Functional Diversity in Woody Organs of Tropical Dry Forests and Implications for Restoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Selection
2.2. Wood Functional Traits
2.3. Bark Functional Traits
2.4. Statistical Analyses
3. Results
3.1. Unidimensional Approach to Functional Diversity
3.2. Multi-Dimensional Approach to Functional Diversity
4. Discussion
4.1. Patterns and Causes of High Functional Diversity in Wood and Bark of Tropical Dry Forests
4.2. Integrating the Functional Diversity of Wood, Bark, and Leaves in Tropical Dry Forests
4.3. Implications of High Functional Diversity in Tropical Dry Forests for Restoration Efforts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gann, G.D.; McDonald, T.; Walder, B.; Aronson, J.; Nelson, C.R.; Jonson, J.; Hallett, J.G.; Eisenberg, C.; Guariguata, M.R.; Liu, J.; et al. International principles and standards for the practice of ecological restoration. Second edition. Restor. Ecol. 2019, 27, S1–S46. [Google Scholar] [CrossRef] [Green Version]
- Lohbeck, M.; Lebrija-Trejos, E.; Martínez-Ramos, M.; Meave, J.A.; Poorter, L.; Bongers, F. Functional trait strategies of trees in dry and wet tropical forests are similar but differ in their consequences for succession. PLoS ONE 2015, 10, e0123741. [Google Scholar]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the holy grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef] [PubMed]
- van der Plas, F. Biodiversity and ecosystem functioning in naturally assembled communities. Biol. Rev. 2019, 94, 1220–1245. [Google Scholar] [CrossRef] [PubMed]
- Brun, P.; Violle, C.; Mouillot, D.; Mouquet, N.; Enquist, B.J.; Munoz, F.; Münkemüller, T.; Ostling, A.; Zimmermann, N.E.; Thuiller, W. Plant community impact on productivity: Trait diversity or key (stone) species effects? Ecol. Lett. 2022, 25, 913–925. [Google Scholar] [CrossRef]
- Martínez-Garza, C.; Bongers, F.; Poorter, L. Are Functional traits good predictors of species performance in restoration plantings in tropical abandoned pastures? For. Ecol. Manag. 2013, 303, 35–45. [Google Scholar] [CrossRef]
- Grime, J.P. Biodiversity and ecosystem function: The debate deepens. Science 1997, 277, 1260–1261. [Google Scholar] [CrossRef] [Green Version]
- Díaz, S.; Lavorel, S.; de Bello, F.; Quétier, F.; Grigulis, K.; Robson, T.M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef] [Green Version]
- de Bello, F.; Lavorel, S.; Díaz, S.; Harrington, R.; Cornelissen, J.H.C.; Bardgett, R.D.; Berg, M.P.; Cipriotti, P.; Feld, C.K.; Hering, D.; et al. Towards an assessment of multiple ecosystem processes and services via functional traits. Biodivers. Conserv. 2010, 19, 2873–2893. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, S.; Sanchez-Azofeifa, A.G.; Duran, S.M.; Espírito-Santo, M.M. Assessing Ecosystem Services in Neotropical Dry Forests: A systematic review. Environ. Conserv. 2017, 44, 34–43. [Google Scholar] [CrossRef]
- Maass, J.M.; Balvanera, P.; Castillo, A.; Daily, G.C.; Mooney, H.A.; Ehrlich, P.; Quesada, M.; Miranda, A.; Jaramillo, V.J.; García-Oliva, F.; et al. Ecosystem services of tropical dry forests: Insights from long-term ecological and social research on the pacific coast of Mexico. Ecol. Soc. 2005, 10, 17. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Raghubanshi, A.S.; Singh, J.S. Plant functional traits with particular reference to tropical deciduous forests: A review. J. Biosci. 2011, 36, 963–981. [Google Scholar] [CrossRef] [PubMed]
- Hulshof, C.M.; Martínez-Yrízar, A.; Burquez, A.; Boyle, B.; Enquist, B.J. Plant functional trait variation in tropical dry forests: A review and synthesis. In Tropical Dry Forests in the Americas: Ecology, Conservation, and Management; Sánchez-Azofeifa, G.A., Powers, J.S., Fernandes, G.W., Eds.; Taylor & Francis Group: Abingdon, UK, 2014; ISBN 978-1-4665-1200-9. [Google Scholar]
- Díaz, S.; Cabido, M. Vive la difference: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Tilman, D. Functional diversity. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Elsevier: New York, NY, USA, 2001; pp. 109–120. ISBN 978-0-12-226865-6. [Google Scholar]
- Laureto, L.M.O.; Cianciaruso, M.V.; Samia, D.S.M. Functional diversity: An overview of its history and applicability. Nat. Conserv. 2015, 13, 112–116. [Google Scholar] [CrossRef] [Green Version]
- de Bello, F.; Carmona, C.P.; Dias, A.T.; Götzenberger, L.; Moretti, M.; Berg, M.P. Handbook of Trait-Based Ecology: From Theory to R Tools; Cambridge University Press: Cambridge, UK, 2021; ISBN 1-108-61301-2. [Google Scholar]
- Trejo, I.; Dirzo, R. Floristic diversity of mexican seasonally dry tropical forests. Biodivers. Conserv. 2002, 11, 2063–2084. [Google Scholar] [CrossRef]
