Application of Microbial Fuel Cell (MFC) for Pharmaceutical Wastewater Treatment: An Overview and Future Perspectives
Abstract
:1. Introduction
2. Basics of Microbial Fuel Cells (MFCs)
3. Treatment of PWW Using Conventional and Advanced Oxidation Processes
3.1. Coagulation
3.2. Adsorption
3.3. Ozonation
3.4. Flotation
3.5. Advanced Oxidation Processes (AOPs)
3.6. Membrane Separation
| SL No. | Conventional Methods | Advantages | Disadvantages | References |
|---|---|---|---|---|
| 1. | Coagulation/sedimentation | Economical | Sludge is produced in large quantities | [16] |
| 2. | Adsorption | A process that is simple, consistent, and straightforward | Adsorbents must be replenished | [21] |
| 3. | Oxidation process | Pollutants are removed quickly | An expensive procedure | [26] |
| 4. | Ozonation process | Variation in volume | The half-life is extremely brief | [20] |
| 5. | Membrane filtration | Metals from pharmaceuticals can be easily removed | Production with concentrated sludge | [36] |
| 6. | Biological treatment | Feasible for eliminating a wide range of pharmaceutical contaminants | Not yet commercialized | [35] |
| 7. | Flocculation | Less sludge settling, dewatering | Costly and high consumption of chemicals | [37] |
| 8. | Membrane distillation | The thermally driven purifying process is cost-effective, especially with respect to waste heat or solar thermal energy | Pore-wetting in membranes | [38] |
| 9. | Microbial electrochemical technology | Electricity production and other important commodities are among the many applications | Upscaling is difficult and expensive | [39] |
| 10. | Nanomaterials | Highly efficient with higher adsorption efficiency, friendly with other techniques | Less ecofriendly, more expensive and hazardous | [40,41] |
4. Treatment of PWW Using MFC
4.1. Removal of Antibiotics
4.2. Removal of Aromatic Compounds
4.3. Other Pharmaceutical Pollutants
| SL No. | Other Pharmaceutical Compounds | Initial Wastewater Concentration | Time | Rate of Antibiotic Elimination | Power Produced | References |
|---|---|---|---|---|---|---|
| 1. | Trimethoprim, lamivudine, levofloxacin, and estrone | 2 g mL−1 | 30 h | ~97% | High energy production | [66] |
| 2. | COD | - | 5.21 h | 93% | 183.06 mW m−2 | [64] |
| 3. | TDS and COD | 800 to 515 mg L−1 (TDS); 5460 to 1060 mg L−1 (COD) | - | 35.2% (TDS) and 80.55% (COD) | High energy production | [8] |
| 4. | Nitrate, phosphate and COD | - | - | 97.12% (nitrate); 93.7% (phosphate); 77.3% (COD) | 838.68 mW m−2 | [69] |
| 5. | COD and NH4+-N | 0.01 g L−1 | - | 39.68% (NH4+-N); 93.68% (COD) | 18.67 W m−3 | [70] |
| 6. | Ciprofloxacin | 10 mg L−1 | 88 h | 99% | - | [61] |
| 7. | Recalcitrant pollutants | 2.52 g COD L−1 | 8 d | 90% (SCOD); 92% (TCOD); 73% (TSS); 82% (COD) | High energy production | [58] |
| 8. | Triclosan | 5.8 mg L−1 | 96 h | 50 to 80% | Intra particle diffusion | [71] |
5. Advantages of MFC over Traditional Processes
