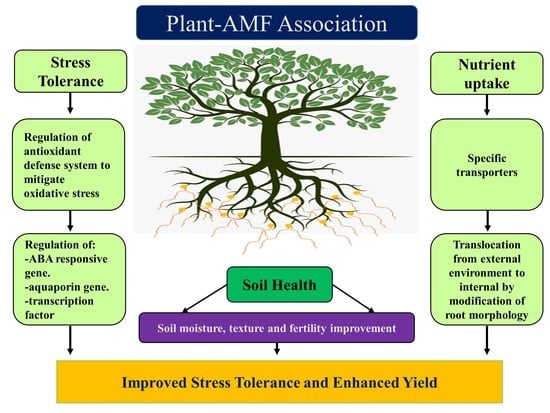
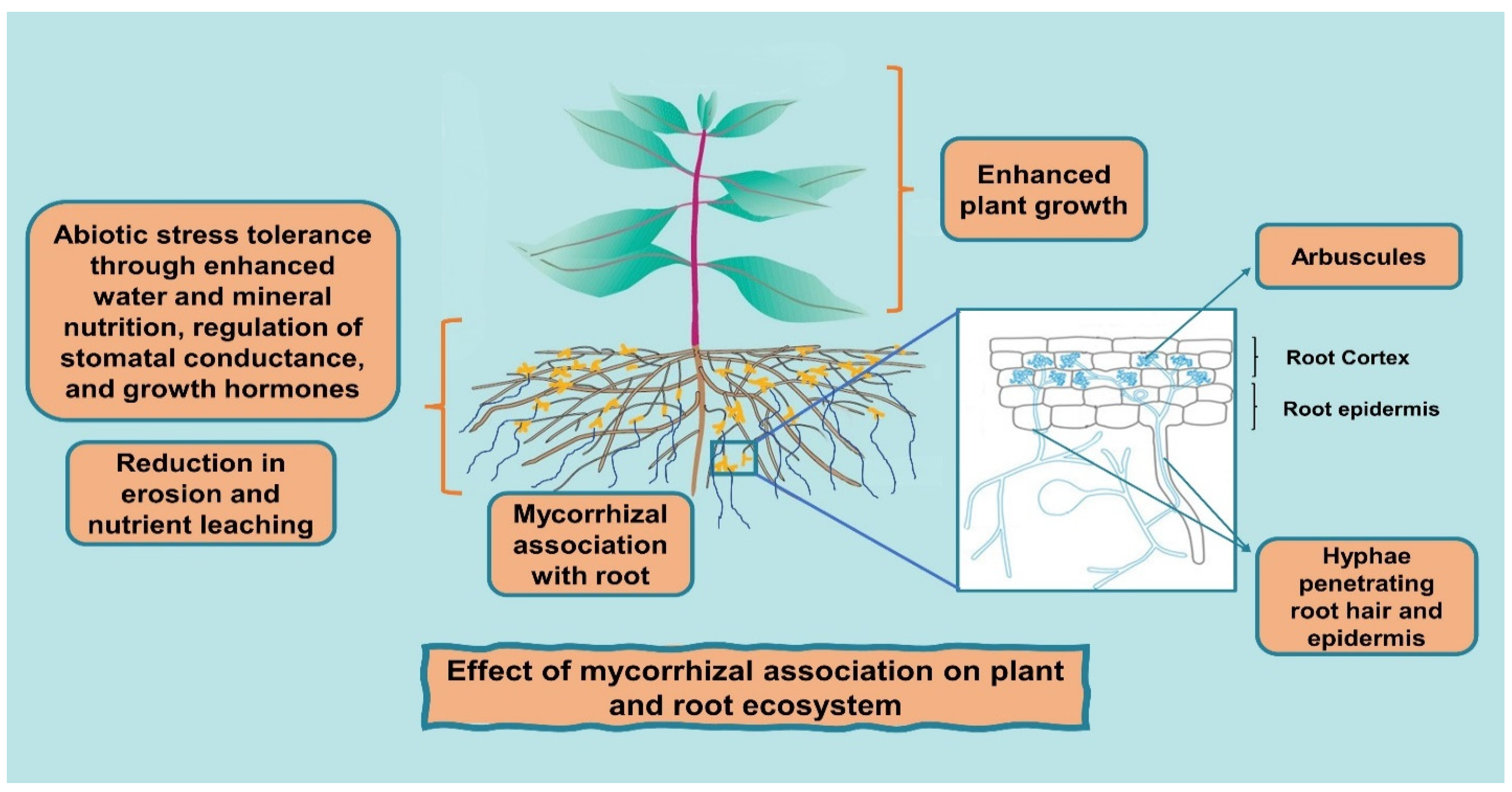
Arbuscular Mycorrhizal Fungi Symbiosis to Enhance Plant–Soil Interaction
Abstract
:1. Introduction
2. AMF and Nutrition Acquisition
Role of AMF in Reducing Erosion and Nutrient Leaching
3. AMF and Abiotic Stresses
3.1. Drought
3.2. Soil Salinity
3.3. Heavy Metals
4. Commercial Production of AMF
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mbodj, D.; Effa-Effa, B.; Kane, A.; Manneh, B.; Gantet, P.; Laplaze, L.; Diedhiou, A.G.; Grondin, A. Arbuscular mycorrhizal symbiosis in rice: Establishment, environmental control and impact on plant growth and resistance to abiotic stresses. Rhizosphere 2018, 8, 12–26. [Google Scholar] [CrossRef]
- Chen, E.C.H.; Morin, E.; Beaudet, D.; Noel, J.; Yildirir, G.; Ndikumana, S.; Charron, P.; St-Onge, C.; Giorgi, J.; Krüger, M.; et al. High intraspecific genome diversity in the model arbuscular mycorrhizal symbiont Rhizophagus irregularis. New Phytol. 2018, 220, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selosse, M.A.; Strullu-Derrien, C.; Martin, F.M.; Kamoun, S.; Kenrick, P. Plants, Fungi and Oomycetes: A 400-million year affair that shapes the biosphere. New Phytol. 2015, 206, 501–506. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wu, Q.S.; Kuča, K. Unravelling the role of arbuscular mycorrhizal fungi in mitigating the oxidative burst of plants under drought stress. Plant Biol. 2021, 23 (Suppl. S1), 50–57. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Summers, W.; Paszkowski, U. Mechanisms underlying establishment of arbuscular mycorrhizal symbioses. Annu. Rev. Phytopathol. 2018, 56, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Aroca, R.; Garcia, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem ii and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 2015, 185, 75–83. [Google Scholar] [CrossRef]
- Strullu-Derrien, C.; Kenrick, P.; Pressel, S.; Duckett, J.G.; Rioult, J.-P.; Strullu, G. Fungal associations in horneophyton ligneri from the rhynie chert (C. 407 million year old) closely resemble those in extant lower land plants: Novel insights into ancestral plant-fungus symbioses. New Phytol. 2014, 203, 964–979. [Google Scholar] [CrossRef]
- Tester, M.; Smith, S.E.; Smith, F.A. The phenomenon of ‘nonmycorrhizal’ plants. Can. J. Bot. 1987, 65, 419–431. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Füzy, A.; Biró, B.; Tóth, T.; Hildebrandt, U.; Bothe, H. Drought, but not salinity, determines the apparent effectiveness of halophytes colonized by arbuscular mycorrhizal fungi. J. Plant Physiol. 2008, 165, 1181–1192. [Google Scholar] [CrossRef]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Minsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fusconi, A.; Mucciarelli, M. How important is arbuscular mycorrhizal colonization in wetland and aquatic habitats? Environ. Exp. Bot. 2018, 155, 128–141. [Google Scholar] [CrossRef]
- Nanjundappa, A.; Bagyaraj, D.J.; Saxena, A.K.; Kumar, M.; Chakdar, H. Interaction between arbuscular mycorrhizal fungi and Bacillus spp. in soil enhancing growth of crop plants. Fungal Biol. Biotechnol. 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primieri, S.; Santos, J.C.P.; Antunes, P.M. Nodule-associated bacteria alter the mutualism between arbuscular mycorrhizal fungi and N2 fixing bacteria. Soil Biol. Biochem. 2021, 154, 108149. [Google Scholar] [CrossRef]
- Ferreira, D.A.; da Silva, T.F.; Pylro, V.S.; Salles, J.F.; Andreote, F.D.; Dini-Andreote, F. Soil microbial diversity affects the plant-root colonization by arbuscular mycorrhizal fungi. Microb. Ecol. 2021, 82, 100–103. [Google Scholar] [CrossRef]
- Roth, R.; Paszkowski, U. Plant carbon nourishment of arbuscular mycorrhizal fungi. Curr. Opin. Plant Biol. 2017, 39, 50–56. [Google Scholar] [CrossRef]
- Jakobsen, I.; Hammer, E.C. Nutrient dynamics in arbuscular mycorrhizal networks. In Mycorrhizal Networks; Horton, T.R., Ed.; Springer: Dordrecht, The Netherlands, 2015; pp. 91–131. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Cameron, D.D.; Neal, A.L.; van Wees, S.C.M.; Ton, J. Mycorrhiza-induced resistance: More than the sum of its parts? Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the impact of arbuscular mycorrhizal symbiosis on tomato tolerance to water stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [Green Version]
