Abstract
Investigating relationships between climatic factors and plant δ13C of both C3 and C4 plants simultaneously is critical for accurately predicting the effects of climate change on plant ecophysiology and ecosystem functioning and reconstructing past vegetation and climate conditions. We selected common C3 and C4 plants in temperate grasslands in Inner Mongolia, China, i.e., Stipa spp., Carex spp., Leymus chinensis and Cleistogenes spp., and investigated the relationships between climatic factors and plant δ13C of each genus/species. The results showed that precipitation, especially growing season precipitation (GSP), was the dominant factor affecting plant δ13C in this region. For C3 plants, there were significantly negative relationships between precipitation and plant δ13C. For C4 plants, plant δ13C of Cleistogenes spp. firstly increased, then decreased with precipitation at a breakpoint GSP 204.84 mm. Our findings emphasize that C4 plant δ13C is sensitive to precipitation, but responses are species-specific and environment-specific, and suggest that C4 plant δ13C can be used as a proxy for water use efficiency (WUE), but care should be taken in evaluating WUE. Moreover, our findings provide basic information for accurately predicting the effects of climate change on ecosystem structure and function and reconstructing past vegetation and climate conditions from bulk materials in arid and semiarid regions.
1. Introduction
Plant carbon isotope composition (δ13C) can reliably record environmental information and comprehensively reflect the physiological and ecological characteristics of plants, and it has been extensively used in studies of plant physiology and ecology, global carbon cycle and global climate change [1,2,3,4]. In order to explore the effects of climate change on plant ecophysiological characteristics and, consequently, on ecosystem functions and services, many studies have been conducted to investigate the relationships between plant δ13C and environmental factors, in particular climatic factors [5,6,7,8]. However, previous studies have mostly concentrated on C3 plants and rarely on C4 plants. For C3 plants, the negative relationships between plant δ13C and precipitation were widely found [6,7,9], while the relationships between plant δ13C and temperature were complex, plant δ13C was found to be positively [10], negatively [11] or not [12] related to temperature.
Few studies have been conducted on C4 plants, mainly because the response of the δ13C of C4 plants to environmental factors is not as sensitive as that of C3 plants. However, this view has been challenged, some studies have found that the δ13C of C4 plants also greatly varied with the change of climatic factors [13,14]. For example, some studies have found that C4 plant δ13C was positively related to precipitation or water availability [7,15,16,17], while other studies found negative relationships between them [13,18,19]. Thus, compared to C3 plants, the responses of the δ13C of C4 plant to increased precipitation or water availability were divergent, because carbon fixation in C4 plants is more complex and δ13C depends on many more factors than in C3 plants [20]. Plant δ13C has been proposed as a time-integrated proxy for plant water use efficiency (WUE) [17,21]. Obviously, the divergent responses of C4 plant δ13C to precipitation or water availability indicates that future research on variations of C4 plant δ13C with precipitation or water availability is essential for correctly estimating C4 plant WUE by C4 plant δ13C. Moreover, paleovegetation, particularly paleo-C3/C4 plant ratio, and paleoclimatic indicators are often obtained from the δ13C of C3 and C4 plants and other carbon sources such as soil organic matter in a two-member mixing model. A single mean C4 end-member δ13C value is usually assumed in the model, as the δ13C of C4 plants was thought to be insensitive to environmental factors and showed less isotopic variability [19,22]. Obviously, this view has been challenged by the variations of the δ13C of C4 plants with the environmental factors mentioned above. Thus, investigating the variation of the δ13C of C4 plants with environmental factors and considering it in the two-member mixing model are essential for correctly inferring C3/C4 ratio in the past and reconstructing a paleoclimate [16,18]. In addition, previous studies mostly integrated the different plant species in the region together to analyze the relationships between their δ13C and environmental factors, and rarely analyzed the relationships between environmental factors and the δ13C of multiple single genera/species at the regional scale. This might confound the interspecies effect with the effect of environmental factors on variations of plant δ13C, and obscure the responses of plant δ13C of some species to environmental changes, especially in the grassland ecosystems where C3 and C4 plants coexist. Thus, further research on climatic factors influencing plant δ13C of multiple single genera/species from both C3 and C4 plants is critical for accurately predicting the effects of climate change on the C3/C4 ratio of plant communities and, consequently, on ecosystem functions and services.
Temperate grassland ecosystems are very sensitive to the global climate change, and are of great significance in the study of global change. The Xilingol League in Inner Mongolia is one of the typical regions of temperate grassland ecosystem in China. C3 and C4 plants coexist in this region, and C3 species are the dominant functional group and contribute about 80% of the net primary productivity of the flora [23]. Thus, the primary objectives of this paper were to (i) investigate the relationship between climatic factors and plant δ13C of common C3 and C4 plants in temperate grasslands in the Xilingol League, Inner Mongolia; (ii) identify the dominant climate factors controlling plant δ13C of common C3 and C4 plants in temperate grasslands.
