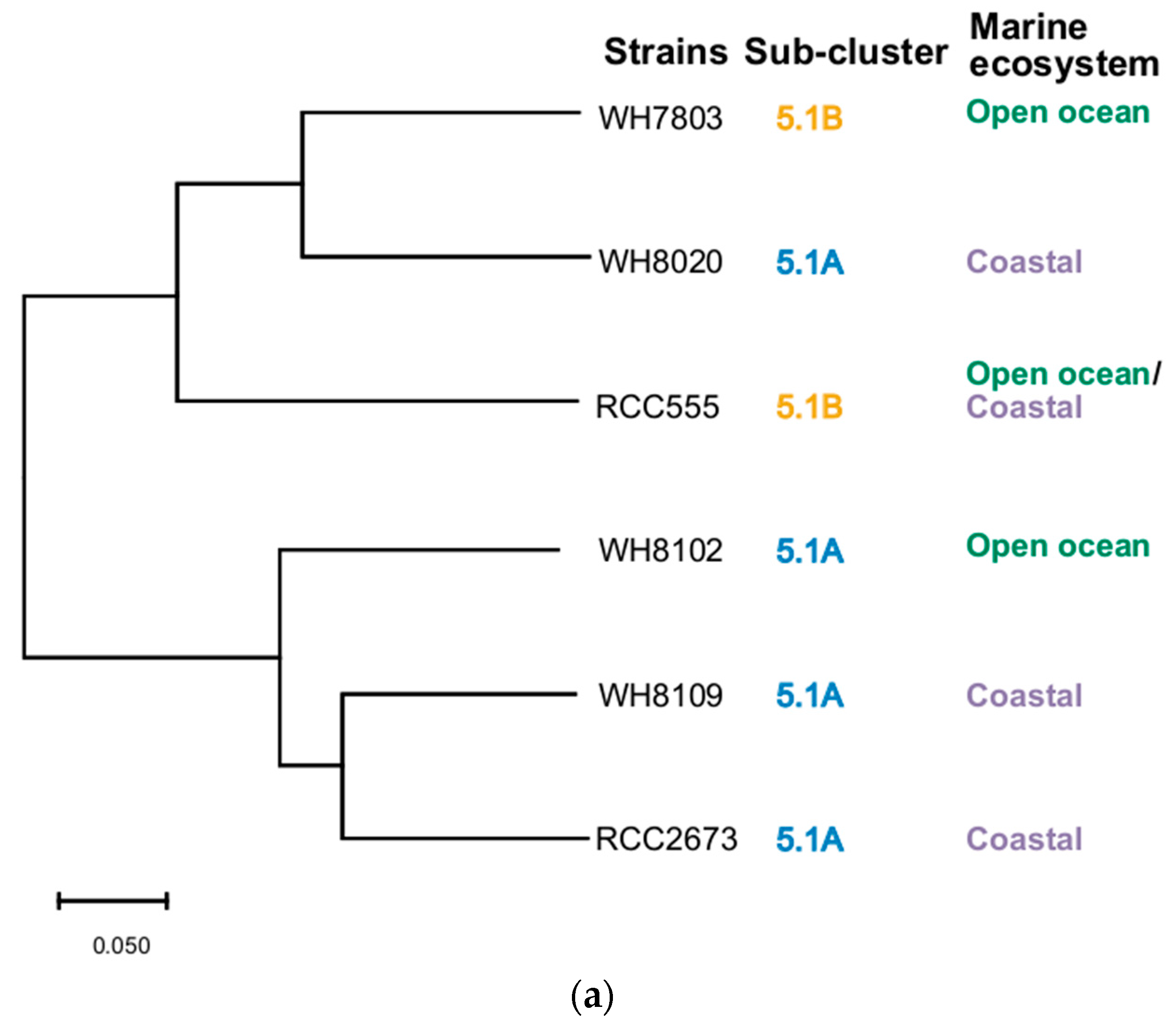
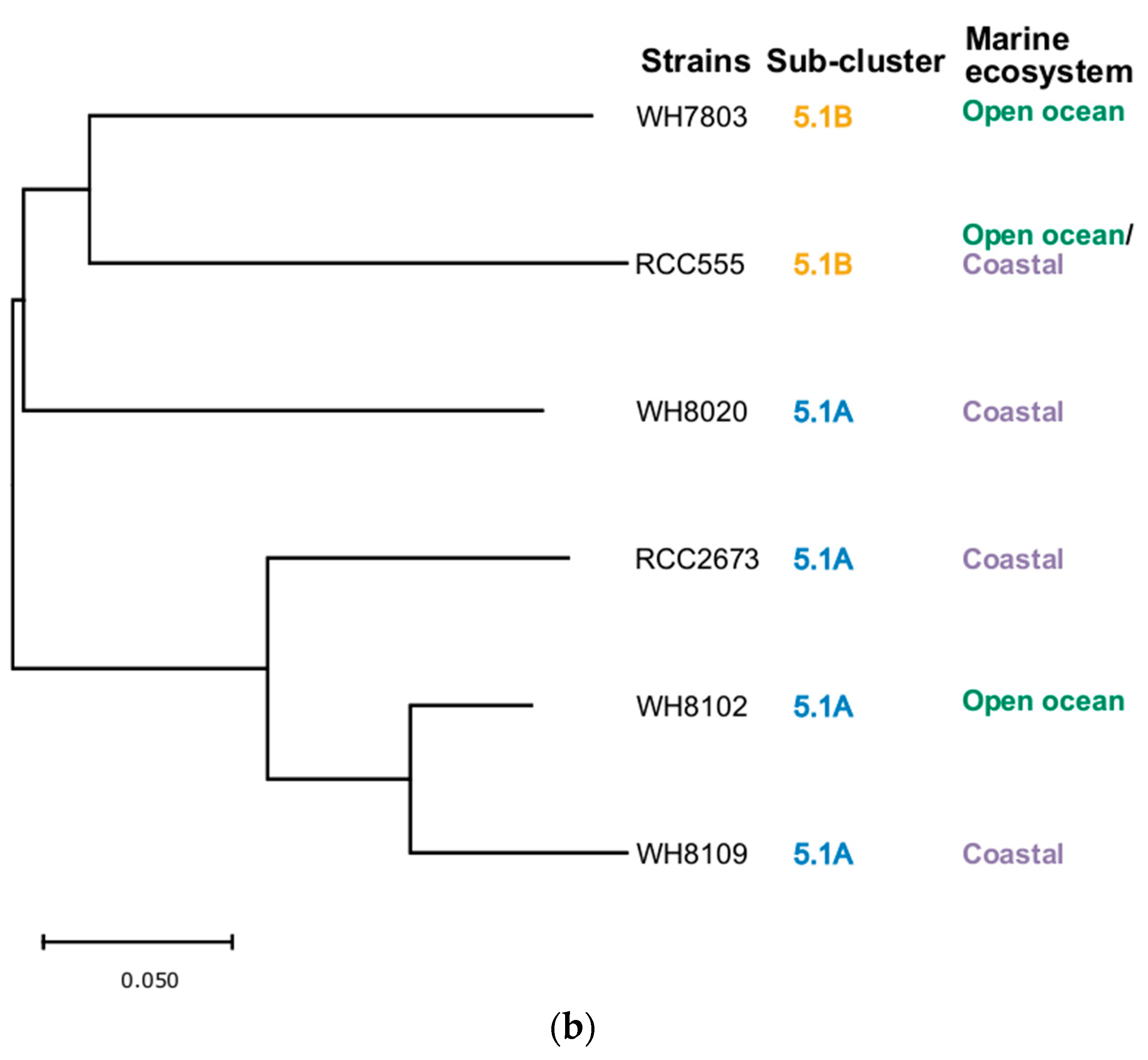
3.5. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, K.; Beardall, J.; Häder, D.-P.; Hall-Spencer, J.M.; Gao, G.; Hutchins, D.A. Effects of Ocean Acidification on Marine Photosynthetic Organisms under the Concurrent Influences of Warming, UV Radiation, and Deoxygenation. Front. Mar. Sci. 2019, 6, 322. [Google Scholar] [CrossRef]
- Carbon Dioxide Now More than 50% Higher than Pre-Industrial Levels. Available online: https://www.noaa.gov/news-release/carbon-dioxide-now-more-than-50-higher-than-pre-industrial-levels#:~:text=Carbon%20dioxide%20measured%20at%20NOAA’s,of%20California%20San%20Diego%20announced (accessed on 17 July 2022).
