Abstract
Given the increasing demands for the quality and safety of animal-derived foods and the strict regulations on the use of antibiotics in animal feed, the use of functional feed additives has attracted increasing research and development. Jujube fruit is an energy-rich food with antioxidant, antibacterial, and antidiarrheal properties. With the expanding areas of cultivation to jujube trees and the intensive processing of jujube in Asia, especially in China, a large number of jujube by-products are produced. These by-products are used widely in animal feed for pigs, chicken, cattle, goats, and fish, as they improve growth performance, promote digestive tract health, and enhance the quality of animal products. This article reviews the nutritional components and benefits of jujube by-products and their potential incorporation in animal feed. The aim of this review is to introduce jujube by-products as a novel supplement or partial dietary replacement in the animal feed industry.
1. Introduction
The jujube (Ziziphus jujuba Mill.), a member of the Rhamnaceae family, consists of approximately 170 species and 12 varieties in the Ziziphus genus worldwide [1]. Cultivated jujube was domesticated originally from wild jujube in the middle and lower drainages of the Yellow River in China [2]. Jujube was cultivated approximately 7000 years ago and was included in the human diet more than 5000 years ago. The large-scale cultivation of jujube trees has been practiced in China for more than 3000 years [2]. It has now been introduced and cultivated in Asia, Europe, Africa, Oceania, and North America [3,4].
Jujube trees are resistant to drought and tolerant of salt-alkaline conditions [4]. The fruit contains a high level of sugars and is rich in vitamin C, iron, and calcium [3]. It also contains active substances such as polyphenols, polysaccharides, and trienoic acid [5], which have been proven to be beneficial, as they possess antioxidant, immunity enhancement, and antitumor properties [3]. Jujube is reported to have a high nutritional value, and its production has earned substantial income for farmers in recent years.
China is the main producer of jujube fruit in the world [3,6]. Jujube trees in China covered 1.3 million ha in 2006 and expanded to approximately 3 million ha in 2019, while the output of jujube increased concomitantly, from 2.46 million tons in 2006 to more than 7.46 million tons in 2019. Based on the field-picking price of 0.8 to 1.3 USD per kilogram in the past five years [7,8], the annual value of the jujube industry in China is estimated at approximately 6 to 10 billion USD.
With the large expansion of jujube production and the development of the jujube processing industry [3], a number of jujube by-products are produced and are used in animal feed [9,10,11]. Research on the nutritional components and active substances of jujube has increased, and the use of jujube as a feed additive or replacement has received increasing attention. This article summarizes the nutrients and active substances in jujube and addresses the current incorporation of jujube by-products in feed and the potential for a broader utilization in animal husbandry.
2. Jujube Distribution and Production
Jujube trees were introduced directly or indirectly from China to more than 50 countries, including South Korea, North Korea, Japan, Afghanistan, India, Pakistan, Iran, the United States, the United Kingdom, and Italy [12]. However, except for South Korea, China is the only country that cultivates jujube trees commercially [13]. Today, China accounts for more than 99% of the total jujube output worldwide, while the second largest output is from South Korea, with close to 0.3% [14]. In 2020, the jujube trees in China were concentrated mainly in Xinjiang, Shaanxi, Hebei, Shanxi, and Shandong, with these five provinces producing more than 90% of the total jujube output in the country. The largest area with jujube trees is in Xinjiang, especially in the Kashgar, Aksu, and Hotan prefectures of southern Xinjiang [12]. The number of tons of jujube produced in China over the past two decades is presented in Figure 1.
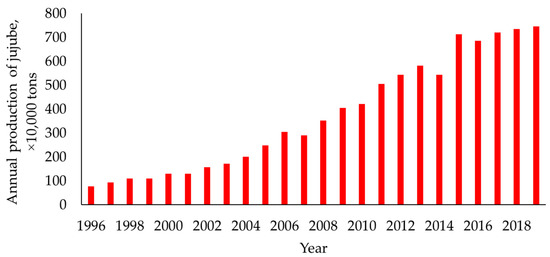
Figure 1.
Jujube produced in China from 1996 to 2019. (Data originated from China Forestry and Grassland Statistical Yearbook [15]).
Common jujube products marketed today include dried jujube, candied jujube, multi-flavored jujube, jujube powder, jujube paste, jujube vinegar, jujube juice, jujube wine, jujube slices, jujube pigment, and jujube flavor [3]. Jujube by-products consist mainly of residual jujube and jujube pomace [3,16]. Approximately 15 to 25% of harvested jujube fruits are classified as residual [16], which consists of defective, low-quality, split, or diseased fruits. More than 7 million tons of dry jujube have been produced in recent years (Figure 1), of which an estimated 1.0 to 1.5 million tons are residual jujube. With improvements in jujube planting technology, the proportion of residual jujube could potentially be reduced to less than 15%, but this amount is still substantial. Jujube pomace is the residue left after making jujube vinegar or wine [3] (Figure 2). The biomass of the pomace is relatively low, depending on the amount of jujube vinegar or wine produced.