2. Materials and Methods
2.1. Study Area
Plant samples were obtained from 29 grassland sites in the Xilingol League (111°25′–119°58′ E and 41°35′–46°46′ N), Inner Mongolia, China (Figure 1). The terrain of this area is high in the south and low in the north, with elevation ranging from 778 to 1957 m a.s.l. [24]. The area is characterized by a temperate continental arid and semi-arid climate with mean annual temperature (MAT) ranging from −3 to 6 °C and mean annual precipitation (MAP) ranging from 118.9 to 486 mm (Figure 1). Most precipitation is in summer, accounting for about 80% of the annual precipitation, and there is a significant negative correlation between MAP and MAT (r2 = 0.74, p < 0.001). Zonal soils are chernozems, kastanozems, calcisols, luvisols and arenosols; intrazonal soil are cambisols, gleysols, solonchaks and solonetz [24]. The main grassland types are temperate meadow steppe, temperate typical steppe and temperate desert steppe. The dominant species of temperate meadow steppe are Stipa grandis, Stipa baicalensis and Leymus chinensis; the dominant species of temperate typical steppe are Stipa krylovii, Stipa grandis, Leymus chinensis, Agropyron cristatum, Artemisia frigida, Cleistogenes squarrosa, Salsola collina and Caragana microphylla; and the dominant species of temperate desert steppe are Stipa klemenzii, Artemisia frigida and Cleistogenes songorica [23].
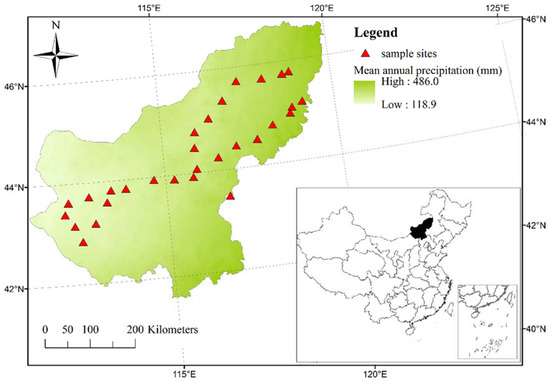
Figure 1.
Locations of 29 sample sites from temperate grasslands in the Xilingol League, Inner Mongolia, China.
2.2. Sampling
Plant samples from 29 sampling sites were collected along the precipitation and temperature gradients in August 2015, which was the peak growing phase. In order to minimize the influences of human disturbance and topographic variation, the selected grasslands were protected by fences, not grazed in the sampling year, far from human habitation and had similar and flat topography. Sampling sites were separated from each other by about 50 km and their geographical information was recorded with a GPS (Garmin, Montana 650, Kansas City, MO, USA). According to dominant species, distribution range and representativeness, e.g., water use strategy and life-form, C3 species Stipa spp., Leymus chinensis and Carex spp. and C4 species Cleistogenes spp. were finally selected as the research objects. In each site, above-ground parts of 10–30 individual plants were randomly sampled for each species. Each sampled species at each site was gathered in an envelope as one sample and transported to a laboratory for analysis.
2.3. Sample Analysis
In the laboratory, plant samples were washed with deionised water to remove dust particles, de-enzymed at 105 °C for 15 min, oven-dried at 80 °C to constant mass, and then were finely ground using a ball mill (Retsch MM400, Haan, Germany). 13C/12C ratios of plant samples were determined by an isotope mass spectrometer (Finnigan MAT-253, Thermo Electron) at the central physico-chemical laboratory of the Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, Beijing. δ13C values of samples were calculated as:
where Rsample and Rstandard are the 13C/12C ratio of the sample and the standard, respectively. The universally accepted standard of Vienna Pee Dee Belemnite (VPDB) was used.
2.4. Meteorological Data
Based on climate monitoring data of 107 climatic stations located across or around Inner Mongolia for the period 1984–2014, databases of mean daily temperature (MDT) and mean daily precipitation (MDP) for Inner Mongolia were obtained by Anuspline interpolation with DEM as a covariate. Using MDT and MDP, databases of mean monthly temperature (MMT) and mean monthly precipitation (MMP) were calculated, and then databases of growing season temperature (GST), growing season precipitation (GSP), mean annual temperature (MAT) and mean annual precipitation (MAP) were further calculated. Lastly, GST, GSP, MAT and MAP of sampling sites were extracted from the databases according to their geographic coordinates. Anuspline interpolation and database calculations were conducted using ArcGIS (ver. 10.7; ESRI Inc., Redlands, CA, USA).
2.5. Data Analysis
The δ13C mean values of different plant species were compared by one-way ANOVA. The LSD test was used for multiple comparisons as the variances among the groups were homogeneous. Univariate regression analyses were conducted to investigate the relationships between plant δ13C and climatic factors (GST, GSP, MAT and MAP). For Cleistogenes spp., the relationships of plant δ13C with climatic factors had potential breakpoints, therefore, piecewise linear regressions were used to fit these relationships. General linear models (GLMs) were used to evaluate integrative effects of climatic factors on plant δ13C and then identify the dominant controlling factors of plant δ13C. Piecewise linear regressions were conducted in Origin (ver. 2018; OriginLab, Northampton, MA, USA) and all other statistics computed in SPSS (ver.19.0; SPSS Inc., Chicago, CA, USA).
