Distinctive Features of Composts of Different Origin: A Thorough Examination of the Characterization Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of the Samples
2.2. Physico-Chemical Characterization
2.2.1. Elemental Characterization
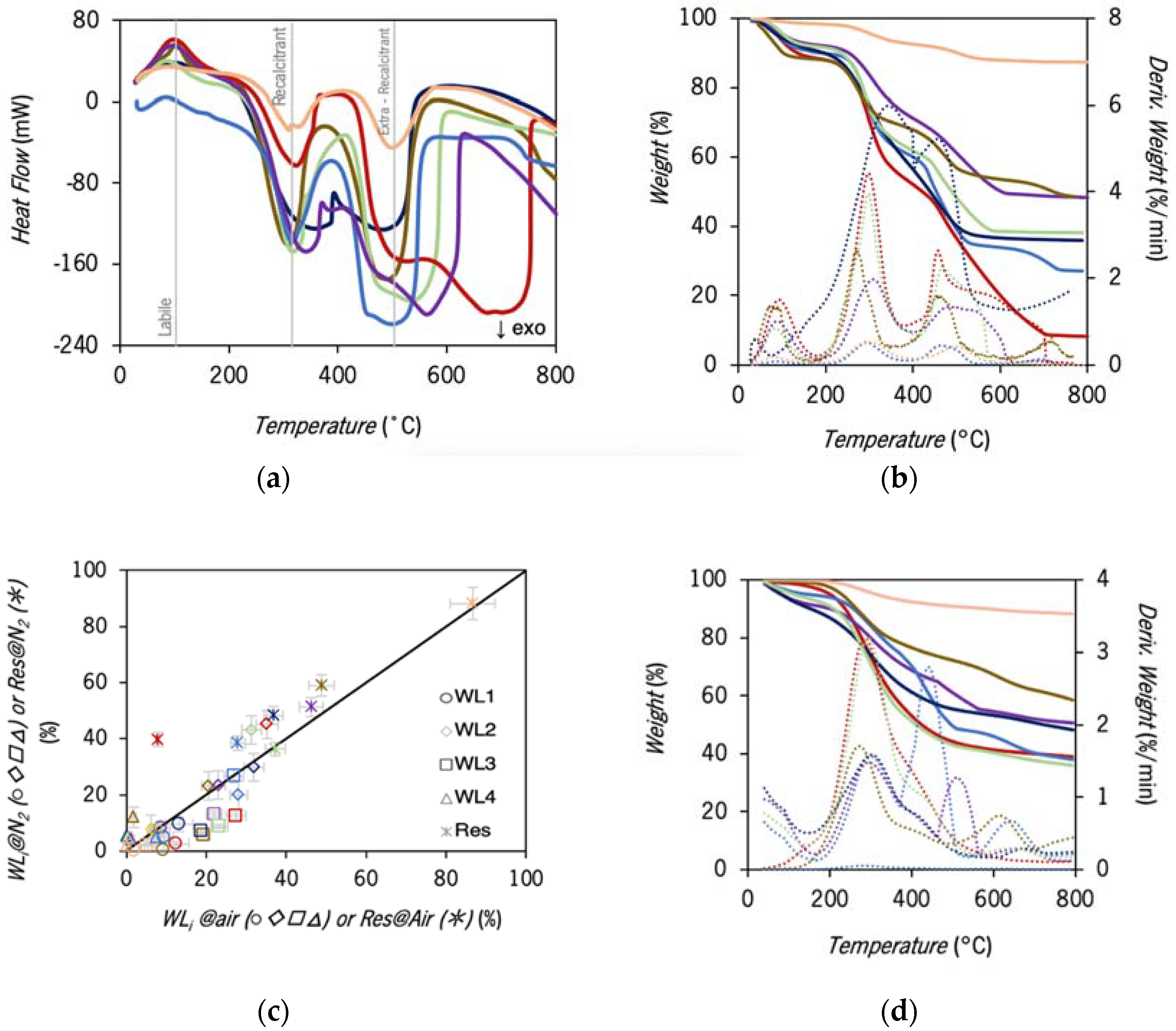
2.2.2. Characterization by TGA and DSC
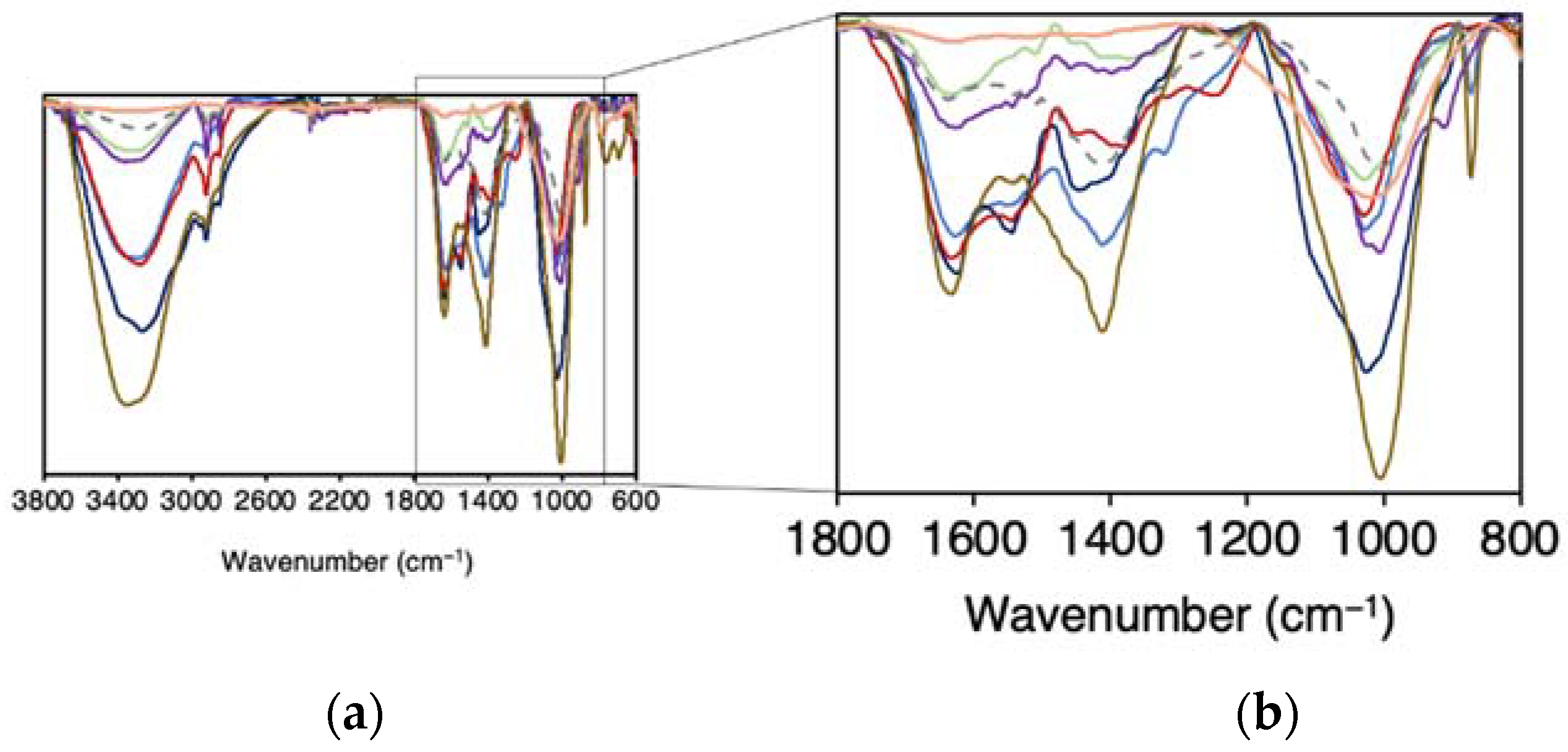
2.2.3. Characterization by ATR–FTIR Spectroscopy
2.2.4. Characterization by SEM and X-ray Diffraction Analysis
2.3. Plant Field Experiment
2.3.1. Biological Material
2.3.2. Experimental Design and Sampling
2.4. Statistical Analyses
3. Results and Discussion
3.1. Elemental Characterization and CEC
3.2. ATR-FTIR Characterization
3.3. DSC and TGA Characterization
3.4. Correlations between Physico-Chemical Parameters
3.5. PCA Analysis
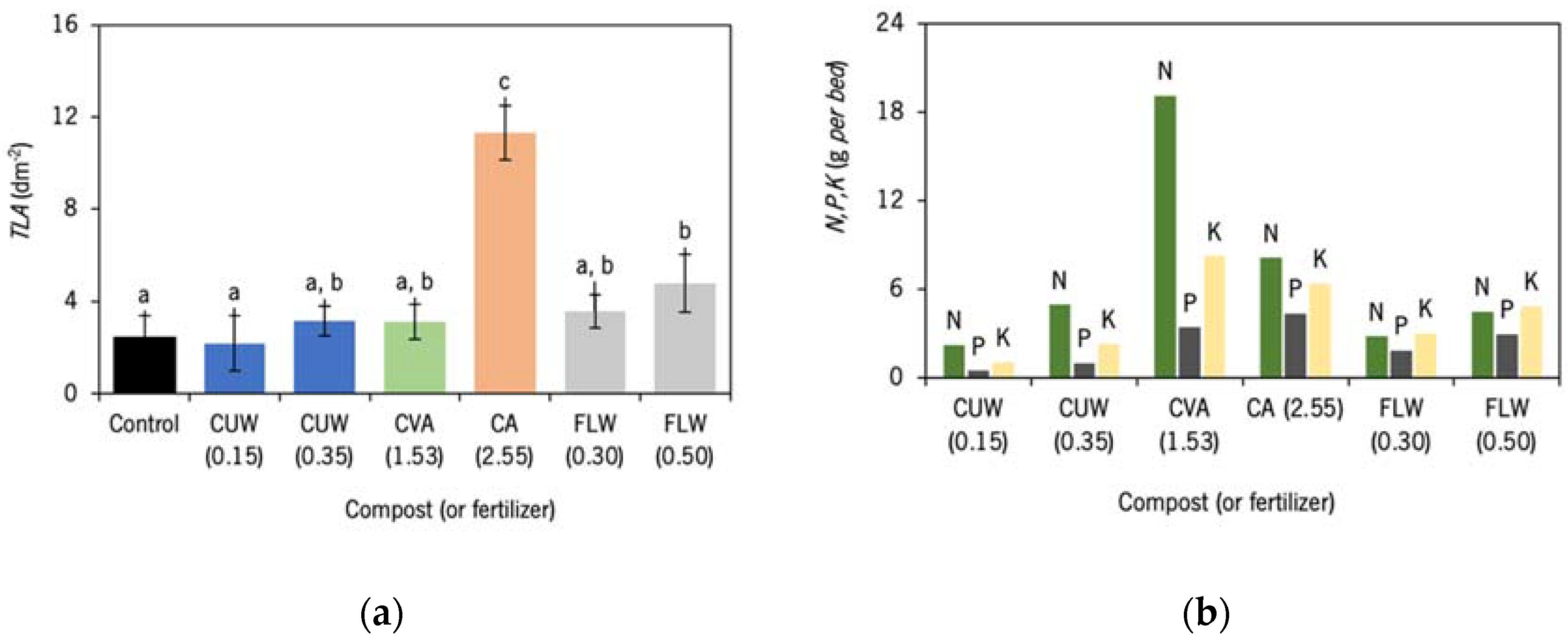
3.6. Impacts of Different Composts and Fertiliser on Lettuce Leaf Area
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valin, H.; Sands, R.D.; van der Mensbrugghe, D.; Nelson, G.C.; Ahammad, H.; Blanc, E.; Bodirsky, B.; Fujimori, S.; Hasegawa, T.; Havlik, P.; et al. The Future of Food Demand: Understanding Differences in Global Economic Models. Agric. Econ. 2014, 45, 51–67. [Google Scholar] [CrossRef]
- United Nations. General Assembly Resolution A/RES/70/1; Transforming Our World, the 2030 Agenda for Sustain—Able Development; FAO: Rome, Italy, 2015. [Google Scholar]
- Derpsch, R.; Friedrich, T.; Kassam, A.; Li, H. Current Status of Adoption of No-till Farming in the World and Some of Its Main Benefits. Int. J. Agric. Bio. Engineer. 2010, 3, 1–25. [Google Scholar]
- Somasundaram, J.; Sinha, N.K.; Dalal, R.C.; Lal, R.; Mohanty, M.; Naorem, A.K.; Hati, K.M.; Chaudhary, R.S.; Biswas, A.K.; Patra, A.K.; et al. No-Till Farming and Conservation Agriculture in South Asia—Issues, Challenges, Prospects and Benefits. Crit. Rev. Plant. Sci. 2020, 39, 236–279. [Google Scholar] [CrossRef]