- Pennington, R.T.; Lavin, M.; Oliveira-Filho, A. Woody Plant Diversity, Evolution, and Ecology in the Tropics: Perspectives from Seasonally Dry Tropical Forests. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 437–457. [Google Scholar] [CrossRef] [Green Version]
- Banda-R, K.; Delgado-Salinas, A.; Dexter, K.G.; Linares-Palomino, R.; Oliveira-Filho, A.; Prado, D.; Pullan, M.; Quintana, C.; Riina, R.; Rodríguez, M.G.M.; et al. Plant diversity patterns in neotropical dry forests and their conservation implications. Science 2016, 353, 1383–1387. [Google Scholar] [CrossRef] [Green Version]
- Huante, P.; Rincon, E.; Acosta, I. Nutrient availability and growth rate of 34 woody species from a tropical deciduous forest in Mexico. Funct. Ecol. 1995, 9, 849–858. [Google Scholar] [CrossRef]
- Medina, E. Diversity of life forms of higher plants in neotropical dry forests. In Seasonally Dry Tropical Forests; Cambridge University Press: Cambridge, UK, 1995; pp. 221–242. [Google Scholar]
- Lohbeck, M.; Poorter, L.; Lebrija-Trejos, E.; Martínez-Ramos, M.; Meave, J.A.; Paz, H.; Pérez-García, E.A.; Romero-Pérez, I.E.; Tauro, A.; Bongers, F. Successional changes in functional composition contrast for dry and wet tropical forest. Ecology 2013, 94, 1211–1216. [Google Scholar] [CrossRef]
- Sanaphre-Villanueva, L.; Dupuy, J.M.; Andrade, J.L.; Reyes-García, C.; Jackson, P.C.; Paz, H. Patterns of plant functional variation and specialization along secondary succession and topography in a tropical dry forest. Environ. Res. Lett. 2017, 12, 055004. [Google Scholar] [CrossRef]
- Borchert, R. Soil and stem water storage determine phenology and distribution of tropical dry forest Trees. Ecology 1994, 75, 1437–1449. [Google Scholar] [CrossRef]
- Medeiros, C.D.; Scoffoni, C.; John, G.P.; Bartlett, M.K.; Inman-Narahari, F.; Ostertag, R.; Cordell, S.; Giardina, C.; Sack, L. An extensive suite of functional traits distinguishes Hawaiian wet and dry forests and enables prediction of species vital rates. Funct. Ecol. 2019, 33, 712–734. [Google Scholar] [CrossRef]
- Reich, P.B.; Luo, Y.; Bradford, J.B.; Poorter, H.; Perry, C.H.; Oleksyn, J. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl. Acad. Sci. USA 2014, 111, 13721–13726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janzen, D.H. Tropical Dry Forests: The most endangered major tropical ecosystem. In Biodiversity; Wilson, E.O., Ed.; National Academy Press: Washington, DC, USA, 1988; pp. 130–137. [Google Scholar]
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Kapos, V.; Gordon, J.E. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar] [CrossRef]
- Dimson, M.; Gillespie, T.W. Trends in active restoration of tropical dry forest: Methods, metrics, and outcomes. For. Ecol. Manag. 2020, 467, 118150. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L.; Paz, H.; Sack, L.; Bongers, F. Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant Cell Environ. 2011, 34, 137–148. [Google Scholar] [CrossRef]
- Méndez-Alonzo, R.; Pineda-García, F.; Paz, H.; Rosell, J.A.; Olson, M.E. Leaf phenology is associated with soil water availability and xylem traits in a tropical dry forest. Trees 2013, 27, 745–754. [Google Scholar] [CrossRef]
- Méndez-Alonzo, R.; Paz, H.; Zuluaga, R.C.; Rosell, J.A.; Olson, M.E. Coordinated evolution of leaf and stem economics in tropical dry forest trees. Ecology 2012, 93, 2397–2406. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From tropics to tundra: Global convergence in plant functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef] [Green Version]
- Olson, M.E.; Rosell, J.A.; Zamora Muñoz, S.; Castorena, M. Carbon limitation, stem growth rate and the biomechanical cause of corner’s rules. Ann. Bot. 2018, 122, 583–592. [Google Scholar] [CrossRef]
- Balvanera, P.; Quijas, S.; Pérez-Jiménez, A. Distribution patterns of tropical dry forest trees along a mesoscale water availability gradient. Biotropica 2011, 43, 414–422. [Google Scholar] [CrossRef]
- Powers, J.S.; Becknell, J.M.; Irving, J.; Pèrez-Aviles, D. Diversity and structure of regenerating tropical dry forests in Costa Rica: Geographic patterns and environmental drivers. For. Ecol. Manag. 2009, 258, 959–970. [Google Scholar] [CrossRef]
- Markesteijn, L.; Iraipi, J.; Bongers, F.; Poorter, L. Seasonal variation in soil and plant water potentials in a bolivian tropical moist and dry forest. J. Trop. Ecol. 2010, 26, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Chave, J.; Muller-Landau, H.C.; Baker, T.R.; Easdale, T.A.; ter Steege, H.; Webb, C.O. Regional and phylogenetic variation of wood density across 2456 neotropical tree species. Ecol. Appl. 2006, 16, 2356–2367. [Google Scholar] [CrossRef] [Green Version]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef]
