Physiological Comparison of Wheat and Maize Seedlings Responses to Water Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants Material, Growth Conditions, Treatments
2.2. Relative Water Content (RWC)
2.3. Photochemical Efficiency
2.4. Gas Exchange
2.5. Chlorophyll Content
2.6. Plant Height Analysis
2.7. Electrolyte Leakage (EL)
2.8. Statistical Analysis
3. Results and Discussion
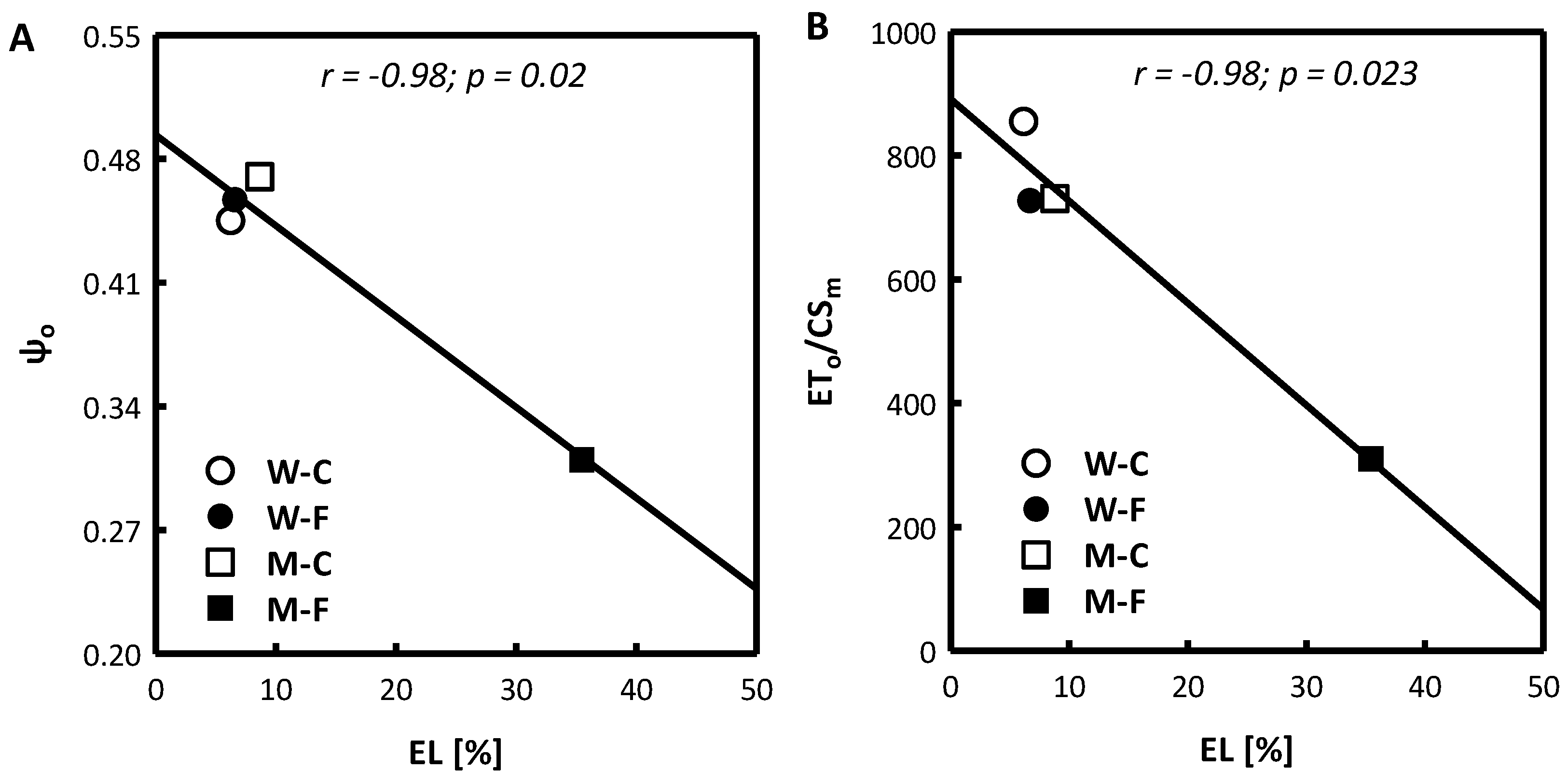
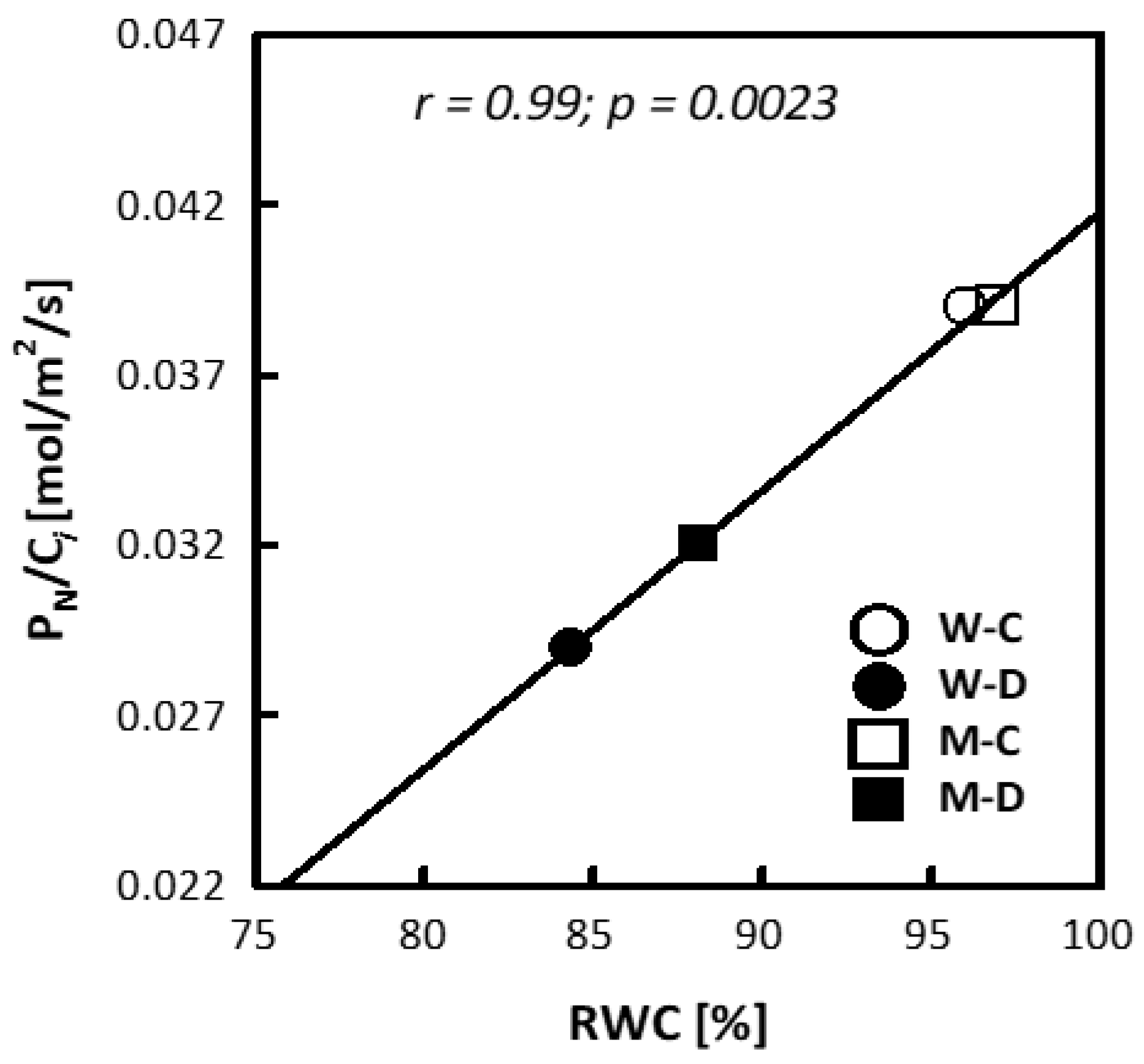
3.1. Plant Height, Relative Water Content (RWC) and Electrolyte Leakage (EL)
3.2. Leaf Gas Exchange and Water Use Efficiency (WUE)
3.3. Photosynthetic Apparatus Activity and Chlorophyll Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borowski, P.F. Nexus between water, energy, food and climate change as challenges facing the modern global, European and Polish economy. AIMS Geosci. 2020, 6, 397–421. [Google Scholar] [CrossRef]
- Eckstein, D.; Künzel, V.; Schäfer, L.; Winges, M. Global climate risk index 2020—Briefing Paper. Germanwatche. V Bonn. 2019. Available online: https://germanwatch.org/sites/default/files/20-2-01e%20Global%20Climate%20Risk%20Index%202020_15.pdf (accessed on 11 March 2022).
- Hura, T.; Tyrka, M.; Hura, K.; Ostrowska, A.; Dziurka, K. QTLs for cell wall-bound phenolics in relation to the photosynthetic apparatus activity and leaf water status under drought stress at different growth stages of triticale. Mol. Genet. Genom. 2017, 292, 415–433. [Google Scholar] [CrossRef]
- Ostrowska, A.; Biesaga-Koscielniak, J.; Grzesiak, M.T.; Hura, T. Physiological responses of spring wheat to 5-aminolevulinic acid under water stress applied at seedling stage. Cereal Res. Commun. 2019, 47, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Hess, T.; Knox, J.; Holman, I.; Sutcliffe, C. Resilience of Primary Food Production to a Changing Climate: On-Farm Responses to Water-Related Risks. Water 2020, 12, 2155. [Google Scholar] [CrossRef]
- Li, Y.; Guan, K.; Schnitkey, G.D.; DeLucia, E.; Peng, B. Excessive rainfall leads to maize yield loss of a comparable magnitude to extreme drought in the United States. Glob. Change Biol. 2019, 25, 2325–2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hura, T.; Hura, K.; Ostrowska, A.; Gadzinowska, J.; Fiust, A. Water stress-induced flag leaf senescence may be accelerated by rehydration. J. Plant Physiol. 