Abstract
Desalination brine is extremely concentrated saline water; it contains various salts, nutrients, heavy metals, organic contaminants, and microbial contaminants. Conventional disposal of desalination brine has negative impacts on natural and marine ecosystems that increase the levels of toxicity and salinity. These issues demand the development of brine management technologies that can lead to zero liquid discharge. Brine management can be productive by adopting economically feasible methodologies, which enables the recovery of valuable resources like freshwater, minerals, and energy. This review focuses on the recent advances in brine management using various membrane/thermal-based technologies and their applicability in water, mineral, and energy recoveries, considering their pros and cons. This review also exemplifies the hybrid processes for metal recovery and zero liquid discharge that may be adopted, so far, as an appropriate futuristic strategy. The data analyzed and outlook presented in this review could definitely contribute to the development of economically achievable future strategies for sustainable brine management.
1. Introduction
Water scarcity has evolved into a global challenge with the population explosion and its demand for industrial and domestic applications. Research has been focused on the technological and material aspects to meet the growing water demand. With the exhaustion of freshwater sources and the abundance of seawater or brackish water, research has been focused on the production of clean water from these resources. Desalination has been an important advancement that has the potential to meet the water crisis, but with the advantages of this process there are disadvantages too. The large production of brine, i.e., the highly concentrated salt stream from the desalination plant, is a major concern as most of the desalination plants dispose of the brine into the original water source. The salt accumulation in brine increases the seawater salinity and consequently it increases the energy needed for desalination for a potable water supply. Brine also contains metals and chemicals (Table 1) that cause negative effects on marine ecosystems. The threats posed by brine discharge lead to socioeconomic and socio-political consequences such as energy demand, water stress, and negative health impacts. Therefore, the increasing pollution of the water resources has to be managed strategically to maintain the balance of the ecosystem.
However, there are socio-political and legal challenges that any management approach should address for the development and the proliferation of brine management. It is severely impacted by a variety of often neglected socio-political factors. These are major factors in the success or failure of many brine management projects around the world, and they are classified into four categories: strengths, weaknesses, opportunities, and dangers. There are links between brine management and society’s critical needs for political stability, better health, economic growth, and water security. For example, proper brine management can result in the commercialization of valuable resources like water, minerals, and energy, which will lower the overall cost and offer a business opportunity that definitely will ensure the economic, social, and environmental stability of countries.
This review focuses on the advancement in brine management and proposes future strategies to overcome the crisis. Hybrid technologies that can be utilized to develop a circular solution of waste to energy or value-added products will also be discussed.
There are several review articles in this area that presented discussions on brine management and treatment-based and technology-based solutions. Bello et al. have recently given an overview of brine management, desalination technologies, life cycle assessment, and recovery methods [1], while Al-Absi et al. provided an update on the use of adsorption processes as a recovery option and discussed the various brine management strategies and technologies [2]. Mavukkandy et al. reviewed recent research and technological development on recovering water, minerals, and energy from desalination brine [3]. Soliman et al. have presented a comprehensive review of the current technologies of various desalination processes and the detailed energy consumption and water production costs of these technologies [4]. However, previous reports lack detailed analysis of future prospects to achieve sustainable brine management. Hence, the present review focuses on the current brine disposal strategies, methods of treatment, hybrid methods for metal recovery, and zero liquid discharge (ZLD). More attention was given to analyzing futuristic developments of a sustainable hybrid strategy for brine management that could open gateways to remarkable water recovery and mineral recovery channels while attaining the near-ZLD approach.

Table 1.
General characteristics of brine from seawater desalination plants [5,6,7].
Table 1.
General characteristics of brine from seawater desalination plants [5,6,7].
| Parameters | Details |
|---|---|
| Physical characteristics | Salinity: above 55,000 mg/L of TDS; conductivity: 0.6 W/mK at 25 °C; temperature: ambient seawater; pH: 7–8. |
| Inorganic salts | Example: sodium chloride (NaCl), calcium chloride (CaCl2), and magnesium chloride (MgCl2) are the major constituents. |
| Metals caused by corrosion | Brine might have high levels of iron, chromium, nickel, and molybdenum if the facility uses low-quality stainless steel. |
| Nutrients | Ammonia, nitrate, and phosphorus. |
| Pretreatment chemicals | Antiscale additive (ethylenediaminetetraacetic acid: EDTA, sodium hexameta phosphate). Biofouling control additives such as chlorine (small quantities)—coagulants. |
| Halogenated organics | Trihalomethanes are common byproducts of chlorine addition (low content). |
| Cleaning chemicals | -Acidic solutions used to adjust the pH of the seawater. -Detergent such as EDTA, oxidants (sodium perborate) and biocides (formaldehyde) are used to clean the membrane. |
1.1. Brine Solution and Characteristics
Brine is a by-product or the end product of a desalination process that consists of various components. A list of typical physical and chemical characteristics of desalination brine is given in Table 1. Brine has a salinity above 55,000 mg/L of total dissolved solids (TDS) in the stream [5]. The chemical characteristics of brine discharge depend on various factors such as the quality of feed water and permeate water, type of desalination process, pre-treatment method, and cleaning procedures used. Each plant has a diverse concentration and components of contaminants in it. The presence of heavy metals, organic contaminants, strong acids/base, antiscalants, coagulants, and biocides add to the complexity of the brine solution.
