Glucose Fuel Cells and Membranes: A Brief Overview and Literature Analysis
Abstract
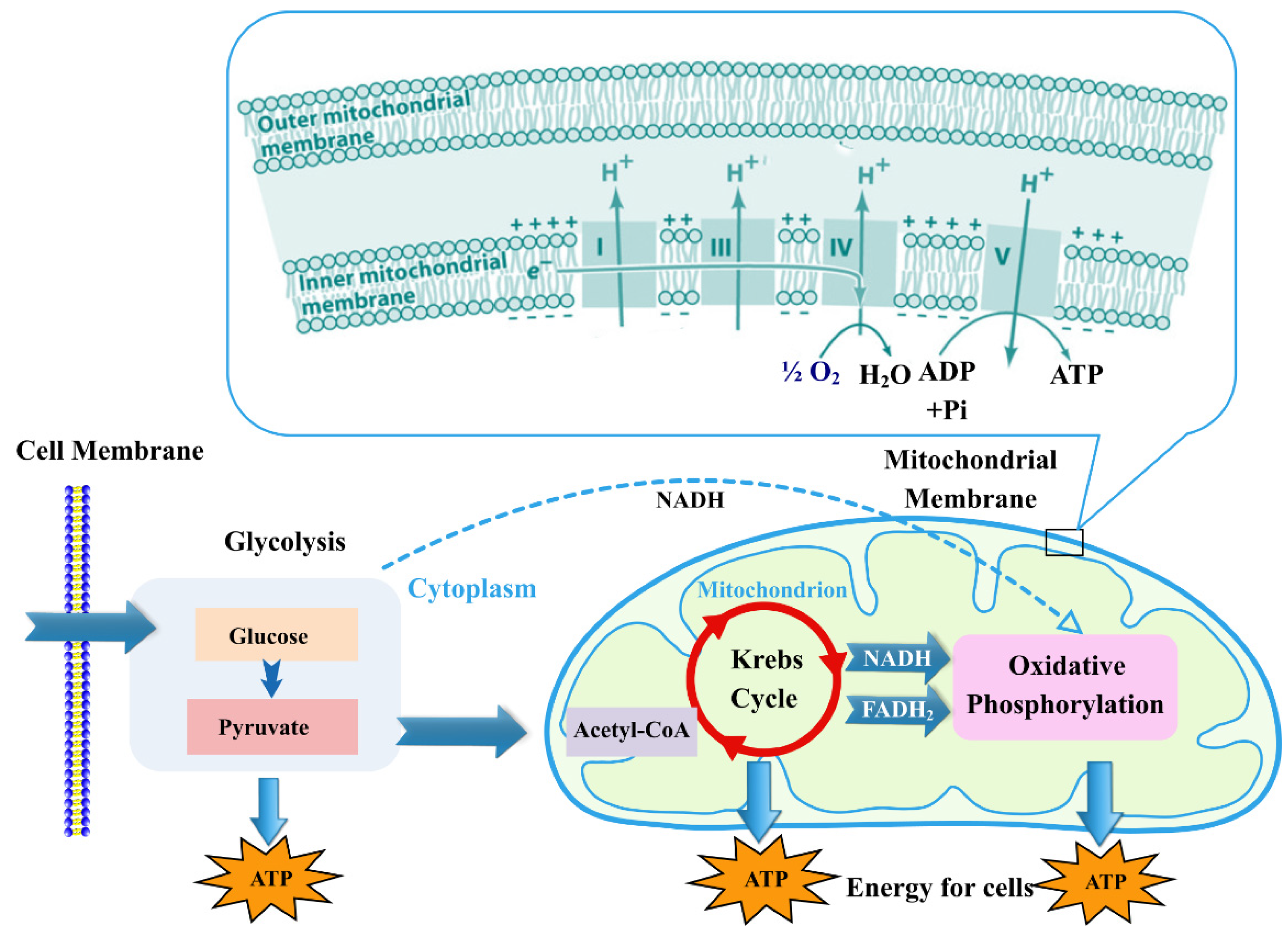
:1. Introduction
2. GFC Types
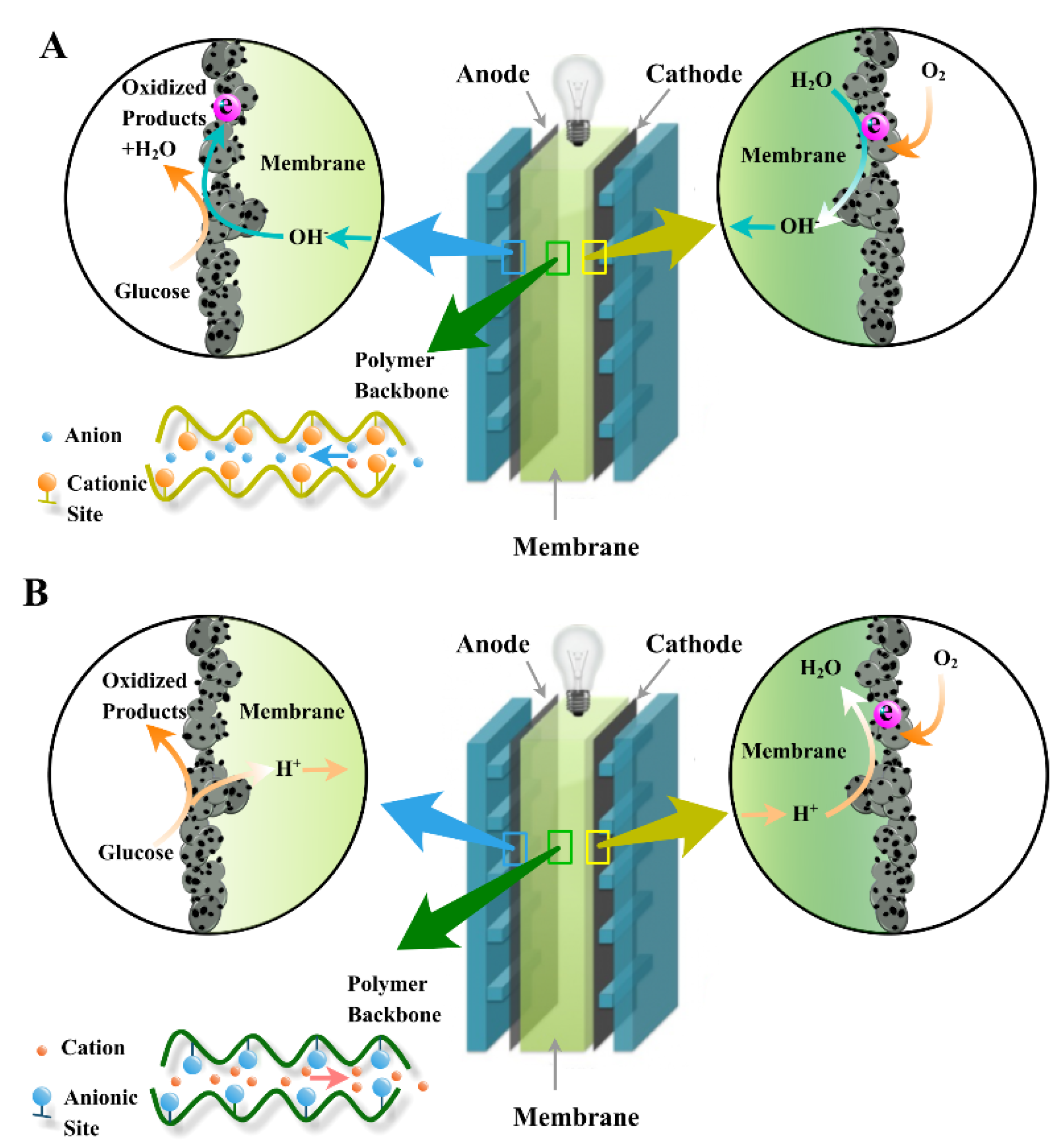
3. Membranes for GFCs
3.1. Role of Membranes in GFCs
3.2. Membranes in GFCs Subsection
3.2.1. PEM
3.2.2. AEM
3.2.3. Other Membranes
4. Literature Analysis
4.1. Data Gathering and Data Analysis
4.2. Literature Analysis of Research Progress