- Vargas, G.; Brodribb, T.J.; Dupuy, J.M.; González-M, R.; Hulshof, C.M.; Medvigy, D.; Allerton, T.A.; Pizano, C.; Salgado-Negret, B.; Schwartz, N.B. Beyond leaf habit: Generalities in plant function across 97 tropical dry forest tree species. New Phytol. 2021, 232, 148–161. [Google Scholar] [CrossRef]
- Olson, M.E.; Soriano, D.; Rosell, J.A.; Anfodillo, T.; Donoghue, M.J.; Edwards, E.J.; León-Gómez, C.; Dawson, T.; Camarero Martínez, J.J.; Castorena, M.; et al. Plant height and hydraulic vulnerability to drought and cold. Proc. Natl. Acad. Sci. USA 2018, 115, 201721728. [Google Scholar] [CrossRef] [Green Version]
- Anfodillo, T.; Carraro, V.; Carrer, M.; Fior, C.; Rossi, S. Convergent tapering of xylem conduits in different woody species. New Phytol. 2006, 169, 279–290. [Google Scholar] [CrossRef]
- Rosell, J.A.; Olson, M.E.; Anfodillo, T. Scaling of xylem vessel diameter with plant size: Causes, predictions, and outstanding questions. Curr. For. Rep. 2017, 3, 46–59. [Google Scholar] [CrossRef]
- Olson, M.; Rosell, J.A.; Martínez-Pérez, C.; León-Gómez, C.; Fajardo, A.; Isnard, S.; Cervantes-Alcayde, M.A.; Echeverría, A.; Figueroa-Abundiz, V.A.; Segovia-Rivas, A. Xylem Vessel-diameter–Shoot-length Scaling: Ecological Significance of Porosity Types and Other Traits. Ecol. Monogr. 2020, 90, e01410. [Google Scholar] [CrossRef]
- Anfodillo, T.; Olson, M.E. Tree Mortality: Testing the link between drought, embolism vulnerability, and xylem conduit diameter remains a priority. Front. For. Glob. Chang. 2021, 4, 704670. [Google Scholar] [CrossRef]
- Rosell, J.A. Bark in woody plants: Understanding the diversity of a multifunctional structure. Integr. Comp. Biol. 2019, 59, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Roth, I. Structural Patterns of Tropical Barks; Encyclopedia of Plant Anatomy; Gebruder Borntraeger: Berlin, Germany, 1981; Volume IX, Part 3. [Google Scholar]
- Poorter, L.; McNeil, A.; Hurtado, V.H.; Prins, H.; Putz, J. Bark traits and life history strategies of tropical dry- and moist forest trees. Funct. Ecol. 2014, 28, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Rosell, J.A. Bark Thickness across the Angiosperms: More than just fire. New Phytol. 2016, 211, 90–102. [Google Scholar] [CrossRef]
- Jackson, J.F.; Adams, D.C.; Jackson, U.B. Allometry of constitutive defense: A model and a comparative test with tree bark and fire regime. Am. Nat. 1999, 153, 614–632. [Google Scholar] [CrossRef]
- Rosell, J.A.; Olson, M.E. The evolution of bark mechanics and storage across habitats in a clade of tropical trees. Am. J. Bot. 2014, 101, 764–777. [Google Scholar] [CrossRef] [Green Version]
- Loram-Lourenço, L.; dos Santos Farnese, F.; de Sousa, L.F.; Alves, R.D.F.B.; de Andrade, M.C.P.; da Silva Almeida, S.E.; de Freitas Moura, L.M.; Costa, A.C.; Silva, F.G.; Galmés, J.; et al. A structure shaped by fire, but also water: Ecological consequences of the variability in bark properties across 31 species from the Brazilian Cerrado. Front. Plant Sci. 2020, 10, 1718. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.; Aakala, T.; Abedi, M. TRY Plant trait database–Enhanced coverage and open access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef] [Green Version]
- Lott, E.J.; Atkinson, T.H. Mexican and Central American Seasonally Dry Tropical Forests: Chamela-Cuixmala, Jalisco, as a Focal Point for Comparison. In Neotropical Savannas and Seasonally Dry Forests; CRC Press: Boca Raton, FL, USA, 2006; ISBN 978-0-429-12425-9. [Google Scholar]
- Fernandes, M.F.; Cardoso, D.; de Queiroz, L.P. An Updated plant checklist of the brazilian caatinga seasonally dry forests and woodlands reveals high species richness and endemism. J. Arid Environ. 2020, 174, 104079. [Google Scholar] [CrossRef]
- Williamson, G.B.; Wiemann, M.C. Measuring wood specific gravity… correctly. Am. J. Bot. 2010, 97, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Kerkhoff, A.J.; Enquist, B.J. Multiplicative by nature: Why logarithmic transformation is necessary in allometry. J. Theor. Biol. 2009, 257, 519–521. [Google Scholar] [CrossRef]
- Vogel, S. Life in Moving Fluids: The Physical Biology of Flow, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 1994; ISBN 0-691-02616-5. [Google Scholar]
- Cornwell, W.K.; Schwilk, D.W.; Ackerly, D.D. A Trait-based test for habitat filtering: Convex hull volume. Ecology 2006, 87, 1465–1471. [Google Scholar] [CrossRef]
- Blonder, B.; Lamanna, C.; Violle, C.; Enquist, B.J. The N-dimensional hypervolume. Glob. Ecol. Biogeogr. 2014, 23, 595–609. [Google Scholar] [CrossRef]
- Blonder, B. Hypervolume concepts in niche-and trait-based ecology. Ecography 2018, 41, 1441–1455. [Google Scholar] [CrossRef]
