Corrosion Behavior of Different Types of Stainless Steel in PBS Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Anodic Behavior of AISI 304L, AISI 316L, and 2205 DSS in PBS Solution
- The potential difference of Edp − Ecorr (in addition to the above marked as ΔE and represents the range of passive potentials) is an indication of the pitting initiation rate; the higher it is, the slower the pitting initiation rate occurs.
- The potential difference of Erp − Ecorr describes how a metallic material can repassivate (pits no longer grow). The more positive this potential difference is, the easier a certain metallic material can repassivate.
- The potential difference of Edp − Erp is the indication of the extent of crevice corrosion resistance (the lower the Edp − Erp, the higher the resistance towards crevice corrosion).
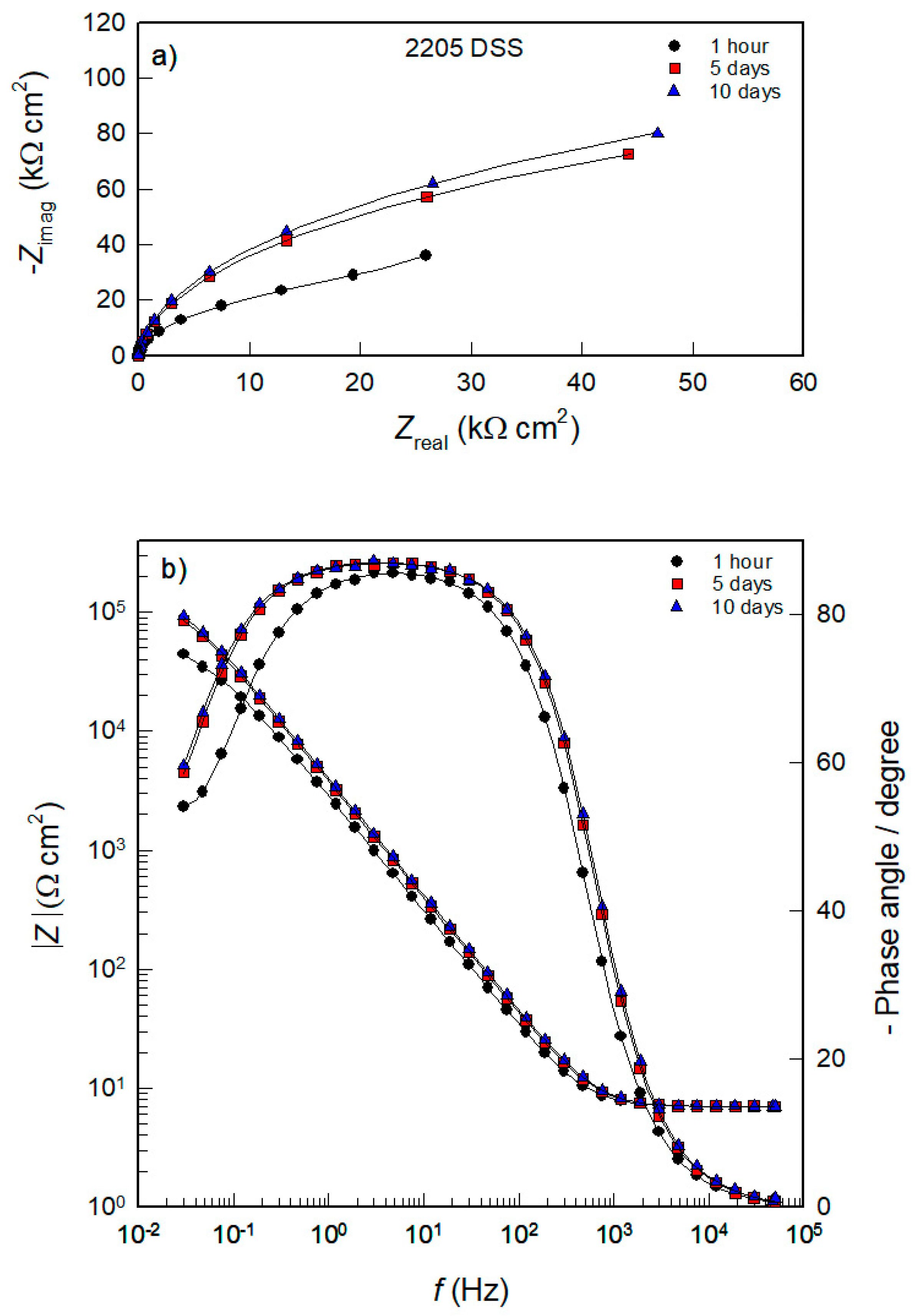
3.2. Spontaneous Corrosion of AISI 304L, AISI 316L, and 2205 DSS in PBS Solution
- (a)
- strength of the oxygen (or OH) chemisorption bond, εM-O, (reflected by the value of the heat of oxygen adsorption, ΔHads(ox)) and
- (b)
- the facility of conversion from the oxygen (or OH) monolayer to a 3D oxide in which a crucial factor is disruption of the metal–metal bonds (reflected by metal-metal bond energy, εM-M) [51].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blackwood, D.J. Biomaterials: Past successes and future problems. Corros. Rev. 2003, 21, 97–124. [Google Scholar] [CrossRef]
- Hashemi, P.M.; Borhani, E.; Nourbakhsh, M.S. A review on nanostructured stainless steel implants for biomedical application. Nanomed J. 2016, 3, 202–216. [Google Scholar]
- Ossowska, A.; Ryl, J.; Sternicki, T. Production and properties of the porous layer obtained by the electrochemical method on the surface of austenitic steel. Materials 2022, 15, 949. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Kim, Y.; Park, Y.; Kim, K. Properties of super stainless steels for orthodontic applications. J. Biomed. Mater. Res. B 2004, 69B, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotman, I. Characteristics of metals used in implants. J. Endourol. 1997, 11, 383–389. [Google Scholar] [CrossRef]