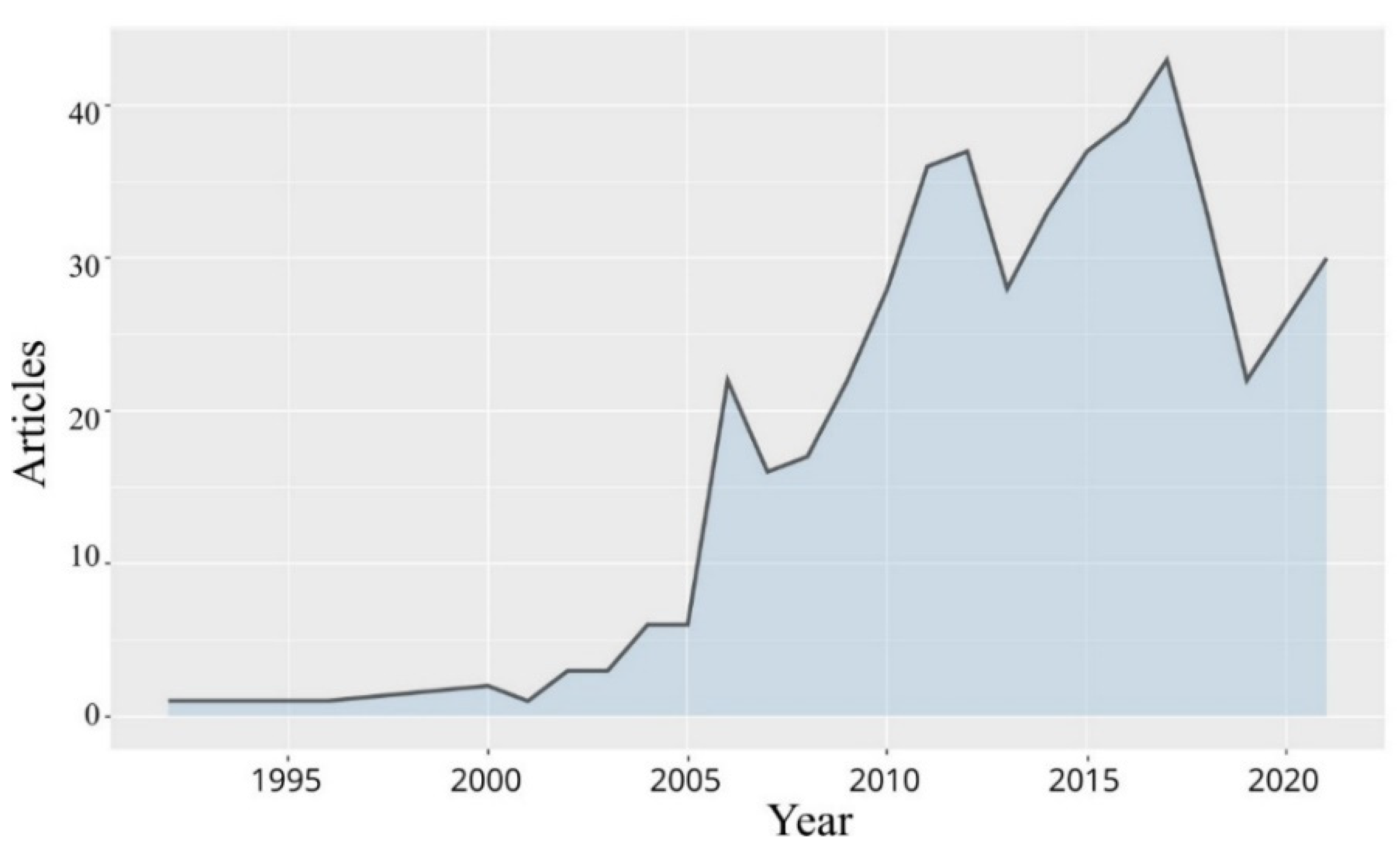
4.2.1. Output of the Research Publications
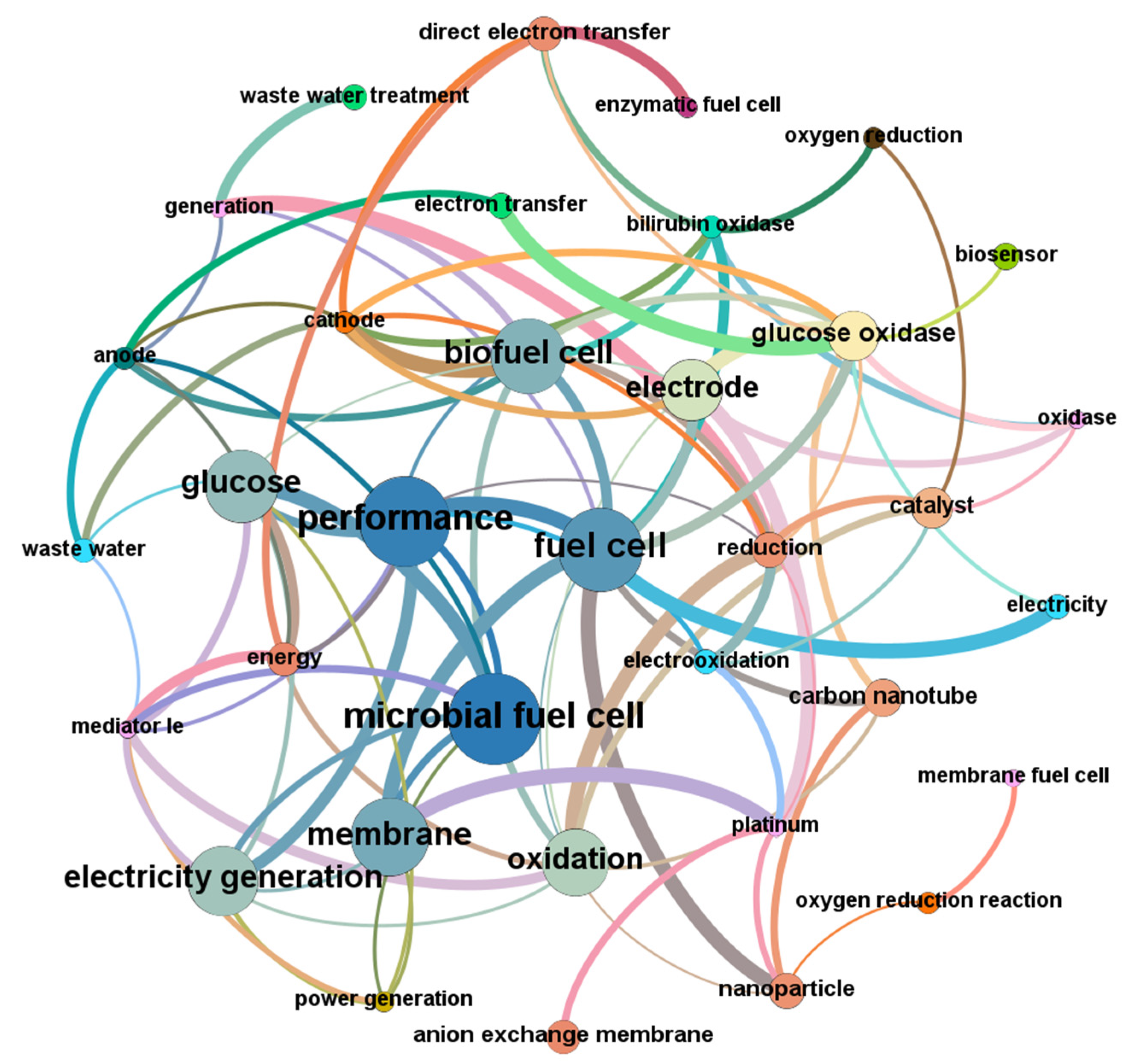
4.2.2. Co-Occurrence Analysis of Keywords
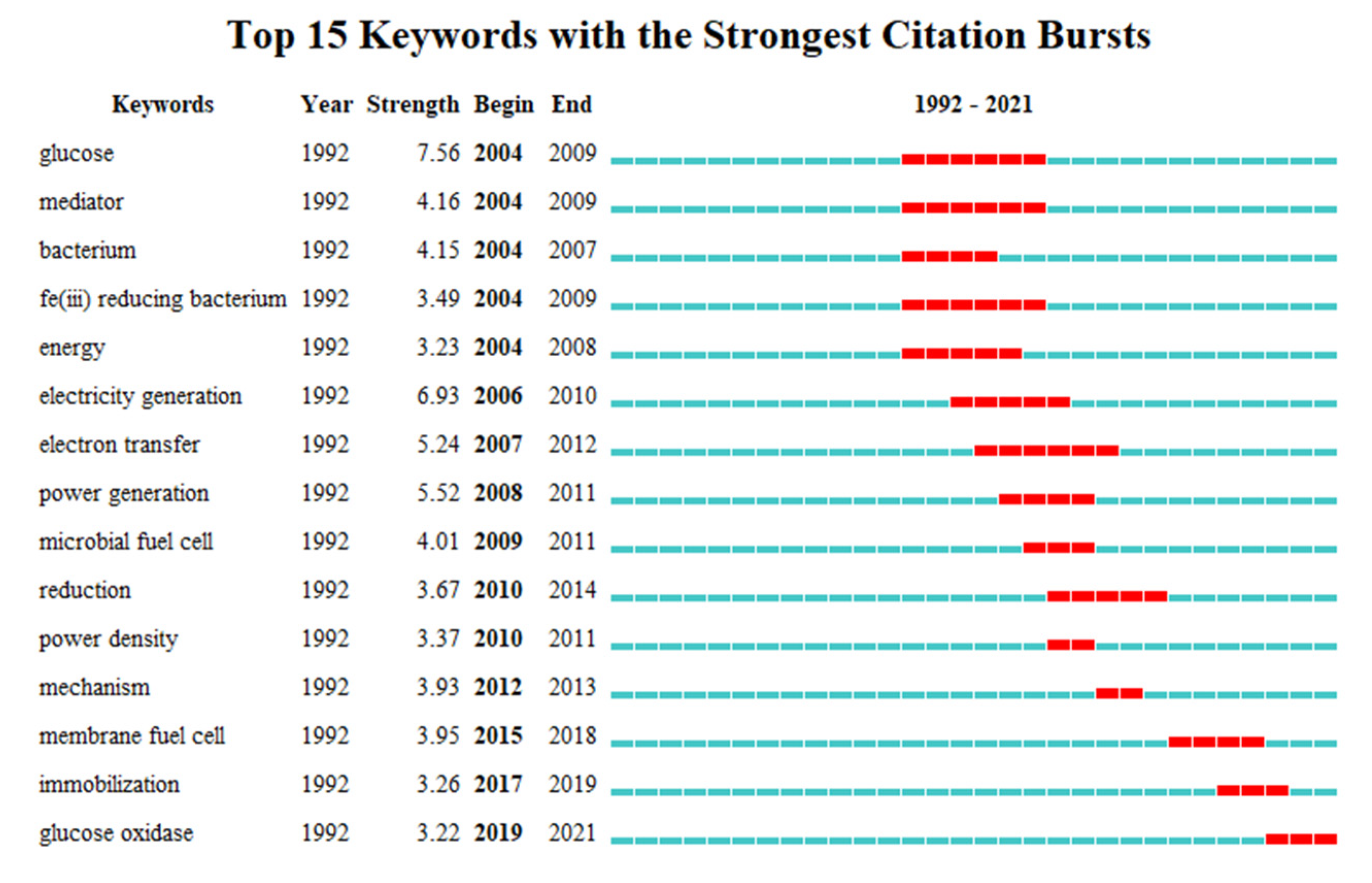
4.2.3. Burst Detection Analysis
4.2.4. Analysis of Leading Countries and International Cooperation
5. Research Challenges
6. Some Latest Solutions for GFCs

7. Future Perspectives
- (1)
- Researchers can draw inspiration from biological systems to find solutions. Researchers have always been fascinated by biological systems because of their complexity and efficiency in accomplishing the tasks required to thrive. Biomimetic membranes are expected to break through existing bottlenecks by using the strategies that nature has evolved over billions of years for in order to improve transport efficiency and specificity.