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Desbiolles, F.; Malleret, L.; Tiliacos, C.; Wong-Wah-Chung, P.; Laffont-Schwob, I. Occurrence and ecotoxicological assessment of pharmaceuticals: Is there a risk for the Mediterranean aquatic environment? Sci. Total Environ. 2018, 639, 1334–1348. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, P. Review on physicochemical, chemical, and biological processes for pharmaceutical wastewater. In Proceedings of the 3rd International Conference on Advances in Energy Resources and Environment Engineering, Harbin, China, 8–10 December 2017; Volume 113, p. 012185. [Google Scholar]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Chen, Z.; Haghighat, F.; Yerushalmi, L. Removal of pharmaceuticals and endocrine disrupting compounds from water by zinc oxide-based photocatalytic degradation: A review. Sustain. Cities Soc. 2016, 27, 407–418. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plant- a review. Environ. Int. 2014, 69, 28–39. [Google Scholar] [CrossRef]
- Rashid, T.; Sher, F.; Hazafa, A.; Hashmi, R.Q.; Zafar, A.; Rasheed, T.; Hussain, S. Design and feasibility study of novel paraboloid graphite based microbial fuel cell for bioelectrogenesis and pharmaceutical wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 104502. [Google Scholar] [CrossRef]
- Mathuriya, A.S. Enhanced tannery wastewater treatment and electricity generation in microbial fuel cell by bacterial strains isolated from tannery waste. Environ. Eng. Manag. J. 2014, 13, 2945–2954. [Google Scholar] [CrossRef]
- Patwardhan, S.B.; Savla, N.; Pandit, S.; Gupta, P.K.; Mathuriya, A.S.; Lahiri, D.; Jadhav, D.A.; Rai, A.K.; Priya, K.; Ray, R.R.; et al. Microbial fuel cell united with other existing technologies for enhanced power generation and efficient wastewater treatment. Appl. Sci. 2021, 11, 10777. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Hiloidhari, M.; Gware, P.; Singh, A.; Pant, D. Development and life cycle assessment of an auto circulating bioelectrochemical reactor for energy positive continuous wastewater treatment. Bioresour. Technol. 2020, 304, 122959. [Google Scholar] [CrossRef]
- Thapa, B.S.; Oh, S.-E.; Peera, S.G.; Singh, L. Fundamentals of bioelectroactive fuel cells. In Bioremediation, Nutrients, and Other Valuable Product Recovery-Using Bioelectrochemical Systems; Singh, L., Mahapatra, D.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–23. [Google Scholar]
- Pandit, S.; Chandrasekhar, K.; Kakarla, R.; Kadier, A.; Jeevitha, V. Basic principles of microbial fuel cell: Technical challenges and economic feasibility. In Microbial Applications Vol. 1; Kalia, V.C., Kumar, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 165–188. [Google Scholar]