1.2. Conventional Methodologies for Disposal of Brine
The conventional strategies involved in the disposal of brine from desalination processes can vary depending on the geographical location, quality, and volume of the brine. Some conventional disposal methods include surface water discharge, deep well injection, land application, evaporation ponds, and conventional crystallizers. There are several factors that influence which option of disposal method can be adopted such as quantity and quality of the brine, geographical location of discharge point, availability and authorization of dump sites, and operational and transportation costs. All these are critical factors to be addressed when a desalination plant needs to be installed. It has been reported that almost 5% to 33% of the total cost will be spent on the disposal processes for the brine. In addition, revenue that can be made from the brine, such as minerals recovery, waste-to-value-added products like fertilizers, etc., are alternatives for a more cost-effective model. The conventional brine disposal strategies have been tabulated in the Table 2.

Table 2.
Conventional brine disposal strategies and its environmental impacts.
1.3. Environmental Impact of Brine
The improper disposal methods of brine can cause several environmental hazards that bring negative impacts to the air and water quality. The toxicity imposed by brine disposal can vary depending on the potential hazardous substances it contains, such as toxic metals (mercury (Hg), cobalt (Co), copper (Cu), iron (Fe), zinc (Zn), and nickel (Ni)), pesticides, and acids, which cause irrevocable changes to the environment. The direct disposal of brine into the ecosystem has also caused severe imbalance to aquatic life by fluctuating the pH, salinity, temperature, eutrophication, etc. There are several reports wherein the direct influence of heavy metals has impacted the flora and fauna [6,7]. The methods for brine disposal vary depending on the geographical location of the desalination plant. The plants that are located in the coastal line usually dispose of the brine back into the seawater, thus affecting its salinity and the marine ecosystem as mentioned earlier, whereas the land-based plants result in the contamination of the groundwater resources and surrounding environment. There are several reports that have highlighted the environmental impacts of brine disposal from desalination plants at specific geological locations [5,7].
Generally, the treatment of brine depends on the composition, such as the removal of all organic matter initially and further removal of salts and other elements. Proper treatment and conversion of brine to value-added substances for industrial and irrigation purposes can be a good brine management strategy [8]. As formerly mentioned, brine has a salinity at least 1.6–2.1 times higher than seawater and at elevated temperature up to 50 °C, which is extremely high compared to the surrounding temperature, thus the potential of affecting the marine flora and fauna. The most devastating effect it can cause is the ‘lethal osmotic shock’ to the fishes, plankton, algae, and seagrass, causing irrevocable damage to their cells, leading to extinction [9,10,11]. Water bodies with abundant marine life such as closed or semi-closed shallow places should not be disposal sites as it greatly affects the marine life because of the change in salinity and lowered dissolved oxygen levels. The seasonal and cumulative effects of brine discharges from desalination plants along the Israeli coast was studied using benthic foraminifera, a known sensitive marine bio-indicator [12]. Another study reported fish survival for three months in raw and calcium-reduced concentrate discharge from the desalination plant [13]. However, a very recent short-term study for six years at two mega-size seawater desalination plants on the Mediterranean coast of Israel has reported that brine discharge has no significant impact on seawater quality. The study presented that it did not impact the oxygen saturation, turbidity, pH, nutrients (except for total organic phosphorus (TOP)), chlorophyll-a, and metal concentrations [5]. An environmental risk assessment is a prerequisite to assess the environmental impacts associated with desalination plants. It studies and processes a proper location for installation of a desalination plant with mitigation strategies and waste disposal methods and their impact on marine and coastal environments [14]. Marine monitoring and assessment should continue for as long as the plants are operational and critically reviewed. The regulations should be re-evaluated periodically for frequency, sampling stations, and parameters measured, and updated when necessary.
