Review of Thermochemical Technologies for Water and Energy Integration Systems: Energy Storage and Recovery
Abstract
:1. Introduction
2. Thermochemical Energy Storage (TCES)
2.1. Overview of TCES Potential
2.2. Description of TCES Technologies
| Technology | Technology Characterization | Operational Conditions | Refs. | |||
|---|---|---|---|---|---|---|
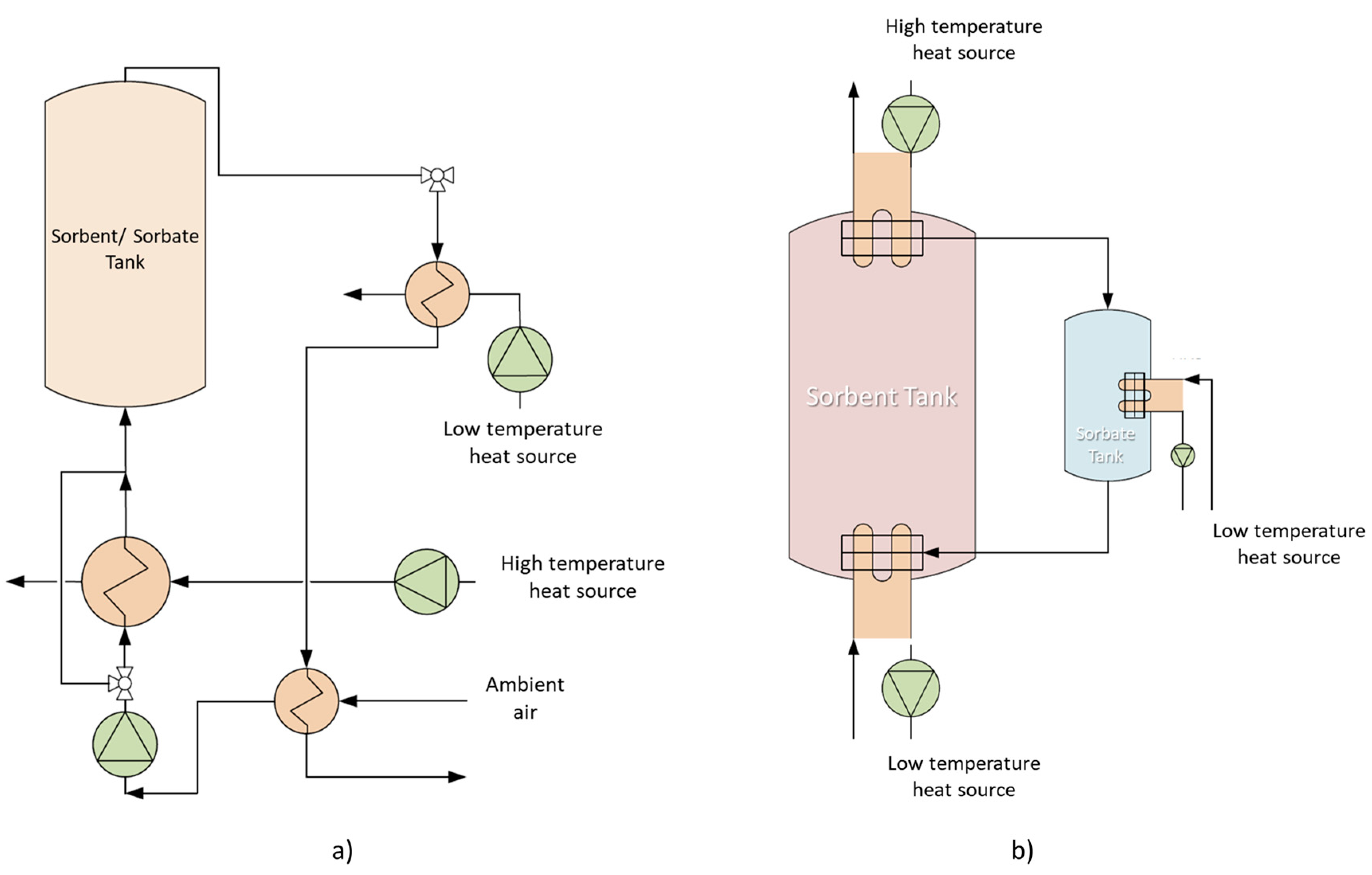
| Adsorption heat storage (AHS) | It is based on the phenomena of desorption (charging) and adsorption (discharging) of an air stream; The configurations for AHS may be classified into open and closed systems, which are pictorially presented in Figure 2; | Adsorption materials include zeolites (for desorption temperatures up to 180 °C and adsorption temperatures up to 80 °C), aluminophosphates/silico-aluminophosphates (for desorption temperatures of 95–140 °C and adsorption temperatures of 30–40 °C) and metal organic frameworks (for desorption temperatures of 90–140 °C and adsorption temperatures of 30–40 °C). | [26,27,28,29,30,31] | |||
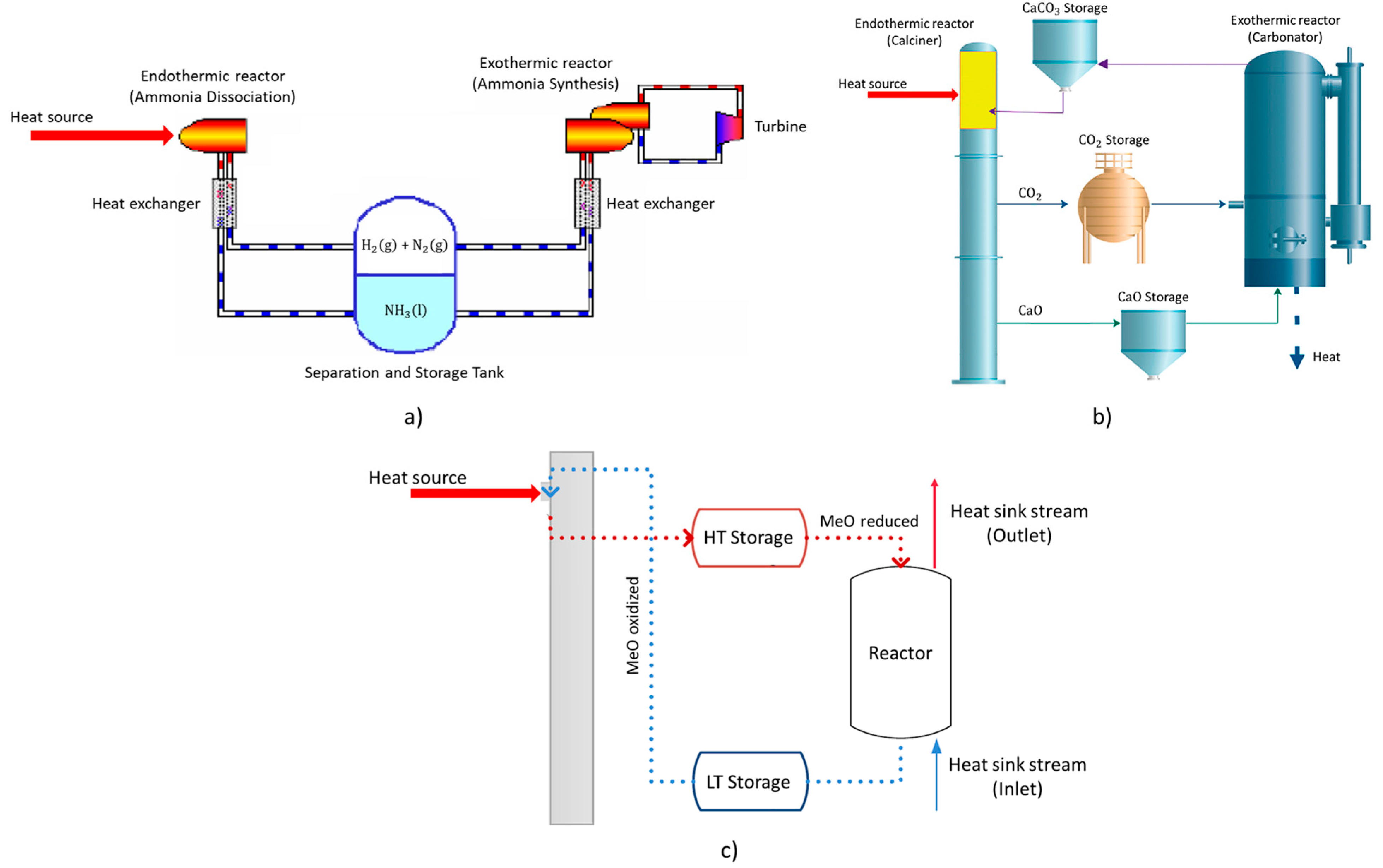
| Ammonia-based energy storage | It is based on the reactions of dissociation/synthesis of ammonia (NH3) into/from nitrogen gas (N2) and hydrogen gas (H2) (as described below); It is overall associated to the following advantages: (i) the reaction is single-step and does not require careful control; (ii) the reactants and products are stable at operating temperatures; (iii) the reactants and products are relatively abundant; (iv) possibility for the storage of liquid phase (NH3) and gas phase (N2 and H2) within the same tank due to density differences; The industrial system typically includes two reaction vessels (for dissociation and synthesis), a separation and storage tank and two heat exchangers—Figure 3a. | Operating temperatures overall vary within the range 400–1000 °C. | [32,33,34,35,36,37,38] | |||
