Biostimulants for Resilient Agriculture: A Preliminary Assessment in Italy
Abstract
:1. Introduction
2. Material & Methods
2.1. Experimental Design
- Improve the photosynthetic activity of the crop and biomass development;
- Ameliorate resistance to biotic and abiotic stressors;
- Improve the overall crop nutrition.
2.1.1. First Experiment
2.1.2. Second Experiment
2.2. Data Collection
2.2.1. First Experiment
2.2.2. Second Experiment
2.3. Data Analysis
2.3.1. Statistical Analysis
2.3.2. Satellite Vegetation Indices
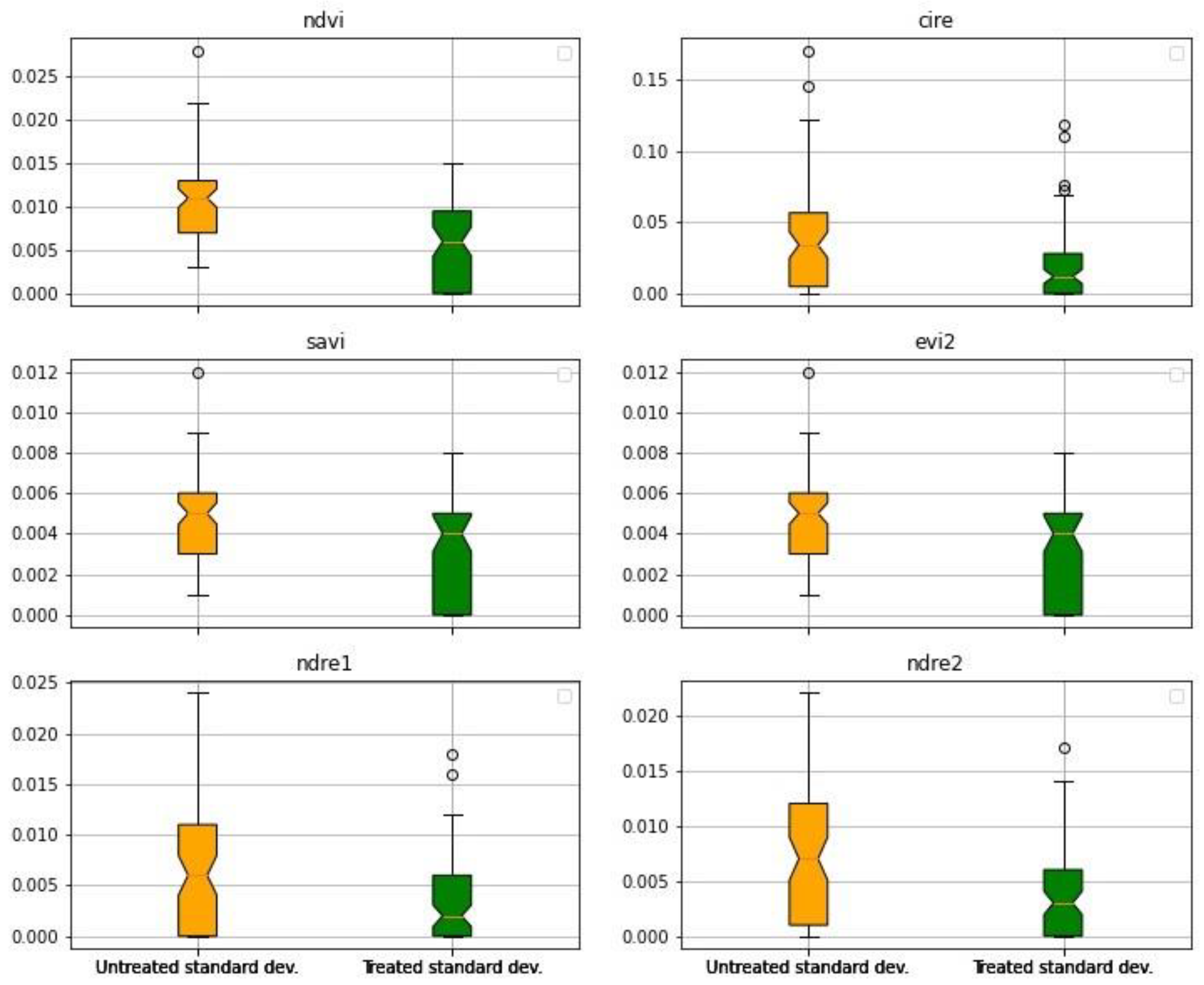
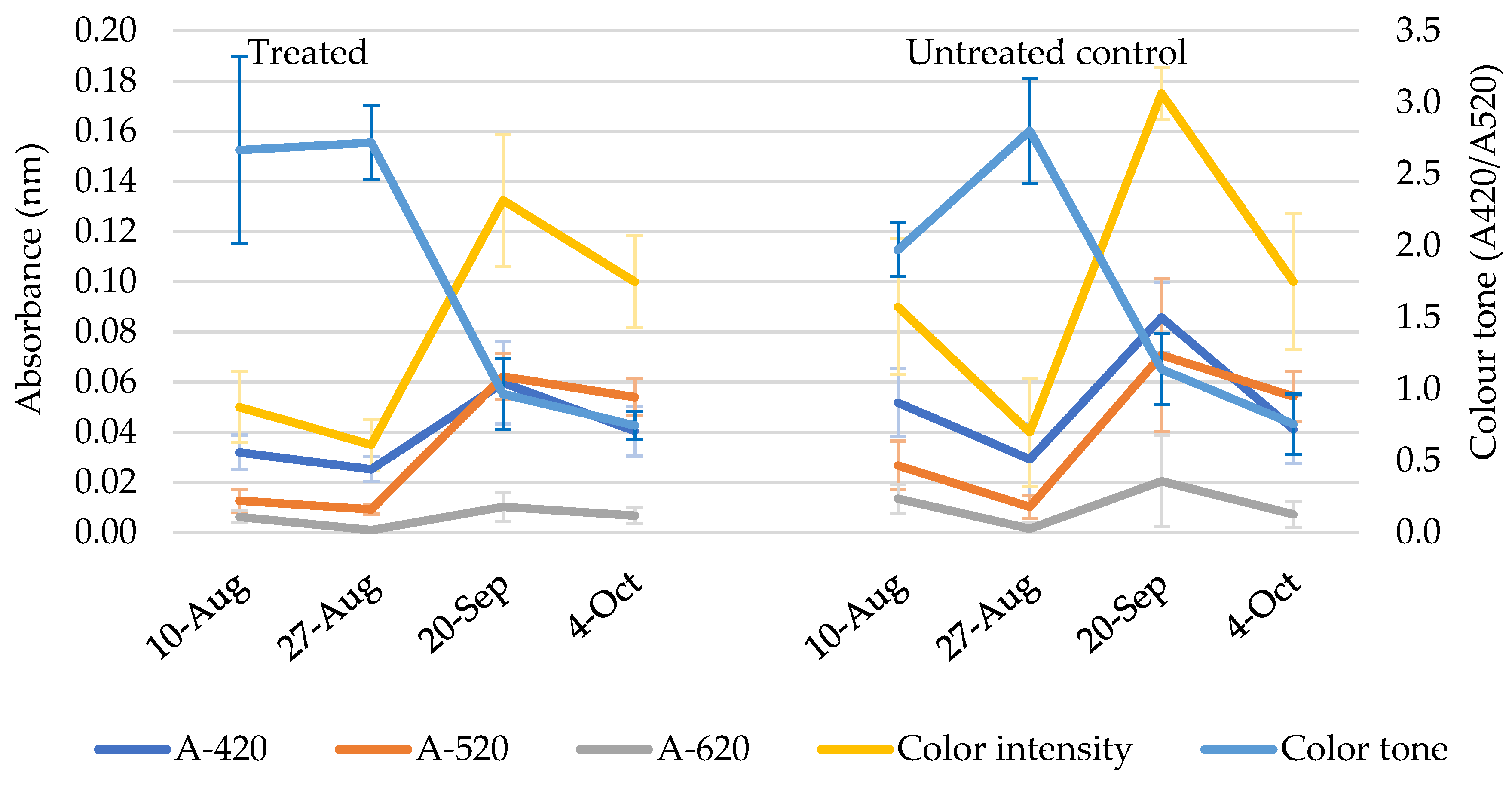
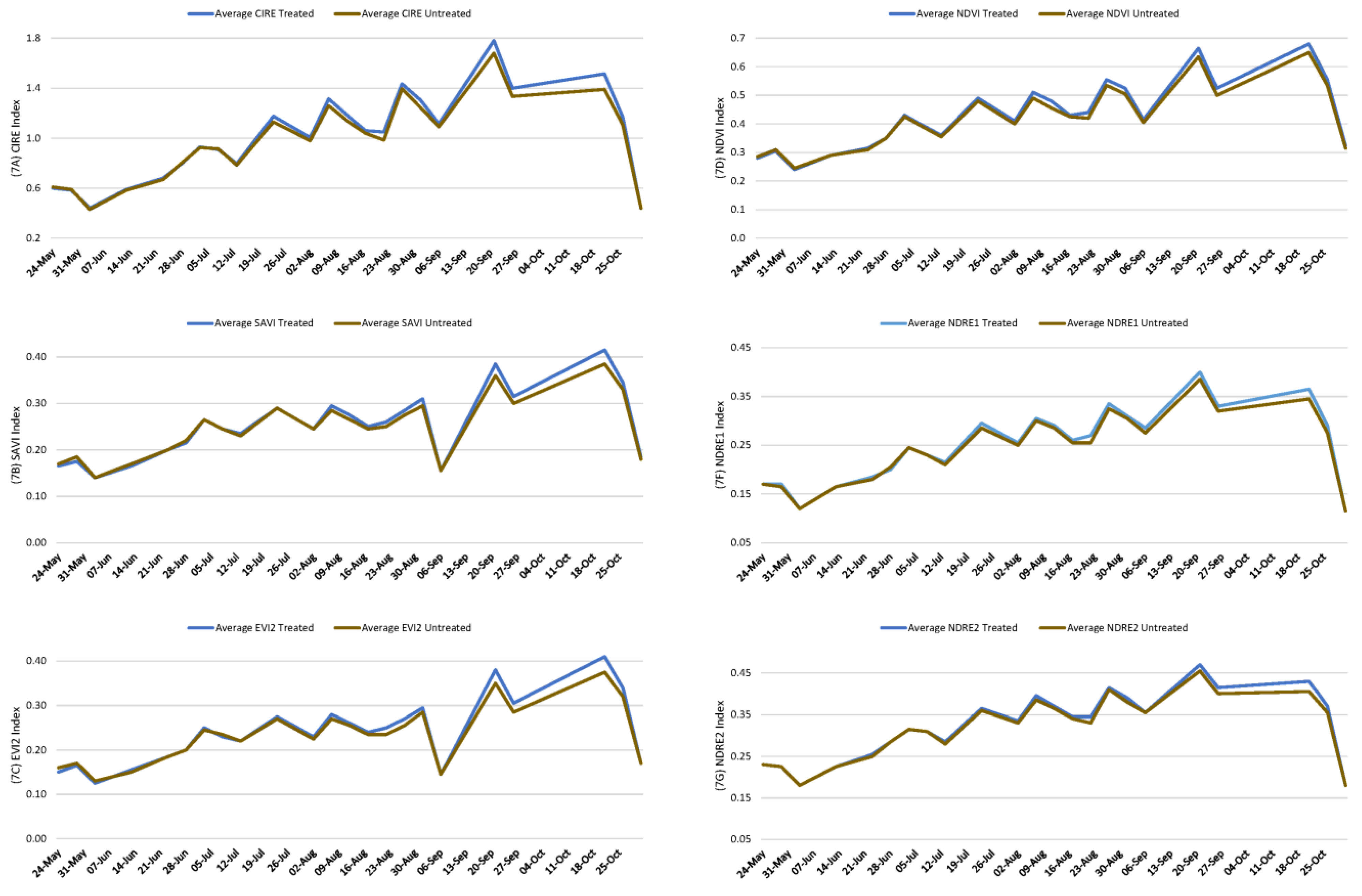
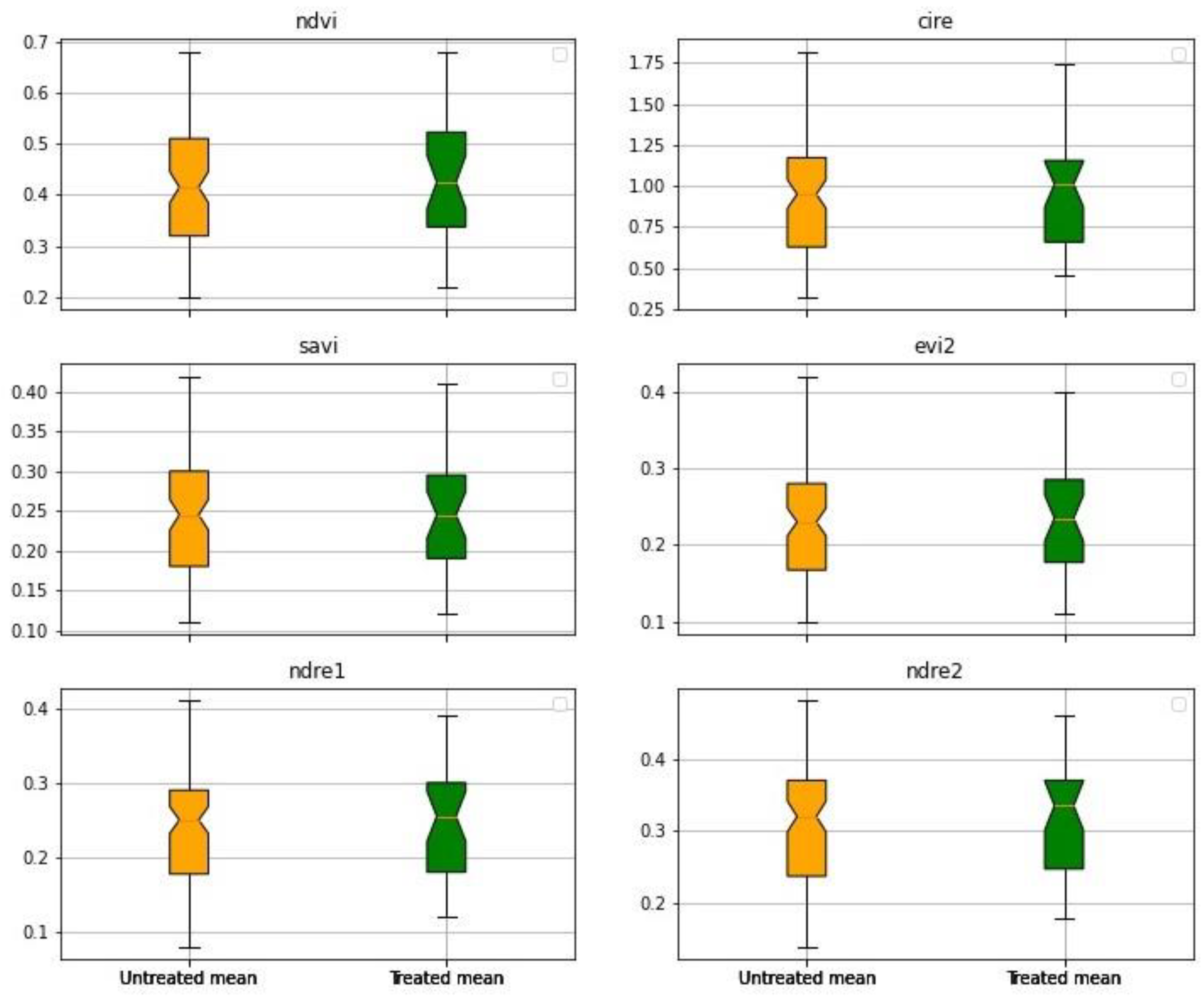
3. Results
3.1. First Experiment
3.2. Second Experiment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiebe, K.; Robinson, S.; Cattaneo, A. Climate Change, Agriculture and Food Security: Impacts and the Potential for Adaptation and Mitigation. In Sustainable Food and Agriculture—An Integrated Approach; Campanhola, C., Pandey, S., Eds.; The Food and Agricultural Organisation of the United Nations (FAO): Rome, Italy, 2019; pp. 55–74. [Google Scholar] [CrossRef]
- UN. Sustainable Development Goals: The 17 Goals; Department of Economic and Social Affairs of the United Nations (UN): New York, NY, USA. Available online: https://sdgs.un.org/goals (accessed on 22 February 2022).