2. Conventional Technologies for Brine Treatment
Proper brine management must be designed to fulfill the criteria of a brine recycling loop. Subjecting desalination brine to chemical/electro chemical coagulation, chemical oxidation, chemical precipitations, and biological assimilation are the traditional ways of brine treatment for decontamination/resource recovery before the most modern technology replaced the conventional techniques.
Among conventional methods, chemical precipitation is mainly used only for inorganic removal, whereas other methods are adopted for organic impurities. For example, electro-/chemical coagulation and chemical precipitations are closely related techniques that involve the summoning of smaller impurities into larger debris to help them settle at the bottom, on top, or on a targeted site. Coagulation manipulates electrostatic charge neutralization on organic impurities, especially non-settleable solids, upon absorption against suitably added chemical agents called coagulants or flocculants (e.g., metal oxides). The types and dosages of coagulants depend upon the nature, concentration, and composition of the brine. Frequently used coagulants are Al3+, Fe3+-based salts, polymerized inorganic metal salts, etc. Polymers like polyamine or polydiallyldimethylammonium chloride containing large numbers of charges may also be found useful as effective coagulants [15,16]. However, state-of-the-art advancements made in this field could achieve only a maximum of 58% dissolved organic content (DOC), so far, with a high dosage of coagulant (8.95 mM Fe3+) [16]. This is because most classes of brines consist of high concentrations of salts containing organic impurities with all ranges of molecular weight (MW), whereas coagulation is effective only in the case of high-MW organics removal. It has been observed that over-dosage of these salts (especially Fe-based or old alum-based salts) in a treatment process may lead to machinery impairment, mandating additional maintenance. Because of this very reason, coagulation/flocculation is not extensively used for brine treatment. Another concern of using a metal-based coagulant is its adverse effect on the ecosystem and human health. Thus, a flawless coagulation technology has a long route ahead to attain an acceptable competence. Mohamed et al. found that Al3+ and Fe3+ ions impregnated onto activated silica, i.e., hydrolyzed poly aluminum ferric chloride plus silicate (PAlFeCl + Si), is a good alternative to conventional coagulants offering removal of 89% COD [17]. In an attempt to reduce the environmental impact, synthetic derivatives of many natural coagulants have also been developed by exploiting a number of biopolymers, viz., lignin, tannin, starch, etc. [18].
In electrocoagulation, an electrochemical reactor deployed with stainless steel and aluminum as electrodes is being used. The elevated electrical conductivity of high saline water is highly suitable to be treated by this method with added the advantage of less electricity consumption. However, the electrodes must be regularly maintained or replaced for consistent performance as the dissolution of metals from electrodes cause the coagulation/flocculation of charged impurity metal ions like Ca, Sr, or non-metal like SiO2, to affect the overall performance [19].
A major affliction that ever retards the working performance of any bulk brine treatment plant is the frequent deposition of scale-forming substances. Thus, the presence of scale precursor ions, viz. Ca2+, Ba2+, Mg2+, Si, Sr2+, etc., are invariably responsible for inefficient water recovery since they tend to form deposits on machinery parts because of their lower solubility limits. An ancient method to resolve this problem is to remove such ions with the aid of chemical agents such as lime-soda, ash, etc., so-called precipitants or softening agents. Lime softening is a widely employed robust technology for eliminating high scale-forming ions. Several studies have long focused on using Ca2+ to remove silica with other metals like Ba or Mg as their hydroxides [20,21]. Recently Boo et al. introduced a thermomorphic hydrophilicity base-induced precipitation strategy for the removal of scalants driven by basic conditions by thermoresponisive amines. The use of diisopropylamine managed to remove ~80% hardness of ultra-high-saline brine with recovery of amines for reuse in warm conditions [22].
Apart from chemical softening methods, there exists another pretreatment approach, known as seeded slurry precipitation, suitable for low-saline brine particularly rich with calcium sulfate [21,23]. The mechanism herein involves the growth of scalants onto the seed crystals. In this procedure, a slurry made out of seed crystals is introduced into the brine. The seed crystals serve as nucleating centers for the deposition of scalants like silica and calcium sulfate. In a more convenient approach called a pellet reactor, the same methodology was applied but in a heterogenous manner with an added advantage of formation of dry sludge [24]. A typical bed fluidized bed reactor contain packed calcium carbon crystals as seeding platforms for preventing super-saturation of scale-forming salts [21].