- Malekmohammadi, S.; Mirbagheri, S.A. A review of the operating parameters on the microbial fuel cell for wastewater treatment and electricity generation. Water Sci. Technol. 2021, 84, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Qi, P.S.; Liu, Y.Z. A review on advanced treatment of pharmaceutical wastewater. In Proceedings of the International Conference on Environmental and Energy Engineering (IC3E 2017), Suzhou, China, 22–24 March 2017; Volume 63, p. 012025. [Google Scholar]
- Xu, X.; Cheng, Y.; Zhang, T.; Ji, F.; Xu, X. Treatment of pharmaceutical wastewater using interior micro-electrolysis/Fenton oxidation-coagulation and biological degradation. Chemosphere 2016, 152, 23–30. [Google Scholar] [CrossRef]
- Changotra, R.; Rajput, H.; Guin, J.P.; Khader, S.A.; Dhir, A. Techno-economical evaluation of coupling ionizing radiation and biological treatment process for the remediation of real pharmaceutical wastewater. J. Clean. Prod. 2020, 242, 118544. [Google Scholar] [CrossRef]
- Ek, M.; Baresel, C.; Magnér, J.; Bergström, R.; Harding, M. Activated carbon for the removal of pharmaceutical residues from treated wastewater. Water Sci. Technol. 2014, 69, 2372–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issaka, E.; Amu-Darko, J.N.O.; Yakubu, S.; Fapohunda, F.O.; Ali, N.; Bilal, M. Advanced catalytic ozonation for degradation of pharmaceutical pollutants—A review. Chemosphere 2022, 289, 133208. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, N.; Guo, W.; Wang, Y.; Huang, X.; Wu, P.; Dang, Z.; Zhang, X.; Xian, J. Simultaneous electricity production and antibiotics removal by microbial fuel cells. J. Environ. Manag. 2018, 217, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.; Lema, J.M.; Omil, F. Pre-treatment of hospital wastewater by coagulation-flocculation and flotation. Bioresour. Technol. 2009, 100, 2138–2146. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.; Vakili, M.; Van Le, H. Removal performance and optimisation of pharmaceutical micropollutants from synthetic domestic wastewater by hybrid treatment. J. Contam. Hydrol. 2020, 235, 103736. [Google Scholar] [CrossRef]
- Ferrer-Polonio, E.; Fernández-Navarro, J.; Iborra-Clar, M.I.; Alcaina-Miranda, M.I.; Mendoza-Roca, J.A. Removal of pharmaceutical compounds commonly-found in wastewater through a hybrid biological and adsorption process. J. Environ. Manag. 2020, 263, 110368. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J.; He, S.; Chen, C.; Wojnárovits, L.; Takács, E. Treatment of pharmaceutical wastewater by ionizing radiation: Removal of antibiotics, antimicrobial resistance genes and antimicrobial activity. J. Hazard. Mater. 2021, 415, 125724. [Google Scholar] [CrossRef]
- Tormo-Budowski, R.; Cambronero-Heinrichs, J.C.; Durán, J.E.; Masís-Mora, M.; Ramirez-Morales, D.; Quirós-Fournier, J.P.; Rodriguez-Rodriguez, C.E. Removal of pharmaceuticals and ecotoxicological changes in wastewater using Trametes versicolor: A comparison of fungal stirred tank and trickle-bed bioreactors. Chem. Eng. J. 2021, 410, 128210. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Mylapilli, S.V.P.; Reddy, S.N. Sub and supercritical water oxidation of pharmaceutical wastewater. J. Environ. Chem. Eng. 2019, 7, 103165. [Google Scholar] [CrossRef]
