Impacts of Ascorbic Acid and Alpha-Tocopherol on Chickpea (Cicer arietinum L.) Grown in Water Deficit Regimes for Sustainable Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experiment Layout
2.2. Soil Assessment
2.3. Germination and Agronomic Characteristics
2.4. Physiological and Biochemical Attributes
2.4.1. Leaf Photosynthetic Pigment
2.4.2. Total Proline Content (TPC) and Soluble Protein Content (SPC)
2.4.3. Soluble Sugar Content (SSC) and Hydrogen Peroxide (H2O2)
2.4.4. Malondialdehyde (MDA) and Glycine Betaine (GB) Assay
2.4.5. Antioxidant Enzymatic Assays
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect on Soil Physicochemical Properties
3.2. Morphological Characteristics
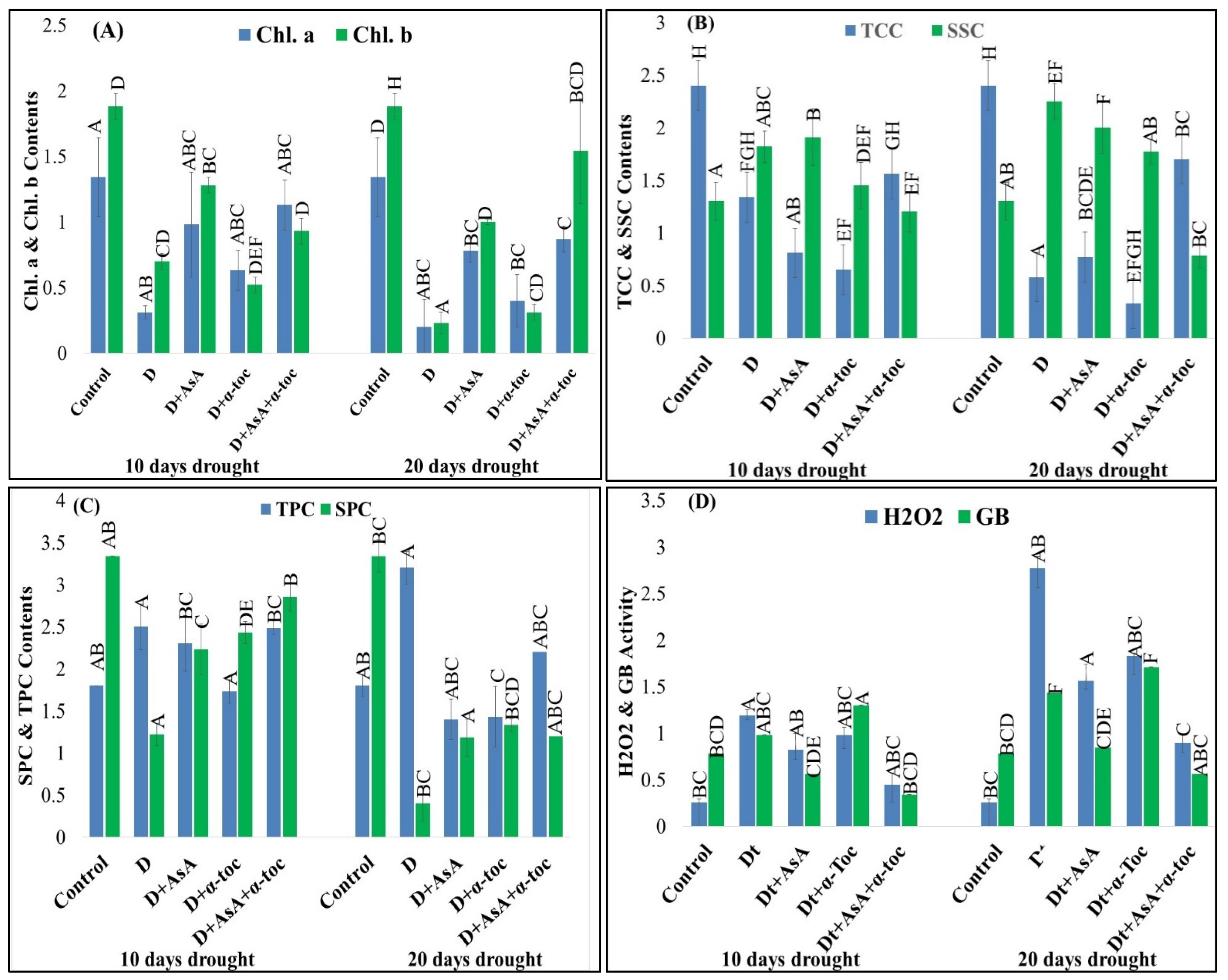
3.3. Effects on Physiological Attributes and Antioxidant Activities
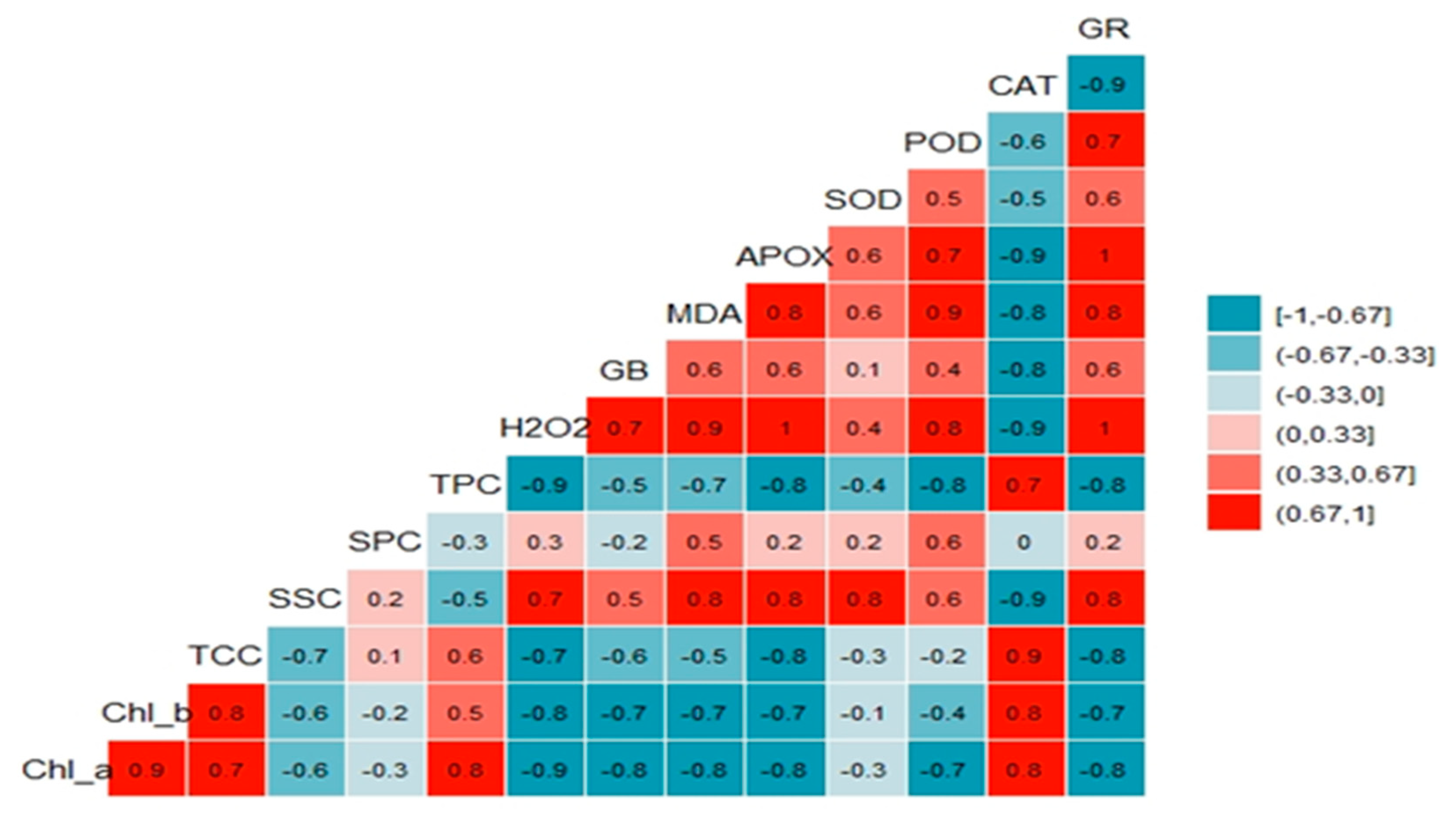
3.4. Principal Components and Correlations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Beltagi, H.S.; Mohamed, H.I.; Sofy, M.R. Role of ascorbic acid, glutathione and proline applied as singly or in sequence combination in improving chickpea plant through physiological change and antioxidant defense under different levels of irrigation intervals. Molecules 2020, 25, 1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Beltagi, H.S.; Ahmed, S.H.; Namich, A.A.M.; Abdel-Sattar, R.R. Effect of salicylic acid and potassium citrate on cotton plant under salt stress. Fresen. Environ. Bull. 2017, 26, 1091–1100. [Google Scholar]
- Ullah, A.; Sadaf, S.; Ullah, S.; Alshaya, H.; Okla, M.K.; Alwasel, Y.A.; Tariq, A. Using halothermal time model to describe barley (Hordeum vulgare L.) seed germination response to water potential and temperature. Life 2022, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ullah, A.; Ullah, S.; Saleem, M.H.; Okla, M.K.; Al-Hashimi, A.; Chen, Y.; Ali, S. Quantifying temperature and osmotic stress impact on seed germination rate and seedling growth of eruca sativa mill. via hydrothermal time model. Life 2022, 12, 400. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Khan, S.; Sulaiman, S.; Muhammad, M.; Badshah, L.; Bussmann, R.W.; Hussain, W. Quantitative study on medicinal plants traded in selected herbal markets of Khyber Pakhtunkhwa, Pakistan. Ethnobot. Res. Appl. 2020, 20, 1–36. [Google Scholar] [CrossRef]
- Mohamed, H.I.; El-Beltagi, H.S.; Aly, A.A.; Latif, H.H. The role of systemic and non-systemic fungicides on the physiological and biochemical parameters in plant: Implications for defense responses. Fresen. Environ. Bull. 2018, 27, 8585. [Google Scholar]
- Showler, A.T.; Shah, S.; Khan, S.; Ullah, S.; Degola, F. Desert locust episode in Pakistan, 2018–2021, and the current status of integrated desert locust management. J. Integr. Pest Manag. 2022, 13, 1. [Google Scholar] [CrossRef]