The above discussed conventional methods may not be well-applicable for the treatment of brine with high salinity, containing organic pollutants such as hormones, pharmaceuticals, personal care products, and soluble microbial products. A special caution must be taken for the removal of such organic contaminants present in trace amounts [25]. A most common way of treating recalcitrant organic contaminants is by converting them into viable smaller fragments. Different combinations of advanced oxidation processes by means of O3 (ozonization), UV-H2O2/O3 [26], UVA-TiO2 [27], electro oxidation [28], non-thermal plasma [29,30], photo-Fenton oxidation [31], etc., have been documented by a number of groups. Almost all these schemes work on the principle of free radical formation by photolysis. However, it is crucial to pretreat the brine prior to this stage because the presence of groups like sulfates in the medium most likely deactivate hydroxyl free radicals for further oxidation. Despite a well-inculcated track record of oxidation methodologies, very few groups have so far really focused on the scaling-up and assessment of hazardous consequences associated with the generated low-molecular-weight fragments/byproducts [32].
A relatively less and inefficiently explored, but far older, technique for removal of sulfate or ammonia is through bioprocessing, wherein useful microbes assimilate them by converting them into remediable forms, but only a low-saline brine could be treated by this method since higher-saline brine contains large varieties of heavy metals that can inhibit/reduce microbial growth. Most of the studies in this area concentrate on the reduction of nitrate to N2 and sulfate to sulfides. A typical example is the conversion of nitrate content into ammonia and then N2 by denitrifying bacteria [33]. Many groups have come up with woodchip bioreactors, known as a convenient treatment method for nitrate removal [34,35,36,37,38]. However, these techniques require the addition or attachment of electron-donating groups such as ethanol or acetate in an attempt to enhance the conversion efficiency. Unfortunately, this procedure causes the increase in DOC, which has to further alleviate serious environmental impacts. Another limitation of this method is its inconsistent performance. This is caused by the high initial DOC content that is due to bacterial multiplication resulting in better performance in the first weeks, as revealed by Díaz-García et al. This trend could be minimized by performing an alternate drying–rewetting cycle while using wood chip reactors [39].
3. Brine Management and Zero Liquid Discharge
It being said that one side of a coin shows cases of the removal of toxic components/elements from brine, the flip side of the coin shows that a large volume of potentially reusable water is abandoned in the form of liquid waste. The recovery the water content thus provides a solution to compensate for water scarcity, and also alleviate the major concern with liquid waste disposal. A cutting-edge technology for brine management and resource recovery is the ZLD scheme. Since the invention of this concept, traced back to the 1970s in the U. S. (put forward for regulating the salinity of the Colorado River, U.S.), ZLD has witnessed tremendous advancement, especially in the last decade [40]. It is a strategic engineering approach for waste management ensuring the complete elimination/recovery of liquid, as well as minerals, from the feed wastewater, leaving solid waste to be disposed of. On the other hand, liquid portions of brine and valuable salts are effectively recovered and reused, enabling them to enter into a circular cycle, entitling effective net zero liquid discharge to the environment. The foremost drive for this innovation is the quest for the maximum recovery/reuse of water in dry lands and the easy and convenient disposal of the solid waste. The major outcome of ZLD, i.e., solid waste, prevents the entering of liquefied contaminants into the main flow stream, making it easier to treat them. Thus, ZLD on one hand averts the effluent-drain-water discharge and associated threats of aquatic environmental pollution; on the other hand, it demands greater overhead because of the involvement of energy-intensive sophisticated technologies. A rough estimation of the global market of ZLD requires funding of a minimum of $100–200 million per annum [40]. This made the execution of this technology limited to economic First World countries such as those in North America and Europe (not 100% execution), while it is prompt to implement in developing countries such as China and India [40]. In First World countries, factories are investing in recovering/recycling of water, implementing ZLD even without regulatory push to achieve better sustainability. Though ZLD negotiates a better balance between waste management and the environment, economization of ZLD technology is often hit by cheaper near-ZLD/close-to-ZLD technology by possible on-site removal/recovery of liquid/water from the effluents at the production site. There are cases wherein near-ZLD technologies are largely put forward to compromise the economical constrains associated with the ZLD technique, often involving incomplete removal of liquid waste/water recovery. They simply achieve lower volumes of brine [40]. Thus, recent studies apparently focus on bridging the gap between economic constraints and the efficacy of the overall ZLD system. Operations like forward osmosis (FO), electrodialysis (ED), and membrane distillation (MD) are majorly performed in conjunction with reverse osmosis (RO) for treating RO brine concentrates to achieve ZLD, since these methods can treat brine of high salinity (>200,000 mg/L) [40]. An ideal ZLD process is designed for the maximum recovery of resources. Regardless, purified water is the first and foremost incentive of any brine treatment process. Forward osmosis, electrodialysis, membrane distillation, and hybrid processes are the major approaches adopted for freshwater recovery, and they are discussed in the proceeding sections. This step is followed by mineral recovery techniques in the subsequent stages. It should be noted that a careful screening of technologies must be made, rendering the concentration and composition of RO brine.