- Swenson, N.G. Functional and Phylogenetic Ecology in R; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 1-4614-9541-5. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; v. 3.5.9; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Carlquist, S. Wood and Bark Anatomy of Caricaceae; Correlations with systematics and habit. IAWA J. 1998, 19, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Santiago, L.S.; Silvera, K.; Andrade, J.L.; Dawson, T.E. Functional strategies of tropical dry forest plants in relation to growth form and isotopic composition. Environ. Res. Lett. 2017, 12, 115006. [Google Scholar] [CrossRef] [Green Version]
- Swenson, N.G.; Enquist, B.J.; Pither, J.; Kerkhoff, A.J.; Boyle, B.; Weiser, M.D.; Elser, J.J.; Fagan, W.F.; Forero-Montaña, J.; Fyllas, N.; et al. The biogeography and filtering of woody plant functional diversity in north and south america. Glob. Ecol. Biogeogr. 2012, 21, 798–808. [Google Scholar] [CrossRef]
- Scholz, F.G.; Bucci, S.J.; Goldstein, G.; Meinzer, F.C.; Franco, A.C.; Miralles-Wilhelm, F. Biophysical properties and functional significance of stem water storage tissues in neotropical savanna Trees. Plant Cell Environ. 2007, 30, 236–248. [Google Scholar] [CrossRef]
- Ogburn, R.M.; Edwards, E.J. The ecological water-use strategies of succulent plants. In Advances in Botanical Research; Kader, J.C., Delseny, M., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 55, pp. 179–225. ISBN 0065-2296 978-0-12-380868-4. [Google Scholar]
- Martínez-Vilalta, J.; Pockman, W.T. The vulnerability to freezing-induced xylem cavitation of Larrea Tridentata (Zygophyllaceae) in the Chihuahuan Desert. Am. J. Bot. 2002, 89, 1916–1924. [Google Scholar] [CrossRef] [Green Version]
- Ball, M.C.; Canny, M.J.; Huang, C.X.; Egerton, J.J.G.; Wolfe, J. Freeze/Thaw-induced embolism depends on nadir temperature: The heterogeneous hydration hypothesis. Plant Cell Environ. 2006, 29, 729–745. [Google Scholar] [CrossRef]
- Sevanto, S.; Holbrook, N.M.; Ball, M.C. Freeze/thaw-induced embolism: Probability of critical bubble formation depends on speed of ice formation. Front. Plant Sci. 2012, 3, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineda-García, F.; Paz, H.; Meinzer, F.C. Drought Resistance in early and late secondary successional species from a tropical dry forest: The interplay between xylem resistance to embolism, sapwood water storage and leaf shedding. Plant Cell Environ. 2013, 36, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Pineda-García, F.; Paz, H.; Meinzer, F.C.; Angeles, G. Exploiting water versus tolerating drought: Water-use strategies of trees in a secondary successional tropical dry forest. Tree Physiol. 2016, 36, 208–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawes, M.J.; Hylton, A.; Russell-Smith, J.; Murphy, B.; Midgley, J.J. How Do Small Savanna Trees Avoid Stem Mortality by Fire? The roles of stem diameter, height and bark thickness. Ecosphere 2011, 2, 1–13. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Geiger, E.L.; Gotsch, S.G.; Rossatto, D.R.; Silva, L.C.R.; Lau, O.L.; Haridasan, M.; Franco, A.C. Ecological thresholds at the savanna-forest boundary: How plant traits, resources and fire govern the distribution of tropical biomes. Ecol. Lett. 2012, 15, 759–768. [Google Scholar] [CrossRef]
- Romero, C. Bark Structure and Functional Ecology. In Bark: Use, Management, and Commerce in Africa; Cunningham, A.B., Campbell, B.M., Luckert, M.K., Eds.; Advances in Economic Botany; The New York Botanical Garden Press: New York, NY, USA, 2014; Volume 17, pp. 5–25. [Google Scholar]
- Graves, S.J.; Rifai, S.W.; Putz, F.E. Outer bark thickness decreases more with height on stems of fire-resistant than fire-sensitive Floridian oaks (Quercus Spp.; Fagaceae). Am. J. Bot. 2014, 101, 2183–2188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosell, J.A.; Castorena, M.; Laws, C.; Westoby, M. Bark Ecology of Twigs vs. Main Stems: Functional traits across 85 species of Angiosperms. Oecologia 2015, 178, 1033–1043. [Google Scholar] [CrossRef]
- Dexter, K.G.; Pennington, R.T.; Oliveira-Filho, A.T.; Bueno, M.L.; Silva de Miranda, P.L.; Neves, D.M. Inserting tropical dry forests into the discussion on biome transitions in the tropics. Front. Ecol. Evol. 2018, 6, 123. [Google Scholar] [CrossRef] [Green Version]
- Edwards, E.J.; Chatelet, D.S.; Chen, B.-C.; Ong, J.Y.; Tagane, S.; Kanemitsu, H.; Tagawa, K.; Teramoto, K.; Park, B.; Chung, K.-F.; et al. Convergence, consilience, and the evolution of temperate deciduous forests. Am. Nat. 2017, 190, S87–S104. [Google Scholar] [CrossRef] [Green Version]