- Shah, S.; Khan, S.; Bussmann, R.W.; Ali, M.; Hussain, D.; Hussain, W. Quantitative ethnobotanical study of Indigenous knowledge on medicinal plants used by the tribal communities of Gokand Valley, District Buner, Khyber Pakhtunkhwa, Pakistan. Plants 2020, 9, 1001. [Google Scholar] [CrossRef]
- Uddin, S.; Ullah, S.; Nafees, M. Effect of seed priming on growth and performance of Vigna radiata L. under induced drought stress. J. Agric. Food Res. 2021, 4, 100140. [Google Scholar] [CrossRef]
- Nafees, M.; Ullah, S.; Ahmed, I. Morphological and elemental evaluation of biochar through analytical techniques and its combined effect along with plant growth promoting rhizobacteria on Vicia faba L. under induced drought stress. Microsc. Res. Tech. 2021, 84, 2947–2959. [Google Scholar] [CrossRef]
- Saeed, S.; Ullah, S.; Nafees, M.; Bibi, F.; Ullah, R. Morphological assessment of glutamate zerovalent iron nanoparticles by scanning electron microscopy and its combined effect with indole acetic acid on amelioration of lead toxicity in maize (Zea mays L.). Microsc. Res. Tech. 2020, 83, 1499–1506. [Google Scholar] [CrossRef]
- Ahmad, I.; Ullah, S.; Nafees, M. Effect of osmopriming and thermopriming on amelioration of mercuric chloride stress tolerance in mungbean (Vigna radiata L.). Plant Physiol. Rep. 2020, 25, 516–528. [Google Scholar] [CrossRef]
- Roy, P.R.; Tahjib-Ul-Arif, M.; Akter, T.; Ray, S.R.; Sayed, M.A. Exogenous ascorbic acid and hydrogen peroxide alleviates salt-induced oxidative stress in rice (Oryza sativa L.) by enhancing antioxidant enzyme activities and proline content. Adv. Environ. Biol. 2016, 10, 148–155. [Google Scholar]
- El-Beltagi, H.S.; Ahmad, I.; Basit, A.; Shehata, W.F.; Hassan, U.; Shah, S.T.; Haleema, B.; Jalal, A.; Amin, R.; Khalid, M.A.; et al. Ascorbic acid enhances growth and yield of sweet peppers (Capsicum annum) by mitigating salinity stress. Gesunde Pflanz. 2022, 74, 423–433. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmad, I.; Basit, A.; Abd El-Lateef, H.M.; Yasir, M.; Shah, S.T.; Ullah, I.; Mohamed, M.E.M.; Ali, I.; Ali, F. Effect of azospirillum and azotobacter species on the performance of cherry tomato under different salinity levels. Gesunde Pflanzen 2022, 74, 487–499. [Google Scholar] [CrossRef]
- Rahman, G.; Ullah, S.; Dawood, M.; Farhan, M.; Moazzam, U.I.; Lee, B.G. Spatio-temporal characteristics of meteorological drought in Khyber Pakhtunkhwa, Pakistan. PLoS ONE 2021, 16, e0249718. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Khan, S.; Sulaiman, S.S.; Shah, A.A. Morphological features of various selected tree species on the greater university campus Peshawar, Pakistan. Int. J. Bot. Stud. 2019, 4, 92–97. [Google Scholar] [CrossRef]