| Reactions | ||||||
| Haber–Bosch synthesis (Endothermic) | (1) | |||||
| Calcium-looping energy storage | It is based on the reactions of calcination/carbonation of calcium carbonate (CaCO3) into/from calcium oxide (CaO) and carbon dioxide (CO2) (as described below); The industrial system encompasses three vessels for carbonate, calcium oxide and carbon dioxide—Figure 3b. | Carbonation occurs at about 650 °C, calcination occurs in much more higher temperatures. | [39,40,41,42,43] | |||
| Reactions | ||||||
| Calcination (Endothermic) | (2) | |||||
| Metal oxide energy storage | It is based on the reactions of oxidation/reduction of metal oxides (as described below); A typical industrial installation includes the supply of a heat source for the occurrence of reduction reaction and a reactor for the occurrence of the oxidation reaction, as represented in Figure 3c; The reaction enthalpy highly varies for different metal oxides. | The operational temperatures for the occurrence of reaction are set in the range of 700–1400 °C. | [44,45,46,47,48,49,50] | |||
| Occurring Reaction | ||||||
| Reduction (Endothermic) | (3) | |||||
3. Energy Recovery from Wastewater
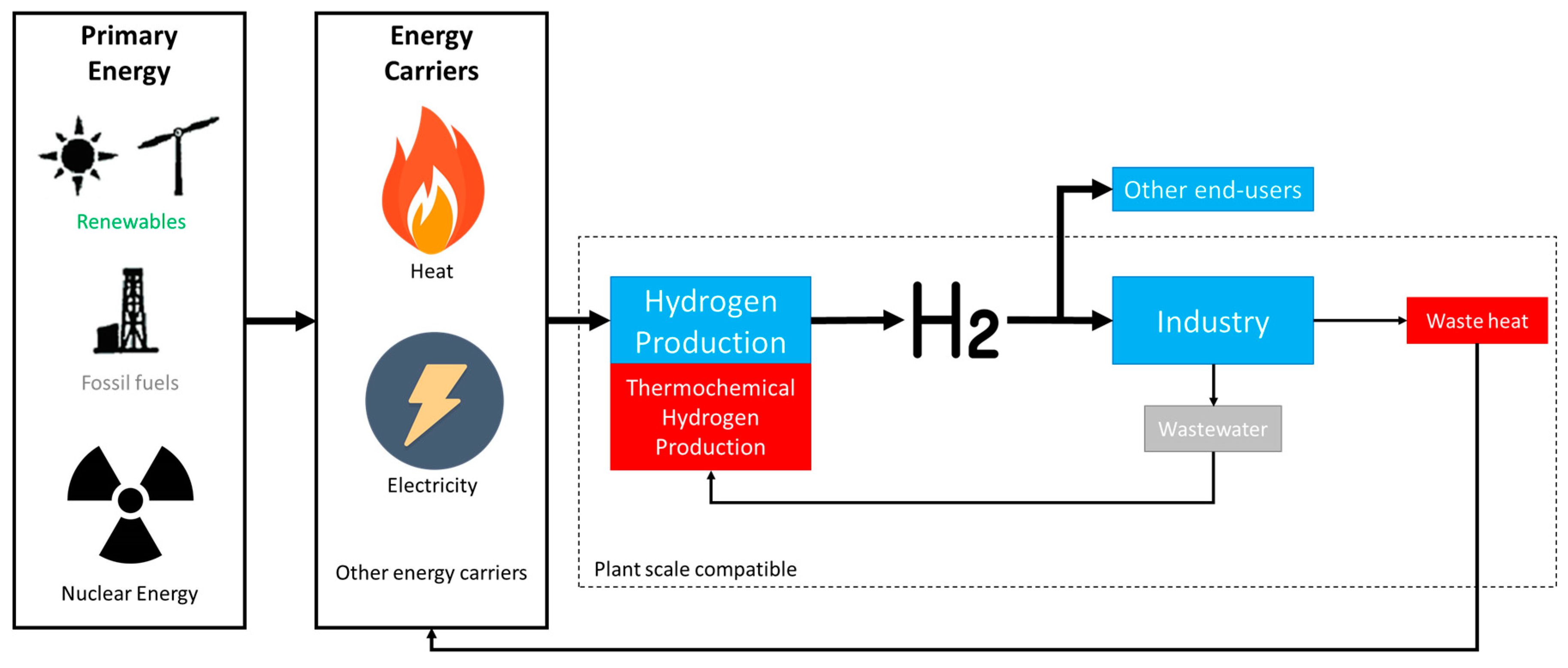
3.1. Framework of Alternative Fuel Production with Focus on Green Hydrogen
- Considerable electricity use (which is already an energy carrier and there is the possibility of additional energy losses by converting it into another energy carrier such as hydrogen);
- Low efficiency of commercial solar panels.
