Geochemical Speciation, Risk Assessment, and Sources Identification of Heavy Metals in Mangrove Surface Sediments from the Nanliu River Estuary of the Beibu Gulf, China
Abstract
1. Introduction
2. Materials and Methods
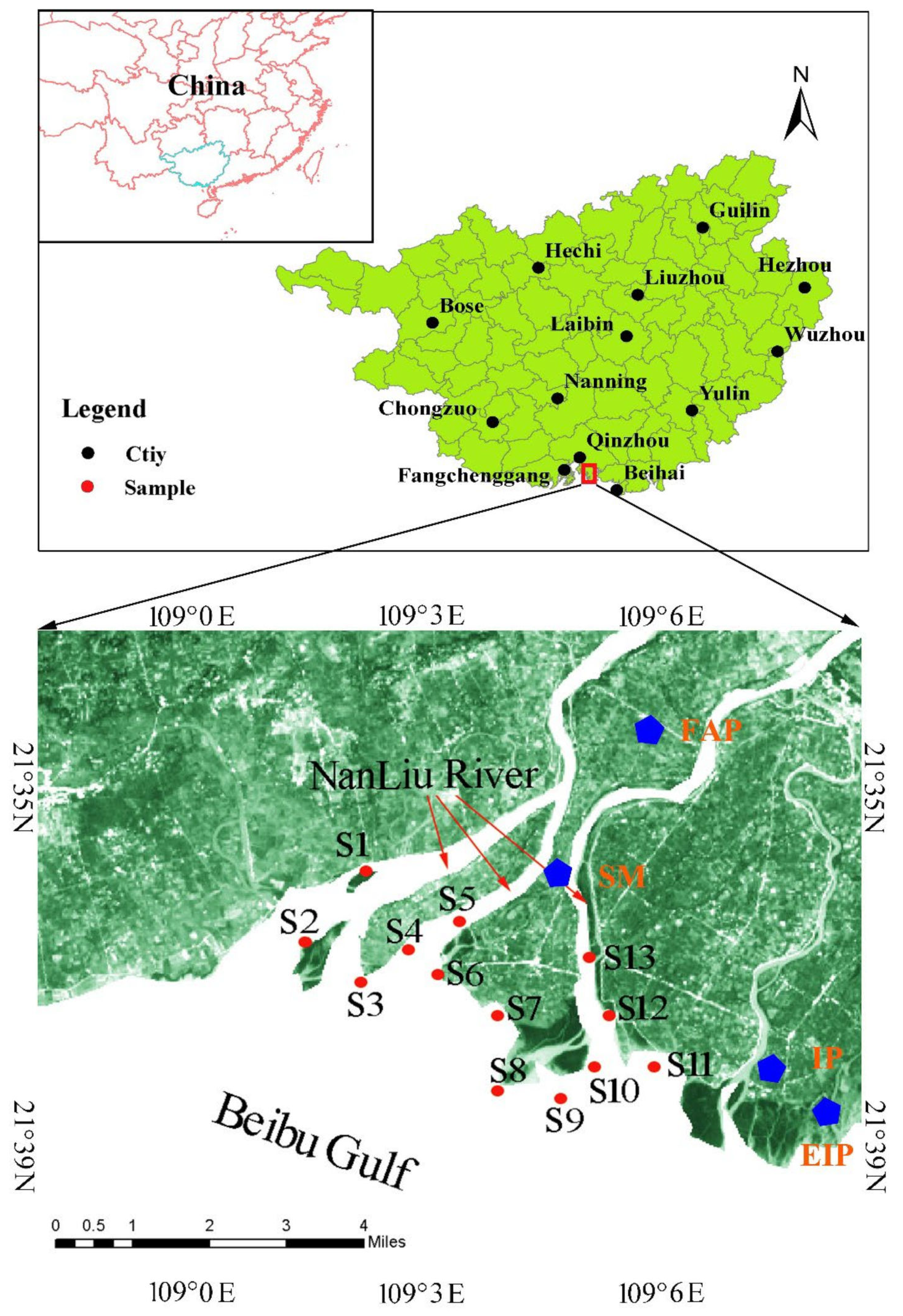
2.1. Sample Collection and Preparation
2.2. Sample Analysis
2.3. Evaluation Methods
2.3.1. Geo-Accumulation Index (Igeo)