- Luginbuehl, L.H.; Menard, G.N.; Kurup, S.; van Erp, H.; Radhakrishnan, G.V.; Breakspear, A.; Oldroyd, G.E.D.; Eastmond, P.J. Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 2017, 356, 1175–1178. [Google Scholar] [CrossRef] [Green Version]
- Nell, M.; Wawrosch, C.; Steinkellner, S.; Vierheilig, H.; Kopp, B.; Lössl, A.; Franz, C.; Novak, J.; Zitterl-Eglseer, K. Root Colonization by symbiotic arbuscular mycorrhizal fungi increases sesquiterpenic acid concentrations in Valeriana officinalis L. Planta Med. 2010, 76, 393–398. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Ravnskov, S.; Liu, F.; Rubæk, G.H.; Andersen, M.N. Arbuscular mycorrhizal fungi alleviate abiotic stresses in potato plants caused by low phosphorus and deficit irrigation/partial root-zone drying. J. Agric. Sci. 2018, 156, 46–58. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.Y.; Grønlund, M.; Jakobsen, I.; Grotemeyer, M.S.; Rentsch, D.; Miyao, A.; Hirochika, H.; Kumar, C.S.; Sundaresan, V.; Salamin, N.; et al. Nonredundant regulation of rice arbuscular mycorrhizal symbiosis by two members of the PHOSPHATE TRANSPORTER1 gene family. Plant Cell 2012, 24, 4236–4251. [Google Scholar] [CrossRef] [Green Version]
- Jeong, K.; Mattes, N.; Catausan, S.; Chin, J.H.; Paszkowski, U.; Heuer, S. Genetic diversity for mycorrhizal symbiosis and phosphate transporters in rice. J. Integr. Plant Biol. 2015, 57, 969–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courty, P.E.; Smith, P.; Koegel, S.; Redecker, D.; Wipf, D. Inorganic nitrogen uptake and transport in beneficial plant root-microbe interactions. Crit. Rev. Plant Sci. 2015, 34, 4–16. [Google Scholar] [CrossRef]
- Koegel, S.; Mieulet, D.; Baday, S.; Chatagnier, O.; Lehmann, M.F.; Wiemken, A.; Boller, T.; Wipf, D.; Bernèche, S.; Guiderdoni, E.; et al. Phylogenetic, structural, and functional characterization of AMT3;1, an ammonium transporter induced by mycorrhization among model grasses. Mycorrhiza 2017, 27, 695–708. [Google Scholar] [CrossRef] [Green Version]
- Leigh, J.; Hodge, A.; Fitter, A.H. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol. 2009, 181, 199–207. [Google Scholar] [CrossRef]
- Battini, F.; Grønlund, M.; Agnolucci, M.; Giovannetti, M.; Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017, 7, 4686. [Google Scholar] [CrossRef]
- Turrini, A.; Bedini, A.; Loor, M.B.; Santini, G.; Sbrana, C.; Giovannetti, M.; Avio, L. Local diversity of native arbuscular mycorrhizal symbionts differentially affects growth and nutrition of three crop plant species. Biol. Fertil. Soils 2018, 54, 203–217. [Google Scholar] [CrossRef]
- Kayama, M.; Yamanaka, T. Growth characteristics of ectomycorrhizal seedlings of Quercus glauca, Quercus salicina, and Castanopsis cuspidata planted on acidic soil. Trees Struct. Funct. 2014, 28, 569–583. [Google Scholar] [CrossRef]
- Balliu, A.; Sallaku, G.; Rewald, B. AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 2015, 7, 15967–15981. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zeng, R.; Liao, H. Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant Biol. 2016, 58, 193–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, R.; Bhola, D.; Akdi, K.; Cruz, C.; KVSS, S.; Tuteja, N.; Varma, A. Introduction to mycorrhiza: Historical development. In Mycorrhiza-Function, Diversity, State of the Art; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–7. [Google Scholar] [CrossRef]
- Haselwandter, K. Structure and function of siderophores produced by mycorrhizal fungi. Mineral. Mag. 2008, 72, 61–64. [Google Scholar] [CrossRef]
- Winkelmann, G. A Search for Glomuferrin: A potential siderophore of arbuscular mycorrhizal fungi of the genus Glomus. BioMetals 2017, 30, 559–564. [Google Scholar] [CrossRef]
- Etesami, H.; Jeong, B.R.; Glick, B.R. Contribution of arbuscular mycorrhizal fungi, phosphate–solubilizing bacteria, and silicon to P uptake by plant. Front. Plant Sci. 2021, 12, 1355. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N.; Sabzalian, M.R. Nutritional status, essential oil changes and water-use efficiency of Rose geranium in response to arbuscular mycorrhizal fungi and water deficiency stress. Symbiosis 2017, 73, 15–25. [Google Scholar] [CrossRef]
- Bagheri, V.; Shamshiri, M.H.; Shirani, H.; Roosta, H.R. Nutrient uptake and distribution in mycorrhizal pistachio seedlings under drought stress. J. Agric. Sci. Technol. 2012, 14, 1591–1604. [Google Scholar]