3.2. Traditional Wastewater-to-Energy Technologies (WWtE)
| Technology | Technology Characterization | Produced Fuel Characterization | Refs. |
|---|---|---|---|
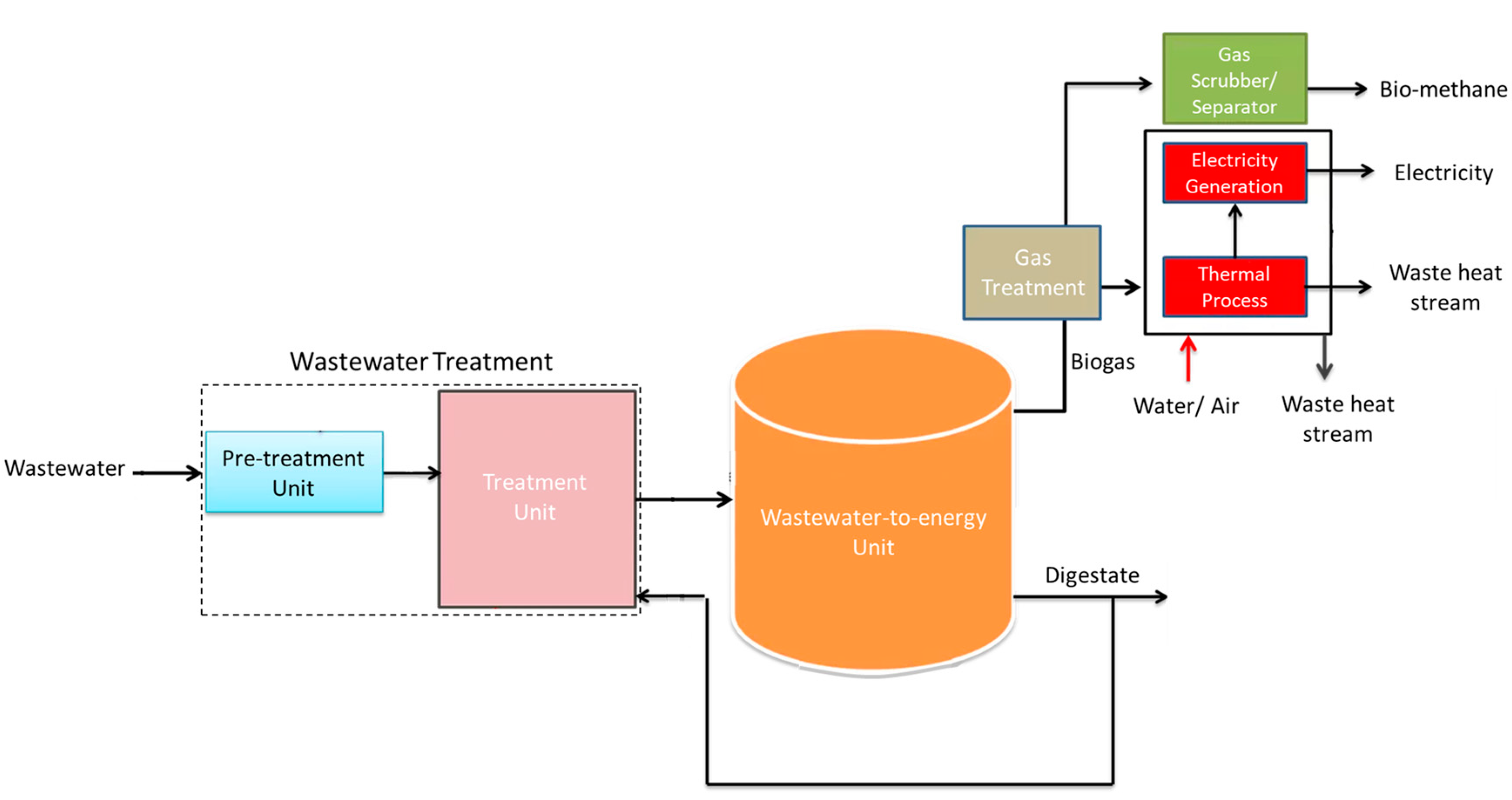
| Anaerobic Digestion | It is a process in which the output is primarily biogas, with a digestate resulting as the by-product; It is prominently applied for the treatment of wastewater streams with a significant load of organic materials, which are considerable prone to biological degradation; The anaerobic digestion process may be integrated in the operation of a WWT unit as represented in Figure 5a. | The produced biogas may be injected in natural gas networks, through the process of separation of carbon dioxide and other contaminants to turn biogas into biomethane. | [60,61,62,63,64,65,66] |
| Gasification | It subsists on the partial oxidation of biodegradable material present in wastewater streams for the production of synthesis gas (syngas), as well as a solid fraction of char as by-product; The gasification process may be integrated in the operation of a WWT unit as represented in Figure 5b. | The produced syngas is commonly composed by hydrogen (H2), carbon monoxide (CO), carbon dioxide (CO2) and methane (CH4); The produced syngas is an intermediate in the production of other fuel gases, such as diesel fuel (by Fischer-Tropch process) and hydrogen (which must be refined for its use in fuel cells). | [67,68,69,70,71,72] |
| Electrolysis | It is a process that uses an electric current to produce hydrogen, based on oxidation-reduction reactions; A set of by-products (such as chlorine and sodium hydroxide) may also be generated, as represented in Figure 5c; Several types of electrolysis processes exist, such as: alkaline water electrolysis, solid oxide electrolysis, microbial electrolysis and PEM water electrolysis; | The produced hydrogen may be directly injected into the natural gas fuel supply to combustion-based processes, through processes of production of hydrogen-enriched natural gas (HENG). | [73,74,75,76,77,78,79,80,81,82] |
3.3. Thermochemical Water Splitting (TWS)
| Technology | Technology Characterization | Operational Conditions | Refs. | |||
|---|---|---|---|---|---|---|
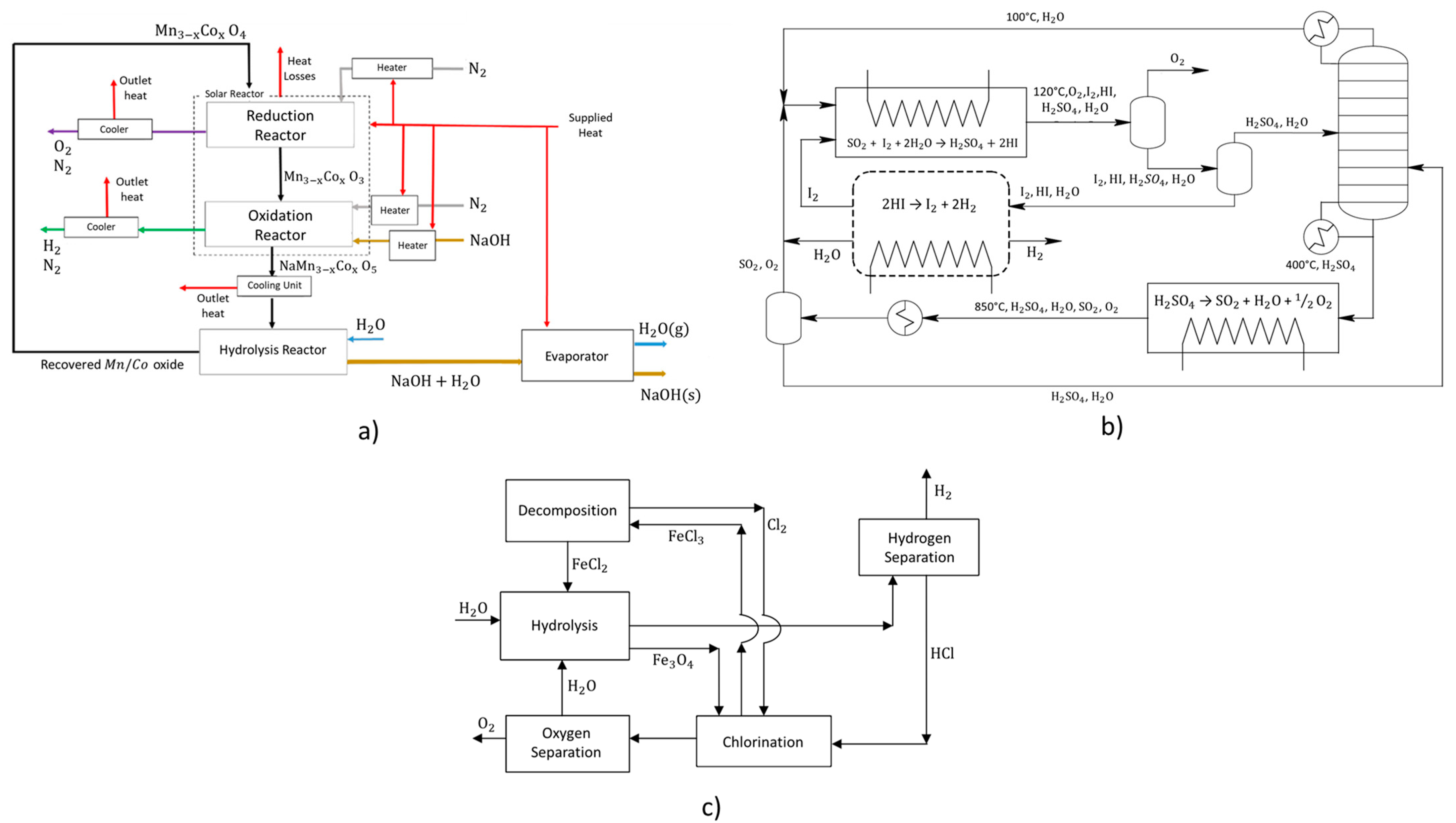
| Metal oxide cycle | It is a two-step thermal cycle based on the redox reactions of metal oxides (as described below); It presents the following advantages in comparison to the remaining thermal cycles: (i) in terms of input-output streams, wastewater and heat are the only inputs and hydrogen and oxygen are the only outputs; (ii) the produced H2 and O2 are separated in different reactions; (iii) the existence of continuous recycling of reactants and products; (iv) the produced H2 gas is pure; A typical installation encompassing this thermal cycle is represented in Figure 6a; | Typical metal oxides implemented for this type of thermal cycle are: CdO/Cd, ZnO/Zn, SnO2/SnO, Mn2O3/MnO, CeO2/Ce2O3 and Fe3O4/FeO; The operational temperatures across the cycle are in the range of 900–2000 °C; The reaction enthalpy highly varies for different metal oxides. | [50,59,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99] | |||
| Reactions | ||||||
| Reduction | (5) | |||||
| Oxidation | (6) | |||||
| Sulfur-iodine cycle | It is three-step thermal cycle based on the use of sulfur and iodine components (as described by the reactions below); The advantage of being a significantly high efficiency hydrogen production system, although it as an associated drawback of the involvement of high corrosive sulfuric and iodic acids; A typical installation of this thermal cycle is represented in Figure 6b. | Reaction (CE7) typically occurs at about 120 °C, (CE8) above 800 °C and (CE9) above 350 °C. | [10,100,101,102,103,104] | |||
| Reactions | ||||||
| Sulfuric acid decomposition | (7) | |||||
| Bunsen reaction | (8) | |||||
| Iodic acid decomposition | (9) | |||||
| Iron-chlorine cycle | It is a four-step thermal cycle based on the use of iron and chlorine components (as described by the reactions below); A typical installation of this thermal cycle is represented in Figure 6c. | Thermal decomposition occurs at 425 °C, the reverse Deacon reaction and hydrolysis in the range 525–925 °C and chlorination at 125 °C. | [105,106,107] | |||
| Reactions | ||||||
| Thermal Decomposition | (10) | |||||
| Reverse Deacon Reaction | (11) | |||||
| Chlorination | (12) | |||||
| Hydrolysis | (13) | |||||
4. Thermochemical Technologies for Water and Energy Integration
| Aspect | Characterization | Refs. |
|---|---|---|
| Thermochemical Energy Storage for Heat Recovery from Thermal Processes | The functioning of TCES units is analyzed in terms of the supply of variable quantities of thermal energy recovered form waste heat streams; The studies are generally focused on:
| [108,109] |
| Use of thermal energy to drive Wastewater-to-energy units | The use of thermal energy (namely the one generated from solar thermal systems) is analyzed for the functioning of WWtE units; These studies are generally focused on:
| [110,111] |
- Analysis of the integration of these units for sustainable fuel generation to be used as additional fuel streams in thermal processes;
- Assessment of TWS integration as a (potentially) more efficient form of hydrogen production in comparison to electrolysis;
- Use of waste heat streams as an alternative heat source for TWS instead of solar thermal.
5. Conclusions