- (2)
- Fundamental and extensive analytical studies must be conducted to understand the processes that occur in GFC membranes in operando. Ion exchange dynamics in the membranes, the effect of carbonation, and the swelling of the polymer upon hydration are some of the fundamental investigations needed to be cultivated in this field. These investigations also need new methods for ex-situ and in-situ characterization.
- (3)
- Composite membrane materials can combine the required characteristic and are promising to address most of the outstanding issues and challenges. A wide range of properties can be available through appropriate doping, structural characteristics adjustment, core-shell formation, and even composite microstructures. For example, heterostructures can be precisely assembled to provide unique ion and electron transport properties. The composite membrane can also provide an appropriate balance among various features.
- (4)
- GFCs are expected to incorporate with various other commercialized technology to realize multiple applications, such as continuous glucose monitoring, drug smart delivery, self-sustained sensing, and targeted therapy for cancer. For implantation devices, the incorporation with GFCs make it possible to remove external power sources, which drastically simplifies the electronics required and allows for miniature designs.
- (5)
- GFC research and its successful commercialization must be achieved through extensive global cooperation among researchers with different expertise. The global cooperation can speed up the resolution of bottleneck issues and promote the innovation ability of the international community.
8. Conclusions and Outlooks
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEM | anion exchange membrane |
| AGFCs | abiotic glucose fuel cells |
| ATP | adenosine triphosphate |
| BOD | bilirubin oxidase |
| CoA | coenzyme A |
| NADH | nicotinamide adenine dinucleotide. |
| FADH2 | flavin adenine dinucleotide |
| MGFCs | microbial glucose fuel cells |
| EGFCs | enzymatic glucose fuel cells |
| GDH | glucose dehydrogenase |
| GFCs | glucose fuel cells |
| GOD | glucose Oxidase |
| GOR | glucose oxidation reaction |
| MEA | membrane electrode assembly |
| ORR | oxygen reduction reaction |
| PEM | proton exchange membrane |
| POM | polyoxometalate |
| PS | polysulfone |
| PVA | Polyvinyl alcohol |
References
- Wang, C.; O’Hagan, M.P.; Willner, B.; Willner, I. Bioinspired Artificial Photosynthetic Systems. Chemistry 2022, 28, e202103595. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E. External abiotic glucose fuel cells. Sustain. Energy Fuels 2021, 5, 5038–5060. [Google Scholar] [CrossRef]
- Lemoine, C.; Holade, Y.; Dubois, L.; Napporn, T.W.; Servat, K.; Kokoh, K.B. New insights on the selective electroconversion of the cellulosic biomass-derived glucose at PtAu nanocatalysts in an anion exchange membrane fuel cell. J. Electroanal. Chem. 2021, 887, 115162. [Google Scholar] [CrossRef]
- Antolini, E. Lignocellulose, Cellulose and Lignin as Renewable Alternative Fuels for Direct Biomass Fuel Cells. Chemsuschem 2021, 14, 189–207. [Google Scholar] [CrossRef]
- Shen, Y.X.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic membranes: A review. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Loose, M.; Schwille, P. Biomimetic membrane systems to study cellular organization. J. Struct. Biol. 2009, 168, 143–151. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Piersma, B.J.; Gileadi, E. Anodic oxidation of cellulose and lower carbohydrates. Electrochim. Acta 1964, 9, 1329–1332. [Google Scholar] [CrossRef]
- Yahiro, A.T.; Lee, S.M.; Kimble, D.O. Bioelectrochemistry: I. Enzyme utilizing bio-fuel cell studies. Biochim. Biophys. Acta BBA-Spec. Sect. Biophys. Subj. 1964, 88, 375–383. [Google Scholar]
- Li, Z.; Liu, X.H.; Liu, P.; Zhang, P.P. The Performance of Electron-Mediator Modified Activated Carbon as Anode for Direct Glucose Alkaline Fuel Cell. Catalysts 2016, 6, 95. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.H.; Hao, M.Q.; Feng, M.N.; Zhang, L.; Zhao, Y.; Du, X.W.; Wang, G.Y. A One-compartment direct glucose alkaline fuel cell with methyl viologen as electron mediator. Appl. Energy 2013, 106, 176–183. [Google Scholar] [CrossRef]
- Liu, X.H.; Li, Z.; Yang, Y.L.; Liu, P.; Zhang, P.P. Electricity generation from a refuelable glucose alkaline fuel cell with a methyl viologen-immobilized activated carbon anode. Electrochim. Acta 2016, 222, 1430–1437. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.H.; Wang, X.; Zhang, P.P.; Shi, J.F. Peony petal-like 3D graphene-nickel oxide nanocomposite decorated nickel foam as high-performance electrocatalyst for direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2017, 42, 29863–29873. [Google Scholar] [CrossRef]
- Li, Y.; Ding, J.; Liu, X.H.; Wang, J.; Jiao, S.P.; Kang, N.; Li, J.Y.; Irfan, M.; Zhang, P.P. Physically mixed Ni2Co/graphene catalyst for enhanced glucose oxidation in a glucose fuel cell. Biomass Convers. Biorefinery 2022, 1–13. [Google Scholar] [CrossRef]