- Xiong, Z.; Jiang, Y.; Wu, Z.; Yao, G.; Lai, B. Synthesis strategies and emerging mechanisms of metal-organic frameworks for sulfate radical-based advanced oxidation process: A review. Chem. Eng. J. 2021, 421, 127863. [Google Scholar] [CrossRef]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key points of advanced oxidation processes (AOPs) for wastewater, organic pollutants and pharmaceutical waste treatment: A mini review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Tekin, H.; Bilkay, O.; Ataberk, S.S.; Balta, T.H.; Ceribasi, I.H.; Sanin, F.D.; Dilek, F.B.; Yetis, U. Use of Fenton oxidation to improve the biodegradability of a pharmaceutical wastewater. J. Hazard. Mater. 2006, 136, 258–265. [Google Scholar] [CrossRef]
- Sathe, S.M.; Chakraborty, I.; Dubey, B.K.; Ghangrekar, M.M. Microbial fuel cell coupled Fenton oxidation for the cathodic degradation of emerging contaminants from wastewater: Applications and challenges. Environ. Res. 2022, 204, 112135. [Google Scholar] [CrossRef]
- Martínez, F.; Molina, R.; Rodríguez, I.; Pariente, M.I.; Segura, Y.; Melero, J.A. Techno-economical assessment of coupling Fenton/biological processes for the treatment of a pharmaceutical wastewater. J. Environ. Chem. Eng. 2018, 6, 485–494. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Shi, H.J. UV/chlorinated cyanurates as an emerging advanced oxidation process for drinking water and potable reuse treatments. Water Res. 2022, 211, 118075. [Google Scholar] [CrossRef]
- Lefebvre, O.; Shi, X.; Wu, C.H.; Ng, H.Y. Biological treatment of pharmaceutical wastewater from the antibiotics industry. Water Sci. Technol. 2014, 69, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. A critical review on membrane separation processes applied to remove pharmaceutically active compounds from water and wastewater. J. Water Process Eng. 2018, 26, 156–175. [Google Scholar] [CrossRef]
- Razzak, S.A.; Farooque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.Z.; Hossain, M.M. A Comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Environ. Adv. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Ibrar, I.; Yadav, S.; Naji, O.; Alanezi, A.A.; Ghaffour, N.; Déon, S.; Subbiah, S.; Altaee, A. Development in forward osmosis-membrane distillation hybrid system for wastewater treatment. Sep. Purif. Technol. 2022, 286, 120498. [Google Scholar] [CrossRef]
- Mohan, V.S.; Srikanth, S.; Velvizhi, G.; Lenin Babu, M. Microbial fuel cells for sustainable bioenergy generation: Principles and perspective applications. In Biofuel Technologies; Gupta, V., Tuohy, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 335–368. [Google Scholar]