- Shah, S.; Ullah, S.; Ali, S.; Khan, A.; Ali, M.; Hassan, S. Using mathematical models to evaluate germination rate and seedlings length of Chickpea seed Cicer arietinum L. to osmotic stress at cardinal temperatures. PLoS ONE 2021, 16, e0260990. [Google Scholar] [CrossRef] [PubMed]
- Lalay, G.; Ullah, S.; Ahmed, I. Physiological and biochemical responses of L. to drought-induced stress by the application of biochar and Plant Growth Promoting Rhizobacteria. Microsc. Res. Tech. 2022, 85, 1267–1281. [Google Scholar] [CrossRef]
- Ahmad, A.; Anis, M. Meta-topolin improves in vitro morphogenesis, rhizogenesis and biochemical analysis in Pterocarpus marsupium Roxb.: A potential drug-yielding tree. J. Plant Growth Regul. 2019, 38, 1007–1016. [Google Scholar] [CrossRef]
- Brugière, N.; Dubois, F.; Limami, A.M.; Lelandais, M.; Roux, Y.; Sangwan, R.S.; Hirel, B. Glutamine synthetase in the phloem plays a major role in controlling proline production. Plant Cell 1999, 11, 1995–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Kan, G.; Hu, Z.; Cheng, H.; Zhang, Y.; Wang, Q.; Wang, H.; Yang, Y.; Li, H.; Hao, D.; et al. Use of single nucleotide polymorphisms and haplotypes to identify genomic regions associated with protein content and water-soluble protein content in soybean. Theor. Appl. Genet. 2014, 127, 1905–1915. [Google Scholar] [CrossRef]
- Johnson, R.R.; Balwani, T.L.; Johnson, L.J.; McClure, K.E.; Dehority, B.A. Corn plant maturity. II. Effect on in vitro cellulose digestibility and soluble carbohydrate content. J. Anim. Sci. 1966, 25, 617–623. [Google Scholar] [CrossRef]
- Velikova, V.; Loreto, E.; Tsonev, T.; Brilli, F.; Edreva, A. Isoprene prevents the negative consequences of high temperature stress in Platanus orientalis leaves. Funct. Plant Biol. 2006, 33, 931–940. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean Vigna radiata L. Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef]
- Flohe, L. Superoxide dismutase assays. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 93–104. [Google Scholar]
- Khan, M.N.; Ali, S.; Yaseen, T.; Ullah, S.; Zaman, A.; Iqbal, M.; Shah, S. Eco-Taxonomic Study of Family Poaceae (Gramineae). RADS J. Biol. Res. Appl. Sci. 2019, 10, 63–75. [Google Scholar] [CrossRef]
- Livingstone, D.; Lips, F.; Martinez, P.G.; Pipe, R.K. Antioxidant enzymes in the digestive gland of the common mussel Mytilus edulis. Mar. Biol. 1992, 112, 265–276. [Google Scholar] [CrossRef]
- Sedri, M.H.; Roohi, E.; Niazian, M.; Niedbała, G. Interactive effects of nitrogen and potassium fertilizers on quantitative-qualitative traits and drought tolerance indices of rainfed wheat cultivar. Agronomy 2021, 12, 30. [Google Scholar] [CrossRef]