- Dai, Y.X.; Ding, J.; Li, J.Y.; Li, Y.; Zong, Y.P.; Zhang, P.P.; Wang, Z.Y.; Liu, X.H. N, S and Transition-Metal Co-Doped Graphene Nanocomposites as High-Performance Catalyst for Glucose Oxidation in a Direct Glucose Alkaline Fuel Cell. Nanomaterials 2021, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Liu, X.H.; Irfan, M.; Yang, L.; Li, S.L.; Ding, J.; Li, Y.; Khan, I.U.; Zhang, P.P. Macaroon-like FeCo2O4 modified activated carbon anode for enhancing power generation in direct glucose fuel cell. Int. J. Hydrogen Energy 2019, 44, 8178–8187. [Google Scholar] [CrossRef]
- Gao, M.Y.; Liu, X.H.; Irfan, M.; Shi, J.F.; Wang, X.; Zhang, P.P. Nickle-cobalt composite catalyst-modified activated carbon anode for direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2018, 43, 1805–1815. [Google Scholar] [CrossRef]
- Hao, M.Q.; Liu, X.H.; Feng, M.N.; Zhang, P.P.; Wang, G.Y. Generating power from cellulose in an alkaline fuel cell enhanced by methyl viologen as an electron-transfer catalyst. J. Power Sources 2014, 251, 222–228. [Google Scholar] [CrossRef]
- Ho, J.; Li, Y.; Dai, Y.X.; Kim, T.; Wang, J.; Ren, J.; Yun, H.; Liu, X.H. Ionothermal synthesis of N-doped carbon supported CoMn2O4 nanoparticles as ORR catalyst in direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2021, 46, 20503–20515. [Google Scholar] [CrossRef]
- Irfan, M.; Khan, I.U.; Wang, J.; Li, Y.; Liu, X.H. 3D porous nanostructured Ni3N-Co3N as a robust electrode material for glucose fuel cell. RSC Adv. 2020, 10, 6444–6451. [Google Scholar] [CrossRef] [Green Version]
- Rismani-Yazdi, H.; Christy, A.D.; Dehority, B.A.; Morrison, M.; Yu, Z.; Tuovinen, O.H. Electricity generation from cellulose by rumen microorganisms in microbial fuel cells. Biotechnol. Bioeng. 2007, 97, 1398–1407. [Google Scholar] [CrossRef]
- Irfan, M.; Liu, X.H.; Li, S.L.; Khan, I.U.; Li, Y.; Wang, J.; Wang, X.; Du, X.W.; Wang, G.Y.; Zhang, P.P. High-performance glucose fuel cell with bimetallic Ni-Co composite anchored on reduced graphene oxide as anode catalyst. Renew. Energy 2020, 155, 1118–1126. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.H.; Li, Y.; Liu, P.; Chen, X.C.; Zhang, P.P.; Wang, Z.Y.; Liu, X.H. Sweet Drinks as Fuels for an Alkaline Fuel Cell with Nonprecious Catalysts. Energies 2021, 14, 206. [Google Scholar] [CrossRef]
- Apblett, C.; Ingersoll, D.; Roberts, G. Development and testing of an air breathing, membrane separated, enzyme anode fuel cell for glucose fuels. Abstr. Pap. Am. Chem. Soc. 2005, 230, U1671. [Google Scholar]
- Ivnitski, D.; Branch, B.; Atanassov, P.; Apblett, C. Glucose oxidase anode for biofuel cell based on direct electron transfer. Electrochem. Commun. 2006, 8, 1204–1210. [Google Scholar] [CrossRef]
- Abreu, C.; Nedellec, Y.; Ondel, O.; Buret, F.; Cosnier, S.; Le Goff, A.; Holzinger, M. Glucose oxidase bioanodes for glucose conversion and H2O2 production for horseradish peroxidase biocathodes in a flow through glucose biofuel cell design. J. Power Sources 2018, 392, 176–180. [Google Scholar] [CrossRef]
- Chu, T.F.; Rajendran, R.; Kuznetsova, I.; Wang, G.J. High-power, non-enzymatic glucose biofuel cell based on a nano/micro hybrid-structured Au anode. J. Power Sources 2020, 453, 227844. [Google Scholar] [CrossRef]
- Do, U.P.; Seland, F.; Johannessen, E.A. A micro fuel cell for abiotical catalysis of glucose. J. Power Sources 2020, 478, 229032. [Google Scholar] [CrossRef]
- Lutkenhaus, J.L.; Hammond, P.T. Electrochemically enabled polyelectrolyte multilayer devices: From fuel cells to sensors. Soft Matter 2007, 3, 804–816. [Google Scholar] [CrossRef]
- Tan, Y.M.; Xie, Q.J.; Huang, J.H.; Duan, W.S.; Ma, M.; Yao, S.Z. Study on glucose biofuel cells using an electrochemical noise device. Electroanalysis 2008, 20, 1599–1606. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.F.; Wu, S.B.; Dong, R.J.; Angelidaki, I. Innovative operation of microbial fuel cell-based biosensor for selective monitoring of acetate during anaerobic digestion. Sci. Total Environ. 2019, 655, 1439–1447. [Google Scholar] [CrossRef]
- Jeerapan, I.; Sempionatto, J.R.; Wang, J. On-Body Bioelectronics: Wearable Biofuel Cells for Bioenergy Harvesting and Self-Powered Biosensing. Adv. Funct. Mater. 2020, 30, 1906243. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, J.; Shinde, S.; Wang, X.; Li, Y.; Dai, Y.; Ren, J.; Zhang, P.; Liu, X. Simultaneous wastewater treatment and energy harvesting in microbial fuel cells: An update on the biocatalysts. RSC Adv. 2020, 10, 25874–25887. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Ringeisen, B.R.; Henderson, E.; Wu, P.K.; Pietron, J.; Ray, R.; Little, B.; Biffinger, J.C.; Jones-Meehan, J.M. High power density from a miniature microbial fuel cell using Shewanella oneidensis DSP10. Environ. Sci. Technol. 2006, 40, 2629–2634. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.; Murano, C. Microbial fuel cells utilising carbohydrates. J. Chem. Technol. Biotechnol. 2007, 82, 92–100. [Google Scholar] [CrossRef]