- Li, W.; Gao, K.; Beardall, J. Interactive effects of ocean acidification and nitrogen-limitation on the diatom Phaeodactylum tricornutum. PLoS ONE 2012, 7, e51590. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.-Q.; Carter, B.R.; Feely, R.A.; Lauvset, S.K.; Olsen, A. Surface ocean pH and buffer capacity: Past, present and future. Sci. Rep. 2019, 9, 18624. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.-X.; Warner, M.E.; Zhang, Y.; Feng, Y.; Hutchins, D.A. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (cyanobacteria)1. J. Phycol. 2007, 43, 485–496. [Google Scholar] [CrossRef]
- Hare, C.E.; Leblanc, K.; Ditullio, G.R.; Kudela, R.; Zhang, Y.; Lee, P.A.; Riseman, S.; Hutchins, D.A. Consequences of increased temperature and CO2 for phytoplankton community structure in the Bering Sea. Mar. Ecol. Prog. Ser. 2007, 352, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Flombaum, P.; Gallegos, J.L.; Gordillo, R.A.; Rincon, J.; Zabala, L.L.; Jiao, N.A.Z.; Karl, D.M.; Li, W.K.W.; Lomas, M.W.; Veneziano, D.; et al. Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. USA 2013, 110, 9824–9829. [Google Scholar] [CrossRef] [Green Version]
- Scanlan, D.J.; Ostrowski, M.; Mazard, S.; Dufresne, A.; Garczarek, L.; Hess, W.R.; Post, A.F.; Hagemann, M.; Paulsen, I.; Partensky, F. Ecological Genomics of Marine Picocyanobacteria. Microbiol. Mol. Biol. Rev. 2009, 73, 249. [Google Scholar] [CrossRef] [Green Version]
- Mackey, K.R.M.; Post, A.F.; McIlvin, M.R.; Saito, M.A. Physiological and proteomic characterization of light adaptations in marine Synechococcus. Environ. Microbiol. 2017, 19, 2348–2365. [Google Scholar] [CrossRef]
- Lovindeer, R.; Abbott, L.; Medina, H.; Mackey, K.R.M. Costs and Limitations of Marine Synechococcus Blue-Green Chromatic Acclimation. Front. Mar. Sci. 2021, 8, 1198. [Google Scholar] [CrossRef]
- Sohm, J.A.; Ahlgren, N.A.; Thomson, Z.J.; Williams, C.; Moffett, J.W.; Saito, M.A.; Webb, E.A.; Rocap, G. Co-occurring Synechococcus ecotypes occupy four major oceanic regimes defined by temperature, macronutrients and iron. ISME J. 2016, 10, 333–345. [Google Scholar] [CrossRef] [Green Version]
- Tai, V.; Palenik, B. Temporal variation of Synechococcus clades at a coastal Pacific Ocean monitoring site. ISME J. 2009, 3, 903–915. [Google Scholar] [CrossRef] [Green Version]
- DuRand, M.D.; Olson, R.J.; Chisholm, S.W. Phytoplankton population dynamics at the Bermuda Atlantic Time-series station in the Sargasso Sea. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 1983–2003. [Google Scholar] [CrossRef]
- Bao, N.; Gao, K. Interactive Effects of Elevated CO2 Concentration and Light on the Picophytoplankton Synechococcus. Front. Mar. Sci. 2021, 8, 634189. [Google Scholar] [CrossRef]
- Barcelos e Ramos, J.; Biswas, H.; Schulz, K.G.; LaRoche, J.; Riebesell, U. Effect of rising atmospheric carbon dioxide on the marine nitrogen fixer Trichodesmium. Glob. Biogeochem. Cycles 2007, 21, GB2028. [Google Scholar] [CrossRef] [Green Version]
- Hutchins, D.A.; Fu, F.-X.; Zhang, Y.; Warner, M.E.; Feng, Y.; Portune, K.; Bernhardt, P.W.; Mulholland, M.R. CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates, and elemental ratios: Implications for past, present, and future ocean biogeochemistry. Limnol. Oceanogr. 2007, 52, 1293–1304. [Google Scholar] [CrossRef] [Green Version]
- Kranz, S.A.; Eichner, M.; Rost, B. Interactions between CCM and N2 fixation in Trichodesmium. Photosynth. Res. 2011, 109, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Levitan, O.; Rosenberg, G.; Setlik, I.; SetlikovA, E.; Grigel, J.; Klepetar, J.; Prasil, O.; Berman-Frank, I. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob. Chang. Biol. 2007, 13, 531–538. [Google Scholar] [CrossRef]