- Sorption and reaction-based technologies present a significantly higher overall potential compared to standard thermal storage technologies owing to the increased energy storage capacity;
- The approached open and closed system Adsorption Heat Storage (AHS) present an apparatus and operational conditions (lower temperature) that are more adequate to industrial waste heat recovery in comparison to reaction-based technologies (which are commonly set to be installed for the heat source component to be a solar thermal system).
- Thermochemical water splitting has a higher operational potential in comparison to the approached traditional technologies (anaerobic digestion, gasification and electrolysis), which is due to the decreased number of steps to produced hydrogen from wastewater (in comparison to anaerobic digestion and gasification) and energy conversion steps (in comparison to electrolysis);
- The required enthalpy to be supplied as the driving force for these technologies is significantly high and requires heat sources which are not compatible with the waste heat potential of industrial sites (they require the supply of thermal energy from nuclear reactors and solar thermal systems instead).
- Thermochemical technologies have been proved to be structurally compatible with the general concept of WEIS, with a small number of studies performing equipment sizing;
- The potential associated to these technologies in terms of environmental benefits is still set to be furtherly calculated (for instance, for the definition of typical and potential values for overall water savings, energy savings and pollutant reduction);
- The existing conceptualization for integration of wastewater treatment and WWtE units for additional energy generation is valid in the scope of the overall concept of WEIS, although it still subsists in a deeper comprehension on energy supply and demand analysis and in terms of the modification of the destination processes for the supply of the new fuels.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| AHS | Adsorption heat storage |
| EU | European Union |
| GHG | Greenhouse gases |
| HENG | Hydrogen-enriched natural gas |
| R&D | Research & Development |
| TCES | Thermochemical energy storage |
| TCT | Thermochemical technology |
| TES | Thermal energy storage |
| TRL | Technology readiness level |
| TWS | Thermochemical water splitting |
| WEIS | Water and Energy Integration Systems |
| WHR | Waste heat recovery |
| WWtE | Wastewater-to-energy |
References
- Hu, M.; Ye, Z.; Zhang, H.; Chen, B.; Pan, Z.; Wang, J. Thermochemical conversion of sewage sludge for energy and resource recovery: Technical challenges and prospects. Environ. Pollut. Bioavailab. 2021, 33, 145–163. [Google Scholar] [CrossRef]
- Hritz, S.; Eusha, M.; Mir, F.R.; Schories, G. Life cycle analysis of the solar thermochemical energy storage scheme SOCRATCES. Open Res. Eur. 2022, 2, 14. [Google Scholar] [CrossRef]
- DOE-USA Hydrogen Production: Thermochemical Water Splitting. 2013; pp. 1–4. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-thermochemical-water-splitting (accessed on 1 June 2022).
- Oliveira, M.C.; Iten, M.; Cruz, P.L.; Monteiro, H. Review on energy efficiency progresses, technologies and strategies in the ceramic sector focusing on waste heat recovery. Energies 2020, 13, 6096. [Google Scholar] [CrossRef]
- Salgot, M.; Folch, M. Wastewater treatment and water reuse. Curr. Opin. Environ. Sci. Health 2018, 2, 64–74. [Google Scholar] [CrossRef]
- Miró, L.; Gasia, J.; Cabeza, L.F. Thermal energy storage (TES) for industrial waste heat (IWH) recovery: A review. Appl. Energy 2016, 179, 284–301. [Google Scholar] [CrossRef] [Green Version]
- Nazari, L.; Sarathy, S.; Santoro, D.; Ho, D.; Ray, M.B.; Xu, C.C. 3-Recent advances in energy recovery from wastewater sludge. In Direct Thermochemical Liquefaction for Energy Applications; Woodhead Publishing Limited: Sawston, Cambridge, UK, 2018; pp. 67–100. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, Q.; Huang, H.; Mao, H.; Liu, Y.; Xiao, Y. Applications of low-temperature thermochemical energy storage systems for salt hydrates based on material classification: A review. Sol. Energy 2021, 214, 149–178. [Google Scholar] [CrossRef]
- Tizzoni, A.C.; Giaconia, A.; Lanchi, M. Splitting water with renewable Heat: Green hydrogen beyond Electrolysis. Energ. Ambiente E Innov. 2021, 88–94. [Google Scholar] [CrossRef]
- Hiroki, N.; Yu, K.; Nobuyuki, T.; Hiroaki, T.; Jin, I.; Seiji, K.; Myagmarjav, O.; Yoshiyuki, I.; Shinji, K. Hydrogen production using thermochemical water-splitting Iodine–Sulfur process test facility made of industrial structural materials: Engineering solutions to prevent iodine precipitation. Int. J. Hydrogen Energy 2021, 46, 22328–22343. [Google Scholar] [CrossRef]
- Felix, C.B.; Ubando, A.T.; Chen, W.H.; Goodarzi, V.; Ashokkumar, V. COVID-19 and industrial waste mitigation via thermochemical technologies towards a circular economy: A state-of-the-art review. J. Hazard. Mater. 2022, 423, 127215. [Google Scholar] [CrossRef] [PubMed]
- Zaini, I.N. Enhancing the Circular Economy: Resource Recovery through Thermochemical Conversion Processes of Landfi ll Waste and Biomass. Doctoral Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2021; pp. 1–85. [Google Scholar]
- European Commission Energy and the Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal/energy-and-green-deal_en (accessed on 8 June 2022).