4. Brine Management: Resource Recovery Technologies
4.1. Freshwater Recovery Technologies
4.1.1. Forward Osmosis
Forward osmosis, as the name implies, is an osmotic pressure-driven membrane process, unlike RO (which uses hydraulic pressure), it uses the osmotic pressure gradient across the membrane to separate the feed water and allow it to permeate. In brine treatment, this method is majorly adopted for water recovery. In principle, as shown in Figure 1, to attain an osmotic pressure gradient, a high-saline solution called a draw solution will be used. During the process, water from feed water (low saline) will pass through the semipermeable membrane to the draw solution (DS), which is highly saline, to achieve the osmotic equilibrium. As the process continues, there will a diluted draw solution and concentrated feed. The freshwater and draw solution can be separated via a regeneration process using RO/evaporation/mechanical methods. The remaining concentrated DS can be reused further. The obtained concentrated brine feed can be subjected to crystallizers/evaporators for minerals recovery.
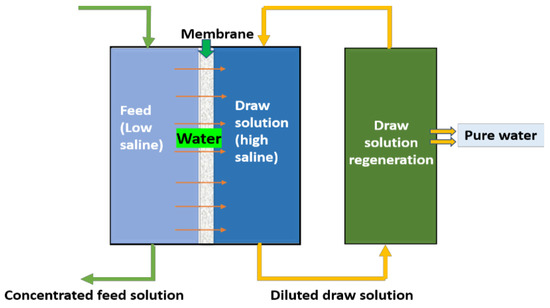
Figure 1.
Schematic diagram for forward osmosis.
In the FO process, the major role was given for a draw solution, since its characteristics will control the water transport through the membrane and the regeneration of potable water. Conventionally, NaCl and MgCl2 are used as DS in RO regeneration, till now, and numerous draw solutions of organic solutes, inorganic solutes, volatile salutes, polyelectrolytes, bio-waste materials, and nanoparticles were studied; however, there is a need to fill some voids to meet the ideal DS. The majorly governed factors for an ideal DS are availability, cost effectiveness, high flux rates, reduced fouling potential, low reverse solute diffusion, non-toxicity, and ease of recovery/regeneration [41]. Hence, most of the current research is focused on developing such an ideal draw solution for FO technology.
One of the main issues associated with DS is the energy utilized for recovery/regeneration; to alleviate this, studies on developing DS with thermolytic, mechanical, and magnetic responsiveness or hybrid solutions for those are under exploration. Recently, liquid fertilizers have also been used as draw solutions. The major goal of liquid fertilizers as DS is there is no need of regeneration; diluted DS can be directly used in irrigation. This technology is referred to as fertilizer-drawn forward osmosis. This methodology is found to be very effective to supply the essential nutrients to crops via irrigation. FO-related studies also paved the way to efficient FO membranes; the governing factors for the same are nature, surface characteristics, thickness modulation, wetting behavior, fouling resistance, etc. [42]. The recent studies on water recovery from brine using FO technology are illustrated in Table 3.

Table 3.
Summary of water recovery studies recently reported using FO process.
Table 3.
Summary of water recovery studies recently reported using FO process.