2.3.2. Potential Ecological Risk Index (RI)
2.3.3. Risk Assessment Code (RAC)
2.3.4. Individual Contamination Factors (ICF) and Global Contamination Factor (GCF)
2.3.5. Pollution Load Index (PLI)
2.4. Data Procession
3. Results and Discussion
3.1. Physical and Chemical Parameters of Sediments and Distribution Characteristics of Heavy Metal Content
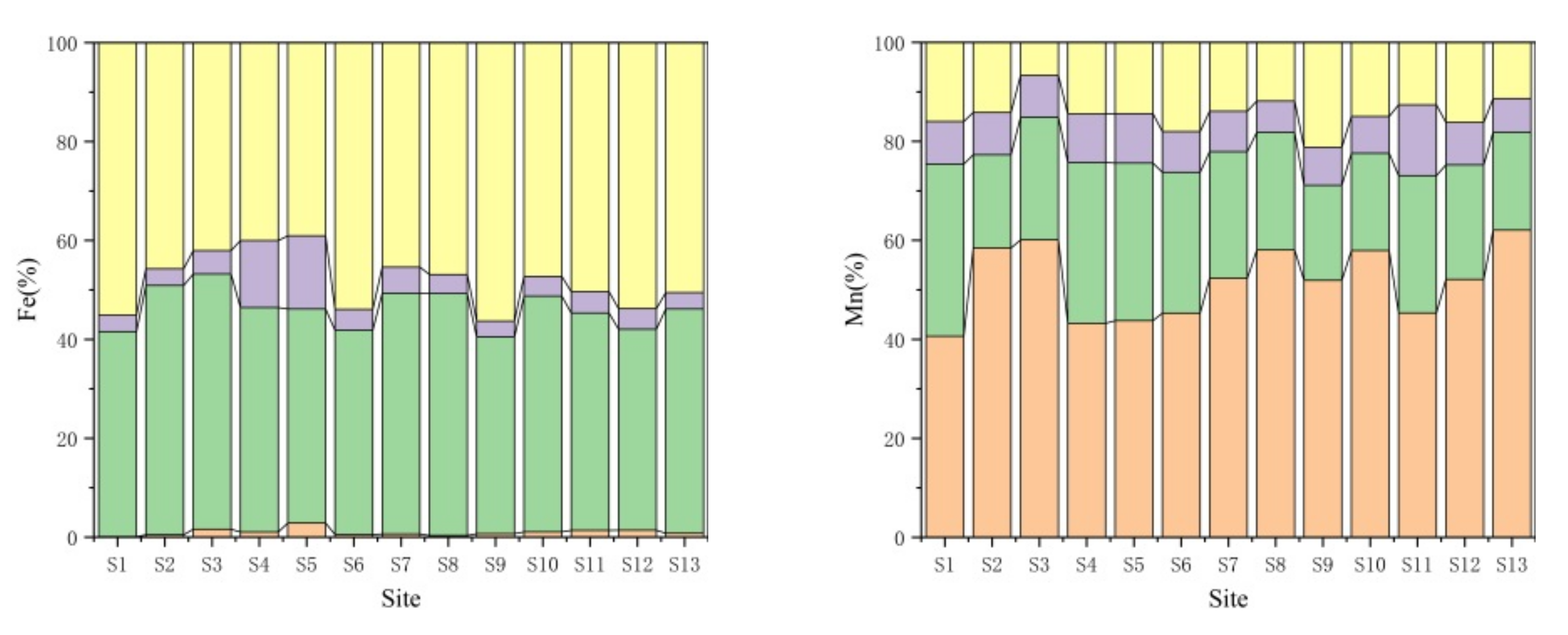
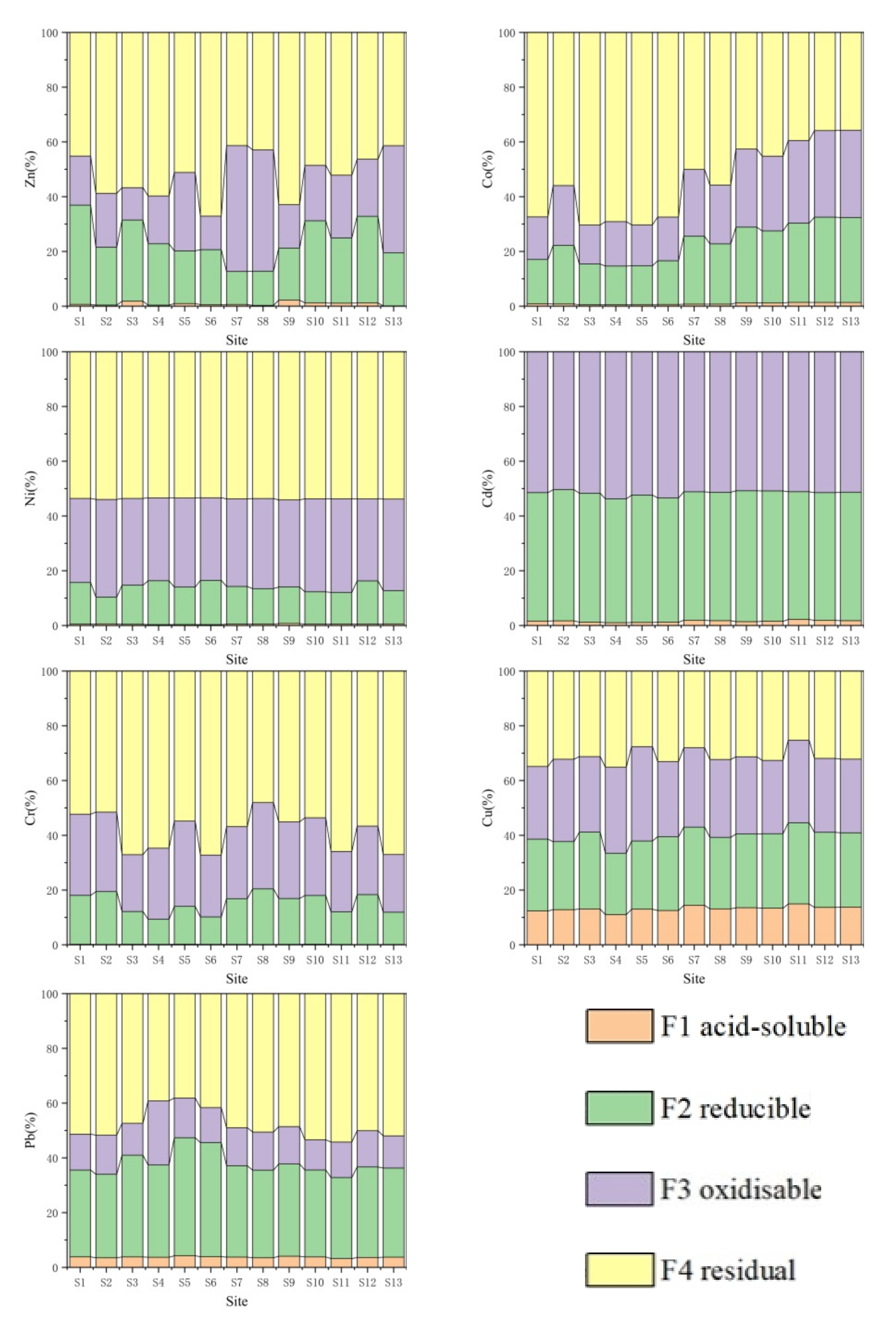