- EU. Farm to Fork Strategy: For a Fair, Healthy and Environmentally-Friendly Food System; EU: Brussels, Belgium, 2020; p. 22. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/f2f_action-plan_2020_strategy-info_en.pdf (accessed on 22 February 2022).
- El Chami, D.; Daccache, A.; El Moujabber, M. How Can Sustainable Agriculture Increase Climate Resilience? A Systematic Review. Sustainability 2020, 12, 3119. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, A.V.; Pilbeam, D.J. Introduction. In Handbook of Plant Nutrition, 2nd ed.; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 3–18. [Google Scholar]
- Jaskulska, I.; Lemanowicz, J.; Breza-Boruta, B.; Siwik-Ziomek, A.; Radziemska, M.; Dariusz, J.; Białek, M. Chemical and Biological Properties of Sandy Loam Soil in Response to Long-Term Organic–Mineral Fertilisation in a Warm-Summer Humid Continental Climate. Agronomy 2020, 10, 1610. [Google Scholar] [CrossRef]
- Agegnehu, G.; Nelson, P.N.; Bird, M.I. Crop yield, plant nutrient uptake and soil physicochemical properties under organic soil amendments and nitrogen fertilization on Nitisols. Soil Tillage Res. 2016, 160, 239–246. [Google Scholar] [CrossRef]
- Singh, Y.V.; Singh, K.K.; Sharma, S.K. Influence of Crop Nutrition on Grain Yield, Seed Quality and Water Productivity under Two Rice Cultivation Systems. Rice Sci. 2013, 20, 129–138. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. The Role of Nutrient Efficient Plants in Improving Crop Yields in the Twenty First Century. J. Plant Nutr. 2008, 31, 1121–1157. [Google Scholar] [CrossRef]
- Howden, S.M.; Soussana, J.-F.; Tubiello, F.N.; Chhetri, N.; Dunlop, M.; Meinke, H. Adapting agriculture to climate change. Proc. Natl. Acad. Sci. USA 2007, 104, 19691–19696. [Google Scholar] [CrossRef] [Green Version]
- El Chami, D. Towards Sustainable Organic Farming Systems. Sustainability 2020, 12, 9832. [Google Scholar] [CrossRef]
- Chami, D.; Galli, F. An Assessment of Seaweed Extracts: Innovation for Sustainable Agriculture. Agronomy 2020, 10, 1433. [Google Scholar] [CrossRef]
- Zhang, X.; Schmidt, R.E. The impact of growth regulators on the α-tocopherol status in water-stressed Poa pratensis L. Int. Turfgrass Soc. Res. J. 1997, 8, 1364–1371. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Wells, E.; Wilkinson, M.; Wood, P.; Scanlan, C. The use of macroalgal species richness and composition on intertidal rocky seashores in the assessment of ecological quality under the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J. The Roman Book of Gardening, 1st ed.; Routledge: Abingdon, UK, 2004; p. 164. [Google Scholar]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Brassica napus Growth is Promoted by Ascophyllum nodosum (L.) Le Jol. Seaweed Extract: Microarray Analysis and Physiological Characterization of N, C, and S Metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Physiological Effects of Liquid Applications of a Seaweed Extract and a Humic Acid on Creeping Bentgrass. J. Am. Soc. Hortic. Sci. 2003, 128, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Billard, V.; Etienne, P.; Jannin, L.; Garnica, M.; Cruz, F.; Garcia-Mina, J.-M.; Yvin, J.-C.; Ourry, A. Two Biostimulants Derived from Algae or Humic Acid Induce Similar Responses in the Mineral Content and Gene Expression of Winter Oilseed Rape (Brassica napus L.). J. Plant Growth Regul. 2013, 33, 305–316. [Google Scholar] [CrossRef]
- Stamatiadis, S.; Evangelou, E.; Yvin, J.-C.; Tsadilas, C.; Mina, J.M.G.; Cruz, F. Responses of winter wheat to Ascophyllum nodosum (L.) Le Jol. extract application under the effect of N fertilization and water supply. J. Appl. Phycol. 2014, 27, 589–600. [Google Scholar] [CrossRef]
- Dobromilska, R.; Mikiciuk, M.; Gubarewicz, K. Evaluation of cherry tomato yielding and fruit mineral composition after using of Bio-algeen S-90 preparation. J. Plant Growth Regul. 2008, 13, 491–499. [Google Scholar]