- Sadegh, H.; Ali, G.A.; Gupta, V.K.; Makhlouf, A.S.H.; Shahryari-Ghoshekandi, R.; Nadagouda, M.N.; Sillanpää, M.; Megiel, E. The role of nanomaterials as effective adsorbents and their applications in wastewater treatment. J. Nanostruct. Chem. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Gautam, P.K.; Singh, A.; Misra, K.; Sahoo, A.K.; Samanta, S.K. Synthesis and applications of biogenic nanomaterials in drinking and wastewater treatment. J. Environ. Manag. 2019, 231, 734–748. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Biological combination processes for efficient removal of pharmaceutically active compounds from wastewater: A review and future perspectives. J. Environ. Chem. Eng. 2017, 5, 3590–3603. [Google Scholar] [CrossRef]
- Wen, Q.; Kong, F.; Zheng, H.; Cao, D.; Ren, Y.; Yin, J. Electricity generation from synthetic penicillin wastewater in an air-cathode single chamber microbial fuel cell. Chem. Eng. J. 2011, 168, 572–576. [Google Scholar] [CrossRef]
- Wang, J.; He, M.F.; Zhang, D.; Ren, Z.; Song, T.S.; Xie, J. Simultaneous degradation of tetracycline by a microbial fuel cell and its toxicity evaluation by zebrafish. RSC Adv. 2017, 7, 44226–44233. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Yu, Q.; Zhai, W.; Wang, F.; Scott, K. High tolerance of and removal of cefazolin sodium in single-chamber microbial fuel cells operation. Bioresour. Technol. 2018, 249, 76–81. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Li, D. Cometabolic degradation of chloramphenicol via a meta-cleavage pathway in a microbial fuel cell and its microbial community. Bioresour. Technol. 2017, 229, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ngo, H.H.; Guo, W.; Lee, D.; Nghiem, D.L.; Zhang, J.; Liang, S.; Varjani, S.; Wang, J. Performance of microbial fuel cell for treating swine wastewater containing sulfonamide antibiotics. Bioresour. Technol. 2020, 311, 123588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shen, C.; Huang, J.; Wang, L.; Pang, Q.; Peng, F.; Hou, J.; Ni, L.; He, F.; Xu, B. The effect of sulfamethoxazole on nitrogen removal and electricity generation in a tidal flow constructed wetland coupled with a microbial fuel cell system: Microbial response. Chem. Eng. J. 2022, 431, 134070. [Google Scholar] [CrossRef]
- Xie, J.; Li, H.; Wang, S.; Chen, H.; Jiang, W.; Zhang, L.; Wang, L.; Wu, Y.; Li, L.; Lu, X. Removal and fate of carbamazepine in the microbial fuel cell coupled constructed wetland system. Environ. Eng. Res. 2022, 27, 158–168. [Google Scholar] [CrossRef]
- Zhao, H.; Kong, C.H. Enhanced removal of p-nitrophenol in a microbial fuel cell after long-term operation and the catabolic versatility of its microbial community. Chem. Eng. J. 2018, 339, 424–431. [Google Scholar] [CrossRef]
- Zhang, L.; Yin, X.; Li, S.F.Y. Bio-electrochemical degradation of paracetamol in a microbial fuel cell-Fenton system. Chem. Eng. J. 2015, 276, 185–192. [Google Scholar] [CrossRef]
- Song, H.; Guo, W.; Liu, M.; Sun, J. Performance of microbial fuel cells on removal of metronidazole. Water Sci. Technol. 2013, 68, 2599–2604. [Google Scholar] [CrossRef]