- Edwards, E.J.; Chatelet, D.S.; Sack, L.; Donoghue, M.J. Leaf life span and the leaf economic spectrum in the context of whole plant architecture. J. Ecol. 2014, 102, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J. Resprouting as a key functional trait: How buds, protection and resources drive persistence after fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villar, R.; Merino, J. Comparison of leaf construction costs in woody species with differing leaf life-spans in contrasting ecosystems. New Phytol. 2001, 151, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Markesteijn, L.; Poorter, L.; Bongers, F. Light-dependent leaf trait variation in 43 tropical dry forest tree species. Am. J. Bot. 2007, 94, 515–525. [Google Scholar] [CrossRef] [Green Version]
- Gotsch, S.G.; Powers, J.S.; Lerdau, M.T. Leaf traits and water relations of 12 evergreen species in Costa Rican wet and dry forests: Patterns of intra-specific variation across forests and seasons. Plant Ecol. 2010, 211, 133–146. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Rosell, J.A.; Gleason, S.; Méndez-Alonzo, R.; Chang, Y.; Westoby, M. Bark Functional Ecology: Evidence for tradeoffs, functional coordination, and environment producing bark diversity. New Phytol. 2014, 201, 486–497. [Google Scholar] [CrossRef]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of degraded tropical forest landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef] [Green Version]
- Laughlin, D.C. Applying trait-based models to achieve functional targets for theory-driven ecological restoration. Ecol. Lett. 2014, 17, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Laughlin, D.C.; Chalmandrier, L.; Joshi, C.; Renton, M.; Dwyer, J.M.; Funk, J.L. Generating species assemblages for restoration and experimentation: A new method that can simultaneously converge on average trait values and maximize functional diversity. Methods Ecol. Evol. 2018, 9, 1764–1771. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, H.; Ziegler, W.; Kolle, O.; Trumbore, S. Thirst Beats Hunger—Declining Hydration during drought prevents carbon starvation in Norway spruce saplings. New Phytol. 2013, 200, 340–349. [Google Scholar] [CrossRef]
- Carlucci, M.B.; Brancalion, P.H.; Rodrigues, R.R.; Loyola, R.; Cianciaruso, M.V. Functional traits and ecosystem services in ecological restoration. Restor. Ecol. 2020, 28, 1372–1383. [Google Scholar] [CrossRef]
- Funk, J.L.; Cleland, E.E.; Suding, K.N.; Zavaleta, E.S. Restoration through reassembly: Plant traits and invasion resistance. Trends Ecol. Evol. 2008, 23, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, R.; Warman, L.; Cordell, S.; Vitousek, P.M. Using plant functional traits to restore Hawaiian rainforest. J. Appl. Ecol. 2015, 52, 805–809. [Google Scholar] [CrossRef]
- Zhang, H.; Ye, W.; Lian, J. Scale-dependent functional redundancy in a tropical forest. Trop. Conserv. Sci. 2019, 12, 1940082919893853. [Google Scholar] [CrossRef] [Green Version]
- Hortal, J.; de Bello, F.; Diniz-Filho, J.A.F.; Lewinsohn, T.M.; Lobo, J.M.; Ladle, R.J. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 523–549. [Google Scholar] [CrossRef] [Green Version]
- Chazdon, R.L.; Guariguata, M.R. Natural regeneration as a tool for large-scale forest restoration in the tropics: Prospects and challenges. Biotropica 2016, 48, 716–730. [Google Scholar] [CrossRef]
- Caso, M.; González-Abraham, C.; Ezcurra, E. Divergent ecological effects of oceanographic anomalies on terrestrial ecosystems of the Mexican pacific coast. Proc. Natl. Acad. Sci. USA 2007, 104, 10530–10535. [Google Scholar] [CrossRef] [Green Version]
- Fajardo, A.; McIntire, E.J.; Olson, M.E. When short stature is an asset in trees. Trends Ecol. Evol. 2019, 34, 193–199. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Strahan, R.T.; Huffman, D.W.; Sánchez Meador, A.J. Using trait-based ecology to restore resilient ecosystems: Historical conditions and the future of montane forests in western North America. Restor. Ecol. 2017, 25, S135–S146. [Google Scholar] [CrossRef]
- Hartung, M.; Carreño-Rocabado, G.; Peña-Claros, M.; van der Sande, M.T. Tropical dry forest resilience to fire depends on fire frequency and climate. Front. For. Glob. Chang. 2021, 4, 755104. [Google Scholar] [CrossRef]
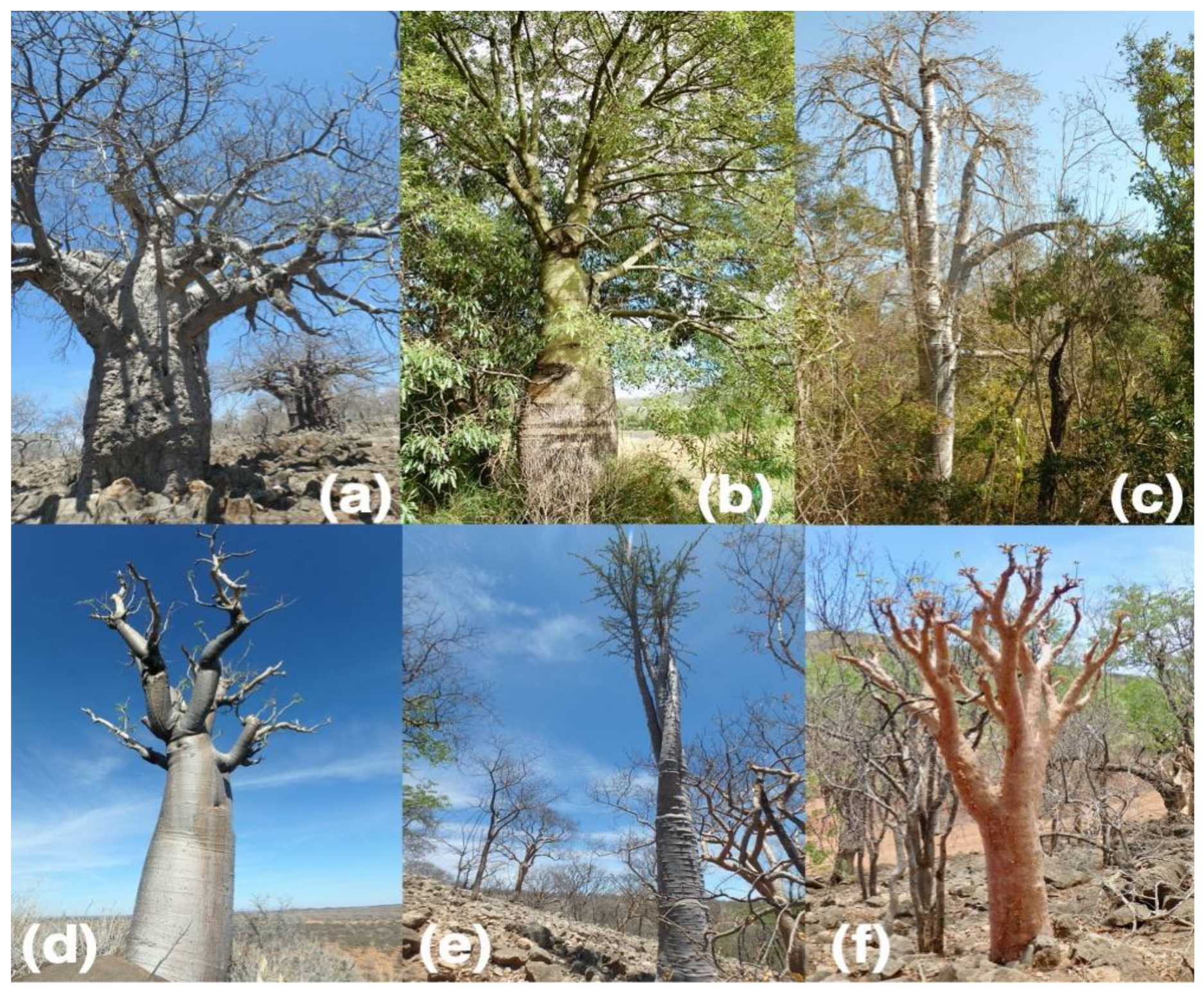
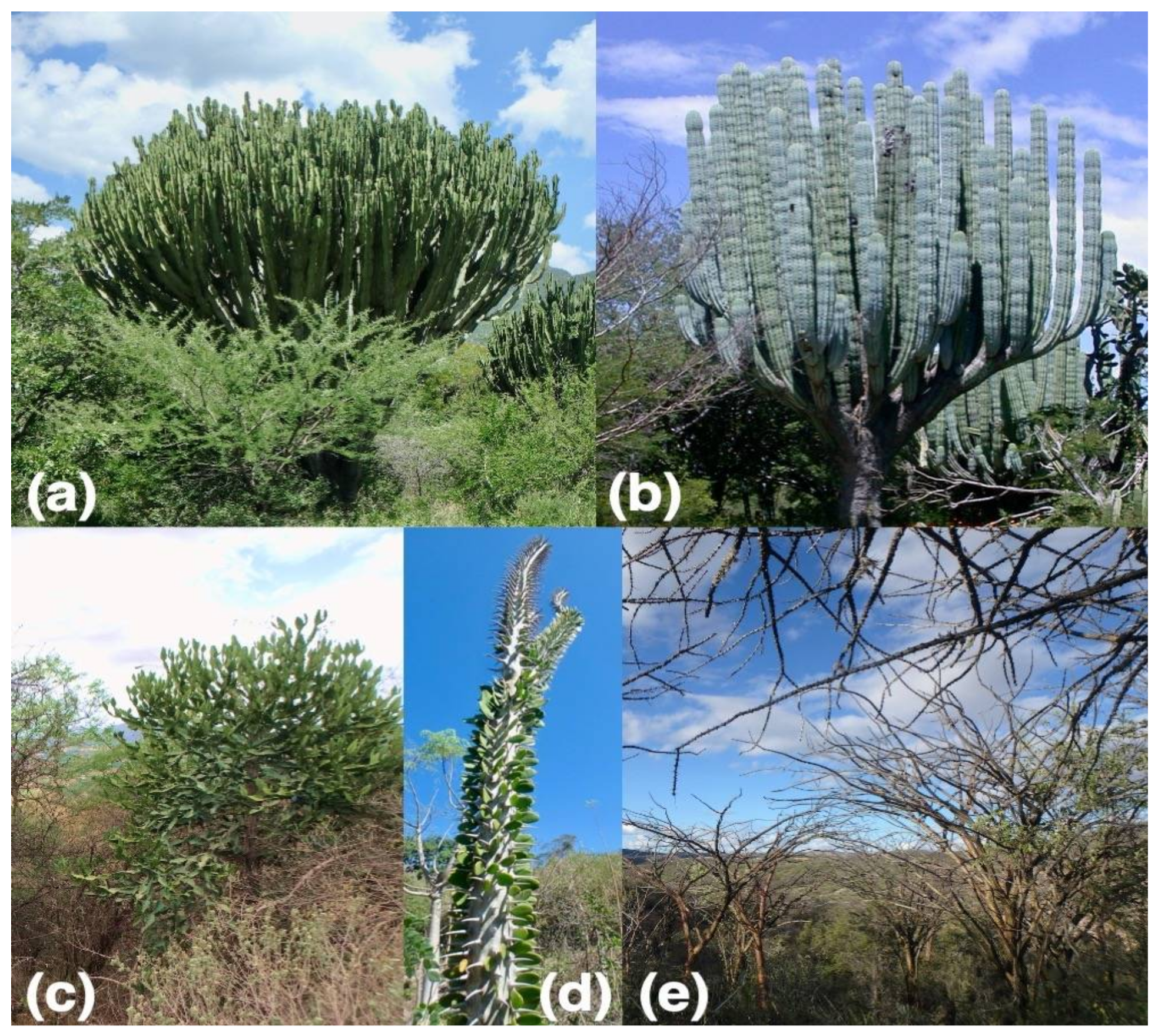
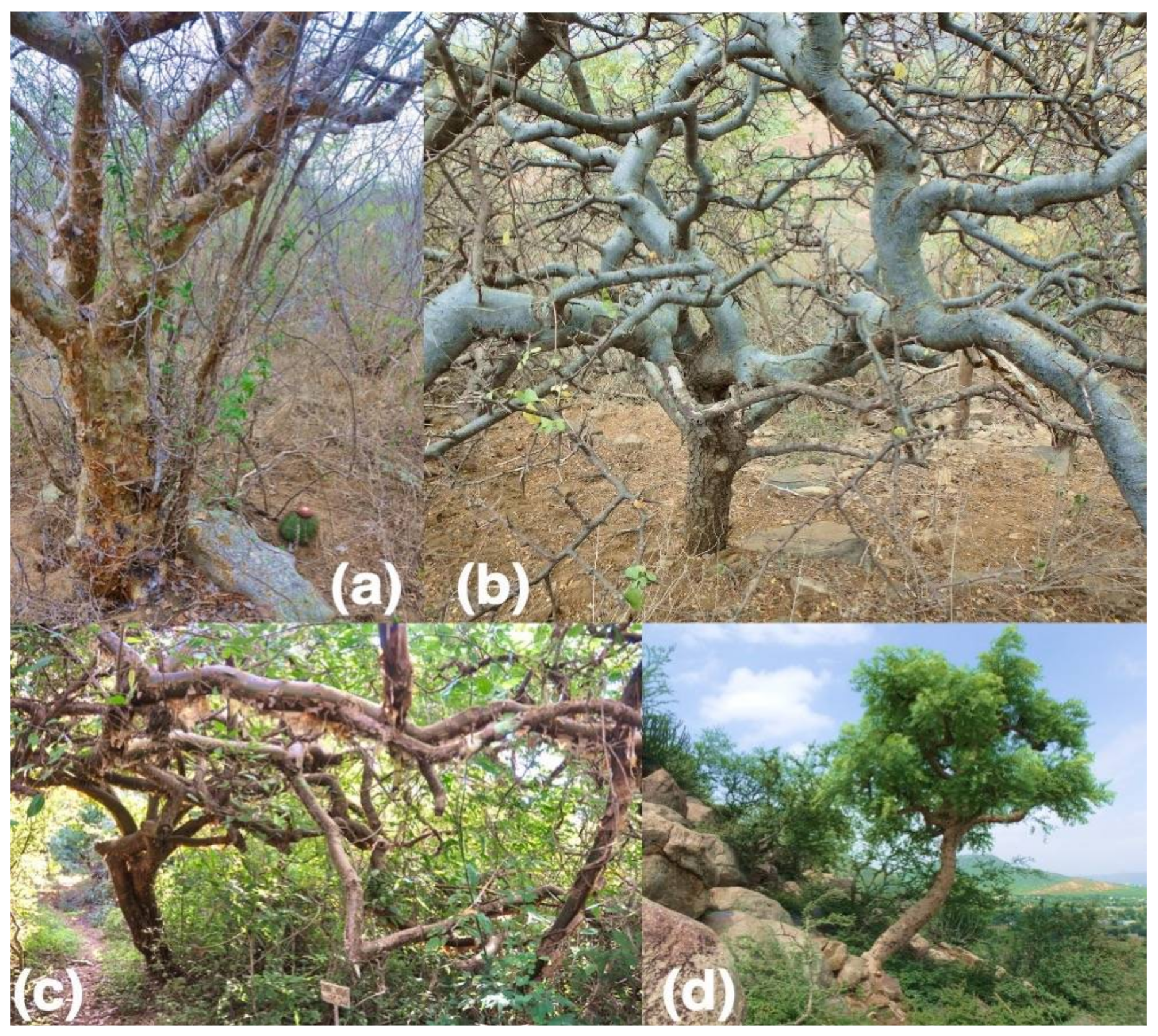

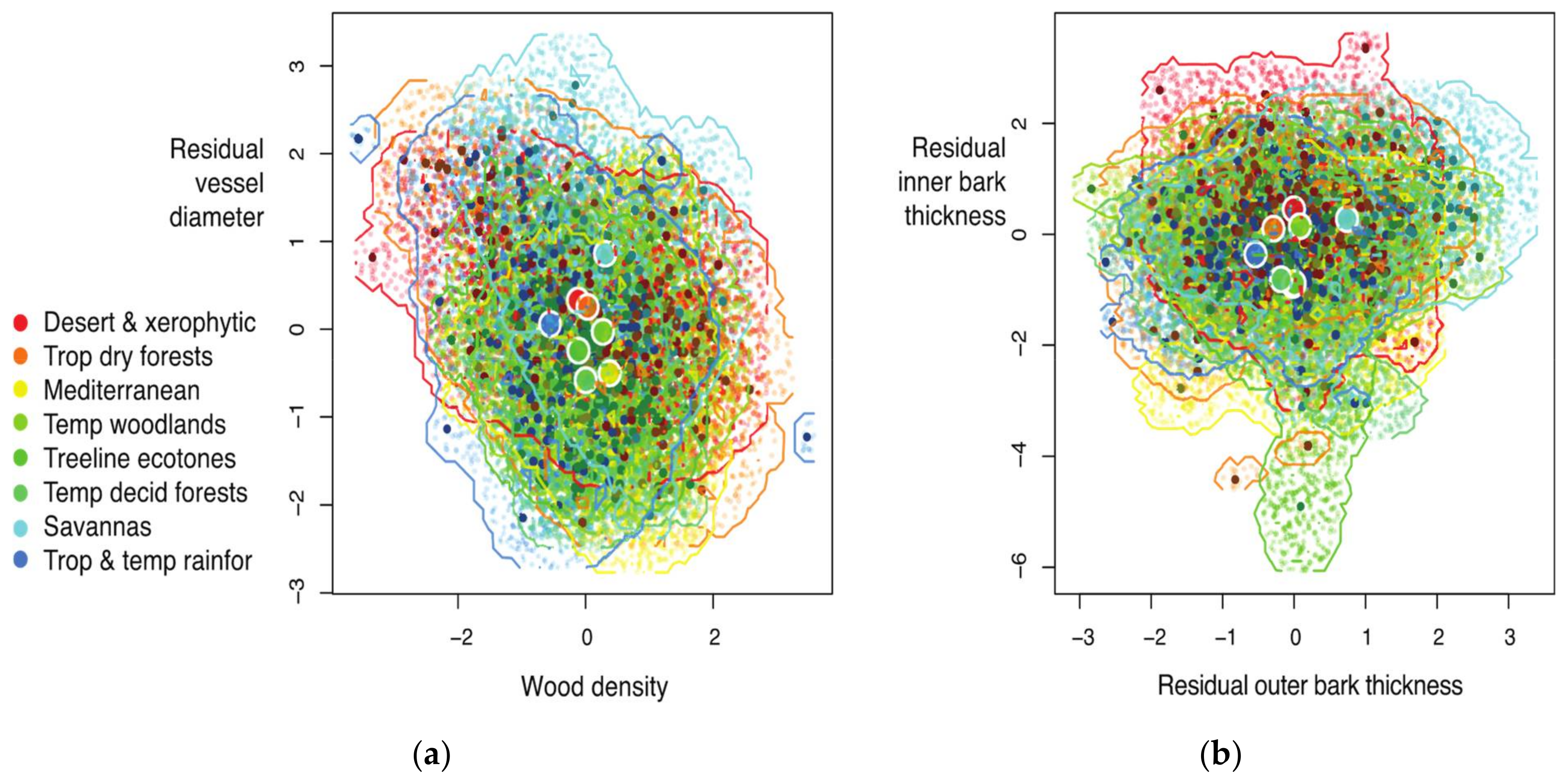

| Major Vegetation Type and Locality | Lat, lon | MAT (°C) | MAP (mm) | Species with Wood Traits | Species with Bark Traits |
|---|---|---|---|---|---|
| Deserts and xerophytic shrublands (Desert & xerophytic) | |||||
| Baja California Cape, Mexico | 23.03° N, 109.72° W | 23.8 | 213 | 40 | 43 |