2019, 236, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on cereal, legume, tuber and root crops production: A review. Agric. Water Manag. 2017, 179, 18–33. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Huang, Z.; Zhu, M.; Li, C.; Zhu, X.; Guo, W. Does cyclic water stress damage wheat yield more than a single stress? PLoS ONE 2018, 13, e0195535. [Google Scholar] [CrossRef] [Green Version]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.; Pintado, M.M.; Vasconcelos, M.W. Preserving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Tadesse, W.; Sanchez-Garcia, M.; Assefa, S.G.; Amri, A.; Bishaw, Z.; Ogbonnaya, F.C.; Baum, M. Genetic gains in wheat breeding and its role in feeding the world. Crop. Breed. Genet. Genom. 2019, 1, e190005. [Google Scholar] [CrossRef] [Green Version]
- Jat, M.L.; Stirling, C.M.; Jat, H.S.; Tetarwal, J.P.; Jat, R.K.; Singh, R.; Lopez-Ridaura, S.; Shirsath, P.B. Soil processes and wheat cropping under emerging climate change scenarios in South Asia. Adv. Agron. 2018, 148, 111–171. [Google Scholar] [CrossRef]
- Djaman, K.; O’Neill, M.; Owen, C.K.; Smeal, D.; Koudahe, K.; West, M.; Allen, S.; Lombard, K.; Irmak, S. Crop evapotranspiration, irrigation water requirement and water productivity of maize from meteorological data under semiarid climate. Water 2018, 10, 405. [Google Scholar] [CrossRef] [Green Version]
- Durodola, O.S.; Mourad, K.A. Modelling maize yield and water requirements under different climate change scenarios. Climate 2020, 8, 127. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity techniques for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Strasser, R.J. Constructive and destructive actions of light on the photosynthetic apparatus. J. Sci. Ind. Res. 1997, 56, 133–148. [Google Scholar]
- Lazar, D. Chlorophyll a fluorescence induction. Biochim. Biophys. Acta 1999, 1412, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Appenroth, K.J.; Stockel, J.; Srivastava, A.; Strasser, R.J. Multiple effects of chromate on the photosynthetic apparatus of Spirodelapolyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environ. Pollut. 2001, 115, 49–64. [Google Scholar] [CrossRef]