3.2. Distribution Characteristics of Geochemical Speciation of Heavy Metals
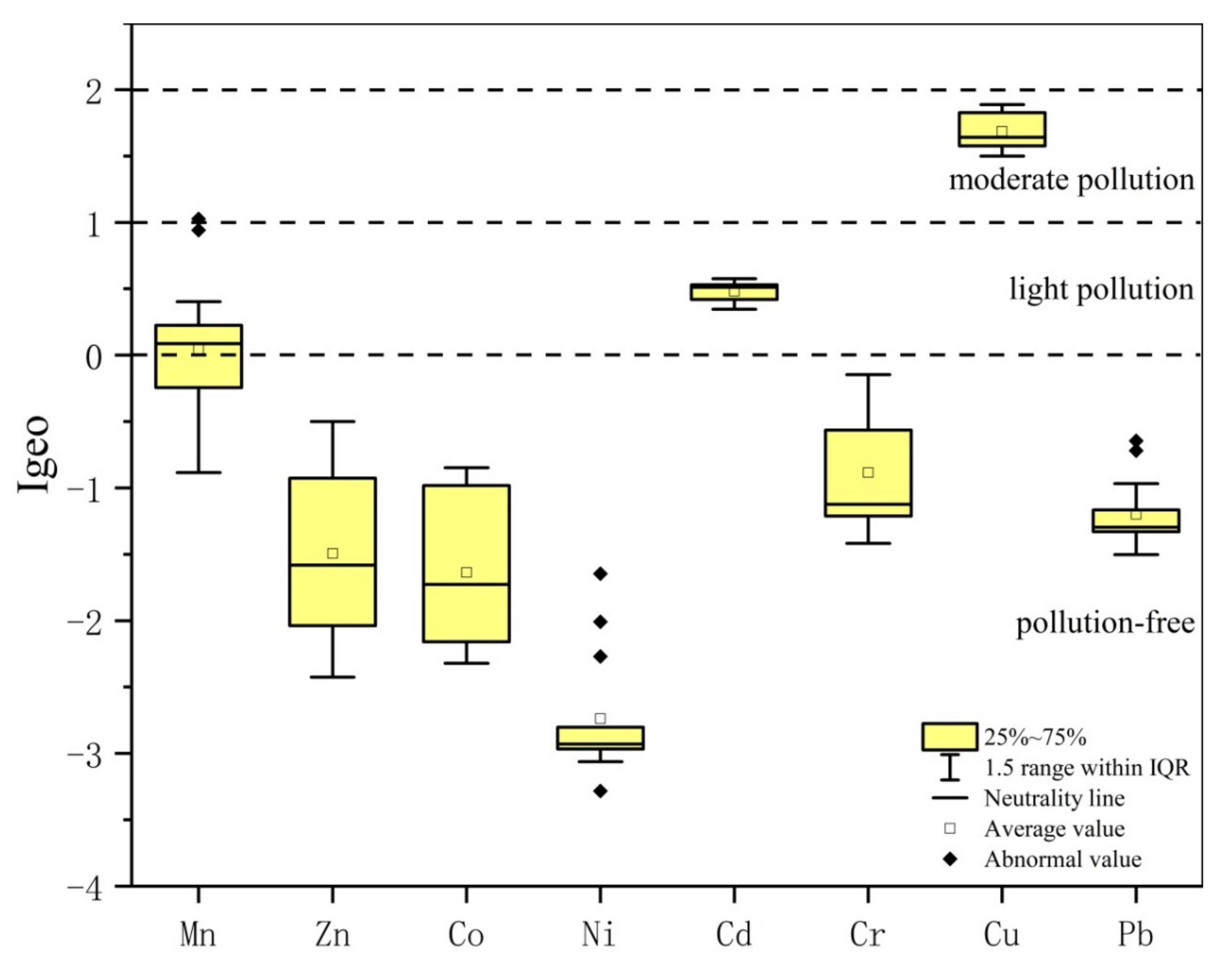
3.3. Geo-Accumulation Index (Igeo)
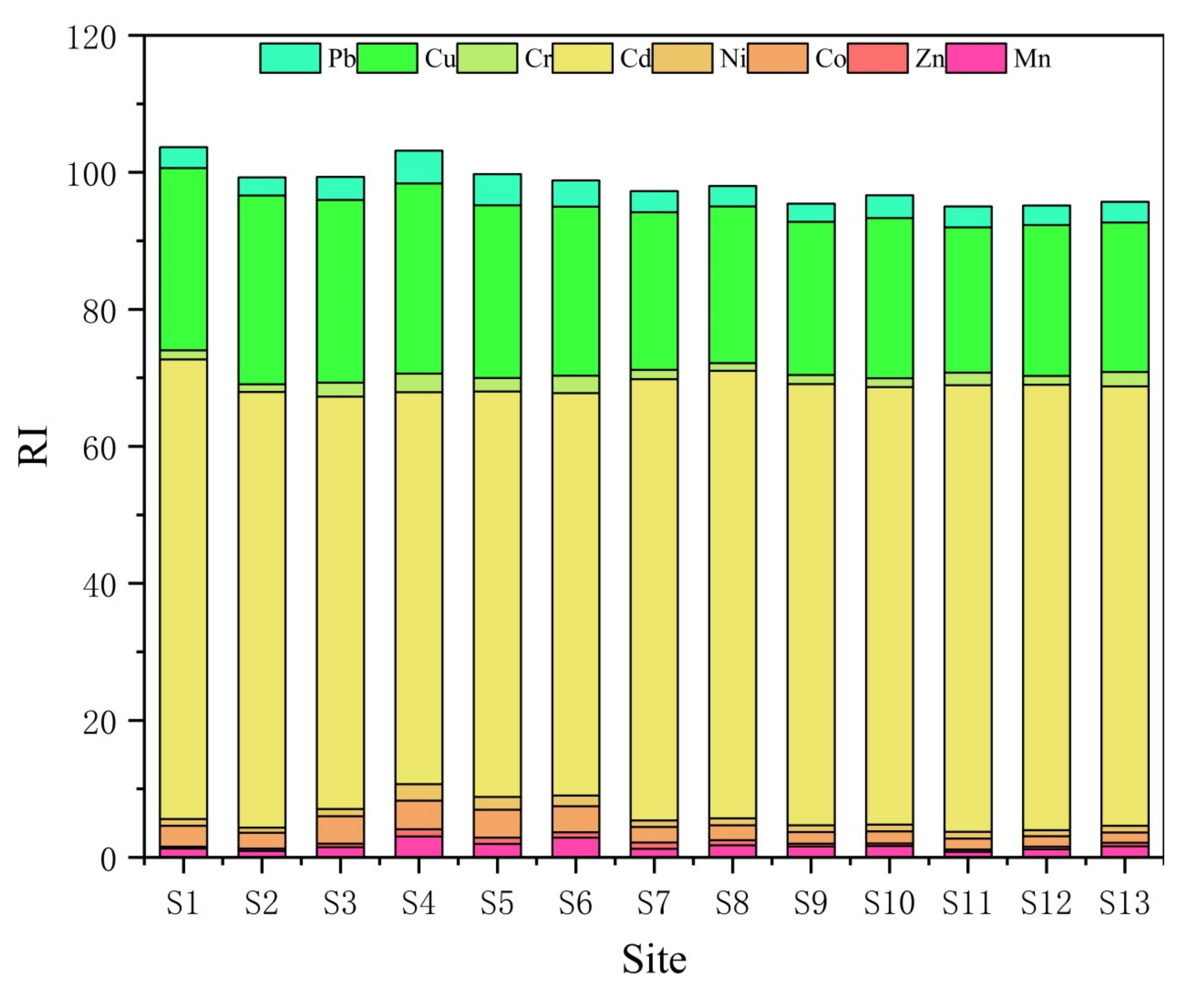
3.4. Potential Ecological Risk Index (RI)
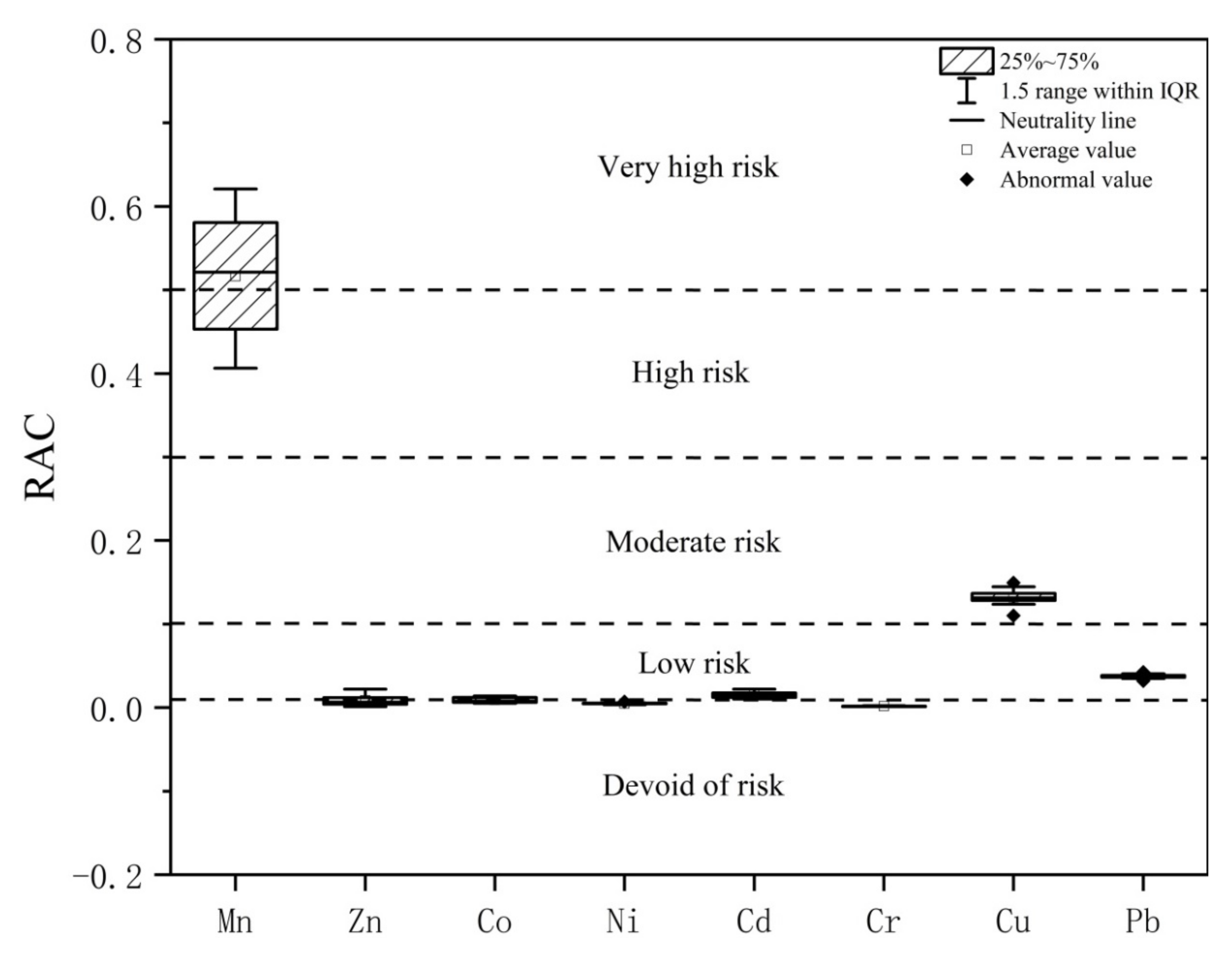
3.5. Risk Assessment Code (RAC)
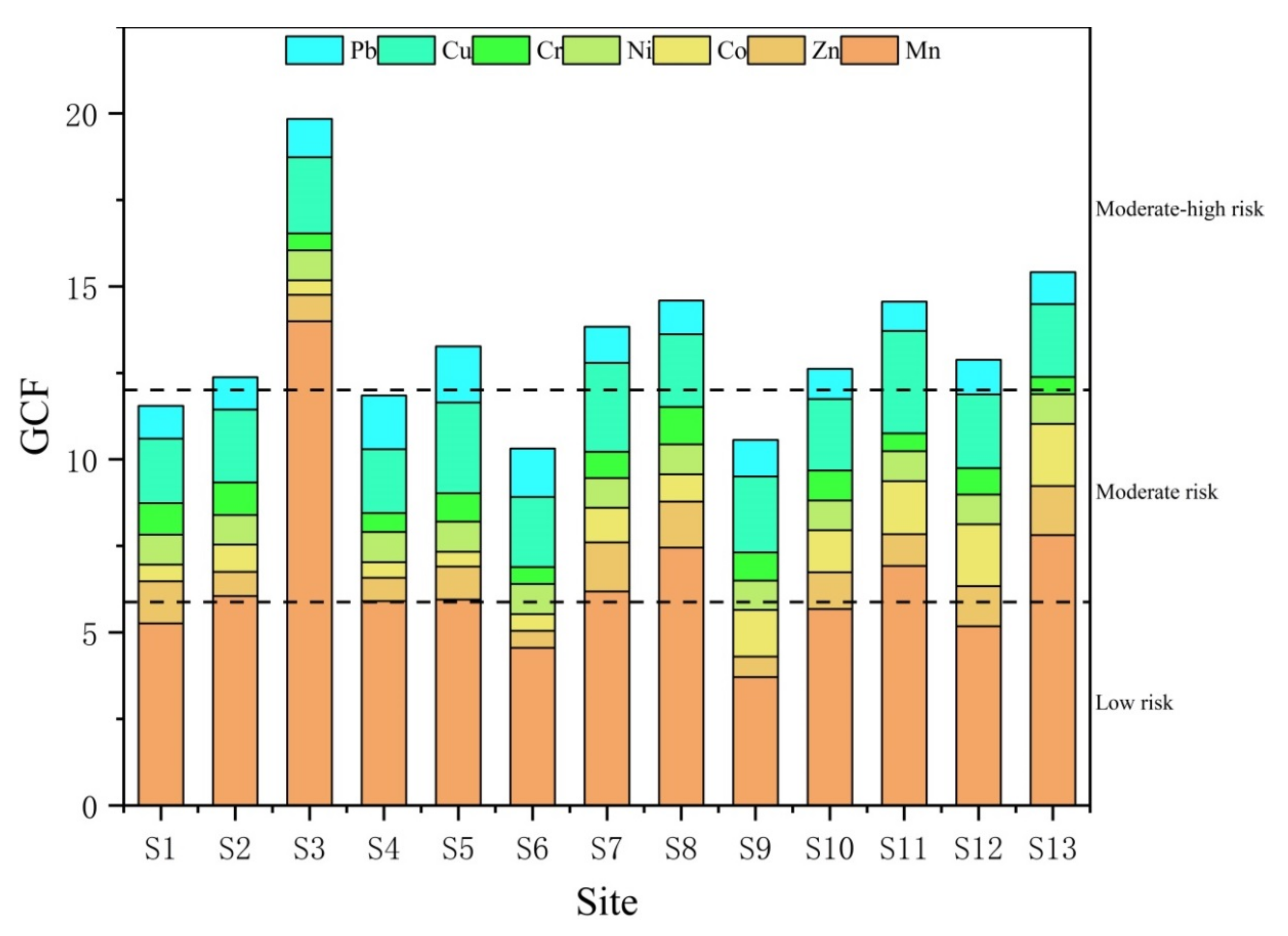