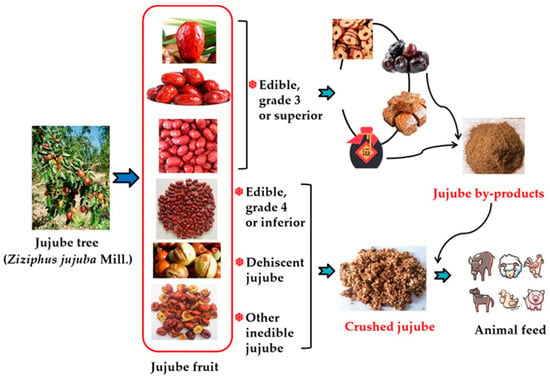
Figure 2.
Schematic diagram of jujube by-product from dried jujube fruit.
Note: The grade classification of dried jujube in China is based on the diameter, sugar content, and weight of the fruit, and these values differ with varieties. In common among varieties, the percentage of defective fruit in grade 3 or superior grade jujube is less than 10% and is processed to edible dried jujube.
3. Nutrients and Active Components in Jujube
3.1. Nutrients
On a dry matter basis, the crude protein content of jujube ranges between 4.5 and 7.7% [17,18], slightly lower than that of corn, wheat, and rice. The ether extract (EE) content varies with variety and ranges between 0.27 to 1.15% [17,18], which is lower than values reported for corn, wheat, or rice, and the crude fiber (CF) content ranges between 3.5 and 9.0%, which is higher than the CF of corn and wheat but lower than or similar to the content in rice. Most varieties of jujube have similar nitrogen-free extract contents as grains, approximately 55 to 65% [17,18]. In our recent study, using the method of Zhang et al. [19], the in vitro dry matter digestibility of jujube ranged between 74.2 and 77.8%, which was slightly lower than the 78.6% reported for corn. The neutral detergent fiber (NDF) content was 12.2% (a range of 11.2 to 12.8%), which was 12 to 28% higher than the 10.0% for corn. The contents of calcium and phosphorus vary with the location of the tree and variety of jujube. The gross energy (GE), which is the amount of heat liberated upon total combustion, ranges between 12 and 15 MJ/kg dry matter for most jujube varieties [3]. These values are similar to the GE of 14.9 MJ/kg dry matter suggested by the US Department of Agriculture [20]. In general, the protein and fat contents in jujube are relatively low, but the total sugar content and gross energy yield are high. Hence, jujube should be considered as a high-energy feed.
3.2. Amino Acid Composition and Characteristics
Up to 26 free amino acids (AAs) were detected in jujube dates [21,22], and their contents varied with the ripening stage and drying process [21,23]. Among the 20 common AAs, tryptophan and cysteine were not reported or were reported at very low concentrations in some species of jujube [21,22]. Furthermore, the concentration of cysteine was reduced after hot-air drying at 140 °C for 40 min [23]. Proline, aspartic acid, and glutamic acid are the three most abundant AAs in ripe jujube [18,21]. Jia et al. [24] reported that the contents of proline and aspartic acid were the highest of all AAs when examining five jujube cultivars, which was consistent with the results reported by Song et al. [21]. Proline is required for the synthesis of collagen and cartilage [25] and plays an important role in wound healing and antioxidant and immune responses [26]. It is also a conditionally essential AA in the early stage of fetal development and protects against fetal intrauterine growth retardation in pigs [27]. Aspartic acid is a neurotransmitter that mediates reproduction and hormone secretion and has a neuroprotective property [28]. These characteristics suggest that jujube is particularly beneficial for females and pregnant livestock. However, the contents of lysine and methionine in jujube is lower than in corn, and these two AAs should be balanced in feed according to the nutrient requirements of the animal species.
3.3. Active Components and Physiological Functions of Jujube
In recent years, several researchers published comprehensive reviews on the biologically active components in jujube and the potential nutritional value of the fruit for humans [6,20,29,30]. The main active components of jujube are polysaccharides, phenols, cyclic nucleotides, and organic acids. Polysaccharides improve the phagocytosis of mononuclear macrophages and affect the expression of proinflammatory cytokines (TNF-α, IL-1β, IL-6) [31,32,33]. The immune-modulatory mechanism of jujube inhibits the phosphorylation of the signaling proteins P-38 and JNK and exerts anti-inflammatory effects through the NF-κB and P38/JNK-MAPK signaling pathways [34]. Jujube polysaccharides also display hypolipidemic activity [35].
The polyphenols in jujube have anticancer and antioxidant properties [20], which are dependent on the variety of jujube [36] and the part of the jujube fruit. For example, the polyphenol content is higher in the skin than in the flesh of the jujube [29]. The biological activities of jujube are attributed partly to flavan-3-ols, which account for 92% of the total polyphenols [37], and to polymeric procyanidins (25.4 mmol/100 g) for their high oxidation activity [38]. The phenols in jujube alleviate dyslipidemia and insulin resistance caused by a high-fat, high-fructose diet [39].