- Eichner, M.; Rost, B.; Kranz, S.A. Diversity of ocean acidification effects on marine N2 fixers. J. Exp. Mar. Biol. Ecol. 2014, 457, 199–207. [Google Scholar] [CrossRef] [Green Version]
- Garcia, N.S.; Fu, F.-X.; Breene, C.L.; Yu, E.K.; Bernhardt, P.W.; Mulholland, M.R.; Hutchins, D.A. Combined effects of CO2 and light on large and small isolates of the unicellular N2-fixing cyanobacterium Crocosphaera watsonii from the western tropical Atlantic Ocean. Eur. J. Phycol. 2013, 48, 128–139. [Google Scholar] [CrossRef]
- King, A.L.; Jenkins, B.D.; Wallace, J.R.; Liu, Y.; Wikfors, G.H.; Milke, L.M.; Meseck, S.L. Effects of CO2 on growth rate, C:N:P, and fatty acid composition of seven marine phytoplankton species. Mar. Ecol. Prog. Ser. 2015, 537, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Macken, A.; Johnsen, B.; Norli, M.; Skogan, O.A.S.; Tollefsen, K.E. The MicroClimate Screen—A microscale climate exposure system for assessing the effect of CO2, temperature and UV on marine microalgae. Mar. Environ. Res. 2022, 179, 105670. [Google Scholar] [CrossRef] [PubMed]
- Delille, B.; Harlay, J.; Zondervan, I.; Jacquet, S.; Chou, L.; Wollast, R.; Bellerby, R.G.J.; Frankignoulle, M.; Borges, A.V.; Riebesell, U.; et al. Response of primary production and calcification to changes of pCO2 during experimental blooms of the coccolithophorid Emiliania huxleyi. Glob. Biogeochem. Cycles 2005, 19, GB2023. [Google Scholar] [CrossRef] [Green Version]
- Paulino, A.I.; Egge, J.K.; Larsen, A. Effects of increased atmospheric CO2 on small and intermediate sized osmotrophs during a nutrient induced phytoplankton bloom. Biogeosciences 2008, 5, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Barakat, K.M.; El-Sayed, H.S.; Khairy, H.M.; El-Sheikh, M.A.; Al-Rashed, S.A.; Arif, I.A.; Elshobary, M.E. Effects of ocean acidification on the growth and biochemical composition of a green alga (Ulva fasciata) and its associated microbiota. Saudi. J. Biol. Sci. 2021, 28, 5106–5114. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, H.S.; Elshobary, M.E.; Barakat, K.M.; Khairy, H.M.; El-Sheikh, M.A.; Czaja, R.; Allam, B.; Senousy, H.H. Ocean acidification induced changes in Ulva fasciata biochemistry may improve Dicentrarchus labrax aquaculture via enhanced antimicrobial activity. Aquaculture 2022, 560, 738474. [Google Scholar] [CrossRef]
- Keys, M.; Tilstone, G.; Findlay, H.S.; Widdicombe, C.E.; Lawson, T. Effects of elevated CO2 and temperature on phytoplankton community biomass, species composition and photosynthesis during an experimentally induced autumn bloom in the western English Channel. Biogeosciences 2018, 15, 3203–3222. [Google Scholar] [CrossRef] [Green Version]
- Lomas, M.; Hopkinson, B.; Losh, J.; Ryan, D.; Shi, D.; Xu, Y.; Morel, F. Effect of ocean acidification on cyanobacteria in the subtropical North Atlantic. Aquat. Microb. Ecol. 2012, 66, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Tortell, P.D.; DiTullio, G.; Sigman, D.; Morel, F.M.M.; Tortell, P.D.; DiTullio, G.R.; Sigman, D.M.; Morel, F.M.M. CO2 effects on taxonomic composition and nutrient utilization in an Equatorial Pacific phytoplankton assemblage. Mar. Ecol. Prog. Ser. 2002, 236, 37–43. [Google Scholar] [CrossRef]
- Basu, S.; Mackey, K. Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate. Sustainability 2018, 10, 869. [Google Scholar] [CrossRef] [Green Version]