- Huang, X.; Liu, C.; Li, K.; Su, J.; Zhu, G.; Liu, L. Performance of vertical up-flow constructed wetlands on swine wastewater containing tetracyclines and tet genes. Water Res. 2015, 70, 109–117. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Li, J.; Ly, Q.V.; Nguyen, T.A.H. Applying a new pomelo peel derived biochar in microbial fell cell for enhancing sulfonamide antibiotics removal in swine wastewater. Bioresour. Technol. 2020, 318, 123886. [Google Scholar] [CrossRef]
- Catal, T.; Yavaser, S.; Enisoglu-Atalay, V.; Bermek, H.; Ozilhan, S. Monitoring of neomycin sulfate antibiotic in microbial fuel cells. Bioresour. Technol. 2018, 268, 116–120. [Google Scholar] [CrossRef]
- Wu, D.; Sun, F.; Zhou, Y. Degradation of chloramphenicol with novel metal foam electrodes in bioelectrochemical systems. Electrochim. Acta 2017, 240, 136–145. [Google Scholar] [CrossRef]
- Ondon, B.S.; Li, S.; Zhou, Q.; Li, F. Simultaneous removal and high tolerance of norfloxacin with electricity generation in microbial fuel cell and its antibiotic resistance genes quantification. Bioresour. Technol. 2020, 304, 122984. [Google Scholar] [CrossRef]
- Pugazhendi, A.; Jamal, M.T.; Al-Mur, B.A.; Jeyakumar, R.B. Bioaugmentation of electrogenic halophiles in the treatment of pharmaceutical industrial wastewater and energy production in microbial fuel cell under saline condition. Chemosphere 2022, 288, 132515. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fu, Y.; Guo, C.; Li, Y.; Jiang, N.; Yin, C. Electricity generation and removal performance of a microbial fuel cell using sulfonated poly (ether ether ketone) as proton exchange membrane to treat phenol/acetone wastewater. Bioresour. Technol. 2018, 260, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zheng, D.; Xie, Z.; He, Q.; Yu, J. The degradation of ibuprofen in a novel microbial fuel cell with PANi@CNTs/SS bio-anode and CuInS2 photocatalytic cathode: Property, efficiency and mechanism. J. Clean. Prod. 2020, 265, 121872. [Google Scholar] [CrossRef]
- Yan, W.; Wang, S.; Ding, R.; Tian, X.; Bai, R.; Gang, H.; Yan, W.; Xiao, Y.; Zhao, F. Long-term operation of electroactive biofilms for enhanced ciprofloxacin removal capacity and anti-shock capabilities. Bioresour. Technol. 2019, 275, 192–199. [Google Scholar] [CrossRef]
- Velvizhi, G.; Mohan, S.V. Biocatalyst behavior under self-induced electrogenic microenvironment in comparison with anaerobic treatment: Evaluation with pharmaceutical wastewater for multi-pollutant removal. Bioresour. Technol. 2011, 102, 10784–10793. [Google Scholar] [CrossRef]
- Xu, H.; Quan, X.; Xiao, Z.; Chen, L. Effect of anodes decoration with metal and metal oxides nanoparticles on pharmaceutically active compounds removal and power generation in microbial fuel cells. Chem. Eng. J. 2018, 335, 539–547. [Google Scholar] [CrossRef]