Jujube is rich in cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) [40], which accumulate in the fruit mainly during late maturity [41]. The contents of cAMP and cGMP are highest in the pericarp, intermediate in the pulp and leaf, and lowest in the hanging stem [42]. The concentration of cAMP in jujube cultivars ranges between 500 and 1000 mg/kg dry matter. The cAMP inhibits the growth of B16F0 and Li-7 tumor cells [43] and, as demonstrated in jujuboside, has potential neuroprotective properties, using a mechanism related to the cAMP-PKA-CREB signal transduction pathway, antioxidant activity, and hormone secretion regulation [29]. The fermentation of jujube powder with yeast increased the content of cAMP and improved the immune function in mice with low immunity [44]. The extraction of cAMP and cGMP from jujube fruit is attracting increasing attention among researchers.
Malic acid (120–509 mg/100 g fresh weight, FW) and citric acid (29.4–181 mg/100 g FW) are the main organic acids in jujube, while iron (5.27–12.5 mg/100 g dry weight, DW), calcium (16.2–30.2 mg/100 g DW), and magnesium (51.2–70.0 mg/100 g DW) are the main minerals [45]. Consequently, jujube is a good source of organic acids, iron, and calcium for animal feed. In addition, botulin, quercetin, and triterpenoids in jujube possess antidiarrheal and antibacterial activities [46]. By enhancing Foxo activity, jujube regulated the expression of genes related to aging in Drosophila flies, extended their lifespan by 11.1%, and increased their tolerance to environmental stress [47]. Jujube is characterized by high yields of energy and concentration of minerals and low levels of crude fiber content and has multiple physiological functions, so it can have widespread use in animal feed.
4. Uses of Jujube in Animal Feed
The large amount available, the relatively low price, and the health benefits are attractive features to include jujube in animal feed. Research on jujube products in animal feed is conducted mostly in China, and, at present, jujube is available as dried powder, pellets, fermented powder, and complete feed. In this section, we discuss relevant advances to convey the potential value of jujube to the feed industry.
4.1. Pig Feed
To date, the effects of feeding residual or fermented jujube have been examined in piglets, growing pigs, finishing pigs, and perinatal sows. It was reported that replacing corn with a 10, 15, or 20% fermented jujube meal increased the feed intake and daily weight gain of growing pigs. The 15% replacement group had a lower ratio of feed consumption to weight gain than the control, 10, and 20% groups and had the highest feed intake [48]. A dietary supplement of 4% unfermented or fermented jujube powder for sows from mid-gestation to day 14 of lactation did not affect feed intake, but it increased the litter weight of piglets at 7 days of age and increased the survival rate of weaned piglets [49]. A supplementary 4% jujube powder reduced the plasma urea nitrogen concentration of the sows on day 109 of gestation and the insulin resistance index on day 14 of lactation. In addition, the jujube powder additive reduced fecal Clostridium levels, perinatal oxidative stress, insulin resistance, and constipation in sows and improved reproductive performance [49]. These studies indicate that the addition of unfermented or fermented jujube powder or replacing corn with jujube powder could improve the production performance of pigs at different physiological stages.
4.2. Poultry Feed
The effects of feeding supplementary jujube powder have been tested in broilers and laying hens. Replacing 10 or 15% of the corn with jujube powder in the diet of Taihang chickens reduced stress, increased the rate of protein synthesis, increased serum globulin content, promoted immune organ growth and development, enhanced pathogen resistance, and improved survival rate [50]. Zhao [51] reported that adding 10% jujube powder to the diet of laying hens improved egg production and reduced cholesterol content in the yolk. Furthermore, Ma et al. [52] reported that substituting 10% of the corn with jujube powder decreased triglycerides and total cholesterol levels, increased the levels of low-density and high-density lipoprotein cholesterol, and enhanced the total antioxidant capacity and immunoglobulin IgG concentration in the serum of Hy-Line Gray laying hens. The addition of 2 to 8% jujube powder increased the utilization rate of nitrogen and calcium [53] and improved the slow growth and low immunity caused by cyclophosphamide in chicks [54]. It can be concluded that jujube powder can improve the meat and egg production of poultry and can be used widely in poultry feed.