- DeVries, T.; Primeau, F.; Deutsch, C. The sequestration efficiency of the biological pump. Geophys. Res. Lett. 2012, 39, L13601. [Google Scholar] [CrossRef] [Green Version]
- Andersson, A.J.; Kline, D.I.; Edmunds, P.J.; Archer, S.D.; Bednaršek, N.; Carpenter, R.C.; Chadsey, M.; Goldstein, P.; Grottoli, A.G.; Hurst, T.P.; et al. Understanding ocean acidification impacts on organismal to ecological scales. Oceanography 2015, 28, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Moreno, A.R.; Martiny, A.C. Ecological Stoichiometry of Ocean Plankton. Annu. Rev. Mar. Sci. 2018, 10, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Waterbury, J.B.; Watson, S.W.; Valois, F.W.; Franks, D.G. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Can. Bull. Fish Aquat. Sci. 1986, 214, 71–120. [Google Scholar]
- Campbell, D.; Hurry, V.; Clarke, A.K.; Gustafsson, P.; Oquist, G. Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol. Mol. Biol. Rev. 1998, 62, 667–683. [Google Scholar] [CrossRef] [Green Version]
- Mackey, K.R.M.; Paytan, A.; Grossman, A.R.; Bailey, S. A photosynthetic strategy for coping in a high-light, low-nutrient environment. Limnol. Oceanogr. 2008, 53, 900–913. [Google Scholar] [CrossRef] [Green Version]
- Barrie, A.; Davies, J.E.; Park, A.J.; Workman, C.T. Continuous-flow stable isotope analysis for biologists. Spectroscopy 1989, 4, 42–52. [Google Scholar]
- Price, G.D.; Badger, M.R.; Woodger, F.J.; Long, B.M. Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): Functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J. Exp. Bot. 2008, 59, 1441–1461. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Roy, A.S.; Mohanty, K.; Ghoshal, A.K. CO2 biofixation and carbonic anhydrase activity in Scenedesmus obliquus SA1 cultivated in large scale open system. Bioresour. Technol. 2014, 164, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Satagopan, S.; Huening, K.A.; Tabita, F.R. Selection of cyanobacterial (Synechococcus sp. strain PCC 6301) RubisCO variants with improved functional properties that confer enhanced CO2-dependent growth of Rhodobacter capsulatus, a photosynthetic bacterium. Mol. Biol. Physiol. 2019, 10, e01537-19. [Google Scholar] [CrossRef] [Green Version]
- Mackey, K.R.M.; Morris, J.J.; Morel, F.M.M.; Kranz, S.A. Response of Photosynthesis to Ocean Acidification. Oceanography 2015, 28, 74–91. [Google Scholar] [CrossRef]
- Mackey, K.R.M.; Paytan, A.; Caldeira, K.; Grossman, A.R.; Moran, D.; McIlvin, M.; Saito, M.A. Effect of temperature on photosynthesis and growth in marine Synechococcus spp. Plant Physiol. 2013, 163, 815–829. [Google Scholar] [CrossRef] [Green Version]
- Bertilsson, S.; Berglund, O.; Karl, D.M.; Chisholm, S.W. Elemental composition of marine Prochlorococcus and Synechococcus: Implications for the ecological stoichiometry of the sea. Limnol. Oceanogr. 2003, 48, 1721–1731. [Google Scholar] [CrossRef]
- Lopez, J.S.; Garcia, N.S.; Talmy, D.; Martiny, A.C. Diel variability in the elemental composition of the marine cyanobacterium Synechococcus. J. Plankton Res. 2016, 38, 1052–1061. [Google Scholar] [CrossRef] [Green Version]
- Garcia, N.S.; Sexton, J.; Riggins, T.; Brown, J.; Lomas, M.W.; Martiny, A.C. High Variability in Cellular Stoichiometry of Carbon, Nitrogen, and Phosphorus within Classes of Marine Eukaryotic Phytoplankton under Sufficient Nutrient Conditions. Front. Microbiol. 2018, 9, 543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martiny, A.C.; Pham, C.T.A.; Primeau, F.W.; Vrugt, J.A.; Moore, J.K.; Levin, S.A.; Lomas, M.W. Strong latitudinal patterns in the elemental ratios of marine plankton and organic matter. Nat. Geosci. 2013, 6, 279–283. [Google Scholar] [CrossRef]