3. Results
3.1. Plant δ13C of Common C3 and C4 Species in Temperate Grasslands
Plant δ13C of Stipa spp. (C3) ranged from −26.85‰ to −24.33‰ with an average value of −25.68‰ ± 0.77, and 65% of these values were within the range −26.5 to −25% (Figure 2A). Plant δ13C of Carex spp. (C3) ranged from −27.67‰ to −24.22‰ with an average value of −26.16‰ ± 0.84, and 68% of these values were within the range −27 to −25.5% (Figure 2B). Plant δ13C of Leymus chinensis (C3) ranged from −27.89‰ to −24.88‰ with an average value of −26.15‰ ± 0.76, and 63% of these values were within the range −26.5 to −25.5% (Figure 2C). Plant δ13C of Cleistogenes spp. (C4) ranged from −17.33‰ to −14.62‰ with an average value of −15.72‰ ± 0.66, and 81% of these values were within the range −16.5 to −15% (Figure 2D). Previous studies have found that the range of plant δ13C for C3 and C4 plants are from −35‰ to −20‰ and from −19‰ to −9‰, respectively [25,26]. Thus, the ranges of plant δ13C of Stipa spp., Carex spp. and Leymus chinensis were all within the range found for C3 plants, and the range of plant δ13C of Cleistogenes spp. was within the range found for C4 plants. The plant δ13C of different plant species were significant different (p < 0.001). The plant δ13C of Cleistogenes spp. was significantly higher than that of Stipa spp., Carex spp. and Leymus chinensis; the plant δ13C of Stipa spp. was significantly higher than that of Carex spp. and Leymus chinensis; there was no significant difference between the plant δ13C of Carex spp. and Leymus chinensis (Figure 3).
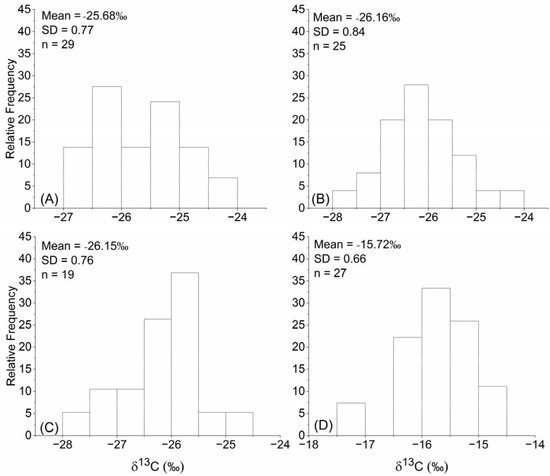
Figure 2.
Frequency distribution of plant δ13C for (A) Stipa spp., (B) Carex spp., (C) Leymus chinensis and (D) Cleistogenes spp.
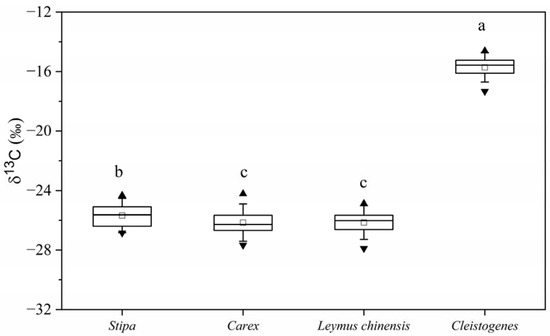
Figure 3.
Box-whisker plot showing the differences of δ13C values among different species. “▲” means maximum value, “□” means average value and “▼” means minimum value. The different lowercase letters indicate significant differences at p < 0.05, and the same lowercase letters mean non-significant differences.
3.2. Relationships between Plant δ13C and Climatic Factors
The plant δ13C of Stipa spp. was significantly positively related with MAT and GST, and negatively related with MAP and GSP (Figure 4A–D). GST and GSP explained more variation in the plant δ13C of Stipa spp. than MAT and MAP, respectively, and the rates at which plant δ13C of Stipa spp. changed with GST and GSP were higher than those with MAT and MAP, respectively (Figure 4A–D). The relationships of the plant δ13C of Carex spp. with climatic factors were similar to those of Stipa spp., while the explanatory ability of climate factors to variation in the plant δ13C of Carex spp. was higher than that of Stipa spp. (Figure 4E–H). The plant δ13C of Leymus chinensis showed no trend with MAT and GST, while it was significantly negatively related with MAP and GSP (Figure 4I–L). The plant δ13C of Cleistogenes spp. showed no trend with MAT (Figure 4M), while it increased with GST when GST was ≤16.27 °C and decreased with GST when GST was >16.27 °C, however the correlations were not significant (Figure 4N). In the sites with MAP ≤ 230.70 mm, the plant δ13C of Cleistogenes spp. had a weak positive correlation with MAP, whereas it had a significant negative correlation with MAP when MAP was >230.70 mm (Figure 4O). The relationship of the plant δ13C of Cleistogenes spp. with GSP was similar to that with MAP, while the breakpoint for GSP was 204.84 mm (Figure 4P).