- Oh, S.E.; Logan, B.E. Proton exchange membrane and electrode surface areas as factors that affect power generation in microbial fuel cells. Appl. Microbiol. Biotechnol. 2006, 70, 162–169. [Google Scholar] [CrossRef]
- Harewood, A.J.T.; Popuri, S.R.; Cadogan, E.I.; Lee, C.H.; Wang, C.C. Bioelectricity generation from brewery wastewater in a microbial fuel cell using chitosan/biodegradable copolymer membrane. Int. J. Environ. Sci. Technol. 2017, 14, 1535–1550. [Google Scholar] [CrossRef]
- Sato, F.; Togo, M.; Islam, M.K.; Matsue, T.; Kosuge, J.; Fukasaku, N.; Kurosawa, S.; Nishizawa, M. Enzyme-based glucose fuel cell using Vitamin K-3-immobilized polymer as an electron mediator. Electrochem. Commun. 2005, 7, 643–647. [Google Scholar] [CrossRef]
- Kuwahara, T.; Oshima, K.; Shimomura, M.; Miyauchi, S. Properties of the enzyme electrode fabricated with a film of polythiophene derivative and its application to a glucose fuel cell. J. Appl. Polym. Sci. 2007, 104, 2947–2953. [Google Scholar] [CrossRef]
- Sue, C.Y.; Tsai, N.C. Human powered MEMS-based energy harvest devices. Appl. Energy 2012, 93, 390–403. [Google Scholar] [CrossRef]
- Jeon, W.Y.; Lee, J.H.; Dasbnyam, K.; Choi, Y.B.; Kim, T.H.; Lee, H.H.; Kim, H.W.; Kim, H.H. Performance of a glucose-reactive enzyme-based biofuel cell system for biomedical applications. Sci. Rep. 2019, 9, 10872. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, W.; Yan, K.; Gao, J.; Zhang, J. Fuel cell-based self-powered electrochemical sensors for biochemical detection. Nano Energy 2019, 61, 173–193. [Google Scholar] [CrossRef]
- Bennett, R.; Leech, D. Improved operational stability of mediated glucose enzyme electrodes for operation in human physiological solutions. Bioelectrochemistry 2020, 133, 107460. [Google Scholar] [CrossRef] [PubMed]
- Mano, N.; Mao, F.; Heller, A. A miniature membrane-less biofuel cell operating at +0.60 V under physiological conditions. Chembiochem 2004, 5, 1703–1705. [Google Scholar] [CrossRef]
- Kim, H.H.; Mano, N.; Zhang, X.C.; Heller, A. A miniature membrane-less biofuel cell operating under physiological conditions at 0.5 V. J. Electrochem. Soc. 2003, 150, A209–A213. [Google Scholar] [CrossRef]
- Kavanagh, P.; Boland, S.; Jenkins, P.; Leech, D. Performance of a Glucose/O-2 Enzymatic Biofuel Cell Containing a Mediated Melanocarpus albomyces Laccase Cathode in a Physiological Buffer. Fuel Cells 2009, 9, 79–84. [Google Scholar] [CrossRef]
- Tung, S.P.; Huang, T.K.; Lee, C.Y.; Chiu, H.T. Electrochemical growth of gold nanostructures on carbon paper for alkaline direct glucose fuel cell. RSC Adv. 2012, 2, 1068–1073. [Google Scholar] [CrossRef]
- Basu, D.; Sood, S.; Basu, S. Performance comparison of Pt-Au/C and Pt-Bi/C anode catalysts in batch and continuous direct glucose alkaline fuel cell. Chem. Eng. J. 2013, 228, 867–870. [Google Scholar] [CrossRef]
- Yan, X.L.; Ge, X.B.; Cui, S.Z. Pt-decorated nanoporous gold for glucose electrooxidation in neutral and alkaline solutions. Nanoscale Res. Lett. 2011, 6, 313. [Google Scholar] [CrossRef] [Green Version]
- Eustis, R.; Tsang, T.M.; Yang, B.; Scott, D.; Liaw, B.Y. Seeking effective dyes for a mediated glucose air alkaline battery/fuel cell. J. Power Sources 2014, 248, 1133–1140. [Google Scholar] [CrossRef]
- Schechner, P.; Kroll, E.; Bubis, E.; Chervinsky, S.; Zussman, E. Silver-plated electrospun fibrous anode for glucose alkaline fuel cells. J. Electrochem. Soc. 2007, 154, B942–B948. [Google Scholar] [CrossRef]
- Liu, S.S.; Liu, X.H.; Wang, Y.; Zhang, P.P. Electricity generation from macroalgae Enteromorpha prolifera hydrolysates using an alkaline fuel cell. Bioresour. Technol. 2016, 222, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, X.H.; Dong, F.; Lin, Q.X.; Tong, Y.D.; Li, Y.; Zhang, P.P. Electricity generation from banana peels in an alkaline fuel cell with a Cu2O-Cu modified activated carbon cathode. Sci. Total Environ. 2018, 631–632, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, X.H.; Wang, M.Y.; Zhang, P.P.; Zong, Y.P.; Zhang, Q.F. A single-chamber microbial fuel cell for rapid determination of biochemical oxygen demand using low-cost activated carbon as cathode catalyst. Environ. Technol. 2018, 39, 3228–3237. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Liu, X.H.; Kim, C.H.; Dong, F.; Li, S.L.; Ding, J.; Li, Y.; Muhammad, I.; Zhang, P.P. Energy extraction. from seaweed under low temperatures by using an alkaline fuel cell. Energy Sources Part A-Recovery Util. Environ. Eff. 2018, 40, 2107–2115. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.H.; Wang, J.; Yang, L.; Chen, X.C.; Wang, X.; Zhang, P.P. Marine Algae-Derived Porous Carbons as Robust Electrocatalysts for ORR. Catalysts 2019, 9, 730. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, X.H.; Ding, J.; Li, S.L.; Dong, F.; Irfan, M.; Li, Y.; Wang, G.Y.; Du, X.W.; Zhang, P.P. Chlorella-derived porous heteroatom-doped carbons as robust catalysts for oxygen reduction reaction in direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2019, 44, 2823–2831. [Google Scholar] [CrossRef]