- Wang, Y.; Wang, M.; Li, Y.; Wu, A.; Huang, J. Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 2018, 13, e0196408. [Google Scholar] [CrossRef]
- Lin, J.; Wang, Y.; Sun, S.; Mu, C.; Yan, X. Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 2017, 576, 234–241. [Google Scholar] [CrossRef]
- Evelin, H.; Giri, B.; Kapoor, R. Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in nacl-stressed Trigonella foenum-Graecum. Mycorrhiza 2012, 22, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops—A meta-analysis. Soil Biol. Biochem. 2015, 81, 147–158. [Google Scholar] [CrossRef]
- Hashem, A.; Alqarawi, A.A.; Radhakrishnan, R.; Al-Arjani, A.B.F.; Aldehaish, H.A.; Egamberdieva, D.; Abd Allah, E.F. Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 2018, 25, 1102–1114. [Google Scholar] [CrossRef]
- Zhu, X.; Song, F.; Liu, S.; Liu, F. Arbuscular mycorrhiza improve growth, nitrogen uptake, and nitrogen use efficiency in wheat grown under elevated CO2. Mycorrhiza 2016, 26, 133–140. [Google Scholar] [CrossRef]
- Briccoli Bati, C.; Santilli, E.; Lombardo, L. Effect of arbuscular mycorrhizal fungi on growth and on micronutrient and macronutrient uptake and allocation in olive plantlets growing under high total mn levels. Mycorrhiza 2015, 25, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, E.; Bedini, S. Enhancing ecosystem services in sustainable agriculture: Biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2014, 68, 429–439. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: Let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [Green Version]
- Lu, F.C.; Lee, C.Y.; Wang, C.L. The influence of arbuscularmycorrhizal fungi inoculation on yam (Dioscorea Spp.) tuber weights and secondary metabolite content. PeerJ 2015, 2015, e1266. [Google Scholar] [CrossRef] [Green Version]
- Hijri, M. Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 2016, 26, 209–214. [Google Scholar] [CrossRef]
- Ortas, I. The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crops Res. 2012, 125, 35–48. [Google Scholar] [CrossRef]
- Gutjahr, C.; Paszkowski, U. Multiple control levels of root system remodeling in arbuscular mycorrhizal symbiosis. Front. Plant Sci. 2013, 4, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; van der Heijden, M.G.A. The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci. 2015, 20, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Yang, S.; Li, F.; Malhi, S.S.; Wang, P.; Suo, D.; Wang, J. Long-term fertilization effects on crop yield and nitrate nitrogen accumulation in soil in northwestern China. Agron. J. 2004, 96, 1039–1049. [Google Scholar] [CrossRef]
- Barrow, C.J. Biochar: Potential for countering land degradation and for improving agriculture. Appl. Geogr. 2012, 34, 21–28. [Google Scholar] [CrossRef]
- Tardieu, F.; Draye, X.; Javaux, M. Root water uptake and ideotypes of the root system: Whole-plant controls matter. Vadose Zone J. 2017, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; Tittal, M.; Mir, R.A.; Agarwal, R. Alleviation of water and osmotic stress-induced changes in nitrogen metabolizing enzymes in Triticum aestivum L. cultivars by potassium. Protoplasma 2017, 254, 1953–1963. [Google Scholar] [CrossRef]
- Tardieu, F.; Granier, C.; Muller, B. Water deficit and growth. co-ordinating processes without an orchestrator? Curr. Opin. Plant Biol. 2011, 14, 283–289. [Google Scholar] [CrossRef]
- Marulanda, A.; Azcón, R.; Ruiz-Lozano, J.M. Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiol. Plant. 2003, 119, 526–533. [Google Scholar] [CrossRef]
- Hallett, P.D.; Feeney, D.S.; Bengough, A.G.; Rillig, M.C.; Scrimgeour, C.M.; Young, I.M. Disentangling the impact of am fungi versus roots on soil structure and water transport. Plant Soil 2009, 314, 183–196. [Google Scholar] [CrossRef]
- Smith, S.E.; Facelli, E.; Pope, S.; Smith, F.A. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 2010, 326, 3–20. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ghalavand, A.; Dolatabadian, A.; Jamshidi, E.; Khodaei-Joghan, A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 2013, 117, 106–114. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Hormonal responses in host plants triggered by arbuscular mycorrhizal fungi. In Arbuscular Mycorrhizas: Physiology and Function; Koltai, H., Kapulnik, Y., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 169–190. [Google Scholar] [CrossRef]