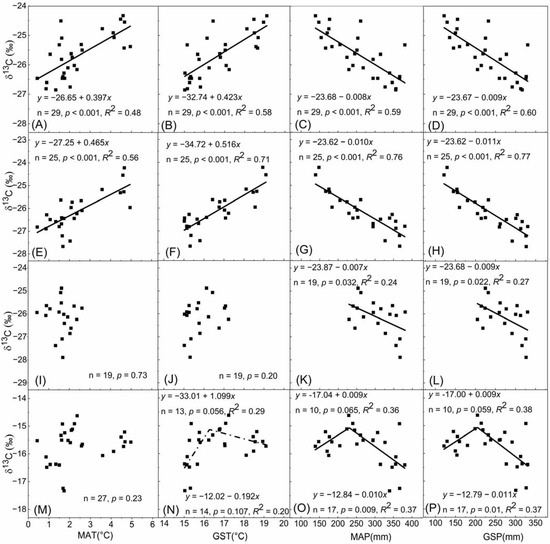
Figure 4.
The relationships between plant δ13C and climatic factors for (A–D) Stipa spp., (E–H) Carex spp., (I–L) Leymus chinensis and (M–P) Cleistogenes spp. MAT, mean annual temperature; GST, growing season temperature; MAP, mean annual precipitation; GSP, growing season precipitation. Black squares represent data points; lines represent regression fitting lines.
3.3. Integrative Effects of Climatic Factors on Plant δ13C
GLMs were conducted to define the proportions of variation in plant δ13C accounted for by GST, GSP, MAT and MAP; the results are presented in Table 1. Although there were significant correlations between plant δ13C and GST, GSP, MAT and MAP for both Stipa spp. and Carex spp. in univariate regressions, GLM analyses showed that GSP was the only influencing climatic factor for the plant δ13C of Stipa spp., with GSP explaining 60.02% of the variation. The plant δ13C of Carex spp. was mainly affected by GSP which explained 77.06% of the variation. Meanwhile, MAP and interaction between GSP and GST had weak effects on the plant δ13C of Carex spp. (Table 1). Moreover, GLM analyses further confirmed that the plant δ13C of Leymus chinensis was affected only by precipitation and not by temperature, and the plant δ13C of Cleistogenes spp. was mainly affected by GSP with a breakpoint (Table 1).

Table 1.
The integrative effects of temperature and precipitation on plant δ13C for Stipa spp., Carex spp., Leymus chinensis and Cleistogenes spp.
4. Discussion
4.1. Characteristics of the Plant δ13C of C3 and C4 Species in Temperate Grasslands
The plant δ13C of Stipa spp. was significantly higher than that of Leymus chinensis. MAP and GSP in the distribution area of Leymus chinensis are 229.18 mm–380.63 mm and 204.84 mm–330.07 mm, respectively, and they are 138.87 mm–380.63 mm and 121.45 mm–330.07 mm, respectively for Stipa spp. Thus, one possible reason for the significant difference in the plant δ13C between Stipa spp. and Leymus chinensis is that the distribution area of Leymus chinensis is wetter than that of Stipa spp., and that the plant δ13C in wet conditions is lower than that in dry conditions for C3 species [6,7,9]. However, it is worth noting that the plant δ13C of Stipa spp. was also significantly higher than that of Carex spp., although the precipitation ranges in their distribution areas were the same. This indicated that, in addition to climatic factors, other factors such as leaf or root morphology, chemical compounds and water use strategy, may influence plant δ13C [27]. The range of plant δ13C of Cleistogenes spp. is wider in the present study (−17.33‰–−14.62‰) than that in a pot experiment (−14.75‰–−13.07‰) carried out at the Inner Mongolia Grassland Ecosystem Research Station [28]. This inconsistency can be attributed to the different precipitation ranges in the two studies. In the pot experiment, only two precipitation levels were set, with GSP 160 mm for high precipitation and GSP 104 mm for low precipitation, while GSP ranged from 121.45 mm to 330.07 mm in the present study. Thus, a wide precipitation range resulted to a wider range of plant δ13C.
4.2. The Relationships between Plant δ13C and Temperature
Temperature can influence plant carbon isotope fractionation through a series of mechanisms. On one hand, temperature can affect the stomatal conductance of plants, and then affect the ratio between the intercellular and atmospheric concentration of CO2 (Ci/Ca). When temperature is high, in order to retain water, plants close some stomata, which leads to a decrease in stomatal conductance and Ci/Ca, and then an increase in plant δ13C [29]. On the other hand, temperature can affect the morphology of plant leaves and the diffusion rate of gas on the leaf surface, then affect Ci/Ca. In order to adapt to a low temperature condition, the leaf thickness of plants will increase, which will then increase the thickness of the palisade tissue and affect the diffusion and fixation of CO2. Meanwhile a low temperature will result in higher fog resistance, weaken the diffusion rate of air on the leaf surface, reduce the diffusion rate of CO2 into mesophyll cells, decrease Ci/Ca, and finally lead to an increase in plant δ13C [30,31]. Moreover, temperature can affect the carboxylase activity of a plant, then affect the carbon isotope fractionation in the process of photosynthesis. Temperatures that are too high or too low will reduce the degree of carbon isotope fractionation in the process of photosynthesis, while different plants have different optimal temperature for photosynthesis. This may be the reason for the complicated relationships between plant δ13C and temperature [32] and the inconsistent understanding of these relationships. For C3 plants, positive [10,33], negative [11,34] and no [35,36] correlations between plant δ13C and temperature were found in previous studies. For C4 plants, there are relatively few studies on the relationship between plant δ13C and temperature, and previous studies mostly found that there was no correlation between temperature and the plant δ13C of C4 plants [33]. In the present study, combining the univariate regression and GLMs analyses, we found that the plant δ13C of C3 and C4 plants was mainly affected by precipitation not by temperature. This may be due to the fact that temperature is not a limiting factor for plant growth in our study area, so it has no significant effect on plant δ13C. The positive correlation between plant δ13C and temperature revealed by univariate analysis is due to the significant negative correlation between temperature and precipitation in this area (p < 0.01) [37]. This view is supported by Li et al. [38] and Schleser et al. [32].