- European Comission EU Strategy on Energy System Integration|Energy. 9 April 2020. Available online: https://ec.europa.eu/energy/topics/energy-system-integration/eu-strategy-energy-system-integration_en (accessed on 8 June 2022).
- European Commission-Press Release Powering a Climate-Neutral Economy: Commission Sets out Plans for the Energy System of the Future and Clean Hydrogen. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_20_1259 (accessed on 8 June 2022).
- Oliveira, M.C.; Iten, M.; Matos, H.A. Assessment of energy efficiency improvement in ceramic industry through waste heat recovery modelling. Comput. Aided Chem. Eng. 2021, 50, 1653–1658. [Google Scholar] [CrossRef]
- Castro Oliveira, M.; Iten, M. Modelling of A Solar Thermal Energy System For Energy Efficiency Improvement In A Ceramic Plant. Renew. Energy Environ. Sustain. 2021, 6, 31. [Google Scholar] [CrossRef]
- Iten, M.; Fernandes, U.; Oliveira, M.C. Framework to assess eco-efficiency improvement: Case study of a meat production industry. Energy Rep. 2021, 7, 7134–7148. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Vieira, S.M.; Iten, M.; Matos, H.A. Optimisation of Water-Energy Networks in Process Industry: Implementation of Non-Linear and Multi-Objective Models. Front. Chem. Eng. 2022, 3, 750411. [Google Scholar] [CrossRef]
- Lizana, J.; Chacartegui, R.; Barrios-Padura, A.; Valverde, J.M.; Ortiz, C. Identification of best available thermal energy storage compounds for low-to-moderate temperature storage applications in buildings. Mater. Constr. 2018, 68, 160. [Google Scholar] [CrossRef] [Green Version]
- Abedin, A.H. A Critical Review of Thermochemical Energy Storage Systems. Open Renew. Energy J. 2011, 4, 42–46. [Google Scholar] [CrossRef] [Green Version]
- Sarbu, I.; Sebarchievici, C. A comprehensive review of thermal energy storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Riffat, S.B. Thermochemical energy storage technologies for building applications: A state-of-the-art review. Int. J. Low-Carbon Technol. 2013, 8, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Rouhani, M. Sorption thermal energy storage for sustainable heating and cooling. Ph.D. Thesis, Simon Fraser University, Burnaby, BC, Canada, 2019. [Google Scholar]
- André, L.; Abanades, S. Recent advances in thermochemical energy storage via solid–gas reversible reactions at high temperature. Energies 2020, 13, 5859. [Google Scholar] [CrossRef]
- Farulla, G.A.; Cellura, M.; Guarino, F.; Ferraro, M. A review of thermochemical energy storage systems for power grid support. Appl. Sci. 2020, 10, 3142. [Google Scholar] [CrossRef]
- Ayaz, H.; Chinnasamy, V.; Yong, J.; Cho, H. Review of technologies and recent advances in low-temperature sorption thermal storage systems. Energies 2021, 14, 6052. [Google Scholar] [CrossRef]
- Krönauer, A.; Lävemann, E.; Brückner, S.; Hauer, A. Mobile sorption heat storage in industrial waste heat recovery. Energy Procedia 2015, 73, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C.M. Sorption heat storage for long-term low-temperature applications: A review on the advancements at material and prototype scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Abedin, A.H.; Rosen, M.A. Closed and open thermochemical energy storage: Energy- and exergy-based comparisons. Energy 2012, 41, 83–92. [Google Scholar] [CrossRef]
- Vasta, S.; Brancato, V.; La Rosa, D.; Palomba, V.; Restuccia, G.; Sapienza, A.; Frazzica, A. Adsorption heat storage: State-of-the-art and future perspectives. Nanomaterials 2018, 8, 522. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Lovegrove, K.M.; Sepulveda, A.; Lavine, A.S. Design and optimization of an ammonia synthesis system for ammonia-based solar thermochemical energy storage. Sol. Energy 2018, 159, 992–1002. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, L.; Kong, M.; Lavine, A.S. Heat recovery from an autothermal ammonia synthesis reactor for solar thermochemical energy storage. Sol. Energy 2018, 176, 256–266. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Qi, C.; Ling, X.; Peng, H. State of the art on the high-temperature thermochemical energy storage systems. Energy Convers. Manag. 2018, 177, 792–815. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, L.; Lavine, A.S. Feasibility of using ammonia-based thermochemical energy storage to produce high-temperature steam or sCO2. Sol. Energy 2018, 176, 638–647. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; Aryafar, H.; Wen, T.; Lavine, A.S. Performance of conical ammonia dissociation reactors for solar thermochemical energy storage. Appl. Energy 2019, 255. [Google Scholar] [CrossRef]
- Xia, Q.; Feng, S.; Kong, M.; Chen, C. Efficiency enhancement of an ammonia-based solar thermochemical energy storage system implemented with hydrogen permeation membrane. Sustainability 2021, 13, 12783. [Google Scholar] [CrossRef]
- IEA ECES ANNEX 23. Applying Energy Storage in Ultra-low Energy Buildings—Final Report. 2014. Available online: https://doi.org/10.13140/RG.2.1.4995.4727 (accessed on 8 June 2022).