- Ali, S.; Rani, A.M.A.; Mufti, R.A.; Azam, F.I.; Hastuty, S.; Baig, Z.; Hussain, M.; Shehzad, N. The influence of nitrogen absorption on microstructure, properties and cytotoxicity assessment of 316l stainless steel alloy reinforced with boron and niobium. Processes 2019, 7, 506. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Irfan, M.; Niazi, U.M.; Rani, A.M.A.; Rashedi, A.; Rahman, S.; Khan, M.K.A.; Alsaiari, M.A.; Legutko, S.; Petru, J.; et al. Microstructure and mechanical properties of modified 316L stainless steel alloy for biomedical applications using powder metallurgy. Materials 2022, 15, 2822. [Google Scholar] [CrossRef]
- Beloti, M.M.; Rollo, J.M.D.A.; Itman Filho, A.; Rosa, A.L. In vitro biocompatibility of duplex stainless steel with and without 0.2% niobium. J. Appl. Biomater. Biomech. 2004, 2, 162–168. [Google Scholar]
- Kocijan, A.; Conradi, M. The corrosion behavior of austenitic and duplex stainless steel in artificial body fluids. Mater. Technol. 2010, 44, 21–24. [Google Scholar]
- Kocijan, A.; Conradi, M.; Schön, P.M. Austenitic and duplex stainless steels in simulated physiological solution characterized by electrochemical and X-ray photoelectron spectroscopy studies. J. Biomed. Mater. Res. Part B 2012, 100B, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Köse, C.; Kaçar, R.; Zorba, A.P.; Bağirova, M.; Abamor, E.Ş.; Allahverdiyev, A.M. Interactions between fibroblast cells and laser beam welded AISI 2205 duplex stainless steel. Mater. Sci. Medzg. 2018, 24, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Platt, J.A.; Guzman, A.; Zuccari, A.; Thornburg, D.W.; Rhodes, B.F.; Oshida, Y.; Moore, B.K. Corrosion behavior of 2205 duplex stainless steel. Am. J. Orthod. Dentofacial Orthop. 1997, 112, 69–79. [Google Scholar] [CrossRef]
- Hammood, A.S.; Noor, A.F.; Alkhafagy, M.T. Effect of heat treatment on corrosion behavior of duplex stainless steel in orthodontic applications. Mater. Res. Express 2017, 4, 126506. [Google Scholar] [CrossRef]
- Pardo, A.; Merino, M.C.; Coy, A.E.; Viejo, F.; Arrabal, R.; Matykina, E. Pitting corrosion behavior of austenitic stainless steels–combining effects of Mn and Mo additions. Corros. Sci. 2008, 50, 1796–1806. [Google Scholar] [CrossRef]
- Valero Vidal, C.; Igual Munoz, A. Electrochemical characterization of biomedical alloys for surgical implants in simulated body fluids. Corros. Sci. 2008, 50, 1954–1961. [Google Scholar] [CrossRef]
- Jafari, E.; Hadianfard, M.J. Influence of surface treatment on the corrosion resistance of stainless steel in simulated human body environment. J. Mater. Sci. Technol. 2009, 25, 611–614. [Google Scholar]