- Khalid, M.F.; Hussain, S.; Ahmad, S.; Ejaz, S.; Zakir, I.; Ali, M.A.; Ahmed, N.; Anjum, M.A. Impacts of abiotic stresses on growth and development of plants. In Plant Tolerance to Environmental Stress; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–8. [Google Scholar]
- Masoumi, Z.; Haghighi, M.; Jalali, S.A.H. Flooding or drought which one is more offensive on pepper physiology and growth? Mol. Biol. Rep. 2021, 48, 4233–4245. [Google Scholar] [CrossRef]
- Feng, Z.; Ding, C.; Li, W.; Wang, D.; Cui, D. Applications of metabolomics in the research of soybean plant under abiotic stress. Food Chem. 2020, 310, 125914. [Google Scholar] [CrossRef]
- Baye, A.; Berihun, B.; Bantayehu, M.; Derebe, B. Genotypic and phenotypic correlation and path coefficient analysis for yield and yield-related traits in advanced bread wheat (Triticum aestivum L.) lines. Cogent Food Agric. 2020, 6, 1752603. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Mutava, R.N.; Prince, K.S.J.; Syed, N.H.; Song, L.; Babu, V.; Chen, W.; Nguyen, H.T. Understanding abiotic stress tolerance mechanisms in soybean: A comparative evaluation of soybean response to drought and flooding stress. Plant Physiol. Biochem. 2015, 86, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, T.C. Plant responses to water stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1973, 24, 519–570. [Google Scholar] [CrossRef]
- Karimpour, M. Effect of drought stress on RWC and chlorophyll content on wheat (Triticum durum L.) genotypes. World. Ess. J. 2019, 7, 52–56. [Google Scholar]
- Azizi, S.; Tabari, M.; Striker, G.G. Growth, physiology, and leaf ion concentration responses to long-term flooding with fresh or saline water of Populus euphratica. S. Afr. J. Bot. 2017, 108, 229–236. [Google Scholar] [CrossRef]
- Garcia-Sanchez, F.; Syvertsen, J.P.; Gimeno, V.; Botia, P.; Perez-Perez, J.G. Responses to flooding and drought stress by two citrus rootstock seedlings with different water-use efficiency. Physiol. Plant. 2007, 130, 532–542. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Y.; Yue, Z.; Chen, X.; Cao, X.; Ai, X.; Jiang, B.; Xing, Y. The leaf-air temperature difference reflects the variation in water status and photosynthesis of sorghum under waterlogged conditions. PLoS ONE 2005, 14, e0219209. [Google Scholar] [CrossRef] [Green Version]