- Toledo, M.; Siles, J.A.; Gutiérrez, M.C.; Martín, M.A. Monitoring of the Composting Process of Different Agroindustrial Waste: Influence of the Operational Variables on the Odorous Impact. Waste Manag. 2018, 76, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Group: Washington, DC, USA, 2018. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2008/98/EC on Waste; European Union: Maastricht, The Netherlands, 2018; pp. 109–140. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste; European Union: Maastricht, The Netherlands, 2008; pp. 3–30. [Google Scholar]
- Rynk, R.; Schwarz, M.; Richard, T.L.; Cotton, M.; Halbach, T.; Siebert, S. Compost Feedstocks. In The Composting Handbook; Elsevier: Amsterdam, The Netherlands, 2022; pp. 103–157. [Google Scholar]
- Haug, R.T. The Practical Handbook of Compost Engineering, 1st ed.; Routledge: London, UK, 1993. [Google Scholar] [CrossRef]
- Michel, F.; O’Neill, T.; Rynk, R.; Bryant-Brown, M.; Calvez, V.; Li, J.; Paul, J. Contained and In-Vessel Composting Methods and Methods Summary. In The Composting Handbook; Elsevier: Amsterdam, The Netherlands, 2022; pp. 271–305. [Google Scholar]
- Michel, F.; O’Neill, T.; Rynk, R.; Gilbert, J.; Smith, M.; Aber, J.; Keener, H. Forced Aeration Composting, Aerated Static Pile, and Similar Methods. In The Composting Handbook; Elsevier: Amsterdam, The Netherlands, 2022; pp. 197–269. [Google Scholar]
- Michel, F.; O’Neill, T.; Rynk, R.; Gilbert, J.; Wisbaum, S.; Halbach, T. Passively Aerated Composting Methods, Including Turned Windrows. In The Composting Handbook; Elsevier: Amsterdam, The Netherlands, 2022; pp. 159–196. [Google Scholar]
- Fernández-Gómez, M.J.; Nogales, R.; Plante, A.; Plaza, C.; Fernández, J.M. Application of a Set of Complementary Techniques to Understand How Varying the Proportion of Two Wastes Affects Humic Acids Produced by Vermicomposting. Waste Manag. 2015, 35, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, M.; González-Gaitano, G.; García-Mina, J.M. The Usefulness of UV-Visible and Fluorescence Spectroscopies to Study the Chemical Nature of Humic Substances from Soils and Composts. Org. Geochem. 2006, 37, 1949–1959. [Google Scholar] [CrossRef]
- Li, X.; Xing, M.; Yang, J.; Huang, Z. Compositional and Functional Features of Humic Acid-like Fractions from Vermicomposting of Sewage Sludge and Cow Dung. J. Hazard. Mater. 2011, 185, 740–748. [Google Scholar] [CrossRef]