- Crouch, I.J.; Beckett, R.P.; Van Staden, J. Effect of seaweed concentrate on the growth and mineral nutrition of nutrient-stressed lettuce. J. Appl. Phycol. 1990, 2, 269–272. [Google Scholar] [CrossRef]
- Mancuso, S.; Azzarello, E.; Mugnai, S.; Briand, X. Marine Bioactive Substances (IPA Extract) Improve Foliar Ion Uptake and Water Stress Tolerance in Potted Vitis vinifera Plants. Adv. Hortic. Sci. 2006, 20, 156–161. [Google Scholar] [CrossRef]
- Möller, M.; Smith, M. The significance of the mineral component of seaweed suspensions on lettuce (Lactuca sativa L.) seedling growth. J. Plant Physiol. 1998, 153, 658–663. [Google Scholar] [CrossRef]
- Shukla, P.S.; Shotton, K.; Norman, E.; Neily, W.; Critchley, A.; Prithiviraj, B. Seaweed extract improve drought tolerance of soybean by regulating stress-response genes. AoB Plants 2018, 10, plx051. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Fan, D.; Hodges, D.M.; Critchley, A.T.; Prithiviraj, B. A Commercial Extract of Brown Macroalga (Ascophyllum nodosum) Affects Yield and the Nutritional Quality of Spinach In Vitro. Commun. Soil Sci. Plant Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- Chouliaras, V.; Tasioula, M.; Chatzissavvidis, C.; Therios, I.; Tsabolatidou, E. The effects of a seaweed extract in addition to nitrogen and boron fertilization on productivity, fruit maturation, leaf nutritional status and oil quality of the olive (Olea europaea L.) cultivar Koroneiki. J. Sci. Food Agric. 2009, 89, 984–988. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Andreotti, C. Use of Biostimulants for Organic Apple Production: Effects on Tree Growth, Yield, and Fruit Quality at Harvest and During Storage. Front. Plant Sci. 2018, 9, 1342. [Google Scholar] [CrossRef]
- Frioni, T.; Sabbatini, P.; Tombesi, S.; Norrie, J.; Poni, S.; Gatti, M.; Palliotti, A. Effects of a biostimulant derived from the brown seaweed Ascophyllum nodosum on ripening dynamics and fruit quality of grapevines. Sci. Hortic. 2018, 232, 97–106. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Biostimulation to Tempranillo grapevines (Vitis vinifera L.) through a brown seaweed during two seasons: Effects on grape juice and wine nitrogen compounds. Sci. Hortic. 2020, 264, 109177. [Google Scholar] [CrossRef]
- Zouari, I.; Mechri, B.; Tekaya, M.; Dabbaghi, O.; Cheraief, I.; Mguidiche, A.; Annabi, K.; Laabidi, F.; Attia, F.; Hammami, M.; et al. Olive oil quality influenced by biostimulant foliar fertilizers. Braz. J. Biol. Sci. 2020, 7, 3–18. [Google Scholar] [CrossRef] [Green Version]
- Chanda, M.; Benhima, R.; Elmernissi, N.; Kasmi, Y.; Karim, L.; Sbabou, L.; Youssef, Z.; ElArroussi, H. Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci. Rep. 2020, 10, 2820. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V., Stocker, T.F., Qin, D., Dokken, D.J., Ebi, K.L., Mastrandrea, M.D., Mach, K.J., Plattner, G.-K., Allen, S.K., et al., Eds.; IPCC: Geneva, Switzerland; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; p. 582. [Google Scholar]
- Wall, E.; Smit, B. Climate Change Adaptation in Light of Sustainable Agriculture. J. Sustain. Agric. 2005, 27, 113–123. [Google Scholar] [CrossRef]
- Istat. Indagine Sulla Struttura e Produzione delle Aziende Agricole: Aziende e Superfici per Coltivazione; Istituto Nazionale di Statistica: Rome, Italy, 2016. [Google Scholar]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.A.; Merzlyak, M.N. Quantitative estimation of chlorophyll-a using reflectance spectra: Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Rouse, W., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the great plains with ERTS. In Proceedings of the Third Earth Resources Technology Satellite-1 Symposium, NASA SP-351, Washington, DC, USA, 10–14 December 1974; pp. 309–317. [Google Scholar]