| Mojave Desert, California, USA | 34.1° N, 116.6° W | 16.3 | 324 | 30 | 27 |
| Pedregal de San Ángel Reserve, Mexico | 19.31° N, 99.19° W | 14.7 | 905 | - | 18 |
| Tropical dry forests | |||||
| Fazenda Almas, Paraíba, Brazil | 7.5° S, 39.9° W | 22.3 | 580 | 40 | 41 |
| Chamela, Jalisco, Mexico | 19.5° N, 105.04° W | 26.2 | 795.7 | 33 | 57 |
| Mediterranean woodlands and shrublands (Mediterranean) | |||||
| Santa Monica Mountains, California, USA | 34.1° N, 118.7° W | 14.9 | 575 | 24 | 24 |
| Migliarino San Rossore Park, Italy | 43.8° N, 10.3° E | 14.7 | 905 | - | 7 |
| Temperate woodlands | |||||
| Bothwell, Tasmania, Australia | 42.4° S, 147° E | 7.1 | 733 | - | 12 |
| Yengo National Park, NSW, Australia | 32.8° S, 150.9° E | 16.3 | 792.3 | - | 23 |
| Sydney area, Australia | 33.8° S, 151.1° E | 16.5 | 1162.2 | 34 | 62 |
| Temperate treeline ecotones (Treeline ecotones) | |||||
| San Pedro Mártir, Baja California, Mexico | 31.0° N, 115.5° W | 7.4 | 763 | 24 | - |
| Coyhaique, Patagonia, Chile | 45.5° S, 72.0° W | 4.1 | 944 | 20 | - |
| Pyrenees highlands, Spain | 42.8° N, 0.3° W | 3.2 | 1263 | 18 | - |
| Mount Field, Tasmania, Australia | 42.7° S, 146.6° E | 4.5 | 1515 | 23 | 21 |
| Temperate deciduous forests (Temp decid forests) | |||||
| Yale Forest, Connecticut, USA | 42.0°N, 72.1° W | 8.1 | 1238 | 29 | - |
| Pyrenee foothills, Spain | 43.2° N, 1.6° E | 12.2 | 1279 | 25 | - |
| Pordenone, Italy | 46.1° N, 12.5° E | 11.7 | 1284 | - | 22 |
| Tropical savannas (Savannas) | |||||
| Botucatu cerrado, São Paulo, Brazil | 22.9° S, 48.5° W | 18.9 | 1331 | 31 | 40 |
| Howard Springs, NT, Australia | 12.5° S, 131.1° E | 27.3 | 1570 | 24 | 24 |
| Tropical and temperate rainforests (Trop & temp rainfor) | |||||
| Loja, Ecuador | 4.0° S, 79.2° W | 14.8 | 1083 | 21 | - |
| Atherton Tablelands, Australia | 17.7° S, 145.5° E | 19 | 1382 | - | 11 |
| New South Wales temperate rainforests, Australia | 34.1° S, 151.0° E | 16.3 | 1419.5 | 26 | 18 |
| Daintree, Queensland, Australia | 16.1°S, 145.45° E | 25.2 | 2081 | 29 | 35 |
| Los Tuxtlas, Veracruz, Mexico | 18.6°N, 95.1° W | 24 | 3356.5 | 41 | 52 |
| Major Vegetation Type and Locality | Stem Diameter (cm) | Plant Height (m) | Vessel Diameter (μm) | Wood Density (g cm−3) | Total Bark Thickness (mm) | Inner Bark Thickness (mm) | Outer Bark Thickness (mm) |
|---|---|---|---|---|---|---|---|
| Deserts and xerophytic shrublands (Desert & xerophytic) | |||||||
| Baja California Cape, Mexico | N = 50 7.7 (0.3, 34.6) | N = 50 2.6 (0.1, 7.7) | N = 40 49.8 (20.0, 104.2) | N = 38 0.52 (0.06, 0.83) | N = 43 5.9 (0.2, 31.4) | N = 31 6.1 (0.3, 30.6) | N = 31 0.8 (0.04, 4.8) |
| Mojave Desert, California, USA | N = 31 2.6 (0.4, 14.4) | N = 31 1.4 (0.1, 4.9) | N = 30 32.4 (13.1, 109.5) | N = 29 0.57 (0.31, 0.83) | N = 27 2.0 (0.5, 7.8) | N = 27 1.0 (0.2, 3.2) | N = 27 1.0 (0.03, 5.5) |
| Pedregal de San Ángel Reserve, Mexico | N = 18 13.2 (0.9, 32.6) | N = 18 4.9 (1.0, 19.0) | - | - | N = 18 8.2 (0.5, 22.1) | N = 17 5.7 (0.3, 15.5) | N = 17 2.3 (0.1, 9.3) |
| Tropical dry forests | |||||||
| Fazenda Almas, Paraíba, Brazil | N = 43 10.7 (0.8, 27.4) | N = 43 5.2 (0.9, 12.6) | N = 40 59.8 (19.5, 144.5) | N = 40 0.64 (0.18, 0.90) | N = 41 5.6 (0.3, 19.0) | N = 41 4.0 (0.1, 14.2) | N = 41 1.6 (0.02, 10.2) |
| Chamela, Jalisco, Mexico | N = 63 18.7 (0.1, 59.4) | N = 63 7.4 (0.2, 20.0) | N = 33 81.1 (20.3, 173.5) | N = 31 0.51 (0.21, 0.80) | N = 57 8.4 (0.3, 48.5) | N = 55 6.8 (0.6, 27.7) | N = 55 1.9 (0.1, 20.8) |
| Mediterranean woodlands and shrublands (Mediterranean) | |||||||
| Santa Monica Mountains, California, USA | N = 26 3.9 (0.4, 15.0) | N = 26 2.7 (0.6, 7.1) | N = 24 32.1 (19.8, 88.0) | N = 24 0.59 (0.30, 0.78) | N = 24 1.6 (0.3, 4.0) | N = 22 1.1 (0.2, 3.8) | N = 22 0.6 (0.1, 2.1) |
| Migliarino San Rossore Park, Italy | N = 7 5.7 (0.2, 11.9) | N = 7 2.3 (0.2, 4.8) | - | - | N = 7 2.4 (0.3, 6.2) | N = 7 1.5 (0.1, 3.7) | N = 7 0.9 (0.2, 2.6) |
| Temperate woodlands | |||||||
| Bothwell, Tasmania, Australia | N = 12 12.0 (0.4, 68.0) | N = 12 4.5 (1.1, 21.0) | - | - | N = 12 4.9 (0.4, 22.1) | N = 12 3.9 (0.3, 18.7) | N = 12 1.0 (0.03, 4.0) |
| Yengo National Park, NSW, Australia | N = 23 9.5 (0.3, 46.0) | N = 23 6.7 (0.3, 35.0) | N = 15 43.6 (15.2, 98.3) | N = 15 0.67 (0.51, 0.82) | N = 23 8.9 (0.4, 52.9) | N = 23 4.2 (0.1, 29.9) | N = 23 4.7 (0.1, 41.3) |
| Sydney area, Australia | N = 63 7.2 (0.2, 119.4) | N = 63 3.5 (0.2, 31.3) | N = 25 47.9 (11.2, 143.6) | N = 25 0.54 (0.31, 0.69) | N = 62 2.7 (0.2, 35.2) | N = 57 1.8 (0.2, 17.2) | N = 57 1.1 (0.05, 22.3) |
| Temperate treeline ecotones (Treeline ecotones) | |||||||