- Barrena, R.; Font, X.; Gabarrell, X.; Sánchez, A. Home Composting versus Industrial Composting: Influence of Composting System on Compost Quality with Focus on Compost Stability. Waste Manag. 2014, 34, 1109–1116. [Google Scholar] [CrossRef] [Green Version]
- Muscolo, A.; Papalia, T.; Settineri, G.; Mallamaci, C.; Jeske-Kaczanowska, A. Are Raw Materials or Composting Conditions and Time That Most Influence the Maturity and/or Quality of Composts? Comparison of Obtained Composts on Soil Properties. J. Clean. Prod. 2018, 195, 93–101. [Google Scholar] [CrossRef]
- Han, W.; Clarke, W.; Pratt, S. Composting of Waste Algae: A Review. Waste Manag. 2014, 34, 1148–1155. [Google Scholar] [CrossRef]
- Dhyani, V.; Kumar Awasthi, M.; Wang, Q.; Kumar, J.; Ren, X.; Zhao, J.; Chen, H.; Wang, M.; Bhaskar, T.; Zhang, Z. Effect of Composting on the Thermal Decomposition Behavior and Kinetic Parameters of Pig Manure-Derived Solid Waste. Bioresour. Technol. 2018, 252, 59–65. [Google Scholar] [CrossRef]
- Soobhany, N.; Gunasee, S.; Rago, Y.P.; Joyram, H.; Raghoo, P.; Mohee, R.; Garg, V.K. Spectroscopic, Thermogravimetric and Structural Characterization Analyses for Comparing Municipal Solid Waste Composts and Vermicomposts Stability and Maturity. Bioresour. Technol. 2017, 236, 11–19. [Google Scholar] [CrossRef]
- Dell’Abate, M.T.; Canali, S.; Trinchera, A.; Benedetti, A.; Sequi, P. Thermal Analysis in the Evaluation of Compost Stability: A Comparison with Humification Parameters. Nutr. Cycl. Agroecosyst. 1998, 51, 217–224. [Google Scholar] [CrossRef]
- Silva, M.E.F.; de Lemos, L.T.; Nunes, O.C.; Cunha-Queda, A.C. Influence of the Composition of the Initial Mixtures on the Chemical Composition, Physicochemical Properties and Humic-like Substances Content of Composts. Waste Manag. 2014, 34, 21–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campitelli, P.; Ceppi, S. Chemical, Physical and Biological Compost and Vermicompost Characterization: A Chemometric Study. Chemom. Intell. Lab. Syst. 2008, 90, 64–71. [Google Scholar] [CrossRef]