4.3. Ruminant Feed
4.3.1. Beef Cattle
Jujube fruits are rich in energy and could increase the energy yield of feed for fattening animals. However, the high sugar content also raises concerns about interference with proper rumen metabolism. Zhao et al. [55] reported an increase in the average daily gain (ADG) of fattening cattle when 18% of ammoniated corn stalk was replaced with jujube. In in vivo and in vitro studies, pelleted feed containing 5 to 25% jujube powder enhanced rumen fermentation, improved feed utilization efficiency, and increased the ADG of beef cattle [56]. A supplementation of 15% jujube was recommended, as this percentage did not affect carcass traits or meat quality indices [56]. In groups of cattle supplemented with 3, 6, or 9% jujube pomace, dry matter intake increased, and the ADG improved by 7.0, 16.4, and 10.9%, respectively [16]. The antioxidant activity of superoxide dismutase and feed utilization efficiency increased in the groups supplemented with 3 and 6% jujube pomace, and the apparent digestibilities of dry matter and nitrogen-free extract were improved in the 6% group; thus, a supplement of 6% jujube pomace was recommended [16]. However, there are little data on the effect of jujube feeding on the apparent digestibilities of crude protein, rumen degradable protein (RDP), and rumen undegradable protein (RUDP). A recent study in finishing bulls reported an increase in the dry matter intake and average daily gain and the apparent digestibilities of crude protein, acid detergent fiber, and non-fiber carbohydrate by replacing corn with 15% jujube powder [57]. Furthermore, there was an increase in the activities of fibrinogen and xylanase, the ruminal concentration of total volatile fatty acids, and the serum concentrations of glucose, total antioxidants, and superoxide dismutase, but there was a decrease in the serum concentration of malondialdehyde [57]. In general, jujube powder can improve forestomach fermentation parameters, hindgut digestion and metabolism, and growth performance in beef cattle. However, there is no effect on meat production or meat quality.
4.3.2. Dairy Cattle
Several reports on the effect of jujube on milk production in ruminants have been published. A combination of jujube and fresh beer dregs compensated for insufficient lysine content in the diet and improved the milk yield of dairy cows [58]. Adding residual jujube to diets improved feed nutrient utilization and the rumen environment and increased the feed intake, milk yield, and milk quality of lactating cows. The optimal supplement of jujube residue was 8% of the dry matter [59]. Jujube, when provided at 10% dry matter in the diet, enabled dairy cows to maintain a normal rumen environment and improved palatability, nutrient digestibility, and lactation performance [60].
4.3.3. Goats
In goats, the addition of 15 to 20% jujube powder derived from crinkled and cracked jujube increased the activities of catalase and superoxide dismutase and the levels of immunoglobulin in blood [61]. The substitution of corn with 5 to 20% jujube powder increased the dry matter intake and ADG; improved the apparent digestibilities of dry matter, organic matter, crude protein, and acid detergent fiber; and increased the net meat yield and dressing percentage in Jinlan cashmere goats [10]. The addition of jujube powder to the diet decreased the Warner–Bratzler shear force, pH, and crude fat content of the longissimus dorsi muscle, but it increased the protein and AA contents of the meat, as well as the contents of Ca, K, Mg, Na, Mn, Fe, and Zn. Fatty acid composition was not affected. A substitution of 15% jujube promoted growth performance and improved meat quality and, consequently, 15% jujube was recommended as optimal for goats [10]. To date, the feeding of jujube in sheep has not been reported. Recently, we conducted studies in fattening lambs and found that jujube intake reduced the blood concentrations of urea nitrogen, total cholesterol, and triglycerides, without affecting the concentrations of blood glucose and insulin [11]. In addition, jujube increased the blood antioxidant capacity of the lambs (unpublished observations).
4.4. Fish Feed
Adding prebiotics, enzyme preparation, herbal extracts, plant polysaccharides, and other active ingredients in feed can promote immunity and reduce mortality in fish. Liu et al. [62] reported that supplementing fish food with 0.25, 1.00, or 4.00% jujube extract had little effect on the growth rate of rainbow trout, but it increased the fat content, spleen body index, and antioxidant capacity. The addition of 0.5% jujube extract promoted renal function, protected the liver, and improved non-specific immune function [63]. Dietary supplementation with 0.5 or 1.0% jujube improved the growth performance of carp and the production performance of carp fry and enhanced skin and mucosal immunity [9]. These studies indicated that jujube can be important to the fish feed industry. Of the 20 AAs available, 10 AAs, including arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine, are considered to be indispensable for the diet of fish and other aquatic animals [64,65]. Cysteine is conditionally indispensable as it can only be synthesized from methionine and serine precursors [64]. Jujube has low concentrations of cysteine and methionine [21,22], and therefore, when jujube by-products are included in fish feed, cysteine and methionine should be supplemented.
4.5. Feed Formulation and Production
The production of good silage requires 8 to 10% (as dry matter) of water-soluble carbohydrates (WSCs), which are especially low in leguminous forages. The soluble sugar content in jujube ranges from 50 to 67% [66]; thus, it can be important as a silage additive to provide WSC for the growth of lactic acid bacteria (LAB). Compared with other commonly used sources of WSC, such as molasses, jujube has advantages of easier transportation, processing, and blending. Supplementation with 7.5% residual jujube powder (WSC content of 59.8%) improved the fermentation parameters and nutritional quality of the silage; increased the relative abundance of LAB; decreased the concentration of aflatoxin and the relative abundances of yeast, mold, and aerobic bacteria; and increased the digestibility and metabolizable energy yield for ruminants [67].