| San Pedro Mártir, Baja California, Mexico | N = 24 3.1 (0.5, 12.4) | N = 24 1.7 (0.1, 11.2) | N = 24 27.0 (11.8, 58.9) | N = 24 0.51 (0.24, 0.72) | - | - | - |
| Coyhaique, Patagonia, Chile | N = 20 2.4 (0.7, 7.3) | N = 20 1.7 (0.7, 7.7) | N = 20 26.1 (17.1, 39.6) | N = 20 0.54 (0.38, 0.69) | - | - | - |
| Pyrenees highlands, Spain | N = 17 5.7 (0.4, 16.8) | N = 17 3.1 (0.4, 7.3) | N = 17 37.4 (17.3, 88.6) | N = 17 0.49 (0.31, 0.63) | - | - | - |
| Mount Field, Tasmania, Australia | N = 23 3.9 (0.4, 33.0) | N = 23 1.8 (0.3, 13.7) | N = 23 29.4 (14.1, 118.0) | N = 21 0.60 (0.40, 0.83) | N = 21 1.8 (0.4, 10.6) | N = 19 1.5 (0.1, 9.8) | N = 19 0.4 (0.1, 1.5) |
| Temperate deciduous forest (Temp decid forests) | |||||||
| Yale Forest, Connecticut, USA | N = 29 17.4 (0.4, 60.6) | N = 29 10.1 (0.7, 30.2) | N = 29 51.2 (14.5, 136.2) | N = 29 0.52 (0.29, 0.71) | - | - | - |
| Pyrenee foothills, Spain | N = 21 11.2 (0.3, 47.3) | N = 21 5.9 (0.7, 18.0) | N = 21 45.6 (16.9, 106.5) | N = 21 0.56 (0.37, 0.77) | - | - | - |
| Pordenone, Italy | N = 22 6.4 (0.4, 16.9) | N = 22 5.6 (0.6, 10.0) | N = 5 35.0 (21.1, 47.7) | N = 5 0.57 (0.50, 0.70) | N = 22 2.4 (0.3, 11.0) | N = 22 1.6 (0.1, 5.2) | N = 22 0.8 (0.1, 5.8) |
| Tropical savannas (Savannas) | |||||||
| Botucatu cerrado, São Paulo, Brazil | N = 46 9.5 (0.8, 39.8) | N = 46 3.2 (0.8, 7.3) | N = 31 62.0 (14.1, 122.8) | N = 31 0.53 (0.32, 0.68) | N = 40 11.6 (0.4, 53.8) | N = 40 5.2 (0.2, 17.0) | N = 40 6.4 (0.04, 38.9) |
| Howard Springs, NT, Australia | N = 24 12.7 (0.3, 35.5) | N = 24 6.7 (0.1, 15.6) | N = 24 86.9 (20.1, 158.1) | N = 24 0.64 (0.32, 0.84) | N = 24 10.9 (0.3, 26.6) | N = 19 6.4 (0.5, 11.7) | N = 19 7.0 (0.3, 19.8) |
| Tropical and temperate rainforests (Trop & temp rainfor) | |||||||
| Loja, Ecuador | N = 21 2.7 (0.6, 12.7) | N = 21 1.2 (0.6, 2.9) | N = 21 23.6 (12.1, 41.2) | N = 20 0.48 (0.40, 0.60) | - | - | - |
| Atherton Tablelands, Australia | N = 11 10.2 (1.9, 22.9) | N = 11 5.7 (2.0, 12.0) | - | - | N = 11 3.6 (0.7, 9.9) | N = 11 3.2 (0.3, 9.7) | N = 11 0.5 (0.1, 1.7) |
| New South Wales temperate rainforests, Australia | N = 22 20.2 (1.3, 63.2) | N = 22 10.9 (1.3, 25.3) | N = 20 65.0 (29.6, 198.7) | N = 20 0.47 (0.18, 0.70) | N = 18 4.9 (0.4, 13.0) | N = 18 3.5 (0.3, 8.6) | N = 18 1.5 (0.1, 6.7) |
| Daintree, Queensland, Australia | N = 35 21.3 (0.7, 72.6) | N = 35 10.9 (0.5, 30.8) | N = 29 85.4 (20.2, 210.4) | N = 29 0.53 (0.33, 0.71) | N = 35 5.3 (0.6, 26.8) | N = 35 4.6 (0.4, 24.3) | N = 35 0.7 (0.1, 3.0) |
| Los Tuxtlas, Veracruz, Mexico | N = 55 36.4 (1.2, 130.2) | N = 55 15.0 (0.9, 32.5) | N = 41 103.2 (24.1, 244.3) | N = 41 0.45 (0.03, 1.03) | N = 52 8.0 (0.9, 21.1) | N = 50 6.8 (0.7, 17.1) | N = 50 1.3 (0.1, 8.6) |
| Vegetation Types | N | Median | Minimum | Maximum | Range | Variance |
|---|---|---|---|---|---|---|
| Residuals vessel diameter ~ height (total N = 512) | ||||||
| Desert & xerophytic | 70 | 0.040 | −0.244 | 0.508 | 0.752 | 0.019 |
| Trop dry forests | 73 | 0.010 | −0.371 | 0.363 | 0.734 | 0.031 |
| Mediterranean | 24 | −0.116 | −0.313 | 0.245 | 0.558 | 0.018 |
| Temp woodlands | 40 | −0.039 | −0.324 | 0.260 | 0.583 | 0.018 |
| Treeline ecotones | 84 | −0.068 | −0.306 | 0.297 | 0.603 | 0.013 |
| Temp decid forests | 55 | −0.139 | −0.322 | 0.218 | 0.540 | 0.016 |
| Savannas | 55 | 0.149 | −0.356 | 0.462 | 0.819 | 0.025 |
| Trop & temp rainfor | 111 | −0.006 | −0.361 | 0.360 | 0.721 | 0.028 |
| Wood density (g/cm3) (total N = 504) | ||||||
| Desert & xerophytic | 67 | 0.58 | 0.06 | 0.83 | 0.77 | 0.032 |
| Trop dry forests | 71 | 0.64 | 0.18 | 0.90 | 0.72 | 0.034 |
| Mediterranean | 24 | 0.60 | 0.30 | 0.78 | 0.48 | 0.011 |
| Temp woodlands | 40 | 0.59 | 0.31 | 0.82 | 0.51 | 0.015 |
| Treeline ecotones | 82 | 0.55 | 0.24 | 0.83 | 0.59 | 0.013 |
| Temp decid forests | 55 | 0.54 | 0.29 | 0.77 | 0.48 | 0.007 |
| Savannas | 55 | 0.58 | 0.32 | 0.84 | 0.53 | 0.016 |
| Trop & temp rainfor | 110 | 0.46 | 0.03 | 1.03 | 1.00 | 0.017 |
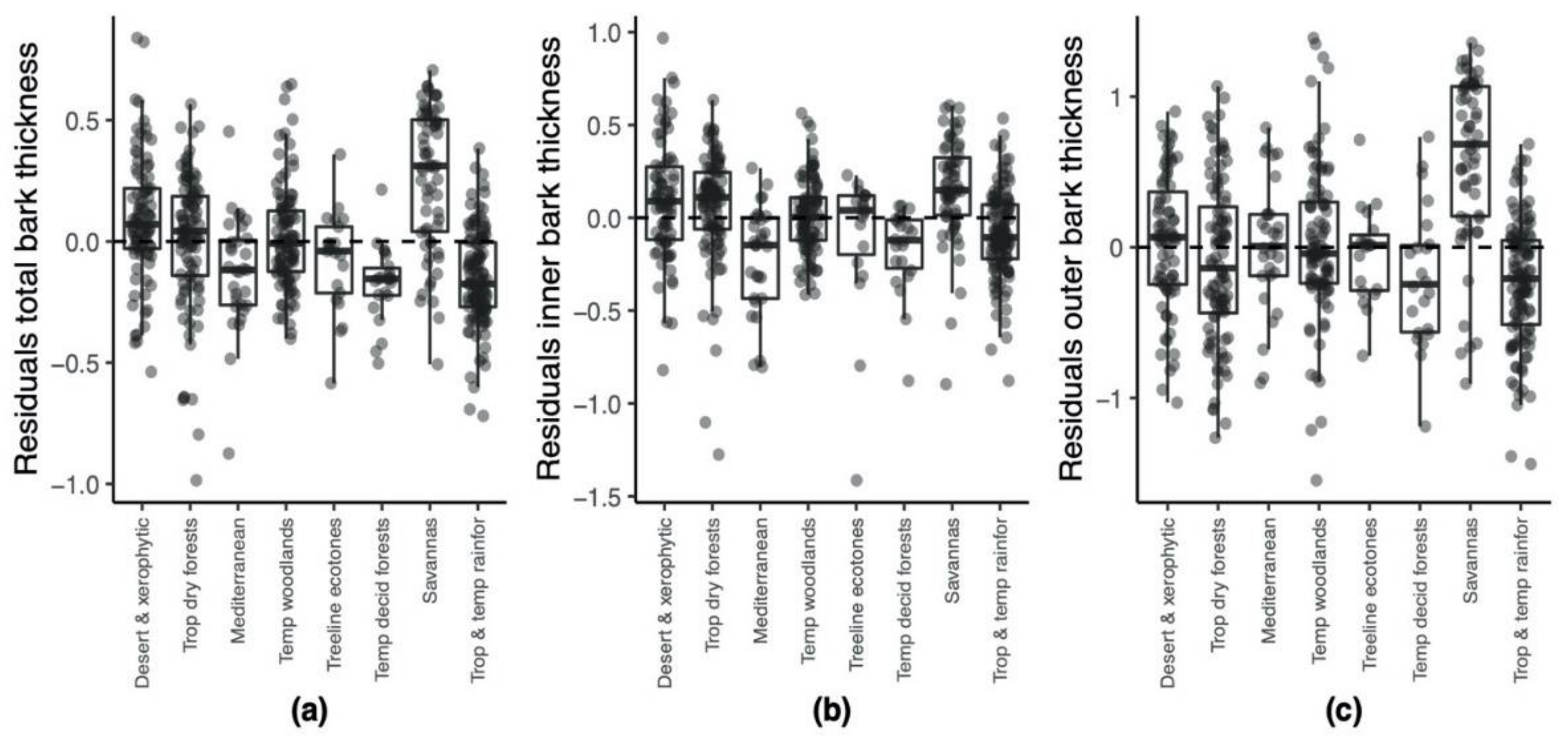
| Residuals total bark thickness ~ stem diameter (total N = 537) | ||||||
| Desert & xerophytic | 88 | 0.070 | −0.538 | 0.839 | 1.377 | 0.067 |
| Trop dry forests | 98 | 0.043 | −0.985 | 0.565 | 1.550 | 0.078 |
| Mediterranean | 31 | −0.117 | −0.874 | 0.453 | 1.328 | 0.055 |
| Temp woodlands | 97 | −0.009 | −0.401 | 0.648 | 1.050 | 0.047 |
| Treeline ecotones | 21 | −0.040 | −0.585 | 0.359 | 0.944 | 0.044 |
| Temp decid forests | 22 | −0.154 | −0.503 | 0.214 | 0.716 | 0.025 |
| Savannas | 64 | 0.311 | −0.507 | 0.706 | 1.213 | 0.085 |
| Trop & temp rainfor | 116 | −0.175 | −0.719 | 0.383 | 1.103 | 0.039 |
| Residuals inner bark thickness ~ stem diameter (total N = 506) | ||||||
| Desert & xerophytic | 75 | 0.089 | −0.821 | 0.968 | 1.789 | 0.103 |