- Ojuederie, O.B.; Olanrewaju, O.S.; Babalola, O.O.J.A. Plant growth promoting rhizobacterial mitigation of drought stress in crop plants: Implications for sustainable agriculture. Agronomy 2019, 9, 712. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.A.; Issa, B.; Rahut, D.B.J.S. Adoption and impact of the maize hybrid on the livelihood of the maize growers: Some policy insights from Pakistan. Scientifica 2020, 2020, 5959868. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Akram, N.A.; Saleem, M.H.; Ashraf, M.; Ahmed, S.; Ali, S.; Alsahli, A.A.; Alyemeni, M.N. Seed treatment with α-tocopherol regulates growth and key physio-biochemical attributes in carrot (Daucus carota L.) plants under water limited regimes. Agronomy 2021, 11, 469. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ali, S.; Iqbal, N.; Javed, M.T.; Rizwan, M.; Khaliq, R.; Shahid, S.; Perveen, R.; Alamri, S.; Alyemeni, M.; et al. Alpha-tocopherol fertigation confers growth physio-biochemical and qualitative yield enhancement in field grown water deficit wheat (Triticum aestivum L.). Sci. Rep. 2019, 9, 12924. [Google Scholar] [CrossRef] [Green Version]
- Kosar, F.; Akram, N.A.; Ashraf, M.; Ahmad, A.; Alyemeni, M.N.; Ahmad, P. Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiol. Plant. 2021, 172, 317–333. [Google Scholar] [CrossRef]
- Afify, A.E.M.M.; El-Beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A. Biochemical changes in phenols, flavonoids, tannins, vitamin E, β–carotene and antioxidant activity during soaking of three white sorghum varieties. Asian Pac. J. Trop. Biomed. 2012, 2, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, M.; Akram, N.A.; Ashraf, M.; Al-Qurainy, F.; Ahmad, F. Alpha-tocopherol-induced regulation of growth and metabolism in plants under non-stress and stress conditions. J. Plant Growth Regul. 2019, 38, 1325–1340. [Google Scholar] [CrossRef]
- Ramadan, K.M.A.; Alharbi, M.M.; Alenzi, A.M.; El-Beltagi, H.S.; Darwish, D.B.; Aldaej, M.I.; Shalaby, T.A.; Mansour, T.A.; El-Gabry, Y.A.E.-G.; Ibrahim, M.F.M. Alpha Lipoic Acid as a Protective Mediator for Regulating the Defensive Responses of Wheat Plants against Sodic Alkaline Stress: Physiological, Biochemical and Molecular Aspects. Plants 2022, 11, 787. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Ma, Y.; Zhang, Z.; Yue, H.; Wang, L.; Li, J.; Jiao, Y. Effects of exogenous γ-aminobutyric acid (GABA) on photosynthesis and antioxidant system in pepper (Capsicum annuum L.) seedlings under low light stress. J. Plant Growth Regul. 2017, 36, 436–449. [Google Scholar] [CrossRef]
- Khan, S.; Hussain, W.; Shah, S.; Hussain, H.; Altyar, A.E.; Ashour, M.L.; Pieroni, A. Overcoming tribal boundaries: The biocultural heritage of foraging and cooking wild vegetables among four pathan groups in the Gadoon valley, NW Pakistan. Biology 2021, 10, 537. [Google Scholar] [CrossRef]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 2018, 9, 2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, U.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Xu, W.; Cai, S.