The production of high-quality fermented concentrate feed requires a sufficient amount of carbon that is readily available. The high sugar and low starch contents in jujube can satisfy this demand. A recent study tested the addition of a mixed fermentation inoculant containing Saccharomyces cerevisiae, Lactobacillus plantarum, and Bacillus subtilis to produce a solid fermented feed consisting of jujube, soybean meal, and bran. Due to the high-soluble sugar content, jujube can function as a natural adhesive in pelleted feed processing for livestock and poultry diets. However, the optimal bacterial agent, water content, and jujube supplementation level still require further research [68].
5. Conclusions
Jujube is palatable and is high in sugar content and energy yield but low in protein content, and therefore, it should be used as a high-energy feed. The contents of lysine, methionine, and cysteine in jujube are low but of proline and aspartic acid are high. The concentrations of calcium and phosphorus vary greatly. Thus, it is necessary to adjust the balances of AAs and minerals in the diet. Jujube is rich in polysaccharides, phenols, vitamin C, triterpenoid acid, and iron and has antidiarrheal, antibacterial, immune promoting, and antioxidant properties. In livestock, poultry, and fish, the proper level of jujube as a supplement or as a replacement can increase feed palatability, feed intake, and nutrient digestibility, reduce the feed-to-gain conversion rate and improve the intestinal microbial system and quality of animal products. Given its ability to improve immunity, and, thereby, to reduce the use of antibiotics, jujube fruit has broad potential use in animal feed as a growth promoter, antioxidant, and flavoring agent. In the future, systematic research on the digestion and metabolism of residual jujube in different animals is needed. Optimal feeding level recommendations, processing technology, and feed product standards and specifications should be improved. In addition, attention should be paid to the feeding safety of microbial populations, mycotoxins, pesticides, and heavy metals in jujube by-products.
Author Contributions
Conceptualization, X.Z. and N.C.; writing—original draft preparation, T.X., S.Z. and A.D.; funding acquisition, J.Y.; writing—review and editing, N.C. and A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Open Project of the State Key Laboratory of Sheep Genetic Improvement and Healthy Production, grant number MYSKLKF201907; the Key Areas of Scientific and Technological Research Projects supported by Bintuan Science and Technology Program, grant number 2020AB016; and the Young and Middle-aged Leading Talent Plan supported by Bingtuan Science and Technology Program, grant number 2020CB026.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Li Yanghui for assistance with data collection and three reviewers for their suggestions on the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, M. Advances in taxonomy study on the genus Ziziphus. Acta Hortic. Sin. 1999, 26, 302–308. [Google Scholar]
- Qu, Z.; Wu, Y. Discussion about the origin of jujube cultivation. J. Beijing Univ. Agric. 1983, 1, 1–5. [Google Scholar]
- Rashwan, A.K.; Karim, N.; Shishir, M.R.I.; Bao, T.; Lu, Y.; Chen, W. Jujube fruit: A potential nutritious fruit for the development of functional food products. J. Funct. Foods 2020, 75, 104205. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Shan, F. Chinese jujube industry takes root in Western Australia. ISHS Acta Hortic. 2016, 1116, 31–34. [Google Scholar] [CrossRef]
- Shi, Q.; Han, G.; Liu, Y.; Jiang, J.; Jia, Y.; Li, X. Nutrient composition and quality traits of dried jujube fruits in seven producing areas based on metabolomics analysis. Food Chem. 2022, 385, 132627. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Bao, T.; Mo, J.; Ni, J.; Chen, W. Research advances in bioactive components and health benefits of jujube (Ziziphus jujuba Mill.) fruit. J. Zhejiang Univ. Sci. B 2021, 22, 431–449. [Google Scholar] [CrossRef]
- Liu, N. Study on Economic Development of Jujube Industry in China under Supply-Side Structural Reform. Ph.D. Thesis, Heibei Agricultural University, Baoding, China, 2018. [Google Scholar]
- Xie, X.; Jin, D.; Dai, J.; Bao, Y.; Zhang, Y.; Wang, S. Research report on the development of jujube industry. China Rural. Sci. Technol. 2021, 10, 54–57. [Google Scholar]
- Hoseinifar, S.H.; Khodadadian Zou, H.; Van Doan, H.; Harikrishnan, R.; Yousefi, M.; Paknejad, H.; Ahmadifar, E. Can dietary jujube (Ziziphus jujuba Mill.) fruit extract alter cutaneous mucosal immunity, immune related genes expression in skin and growth performance of common carp (Cyprinus carpio)? Fish Shellfish Immunol. 2019, 94, 705–710. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, C.X.; Zhang, J.X.; Dong, K.H.; Wang, P.J.; Ren, Y.S.; Song, Z.P.; Yan, Z.W. Growth performance, nutrient digestibility, carcass traits, body composition, and meat quality of goat fed Chinese jujube (Ziziphus jujuba Mill) fruit as a replacement for maize in diet. Anim. Feed. Sci. Technol. 2018, 246, 127–136. [Google Scholar] [CrossRef]
- Hui, H.; Yang, J.; Liu, F.; Chen, N.; Liu, L.; Zhou, X. Effects of replacement of maize with residual jujube on growth performance, blood biochemistry and hormone indices of Karakul sheep. Chin. J. Anim. Sci. 2022, 4, 1–9. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Wang, L.; Liu, P.; Zhao, J.; Zhao, Z.; Yao, S.; Stănică, F.; Liu, Z.; Wang, L.; et al. The historical and current research progress on jujube-a superfruit for the future. Hortic. Res. 2020, 7, 119. [Google Scholar] [CrossRef]
- Liu, M. The present status, problems and countermeasures of Chinese jujube production. Rev. China Agric. Sci. Technol. 2000, 2, 76–80. [Google Scholar]
- Song, L.; Meinhardt, L.W.; Bailey, B.; Zhang, D. Genetic improvement of Chinese jujube for disease resistances: Status, knowledge gaps and research needs. Crop Breed. Genet. Genom. 2019, 1, e190015. [Google Scholar]
- National Forestry and Grassland Administration. China Forestry and Grassland Statistical Yearbook; China Forestry Publishing House: Beijing, China, 2020; pp. 34–37.