- Reyes-Torres, M.; Oviedo-Ocaña, E.R.; Dominguez, I.; Komilis, D.; Sánchez, A. A Systematic Review on the Composting of Green Waste: Feedstock Quality and Optimization Strategies. Waste Manag. 2018, 77, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Jakubus, M. A Comparative Study of Composts Prepared from Various Organic Wastes Based on Biological and Chemical Parameters. Agronomy 2020, 10, 869. [Google Scholar] [CrossRef]
- Zmora-Nahum, S.; Hadar, Y.; Chen, Y. Physico-Chemical Properties of Commercial Composts Varying in Their Source Materials and Country of Origin. Soil Biol. Biochem. 2007, 39, 1263–1276. [Google Scholar] [CrossRef]
- Dresbøll, D.B.; Thorup-Kristensen, K. Structural Differences in Wheat (Triticum Aestivum), Hemp (Cannabis Sativa) and Miscanthus (Miscanthus Ogiformis) Affect the Quality and Stability of Plant Based Compost. Sci. Hortic. 2005, 107, 81–89. [Google Scholar] [CrossRef]
- Smidt, E.; Tintner, J. Application of Differential Scanning Calorimetry (DSC) to Evaluate the Quality of Compost Organic Matter. Thermochim. Acta 2007, 459, 87–93. [Google Scholar] [CrossRef]
- López, R.; Antelo, J.; Silva, A.C.; Bento, F.; Fiol, S. Factors That Affect Physicochemical and Acid-Base Properties of Compost and Vermicompost and Its Potential Use as a Soil Amendment. J. Environ. Manag. 2021, 300, 113702. [Google Scholar] [CrossRef] [PubMed]
- Campitelli, P.; Ceppi, S. Effects of Composting Technologies on the Chemical and Physicochemical Properties of Humic Acids. Geoderma 2008, 144, 325–333. [Google Scholar] [CrossRef]
- Walkley, A. A Critical Examination of a Rapid Method for Determining Organic Carbon in Soils—Effect of Variations in Digestion Conditions and of Inorganic Soil Constituents. Soil Sci. 1947, 63, 251–264. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Sumner, M.E.; Miller, W.P. Cation Exchange Capacity and Exchange Coefficients. In Methods of Soil Analysis Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 201–1229. [Google Scholar]
- Lovie, P. Coefficient of Variation. In Encyclopedia of Statistics in Behavioral Science; Everitt, B.S., Howell, D.C., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2005. [Google Scholar]