- Jiang, Z.; Huete, A.R.; Didan, K.; Miura, T. Development of a two-band enhanced vegetation index without a blue band. Remote Sens. Environ. 2008, 112, 3833–3845. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.L.; et al. Coincident Detection of Crop Water Stress, Nitrogen Status and Canopy Density using Ground-Based Multispectral Data. In Proceedings of the 5th International Conference on Precision Agriculture, Bloomington, IN, USA, 16–19 July 2000; Robert, P.C., Rust, R.H., Larson, W.E., Eds.; 2000. [Google Scholar]
- Giuffrè, A.M. Evolution of Fatty Alcohols in Olive Oils produced in Calabria (Southern Italy) during Fruit Ripening. J. Oleo Sci. 2014, 63, 485–496. [Google Scholar] [CrossRef] [Green Version]
- Giuffrè, A.M. Influence of cultivar and harvest year on triglyceride composition of olive oils produced in Calabria (Southern Italy). Eur. J. Lipid Sci. Technol. 2013, 115, 928–934. [Google Scholar] [CrossRef]
- Uceda, M.; Hermoso, M.; Aguilera, M.P. The Quality of Olive Oil. In El Cultivo del Olivo; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Mundi-Prensa: Madrid, Spain, 2004; pp. 657–684. [Google Scholar]
- Almadi, L.; Paoletti, A.; Cinosi, N.; Daher, E.; Rosati, A.; Di Vaio, C.; Famiani, F. A Biostimulant Based on Protein Hydrolysates Promotes the Growth of Young Olive Trees. Agriculture 2020, 10, 618. [Google Scholar] [CrossRef]
- Ali, A.H.; Aboohanah, M.A.; Abdulhussein, M.A. Impact of foliar application with dry yeast suspension and amino acid on vegetative growth, yield and quality characteristics of olive. Kufa J. Agric. Sci. 2019, 11, 33–42. [Google Scholar]
- Gamboa, G.G.; Romanazzi, G.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. A review of the use of biostimulants in the vineyard for improved grape and wine quality: Effects on prevention of grapevine diseases. J. Sci. Food Agric. 2019, 99, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Lisek, J.; Sas-Paszt, L.; Derkowska, E.; Mrowicki, T.; Przybył, M.; Frąc, M. Growth, Yielding and Healthiness of Grapevine Cultivars ‘Solaris’ and ‘Regent’ in Response to Fertilizers and Biostimulants. J. Hortic. Res. 2016, 24, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.S.; Ahmad, B.; Jaskani, M.J.; Ahmad, R.; Malik, A.U. Foliar application of mixture of amino acids and seaweed (Ascophyllum nodosum) extract improve growth and physicochemical properties of grapes. Int. J. Agric. Biol. 2012, 14, 383–388. [Google Scholar]
- Norrie, J.; Branson, T.; Keathley, P.E. Marine plant extracts impact on grape yield and quality. Acta Hortic. 2002, 315–319. [Google Scholar] [CrossRef]
- Norrie, J.; Keathley, J.P. Benefits of ascophyllum nodosum marine-plant extract applications to ‘thompson seedless´ grape production. Acta Hortic. 2006, 243–248. [Google Scholar] [CrossRef]
- Weber, N.; Schmitzer, V.; Jakopic, J.; Stampar, F. First fruit in season: Seaweed extract and silicon advance organic strawberry (Fragaria×ananassa Duch.) fruit formation and yield. Sci. Hortic. 2018, 242, 103–109. [Google Scholar] [CrossRef]
- Tanou, G.; Ziogas, V.; Molassiotis, A. Foliar Nutrition, Biostimulants and Prime-Like Dynamics in Fruit Tree Physiology: New Insights on an Old Topic. Front. Plant Sci. 2017, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Kunicki, E.; Grabowska, A.; Sękara, A.; Wojciechowska, R. The effect of cultivar type, time of cultivation, and biostimulant treatment on the yield of spinach (Spinacia oleracea L.). Folia Hortic. 2010, 22, 9–13. [Google Scholar] [CrossRef] [Green Version]
- EBIC. Promoting the Biostimulant Industry and the Role of Plant Biostimulants in Making Agriculture More Sustainable; European Biostimulants Industry Council. Available online: http://www.biostimulants.eu/ (accessed on 30 March 2020).