- Wen, X.; Tang, X.; Zhang, S.; Wang, Y.; Song, Z.; Zhang, Y. Effect of jujube residue on apparent nutrient digestibility, blood biochemical indexes and fattening performance of beef cattle. Heilongjiang Anim. Sci. Vet. Med. 2019, 10, 124–127. [Google Scholar] [CrossRef]
- Han, Z.; Liu, B.; Cao, Y. Analysis and evaluation on nutritive composition of Chinese dates in different producing areas of north shaanxi. J. Anhui Agric. Sci. 2007, 35, 9830–9831. [Google Scholar]
- Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Jiao, Z. Comparison of nutritional composition of jujube from Xinjiang. Food Nutr. China 2018, 24, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, M.; Rong, Z.; Long, D.; Mao, H. Urea plus nitrate pretreatment of rice and wheat straws enhances degradation and reduces methane production in in vitro ruminal culture. J. Sci. Food Agric. 2018, 98, 5205–5211. [Google Scholar] [CrossRef]
- Gao, Q.H.; Wu, C.S.; Wang, M. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2013, 61, 3351–3363. [Google Scholar] [CrossRef]
- Song, J.; Bi, J.; Chen, Q.; Wu, X.; Lyu, Y.; Meng, X. Assessment of sugar content, fatty acids, free amino acids, and volatile profiles in jujube fruits at different ripening stages. Food Chem. 2019, 270, 344–352. [Google Scholar] [CrossRef]
- Choi, S.H.; Ahn, J.B.; Kozukue, N.; Levin, C.E.; Friedman, M. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of jujube (Ziziphus jujuba Mill.) fruits and seeds harvested from plants grown in Korea. J. Agric. Food Chem. 2011, 59, 6594–6604. [Google Scholar] [CrossRef]
- Xu, L.; Huang, G.; Liu, C.; Zhang, L.; Liang, M.; Xu, G.; Li, R.; Wu, Z.; Gao, Y. Effect of high temperature hot air drying on the content of free amino acids in jujube. Food Ferment. Ind. 2022, 6, 1–9. [Google Scholar] [CrossRef]
- Jia, X.; Yang, Y.; He, Y.; Pu, B. Analysis and evaluation of nutritive composition of jujube in producing areas of Aksu. Mod. Food Sci. Technol. 2011, 27, 847–849. [Google Scholar]
- Li, P.; Wu, G. Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 2018, 50, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Kim, S.W.; Knabe, D.A.; Li, P.; Li, X.; McKnight, J.R.; Satterfield, M.C.; et al. Proline and hydroxyproline metabolism: Implications for animal and human nutrition. Amino Acids 2011, 40, 1053–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q. Preliminary Study on the Regulated Molecular Mechanism of Proline in the Fetus of Huan Jiang sows. Master’s Thesis, Guangxi University, Nanning, China, 2017. [Google Scholar]
- Li, Y.; Han, H.; Yin, J.; Li, T.; Yin, Y. Role of D-aspartate on biosynthesis, racemization, and potential functions: A mini-review. Anim. Nutr. 2018, 4, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Li, Z.; Qi, A.; Yao, P.; Zhou, Z.; Dong, T.T.X.; Tsim, K.W.K. A review of dietary Ziziphus jujuba fruit (Jujube): Developing health food supplements for brain protection. Evid.-Based Complement. Altern. Med. eCAM 2017, 2017, 3019568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Peng, Q.; Yuan, Y.; Shen, J.; Xie, X.; Wang, M. Isolation, structures and bioactivities of the polysaccharides from jujube fruit (Ziziphus jujuba Mill.): A review. Food Chem. 2017, 227, 349–357. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Wang, Y.; Liu, G.; Zhang, Z.; Zhao, Z.; Cheng, H. In vitro antioxidative and immunological activities of polysaccharides from Zizyphus jujuba cv. Muzao. Int. J. Biol. Macromol. 2017, 95, 1119–1125. [Google Scholar] [CrossRef]
- Zou, M.; Chen, Y.; Sun-Waterhouse, D.; Zhang, Y.; Li, F. Immunomodulatory acidic polysaccharides from Zizyphus jujuba cv. Huizao: Insights into their chemical characteristics and modes of action. Food Chem. 2018, 258, 35–42. [Google Scholar] [CrossRef]