- De Sa, M.H.; Brandao, L. Non-enzymatic direct glucose fuel cells (DGFC): A novel principle towards autonomous electrochemical biosensors. Int. J. Hydrogen Energy 2020, 45, 29749–29762. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zheng, H.; Kang, J.; Yang, F.; Cao, Y.; Xiang, M. An alkaline direct oxidation glucose fuel cell using three-dimensional structural Au/Ni-foam as catalytic electrodes. RSC Adv. 2017, 7, 3035–3042. [Google Scholar] [CrossRef] [Green Version]
- Maeng, B.; Park, J. Characterization of Polymer Electrolyte Membranes for Application of Glucose Biofuel Cell. In Proceedings of the 15th International Conference on Control, Automation and Systems (ICCAS), Busan, Korea, 13–16 October 2015; pp. 1846–1847. [Google Scholar]
- Freijanes, Y.; Barragan, V.M.; Munoz, S. Chronopotentiometric study of a Nafion membrane in presence of glucose. J. Membr. Sci. 2016, 510, 79–90. [Google Scholar] [CrossRef]
- Bahar, T. Development of Reasonably Stable Chitosan Based Proton Exchange Membranes for a Glucose Oxidase Based Enzymatic Biofuel Cell. Electroanalysis 2020, 32, 536–545. [Google Scholar] [CrossRef]
- Cha, H.; Kwon, O.; Kim, J.; Choi, H.; Yoo, H.; Kim, H.; Park, T. Effects of the Anode Diffusion Layer on the Performance of a Nonenzymatic Electrochemical Glucose Fuel Cell with a Proton Exchange Membrane. ACS Omega 2021, 6, 34752–34762. [Google Scholar] [CrossRef] [PubMed]
- Changkhamchom, S.; Kunanupatham, P.; Phasuksom, K.; Sirivat, A. Anion exchange membranes composed of quaternized polybenzimidazole and quaternized graphene oxide for glucose fuel cell. Int. J. Hydrogen Energy 2021, 46, 5642–5652. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, K.H. Glucose oxidase nanotube-based enzymatic biofuel cells with improved laccase biocathodes. Phys. Chem. Chem. Phys. 2013, 15, 3510–3517. [Google Scholar] [CrossRef] [PubMed]
- Touahar, I.E.; Haroune, L.; Ba, S.; Bellenger, J.P.; Cabana, H. Characterization of combined cross-linked enzyme aggregates from laccase, versatile peroxidase and glucose oxidase, and their utilization for the elimination of pharmaceuticals. Sci. Total Env. 2014, 481, 90–99. [Google Scholar] [CrossRef]
- Zheng, W.; Ma, J.Y.; Guo, F.; Li, J.; Zhou, H.M.; Xu, X.X.; Li, L.; Zheng, Y.F. A novel biofuel cell based on electrospun collagen-carbon nanotube nanofibres. Bio-Med. Mater. Eng. 2014, 24, 229–235. [Google Scholar] [CrossRef]
- Yang, Y.L.; Liu, X.H.; Hao, M.Q.; Zhang, P.P. Performance of a low-cost direct glucose fuel cell with an anion-exchange membrane. Int. J. Hydrogen Energy 2015, 40, 10979–10984. [Google Scholar] [CrossRef]
- Ogungbemi, E.; Ijaodola, O.; Khatib, F.N.; Wilberforce, T.; El Hassan, Z.; Thompson, J.; Ramadan, M.; Olabi, A.G. Fuel cell membranes—Pros and cons. Energy 2019, 172, 155–172. [Google Scholar] [CrossRef] [Green Version]
- El Ichi-Ribault, S.; Zebda, A.; Laaroussi, A.; Reverdy-Bruas, N.; Chaussy, D.; Belgacem, M.N.; Suherman, A.L.; Cinquin, P.; Martin, D.K. Laccase-based biocathodes: Comparison of chitosan and Nafion. Anal. Chim. Acta 2016, 937, 43–52. [Google Scholar] [CrossRef]
- Kook, L.; Nemestothy, N.; Bakonyi, P.; Zhen, G.Y.; Kumar, G.; Lu, X.Q.; Su, L.H.; Saratale, G.D.; Kim, S.H.; Gubicza, L. Performance evaluation of microbial electrochemical systems operated with Nafion and supported ionic liquid membranes. Chemosphere 2017, 175, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Chae, K.J.; Choi, M.; Ajayi, F.F.; Park, W.; Chang, I.S.; Kim, I.S. Mass transport through a proton exchange membrane (Nafion) in microbial fuel cells. Energy Fuels 2008, 22, 169–176. [Google Scholar] [CrossRef]
- Li, J.; Li, X.J.; Yu, S.C.; Hao, J.K.; Lu, W.T.; Shao, Z.G.; Yi, B.L. Porous polybenzimidazole membranes doped with phosphoric acid: Preparation and application in high-temperature proton-exchange-membrane fuel cells. Energy Convers. Manag. 2014, 85, 323–327. [Google Scholar] [CrossRef]
- Chendake, Y.J.; Kharul, U.K. Transport of organic acids through polybenzimidazole based membranes by ‘Chemodialysis’. J. Membr. Sci. 2014, 451, 243–251. [Google Scholar] [CrossRef]
- Hoskins, D.L.; Zhang, X.; Hickner, M.A.; Logan, B.E. Spray-on polyvinyl alcohol separators and impact on power production in air-cathode microbial fuel cells with different solution conductivities. Bioresour. Technol. 2014, 172, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Abu Sayeed, M.D.; Talukdar, K.; Kim, H.J.; Park, Y.; Gopalan, A.I.; Kim, Y.H.; Lee, K.P.; Choi, S.J. Passive approach for the improved dispersion of polyvinyl alcohol-based functionalized multi-walled carbon nanotubes/Nafion membranes for polymer electrolyte membrane fuel cells. J. Nanosci. Nanotechnol. 2014, 14, 9329–9334. [Google Scholar] [CrossRef]
- Cho, E.B.; Kim, H.; Kim, D. Effect of morphology and pore size of sulfonated mesoporous benzene-silicas in the preparation of poly(vinyl alcohol)-based hybrid nanocomposite membranes for direct methanol fuel cell application. J. Phys. Chem. B 2009, 113, 9770–9778. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Hu, T.Y.; Kumar, S.R.; Chang, C.H.; Wu, K.C.; Tung, K.L.; Lue, S.J. Highly Zeolite-Loaded Polyvinyl Alcohol Composite Membranes for Alkaline Fuel-Cell Electrolytes. Polymers 2018, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Vinodh, R.; Atchudan, R.; Kim, H.J.; Yi, M. Recent Advancements in Polysulfone Based Membranes for Fuel Cell (PEMFCs, DMFCs and AMFCs) Applications: A Critical Review. Polymers 2022, 14, 300. [Google Scholar] [CrossRef]