- Asaduzzaman, M.D.; Mohammad Mustafa, C.; Islam, M. Effect of concentration of sodium chloride solution on the pitting corrosion behavior of AISI 304L austenitic stainless steel. CICEQ 2011, 17, 477–483. [Google Scholar] [CrossRef]
- Brajković, T.; Juraga, I.; Šimunović, V. Influence of surface treatment on corrosion resistance of Cr-Ni steel. Eng. Rev. 2013, 33, 129–134. [Google Scholar]
- Kocijan, A.; Donik, Č.; Jenko, M. The electrochemical study of duplex stainless steel in chloride solutions. Mater. Technol. 2009, 43, 39–42. [Google Scholar]
- Hakiki, N.E.; Boudin, S.; Rondot, B.; Da Cunha Belo, M. The electronic structure of passive films formed on stainless steels. Corros. Sci. 1995, 37, 1809–1822. [Google Scholar] [CrossRef]
- Ramya, S.; Anita, T.; Shaikh, H.; Dayal, R.K. Laser Raman microscopic studies of passive films formed on type 316LN stainless steels during pitting in chloride solution. Corros. Sci. 2010, 52, 2114–2121. [Google Scholar] [CrossRef]
- Ihrzo, A.; Segui, Y.; Bui, N.; Dabosi, F. On the conduction mechanisms of passive films on molybdenum-containing stainless steel. Corrosion 1986, 42, 141–146. [Google Scholar]
- Wegrelius, L.; Falkenberg, F.; Olefjord, I. Passivation of stainless steels in hydrochloric acid. J. Electrochem. Soc. 1999, 146, 1397–1406. [Google Scholar] [CrossRef]
- Malik, A.U.; Siddiqi, N.A.; Ahmad, S.; Andijani, I.N. The effect of dominant alloy additions on the corrosion behavior of some conventional and high alloy stainless steels in seawater. Corros. Sci. 1995, 37, 1521–1535. [Google Scholar] [CrossRef]
- Potgieter, J.H.; Olubambi, P.A.; Cornish, L.; Machio, C.N.; El-Sayed Sherif, M. Influence of nickel additions on the corrosion behavior of low nitrogen 22% Cr series of duplex stainless steels. Corros. Sci. 2008, 50, 2572–2579. [Google Scholar] [CrossRef]
- Rossi, A.; Elsener, B. XPS study of passive films on stainless steels in neutral solutions. In Proceedings of the 12th International Corrosion Congress, Houston, TX, USA, 19–24 September 1993; pp. 2120–2130. [Google Scholar]
- Lorang, G.; Da Cunha Belo, M.; Sinoes, A.M.P.; Ferreira, M.G.S. Chemical composition of passive films on AISI 304 stainless steel. J. Electrochem. Soc. 1994, 141, 3347–3356. [Google Scholar] [CrossRef]
- Milošev, I.; Strehblow, H.H. The behavior of stainless steels in physiological solution containing complexing agent studied by X-ray photoelectron spectroscopy. J. Biomed. Res. 2000, 52, 404–412. [Google Scholar] [CrossRef]