- Kettaneh, N.; Berglund, A.; Wold, S. PCA and PLS with Very Large Data Sets. Comput. Stat. Data Anal. 2005, 48, 69–85. [Google Scholar] [CrossRef]
- Vilas-Boas, Â.; Valderrama, P.; Fontes, N.; Geraldo, D.; Bento, F. Evaluation of Total Polyphenol Content of Wines by Means of Voltammetric Techniques: Cyclic Voltammetry vs Differential Pulse Voltammetry. Food Chem. 2019, 276, 719–725. [Google Scholar] [CrossRef]
- Harada, Y.; Inoko, A. Relationship between Cation-Exchange Capacity and Degree of Maturity of City Refuse Composts. Soil Sci. Plant. Nutr. 1980, 26, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Baddi, G.A.; Alburquerque, J.A.; Gonzálvez, J.; Cegarra, J.; Hafidi, M. Chemical and Spectroscopic Analyses of Organic Matter Transformations during Composting of Olive Mill Wastes. Int. Biodeterior. Biodegrad. 2004, 54, 39–44. [Google Scholar] [CrossRef]
- Kaiser, M.; Ellerbrock, R.H. Functional Characterization of Soil Organic Matter Fractions Different in Solubility Originating from a Long-Term Field Experiment. Geoderma 2005, 127, 196–206. [Google Scholar] [CrossRef]
- Fuentes, M.; Baigorri, R.; González-Gaitano, G.; García-Mina, J.M. The Complementary Use of 1H-NMR, 13C-NMR, FTIR and Size Exclusion Chromatography to Investigate the Principal Structural Changes Associated with Composting of Organic Materials with Diverse Origin. Org. Geochem. 2007, 38, 2012–2023. [Google Scholar] [CrossRef]
- Smidt, E.; Meissl, K.; Schwanninger, M.; Lechner, P. Classification of Waste Materials Using Fourier Transform Infrared Spectroscopy and Soft Independent Modeling of Class Analogy. Waste Manag. 2008, 28, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Inbar, Y.; Chen, Y.; Hadar, Y. Solid-State Carbon-13 Nuclear Magnetic Resonance and Infrared Spectroscopy of Composted Organic Matter. Soil Sci. Soc. Am. J. 1989, 53, 1695–1701. [Google Scholar] [CrossRef]
- Plante, A.F.; Fernández, J.M.; Leifeld, J. Application of Thermal Analysis Techniques in Soil Science. Geoderma 2009, 153, 1–10. [Google Scholar] [CrossRef]