- Tregambi, C.; Montagnaro, F.; Salatino, P.; Solimene, R. A model of integrated calcium looping for CO2 capture and concentrated solar power. Sol. Energy 2015, 120, 208–220. [Google Scholar] [CrossRef]
- Valverde, J.M.; Barea-López, M.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Effect of Thermal Pretreatment and Nanosilica Addition on Limestone Performance at Calcium-Looping Conditions for Thermochemical Energy Storage of Concentrated Solar Power. Energy Fuels 2017, 31, 4226–4236. [Google Scholar] [CrossRef]
- Pillai, B.B.K.; Surywanshi, G.D.; Patnaikuni, V.S.; Anne, S.B.; Vooradi, R. Performance analysis of a double calcium looping-integrated biomass-fired power plant: Exploring a carbon reduction opportunity. Int. J. Energy Res. 2019, 43, 5301–5318. [Google Scholar] [CrossRef]
- Ortiz, C.; Valverde, J.M.; Chacartegui, R.; Pérez-Maqueda, L.A.; Gimenez-Gavarrell, P. Scaling-up the calcium-looping process for co2 capture and energy storage. KONA Powder Part. J. 2021, 38, 189–208. [Google Scholar] [CrossRef] [Green Version]
- Valverde, J.M. The ca-looping process for CO2 capture and energy storage: Role of nanoparticle technology. J. Nanoparticle Res. 2018, 20, 1–16. [Google Scholar] [CrossRef]
- Preisner, N.C.; Bürger, I.; Wokon, M.; Linder, M. Numerical investigations of a counter-current moving bed reactor for thermochemical energy storage at high temperatures. Energies 2020, 13, 772. [Google Scholar] [CrossRef] [Green Version]
- Block, T.; Schmücker, M. Metal oxides for thermochemical energy storage: A comparison of several metal oxide systems. Sol. Energy 2016, 126, 195–207. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Nellore, R.; Moghtaderi, B. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle. Energy Convers. Manag. 2018, 168, 421–453. [Google Scholar] [CrossRef]
- Furtado, O.R. Metal oxides and the thermochemical storage of solar energy. Chim. Oggi/Chem. Today 2019, 37, 16–18. [Google Scholar]
- Moya, J.; Marugán, J.; Orfila, M.; Díaz-Pérez, M.A.; Serrano-Ruiz, J.C. Improved thermochemical energy storage behavior of manganese oxide by molybdenum doping. Molecules 2021, 26, 583. [Google Scholar] [CrossRef]
- Miller, J.E.; Ambrosini, A.; Babiniec, S.M.; Coker, E.N.; Ho, C.K.; Al-Ansary, H.; Jeter, S.M.; Loutzenhiser, P.G.; Johnson, N.G.; Stechel, E.B. High performance reduction/oxidation metal oxides for thermochemical energy storage (PROMOTES). In Proceedings of the ASME 2016 10th International Conference on Energy Sustainability Collocated with the ASME 2016 Power Conference and the ASME 2016 14th International Conference on Fuel Cell Science, Engineering and Technology, Charlotte, NC, USA, 26–30 June 2016; Volume 1. [Google Scholar] [CrossRef]
- Dessie, Y.G.; Hong, Q.; Lougou, B.G.; Zhang, J.; Jiang, B.; Anees, J.; Tegegne, E.B. Thermochemical energy storage performance analysis of (Fe,co,mn)ox mixed metal oxides. Catalysts 2021, 11, 362. [Google Scholar] [CrossRef]
- Bora, R.R.; Richardson, R.E.; You, F. Resource recovery and waste-to-energy from wastewater sludge via thermochemical conversion technologies in support of circular economy: A comprehensive review. BMC Chem. Eng. 2020, 2, 8. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Sheffield, J.W.; Martin, K.B.; Folkson, R. Electricity and hydrogen as energy vectors for transportation vehicles. In Alternative Fuels and Advanced Vehicle Technologies for Improved Environmental Performance; Woodhead Publishing: Sawston, UK, 2014; pp. 117–137. [Google Scholar] [CrossRef]
- Governo de Portugal Estratégia Nacional Para o Hidrogénio; Diário da República: Lisbon, Portugal, 2019; pp. 5–13.
- AFDC Hydrogen Basics. Available online: https://afdc.energy.gov/fuels/hydrogen_basics.html (accessed on 8 June 2022).
- Rievaj, V.; Gaňa, J.; Synák, F. Is hydrogen the fuel of the future? Transp. Res. Procedia 2019, 40, 469–474. [Google Scholar] [CrossRef]
- Boretti, A.; Nayfeh, J.; Al-Maaitah, A. Hydrogen Production by Solar Thermochemical Water-Splitting Cycle via a Beam Down Concentrator. Front. Energy Res. 2021, 9, 666191. [Google Scholar] [CrossRef]
- Boretti, A. Technology Readiness Level of Solar Thermochemical Splitting Cycles. ACS Energy Lett. 2021, 6, 1170–1174. [Google Scholar] [CrossRef]
- Safari, F.; Dincer, I. A review and comparative evaluation of thermochemical water splitting cycles for hydrogen production. Energy Convers. Manag. 2020, 205, 112182. [Google Scholar] [CrossRef]
- Sevillano, C.A.; Pesantes, A.A.; Peña Carpio, E.; Martínez, E.J.; Gómez, X. Anaerobic digestion for producing renewable energy-the evolution of this technology in a new uncertain scenario. Entropy 2021, 23, 145. [Google Scholar] [CrossRef]
- Saltworks Anaerobic Digester Wastewater Management during Biogas Production. Available online: https://www.saltworkstech.com/articles/anaerobic-digester-wastewater-management-during-biogas-production/ (accessed on 8 June 2022).
- Park, C.; Chon, D.H.; Brennan, A.; Eom, H. Investigation of sludge reduction and biogas generation in high-rate anaerobic side-stream reactors for wastewater treatment. Environ. Sci. Water Res. Technol. 2018, 4, 1829–1838. [Google Scholar] [CrossRef]
- Wan, J.; Gu, J.; Zhao, Q.; Liu, Y. COD capture: A feasible option towards energy self-sufficient domestic wastewater treatment. Sci. Rep. 2016, 6, 25054. [Google Scholar] [CrossRef]
- Vicentin, R.; Fdz-Polanco, F.; Fdz-Polanco, M. Energy integration in wastewater treatment plants by anaerobic digestion of urban waste: A process design and simulation study. Int. J. Chem. Eng. 2019, 2019, 2621048. [Google Scholar] [CrossRef]
- Chow, W.L.; Chong, S.; Lim, J.W.; Chan, Y.J.; Chong, M.F.; Tiong, T.J.; Chin, J.K.; Pan, G.T. Anaerobic co-digestion of wastewater sludge: A review of potential co-substrates and operating factors for improved methane yield. Processes 2020, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Savickis, J.; Zemite, L.; Zeltins, N.; Bode, I.; Jansons, L.; Dzelzitis, E.; Koposovs, A.; Selickis, A.; Ansone, A. The biomethane injection into the natural gas networks: The EU’s gas synergy path. Latv. J. Phys. Tech. Sci. 2020, 57, 34–50. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. Gasification of sewage sludge. In Industrial and Municipal Sludge Industrial and Municipal Sludge Emerging Concerns and Scope for Resource Recovery; Butterworth-Heinemann: Oxford, UK, 2019; pp. 575–593. [Google Scholar] [CrossRef]
- Migliaccio, R.; Brachi, P.; Montagnaro, F.; Papa, S.; Tavano, A.; Montesarchio, P.; Ruoppolo, G.; Urciuolo, M. Sewage Sludge Gasification in a Fluidized Bed: Experimental Investigation and Modeling. Ind. Eng. Chem. Res. 2021, 60, 5034–5047. [Google Scholar] [CrossRef]
- Werle, S. Sewage sludge gasification process for clean and sustainable environment. Renew. Energy Environ. Sustain. 2016, 1, 35. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. Influence of wastewater treatment and the method of sludge disposal on the gasification process. Ecol. Chem. Eng. S 2014, 21, 255–268. [Google Scholar] [CrossRef] [Green Version]
- Metawater Wastewater Sludge Gasification Power Generation System. Available online: http://www.metawater.co.jp/eng/product/plant/sewer/gas_convert/ (accessed on 8 June 2022).
- Capodaglio, A.G.; Bolognesi, S. Ecofuel feedstocks and their prospects. In Advances in Eco-Fuels for a Sustainable Environment; Woodhead Publishing: Sawston, UK, 2019; pp. 15–51. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, J.; Meng, L.; Liu, L. Efficient hydrogen production by saline water electrolysis at high current densities without the interfering chlorine evolution. J. Mater. Chem. A 2021, 9, 22248–22253. [Google Scholar] [CrossRef]
- Chlorine Chemistry Chlorine Manufacturing and Production. Available online: https://www.chlorine.org/what-is-chlorine/manufacturing/ (accessed on 10 June 2022).