-Y.; Zhang, Y.; Wang, Y.; Ahammed, G.J.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Reiter, R.J.; et al. Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J. Pineal Res. 2016, 61, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.; Zhao, Y.; Pan, Y.; Chen, X.; Wang, Y.; Lin, J.; Wang, J.; Yang, Q. Exogenous Applications of Spermidine Improve Drought Tolerance in Seedlings of the Ornamental Grass Hordeum jubatum in Northeast China. Agronomy 2022, 12, 1180. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Yu, Y.; Zeng, H.; Deng, L.; Zhu, L.; Chen, G.; Wang, Y. Melatonin-Induced Resilience Strategies against the Damaging Impacts of Drought Stress in Rice. Agronomy 2022, 12, 813. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Akladious, S.A.; El-Beltagi, H.S. Mitigation the harmful effect of salt stress on physiological, biochemical and anatomical traits by foliar spray with trehalose on wheat cultivars. Fresenius Env. Bull 2018, 27, 7054–7065. [Google Scholar]
- Latif, M.; Akram, N.A.; Ashraf, M. Regulation of some biochemical attributes in drought-stressed cauliflower (Brassica oleracea L.) by seed pre-treatment with ascorbic acid. J. Hortic. Sci. Biotechnol. 2016, 91, 29–137. [Google Scholar] [CrossRef]
- Elansary, O.H.; Mahmoud, E.A.; El-Ansary, D.O.; Mattar, M.A. Effects of water stress and modern biostimulants on growth and quality characteristics of mint. Agronomy 2019, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, G.; Faried, H.N.; Razzaq, K.; Ullah, S.; Wattoo, F.M.; Shehzad, M.A.; Sajjad, Y.; Ahsan, M.; Javed, T.; Dessoky, E.S.; et al. Chitosan-Induced Physiological and Biochemical Regulations Confer Drought Tolerance in Pot Marigold (Calendula officinalis L.). Agronomy 2022, 12, 474. [Google Scholar] [CrossRef]
- Shalaby, T.A.; Taha, N.A.; Taher, D.I.; Metwaly, M.M.; El-Beltagi, H.S.; Rezk, A.A.; El-Ganainy, S.M.; Shehata, W.F.; El-Ramady, H.R.; Bayoumi, Y.A. Paclobutrazol improves the quality of tomato seedlings to be resistant to Alternaria solani blight disease: Biochemical and histological perspectives. Plants 2022, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- El-Beltagi, H.S.; Mohamed, H.I.; Aldaej, M.I.; Al-Khayri, J.M.; Rezk, A.A.; Al-Mssallem, M.Q.; Sattar, M.N.; Ramadan, K.M.A. Production and antioxidant activity of secondary metabolites in Hassawi rice (Oryza sativa L.) cell suspension under salicylic acid, yeast extract, and pectin elicitation. Vitr. Cell Dev. Biol. Plant 2022, 1–15. [Google Scholar] [CrossRef]
- Mohamed, A.A.; El-Beltagi, H.S.; Rashed, M.M. Cadmium stress induced change in some hydrolytic enzymes, free radical formation and ultrastructural disorders in radish plant. Electron. J. Environ. Agric. Food Chem. 2009, 8, 969–983. [Google Scholar]