- Sathi, K.S.; Masud, A.A.C.; Falguni, M.R.; Ahmed, N.; Rahman, K.; Hasanuzzaman, M. Screening of soybean genotypes for waterlogging stress tolerance and understanding the physiological mechanisms. Adv. Agric. 2022, 2022, 5544665. [Google Scholar] [CrossRef]
- Grzesiak, M.T.; Janowiak, F.; Szczyrek, P.; Kaczanowska, K.; Ostrowska, A.; Rut, G.; Hura, T.; Rzepka, A.; Grzesiak, S. Impact of soil compaction stress combined with drought or waterlogging on physiological and biochemical markers in two maize hybrids. Acta Physiol. Plant 2016, 38, 109. [Google Scholar] [CrossRef] [Green Version]
- Lama, R.; Jaishee, N.; Chakraborty, U.; Roy, A. Responses of seven maize genotypes during flooding stress and identification of cultivars most tolerant to flooding conditions. Plant Arch. 2020, 20, 3244–3249. [Google Scholar]
- Tian, L.; Bi, W.; Liu, X.; Sun, L.; Li, J. Effects of waterlogging stress on the physiological response and grain-filling characteristics of spring maize (Zea mays L.) under field conditions. Acta Physiol. Plant. 2019, 41, 1–14. [Google Scholar] [CrossRef]
- Xiong, R.; Sang, L.; Liu, R.; Cheng, R.; Li, P.; Huang, L.; Cao, G. Effects of waterlogging on maize seedling growth during seed germination. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 598, p. 012075. [Google Scholar]
- Paradiso, A.; Caretto, S.; Leone, A.; Bove, A.; Nisi, R.; De Gara, L. ROS production and scavenging under anoxia and re-oxygenation in Arabidopsis cells: A balance between redox signalling and impairment. Front. Plant Sci. 2016, 7, 1803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdomo, J.A.; Capo-Bauca, S.; Carmo-Silva, E.; Galmes, J. Rubisco and Rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef] [Green Version]
- Todorova, D.; Aleksandrov, V.; Anev, S.; Sergiev, I. Photosynthesis alterations in wheat plants induced by herbicide, soil drought or flooding. Agronomy 2022, 12, 390. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, H.; Song, X.; Jin, J.; Zhang, X. The responses of plant leaf CO2/H2O exchange and water use efficiency to drought: A meta-analysis. Sustainability 2018, 10, 551. [Google Scholar] [CrossRef] [Green Version]