- Naimi, Y.; Antar, A. Hydrogen Generation by Water Electrolysis. In Advances in Hydrogen Generation Technologies; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Coskun Avci, A.; Toklu, E. A new analysis of two phase flow on hydrogen production from water electrolysis. Int. J. Hydrogen Energy 2021, 47, 6986–6995. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis–A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Scott, K. Introduction to Electrolysis, Electrolysers and Hydrogen Production. In Electrochemical Methods for Hydrogen Production; Royal Society of Chemistry: London, UK, 2020; pp. 1–27. [Google Scholar]
- Cavaliere, P.D.; Perrone, A.; Silvello, A. Water electrolysis for the production of hydrogen to be employed in the ironmaking and steelmaking industry. Metals 2021, 11, 1816. [Google Scholar] [CrossRef]
- Ahmad Kamaroddin, M.F.; Sabli, N.; Tuan Abdullah, T.A.; Siajam, S.I.; Abdullah, L.C.; Abdul Jalil, A.; Ahmad, A. Membrane-based electrolysis for hydrogen production: A review. Membranes 2021, 11, 810. [Google Scholar] [CrossRef] [PubMed]
- Schiro, F.; Stoppato, A.; Benato, A. Modelling and analyzing the impact of hydrogen enriched natural gas on domestic gas boilers in a decarbonization perspective. Carbon Resour. Convers. 2020, 3, 122–129. [Google Scholar] [CrossRef]
- Atlantic Hydrogen Inc. Hydrogen-Enriched Natural Gas Bridge to an Ultra-Low Carbon World; Atlantic Hydrogen Inc.: Fredericton, NB, Canada, 2009. [Google Scholar]
- Mehrpooya, M.; Habibi, R. A review on hydrogen production thermochemical water-splitting cycles. J. Clean. Prod. 2020, 275, 123836. [Google Scholar] [CrossRef]
- Chambon, M.; Abanades, S.; Flamant, G. Design of a lab-scale rotary cavity-type solar reactor for continuous thermal reduction of volatile oxides under reduced pressure. In Proceedings of the ASME 2009 3rd International Conference on Energy Sustainability Collocated with the Heat Transfer and InterPACK09 Conferences, San Francisco, CA, USA, 19–23 July 2009; ES2009. Volume 2, pp. 507–515. [Google Scholar] [CrossRef]
- Chambon, M.; Abanades, S.; Flamant, G. Thermal dissociation of compressed ZnO and SnO2 powders in a moving-front solar thermochemical reactor. AIChE J. 2011, 57, 2264–2273. [Google Scholar] [CrossRef]
- Chambon, M.; Abanades, S.; Flamant, G. Solar thermal reduction of ZnO and SnO2: Characterization of the recombination reaction with O2. Chem. Eng. Sci. 2010, 65, 3671–3680. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S. Kinetic analysis of high-temperature solid-gas reactions by an inverse method applied to ZnO and SnO2 solar thermal dissociation. Chem. Eng. J. 2013, 217, 139–149. [Google Scholar] [CrossRef]
- Koepf, E.; Villasmil, W.; Meier, A. Pilot-scale solar reactor operation and characterization for fuel production via the Zn/ZnO thermochemical cycle. Appl. Energy 2016, 165, 1004–1023. [Google Scholar] [CrossRef]
- Lindemer, M.D.; Advani, S.G.; Prasad, A.K. Experimental investigation of heterogeneous hydrolysis with Zn vapor under a temperature gradient. Int. J. Hydrogen Energy 2017, 42, 7847–7856. [Google Scholar] [CrossRef]
- Villafán-Vidales, H.I.; Abanades, S.; Montiel-González, M.; Romero-Paredes, H.; Arancibia-Bulnes, C.A.; Estrada, C.A. Transient heat transfer simulation of a 1 kWth moving front solar thermochemical reactor for thermal dissociation of compressed ZnO. Chem. Eng. Res. Des. 2015, 93, 174–184. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S. Investigation of thermal and carbothermal reduction of volatile oxides (ZnO, SnO2, GeO2, and MgO) via solar-driven vacuum thermogravimetry for thermochemical production of solar fuels. Thermochim. Acta 2015, 605, 86–94. [Google Scholar] [CrossRef]
- Pérez, A.; Orfila, M.; Linares, M.; Sanz, R.; Marugán, J.; Molina, R.; Botas, J.A. Hydrogen production by thermochemical water splitting with La0.8Al0.2MeO3-δ (Me= Fe, Co, Ni and Cu) perovskites prepared under controlled pH. Catal. Today 2021. [Google Scholar] [CrossRef]
- de la Calle, A.; Ermanoski, I.; Stechel, E.B. Towards chemical equilibrium in thermochemical water splitting. Part 1: Thermal reduction. Int. J. Hydrogen Energy 2021, 47, 10474–10482. [Google Scholar] [CrossRef]
- Mao, Y.; Gao, Y.; Dong, W.; Wu, H.; Song, Z.; Zhao, X.; Sun, J.; Wang, W. Hydrogen production via a two-step water splitting thermochemical cycle based on metal oxide—A review. Appl. Energy 2020, 267, 114860. [Google Scholar] [CrossRef]
- Cui, B.; Zhang, J.; Liu, S.; Liu, X.; Zhang, Z.; Sun, J. A low-temperature electro-thermochemical water-splitting cycle for hydrogen production based on LiFeO2/Fe redox pair. Int. J. Hydrogen Energy 2020, 45, 20800–20807. [Google Scholar] [CrossRef]
- Orfila, M.; Linares, M.; Molina, R.; Marugán, J.; Botas, J.Á.; Sanz, R. Hydrogen production by water splitting with Mn3-xCoxO4 mixed oxides thermochemical cycles: A thermodynamic analysis. Energy Convers. Manag. 2020, 216, 112945. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Mukthar Ali, M.; Dhua, S.; Sundararajan, T.; Ranga Rao, G. Thermochemical hydrogen production using Rh/CeO2/γ-Al2O3 catalyst by steam reforming of ethanol and water splitting in a packed bed reactor. Int. J. Hydrogen Energy 2021, 46, 19254–19269. [Google Scholar] [CrossRef]