- Fernández, J.M.; Plaza, C.; Polo, A.; Plante, A.F. Use of Thermal Analysis Techniques (TG-DSC) for the Characterization of Diverse Organic Municipal Waste Streams to Predict Biological Stability Prior to Land Application. Waste Manag. 2012, 32, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Dell’Abate, M.T.; Benedetti, A.; Trinchera, A.; Dazzi, C. Humic Substances along the Profile of Two Typic Haploxerert. Geoderma 2002, 107, 281–296. [Google Scholar] [CrossRef]
- Merino, A.; Fonturbel, M.T.; Fernández, C.; Chávez-Vergara, B.; García-Oliva, F.; Vega, J.A. Inferring Changes in Soil Organic Matter in Post-Wildfire Soil Burn Severity Levels in a Temperate Climate. Sci. Total Environ. 2018, 627, 622–632. [Google Scholar] [CrossRef]
- King, S.G.; McCafferty, L.; Stolojan, V.; Silva, S.R.P. Highly Aligned Arrays of Super Resilient Carbon Nanotubes by Steam Purification. Carbon 2015, 84, 130–137. [Google Scholar] [CrossRef]
- Som, M.P.; Lemée, L.; Amblès, A. Stability and Maturity of a Green Waste and Biowaste Compost Assessed on the Basis of a Molecular Study Using Spectroscopy, Thermal Analysis, Thermodesorption and Thermochemolysis. Bioresour. Technol. 2009, 100, 4404–4416. [Google Scholar] [CrossRef]
- Lim, S.L.; Wu, T.Y. Determination of Maturity in the Vermicompost Produced from Palm Oil Mill Effluent Using Spectroscopy, Structural Characterization and Thermogravimetric Analysis. Ecol. Eng. 2015, 84, 515–519. [Google Scholar] [CrossRef]
- Haumaier, L.; Zech, W. Black Carbon—Possible Source of Highly Aromatic Components of Soil Humic Acids. Org. Geochem. 1995, 23, 191–196. [Google Scholar] [CrossRef]
- Dong, Y.; Wan, L.; Cai, J.; Fang, Q.; Chi, Y.; Chen, G. Natural Carbon-Based Dots from Humic Substances. Sci. Rep. 2015, 5, 10037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, M.M.C.; Francisco, C.R.L.; de Oliveira, A.; de Campos, S.S.; Bilck, A.P.; Fuchs, R.H.B.; Gonçalves, O.H.; Valderrama, P.; Genena, A.K.; Leimann, F.V. Textural, Color, Hygroscopic, Lipid Oxidation, and Sensory Properties of Cookies Containing Free and Microencapsulated Chia Oil. Food Bioprocess Technol. 2018, 11, 926–939. [Google Scholar] [CrossRef]