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Mohammadipour, N.; Souri, M.K. Beneficial effects of glycine on growth and leaf nutrient concentrations of coriander (Coriandrum sativum) plants. J. Plant Nutr. 2019, 42, 1637–1644. [Google Scholar] [CrossRef]
- Souri, M.K.; Bakhtiarizade, M. Biostimulation effects of rosemary essential oil on growth and nutrient uptake of tomato seedlings. Sci. Hortic. 2019, 243, 472–476. [Google Scholar] [CrossRef]








| Indicator | Italy | Apulia |
|---|---|---|
| Average Farm Size (ha) | 11.0 | 6.6 |
| Total Agricultural Area (ha) | 12,598,161 | 1,285,274 |
| Grape Agricultural Area (ha) † | 614,958 | 92,038 |
| Olive Agricultural Area (ha) ‡ | 1,032,858 | 366,897 |
| Experiment 1: Olive Orchard | Experiment 2: Grape Orchard | |
|---|---|---|
| Location | Carovigno | Ostuni |
| Coordinates | 40°43′33.8″ N 17°43′30.5″ E | 40°42′29.8″ N 17°23′27.2″ E |
| Variety | Leccino | Montepulciano |
| Age | 50 | 31 |
| Soil Type | Loam | Clay Loam |
| Soil pH | 7.9 | 7.3 |
| EC (μS cm−1) | 0.63 | 0.14 |
| % Sand (2–0.05 mm) | 36.0 | 37.5 |
| % Lime (0.05–0.002 mm) | 38.3 | 31.7 |
| % Clay (<0.002 mm) | 25.7 | 30.8 |
| Total Calcium (g/kg) | 20 | 16 |
| Treatments | Yield kg plant−1 | Acidity % | No. Peroxides meq O2 kg−1 | K232 | K270 | Total Polyphenol mg kg−1 |
|---|---|---|---|---|---|---|
| Untreated control | 75.27 a A | 0.23 a A | 5.95 a A | 1.72 a A | 0.09 b A | 50.50 b B |
| Biostimulant | 80.85 a A | 0.21 b A | 6.20 a A | 1.78 a A | 0.13 a A | 181.00 a A |
| Treatments | 7 June 2021 | 21 June 2021 | 6 July 2021 |
|---|---|---|---|
| Pre-Flowering Stage | Post-Fruit Set | Berries Beginning to Touch | |
| Untreated control | 10.8 a A | 13.3 a A | 13.7 b B |
| Biostimulant | 11.3 a A | 14.5 a A | 15.0 a A |
| Treatments | Number of Bunches Plant−1 | Bunch Weight (Std) (g) |
|---|---|---|
| Untreated control | 34.85 a A | 195.1 (89.6) b A |
| Biostimulant | 34.3 a A | 214.4 (97.3) a A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leogrande, R.; El Chami, D.; Fumarola, G.; Di Carolo, M.; Piegari, G.; Elefante, M.; Perrelli, D.; Dongiovanni, C. Biostimulants for Resilient Agriculture: A Preliminary Assessment in Italy. Sustainability 2022, 14, 6816. https://doi.org/10.3390/su14116816
Leogrande R, El Chami D, Fumarola G, Di Carolo M, Piegari G, Elefante M, Perrelli D, Dongiovanni C. Biostimulants for Resilient Agriculture: A Preliminary Assessment in Italy. Sustainability. 2022; 14(11):6816. https://doi.org/10.3390/su14116816
Chicago/Turabian StyleLeogrande, Rita, Daniel El Chami, Giulio Fumarola, Michele Di Carolo, Giuseppe Piegari, Mario Elefante, Donato Perrelli, and Crescenza Dongiovanni. 2022. "Biostimulants for Resilient Agriculture: A Preliminary Assessment in Italy" Sustainability 14, no. 11: 6816. https://doi.org/10.3390/su14116816
APA StyleLeogrande, R., El Chami, D., Fumarola, G., Di Carolo, M., Piegari, G., Elefante, M., Perrelli, D., & Dongiovanni, C. (2022). Biostimulants for Resilient Agriculture: A Preliminary Assessment in Italy. Sustainability, 14(11), 6816. https://doi.org/10.3390/su14116816