- Shalaby, T.A.; Taha, N.A.; Rakha, M.T.; El-Beltagi, H.S.; Shehata, W.F.; Ramadan, K.M.A.; El-Ramady, H.; Bayoumi, Y.A. Can Grafting Manage Fusarium Wilt Disease of Cucumber and Increase Productivity under Heat Stress? Plants 2022, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.E.M.M.; El-Beltagi, H.S.; Aly, A.A.; El-Ansary, A.E. Antioxidant enzyme activities and lipid peroxidation as biomarker for potato tuber stored by two essential oils from Caraway and Clove and its main component carvone and eugenol. Asian Pac. J. Trop. Biomed. 2012, 2, S772–S780. [Google Scholar] [CrossRef]
- Shalaby, T.A.; El-Newiry, N.A.; El-Tarawy, M.; El-Mahrouk, M.E.; Shala, A.Y.; El-Beltagi, H.S.; Rezk, A.A.; Ramadan, K.M.A.; Shehata, W.F.; El-Ramady, H. Biochemical and physiological response of Marigold (Tagetes Erecta L.) to foliar application of salicylic acid and potassium humate in different soil growth media. Gesunde Pflanz. 2022, 11, 14. [Google Scholar] [CrossRef]
- Kobeasy, M.I.; El-Beltagi, H.S.; El-Shazly, M.A.; Khattab, E.A.H. Induction of resistance in Arachis hypogaea L. Against Peanut mottle virus by nitric oxide and salicylic acid. Physiol. Mol. Plant Pathol. 2011, 76, 112–118. [Google Scholar] [CrossRef]
- Lazić, D.; Putnik-Delić, M.; Daničić, M.; Župunski, M.; Arsenov, D.; Vuković, S.; Maksimović, I. Efficiency of Si in alleviating NaCl-induced stress in oilseed rape. Pak. J. Agric. Sci. 2020, 57, 901–907. [Google Scholar] [CrossRef]




| Treatment | T (°C) | pH | ORP (mV) | Resistivity (Ω·m) | EC (mS/m) | TDS (mg/L) | Salinity | DO |
|---|---|---|---|---|---|---|---|---|
| Control | 18.6 | 7.96 | 99.5 | 1923 | 521 | 260 | 0.25 | 11.2 |
| 10-day drought | 21.3 | 6.6 | 90.9 | 2953 | 290 | 256 | 0.25 | 11.2 |
| D + AsA | 19.6 | 7.2 | 88.1 | 2984 | 151 | 129 | 0.24 | 11.2 |
| D + α-toc | 19.6 | 7.0 | 86.9 | 2681 | 595 | 298 | 0.29 | 11.2 |
| D (AsA + α-toc) | 19.6 | 6.8 | 85.2 | 2890 | 529 | 264 | 0.26 | 11.2 |
| 20-day drought | 24.4 | 7.15 | 82.2 | 1100 | 522 | 160 | 0.11 | 11.2 |
| D + AsA | 19.4 | 7.4 | 82.4 | 1900 | 429 | 129 | 0.15 | 11.2 |
| D + α-toc | 19.4 | 8.33 | 82.9 | 1789 | 586 | 133 | 0.17 | 11.2 |
| D (AsA + α-toc) | 19.4 | 7.13 | 92.3 | 1823 | 501 | 145 | 0.14 | 11.2 |
| Treatments | MGT | GRI | GE | TGI | CVG | WUE |
|---|---|---|---|---|---|---|
| Control | 6.4 ± 0.21 a | 75.5 ± 3.5 b | 10.1 ± 1.2 a | 55.4 ± 3.4 b | 6.3 ± 1.2 a | 2.3 ± 0.5 a |
| 10-day drought | 6.1 ± 0.56 ab | 66.2 ± 2.3 ab | 7.4 ± 1.0 bc | 54.2 ± 5.2 a | 5.6 ± 0.6 a | 3.5 ± 1.2 cd |
| D + AsA | 5.5 ± 0.34 cd | 61.2 ± 3.3 ab | 4.6 ± 0.8 cd | 54.6 ± 2.5 c | 4.3 ± 0.8 ab | 6.2 ± 0.5 bc |
| D + α-toc | 5.8 ± 0.22 a | 71.7 ± 1.9 ef | 5.2 ± 0.5 ac | 56.2 ± 4.3 d | 4.6 ± 1.5 bc | 5.1 ± 1.8 ac |
| D (AsA + α-toc) | 5.7 ± 0.12 c | 65.3 ± 2.7 d | 6.1 ± 1.5 ab | 56.8 ± 6.4 cd | 5.4 ± 0.9 bc | 5.2 ± 2.1 ae |