- Else, M.A.; Coupland, D.; Dutton, L.; Jackson, M.B. Decreased root hydraulic conductivity reduces leaf water potential, initiates stomatal closure and slows leaf expansion in flooded plants of castor oil (Ricinus communis) despite diminished delivery of ABA from the roots to shoots in xylem sap. Physiol. Plant. 2001, 111, 46–54. [Google Scholar] [CrossRef]
- Tong, C.; Hill, C.B.; Zhou, G.; Zhang, X.Q.; Jia, Y.; Li, C. Opportunities for improving waterlogging tolerance in cereal crops—physiological traits and genetic mechanisms. Plants 2021, 10, 1560. [Google Scholar] [CrossRef]
- Bussotti, F.; Gerosa, G.; Digrado, A.; Pollastrini, M. Selection of chlorophyll fluorescence parameters as indicators of photosynthetic efficiency in large scale plant ecological studies. Ecol. Indic. 2020, 108, 105686. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Rastogi, A.; Živčák, M.; Brestic, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lotfi, R.; Stypiński, P.; Samborska, I.A.; et al. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Yin, C.Y.; Berninger, F.; Li, C.Y. Photosynthetic responses of Populus przewalski subjected to drought stress. Photosynthetica 2006, 44, 62–68. [Google Scholar] [CrossRef]
- Gadzinowska, J.; Ostrowska, A.; Hura, K.; Dziurka, M.; Pawlowska, B.; Hura, T. Physiological traits determining high adaptation potential of sweet briar (Rosa rubiginosa L.) at early stage of growth to dry lands. Sci. Rep. 2019, 9, 19390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hura, T.; Hura, K.; Ostrowska, A.; Dziurka, K. Rapid plant rehydration initiates permanent and adverse changes in the photosynthetic apparatus of triticale. Plant Soil 2015, 397, 127–145. [Google Scholar] [CrossRef] [Green Version]
- Biehler, K.; Fock, H. Evidence for the contribution of the Mehler-peroxidase reaction in dissipating excess electrons in drought-stressed wheat. Plant Physiol. 1996, 112, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Noctor, G. Oxygen processing in photosynthesis: Regulation and signalling. New Phytol. 2000, 146, 359–388. [Google Scholar] [CrossRef] [Green Version]