- Choudhary, S.; Cruz, V.; Pandey, A.; Thomas, S.; Birbilis, N. Element-resolved electrochemical analysis of the passivity of additively manufactured stainless steel 316L. Corros. Sci. 2021, 189, 109576. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.; Lin, C.; Pan, C.; Chen, S.; Yang, T.; Lin, D.; Lin, H.; Jang, J. Anisotropic response of Ti-6Al-4V alloy fabricated by 3D printing selective laser melting. Mater. Sci. Eng. A 2017, 682, 389–395. [Google Scholar] [CrossRef]
- Williams, D.E.; Wright, G.A. Nucleation and growth of anodic oxide films on bismuth—I. Cyclic voltammetry. Electrochim. Acta 1976, 21, 1009–1019. [Google Scholar] [CrossRef]
- Tait, W.S. An Introduction to Electrochemical Corrosion Testing for Practicing Engineers and Scientists; Pair O Docks Publications: St. Clair. Racine, WI, USA, 1994; pp. 65–67. [Google Scholar]
- Pal, S.; Finšgar, M.; Bončina, T.; Lojen, G.; Brajlih, T.; Drstvenšek, I. Effect of surface powder particles and morphologies on corrosion of Ti-6Al-4V fabrocated with different energy densities in selective laser melting. Mater. Des. 2011, 211, 1–17. [Google Scholar] [CrossRef]
- Wilde, B.E. Critical appraisal of some popular laboratory electrochemical tests for predicting the localized corrosion resistance of stainless alloys in sea water. Corrosion 1972, 28, 283–291. [Google Scholar] [CrossRef]
- Silverman, D.C. Electrochemical techniques-simple tests for complex predictions in the chemical process industries. Corros. Rev. 1992, 10, 31–78. [Google Scholar] [CrossRef]
- Quin, P.; Lui, Y.; Sercombe, T.B.; Li, Y.; Zhang, C.; Cao, C.; Sun, H.; Zhang, L.C. Improved corrosion resistance on selective laser melting procedure Ti-5Cu alloy after heat treatment. ACS Biomater. Sci. Eng. 2018, 4, 2633–2642. [Google Scholar] [CrossRef]
- El-Taib Heakal, F.; Shehata, O.S.; Tantawy, N.S. Integrity of metallic medical implants in physiological solutions. Int. J. Electrochem. Sci. 2014, 9, 1986–2004. [Google Scholar]
- Ha, H.-Y.; Lee, T.-H.; Bae, J.-H.; Chun, D.W. Molybdenum effects on pitting corrosion resistance of FeCrMnMoNC austenitic stainless steel. Metals 2018, 8, 653. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Di-Franco, F.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Passivation-induced physicochemical alterations of the native surface oxide film on 316L austenitic stainless steel. J. Electrochem. Soc. 2019, 166, C3376–C3388. [Google Scholar] [CrossRef]