| 20-day drought | 5.9 ± 0.56 de | 67.4 ± 3.5 b | 11.3 ± 2.0 b | 53.2 ± 3.4 ab | 6.4 ± 0.4 cd | 4.2 ± 0.5 de |
| D + AsA | 6.2 ± 0.76 cd | 64.2 ± 4.0 a | 6.7 ± 1.1 cd | 51.9 ± 7.3 bc | 3.7 ± 1.1 c | 3.2 ± 0.7 bc |
| D + α-toc | 6.6 ± 0.23 c | 79.4 ± 3.8 cd | 5.9 ± 1.0 a | 57.2 ± 4.9 a | 6.2 ± 0.9d e | 6.1 ± 0.3 bc |
| D (AsA + α-toc) | 6.0 ± 0.33 a | 77.3 ± 3.1 a | 10.0 ± 2.3 ab | 53.4 ± 4.5 a | 5.4 ± 0.5 a | 4.3 ± 0.8 a |
| Treatments | GP | SVI-I | SVI-II | RMC | T50% |
|---|---|---|---|---|---|
| Control | 96.15.16 ab | 2427458.7 d | 11,493.3598 ab | 86.41.2 a | 5.30.2 ab |
| 10-day drought | 93.34.71 c | 93749.253 c | 6616.9153.2 ab | 83.80.4 de | 5.30.2 cd |
| D + AsA | 90.64.71 d | 1261107.0 c | 6619.2357.1 bc | 67.30.4 ef | 5.60.3 de |
| D + α-toc | 94.64.71 a | 1217160.0 b | 7322.4150.4 de | 88.70.8 d | 5.0a |
| D (AsA + α-toc) | 96.34.71 c | 159988.41 c | 10761200.5 ab | 87.30.5 c | 4.80.2 b |
| 20-day drought | 96.64.71 ab | 114350.70 a | 7026161.90 bc | 71.210.8 a | 4.30.2 cd |
| D + AsA | 93.3ab | 1604.112.2 a | 11,967345.4 ab | 82.080.8 bc | 5.10.2 ef |
| D + α-toc | 96.39.43 a | 147117.58 ab | 752976.183 bc | 83.60.6 a | 5.10.4 a |
| D (AsA + α-toc) | 86.34.71 bc | 331937.66 a | 12,531308.2 ab | 79.80.9 ab | 4.60.2 cd |
| Variables | Variation Source | SS | DF | MS | F | p |
|---|---|---|---|---|---|---|
| Chl. a | Treatment | 0.067 | 9 | 0.231 | 3.140 | 0.005 ** |
| Genotype | 0.023 | 2 | 0.563 | 2.451 | 0.000 *** | |
| Treatment × Genotype | 0.080 | 9 | 1.230 | 6.340 | 0.000 *** | |
| Error | 0.052 | 54 | 1.110 | - | - | |
| Chl. b | Treatment | 0.570 | 9 | 0.781 | 13.101 | 0.005 ** |
| Genotype | 0.110 | 2 | 0.881 | 6.231 | 0.002 ** | |
| Treatment × Genotype | 0.067 | 9 | 1.238 | 9.671 | 0.000 *** | |
| Error | 0.089 | 54 | 1.200 | - | - | |
| TCC | Treatment | 0.381 | 9 | 2.134 | 2.341 | 0.000 *** |
| Genotype | 0.182 | 2 | 0.714 | 1.776 | 0.015 | |
| Treatment × Genotype | 0.116 | 9 | 0.891 | 2.341 | 0.018 | |
| Error | 0.667 | 54 | 0.114 | - | - | |
| SSC | Treatment | 0.836 | 9 | 0.843 | 4.674 | 0.011 |
| Genotype | 0.780 | 2 | 0.341 | 2.110 | 0.000 *** | |
| Treatment × Genotype | 0.201 | 9 | 1.349 | 7.890 | 0.010 ** | |
| Error | 0.052 | 54 | 1.220 | - | - | |
| TPC | Treatment | 0.446 | 9 | 1.989 | 11.98 | 0.005 ** |
| Genotype | 0.743 | 2 | 0.231 | 2.778 | 0.000 *** | |
| Treatment × Genotype | 0.890 | 9 | 1.228 | 7.891 | 0.000 *** | |
| Error | 0.520 | 54 | 0.231 | - | - | |
| SPC | Treatment | 0.667 | 9 | 0.667 | 8.219 | 0.000 *** |
| Genotype | 0.211 | 2 | 1.563 | 9.220 | 0.005 ** | |
| Treatment × Genotype | 0.320 | 9 | 1.231 | 2.667 | 0.000 *** | |
| Error | 0.520 | 54 | 1.789 | - | - | |
| H2O2 | Treatment | 0.289 | 9 | 2.452 | 11.231 | 0.001 * |
| Genotype | 0.211 | 2 | 1.561 | 1.781 | 0.000 *** | |
| Treatment × Genotype | 0.856 | 9 | 1.892 | 2.776 | 0.000 *** | |