3.6. Individual Contamination Factors (ICF) and Global Contamination Factor (GCF)
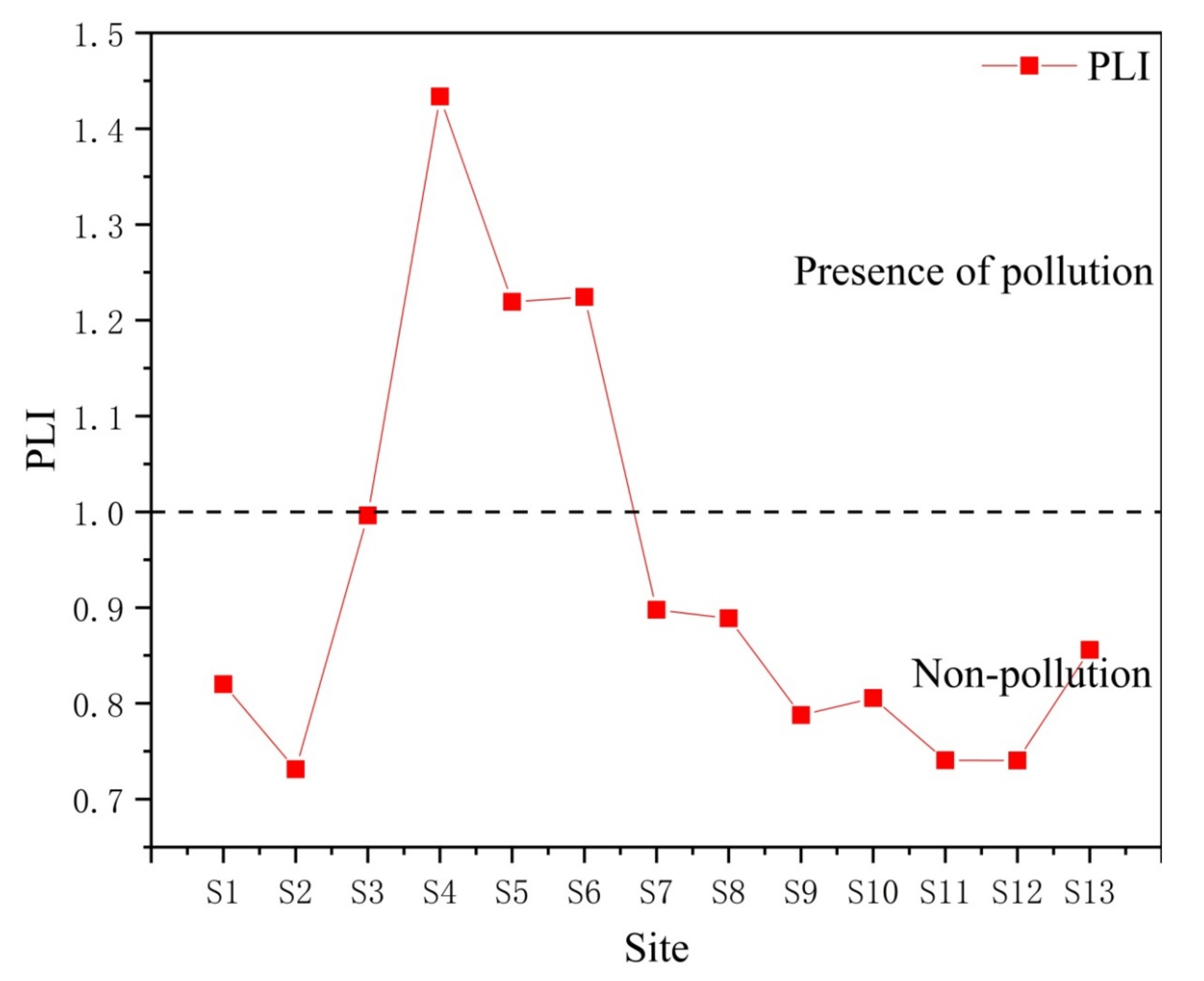
3.7. Pollution Load Index (PLI)
3.8. Heavy Metal Pollution Source Analysis
3.8.1. Correlation Analysis
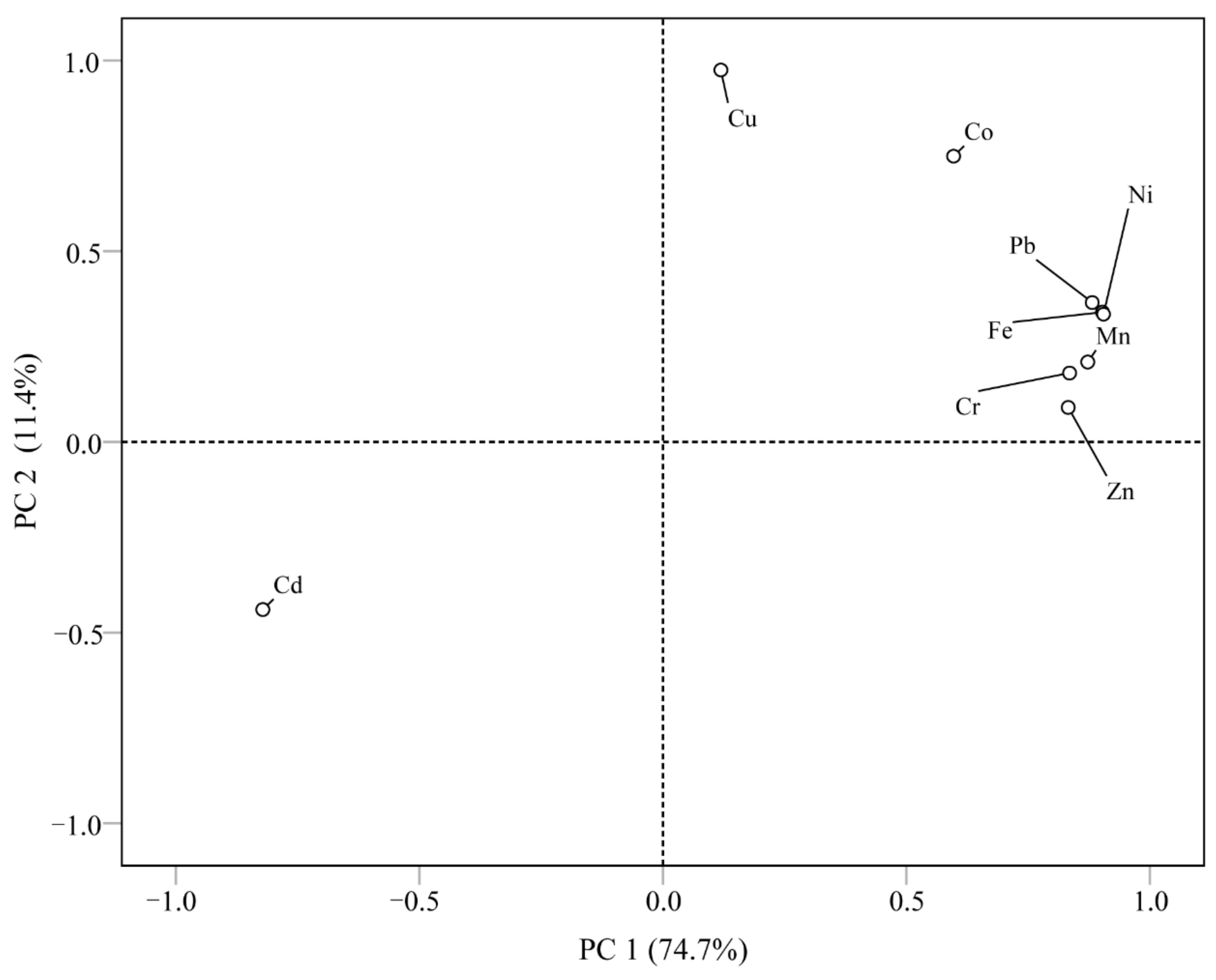
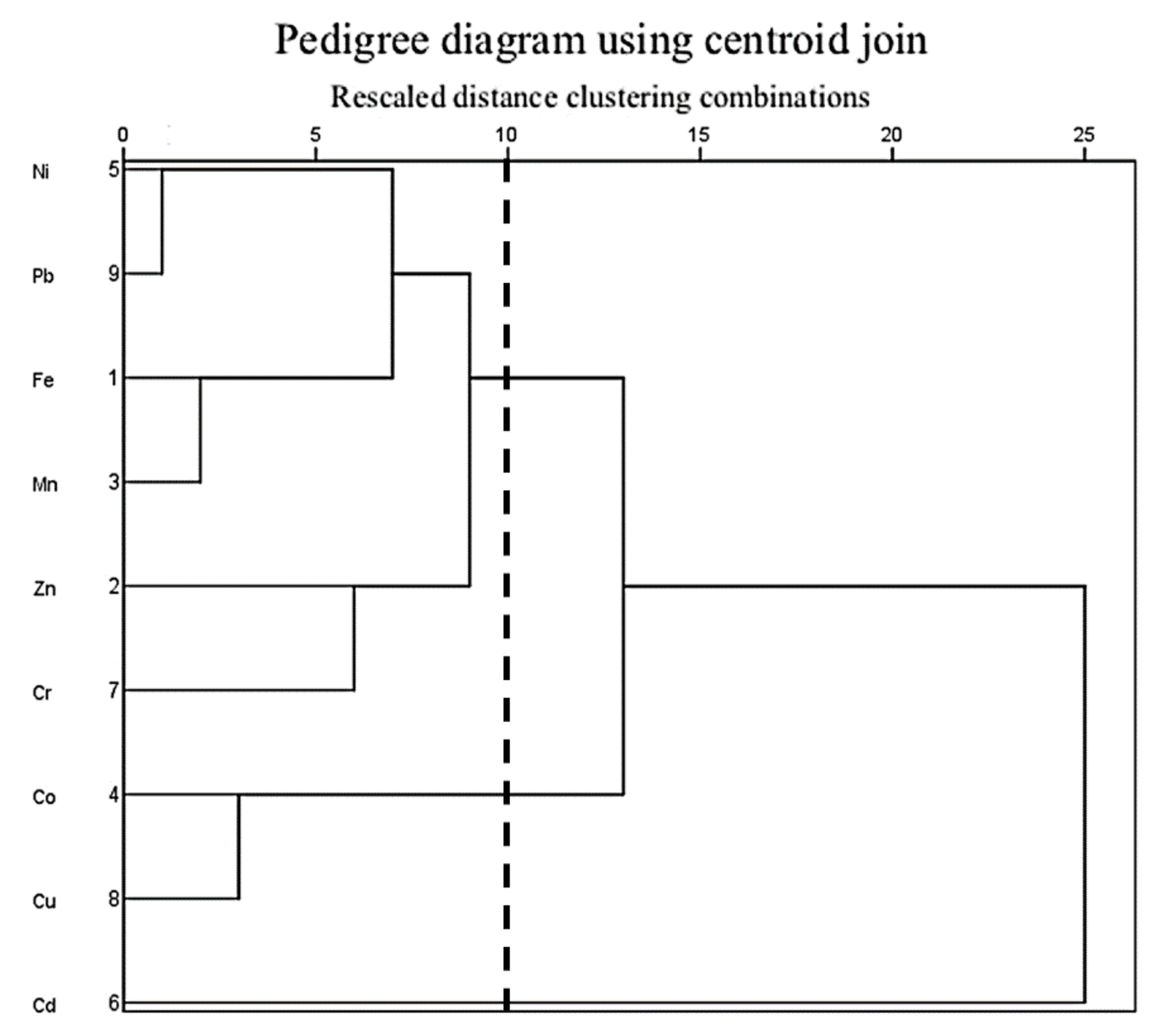
3.8.2. Principal Component Analysis (PCA) and Cluster Analysis (CA)
4. Conclusions
- (1)
- The mean values of Fe, Mn, Zn, Ni, Co, Cd, Cr, Cu, and Pb in the mangrove sediments of Nanliu River were as follows: Fe > Mn > Cu > Zn > Cr > Pb > Co > Ni > Cd. Fe, Zn, Co, Ni, Cr, Cu, and Pd are mainly in the residual speciation and are difficult to release, while Mn is mainly in the weakly acid-soluble speciation, and Cd is mainly in the reducible speciation, both of which have a significant secondary release potential.