- Wang, Z.; Paschalidou, E.-M.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Mechanisms of Cr and Mo enrichments in the passive oxide film on 316L austenitic stainless steel. Front. Mater. 2019, 6, 232. [Google Scholar] [CrossRef] [Green Version]
- Antunes, A.; De Oliveira, M.C.L.; Costa, I. Study of the correlation between corrosion resistance and semi-conducting properties of the passive film of AISI 316L stainless steel in physiological solution. Mater. Corros. 2012, 63, 586–592. [Google Scholar] [CrossRef]
- Rondelli, G.; Torricelli, P.; Giardino, M. In vitro corrosion study by EIS of a nickel-free stainless steel for orthopaedic. Appl. Biomater. 2005, 26, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Tudose, A.E.; Demetrescu, I.; Golgovici, F.; Fulger, M. Oxidation behavior of an austenitic steel (Fe, Cr and Ni), the 310 H, in a deaerated supercritical water static system. Metals 2021, 11, 571. [Google Scholar] [CrossRef]
- Li, D.G.; Wang, J.D.; Chen, D.R. Influence of pH value on the structure and electronic property of the passive film on 316L SS in the simulated cathodic environment of proton exchange membrane fuel cell (PEMFC). Int. J. Hydrog. Energy 2014, 39, 20105–20115. [Google Scholar] [CrossRef] [Green Version]
- Raistrick, I.D.; Macdonald, J.R.; Franceschetti, D.R. Impedance Spectroscopy; Macdonald, J.R., Ed.; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Rammelt, U.; Reinhard, G. On the applicability of a constant phase element (CPE) to the estimation of roughness of solid metal electrodes. Electrochim. Acta 1990, 35, 1045–1049. [Google Scholar] [CrossRef]
- Stoynov, Z. Impedance modelling and data processing: Structural and parametrical estimation. Electrochim. Acta 1990, 35, 1493–1499. [Google Scholar] [CrossRef]
- Boudalia, M.; Guenbour, A.; Bellaouchou, A.; Fernandez-Domene, R.M.; Garcia-Anton, J. Corrosion behaviour of a highly alloyed austenitic Alloy UB6 in contaminated phosphoric acid. Int. J. Corros. 2013, 2013, 363826. [Google Scholar] [CrossRef] [Green Version]
- Makhlouf, A.S.H.; Aliofkhazreaei, M. Handbook of Materials Failure Analysis with Case Studies from Oil and Gas Industry; Butterworth Heinemann: Oxford, UK, 2016; pp. 39–54. [Google Scholar]
- Marcus, P. On some fundamental factors in the effect of alloying elements on passivation of alloys. Coros. Sci. 1994, 36, 2155–2158. [Google Scholar] [CrossRef]