4.3. The Relationships between Plant δ13C and Precipitation
For C3 plants, previous studies have established a consensus on the relationship between precipitation and plant δ13C, i.e., plant δ13C is significantly negatively correlated with precipitation under drought stress [7,9,39,40]. This is because, as precipitation decreases, soil water content decreases and drought stress increases. In order to reduce water evaporation and improve water use efficiency, plants will close some stomata, and the closure of stomata will lead to a decrease in Ci/Ca, and then an increase in plant δ13C [41]. Consistent with previous findings, the plant δ13C of C3 plants was negatively correlated with precipitation in this study. Meanwhile, the correlations between plant δ13C and GSP were stronger than those between plant δ13C and MAP, especially for Stipa spp. When the integrative effects of temperature (MAT and GST) and precipitation (MAP and GSP) were analyzed using GLMs, plant δ13C was only affected by GSP not by MAP. This indicates that the effect of precipitation on plant δ13C is mainly caused by GSP. Moreover, the correlation between plant δ13C and GSP for Carex spp. was stronger than those for Stipa spp. and Leymus chinensis, and the rate of plant δ13C decrease with GSP for Carex spp. (1.1‰/100 mm) was higher than that for Stipa spp. and Leymus chinensis (0.9‰/100 mm). This indicates that the sensitivity of plant δ13C to precipitation is species-specific.
For C4 plants, the plant δ13C of Cleistogenes spp. was not linearly related to precipitation in the present study, i.e., it firstly increased, then decreased with precipitation at a breakpoint of 204.84 mm for GSP and 230.7 mm for MAP. Unlike C3 plants, the plant δ13C of C4 plants is not only determined by the Ci/Ca, but also by the CO2 leakage rate of the bundle sheath (φ) [42,43]. Using the C4 plant isotope fractionation model proposed by Henderson et al. [43], Wang et al. [44] found that at 30 °C, when φ > 0.34, plant δ13C was negatively correlated with Ci/Ca, otherwise plant δ13C was positively correlated with Ci/Ca. In other words, for C4 plants, the decrease in Ci/Ca caused by decreased precipitation may increase the plant δ13C, which is consistent with the rule for C3 plants, or decrease the plant δ13C, which is contrary to the rule for C3 plants. Positive or negative correlations between precipitation and plant δ13C for C4 plants depend on φ, which is an important parameter of C4 photosynthesis and is mainly affected by the CO2 concentration gradient between the bundle sheath and mesophyll cells, and thus by factors influencing the activity ratio of Rubisco to phosphenolpyruvate carboxylase (PEPC). Water availability such as precipitation may be an influencing factor for φ, however previous studies indicated that the responses of φ to water availability are species-specific, φ is not responsive to water availability in Sorghum bicolor [17], while water availability decreases φ in Saccharum officinarum [45] and increases φ in summer maize [46]. Although the response of φ in Cleistogenes spp. to water availability was not determined in the present study, φ in Cleistogenes spp. probably increases with water availability. Thus, φ is lower than 0.34 in the areas with lower precipitation, and there is a weak positive correlation between plant δ13C and Ci/Ca, resulting in the weak positive correlation between precipitation and plant δ13C. Meanwhile, φ is higher than 0.34 in the areas with higher precipitation, and there is a negative correlation between plant δ13C and Ci/Ca, resulting in the negative correlation between precipitation and plant δ13C. This may be the reason why there is a breakpoint in the effect of precipitation on the δ13C of C4 plants, and why the relationships between precipitation and the δ13C of C4 plants are inconsistent in different studies. This emphasizes the fact that the response of the δ13C of C4 plants to environmental factors may vary in different environmental conditions. Liu et al. [13] found that there was a negative correlation between precipitation and the plant δ13C of Bothriochloa ischaemum (a C4 plant) across a precipitation gradient from 350 mm to 700 mm in arid northwestern China. The breakpoint for the effect of precipitation on the plant δ13C of C4 plant was 230.70 mm in our study. Obviously, the precipitation gradient in Liu’s study was within the MAP > 230.70 mm and did not cover precipitation lower than 230.70 mm. Furthermore, their results support our finding that the plant δ13C of C4 plants is negatively correlated with precipitation in areas with MAP higher than 230.70 mm. Meanwhile, this also emphasizes that establishing a precipitation gradient wide enough to cover lower precipitation is important to accurately evaluate the effect of precipitation on the plantδ13C of C4 plants.