- (2)
- The results of the Igeo index method show that Cu is moderately polluted, and Cd and Mn are slightly polluted. However, the results of RI showed that Cd > Cu > Pb > Co > Cr > Mn > Ni > Zn, and the potential environmental harm of Cd pollution is predominant. The distinction between the two evaluation methods is mainly caused by the high toxicity coefficient of Cd.
- (3)
- The results of the RAC analysis show that Mn is at a very high-risk level, Cu is at a medium-risk level, Cd and Pb are at a low-risk level, and Co, Zn, Ni, and Cr are at a risk-free level. A combination of heavy metal speciation, Igeo, RI, RAC, and ICF analysis suggests that Mn and Cd, which are predominantly in unstable speciation, are easily released in the environment and are therefore of high ecological risk, while Cu and Pb are also of some ecological risk and require precaution.
- (4)
- The results of the source analysis, based on correlation analysis and cluster analysis, show that Fe, Mn, Zn, Ni, Cr, and Pb may mainly originate from natural sources and the transportation industry, Co and Cu may mainly originate from ship manufacturing industrial activities, and Cd has a different source than heavy metal elements and may mainly originate from agriculture and aquaculture. The GCF and PLI results suggest that heavy metal enrichment and considerable ecological risk samples are located in the upper part of the region where there are frequent industrial and agricultural activities of the upstream ship manufacturing plant, as well as neighboring farmland and aquaculture ponds.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, J.K.; Yadav, A.K.; Gupta, S.; Verma, R. Heavy metal pollution in coastal environment and its remediation using mangroves: An eco-sustainable approach. Wetlands Conservation: Current Challenges and Future Strategies; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2021; Volume 8, pp. 201–228. [Google Scholar]
- Masnavi, M.R.; Amani, N.; Ahmadzadeh, A. Ecological landscape planning and design strategies for mangrove communities (Hara forests) in South-Pars special economic energy zone, Asalouyeh-Iran. Environ. Nat. Resour. Res. 2016, 6, 44–57. [Google Scholar] [CrossRef][Green Version]
- Maurya, P.; Kumari, R. Toxic metals distribution, seasonal variations and environmental risk assessment in surficial sediment and mangrove plants (A. marina), Gulf of Kachchh (India). J. Hazard. Mater. 2021, 413, 125345. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Ramanathan, A.L.; Kumar, A.; Singhal, R.k.; Datta, D.; Venkateshm, M. Trace metal distribution, assessment and enrichment in the surface sediments of Sundarban mangrove ecosystem in India and Bangladesh. Mar. Pollut. Bull. 2018, 127, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Senthil Kumar, C.K.; Sathish, V.; Manigandan, S.; Tamilarasi, A. Effect of minerals and heavy metals in sand samples of Ponnai river, Tamil Nadu, India. Sci. Rep. 2021, 11, 1–14. [Google Scholar]
- Liu, Y.; Wang, Q.; Zhuang, W.; Yuan, Y.; Yuan, Y.; Jiao, K.; Wang, M.; Chen, Q. Calculation of Thallium’s toxicity coefficient in the evaluation of potential ecological risk index: A case study. Chemosphere 2018, 194, 562–569. [Google Scholar] [CrossRef]
- Aigberua, A.; Tarawou, T. Speciation and mobility of selected heavy metals in sediments of the nun river system, Bayelsa State, Nigeria. Environ. Toxicol. Stud. J. 2018, 2, 1–9. [Google Scholar]
- Ma, L.; Abuduwaili, J.; Liu, W. Spatial distribution and ecological risks of the potentially-toxic elements in the surface sediments of lake Bosten, China. Toxics 2020, 8, 77. [Google Scholar] [CrossRef]
- Guan, J.; Wang, J.; Pan, H.; Yang, C.; Qu, J.; Lu, N.; Yuan, X. Heavy metals in Yinma River sediment in a major Phaeozems zone, Northeast China: Distribution, chemical fraction, contamination assessment and source apportionment. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Jafarabadi, A.R.; Bakhtiyari, A.R.; Toosi, A.S.; Jadot, C. Spatial distribution, ecological and health risk assessment of heavy metals in marine surface sediments and coastal seawaters of fringing coral reefs of the Persian Gulf, Iran. Chemosphere 2017, 185, 1090–1111. [Google Scholar] [CrossRef]
- Nazeer, S.; Hashmi, M.Z.; Malik, R.N. Distribution, risk assessment, and source identification of heavy metals in surface sediments of River Soan, Pakistan. Clean–Soil Air Water 2016, 44, 1250–1259. [Google Scholar] [CrossRef]
- Saadati, M.; Soleimani, M.; Sadeghsaba, M.; Hemami, M.R. Bioaccumulation of heavy metals (Hg, Cd and Ni) by sentinel crab (Macrophthalmus depressus) from sediments of Mousa Bay, Persian Gulf. Ecotoxicol. Environ. Saf. 2020, 191, 211–228. [Google Scholar] [CrossRef]
- Khan, Y.K.; Toqeer, M.; Shah, M.H. Mobility, bioaccessibility, pollution assessment and risk characterization of potentially toxic metals in the urban soil of Lahore, Pakistan. Environ. Geochem. Health 2022, 45, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Das, P.K.; Ghosh, P. The extent of heavy metal pollution by chemical partitioning and risk assessment code of sediments of sewage-fed fishery ponds at East Kolkata Wetland, a Ramsar Site, India. Bull. Environ. Contam. Toxicol. 2022, 108, 731–736. [Google Scholar] [CrossRef]
- Gan, H.; Lin, J.; Liang, K.; Xia, Z. Selected trace metals (As, Cd and Hg) distribution and contamination in the coastal wetland sediment of the northern Beibu Gulf, South China Sea. Mar. Pollut. Bull. 2013, 66, 252–258. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Zhao, M.; Chen, B.; Dada, O.A.; Chu, Z. Spatial distribution and historical trends of heavy metals in the sediments of petroleum producing regions of the Beibu Gulf, China. Mar. Pollut. Bull. 2015, 91, 87–95. [Google Scholar] [CrossRef]
- Lao, Q.; Su, Q.; Liu, G.; Shen, Y.; Chen, F.; Lei, X.; Qing, S.; Wei, C.; Zhang, C.; Gao, J. Spatial distribution of and historical changes in heavy metals in the surface seawater and sediments of the Beibu Gulf, China. Mar. Pollut. Bull. 2019, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, R.; Wang, W.; Ge, Q.; Zhang, W.; Chen, L.; Chu, F. Tracing the source of Pb using stable Pb isotope ratios in sediments of eastern Beibu Gulf, South China Sea. Mar. Pollut. Bull. 2019, 141, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Xu, D.; Ge, Q. Modern modes of sediment distribution and the anthropogenic heavy metal pollution record in northeastern Beibu Gulf, south China sea. Mar. Pollut. Bull. 2020, 150, 110694. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, S.; Chen, L.; Chen, B.; Li, M.; Huang, H. Distribution, source, and ecological risk assessment of potentially toxic elements in surface sediments from Qingfeng River, Hunan, China. J. Soils. Sediments 2021, 21, 2686–2698. [Google Scholar] [CrossRef]