- Fghire, R.; Anaya, F.; Ali, O.I.; Benlhabib, O.; Ragab, R.; Wahbi, S. Physiological and photosynthetic response of quinoa to drought stress. Chil. J. Agric. Res. 2015, 75, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Golebiowska-Pikania, G.; Kopec, P.; Surowka, E.; Janowiak, F.; Krzewska, M.; Dubas, E.; Nowicka, A.; Kasprzyk, J.; Ostrowska, A.; Malaga, S.; et al. Changes in protein abundance and activity induced by drought during generative development of winter barley (Hordeum vulgare L.). J. Proteom. 2017, 169, 73–86. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Niu, J.; Wang, R.; Song, J.; Lv, J.; Zong, X.; Wang, S. Interaction of drought and 5-aminolevulinic acid on growth and drought resistance of Leymuschinensis seedlings. Acta Ecol. Sin. 2016, 36, 180–188. [Google Scholar] [CrossRef]
- Dernetriou, G.; Neonaki, C.; Navakoudis, E.; Kotzabasis, K. Salt stress impact on the molecular structure and function of the photosynthetic apparatus: The protective role of polyamines. Biochim. Biophys. Acta 2007, 1767, 272–280. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Govindjee; Bosa, K.; Koscielniak, J.; Zuk-Golaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Lu, C.; Vonshak, A. Effects of salinity stress on photosystem II function in cyanobacterial Spirulina platensis cells. Physiol. Plant. 2002, 114, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Antonkiewicz, J.; Rapacz, M. Assessment of photosynthetic activity of plants grown on stubble sediments and furnaceash. Zesz. Probl. Post. NaukRol. 2006, 509, 187–196. (In Polish) [Google Scholar]
- Kudoh, H.; Sonoike, K. Irreversible damage to photosystem I by chilling in the light: Cause of the degradation of chlorophyll after returning to normal growth temperature. Planta 2002, 215, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.M.; Zhang, J.H. Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci. 2000, 151, 135–143. [Google Scholar] [CrossRef]
- Park, S.U.; Lee, C.J.; Kim, S.E.; Lim, Y.H.; Lee, H.U.; Nam, S.S.; Kim, H.S.; Kwak, S.S. Selection of flooding stress tolerant sweetpotato cultivars based on biochemical and phenotypic characterization. Plant Physiol. Biochem. 2020, 155, 243–251. [Google Scholar] [CrossRef]
| Parameter | Treatment | Drought | Flooding | ||
|---|---|---|---|---|---|
| Wheat | Maize | Wheat | Maize | ||
| Plant height | Control | 46.9 ± 0.90 bc | 64.1 ± 1.63 a | 46.9 ±0.90 c | 64.1 ± 1.63 a |
| Stress | 43.6 ± 0.85 c | 49.3 ± 1.66 b | 44.5 ± 0.85 c | 59.0 ± 1.78 b | |
| RWC | Control | 96.0 ± 1.11 a | 97.0 ± 1.09 a | 96.0 ± 1.11 a | 97.0 ± 1.09 a |