| Source of Brine | Draw Solution and FO Membrane | Water Recovery and Salinity Level | Ref. |
|---|---|---|---|
| High-saline water | NH3/CO2 as DS and polyamide FO thin film composite membrane | 64% water recovery with 300 mg/L TDS | [43] |
| Reverse osmosis brine | NaCl as DS and flat-sheet cellulose triacetate membrane | 90% water recovery | [44] |
| NaCl-based synthetic brine | Industrial-grade fertilizer ammonium sulfate as DS and commercial FO membrane | 12.7% water recovery | [45] |
| RO brine | 3 M MgCl2 as DS; cellulose-based polymers with an embedded polyester mesh | 50% water recovery | [46] |
| Synthetic brine | Fructose as DS; hydrophilic cotton-derived cellulose-ester plastics embedded on top of a microfiltration membrane | 56.8% recovery with 5 M Fructose; 61.4% recovery with 6 M Fructose | [47] |
| Brine from multi-effect distillation systems | 3 mol/L NaCl as DS; cellulose triacetate membrane and polyamide thin film composite membranes | Brine volume reduced to 54.9% | [48] |
| Four source of high-saline wastewater | Sodium alginate sulfate as DS | - | [49] |
| RO concentrate produced from coal chemical industry | DS: NaCl; membrane: active rejection layer made of cellulose triacetate (CTA) as well as a polyester support layer | 72.1% (4.6g/L TDS), 84.3%, 90.9% and 92.5% (17.4 g/L TDS) water recovery using 1 M, 2 M, 3 M and 4 M DS | [50] |
| Anaerobic palm oil mill effluent | DS: 3 reagent-grade fertilizers (i.e., (NH4)2SO4, monoammonium phosphate (MAP) and KCl) and three commercial grade chemical fertilizers (i.e., (NH4)2SO4-f, monoammonium phosphate-f and muriate of potash; membrane: cellulose triacetate | Highest recovery with MAP, 5.9% for a 4 h operation | [51] |
Among the several brine treatment methods, being an energy-efficient methodology, FO has numerous advantages compared to RO, such as cost effectiveness, low energy consumption, reduced membrane fouling, high water flux, and remarkable rejection rates, and it can be applied to high-saline brine (<200 g/L). Generally, FO technology utilizes low energy (energy cost can be low as 0.02 kWh/m3) compared to other approaches such as RO (2–2.92 kWh/m3) and mechanical vapor compression (20 kWh/m3) [52,53,54]; further cost reduction can be achieved by using a more concentrated draw solution as suggested by Gulied et al. [55]. Therefore, FO is considered as the most suitable brine resource recovery method at present [45].
Although FO has several goals, there are several lab-scale implementations; however, full-scale implementation is still in the growing stage. The world’s first commercial FO plant based on ZLD was deployed in 2016 in China (the Changxing power plant in Zhejiang Province). The system transforms 630 m3/day of used industrial wastewater with the utilization of 90 kWht of energy per m3 of wastewater treatment. The feed wastewater from flue-gas desulfurization is subjected to pre-concentrating RO followed by a membrane brine concentrator (MBC) system. The pretreatment results in the concentration of ~60,000 mg/L; the FO MBC system further concentrates the RO brine to <220,000 mg/L using a NH3/CO2 draw solution. The MBC draw solution subjected to recovery and pass-through RO system finally produces high-quality product water of <100 mg/L TDS. The implemented MBC can recover up to 23 m3/h, having 87% recovery. In 2019, another FO plant was industrialized by Forward Water Technologies, Canada. They developed a thermolytic FO DS for wastewater treatment and achieved the treatment of 15 m3/day.
4.1.2. Electrodialysis Technologies
In electrodialysis, an alternating series of cation and anion selective semipermeable membranes (ion exchange membranes—IEM) are placed in between cathode and anode; clean water is produced by the electrochemical separation of ions, i.e., ions in solution are separated by the influence of electric potential. A schematic diagram illustrating the principle of electrodialysis process is shown in Figure 2. The brine solution is passed through into the cells in the ED system; the voltage gradient makes the movement of anions and cations through the selective membranes to anode and cathode, respectively. The cation-exchange membranes (CEM) allow the cations to block the anions; similarly, anions get passed through anion-exchange membranes (AEM) and cations are blocked. This leads to the complete separation of ions in brine, and ends up in ion enrichment at one side and freshwater recovery in another side. All cations such as Na+, K+, Mg2+, and Ca2+ and all anions such as chlorides, sulfates, and nitrates are found to be separated effectively from brine using ED technology.
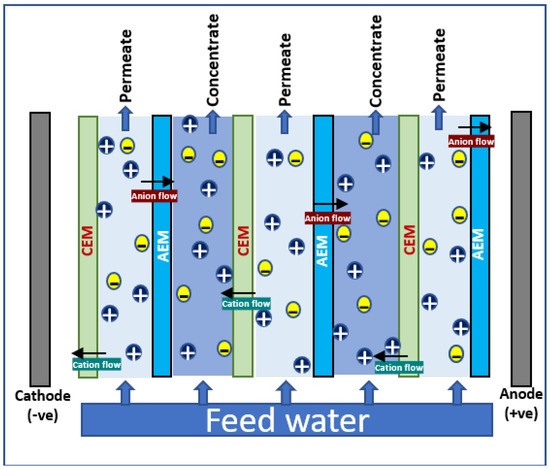
Figure 2.
Schematic diagram for electrodialysis.