- Wu, Q.S.; Zou, Y.N. Arbuscular mycorrhizal fungi and tolerance of drought stress in plants. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Wu, Q.S., Ed.; Springer: Singapore, 2017; pp. 25–41. [Google Scholar] [CrossRef]
- Kong, C.; Camps-Arbestain, M.; Clothier, B.; Bishop, P.; Vázquez, F.M. Use of either pumice or willow-based biochar amendments to decrease soil salinity under arid conditions. Environ. Technol. Innov. 2021, 24, 101849. [Google Scholar] [CrossRef]
- Hussain, S.; Shaukat, M.; Ashraf, M.; Zhu, C.; Jin, Q.; Zhang, J. Salinity stress in arid and semi-arid climates: Effects and management in field crops. In Climate Change and Agriculture; Hussain, S., Ed.; InTechOpen: London, UK, 2019; p. 198. [Google Scholar] [CrossRef] [Green Version]
- FAO. FAOSTAT 2015. Available online: http://faostat3.fao.org/browse/area/159/E (accessed on 14 March 2022).
- Wang, Y.; Wu, W.H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013, 64, 451–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [Green Version]
- Ismail, A.M.; Horie, T. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Ann. Rev. Plant Biol. 2017, 68, 405–434. [Google Scholar] [CrossRef] [Green Version]
- Yamato, M.; Ikeda, S.; Iwase, K. Community of arbuscular mycorrhizal fungi in a coastal vegetation on okinawa island and effect of the isolated fungi on growth of sorghum under salt-treated conditions. Mycorrhiza 2008, 18, 241–249. [Google Scholar] [CrossRef]
- Giri, B.; Kapoor, R.; Mukerji, K.G. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb. Ecol. 2007, 54, 753–760. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Azcon, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. new perspectives for molecular studies. Mycorrhiza 2003, 13, 309–317. [Google Scholar] [CrossRef]
- Khalid, M.; Ur-Rahman, S.; Hassani, D.; Hayat, K.; Zhou, P.; Hui, N. Advances in fungal-assisted phytoremediation of heavy metals: A review. Pedosphere 2021, 31, 475–495. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Chen, B.; Wu, Z.; Li, T.; Hu, Y.; Sun, Y.; Wang, Y. chromium immobilization by extraradical mycelium of arbuscular mycorrhiza contributes to plant chromium tolerance. Environ. Exp. Bot. 2016, 122, 10–18. [Google Scholar] [CrossRef]
- Zhan, F.; Li, B.; Jiang, M.; Yue, X.; He, Y.; Xia, Y.; Wang, Y. Arbuscular mycorrhizal fungi enhance antioxidant defense in the leaves and the retention of heavy metals in the roots of maize. Environ. Sci. Pollut. Res. 2018, 25, 24338–24347. [Google Scholar] [CrossRef] [PubMed]
- Hristozkova, M.; Geneva, M.; Stancheva, I.; Boychinova, M.; Djonova, E. Contribution of arbuscular mycorrhizal fungi in attenuation of heavy metal impact on Calendula officinalis development. Appl. Soil Ecol. 2016, 101, 57–63. [Google Scholar] [CrossRef]
- Chen, X.W.; Wu, F.Y.; Li, H.; Chan, W.F.; Wu, C.; Wu, S.C.; Wong, M.H. Phosphate transporters expression in rice (Oryza sativa L.) associated with arbuscular mycorrhizal fungi (AMF) colonization under different levels of arsenate stress. Environ. Exp. Bot. 2013, 87, 92–99. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Hu, Y.; Su, D.; Lv, J.; Li, G.; Zhang, Z.; et al. Transformation and immobilization of chromium by arbuscular mycorrhizal fungi as revealed by SEM-EDS, TEM-EDS, and XAFS. Environ. Sci. Technol. 2015, 49, 14036–14047. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Kumar Panda, S.; Bhattacharjee, M.K.; Dutta, S. Hrishikesh Upadhyaya. Role of arbuscular mycorrhiza in heavy metal tolerance in plants: Prospects for phytoremidiation. J. Phytol. 2010, 2010, 16–27. [Google Scholar]
- Pavithra, D.; Yapa, N. Arbuscular mycorrhizal fungi inoculation enhances drought stress tolerance of plants. Groundw. Sustain. Dev. 2018, 7, 490–494. [Google Scholar] [CrossRef]
- Pal, A.; Pandey, S. Role of arbuscular mycorrhizal fungi on plant growth and reclamation of barren soil with wheat (Triticum aestivum L.) crop. Int. J. Soil Sci. 2016, 12, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Goicoechea, N.; Antolín, M.C. Increased nutritional value in food crops. Microb. Biotechnol. 2017, 10, 1004–1007. [Google Scholar] [CrossRef] [Green Version]