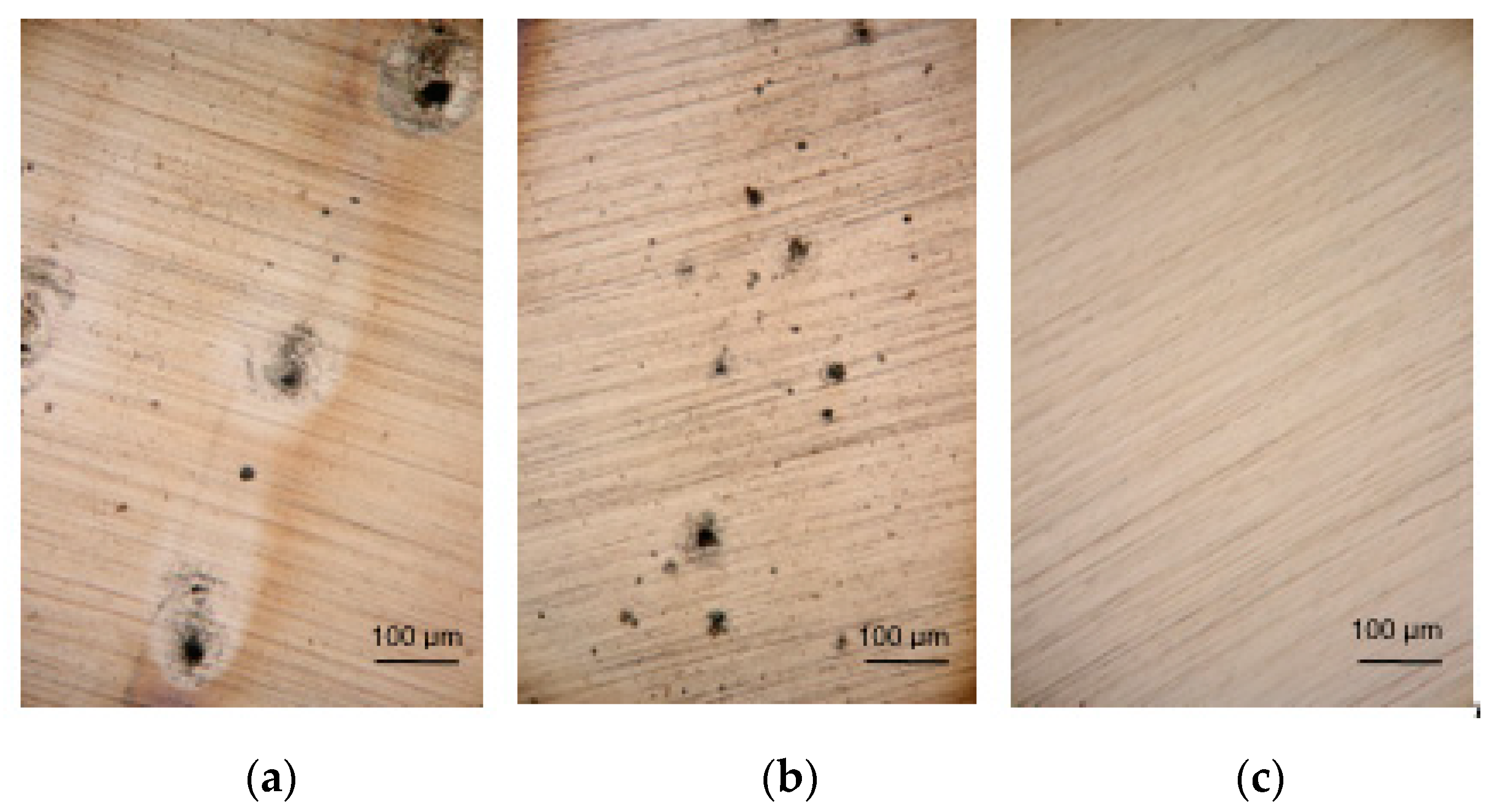










| Chemical Composition (wt.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Cr | Ni | Mn | Mo | Si | Nb | Cu | V | C | Fe |
| AISI 304L | 16.76 | 8.66 | 2.23 | 0.11 | 0.52 | - | 0.38 | - | 0.03 | 71.58 |
| AISI 316L | 16.47 | 10.54 | 1.16 | 2.53 | 0.47 | - | 0.27 | - | 0.002 | 68.55 |
| 2205 DSS | 22.21 | 5.47 | 1.52 | 3.14 | 0.33 | 0.012 | 0.26 | 0.035 | 0.012 | 67.00 |
| Sample | Ecorr (V) | icorr (µA cm−2) | ip (µA cm−2) | Edp (V) | Erp (V) | Edp − Ecorr (V) | Erp − Ecorr (V) | Edp − Erp (V) |
|---|---|---|---|---|---|---|---|---|
| AISI 304L | −0.286 ± 0.014 | 1.31 ± 0.05 | 8.43 ± 0.44 | 0.208 ± 0.013 | −0.098 ± 0.015 | 0.494 ± 0.001 | 0.188 ± 0.001 | 0.306 ± 0.002 |
| AISI 316L | −0.238 ± 0.012 | 0.88 ± 0.04 | 2.61 ± 0.10 | 0.559 ± 0.029 | −0.011 ± 0.010 | 0.797 ± 0.017 | 0.227 ± 0.002 | 0.570 ± 0.019 |
| 2205 DSS | −0.108 ± 0.008 | 0.33 ± 0.02 | 1.35 ± 0.04 | 1.120 ± 0.041 | 0.790 ± 0.020 | 1.228 ± 0.033 | 0.898 ± 0.012 | 0.330 ± 0.021 |
| t (Days) | Qp × 106 (Ω−1 sn cm−2) | np | Rp (kΩ cm2) | Qb × 106 (Ω−1 sn cm−2) | nb | Rb (kΩ cm2) |
|---|---|---|---|---|---|---|
| AISI 304L | ||||||
| 0 | 156.78 ± 4.23 | 0.93 ± 0.02 | 3.91 ± 0.21 | 299.96 ± 4.67 | 0.94 ± 0.02 | 29.51 ± 2.83 |
| 0.24 | 121.99 ± 3.73 | 0.94 ± 0.01 | 8.05 ± 0.53 | 275.14 ± 4.12 | 0.96 ± 0.01 | 81.05 ± 4.05 |
| 1 | 102.89 ± 3.44 | 0.94 ± 0.01 | 12.57 ± 1.58 | 256.52 ± 3.89 | 0.95 ± 0.02 | 121.96 ± 10.11 |
| 2 | 94.74 ± 2.99 | 0.93 ± 0.01 | 21.79 ± 1.44 | 247.04 ± 4.01 | 0.95 ± 0.03 | 160.92 ± 12.02 |
| 3 | 90.32 ± 2.78 | 0.94 ± 0.03 | 25.96 ± 1.98 | 240.59 ± 3.75 | 0.96 ± 0.01 | 147.44 ± 10.11 |
| 4 | 86.05 ± 4.09 | 0.95 ± 0.01 | 35.52 ± 2.36 | 237.85 ± 3.39 | 0.97 ± 0.01 | 160.92 ± 12.37 |
| 5 | 84.83 ± 3.37 | 0.95 ± 0.01 | 35.64 ± 2.11 | 235.64 ± 3.68 | 0. 98 ± 0.01 | 188.72 ± 10.14 |
| 6 | 81.49 ± 3.40 | 0.96 ± 0.01 | 40.80 ± 3.06 | 234.34 ± 3.22 | 0.97 ± 0.01 | 164.79 ± 14.83 |
| 7 | 79.41 ± 3.35 | 0.95 ± 0.02 | 40.23 ± 3.17 | 233.71 ± 4.12 | 0.96 ± 0.02 | 192.48 ± 12.86 |
| 8 | 77.82 ± 2.65 | 0.96 ± 0.01 | 44.31 ± 4.23 | 232.87 ± 4.39 | 0.96 ± 0.01 | 199.67 ±13.59 |
| 9 | 75.17 ± 2.77 | 0.96 ± 0.01 | 44.06 ± 2.41 | 231.38 ± 3.71 | 0.97 ± 0.01 | 189.16 ± 12.36 |
| 10 | 74.16 ± 3.16 | 0.96 ± 0.01 | 47.83 ± 1.79 | 231.17 ± 3.45 | 0.98 ± 0.01 | 200.48 ± 13.02 |
| AISI 316L | ||||||
| 0 | 128.14 ± 5.11 | 0.94± 0.03 | 8.74 ± 0.49 | 245.48 ± 3.97 | 0.95 ± 0.02 | 49.79 ± 3.82 |