Compared to RO, ED has several advantages such as simple operation, high water-recovery rate, long life for membranes, low fouling (since it not pressure driven as RO), and no need of pre/post treatments. The performance of transporting ions in ED majorly depends on the characteristics of the exchange membranes, concentration and nature of ions in feeds, ion density, etc. The polymers such as polyethylene, polysulphone, and polystyrene with charged ions are commonly used as IEMs. The positive charges such as ammonium ions, amines, etc. are used for the preparation of AEM, and sulfonic acid, phosphonic acid, phosphoryl, and carboxylic acid groups are commonly seen in CEM. Depending upon the wetting behavior, electrical, and surface characteristics, IEMs can be homogenous and heterogenous in nature. Novel hybrid membranes such as bipolar membranes, monovalent selective membranes, etc. are emerged to extend the application scenario of ED in brine treatments [56,57].
The degradation/depletion of IEMs membranes over time is the major obstacle in the application of ED. It is found that suspended molecules with 200–700 Da, surface deposition of metal cations, etc. can induce clogging in IEMs, which reduces the overall separation efficiency. To reduce fouling and scaling, some modifications are adopted in ED, known as electrodialysis reversal (EDR) and electrodialysis metathesis. Increased resistance owing to fouling can be overcome by electrodialysis reversal. In EDR, for a certain time interval the electric polarity of the electrodes is reversed to have movement of deposited ions in opposite directions. As a result, clogging can be reduced by the reduction of polarization boundary-layer thickness, thereby improving the efficiency of the system. EDR is considered as ED/EDR and can be used for concentrating high salinity of approximately >100,000 mg/L, utilizing maximum energy of 15 kWh/m3 of feedwater; this energy is less compared to conventional methods [16].
Using ED systems, the water recovery rate is found to be 70–90% depending on the feed water. ED systems are also be used for treating RO concentrate; because of the high salinity of feed water, there is high electrical resistance, voltage drop, and also high energy consumption; hence, most of the studies suggest a hybrid system for a water recovery process. Recently, Bader et al. reported a case study in Kuwait utilizing a pilot-scale high-current-density electrodialysis-evaporator hybrid system for brine management; they reported a 77% water recovery rate [58]. The recent studies on water recovery from brine using ED are illustrated in Table 4.

Table 4.
Summarized reports of ED systems utilized for water recovery from various sources of brine.
Table 4.
Summarized reports of ED systems utilized for water recovery from various sources of brine.
| Source of Brine and Salinity Level | IEMs and Conditions of ED Technologies | Water Recovery Rate | Ref. |
|---|---|---|---|
| RO concentrate discharged from RO plant | Series of ion exchange membranes such as FAS-PET-130, FKS-PET-130, Neosepta-CMX, Neosepta-AMX, LabAM-NR, LabCM-NR were used | 67.78% | [56] |
| RO brine concentrate | RO-ED integrated system | 95% | [59] |
| Brackish water RO concentrate | Lab-scale EDR system with three cell pairs of AEM and CEM | 85% | [60] |
| Synthetic brine | Electrodialyzer with 25 cell pairs of monovalent selective AEM and CEM | 70% | [58] |
| Brackish Water RO brine | Bipolar membrane electrodialysis (BMED) | Acid (0.7 mol/L) and base (0.6 mol/L) recovery | [61] |
| Seawater reverse osmosis brine | Monovalent selective electrodialysis (S-ED) | 55% | [62] |
4.1.3. Membrane Distillation
In MD, thermal-driven separation resulted in the production of potable water from feed, utilizing a hydrophobic microporous membrane. The hydrophobicity of the membrane prevents the movement of water molecules, while the porosity allows permeation of vapors. MD was introduced in 1963 by Bodell; the vapor pressure gradient across the membrane triggered by the temperature difference is the principle behind this separation technique. Compared to conventional distillation, the freshwater production using MD is economically viable, even though there is membrane fouling [2,8,63]. In the MD process, the vapor pressure difference across the membrane is sustained using hot feed water and cold permeate solution. Vapor pressure and temperature differences induce mass transfer via evaporation of the feed water; vapors are diffused through the pores of membrane, which condenses into freshwater at the permeate side. MD have a 50–99% rejection rate and high-quality freshwater recovery with lower cost because of the moderate temperature and pressure conditions [64].