- Cai, Y.; Zhou, X.; Han, A.; Chen, P.; Bai, H. In vitro immunological and anti-complementary activities of two water-soluble lignins from Zizyphus jujube cv. Jinchangzao. Int. J. Biol. Macromol. 2017, 105, 204–212. [Google Scholar] [CrossRef]
- Zhan, R.; Xia, L.; Shao, J.; Wang, C.; Chen, D. Polysaccharide isolated from Chinese jujube fruit (Zizyphus jujuba cv. Junzao) exerts anti-inflammatory effects through MAPK signaling. J. Funct. Foods 2018, 40, 461–470. [Google Scholar] [CrossRef]
- Ji, X.; Liu, F.; Peng, Q.; Wang, M. Purification, structural characterization, and hypolipidemic effects of a neutral polysaccharide from Ziziphus jujuba cv. Muzao. Food Chem. 2018, 245, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Chen, Q.; Li, X.; Li, M.; Kan, C.; Chen, B.; Zhang, Y.; Xue, Z. Quantitative assessment of bioactive compounds and the antioxidant activity of 15 jujube cultivars. Food Chem. 2015, 173, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Carbonell-Barrachina, Á.A.; Legua, P.; Hernández, F. Phenolic composition, ascorbic acid content, and antioxidant capacity of Spanish jujube (Ziziphus jujube Mill.) fruits. Food Chem. 2016, 201, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Zozio, S.; Servent, A.; Cazal, G.; Mbéguié-A-Mbéguié, D.; Ravion, S.; Pallet, D.; Abel, H. Changes in antioxidant activity during the ripening of jujube (Ziziphus mauritiana Lamk). Food Chem. 2014, 150, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Jeong, O.; Kim, H.-S. Dietary chokeberry and dried jujube fruit attenuates high-fat and high-fructose diet-induced dyslipidemia and insulin resistance via activation of the IRS-1/PI3K/Akt pathway in C57BL/6 J mice. Nutr. Metab. 2019, 16, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiskirchen, R. Hepatoprotective and anti-fibrotic agents: It’s time to take the next step. Front. Pharmacol. 2015, 6, 303. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Duan, J.-A.; Qian, D.; Tang, Y.; Wu, D.; Su, S.; Wang, H.; Zhao, Y. Content variations of triterpenic acid, nucleoside, nucleobase, and sugar in jujube (Ziziphus jujuba) fruit during ripening. Food Chem. 2015, 167, 468–474. [Google Scholar] [CrossRef]
- Zhao, A.; Li, D.; Wang, Y.; Sui, C.; Cao, Y.; Liang, Q. Study on the contents of cAMP and cGMP in different cultivars, growing periods and organs in China. Acta Hortic. Sin. 2009, 36, 1134–1139. [Google Scholar]
- Liu, D. The Diversity of cAMP in Southern Xinjiang Jujube Cultivars and Preparation of Concentrated Product. Master’s Thesis, Tarim University, Alaer, China, 2016. [Google Scholar]
- Ji, Y. Chemical Compositions Analysis of Fermented Winter Jujube Powder and Its Effect on the Immune Function of Mouse. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2014. [Google Scholar]
- Wang, L.; Fu, H.; Wang, W.; Wang, Y.; Zheng, F.; Ni, H.; Chen, F. Analysis of reducing sugars, organic acids and minerals in 15 cultivars of jujube (Ziziphus jujuba Mill.) fruits in China. J. Food Compos. Anal. 2018, 73, 10–16. [Google Scholar] [CrossRef]
- Mesaik, A.M.; Poh, H.W.; Bin, O.Y.; Elawad, I.; Alsayed, B. In vivo anti-iinflammatory, anti-bacterial and anti-diarrhoeal activity of Ziziphus jujuba fruit extract. Open Access Maced. J. Med. Sci. 2018, 6, 757–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghimire, S.; Kim, M.S. Jujube (Ziziphus jujuba Mill.) fruit feeding extends lifespan and increases tolerance to environmental stresses by regulating aging-associated gene expression in Drosophila. Biogerontol. 2017, 18, 263–273. [Google Scholar] [CrossRef]
- Ji, J.; Wang, J.; Mu, C.; Wang, H. The influence of biological ferment jujube powder on the production performance in growing pigs. Guizhou J. Anim. Husb. Vet. Med. 2015, 39, 8–9. [Google Scholar]
- Huang, Y. Effects of Jujube Powder and Fermented Jujube Powder on Production Performance, Blood Indicators and Fecal Microbes Flora in Sows. Master’s Thesis, Northwest A & F University, Xianyang, China, 2018. [Google Scholar]