- Moreira, I.; Scarminio, I.S. Chemometric Discrimination of Genetically Modified Coffea Arabica Cultivars Using Spectroscopic and Chromatographic Fingerprints. Talanta 2013, 107, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Pavlou, G.C.; Ehaliotis, C.D.; Kavvadias, V.A. Effect of Organic and Inorganic Fertilizers Applied during Successive Crop Seasons on Growth and Nitrate Accumulation in Lettuce. Sci. Hortic. 2007, 111, 319–325. [Google Scholar] [CrossRef]
- Santos, F.T.; Goufo, P.; Santos, C.; Botelho, D.; Fonseca, J.; Queiros, A.; Costa, M.S.S.M.; Trindade, H. Comparison of Five Agro-Industrial Waste-Based Composts as Growing Media for Lettuce: Effect on Yield, Phenolic Compounds and Vitamin C. Food Chem. 2016, 209, 293–301. [Google Scholar] [CrossRef] [PubMed]



| Sample | Composting Methodology | Raw Material |
|---|---|---|
| Compost of urban waste (CUW) | Tunnel composting | Selective collection of household food waste, food waste from restaurants, canteen, markets, fairs, festivities, pilgrimages and events, green waste from cemetery and household |
| Vermicompost of algae (CVA) | Vermicomposting | 60% animal waste and 40% vegetable remains (fruits and algae), digested by Eisenia foetida earthworms. |
| Vermicompost of domestic waste (CVDW) | Green waste (flowers, leaves, grass, fruit peels) and brown waste (straw, dry leaves, dry grass), digested by Eisenia foetida or Lumbricus rubellus earthworms. | |
| Compost of livestock waste (CLW) | Pile composting | 100% animal waste (a mixture of 5% sheep manure without straw, 25% chicken manure and 70% pig manure) |
| Compost of algae (CA) | 60% animal waste and 40% vegetable remains (fruits and algae). | |
| Compost of sewage sludge (CSS) | Forestry waste, sludge from urban wastewater treatment and sludge from local effluent treatment. | |
| Domestic compost of domestic waste (CDDW) | Domestic Composting | Green waste (flowers, leaves, grass, fruit peels) and brown waste (straw, dry leaves, dry grass). |
| Organic Fertiliser | ||
| Fertiliser of livestock waste (FLW) | - | 100% animal waste (chicken manure). Mixture subjected to a high temperature to eliminate pathogens. |