- Goicoechea, N.; Bettoni, M.M.; Fuertes-Mendizábal, T.; González-Murua, C.; Aranjuelo, I.; Goicoechea, N.; Bettoni, M.M.; Fuertes-Mendizábal, T.; González-Murua, C.; Aranjuelo, I. Durum wheat quality traits affected by mycorrhizal inoculation, water availability and atmospheric CO2 concentration. Crop Pasture Sci. 2016, 67, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Ouledali, S.; Ennajeh, M.; Zrig, A.; Gianinazzi, S.; Khemira, H. Estimating the contribution of arbuscular mycorrhizal fungi to drought tolerance of potted olive trees (Olea europaea). Acta Physiol. Plant 2018, 40, 81. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, W.; Bi, N.; Guo, J.; Wang, L.; Zhao, J.; Zhang, J. Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl. Soil Ecol. 2015, 88, 41–49. [Google Scholar] [CrossRef]
- Boyer, L.R.; Brain, P.; Xu, X.M.; Jeffries, P. Inoculation of drought-stressed strawberry with a mixed inoculum of two arbuscular mycorrhizal fungi: Effects on population dynamics of fungal species in roots and consequential plant tolerance to water deficiency. Mycorrhiza 2015, 25, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Asrar, A.A.; Abdel-Fattah, G.M.; Elhindi, K.M. Improving growth, flower yield, and water relations of snapdragon (Antirhinum majus L.) plants grown under well-watered and water-stress conditions using arbuscular mycorrhizal fungi. Photosynthetica 2012, 50, 305–316. [Google Scholar] [CrossRef]
- Tsoata, E.; Njock, S.R.; Youmbi, E.; Nwaga, D. Early effects of water stress on some biochemical and mineral parameters of mycorrhizal Vigna subterranea (L.) Verdc. (Fabaceae) cultivated in cameroon. Int. J. Agron. Agric. Res. 2015, 7, 21–35. [Google Scholar]
- Zhang, F.; He, J.D.; Ni, Q.D.; Wu, Q.S.; Zou, Y.N. Enhancement of drought tolerance in trifoliate orange by mycorrhiza: Changes in root sucrose and proline metabolisms. Not. Bot. Horti Agrobot. 2018, 46, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Pedranzani, H.; Rodríguez-Rivera, M.; Gutiérrez, M.; Porcel, R.; Hause, B.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 2016, 26, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Yooyongwech, S.; Samphumphuang, T.; Tisarum, R.; Theerawitaya, C.; Cha-Um, S. Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci. Hortic. 2016, 198, 107–117. [Google Scholar] [CrossRef]
- Mirshad, P.P.; Puthur, J.T. Arbuscular mycorrhizal association enhances drought tolerance potential of promising bioenergy grass (Saccharum arundinaceum Retz.). Environ. Monit. Assess. 2016, 188, 425. [Google Scholar] [CrossRef]
- Amiri, R.; Nikbakht, A.; Etemadi, N. Alleviation of drought stress on Rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015, 197, 373–380. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, M.; Sulpice, R.; Chen, H.; Tian, S.; Ban, Y. Arbuscular mycorrhizal fungi alter fractal dimension characteristics of Robinia pseudoacacia L. seedlings through regulating plant growth, leaf water status, photosynthesis, and nutrient concentration under drought stress. J. Plant Growth Regul. 2014, 33, 612–625. [Google Scholar] [CrossRef]
- Zardak, S.G.; Dehnavi, M.M.; Salehi, A.; Gholamhoseini, M. Effects of using arbuscular mycorrhizal fungi to alleviate drought stress on the physiological traits and essential oil yield of fennel. Rhizosphere 2018, 6, 31–38. [Google Scholar] [CrossRef]
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.; Jing, G.; Li, C.; Ma, F. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 2020, 149, 245–255. [Google Scholar] [CrossRef]
- Arpanahi, A.A.; Feizian, M.; Mehdipourian, G.; Khojasteh, D.N. Arbuscular mycorrhizal fungi inoculation improve essential oil and physiological parameters and nutritional values of Thymus daenensis Celak and Thymus vulgaris L. under normal and drought stress conditions. Eur. J. Soil Biol. 2020, 100, 103217. [Google Scholar] [CrossRef]
- Khalloufi, M.; Martínez-Andújar, C.; Lachaâl, M.; Karray-Bouraoui, N.; Pérez-Alfocea, F.; Albacete, A. The interaction between foliar GA3 application and arbuscular mycorrhizal fungi inoculation improves growth in salinized tomato (Solanum lycopersicum L.) plants by modifying the hormonal balance. J. Plant Physiol. 2017, 214, 134–144. [Google Scholar] [CrossRef]
- Hajiboland, R.; Dashtebani, F.; Aliasgharzad, N. Physiological responses of halophytic C4 grass Aeluropus littoralis to salinity and arbuscular mycorrhizal fungi colonization. Photosynthetica 2015, 53, 572–584. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, M.; Li, Y.; Che, Y.; Xiao, Y. Effects of arbuscular mycorrhizal fungi, biochar and cadmium on the yield and element uptake of Medicago sativa. Sci. Total Environ. 2019, 655, 1150–1158. [Google Scholar] [CrossRef]