- Abouzari-Lotf, E.; Jacob, M.V.; Ghassemi, H.; Zakeri, M.; Nasef, M.M.; Abdolahi, Y.; Abbasi, A.; Ahmad, A. Highly conductive anion exchange membranes based on polymer networks containing imidazolium functionalised side chains. Sci. Rep. 2021, 11, 3764. [Google Scholar] [CrossRef]
- Simons, P.; Torres, K.P.; Rupp, J.L.M. Careful Choices in Low Temperature Ceramic Processing and Slow Hydration Kinetics Can Affect Proton Conduction in Ceria. Adv. Funct. Mater. 2021, 31, 2009630. [Google Scholar] [CrossRef]
- Frattini, D.; Accardo, G.; Kwon, Y. Perovskite ceramic membrane separator with improved biofouling resistance for yeast-based microbial fuel cells. J. Membr. Sci. 2020, 599, 117843. [Google Scholar] [CrossRef]
- Simons, P.; Schenk, S.A.; Gysel, M.A.; Olbrich, L.F.; Rupp, J.L.M. A Ceramic-Electrolyte Glucose Fuel Cell for Implantable Electronics. Adv. Mater. 2022, 34, e2109075. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, S.; Mosquera, M.E.G. Conducting Polymer-Based Nanohybrids for Fuel Cell Application. Polymers 2020, 12, 2993. [Google Scholar] [CrossRef]
- Synnestvedt, M.B.; Chen, C.; Holmes, J.H. CiteSpace II: Visualization and knowledge discovery in bibliographic databases. AMIA Annu. Symp. Proc. Am. Med. Inform. Assoc. 2005, 724–728. [Google Scholar]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, D.; Li, P.; Liang, S.; Zhang, Z. A Bibliometric and Visual Analysis of Global Community Resilience Research. Int. J. Env. Res. Public Health 2021, 18, 10857. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanovic, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef]
- Yazici, M.S.; Bahar, T. Effects of cathode gas diffusion layer type and membrane electrode assembly preparation on the performance of immobilized glucose oxidase-based enzyme fuel cell. Asia-Pac. J. Chem. Eng. 2021, 16, e2686. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.K.; Park, J.H. Fabrication and Measurement of Photosynthetic Microbial Fuel Cell Using Nafion-Casted Membrane Electrode Assembly. Nanosci. Nanotechnol. Lett. 2015, 7, 984–988. [Google Scholar] [CrossRef]
- Jeyaraman, S.N.; Slaughter, G. Membranes, immobilization, and protective strategies for enzyme fuel cell stability. Curr. Opin. Electrochem. 2021, 29, 100753. [Google Scholar] [CrossRef]
- Mecheri, B.; D’Epifanio, A.; Geracitano, A.; Campana, P.T.; Licoccia, S. Development of glucose oxidase-based bioanodes for enzyme fuel cell applications. J. Appl. Electrochem. 2013, 43, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Mu, W.; Liu, M.; Zhang, X.; Cai, H.; Deng, Y. Solar-induced direct biomass-to-electricity hybrid fuel cell using polyoxometalates as photocatalyst and charge carrier. Nat. Commun. 2014, 5, 3208. [Google Scholar] [CrossRef]
- Li, Y.; Dong, F.; Jiao, S.; Wang, J.; Dai, Y.; Irfan, M.; Liu, X. Co2+-P(W3O10)43− modified activated carbon as an efficient anode catalyst for direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2022, 47, 22952–22962. [Google Scholar] [CrossRef]
- Hayashi, K.; Arata, S.; Murakami, S.; Nishio, Y.; Kobayashi, A.; Niitsu, K. A 6.1-nA Fully Integrated CMOS Supply Modulated OOK Transmitter in 55-nm DDC CMOS for Glasses-Free, Self-Powered, and Fuel-Cell-Embedded Continuous Glucose Monitoring Contact Lens. IEEE Trans. Circuits Syst. II Express Briefs 2018, 65, 1360–1364. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, Z.; Hong, X.; Wu, H.; Ma, S.; Li, Y.; Chen, D.; Yu, H.; Huang, X.J. Top-Down Strategy of Implantable Biosensor Using Adaptable, Porous Hollow Fibrous Membrane. ACS Sens. 2019, 4, 931–937. [Google Scholar] [CrossRef]
- Serag, E.; El-Maghraby, A.; El Nemr, A. Recent developments in the application of carbon-based nanomaterials in implantable and wearable enzyme-biofuel cells. Carbon Lett. 2022, 32, 395–412. [Google Scholar] [CrossRef]
- Wang, C.; Shim, E.; Chang, H.K.; Lee, N.; Kim, H.R.; Park, J. Sustainable and high-power wearable glucose biofuel cell using long-term and high-speed flow in sportswear fabrics. Biosens. Bioelectron. 2020, 169, 112652. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T. Glucose Fuel Cells and Membranes: A Brief Overview and Literature Analysis. Sustainability 2022, 14, 8376. https://doi.org/10.3390/su14148376
Liu T. Glucose Fuel Cells and Membranes: A Brief Overview and Literature Analysis. Sustainability. 2022; 14(14):8376. https://doi.org/10.3390/su14148376
Chicago/Turabian StyleLiu, Tong. 2022. "Glucose Fuel Cells and Membranes: A Brief Overview and Literature Analysis" Sustainability 14, no. 14: 8376. https://doi.org/10.3390/su14148376
APA StyleLiu, T. (2022). Glucose Fuel Cells and Membranes: A Brief Overview and Literature Analysis. Sustainability, 14(14), 8376. https://doi.org/10.3390/su14148376