Overall, there was a negative relationship between precipitation and the plant δ13C of Cleistogenes spp. among most sites in our study area. This is supported by the finding by Yang et al. [19] that carbon isotope discrimination of Cleistogenes squarrosa increased with precipitation in a similar study area to the present study. However, positive relationships between plant δ13C and precipitation were found for other C4 plants such as Sorghum bicolor [17] and Setaria italica [47]. Gong et al. [48] reported extremely high φ values (0.6–0.7) in Cleistigenes squarrosa compared with previous reports for other C4 plants. This may explain the negative correlation between plant δ13C and precipitation for Cleistigenes squarrosa, but the positive correlation for Sorghum bicolor and Setaria italica. Our results indicate that the plant δ13C of C4 plants is also sensitive to precipitation, but the responses are species-specific and even environment-specific. Moreover, unlike C3 plants and C4 plants which have a negative correlation between plant δ13C and precipitation, there is a negative correlation between plant δ13C and WUE for C4 plants with positive correlation between plant δ13C and precipitation [17]. Thus, the plant δ13C of C4 plants can be used as a proxy for C4 plant WUE, but care should be taken in evaluating WUE using the plant δ13C of C4 plants. Furthermore, knowing the relationship between the plant δ13C of C4 plants and precipitation is critical for accurately evaluating C4 plant WUE.
5. Conclusions
Firstly, our study confirmed that precipitation, especially GSP, was the dominant controlling factor for the plant δ13C of C3 plants in temperate grasslands of Inner Mongolia China. The plant δ13C of C3 plants decreased with precipitation, the response sensitivity of plant δ13C to precipitation differed among different C3 species, and the plant δ13C of Carex spp. had the strongest correlation and highest change rate with precipitation among three C3 species. Secondly, the plant δ13C of C4 plants was also mainly affected by precipitation, however, the plant δ13C of C4 plants was not linearly related to precipitation. The plant δ13C was weakly positively related to precipitation in low rainfall area, and significantly negatively related to precipitation in high rainfall area. This indicates that the plant δ13C of C4 plants is also sensitive to precipitation, and its relationship with precipitation is more complicated, because the plant δ13C of C4 plants is affected by both Ci/Ca and φ. Overall, our study emphasizes that the responses of plant δ13C of C4 plants to precipitation are species-specific and even environment-specific and suggests that care should be taken in evaluating WUE for C4 plants using the plant δ13C. Moreover, our findings provide basic information for accurately predicting the effects of climate change on ecosystem structure and function and reconstructing past vegetation and climate conditions from bulk materials in arid and semiarid region.
Author Contributions
Conceptualization, Y.Z.; methodology, Y.Z. and X.X.; Investigation, Y.Z. and H.L.; Formal analysis, Y.Z. and Y.L.; Writing—original draft, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China [grant number 31400413]; the Fundamental Research Funds for the Central Universities [grant number N2201011, N130301001].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
We thank Hongli Zhang and Wenbo Zhang for their assistance with field sampling and laboratory analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spangenberg, J.E.; Schweizer, M.; Zufferey, V. Carbon and nitrogen stable isotope variations in leaves of two grapevine cultivars (Chasselas and Pinot noir): Implications for ecophysiological studies. Plant Physiol. Biochem. 2021, 163, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Song, W.; Zhou, Y. Linking leaf δ15N and δ13C with soil fungal biodiversity, ectomycorrhizal and plant pathogenic abundance in forest ecosystems of China. Catena 2021, 200, 105176. [Google Scholar] [CrossRef]
- Song, W.; Liu, Y. Survival strategy of the endangered tree Acer catalpifolium Rehd., based on 13C fractionation. Ecol. Evol. 2020, 10, 8532–8537. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, W.; Cheng, X.; Harris, W.; Schaeffer, S.M.; Xu, X.; Zhao, B. Factors affecting 13C enrichment of vegetation and soil in temperate grasslands in Inner Mongolia, China. J. Soils Sediments 2019, 19, 2190–2199. [Google Scholar] [CrossRef]
- Yan, G.; Han, S.; Zhou, M.; Sun, W.; Huang, B.; Wang, H.; Xing, Y.; Wang, Q. Variations in the natural 13C and 15N abundance of plants and soils under long-term N addition and precipitation reduction: Interpretation of C and N dynamics. For. Ecosyst. 2020, 7, 49. [Google Scholar] [CrossRef]