| 0.24 | 107.18 ± 3.72 | 0.94 ± 0.02 | 24.40 ± 2.32 | 220.88 ± 4.23 | 0.97 ± 0.01 | 131.76 ± 9.17 |
| 1 | 90.61 ± 2.93 | 0.94 ± 0.02 | 42.28 ± 2.95 | 208.96 ± 3.54 | 098 ± 0.01 | 260.63 ± 12.35 |
| 2 | 81.01 ± 4.22 | 0.95 ± 0.01 | 49.21 ± 2.93 | 201.83 ± 3.60 | 0.97 ± 0.01 | 344.34 ± 10.37 |
| 3 | 75.72 ± 2.85 | 0.95 ± 0.01 | 52.87 ± 3.01 | 192.28 ± 3.82 | 0.96 ± 0.02 | 429.08 ± 12.13 |
| 4 | 73.49 ± 2.36 | 0.95 ± 0.01 | 59.81 ± 3.23 | 186.61 ± 3.67 | 0.98 ± 0.01 | 494.77 ± 12.88 |
| 5 | 72.95 ± 3.15 | 0.95 ± 0.01 | 58.86 ± 2.88 | 184.99 ± 3.28 | 0. 98 ± 0.01 | 517.67 ± 13.28 |
| 6 | 72.06 ± 3.66 | 0.94 ± 0.03 | 67.66 ± 2.64 | 182.62 ± 3.17 | 0.98 ± 0.01 | 506.64 ± 12.95 |
| 7 | 70.25 ± 2.84 | 0.97 ± 0.01 | 70.06 ± 2.01 | 180.51 ± 3.59 | 0.98 ± 0.01 | 520.32 ± 13.78 |
| 8 | 68.87 ± 3.65 | 0.95 ± 0.02 | 68.26 ± 2.77 | 179.25 ± 3.93 | 0.97 ± 0.01 | 528.74 ± 14.79 |
| 9 | 67.24 ± 3.80 | 0.96 ± 0.01 | 73.37 ± 1.98 | 176.38 ± 4.05 | 0.97 ± 0.02 | 521.42 ± 12.37 |
| 10 | 66.57 ± 3.45 | 0.97 ± 0.01 | 76.36 ± 3.09 | 175.10 ± 3.48 | 0.99 ± 0.01 | 530.14 ± 13.23 |
| 2205 DSS | ||||||
| 0 | 109.84 ± 3.89 | 0.95 ± 0.02 | 10.30 ± 0.95 | 212.38 ± 3.97 | 0.97 ± 0.02 | 65.61 ± 5.19 |
| 0.24 | 85.02 ± 2.71 | 0.96 ± 0.01 | 27.27 ± 2.35 | 194.09 ± 3.37 | 0.98 ± 0.01 | 222.93 ± 10.07 |
| 1 | 72.04 ± 2.16 | 0.96 ± 0.01 | 50.09 ± 2.94 | 179.25 ± 3.06 | 0.98 ± 0.01 | 381.95 ± 13.13 |
| 2 | 64.02 ± 3.28 | 0.96 ± 0.01 | 64.65 ± 2.77 | 172.03 ± 4.13 | 0.97 ± 0.01 | 470.39 ± 11.84 |
| 3 | 63.53 ± 3.01 | 0.95 ± 0.02 | 71.27 ± 3.29 | 168.39 ± 3.54 | 0.99 ± 0.01 | 634.18 ± 14.14 |
| 4 | 62.44 ± 3.05 | 0.96 ± 0.01 | 83.01 ± 3.34 | 163.86 ± 3.23 | 0.98 ± 0.01 | 690.96 ± 15.23 |
| 5 | 59.24 ± 2.84 | 0.97 ± 0.01 | 88.20 ± 3.17 | 162.89 ± 3.76 | 0.99 ± 0.01 | 721.51 ± 14.46 |
| 6 | 58.61 ± 3.71 | 0.97 ± 0.01 | 91.88 ± 3.40 | 160.81 ± 3.17 | 0.99 ± 0.01 | 761.70 ± 15.43 |
| 7 | 58.32 ± 3.42 | 0.97 ± 0.02 | 93.31 ± 3.37 | 158.95 ± 4.20 | 0.98 ± 0.01 | 751.10 ± 16.02 |
| 8 | 57.39 ± 2.64 | 0.97 ± 0.01 | 95.51 ± 3.04 | 157.84 ± 3.79 | 0.99 ± 0.01 | 769.41 ± 13.52 |
| 9 | 56.64 ± 2.89 | 0.98 ± 0.01 | 96.73 ± 2.92 | 157.61 ± 3.55 | 0.98 ± 0.02 | 761.70 ± 14.29 |
| 10 | 56.62 ± 3.11 | 0.97 ± 0.01 | 98.72 ± 3.52 | 157.25 ± 3.22 | 0.99 ± 0.01 | 765.47 ± 14.02 |
| AISI 304L | Spectrum | ||||||
| Element | 1 | 2 | 3 | 4 | 5 | 6 | |
 | O | 10.26 | 7.15 | 1.46 | 2.76 | 2.77 | 0.64 |