- Chen, X.W.; Wu, L.; Luo, N.; Mo, C.H.; Wong, M.H.; Li, H. Arbuscular mycorrhizal fungi and the associated bacterial community influence the uptake of cadmium in rice. Geoderma 2019, 337, 749–757. [Google Scholar] [CrossRef]
- Baghaie, A.H.; Aghili, F.; Jafarinia, R. Soil-indigenous arbuscular mycorrhizal fungi and zeolite addition to soil synergistically increase grain yield and reduce cadmium uptake of bread wheat (through improved nitrogen and phosphorus nutrition and immobilization of Cd in roots). Environ. Sci. Pollut. Res. 2019, 26, 30794–30807. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, J.; Wang, S.; Lin, Q.; Ruan, D.; Chi, H.; Zheng, M.; Chao, Y.; Qiu, R.; Yang, Y. Effects of cadmium-resistant plant growth-promoting rhizobacteria and Funneliformis mosseae on the cadmium tolerance of tomato (Lycopersicon esculentum L.). Int. J. Phytoremediat. 2020, 22, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Rafique, M.; Ortas, I.; Rizwan, M.; Sultan, T.; Chaudhary, H.J.; Işik, M.; Aydin, O. Effects of Rhizophagus clarus and biochar on growth, photosynthesis, nutrients, and cadmium (Cd) concentration of maize (Zea mays) grown in Cd-spiked soil. Environ. Sci. Pollut. Res. 2019, 26, 20689–20700. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, R.E.; Metwally, R.A. Alleviation of cadmium stress by arbuscular mycorrhizal symbiosis. Int. J. Phytoremediat. 2019, 21, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, X.; Yang, W.; Xu, Z.; Zhang, D.; Gao, S. The Effects of arbuscular mycorrhizal fungi on sex-specific responses to Pb pollution in Populus cathayana. Ecotoxicol. Environ. Saf. 2015, 113, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Wang, L.; Zhao, L.; Huang, X.C.; Ma, F. Arbuscular mycorrhizal fungi effect growth and photosynthesis of Phragmites australis (Cav.) Trin Ex. Steudel under copper stress. Plant Biol. 2020, 22, 62–69. [Google Scholar] [CrossRef]
- Ren, C.G.; Kong, C.C.; Wang, S.X.; Xie, Z.H. Enhanced phytoremediation of uranium-contaminated soils by arbuscular mycorrhiza and rhizobium. Chemosphere 2019, 217, 773–779. [Google Scholar] [CrossRef]
- Wang, S.; Pan, S.; Shah, G.M.; Zhang, Z.; Yang, L.; Yang, S. Enhancement in arsenic remediation by maize (Zea mays L.) Using EDTA in combination with arbuscular mycorrhizal fungi. Appl. Ecol. Environ. Res. 2018, 16, 5987–5999. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Oliveira, R.S.; Zhang, C.; Freitas, H. Potential of Plant Beneficial Bacteria and Arbuscular Mycorrhizal Fungi in Phytoremediation of Metal-Contaminated Saline Soils. J. Hazard. Mater. 2019, 379, 120813. [Google Scholar] [CrossRef]
- Debeljak, M.; van Elteren, J.T.; Špruk, A.; Izmer, A.; Vanhaecke, F.; Vogel-Mikuš, K. The role of arbuscular mycorrhiza in mercury and mineral nutrient uptake in maize. Chemosphere 2018, 212, 1076–1084. [Google Scholar] [CrossRef]
- Ferreira, P.A.A.; Ceretta, C.A.; Tiecher, T.; Facco, D.B.; Garlet, L.P.; Soares, C.R.F.S.; Soriani, H.H.; Nicoloso, F.T.; Giachini, A.J.; Brunetto, G.; et al. Rhizophagus clarus and phosphorus in Crotalaria Juncea: Growth, glomalin content and acid phosphatase activity in a copper-contaminated soil. Rev. Bras. Ciênc. Solo 2018, 42, e0170245. [Google Scholar] [CrossRef] [Green Version]
- de Andrade, S.A.L.; Domingues, A.P.; Mazzafera, P. Photosynthesis is induced in rice plants that associate with arbuscular mycorrhizal fungi and are grown under arsenate and arsenite stress. Chemosphere 2015, 134, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.M.M.; Mazen, M.B.E.D.; Nafady, N.A.; Monsef, O.A. bioavailability of cadmium and nickel to Daucus carota L. and Corchorus olitorius L. treated by compost and microorganisms. Soil Environ. 2017, 36, 01–12. [Google Scholar] [CrossRef]
- Garg, N.; Singh, S. Arbuscular mycorrhiza Rhizophagus irregularis and silicon modulate growth, proline biosynthesis and yield in Cajanus cajan L. Millsp. (Pigeonpea) genotypes under cadmium and zinc stress. J. Plant Growth Regul. 2018, 37, 46–63. [Google Scholar] [CrossRef]
- Singh, G.; Pankaj, U.; Chand, S.; Verma, R.K. Arbuscular mycorrhizal fungi-assisted phytoextraction of toxic metals by Zea mays L. from tannery sludge. Soil Sediment Contam. 2019, 28, 729–746. [Google Scholar] [CrossRef]
- Kafil, M.; Boroomand Nasab, S.; Moazed, H.; Bhatnagar, A. Phytoremediation potential of vetiver grass irrigated with wastewater for treatment of metal contaminated soil. Int. J. Phytoremediat. 2019, 21, 92–100. [Google Scholar] [CrossRef]