- Birjandi, N.; Younesi, H.; Ghoreyshi, A.A.; Rahimnejad, M. Enhanced medicinal herbs wastewater treatment in continuous flow bio-electro-Fenton operations along with power generation. Renew. Energy 2020, 155, 1079–1090. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; Li, Y.; Gao, J.; Yu, C.-P. Enhancement of emerging contaminants removal using Fenton reaction driven by H2O2-producing microbial fuel cells. Chem. Eng. J. 2017, 307, 679–686. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Kumar, D.; Mutnuri, S. Probing the degradation of pharmaceuticals in urine using MFC and studying their removal efficiency by UPLC-MS/MS. J. Pharm. Anal. 2021, 11, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Sogani, M.; Pankan, A.O.; Dongre, A.; Yunus, K.; Fisher, A.C. Augmenting the biodegradation of recalcitrant ethinylestradiol using Rhodopseudomonas palustris in a hybrid photo-assisted microbial fuel cell with enhanced bio-hydrogen production. J. Hazard. Mater. 2021, 408, 124421. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gao, C.; Zhao, Y.G.; Wang, A.; Lu, S.; Wang, M.; Maqbool, F.; Huang, Q. Biological treatment of steroidal drug industrial effluent and electricity generation in the microbial fuel cells. Bioresour. Technol. 2012, 123, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Nayak, J.K.; Ghosh, U.K. Post treatment of microalgae treated pharmaceutical wastewater in photosynthetic microbial fuel cell (PMFC) and biodiesel production. Biomass Bioenergy 2019, 131, 105415. [Google Scholar] [CrossRef]
- Shen, F.; Tian, D.; Yang, G.; Deng, S.; Shen, F.; He, J.; Zhu, Y.; Huang, C.; Hu, J. Deacetylation processing of waste cigarette butts for high-titer bioethanol production toward a clean recycling process. ACS Sustain. Chem. Eng. 2020, 8, 11253–11262. [Google Scholar] [CrossRef]
- Chen, X.; Gu, X.; Bao, L.; Ma, S.; Mu, Y. Comparison of adsorption and desorption of triclosan between microplastics and soil particles. Chemosphere 2021, 263, 127947. [Google Scholar] [CrossRef]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total Environ. 2021, 754, 142429. [Google Scholar] [CrossRef]
- Gude, V.G. Wastewater treatment in microbial fuel cells- an overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Trapero, J.R.; Horcajada, L.; Linares, J.J.; Lobato, J. Is microbial fuel cell technology ready? An economic answer towards industrial commercialization. Appl. Energy 2017, 185, 698–707. [Google Scholar] [CrossRef]
- Mansoorian, H.J.; Mahvi, A.H.; Jafari, A.J.; Amin, M.M.; Rajabizadeh, A.; Khanjani, N. Bioelectricity generation using two chamber microbial fuel cell treating wastewater from food processing. Enzyme Microb. Technol. 2013, 52, 352–357. [Google Scholar] [CrossRef]
- Bagchi, S.; Behera, M. Pharmaceutical wastewater treatment in microbial fuel cell. In Integrated Microbial Fuel Cells for Wastewater Treatment, 1st ed.; Abbassi, R., Yadav, A.K., Khan, F., Garaniya, V., Eds.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 135–155. [Google Scholar]