| Stress | 84.3 ± 2.09 b | 88.1 ± 1.41 b | 90.7 ± 2.08 b | 90.9 ± 1.15 b | |
| EL | Control | 6.2 ± 0.28 c | 8.7 ± 0.45 bc | 6.2 ± 0.28 c | 8.7 ± 0.45 b |
| Stress | 9.0 ± 0.38 b | 11.6 ± 1.68 a | 6.6 ± 0.54 bc | 35.4 ± 1.46 a | |
| Parameters | Treatments | Drought | Flooding | ||
|---|---|---|---|---|---|
| Wheat | Maize | Wheat | Maize | ||
| PN | Control | 14.25 ± 2.09 ab | 17.85 ± 1.58 a | 14.25 ± 2.09 b | 17.85 ± 1.58 a |
| Stress | 10.47 ± 1.79 c | 12.64 ± 1.20 b | 13.46 ± 1.91 b | 16.59 ± 1.85 a | |
| E | Control | 4.95 ± 0.75 b | 5.67 ± 0.83 a | 4.95 ± 0.75 ab | 5.67 ± 0.83 a |
| Stress | 3.17 ± 0.43 c | 3.12 ± 0.54 c | 4.34 ± 0.28 b | 4.93 ± 0.40 ab | |
| gs | Control | 0.046 ± 0.0036 b | 0.064 ± 0.0042 a | 0.046 ± 0.0036 b | 0.064 ± 0.0042 ab |
| Stress | 0.025 ± 0.0045 c | 0.036 ± 0.0021 b | 0.039 ± 0.0021c | 0.075 ± 0.0062 a | |
| Ci | Control | 371.3 ± 23.12 bc | 468.3 ± 31.43 a | 371.3 ± 23.12 c | 468.3 ± 31.43 a |
| Stress | 369.2 ± 19.51 c | 406.3 ± 25.18 ab | 382.2 ± 31.65 c | 411.2 ± 27.78 b | |
| WUE | Control | 2.88 ± 0.47 b | 3.15 ± 0.27 ab | 2.88 ± 0.47 b | 3.15 ± 0.27 ab |
| Stress | 3.30 ± 0.39 ab | 4.05 ± 0.62 a | 3.10 ± 0.31 ab | 3.37 ± 0.19a | |
| PN/Ci | Control | 0.038 ± 0.0051 a | 0.039 ± 0.0047 a | 0.038 ± 0.0051 a | 0.039 ± 0.0047 a |
| Stress | 0.029 ± 0.0039 b | 0.032 ± 0.0035 ab | 0.036 ± 0.0029 a | 0.041 ± 0.0040 a | |
| Parameters | Treatments | Drought | Flooding | ||
|---|---|---|---|---|---|
| Wheat | Maize | Wheat | Maize | ||
| ABS/CSm | Control | 2409 ± 61.3 a | 1945 ± 52.0 b | 2409 ± 61.3 a | 1945 ± 52.0 b |
| Stress | 2187 ± 116.9 a | 1481 ± 71.3 c | 2248 ± 68.5 a | 1426 ± 69.9 c | |
| TRo/CSm | Control | 1914 ± 52.9 a | 1553 ± 44.8 b | 1914 ± 52.9 a | 1553 ± 44.8 b |
| Stress | 1453 ± 85.4 b | 959 ± 72.7 c | 1606 ± 56.9 b | 940 ± 67.7 c | |
| ETo/CSm | Control | 857 ± 38.6 a | 731 ± 28.5 b | 857 ± 38.6 a | 731 ± 28.5 b |
| Stress | 619 ± 46.6 c | 304 ± 41.2 d | 729 ± 29.8 b | 310 ± 34.2 c | |
| DIo/CSm | Control | 496 ± 15.1 b | 393 ± 17.9 c | 496 ± 15.1 b | 393 ± 17.9 c |
| Stress | 734 ± 49.9 a | 522 ± 14.1 b | 642 ± 26.6 a | 487 ± 30.1 b | |
| RC/CSm | Control | 687 ± 27.0 a | 650 ± 17.4 a | 687 ± 27.0 a | 650± 17.4 a |
| Stress | 425 ± 29.9 b | 383 ± 23.9 b | 514 ± 25.4 b | 368 ± 27.7 c | |