- Pasbani, B.; Salimi, A.; Aliasgharzad, N.; Hajiboland, R. Colonization with arbuscular mycorrhizal fungi mitigates cold stress through improvement of antioxidant defense and accumulation of protecting molecules in eggplants. Sci. Hortic. 2020, 272, 109575. [Google Scholar] [CrossRef]
- Calvo-Polanco, M.; Sánchez-Romera, B.; Aroca, R.; Asins, M.J.; Declerck, S.; Dodd, I.C.; Martínez-Andújar, C.; Albacete, A.; Ruiz-Lozano, J.M. Exploring the use of recombinant inbred lines in combination with beneficial microbial inoculants (AM Fungus and PGPR) to improve drought stress tolerance in tomato. Environ. Exp. Bot. 2016, 131, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi—From ecology to application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Atieno, M.; Herrmann, L.; Nguyen, H.T.; Phan, H.T.; Nguyen, N.K.; Srean, P.; Than, M.M.; Zhiyong, R.; Tittabutr, P.; Shutsrirung, A.; et al. Assessment of biofertilizer use for sustainable agriculture in the Great Mekong Region. J. Environ. Manag. 2020, 275, 111300. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy. 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Kisiriko, M.; Anastasiadi, M.; Terry, L.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules 2021, 26, 6343. [Google Scholar] [CrossRef] [PubMed]
- Basiru, S.; Mwanza, H.P.; Hijri, M. Analysis of arbuscular mycorrhizal fungal inoculant benchmarks. Miroorganisms 2021, 9, 81. [Google Scholar] [CrossRef] [PubMed]

| Stress | Host Species | Fungus | Mechanism Involved | References |
|---|---|---|---|---|
| Drought stress | Glycine max | Arbuscular mycorhizal fungi | Increased seed fresh and dry weight and photosynthesis | [84] |
| Triticum aestivum | Gigaspora decipiens, Glomus mosseae | Enhanced growth, chlorophyll content | [85] | |
| Triticum durum | Rhizophagus intraradices | In grains, increased levels of Zn, manganese, Fe, and copper (Cu) | [86,87] | |
| Olea europaea | Arbuscular mycorrhiza | Increased uptake of minerals | [88] | |
| Zea mays | Rhizophagus intraradices | Enhanced K, N, and P uptake | [89] | |
| Fragaria ananassa | Funneliformis geosporus BEG11 | Enhanced water usage efficiency | [90] | |
| Antirrhinum majus | Glomus deserticola | Enhanced level of proline and water, number of leaves | [91] | |
| Vigna subterranea | Gigaspora gregaria | Enhanced level of minerals and lower level of proline | [92] | |
| Pontius trifoliata | Paraglomus occultum | Improved rate of water absorption and length of the hypha | [93] | |
| Digitaria eriantha | Rhizophagus irregularis | Improve conductivity of stomata and dry matter of shoot | [94] | |
| Ipomoea batatas | Glomus species | Osmotic potential adjustment | [95] | |
| Saccharum arundinaceum | Glomus species | Improve the uptake of water, metabolites, phenolic, and glutathione levels | [96] | |
| Pelargonium graveolens | Funneliformis mosseae | Increase the contents of nutrients, essential oil, and biomass of plants | [97] | |
| Robinia pseudoacacia | Rhizophagus intraradices | Enhanced rate of photosynthesis and water-use efficiency | [98] | |
| Foeniculum vulgare | Arbuscular mycorhizal fungi | High production of essential oil, main the concentration of salts | [99] | |
| Malus domestica | Arbuscular mycorhizal fungi | Increasing the capacity of gaseous exchange, improving the fluorescence parameters of chlorophyll | [100] | |
| Thymus species | Arbuscular mycorhizal fungi | Increases dry weight of root and shoot, pigments of photosynthesis | [101] | |
| Salinity stress | Cucumis sativus | Glomus intraradices | Improved level of antioxidant enzymes | [45] |
| Oryza sativa | Claroideoglomus etunicalum | The increased conductivity of stomata and the rate of photosynthesis | [6] | |
| Solanum lycopersicum | Rhizophagus irregularis | Increased fresh weight of roots and shoots and number of leaves | [102] | |
| Aleurites moluccanus | Claroideoglomus etunicalum | Enhanced conductivity of stomata and level of soluble sugars | [103] | |
| Acacia species | Glomus fasciculate | Increased level of Cu, Zn, and P | [74] | |
| Aeluropus littoralis | Claroideoglomus etunicatum | Enhance the dry mass of roots and shoots, and conductivity of stomata | [103] | |
| Acacia nilotica | Glomus fasciculate | Enhance biomass of root and shoot | [74] | |
| Cd toxicity | Sesbania rostrata | Glomus mosseae | Enhances concentration of N and P | [42] |