- Cuong, D.T.; Obbard, J.P. Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl. Geochem. 2006, 21, 1335–1346. [Google Scholar] [CrossRef]
- Chowdhury, A.; Naz, A.; Maiti, S.K. Bioaccumulation of potentially toxic elements in three mangrove species and human health risk due to their ethnobotanical uses. Environ. Sci. Pollut. Res. 2021, 28, 33042–33059. [Google Scholar] [CrossRef]
- Muller, G. Index of geoaccumulation in sediments of the Rhine River. Geojournal 1969, 2, 108–118. [Google Scholar]
- Lin, H.; Lan, W.; Feng, Q.; Zhu, X.; Zhao, B. Pollution and ecological risk assessment, and source identification of heavy metals in sediment from the Beibu Gulf, South China Sea. Mar. Pollut. Bull. 2021, 168, 112403. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Huang, S.; Wang, W.; Liu, Y.; Lin, C. Heavy metal pollution and ecological risk assessment in the Maowei sea mangrove, China. Mar. Pollut. Bull. 2020, 161, 111816. [Google Scholar] [CrossRef]
- Mondal, P.; Schintu, M.; Marras, B.; Bettoschi, A.; Biswas, J. Geochemical fractionation and risk assessment of trace elements in sediments from tide-dominated Hooghly (Ganges) River Estuary, India. Chem. Geol. 2020, 532, 119373. [Google Scholar] [CrossRef]
- Srilert, C.; Van, T.P. Spatial and temporal variabilities of surface water and sediment pollution at the main tidal-influenced river in Ca Mau Peninsular, Vietnamese Mekong Delta. J. Hydrol. Reg. Stud. 2022, 41, 101–133. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Huang, K.; Ma, L.; Abuduwaili, J.; Liu, W.; Issanova, G.; Saparov, G.; Lin, L. Human-induced enrichment of potentially toxic elements in a sediment core of lake Balkhash, the largest lake in Central Asia. Sustainability 2020, 12, 4717. [Google Scholar] [CrossRef]
- Perin, G.; Craboledda, L.; Lucchese, M.; Cirillo, R.; Orio, A.A. Heavy metal speciation in the sediments of northern Adriatic Sea. A new approach for environmental toxicity determination. Heavy Metals Environ. 1985, 2, 454–456. [Google Scholar]
- Zhang, G.; Bai, J.; Xiao, R.; Zhao, Q.; Jia, J.; Cui, B.; Liu, X. Heavy metal fractions and ecological risk assessment in sediments from urban, rural and reclamation-affected rivers of the Pearl River Estuary, China. Chemosphere 2017, 184, 278–288. [Google Scholar] [CrossRef]
- Ikem, A.; Egiebor, N.O.; Nyavor, K. Trace elements in water, fish and sediment from Tuskegee Lake, Southeastern USA. Water Air Soil Pollut. 2003, 149, 51–75. [Google Scholar] [CrossRef]
- Yao, Z.G.; Gao, P.; Zhang, J.L.; Li, B.L. Speciation of trace elements in sediments from Dongting Lake, central China. Water Pollution VIII: Modelling, Monitoring and Management; WIT Press: Chilworth, UK, 2006; Volume 95, pp. 119–128. [Google Scholar]
- Zhao, S.; Feng, C.; Yang, Y.; Niu, J.; Shen, Z. Risk assessment of sedimentary metals in the Yangtze Estuary: New evidence of the relationships between two typical index methods. J. Hazard. Mater. 2012, 241, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.L.; Wilson, J.G.; Harris, C.R.; Jeffrey, D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgol. Meeresunters. 1980, 33, 566–575. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kaur, P.; Sidu, G.P.S.; Bali, A.S.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. Pollution assessment of heavy metals in soils of India and ecological risk assessment: A state-of-the-art. Chemosphere 2019, 216, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Ding, H.; Zan, Q.; Li, R. Spatial variation and ecological risk assessment of heavy metals in mangrove sediments across China. Mar. Pollut. Bull. 2019, 143, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qiu, G.Y.; Chai, M.; Shen, X.; Zan, Q. Effects of conversion of mangroves into Gei Wai ponds on accumulation, speciation and risk of heavy metals in intertidal sediments. Environ. Geochem. Health 2019, 41, 159–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zhang, Z.; Liu, C.; Sun, C.; Zhang, W.; Marhaba, T. pH effect on heavy metal release from a polluted sediment. J. Chem. 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Sun, X.; Hu, Z.; Fan, D. Accumulation and transformation of heavy metals in surface sediments from the Yangtze River estuary to the East China Sea shelf. Environ. Pollut. 2019, 245, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Naz, A.; Chowdhury, A.; Mishra, B.K.; Karthikeyan, K. Distribution of heavy metals and associated human health risk in mine, agricultural and roadside soils at the largest chromite mine of India. Environ, Geochem. Health 2018, 40, 1–21. [Google Scholar] [CrossRef]
- Chai, M.; Li, R.; Ding, H.; Zan, Q. Occurrence and contamination of heavy metals in urban mangroves: A case study in Shenzhen, China. Chemosphere 2019, 219, 165–173. [Google Scholar] [CrossRef]
- Gopalakrishnan, G.; Wang, S.; Mo, L.; Zou, J. Distribution determination, risk assessment, and source identification of heavy metals in mangrove wetland sediments from Qi’ao Island, South China. Reg. Stud. Mar. Sci. 2020, 33, 100–112. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.P.; Song, Z. Heavy Metal Contamination and Ecological Risk Assessments in Urban Mangrove Sediments in Zhanjiang Bay, South China. ACS Omega. 2022, 24, 21306–21316. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Kumar, P.; Kumar, R. Ecological risk assessment of heavy metal contamination in mangrove forest sediment of Gulf of Khambhat region, West Coast of India. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Sankarappan, R.; Gopalakrishnan, G.; Shanmugam, R.; Magalingam, R.; Ayyamperumal, R. Diffusion, textural characteristics, and source identification of the heavy metals in the Karankadu mangrove sediments, South India. Arab. J. Geosci. 2021, 14, 1–11. [Google Scholar] [CrossRef]
- Mondal, P.; Reichelt-Brushett, A.J.; Jonathan, M.P.; Babu, S.S.; Sarkar, S.K. Pollution evaluation of total and acid-leachable trace elements in surface sediments of Hooghly River Estuary and Sundarban Mangrove Wetland (India). Environ. Sci. Pollut. Res. 2018, 25, 5681–5699. [Google Scholar] [CrossRef] [PubMed]