| Si | 0.18 | 0.23 | 0.31 | 0.37 | 0.37 | 0.35 | |
| P | 4.24 | 3.25 | 1.08 | 1.05 | |||
| Cl | 0.91 | 0.67 | 0.29 | 0.73 | |||
| Cr | 16.37 | 16.96 | 18.05 | 16.80 | 17.25 | 18.54 | |
| Mn | 18.28 | 14.35 | 1.89 | 1.60 | 1.89 | 1.82 | |
| Fe | 42.06 | 46.79 | 70.97 | 65.77 | 63.55 | 70.73 | |
| Ni | 3.84 | 4.75 | 7.32 | 7.50 | 8.04 | 7.91 | |
| Cu | 3.86 | 6.00 | 3.83 | 4.34 | |||
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |
| AISI 316L | Spectrum | ||||||
| Element | 1 | 2 | 3 | 4 | 5 | 6 | |
 | O | 11.62 | 1.07 | 0.68 | 4.41 | 0.82 | 0.68 |
| Na | 4.84 | ||||||
| Si | 1.24 | 0.33 | 0.42 | 0.53 | 0.44 | 0.43 | |
| P | 0.40 | 0.11 | 0.14 | 0.06 | |||
| Cl | 2.68 | 0.15 | 0.05 | 0.65 | |||
| Cr | 13.23 | 17.28 | 17.01 | 17.30 | 17.32 | 17.68 | |
| Mn | 0.86 | 0.95 | 1.19 | 1.04 | 1.38 | 1.23 | |
| Fe | 56.07 | 69.74 | 68.48 | 65.17 | 67.39 | 66.75 | |
| Ni | 7.04 | 8.54 | 10.08 | 8.62 | 10.26 | 10.67 | |
| Mo | 2.02 | 1.82 | 2.08 | 2.14 | 2.33 | 2.55 | |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | |
| 2205 DSS | Spectrum | ||||||
| Element | 1 | 2 | 3 | 4 | 5 | ||
 | O | 4.90 | 1.86 | 1.80 | 0.66 | 0.74 | |
| Na | 1.41 | 0.93 | 1.08 | ||||
| Si | 0.53 | 0.42 | 0.36 | 0.25 | 0.28 | ||
| P | 0.14 | 0.12 | 0.35 | 0.07 | 0.02 | ||
| Cl | 0.40 | 0.07 | |||||
| Cr | 22.55 | 23.66 | 23.63 | 24.32 | 21.13 | ||
| Mn | 1.01 | 1.26 | 1.57 | 1.37 | 1.56 | ||
| Fe | 61.51 | 63.88 | 63.00 | 66.05 | 67.58 | ||
| Ni | 3.69 | 3.88 | 3.83 | 3.41 | 6.22 | ||
| Mo | 3.87 | 3.60 | 4.11 | 3.83 | 2.32 | ||
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudić, S.; Nagode, A.; Šimić, K.; Vrsalović, L.; Jozić, S. Corrosion Behavior of Different Types of Stainless Steel in PBS Solution. Sustainability 2022, 14, 8935. https://doi.org/10.3390/su14148935
Gudić S, Nagode A, Šimić K, Vrsalović L, Jozić S. Corrosion Behavior of Different Types of Stainless Steel in PBS Solution. Sustainability. 2022; 14(14):8935. https://doi.org/10.3390/su14148935
Chicago/Turabian StyleGudić, Senka, Aleš Nagode, Kristina Šimić, Ladislav Vrsalović, and Sonja Jozić. 2022. "Corrosion Behavior of Different Types of Stainless Steel in PBS Solution" Sustainability 14, no. 14: 8935. https://doi.org/10.3390/su14148935
APA StyleGudić, S., Nagode, A., Šimić, K., Vrsalović, L., & Jozić, S. (2022). Corrosion Behavior of Different Types of Stainless Steel in PBS Solution. Sustainability, 14(14), 8935. https://doi.org/10.3390/su14148935