| Error | 0.052 | 54 | 0.553 | - | - | |
| GB | Treatment | 0.911 | 9 | 0.875 | 6.889 | 0.000 *** |
| Genotype | 0.909 | 2 | 0.167 | 3.667 | 0.005 ** | |
| Treatment × Genotype | 0.800 | 9 | 0.796 | 2.891 | 0.017 ** | |
| Error | 0.775 | 54 | 0.231 | - | - | |
| MDA | Treatment | 0.553 | 9 | 0.223 | 8.990 | 0.000 *** |
| Genotype | 0.218 | 2 | 0.190 | 4.781 | 0.000 *** | |
| Treatment × Genotype | 0182 | 9 | 1.231 | 2.990 | 0.000 *** | |
| Error | 0.562 | 54 | 1.681 | - | - | |
| APOX | Treatment | 0.239 | 9 | 0.990 | 2.887 | 0.000 *** |
| Genotype | 0.918 | 2 | 1.230 | 4.871 | 0.005 ** | |
| Treatment × Genotype | 0.802 | 9 | 0.872 | 1.091 | 0.000 *** | |
| Error | 0.921 | 54 | 0.664 | - | - | |
| SOD | Treatment | 0.222 | 9 | 0.332 | 2.998 | 0.000 *** |
| Genotype | 0.181 | 2 | 0.013 | 2.871 | 0.018 | |
| Treatment × Genotype | 0.272 | 9 | 0.123 | 6.889 | 0.080 | |
| Error | 0.653 | 54 | 0.771 | - | - | |
| POD | Treatment | 0.560 | 9 | 0.010 | 17.870 | 0.010 ** |
| Genotype | 0.230 | 2 | 0.451 | 13.761 | 0.019 | |
| Treatment × Genotype | 0.080 | 9 | 0.087 | 7.891 | 0.000 *** | |
| Error | 0.052 | 54 | 0.171 | - | - | |
| CAT | Treatment | 0.521 | 9 | 0.871 | 4.651 | 0.005 ** |
| Genotype | 0.257 | 2 | 0.776 | 3.981 | 0.005 ** | |
| Treatment × Genotype | 0.871 | 9 | 1.881 | 2.991 | 0.004 ** | |
| Error | 0.233 | 54 | 1.761 | - | - | |
| GR | Treatment | 0.791 | 9 | 0.910 | 21.2 | 0.001 * |
| Genotype | 0.270 | 2 | 0.334 | 12.8 | 0.000 *** | |
| Treatment × Genotype | 0.080 | 9 | 0.008 | - | - | |
| Error | 0.451 | 54 | 0.430 | 3.87 | 0.005 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Beltagi, H.S.; Shah, S.; Ullah, S.; Sulaiman; Mansour, A.T.; Shalaby, T.A. Impacts of Ascorbic Acid and Alpha-Tocopherol on Chickpea (Cicer arietinum L.) Grown in Water Deficit Regimes for Sustainable Production. Sustainability 2022, 14, 8861. https://doi.org/10.3390/su14148861
El-Beltagi HS, Shah S, Ullah S, Sulaiman, Mansour AT, Shalaby TA. Impacts of Ascorbic Acid and Alpha-Tocopherol on Chickpea (Cicer arietinum L.) Grown in Water Deficit Regimes for Sustainable Production. Sustainability. 2022; 14(14):8861. https://doi.org/10.3390/su14148861
Chicago/Turabian StyleEl-Beltagi, Hossam S., Sikandar Shah, Sami Ullah, Sulaiman, Abdallah Tageldein Mansour, and Tarek A. Shalaby. 2022. "Impacts of Ascorbic Acid and Alpha-Tocopherol on Chickpea (Cicer arietinum L.) Grown in Water Deficit Regimes for Sustainable Production" Sustainability 14, no. 14: 8861. https://doi.org/10.3390/su14148861
APA StyleEl-Beltagi, H. S., Shah, S., Ullah, S., Sulaiman, Mansour, A. T., & Shalaby, T. A. (2022). Impacts of Ascorbic Acid and Alpha-Tocopherol on Chickpea (Cicer arietinum L.) Grown in Water Deficit Regimes for Sustainable Production. Sustainability, 14(14), 8861. https://doi.org/10.3390/su14148861