- Al-Kahtany, K.; El-Sorogy, A.; Al-Kahtany, F.; Youssef, M. Heavy metals in mangrove sediments of the central Arabian Gulf shoreline, Saudi Arabia. Arab. J. Geosci. 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Sun, J.; Yu, R.; Hu, G.; Su, G.; Zhang, Y. Tracing of heavy metal sources and mobility in a soil depth profile via isotopic variation of Pb and Sr. Catena 2018, 171, 440–449. [Google Scholar] [CrossRef]
- Bothner, M.H.; Ten Brink, M.B.; Manheim, F.T. Metal concentrations in surface sediments of Boston Harbor—Changes with time. Mar. Environ. Res. 1998, 45, 127–155. [Google Scholar] [CrossRef]
- Setia, R.; Marschner, P.; Baldock, J.; Chittleborough, D.; Smith, P.; Simth, J. Salinity effects on carbon mineralization in soils of varying texture. Soil Biol. Biochem. 2011, 43, 1908–1916. [Google Scholar] [CrossRef]
- Lin, C.; He, M.; Liu, X.; Guo, W.; Liu, S. Distribution and contamination assessment of toxic trace elements in sediment of the Daliao River System, China. Environ. Earth Sci. 2013, 70, 3163–3173. [Google Scholar] [CrossRef]
- Cao, Y.; Lei, K.; Zhang, X.; Xu, L.; Lin, C.; Yang, Y. Contamination and ecological risks of toxic metals in the Hai River, China. Ecotoxicol. Environ. Saf. 2018, 164, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Badr, N.B.E.; El-Fiky, A.A.; Mostafa, A.R.; Al-Mur, B. Metal pollution records in core sediments of some Red Sea coastal areas, Kingdom of Saudi Arabia. Environ. Monit. Asses. 2009, 155, 509–526. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, F.G.; Sharma, V.K. Major and trace elements in sediments of the Campeche Sound, southeast Gulf of Mexico. Mar. Pollut. Bull. 2004, 48, 87–90. [Google Scholar] [CrossRef]
| Step | Power (W) | Warming up Time (Min) | Temperature (°C) | Retention Time (Min) |
|---|---|---|---|---|
| 1 | 1200 | 6 | 120 | 6 |
| 2 | 1200 | 6 | 180 | 15 |
| Step (Fraction) | Reagent | Procedure |
|---|---|---|
| 1 (F1: Exchangeable acid-soluble) | 0.11 mol/L CH3COOH | 20 mL CH3COOH + 1 g sample Shake at 220 rpm for 16 h |
| 2 (F2: Reducible) | 0.5 mol/L NH2OH·HCl (pH = 1.5) | 20 mL NH2OH·HCl + residue from step1and shake at 220 rpm for 16 h |
| 3 (F3: Oxidizable) | 30%H2O2, 1.0 mol/L NH4OAC (pH = 2) | 10 mL 30% H2O2 + residue from step2, 85 °C reaction for until nearly dry + 25 mL NH4OAC (pH = 2), shake at 220 rpm for 16 h |
| 4 (F4: Residual) | HNO3, HCl | 6 mL HNO3 + 3 mL HCl, microwave digestion |
| Value | Grades of Ecological Risk for a Single Metal | Value | Grades of Ecological Risk for a Single Metal |
|---|---|---|---|
| ≤ 30 | Low risk | RI ≤ 70 | Low risk |
| ≤ 60 | Moderate risk | 70 < RI ≤ 140 | Moderate risk |
| ≤ 120 | Considerable risk | 140 < RI ≤ 280 | Considerable risk |
| ≤ 240 | High risk | RI ≥ 280 | Very high risk |
| ≥ 240 | Very high risk |
| Item | μg/g | % | pH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Mn | Zn | Co | Ni | Cd | Cr | Cu | Pb | SAL | TOC | ||
| Mean | 6148.18 | 264.34 | 28.18 | 7.60 | 5.70 | 0.16 | 18.18 | 54.80 | 13.58 | 0.42 | 3.97 | 7.16 |
| SD | 2952.76 | 106.22 | 12.68 | 3.11 | 2.25 | 0.01 | 5.75 | 5.21 | 2.76 | 0.13 | 2.20 | 0.33 |
| Min | 3546.83 | 129.46 | 13.48 | 4.38 | 3.70 | 0.16 | 12.03 | 47.96 | 10.82 | 0.23 | 2.13 | 6.38 |
| Max | 11,809.07 | 487.12 | 51.21 | 12.16 | 11.51 | 0.14 | 29.01 | 62.74 | 19.61 | 0.75 | 9.73 | 7.77 |
| CV/% | 48.03 | 40.18 | 45.01 | 40.91 | 39.42 | 5.10 | 31.65 | 9.51 | 20.29 | 31.15 | 55.26 | 4.59 |
| Location | Fe | Mn | Zn | Co | Ni | Cd | Cr | Cu | Pb |
|---|---|---|---|---|---|---|---|---|---|
| Present study | 6148.18 | 264.34 | 28.18 | 7.60 | 5.70 | 0.16 | 18.18 | 54.80 | 13.58 |
| Maowei sea, China [25] | - | 540 | 166 | 20.1 | 50.7 | 0.790 | 96.5 | 61.9 | 48.9 |
| Shenzhen Futian, China [42] | - | 2351 | 351 | - | 117 | 5.70 | 49.8 | 82.6 | 105 |
| Qi’ao Island, China [43] | - | 1346.3 | 241.7 | - | 50.4 | 9.5 | 389.2 | 81.5 | 70.6 |
| Zhanjiang Bay, China [44] | - | - | - | 2.91 | 7.86 | 0.19 | - | 18.24 | 20.07 |
| Khambhat region Gulf, India [45] | - | 669.016 | 84.022 | 0.246 | 34.655 | 0.086 | 48.189 | 11.641 | 7.135 |
| Karankadu, India [46] | 42,798 | 485 | 194 | - | 24 | 5 | - | 47 | 27 |
| Hooghly River Estuary and Sundarban, India [47] | 25,050 | 517 | 53.76 | - | 27.72 | 0.17 | 29.71 | 33.88 | 14.27 |
| Central Arabian Gulf shoreline, Saudi Arabia [48] | 7946.9 | 122.5 | 28.35 | 3.94 | 81.05 | 1.67 | 50.65 | 209.8 | 4.395 |
| pH | SAL | TOC | Fe | Mn | Zn | Co | Ni | Cd | Cr | Cu | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1 | −0.733 ** | −0.749 ** | −0.713 ** | −0.425 | −0.484 | −0.305 | −0.711 ** | 0.221 | −0.352 | −0.072 | −0.690 ** |
| SAL | 1 | 0.870 ** | 0.730 ** | 0.744 ** | 0.584 * | 0.449 | 0.817 ** | −0.331 | 0.499 | 0.374 | 0.708 ** | |
| TOC | 1 | 0.939 ** | 0.842 ** | 0.761 ** | 0.703 ** | 0.991 ** | −0.635 * | 0.734 ** | 0.432 | 0.950 ** | ||
| Fe | 1 | 0.838 ** | 0.727 ** | 0.799 ** | 0.949 ** | −0.874 ** | 0.782 ** | 0.417 | 0.947 ** | |||
| Mn | 1 | 0.707 ** | 0.647 * | 0.848 ** | −0.789 ** | 0.734 ** | 0.342 | 0.785 ** | ||||
| Zn | 1 | 0.598 * | 0.768 ** | −0.688 ** | 0.553 | 0.218 | 0.748 ** | |||||
| Co | 1 | 0.754 ** | −0.803 ** | 0.637 * | 0.762 ** | 0.792 ** | ||||||
| Ni | 1 | −0.842 ** | 0.773 ** | 0.449 | 0.963 ** | |||||||
| Cd | 1 | −0.815 ** | −0.514 | −0.847 ** | ||||||||
| Cr | 1 | 0.265 | 0.757 ** | |||||||||
| Cu | 1 | 0.449 | ||||||||||
| Pb | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; He, R.; Cai, P.; Huang, G.; Wang, F. Geochemical Speciation, Risk Assessment, and Sources Identification of Heavy Metals in Mangrove Surface Sediments from the Nanliu River Estuary of the Beibu Gulf, China. Sustainability 2022, 14, 9112. https://doi.org/10.3390/su14159112
Chen B, He R, Cai P, Huang G, Wang F. Geochemical Speciation, Risk Assessment, and Sources Identification of Heavy Metals in Mangrove Surface Sediments from the Nanliu River Estuary of the Beibu Gulf, China. Sustainability. 2022; 14(15):9112. https://doi.org/10.3390/su14159112
Chicago/Turabian StyleChen, Bo, Renqiang He, Pingxiong Cai, Guiqiu Huang, and Feng Wang. 2022. "Geochemical Speciation, Risk Assessment, and Sources Identification of Heavy Metals in Mangrove Surface Sediments from the Nanliu River Estuary of the Beibu Gulf, China" Sustainability 14, no. 15: 9112. https://doi.org/10.3390/su14159112
APA StyleChen, B., He, R., Cai, P., Huang, G., & Wang, F. (2022). Geochemical Speciation, Risk Assessment, and Sources Identification of Heavy Metals in Mangrove Surface Sediments from the Nanliu River Estuary of the Beibu Gulf, China. Sustainability, 14(15), 9112. https://doi.org/10.3390/su14159112