- Abanades, S. Metal oxides applied to thermochemical water-splitting for hydrogen production using concentrated solar energy. ChemEngineering 2019, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.C.; Besenbruch, G.E.; Lentsch, R.D.; Schultz, K.R.; Funk, J.F.; Pickard, P.S.; Marshall, A.C.; Showalter, S.K. High Efficiency Generation of Hydrogen Fuels Using Nuclear Power; General Atomics: San Diego, CA, USA, 2003; pp. 29–30. [Google Scholar]
- Tanaka, N.; Takegami, H.; Noguchi, H.; Kamiji, Y.; Myagmarjav, O.; Kubo, S. Introduction of loop operating system to improve the stability of continuous hydrogen production for the thermochemical water-splitting iodine–sulfur process. Int. J. Hydrogen Energy 2021, 46, 27891–27904. [Google Scholar] [CrossRef]
- Corgnale, C.; Gorensek, M.B.; Summers, W.A. Review of sulfuric acid decomposition processes for sulfur-based thermochemical hydrogen production cycles. Processes 2020, 8, 1383. [Google Scholar] [CrossRef]
- Brown, L.C.; Besenbruch, G.E.; Schultz, K.R.; Showalter, S.K.; Marshall, A.C.; Pickard, P.S.; Funk, J.F. High Efficiency Generation of Hydrogen Fuels Using Thermochemical Cycles and Nuclear Power; American Institute of Chemical Engineers: New York, NY, USA, 2002; pp. 1–31. [Google Scholar]
- Moore, R.C. Design Options for a Bunsen Reactor; Sandia National Laboratories (SNL-NM): Albuquerque, NM, USA, 2012; pp. 1–19. [Google Scholar]
- Parma, E.J.; Vernon, M.E.; Gelbard, F.; Moore, R.C.; Stone, H.B.J.; Pickard, P.S. Modeling the sulfuric acid decomposition section for hydrogen production. In ANS Embedded Topical Meeting on the Safety and Technology of Nuclear Hydrogen Production, Control and Management; Sandia National Laboratories (SNL-NM): Albuquerque, NM, USA, 2007; pp. 154–160. [Google Scholar]
- Safari, F.; Dincer, I. A study on the Fe–Cl thermochemical water splitting cycle for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 18867–18875. [Google Scholar] [CrossRef]
- Canavesio, C.; Nassini, H.E.; Bohé, A.E. Evaluation of an iron-chlorine thermochemical cycle for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 8620–8632. [Google Scholar] [CrossRef]
- Utgikar, V.; Ward, B. Life cycle assessment of ISPRA Mark 9 thermochemical cycle for nuclear hydrogen production. J. Chem. Technol. Biotechnol. 2006, 81, 1753–1759. [Google Scholar] [CrossRef]
- Energie, V.D.F.; Biotechnik, V. Combined Thermochemical Energy Storage and Heat Transformation for Industrial Waste Heat Recovery; Fakultät für Energie-, Verfahrens- und Biotechnik der Universität Stuttgart: Stuttgart, Germany, 2021. [Google Scholar]
- Stengler, J.; Richter, M.; Linder, M. Thermochemical energy storage and heat transformation based on SrBr2: Generic reactor concept for validation experiments. In Proceedings of the Materials Science and Engineering, Darmstadt, Germany, 27–29 September 2016; pp. 27–29. [Google Scholar]
- Jiang, H.; Wang, X.; Li, C.; Gu, D.; Jiang, T.; Nie, C.; Yuan, D.; Wu, H.; Wang, B. An alternative electron-donor and highly thermo-assisted strategy for solar-driven water splitting redox chemistry towards efficient hydrogen production plus effective wastewater treatment. Renew. Energy 2021, 176, 388–401. [Google Scholar] [CrossRef]
- Sahu, P.; Wagh, V.; Zodge, S.; Patil, S.; Suryawanshi, K. Hydrogen Generation from Waste Water by using Solar Energy. J. Res. Vol. 2018, 3, 13–19. [Google Scholar]
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A review of sludge-to-energy recovery methods. Energies 2019, 12, 60. [Google Scholar] [CrossRef] [Green Version]
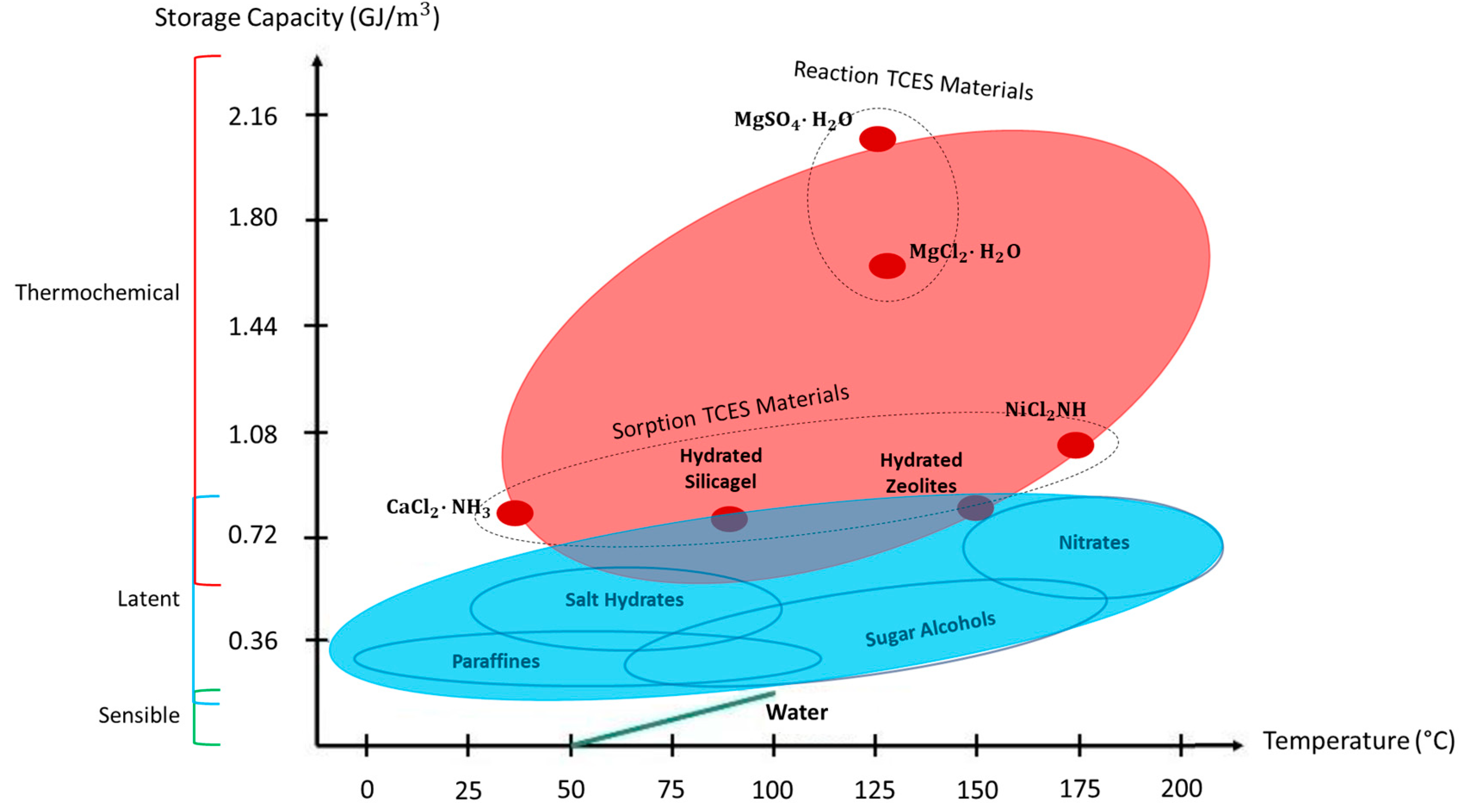

| Parameter | Thermal Storage Type | ||
|---|---|---|---|
| Sensible | Latent | Thermochemical | |
| Temperature range (Examples) | Up to 110 °C (Water Tanks) | 20–40 °C (paraffins) 30–80 °C (salt hydrates) | 20–200 °C |
| Storage capacity | 0.2 GJ/m3 | 0.3–0.5 GJ/m3 | 0.5–3 GJ/m3 |
| Lifetime | Long | Limited | Dependable (on reactant degradation and side reactions) |
| Technology status | Commercially available | Partially commercially available | Generally not available |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro Oliveira, M.; Iten, M.; Matos, H.A. Review of Thermochemical Technologies for Water and Energy Integration Systems: Energy Storage and Recovery. Sustainability 2022, 14, 7506. https://doi.org/10.3390/su14127506
Castro Oliveira M, Iten M, Matos HA. Review of Thermochemical Technologies for Water and Energy Integration Systems: Energy Storage and Recovery. Sustainability. 2022; 14(12):7506. https://doi.org/10.3390/su14127506
Chicago/Turabian StyleCastro Oliveira, Miguel, Muriel Iten, and Henrique A. Matos. 2022. "Review of Thermochemical Technologies for Water and Energy Integration Systems: Energy Storage and Recovery" Sustainability 14, no. 12: 7506. https://doi.org/10.3390/su14127506
APA StyleCastro Oliveira, M., Iten, M., & Matos, H. A. (2022). Review of Thermochemical Technologies for Water and Energy Integration Systems: Energy Storage and Recovery. Sustainability, 14(12), 7506. https://doi.org/10.3390/su14127506