- Murphy, B.P.; Bowman, D.M.J.S. The carbon and nitrogen isotope composition of Australian grasses in relation to climate. Funct. Ecol. 2009, 23, 1040–1049. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, J.; Zhang, W.; Harris, W.; Zhong, H.; Hu, Z.; Song, L. Factors influencing altitudinal patterns of C3 plant foliar carbon isotope composition of grasslands on the Qinghai-Tibet Plateau, China. Alp. Bot. 2011, 121, 79–90. [Google Scholar] [CrossRef]
- Ma, F.; Liang, W.; Zhou, Z.; Xiao, G.; Liu, J.; He, J.; Jiao, B.; Xu, T. Spatial variation in leaf stable carbon isotope composition of three Caragana species in Northern China. Forests 2018, 9, 297. [Google Scholar] [CrossRef] [Green Version]
- Loader, N.; Hemming, D. Spatial variation in pollen δ13C correlates with temperature and seasonal development timing. Holocene 2001, 11, 587–592. [Google Scholar] [CrossRef]
- Zheng, S.; Shangguan, Z. Spatial patterns of foliar stable carbon isotope compositions of C3 plant species in the Loess Plateau of China. Ecol. Res. 2007, 22, 342–353. [Google Scholar] [CrossRef]
- Morecroft, M.D.; Woodward, F.I.; Marrs, R.H. Altitudinal trends in leaf nutrient contents, leaf size and δ13C of Alchemilla alpina. Funct. Ecol. 1992, 6, 730–740. [Google Scholar] [CrossRef]
- Liu, W.-G.; Feng, X.-H.; Ning, Y.-F.; Zhang, Q.-L.; Cao, Y.-N.; An, Z.-S. δ13C variation of C3 and C4 plants across an Asian monsoon rainfall gradient in arid northwestern China. Glob. Chang. Biol. 2005, 11, 1094–1100. [Google Scholar] [CrossRef]
- Buchmann, N.; Brooks, J.R.; Rapp, K.D.; Ehleringer, J.R. Carbon isotope composition of C4 grasses is influenced by light and water supply. Plant Cell Environ. 1996, 19, 392–402. [Google Scholar] [CrossRef]
- Wang, G.; Han, J.; Zhou, L.; Xiong, X.; Wu, Z. Carbon isotope ratios of plants and occurrences of C4 species under different soil moisture regimes in arid region of Northwest China. Physiol. Plant. 2005, 125, 74–81. [Google Scholar] [CrossRef]
- Yang, Q.; Li, X. Investigation of the controlled factors influencing carbon isotope composition of foxtail and common millet on the Chinese Loess Plateau. Sci. China Earth Sci. 2015, 58, 2296–2308. [Google Scholar] [CrossRef]
- Sonawane, B.V.; Cousins, A.B. Mesophyll CO2 conductance and leakiness are not responsive to short- and long-term soil water limitations in the C4 plant Sorghum bicolor. Plant J. 2020, 103, 1590–1602. [Google Scholar] [CrossRef]
- Luo, W.; Wang, X.; Auerswald, K.; Wang, Z.; Bird, M.I.; Still, C.J.; Lu, X.-T.; Han, X. Effects of plant intraspecific variation on the prediction of C3/C4 vegetation ratio from carbon isotope composition of topsoil organic matter across grasslands. J. Plant Ecol. 2021, 14, 628–637. [Google Scholar] [CrossRef]
- Yang, H.; Auerswald, K.; Bai, Y.; Wittmer, M.H.; Schnyder, H. Variation in carbon isotope discrimination in Cleistogenes squarrosa (Trin.) Keng: Patterns and drivers at tiller, local, catchment, and regional scales. J. Exp. Bot. 2011, 62, 4143–4152. [Google Scholar] [CrossRef] [Green Version]
- Eggels, S.; Blankenagel, S.; Schn, C.C.; Avramova, V. The carbon isotopic signature of C4 crops and its applicability in breeding for climate resilience. Theor. Appl. Genet. 2021, 134, 1663–1675. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Richards, R.A. Isotope composition of plant carbon correlates with warter-use efficiency of wheat genotypes. Aust. J. Plant Physiol. 1984, 11, 539–552. [Google Scholar] [CrossRef]
- Murphy, B.P.; Bowman, D.M. Seasonal water availability predicts the relative abundance of C3 and C4 grasses in Australia. Glob. Ecol. Biogeogr. 2007, 16, 160–169. [Google Scholar] [CrossRef]
- Department of Animal Husbandry and Veterinary, Institute of Grasslands, Chinese Academy of Agricultural Sciences (DAHV); Commission for Integrated Survey of Natural Resources, Chinese Academy of Sciences (CISNR). Rangeland Resources of China; China Agricultural Science and Technology Press: Beijing, China, 1996. [Google Scholar]
- Fu, Y.; Yu, Y.; Yao, H. Changes of soil organic carbon of grassland in the Xilinguole, Inner Mongolia from 2000 to 2007. Pr. Sci. 2011, 28, 1589–1597. [Google Scholar]
- O’Leary, M. Carbon isotope fractionation in plants. Phytochemistry 1981, 20, 553–567. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Song, L.; Fan, J.; Harris, W.; Wu, S.; Zhong, H.; Zhou, Y.; Wang, N.; Zhu, X. Adaptive characteristics of grassland community structure and leaf traits along an altitudinal gradient on a subtropical mountain in Chongqing, China. Plant Ecol. 2012, 213, 89–101. [Google Scholar] [CrossRef]