| Medicago sativa | Glomus aggregation | Enhanced concentration of N and P in shoots and reduced cadmium concentration in shoots | [104] | |
| Oryza sativa | Funneliformis mosseae | Decreased uptake of cadmium | [105] | |
| Triticum aestivum | Indigenous | Enhanced growth in plant and decreased uptake of Cd | [106] | |
| Lycopersicon esculentum L. | Funneliformis mosseae | Increased growth in plant and restricted translocation of Cd from root to shoot | [107] | |
| Zea mays | Rhizophagus clarus | Enhanced dry matter production | [108] | |
| Trigonella foenum-graceum. | Glomus clarum, Acavlospora laevis | Enhances the function of antioxidant enzymes | [109] | |
| Pb toxicity | Populus cathayana | Funneliformis mosseae | Enhanced P uptake under stress | [110] |
| Cu toxicity | Phragmites australis | Rhizophagus irregularis | Improved plant growth and development and also enhanced the rate of photosynthesis | [111] |
| Uranium toxicity | Sesbania rostarataa | Glomus etunicatum | Increased biomass of plant | [112,113] |
| Arsenic (As) toxicity | Trifolium repens L. | Glomus versiforme | Increased antioxidant enzymes and dry biomass of plants | [113] |
| Nickel (Ni) toxicity | Helianthus annuus L. | Claroideoglomus claroideum | increased growth in plant | [114] |
| Mercury toxicity | Zea mays | Glomus sp., fungi from Glomeromycota | Enhanced biomass of plant and increased content of mercury in central cylinder of AMF colonized plants | [115] |
| Cu toxicity | Carotalaria juncea | Rhizophagus clarus | Increased plant growth and reduced phytotoxicity | [116] |
| As(III), As (IV) toxicity | Oryza sativa | Rhizophagus irregularis | Increased water use efficiency and chlorophyll concentration | [117] |
| Ni, Cd toxicity | Daucus carota L., Corchorus olitorius L. | Glomus mosseae, Gigaspora margarita | Improved plant growth and decreased accumulation of metals | [118] |
| Cd, Zn toxicity | Canjanus cajan | Rhizophagus irregularis | Improved fresh weight of root and shoot and area and leaf number | [119] |
| Cr, Ni, Cd, Pb toxicity | Zea mays | Rhizophagus intraradices, Rhizophagus fasciculatus | Enhanced concentration of chlorophyll and P and improved length of root and shoot | [120] |
| Pb, Cd, Cu, Zn toxicity | Vetiveria zizaniodes | Glomus mosseae | Increased biomass and decreased stress | [121] |
| Ni, Cd, Cr, Cu, Cd toxicity | Helianthus annuus L. | Funneliformis caledonium | Increased plant growth. Absorption of P and reduced concentration of heavy metal in shoots | [93] |
| Cold stress | Solanum melongena | Rhizophagus irregulars, Funneliformis mosseae | Improving photochemical reactions, reducing the damage in the membrane, and activating the antioxidants defense system | [122] |
| Heat stress | Solanum lycopersicum | Rhizophagus irregularis | Increased plant photosynthetic efficiency | [123] |
| Salinity-Alkali | Legmus Chinensis | Glomus mosseae | Enhanced water, P, and N concentration | [42] |
| Drought and salt stress | Ricinus communis | Arbuscular mycorhizal fungi | Activating the growth of plant and enhancing the net stomatal conductivity, rate of transpiration, and photosynthesis, and reducing the intercellular concentration of carbon dioxide. | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaliq, A.; Perveen, S.; Alamer, K.H.; Zia Ul Haq, M.; Rafique, Z.; Alsudays, I.M.; Althobaiti, A.T.; Saleh, M.A.; Hussain, S.; Attia, H. Arbuscular Mycorrhizal Fungi Symbiosis to Enhance Plant–Soil Interaction. Sustainability 2022, 14, 7840. https://doi.org/10.3390/su14137840
Khaliq A, Perveen S, Alamer KH, Zia Ul Haq M, Rafique Z, Alsudays IM, Althobaiti AT, Saleh MA, Hussain S, Attia H. Arbuscular Mycorrhizal Fungi Symbiosis to Enhance Plant–Soil Interaction. Sustainability. 2022; 14(13):7840. https://doi.org/10.3390/su14137840
Chicago/Turabian StyleKhaliq, Abdul, Shaista Perveen, Khalid H. Alamer, Muhammad Zia Ul Haq, Zaiba Rafique, Ibtisam M. Alsudays, Ashwaq T. Althobaiti, Muneera A. Saleh, Saddam Hussain, and Houneida Attia. 2022. "Arbuscular Mycorrhizal Fungi Symbiosis to Enhance Plant–Soil Interaction" Sustainability 14, no. 13: 7840. https://doi.org/10.3390/su14137840
APA StyleKhaliq, A., Perveen, S., Alamer, K. H., Zia Ul Haq, M., Rafique, Z., Alsudays, I. M., Althobaiti, A. T., Saleh, M. A., Hussain, S., & Attia, H. (2022). Arbuscular Mycorrhizal Fungi Symbiosis to Enhance Plant–Soil Interaction. Sustainability, 14(13), 7840. https://doi.org/10.3390/su14137840