- Wu, Q.; Jiao, S.; Ma, M.; Peng, S. Microbial fuel cell system: A promising technology for pollutant removal and environmental remediation. Environ. Sci. Pollut. Res. 2020, 27, 6749–6764. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Feng, X.; Li, X. Bioelectrochemical approach for control of methane emission from wetlands. Bioresour. Technol. 2017, 241, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Escapa, A.; Mateos, R.; Martínez, E.J.; Blanes, J. Microbial electrolysis cells: An emerging technology for wastewater treatment and energy recovery. From laboratory to pilot plant and beyond. Renew. Sust. Energy Rev. 2016, 55, 942–956. [Google Scholar] [CrossRef]
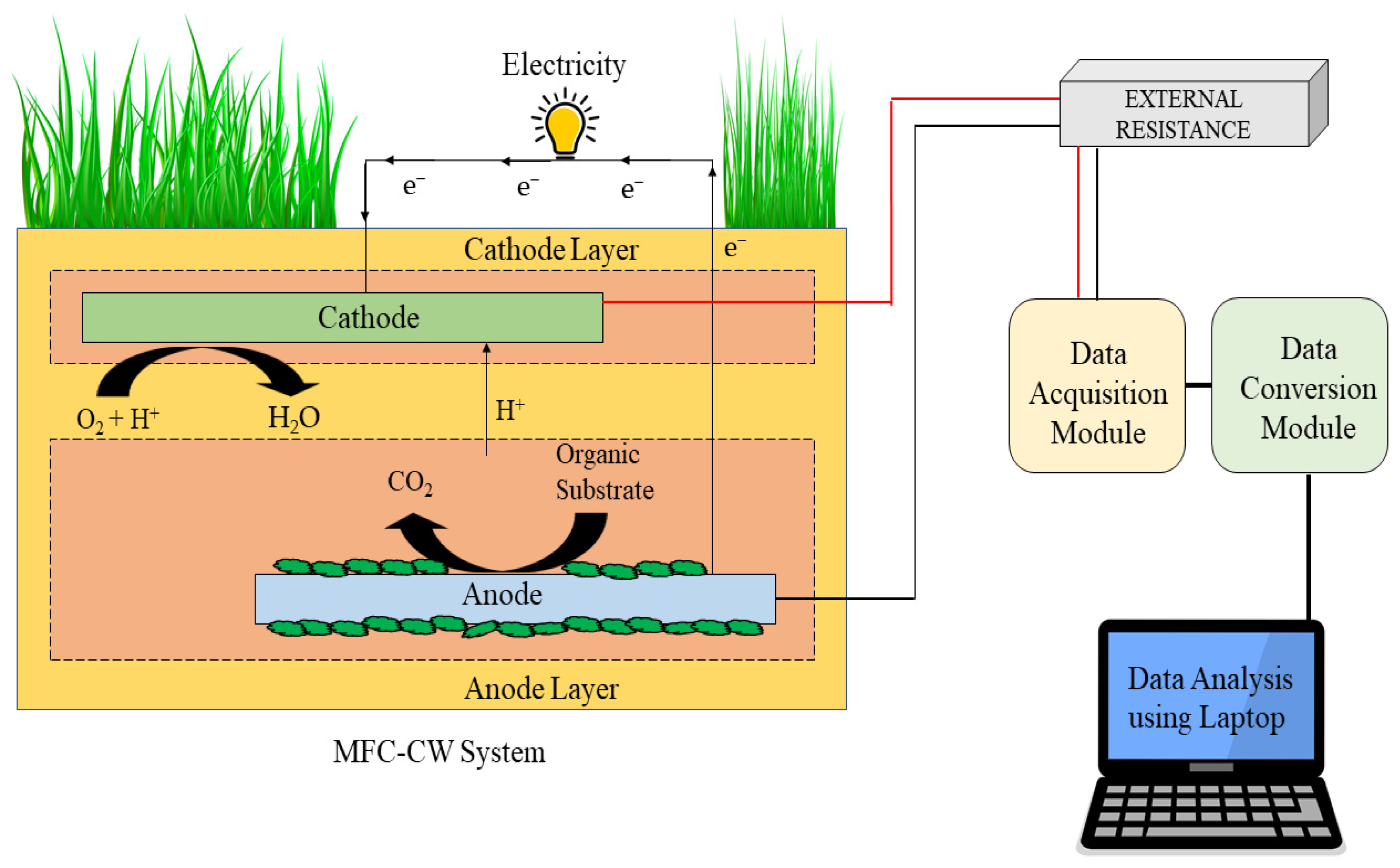
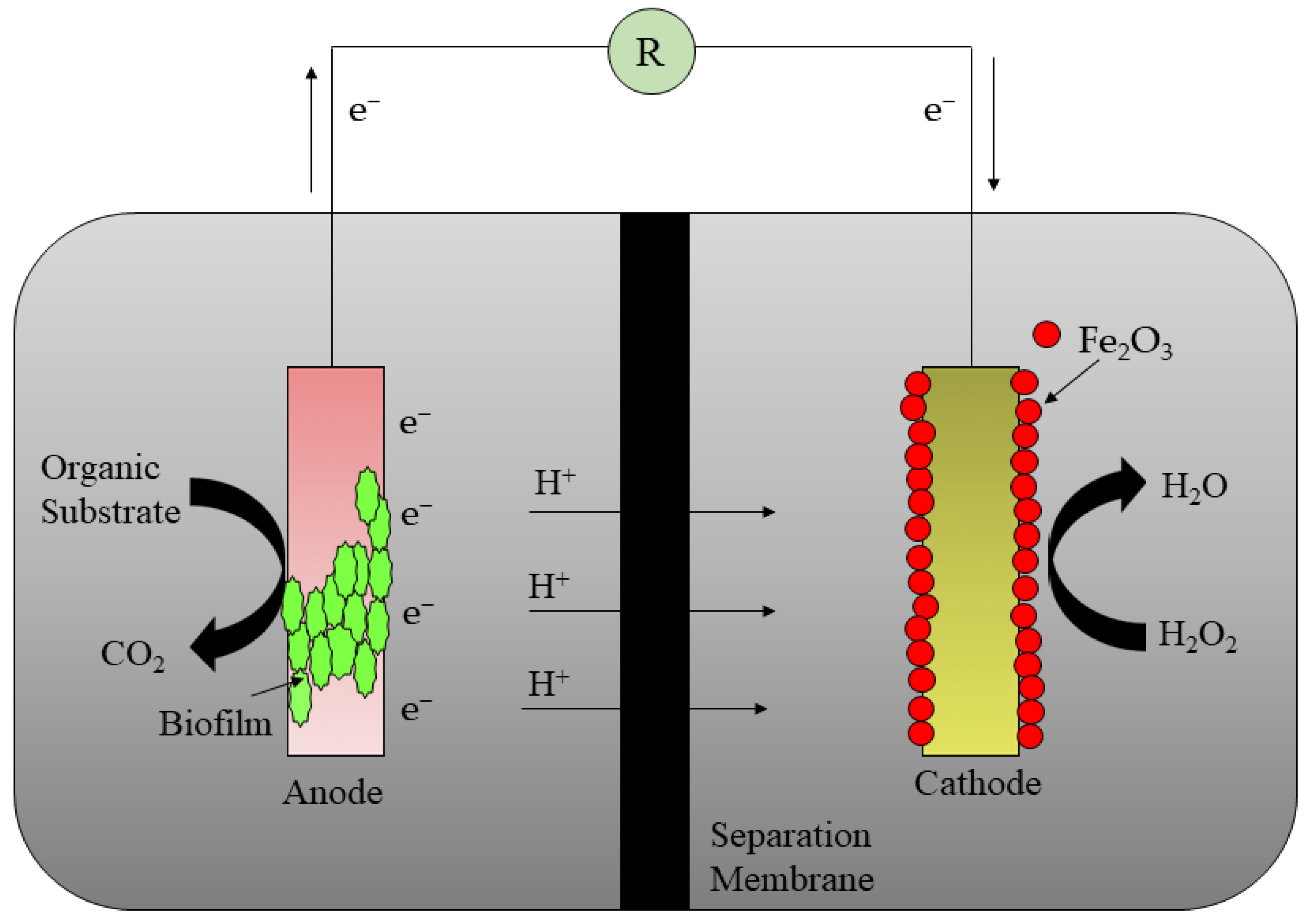

| SL No. | Antibiotics | Initial Wastewater Concentration | Time | Rate of Antibiotic Elimination | Power Density Produced | References |
|---|---|---|---|---|---|---|
| 1. | Penicillin | 50 mg L−1 | 24 h | 98% | 101.7 W m−3 | [43] |
| 2. | Tetracycline | 50 mg L−1 | 168 h | 80% | 2.5 W m−3 | [44] |
| 3. | Cefazolin sodium | 50 mg L−1 | 30 h | ~70% | 30.4 W m−3 | [45] |
| 4. | Chloramphenicol | 80 mg L−1 | 48 h | 61% | 0.86 W m−3 | [46] |
| 5. | Sulfamethoxazole | 200 mg L−1 | 24 h | 70% | - | [43] |
| 6. | Ceftriaxone | 50 mg L−1 | 24 h | 91% | 113 W m−3 | [43] |
| 7. | Sulfanilamide | 30 mg L−1 | 96 h | 90% | - | [47] |
| 8 | Sulfamethoxazole | 10 mg L−1 | 240 d | 80.3% | 524.5 mv | [48] |
| 9. | Carbamazepine | 10 mg L−1 | 10 d | 99% | 0.330 W m−2 | [49] |
| 10. | p-nitrophenol | 50 mg L−1 | 24 h | 81% | - | [50] |
| 11. | Paracetamol | 5 mg L−1 | 9 h | 71% | - | [51] |
| 12. | Glucose–ceftriaxone sodium | 50 mg L−1 | 24 h | 91% | 11 W m−3 | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thapa, B.S.; Pandit, S.; Patwardhan, S.B.; Tripathi, S.; Mathuriya, A.S.; Gupta, P.K.; Lal, R.B.; Tusher, T.R. Application of Microbial Fuel Cell (MFC) for Pharmaceutical Wastewater Treatment: An Overview and Future Perspectives. Sustainability 2022, 14, 8379. https://doi.org/10.3390/su14148379
Thapa BS, Pandit S, Patwardhan SB, Tripathi S, Mathuriya AS, Gupta PK, Lal RB, Tusher TR. Application of Microbial Fuel Cell (MFC) for Pharmaceutical Wastewater Treatment: An Overview and Future Perspectives. Sustainability. 2022; 14(14):8379. https://doi.org/10.3390/su14148379
Chicago/Turabian StyleThapa, Bhim Sen, Soumya Pandit, Sanchita Bipin Patwardhan, Sakshi Tripathi, Abhilasha Singh Mathuriya, Piyush Kumar Gupta, Ram Bharosay Lal, and Tanmoy Roy Tusher. 2022. "Application of Microbial Fuel Cell (MFC) for Pharmaceutical Wastewater Treatment: An Overview and Future Perspectives" Sustainability 14, no. 14: 8379. https://doi.org/10.3390/su14148379
APA StyleThapa, B. S., Pandit, S., Patwardhan, S. B., Tripathi, S., Mathuriya, A. S., Gupta, P. K., Lal, R. B., & Tusher, T. R. (2022). Application of Microbial Fuel Cell (MFC) for Pharmaceutical Wastewater Treatment: An Overview and Future Perspectives. Sustainability, 14(14), 8379. https://doi.org/10.3390/su14148379