- Yang, H.; Yu, Q.; Sheng, W.; Li, S.; Tian, J. Determination of leaf carbon isotope discrimination in C4 plants under variable N and water supply. Sci. Rep. 2017, 7, 351. [Google Scholar] [CrossRef] [Green Version]
- Li, C.-Y.; Wu, C.-C.; Duan, B.-L.; Korpelainen, H.; Luukkanen, O. Age-related nutrient content and carbon isotope composition in the leaves and branches of Quercus aquifolioides along an altitudinal gradient. Trees 2009, 23, 1109–1121. [Google Scholar] [CrossRef]
- Körner, C.; Farquhar, G.; Wong, S. Carbon isotope discrimination by plants follows latitudinal and altitudinal trends. Oecologia 1991, 88, 30–40. [Google Scholar] [CrossRef]
- Grace, J.; Berninger, F.; Nagy, L. Impacts of climate change on the tree line. Ann. Bot. 2002, 90, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Schleser, G.; Helle, G.; Lucke, A.; Vos, H. Isotope signals as climate proxies: The role of transfer functions in the study of terrestrial archives. Quat. Sci. Rev. 1999, 18, 927–943. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Liu, X.; Li, X. Variations in carbon isotope ratios of plants across a temperature gradient along the 400 mm isoline of mean annual precipitation in north China and their relevance to paleovegetation reconstruction. Quat. Sci. Rev. 2013, 63, 83–90. [Google Scholar] [CrossRef]
- Wang, G.; Han, J.; Zhou, L. Relationship between δ13C value of C3 plant and the annual average temperature in northern China. Geol. China 2002, 29, 55–57. [Google Scholar]
- Zhang, C.; Chen, F.J.M. Study on modern plant C-13 in western China and its significance. Chin. J. Geochem. 2003, 22, 97–106. [Google Scholar]
- Wang, L.; Li, X.; Guo, L. The distribution of δ13C value of C3 plant and its responses to climate in arid and semiarid Central East Asia. Quat. Sci. 2006, 26, 955–961. [Google Scholar]
- Zhou, Y.-C.; Cheng, X.-L.; Fan, J.-W.; Xu, X.-Y. Spatial pattern of foliar δ13C in C3-dominated grasslands and its responses to climatic factors in Inner Mongolia, China. J. Northeast. Univ. Nat. Sci. 2016, 37, 273–279. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Liu, X.; Han, J.; Liu, M.; Liu, X. Variations in carbon isotope ratios of C3 plants and distribution of C4 plants along an altitudinal transect on the eastern slope of Mount Gongga. Sci. China Ser. D Earth Sci. 2009, 52, 1714–1723. [Google Scholar] [CrossRef]
- Wittmer, M.; Auerswald, K.; Tungalag, R.; Bai, Y.F.; Schaufele, R.; Schnyder, H. Carbon isotope discrimination of C3 vegetation in Central Asian grassland as related to long-term and short-term precipitation patterns. Biogeosciences 2008, 5, 913–924. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.-A.; Han, J.-M.; Liu, D.-S. The carbon isotope composition of C3 herbaceous plants in loess area of northern China. Sci. China Ser. D Earth Sci. 2003, 46, 1069–1076. [Google Scholar] [CrossRef]
- Van de Water, P.; Leavitt, S.; Betancourt, J. Trends in stomatal density and 13C/12C ratios of Pinus flexilis needles during last glacial-interglacial cycle. Science 1994, 264, 239. [Google Scholar] [CrossRef]
- Farquhar, G. On the nature of carbon isotope discrimination in C4 species. Aust. J. Plant Physiol. 1983, 10, 205–226. [Google Scholar] [CrossRef]
- Henderson, S.; Von Caemmerer, S.; Farquhar, G. Short-term measurements of carbon isotope discrimination in several C4 species. Aust. J. Plant Physiol. 1992, 19, 263–285. [Google Scholar] [CrossRef]
- Wang, G.; Han, J.; Zhou, L.; Xiong, X.; Tan, M.; Wu, Z.; Peng, X. The carbon isotope composition of C4 herbaceous plants in loess area of northern China. Sci. China Ser. D Earth Sci. 2005, 35, 1174–1179. [Google Scholar]
- Saliendra, N.Z.; Meinzer, F.C.; Perry, M.; Thom, M. Associations between partitioning of carboxylase activity and bundle sheath leakiness to CO2, carbon isotope discrimination, photosynthesis, and growth in sugarcane. J. Exp. Bot. 1996, 47, 907–914. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhu, Q. Intercellular CO2 concentration, CO2 leakage from bundle sheath cell and stable carbon isotope in leaf of summer maize under water stress. Acta Pedol. Sin. 2009, 46, 1040–1049. [Google Scholar] [CrossRef]
- Lightfoot, E.; Ustunkaya, M.C.; Przelomska, N.; O’Conne, T.C.; Hunt, H.V.; Jones, M.K.; Petrie, C.A. Carbon and nitrogen isotopic variability in foxtail millet (Setaria italica) with watering regime. Rapid Commun. Mass Spectrom. 2020, 34, e8615. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.Y.; Schaeufele, R.; Schnyder, H. Bundle-sheath leakiness and intrinsic water use efficiency of a perennial C-4 grass are increased at high vapour pressure deficit during growth. J. Exp. Bot. 2017, 68, 321–333. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).