| Trop dry forests | 96 | 0.111 | −1.275 | 0.634 | 1.909 | 0.090 |
| Mediterranean | 29 | −0.147 | −0.804 | 0.268 | 1.071 | 0.083 |
| Temp woodlands | 92 | 0.003 | −0.414 | 0.563 | 0.977 | 0.042 |
| Treeline ecotones | 19 | 0.040 | −1.414 | 0.229 | 1.643 | 0.160 |
| Temp decid forests | 22 | −0.121 | −0.878 | 0.064 | 0.942 | 0.051 |
| Savannas | 59 | 0.149 | −0.896 | 0.605 | 1.501 | 0.077 |
| Trop & temp rainfor | 114 | −0.106 | −0.878 | 0.536 | 1.414 | 0.056 |
| Residuals outer bark thickness ~ stem diameter (total N = 506) | ||||||
| Desert & xerophytic | 75 | 0.067 | −1.031 | 0.901 | 1.932 | 0.201 |
| Trop dry forests | 96 | −0.138 | −1.264 | 1.068 | 2.332 | 0.289 |
| Mediterranean | 29 | 0.008 | −0.901 | 0.793 | 1.694 | 0.192 |
| Temp woodlands | 92 | −0.042 | −1.546 | 1.389 | 2.935 | 0.268 |
| Treeline ecotones | 19 | 0.014 | −0.720 | 0.713 | 1.433 | 0.102 |
| Temp decid forests | 22 | −0.246 | −1.189 | 0.733 | 1.922 | 0.215 |
| Savannas | 59 | 0.684 | −0.905 | 1.356 | 2.262 | 0.309 |
| Trop & temp rainfor | 114 | −0.208 | −1.438 | 0.685 | 2.123 | 0.168 |
| Vegetation Type | Wood Traits (Vessel Diameter and Density) | Bark Traits (Inner and Outer Bark Thickness) | Wood and Bark Traits | |||
|---|---|---|---|---|---|---|
| N | Hypervolume | N | Hypervolume | N | Hypervolume | |
| Desert & xerophytic | 67 | 19.09 (14.65, 21.45) | 75 | 20.65 (14.99, 23.44) | 49 | 314.08 (164.40, 342.38) |
| Trop dry forests | 71 | 24.15 (19.73, 26.75) | 96 | 20.62 (14.69, 24.45) | 63 | 452.92 (234.38, 540.90) |
| Mediterranean | 24 | 13.35 (6.51,16.66) | 29 | 18.14 (9.79, 22.21) | 21 | 232.11 (71.16, 294.24) |
| Temp woodlands | 40 | 14.40 (9.99, 16.26) | 92 | 16.35 (11.46, 17.92) | 36 | 218.07 (103.39, 241.02) |
| Treeline ecotones | 82 | 11.83 (8.50, 13.34) | 19 | 17.82 (5.05, 24.68) | 18 | 113.79 (24.62, 146.36) |
| Temp decid forests | 55 | 9.73 (6.48, 11.27) | 22 | 14.80 (6.29, 19.61) | 5 | 12.69 (0.13, 12.90) |
| Savannas | 55 | 16.70 (11.78, 19.23) | 59 | 19.72 (13.77, 23.41) | 44 | 288.41 (120.68, 346.02) |
| Trop & temp rainfor | 110 | 18.53 (13.28, 22.00) | 114 | 14.12 (10.41, 15.72) | 82 | 238.00 (126.76, 269.17) |
| Desert & Xerophytic | Trop Dry Forests | Mediterr | Temp Woodlands | Treeline Ecotones | Temp Decid Forests | Savannas | Trop & Temp Rainfor | |
|---|---|---|---|---|---|---|---|---|
| Desert & xerophytic | - | 0.709 | 0.688 | 0.690 | 0.694 | 0.612 | 0.705 | 0.655 |
| Trop dry forests | 0.790 | - | 0.602 | 0.741 | 0.634 | 0.620 | 0.671 | 0.700 |
| Mediterranean | 0.620 | 0.653 | - | 0.659 | 0.705 | 0.731 | 0.630 | 0.698 |
| Temp woodlands | 0.742 | 0.711 | 0.767 | - | 0.638 | 0.621 | 0.723 | 0.690 |
| Treeline ecotones | 0.664 | 0.614 | 0.735 | 0.776 | - | 0.650 | 0.624 | 0.675 |
| Temp decid forests | 0.511 | 0.532 | 0.713 | 0.677 | 0.760 | - | 0.567 | 0.757 |
| Savannas | 0.652 | 0.673 | 0.548 | 0.649 | 0.565 | 0.472 | - | 0.596 |
| Trop & temp rainfor | 0.713 | 0.729 | 0.629 | 0.693 | 0.713 | 0.618 | 0.609 | - |
| Desert & Xerophytic | Trop Dry Forests | Mediterr | Temp Woodlands | Treeline Ecotones | Temp Decid Forests | Savannas | Trop & Temp Rainfor | |
|---|---|---|---|---|---|---|---|---|
| Desert & xerophytic | - | - | - | - | - | - | - | - |
| Trop dry forests | 0.661 | - | - | - | - | - | - | - |
| Mediterranean | 0.524 | 0.465 | - | - | - | - | - | - |
| Temp woodlands | 0.642 | 0.547 | 0.608 | - | - | - | - | - |
| Treeline ecotones | 0.412 | 0.308 | 0.506 | 0.473 | - | - | - | - |
| Temp decid forests | NA | NA | NA | NA | NA | - | - | - |
| Savannas | 0.489 | 0.529 | 0.453 | 0.524 | 0.395 | NA | - | - |
| Trop & temp rainfor | 0.599 | 0.523 | 0.524 | 0.587 | 0.409 | NA | 0.414 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosell, J.A.; Olson, M.E.; Martínez-Garza, C.; Martínez-Méndez, N. Functional Diversity in Woody Organs of Tropical Dry Forests and Implications for Restoration. Sustainability 2022, 14, 8362. https://doi.org/10.3390/su14148362
Rosell JA, Olson ME, Martínez-Garza C, Martínez-Méndez N. Functional Diversity in Woody Organs of Tropical Dry Forests and Implications for Restoration. Sustainability. 2022; 14(14):8362. https://doi.org/10.3390/su14148362
Chicago/Turabian StyleRosell, Julieta A., Mark E. Olson, Cristina Martínez-Garza, and Norberto Martínez-Méndez. 2022. "Functional Diversity in Woody Organs of Tropical Dry Forests and Implications for Restoration" Sustainability 14, no. 14: 8362. https://doi.org/10.3390/su14148362
APA StyleRosell, J. A., Olson, M. E., Martínez-Garza, C., & Martínez-Méndez, N. (2022). Functional Diversity in Woody Organs of Tropical Dry Forests and Implications for Restoration. Sustainability, 14(14), 8362. https://doi.org/10.3390/su14148362