- Bauersachs, T.; Schouten, S.; Compaoré, J.; Wollenzien, U.; Stal, L.J.; Sinninghe Damsteé, J.S. Nitrogen isotopic fractionation associated with growth on dinitrogen gas and nitrate by cyanobacteria. Limnol. Oceanogr. 2009, 54, 1403–1411. [Google Scholar] [CrossRef]
- Mackey, K.R.M.; Post, A.F.; McIlvin, M.R.; Cutter, G.A.; John, S.G.; Saito, M.A. Divergent responses of Atlantic coastal and oceanic Synechococcus to iron limitation. Proc. Natl. Acad. Sci. USA 2015, 112, 9944–9949. [Google Scholar] [CrossRef] [Green Version]
- Zwirglmaier, K.; Jardillier, L.; Ostrowski, M.; Mazard, S.; Garczarek, L.; Vaulot, D.; Not, F.; Massana, R.; Ulloa, O.; Scanlan, D.J. Global phylogeography of marine Synechococcus and Prochlorococcus reveals a distinct partitioning of lineages among oceanic biomes. Environ. Microbiol. 2008, 10, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wilhelm, S.; Harvey, H.; Taylor, K.; Jiao, N.; Chen, F. Novel lineages of Prochlorococcus and Synechococcus in the global oceans. ISME J. 2011, 6, 285–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauri, C.; Gruber, N.; Plattner, G.-K.; Alin, S.; Feely, R.; Hales, B.; Wheeler, P. Ocean Acidification in the California Current System. Oceanography 2009, 22, 60–71. [Google Scholar] [CrossRef] [Green Version]
| Strain | Taxonomy | Isolation Details |
|---|---|---|
| WH7803 | Marine sub-cluster 5.1B, clade V | 33.7423° N 67.4913° W; 25 m depth; Sargasso Sea, North Atlantic Ocean; 1978 |
| WH8020 | Marine sub-cluster 5.1A, clade Ia | 38.68° N, 69.3° W; 50 m depth; northwestern Atlantic Ocean slope water; 26 June 1980 |
| WH8102 | Marine sub-cluster 5.1A, clade, IIIa | 22.495° N, 65.6° W; surface; Sargasso Sea, North Atlantic, from Oceanus cruise 92; 15 March 1981 |
| WH8109 | Marine sub-cluster 5.1A, clade IIa | 39.47° N, 70.45° W; northwestern Atlantic Ocean slope water; June 1981 |
| RCC555 | Marine sub-cluster 5.1B, clade IX | 29.47° N 34.92° E; 10 m depth;Gulf of Aqaba; Red Sea; 1999 |
| RCC2673 | Marine sub-cluster 5.1A, clade IV | 32.87° N–117.26° W; 5 m depth; California current; Pacific Ocean; 1999 |
| Strain | Pearson’s Product–Moment Correlation between δ15NAir (‰) and µ (d−1) |
|---|---|
| WH7803 | −0.75 (p = 0.02) * |
| WH8020 | −0.45 (p = 0.23) |
| WH8102 | 0.28 (p = 0.47) |
| WH8109 | 0.68 (p = 0.04) * |
| RCC555 | −0.36 (p = 0.34) |
| RCC2673 | 0.21 (p = 0.59) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basu, S.; Mackey, K.R.M. Effect of Rising Temperature and Carbon Dioxide on the Growth, Photophysiology, and Elemental Ratios of Marine Synechococcus: A Multistressor Approach. Sustainability 2022, 14, 9508. https://doi.org/10.3390/su14159508
Basu S, Mackey KRM. Effect of Rising Temperature and Carbon Dioxide on the Growth, Photophysiology, and Elemental Ratios of Marine Synechococcus: A Multistressor Approach. Sustainability. 2022; 14(15):9508. https://doi.org/10.3390/su14159508
Chicago/Turabian StyleBasu, Samarpita, and Katherine R. M. Mackey. 2022. "Effect of Rising Temperature and Carbon Dioxide on the Growth, Photophysiology, and Elemental Ratios of Marine Synechococcus: A Multistressor Approach" Sustainability 14, no. 15: 9508. https://doi.org/10.3390/su14159508
APA StyleBasu, S., & Mackey, K. R. M. (2022). Effect of Rising Temperature and Carbon Dioxide on the Growth, Photophysiology, and Elemental Ratios of Marine Synechococcus: A Multistressor Approach. Sustainability, 14(15), 9508. https://doi.org/10.3390/su14159508