- Hao, Y.; Li, S.; Zhao, G.; Zhang, C.; Liu, G.; Zhang, Z. Effect of jujube powder on anti-stress ability of Taihang chickens. China Poult. 2018, 40, 31–34. [Google Scholar]
- Zhao, Y. Study of Application of Jujube Powder in Laying Hens Diet. Master’s Thesis, Hebei Agricultural University, Baoding, China, 2012. [Google Scholar]
- Ma, K.; Zhao, Y.; Liu, Y.; Zhang, Z.; Hao, Y.; Zhao, G. Effect of jujube powder on lipid metabolism, antioxidant performance and immune function of layers. China Feed 2017, 19, 5–8. [Google Scholar] [CrossRef]
- Jia, S.; Gao, Q.; Du, B.; Nian, F.; Tang, D. Effects of organic selenium and jujube powder on growth performance and nutrient utilization of broilers. China Feed 2021, 9, 124–128. [Google Scholar] [CrossRef]
- Guo, L.; Ma, K.; Feng, Z.; Liu, Y.; Liu, G.; Li, Q.; Gong, S.; Li, S.; Zhao, G. Effects of jujube polysaccharide on growth performance and immune indexes of immunosuppressive egg-type chicks. China Anim. Husb. Vet. Med. 2021, 48, 3264–3272. [Google Scholar]
- Zhao, J.; Jiao, J.; Zhao, Y.; Liu, P.; Tao, W.; Tao, D. Feasibility analysis of incorporating disabled dates into fattening cattle feed. Contemp. Anim. Husb. 2015, 18, 48–50. [Google Scholar]
- Bo, Y. The Effect of Wasted Chinese Jujube on Ruminal Fermentation and Productive Performance in Beef Cattle. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2015. [Google Scholar]
- Liu, Y.; Liu, C.; Feng, Z.; Zhang, k.; Liu, Q. Effects of jujube power and coated methionine on growth performance, nutrient apparent digestibility, rumen fermentation and serum biochemical indexes in finishing bulls. Chin. J. Anim. Nutr. 2022, 34, 1076–1086. [Google Scholar]
- Wang, X.; Zhao, H.; You, W.; Cheng, H.; Wu, L. The effect of adding feed date, soybean hulls or fresh beer residue on milk performance of dairy cows. Feed Ind. 2010, 31, 27–29. [Google Scholar]
- Zhu, K. The Effect of Jujube Residue in Diet on Rumen Fermentation and Milk Performance of Dairy Cows. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2019. [Google Scholar]
- Chen, X. The Effect of Wasted Chinese Jujube on Ruminal Fermentation and Milk Performance in Dairy Cows. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2019. [Google Scholar]
- Yan, Z. Effects of Supplementation of Different Level Substandard Chinese Jujube in Diet on Productive Performance and Indexes of Physiological and Biochemical of Blood in Bucks. Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2016. [Google Scholar]
- Liu, F.; Liu, X.; Wang, F.; Cui, C.; Su, M.; Gao, B. Effects of jujube extracts on growth and antioxidant capacity of rainbow trout (Oncorhynchus mykiss). China Feed 2020, 9, 98–103. [Google Scholar] [CrossRef]
- Liu, F.; Wang, F.; Li, C.; Ma, Y.; Zhu, R.; Huang, Q. Effects of jujube extracts on serum biochemical indexes and immune-related gene expression in the head kidney of rainbow trout (Oncorhynchus mykiss). Freshw. Fish. 2020, 50, 15–21. [Google Scholar]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Wu, G. Nutrition and functions of amino acids in fish. In Amino Acids in Nutrition and Health; Wu, G., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Abudureyimu, A.; Zhang, Z.; Huang, Y.; Tuerxunjiang, A.; Chen, K.; Li, H. Construction of dynamic model of soluble sugar content in dried jujube. Xinjiang Agric. Sci. 2021, 58, 1342–1354. [Google Scholar]
- Zhang, L. Study on the Quality and Aerobic Stability of Inferior Jujube Powder and Alfalfa Silage. Master’s Thesis, Shihezi University, Shihezi, China, 2019. [Google Scholar]
- Xing, X.; Zhao, L.; Huang, X.; Zhang, X.; Zhang, Y.; Zhang, E. Effects of solid-state fermentation with compound bacteria on nutritional quality of jujube powder. Chin. J. Anim. Nutr. 2021, 33, 5989–6000. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).