| ABS/RC | Control | 3.54 ± 0.076 c | 3.00 ± 0.044 d | 3.54 ± 0.076 c | 3.00 ± 0.044 d |
| Stress | 5.30 ± 0.230 a | 3.92 ± 0.083 b | 4.45 ± 0.141 a | 3.98 ± 0.128 b | |
| TRo/RC | Control | 2.81 ± 0.047 b | 2.39 ± 0.035 c | 2.81 ± 0.047 b | 2.39 ± 0.035 d |
| Stress | 3.47 ± 0.076 a | 2.48 ± 0.052 c | 3.16 ± 0.059 a | 2.56 ± 0.055 c | |
| ETo/RC | Control | 1.24 ± 0.020 b | 1.12 ± 0.022 b | 1.24 ± 0.020 b | 1.12 ± 0.022 b |
| Stress | 1.47 ± 0.083 a | 0.79 ± 0.049 c | 1.45 ± 0.087 a | 0.78 ± 0.057 c | |
| DIo/RC | Control | 0.73 ± 0.030 c | 0.61 ± 0.011 c | 0.73 ± 0.030 b | 0.61 ± 0.011 b |
| Stress | 1.83 ± 0.158 a | 1.44 ± 0.105 b | 1.29 ± 0.088 a | 1.41 ± 0.143 a | |
| Fv/Fm | Control | 0.79 ± 0.005 a | 0.80 ± 0.002 a | 0.79 ± 0.005 a | 0.80 ± 0.002 a |
| Stress | 0.66 ± 0.016 b | 0.64 ± 0.020 b | 0.71 ± 0.010 b | 0.63 ± 0.024 c | |
| ψo | Control | 0.45 ± 0.012 ab | 0.47 ± 0.011 a | 0.45 ± 0.012 ab | 0.47 ± 0.011 a |
| Stress | 0.42 ± 0.019 b | 0.30 ± 0.018 c | 0.46 ± 0.021 a | 0.31 ± 0.025 b | |
| φEo | Control | 0.35 ± 0.010 a | 0.38 ± 0.009 a | 0.35 ± 0.010 ab | 0.38 ± 0.019 a |
| Stress | 0.28 ± 0.013 b | 0.20 ± 0.015 c | 0.32 ± 0.012 b | 0.20 ± 0.020 c | |
| PI | Control | 0.92 ± 0.083 b | 1.19 ± 0.062 a | 0.92 ± 0.083 b | 1.19 ± 0.062 a |
| Stress | 0.31 ± 0.040 c | 0.24 ± 0.039 c | 0.50 ± 0.039 c | 0.27 ± 0.055 d | |
| Chl | Control | 32.1 ± 0.37 bc | 38.0 ± 0.79 a | 32.1 ± 0.37 c | 38.0 ± 0.79 a |
| Stress | 31.1 ± 0.79 c | 34.2 ± 0.98 b | 30.3 ± 0.95 c | 35.4 ± 0.32 b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostrowska, A.; Hura, T. Physiological Comparison of Wheat and Maize Seedlings Responses to Water Stresses. Sustainability 2022, 14, 7932. https://doi.org/10.3390/su14137932
Ostrowska A, Hura T. Physiological Comparison of Wheat and Maize Seedlings Responses to Water Stresses. Sustainability. 2022; 14(13):7932. https://doi.org/10.3390/su14137932
Chicago/Turabian StyleOstrowska, Agnieszka, and Tomasz Hura. 2022. "Physiological Comparison of Wheat and Maize Seedlings Responses to Water Stresses" Sustainability 14, no. 13: 7932. https://doi.org/10.3390/su14137932
APA StyleOstrowska, A., & Hura, T. (2022). Physiological Comparison of Wheat and Maize Seedlings Responses to Water Stresses. Sustainability, 14(13), 7932. https://doi.org/10.3390/su14137932