| Technique | Parameter | CVA | CVDW | CLW | CSS | CDDW | CUW | CA | FLW |
|---|---|---|---|---|---|---|---|---|---|
| EA | C (%) | 29.7 | 43.9 | 22.8 | 31.3 | 23.3 | 34.0 | 6.0 | 22.5 |
| N (%) | 2.4 | 4.8 | 1.8 | 3.6 | 2.3 | 2.7 | 0.6 | 1.7 | |
| S (%) | 0.4 | 0.4 | 0.7 | 1.1 | 0.2 | 0.2 | 0.23 | 0.1 | |
| C/N | 12.5 | 9.2 | 12.8 | 8.6 | 10.3 | 12.6 | 9.97 | 13.2 | |
| ICP-OES | P (g kg−1) | 4.2 | 5.5 | 13.5 | 9.9 | 2.5 | 5.0 | 3.2 | 11.3 |
| Ca (g kg−1) | 13.1 | 9.6 | 78.0 | 19.9 | 16.1 | 77.5 | 11.8 | 212 | |
| Mg (g kg−1) | 4.5 | 4.4 | 18.2 | 5.2 | 3.6 | 3.7 | 3.1 | 6.2 | |
| Na (g kg−1) | 1.8 | 1.1 | 10.5 | 2.2 | 0.6 | 4.8 | 1.5 | 3.0 | |
| K (g kg−1) | 10.2 | 14.6 | 30.2 | 4.3 | 7.6 | 12.2 | 4.7 | 18.3 | |
| Cation exchange capacity | CEC (cmol (+) kg−1) | 137 | 98.9 | 150 | 51.6 | 95.3 | 167 | 46 | 101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.C.; Teixeira, A.; Antelo, J.; Valderrama, P.; Oliveira, R.; Cunha, A.; Gley, R.; Pinheiro, J.P.; Fiol, S.; Bento, F. Distinctive Features of Composts of Different Origin: A Thorough Examination of the Characterization Results. Sustainability 2022, 14, 7449. https://doi.org/10.3390/su14127449
Silva AC, Teixeira A, Antelo J, Valderrama P, Oliveira R, Cunha A, Gley R, Pinheiro JP, Fiol S, Bento F. Distinctive Features of Composts of Different Origin: A Thorough Examination of the Characterization Results. Sustainability. 2022; 14(12):7449. https://doi.org/10.3390/su14127449
Chicago/Turabian StyleSilva, Ana Catarina, Ana Teixeira, Juan Antelo, Patrícia Valderrama, Rui Oliveira, Ana Cunha, Renaud Gley, José Paulo Pinheiro, Sarah Fiol, and Fátima Bento. 2022. "Distinctive Features of Composts of Different Origin: A Thorough Examination of the Characterization Results" Sustainability 14, no. 12: 7449. https://doi.org/10.3390/su14127449
APA StyleSilva, A. C., Teixeira, A., Antelo, J., Valderrama, P., Oliveira, R., Cunha, A., Gley, R., Pinheiro, J. P., Fiol, S., & Bento, F. (2022). Distinctive Features of Composts of Different Origin: A Thorough Examination of the Characterization Results. Sustainability, 14(12), 7449. https://doi.org/10.3390/su14127449