There are different configurations that have emerged for MD; a schematic representation is presented in Figure 3 [6] to enforce lower vapor pressure at the permeate side. These includes direct-contact membrane distillation (Figure 3a), in which the cold permeate has direct contact with the membrane, and the air-gap membrane distillation (Figure 3b) has an air gap interleaved in between the membrane and permeate side to reduce the conduction loss. A sweeping gas membrane distillation (Figure 3c), a cold sweep gas on permeated side, and finally a vacuum membrane distillation (Figure 3d) vacuum is applied on permeate side. MD use low temperature conditions (40–90 °C), which enable the utilization of low-grade heat stream or energy sources such as sun power or geothermal power. Hydrophobic polymers are employed for membrane fabrication, such as polysulfone/polyether sulfone [65], polypropylene [66], polyethylene [67,68], polytetrafluoroethylene [69,70,71], poly (vinylidene fluoride) [72,73,74], etc. Apart from polymers, ceramic-based and hydrophobic-material-coated, omniphobic, Janus, and sandwiched porous membranes have also emerged for use in MD [75].
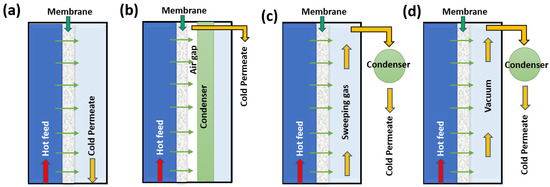
Figure 3.
Schematic representation of various configurations of MD, (a) direct contact, (b) air gap, (c) cold sweep gas, and (d) vacuum membrane distillation.
The efficacy of MD for treating brine solution is already established; it can treat high brine with TDS up to 350,000 mg/L. MD use low temperature conditions (30–90 °C), which thus enables the utilization of a low-grade heat stream or energy sources such as sun power or geothermal power [2]. In 2009, Martinetti et al. [44] reported excellent water recovery from RO brine using vacuum membrane distillation. The authors used two RO brine samples (7500 mg/L and 17,500 mg/L TDS) as feed and two 0.22 µm pore-size flat membranes made of PTFE and PP. The feed was subjected 40–60 °C heating and 2 L deionized water was cooled to 20 °C as permeate. Vacuum membrane distillation recovered 62% and 80% freshwater from brine with 7500 mg/L and 17,500 mg/L TDS, respectively. In another report, 82% water was recovered from artificial RO brine using multistage air gap MD using PP hollow fiber membranes [76]. Recently, Amanda et al. recovered 95% freshwater from synthetic-lithium-rich brine employing multi-stage distillation-membrane crystallization via a fractionation process [77]. The major drawback of MD is the scaling of membranes; for example, calcium-based or silica-based compounds or organic molecules from brine can be precipitated on hydrophobic membrane, which leads to deterioration and reduction in membrane flux. These can be overcome by hydraulic membrane washing with water or by developing fouling-resistant membranes.
4.1.4. Hybrid Processes
A hybrid desalination system is the integration of two or more desalination techniques to enhance performance, provide better environmental solutions, and reduce operational costs compared to standalone systems. Using a hybrid approach, it is feasible to achieve effective brine management. One option to reduce energy usage and satisfy water-demand targets is to combine the strengths of two or more treatment processes. Much effort was put into establishing cost-effective methods for integrating the benefits of individual processes to enhance the water recovery rate of finished products. This section discusses the main hybrid technologies to optimize the overall desalination efficiency while minimizing the reject brine.
Most experts believe that RO is the most energy-efficient desalination technology currently available compared to other industrially implemented methods such as multistage flash (MSF) and multiple effect distillation (MED). However, membrane scalability and a maximum feed-water salinity of 75 g/L restrict the utilization of RO for high water recovery [6]. Silica and other inorganic ions (Ca2+, Mg2+, CO32−, SO42−, Ba2+, Sr2+, etc.) that are sparingly soluble in RO feed water continue to accumulate as water recovery increases. Scale-forming salts such as calcium sulphate or carbonate, barium sulphate, and strontium sulfate are present in RO concentrate and could deposit on the surface of the RO membrane when their solubility is exceeded [78]. These salts reduce the permeate flow and ultimately shorten the useful life of membranes [79]. Antiscalants are less efficient in preventing the precipitation of sparingly soluble salts when the feed water is intensely concentrated [80]. In addition, a high TDS level in RO concentrate may further restrict the utilization of reverse osmosis (RO) technology for the treatment of brine because of the high osmotic pressure requirements for RO. Thus, prior to RO brine treatment, the brine is subjected to a variety of pretreatment methods. Chemical softening by ED, ion exchange (IEX), seeded precipitation (SP), and chemical precipitation (CP) are examples of these methods. Improved treatment efficiency may be achieved with combined technologies with RO. Table 5 and Table 6 present a summary of selected reviewed studies on brine management for the past 10 years using combined techniques with RO from low- and high-salinity water feeds, respectively targeted at increasing water recovery and minimizing brine volume. These hybrid technologies will be further discussed in the subsequent sections.

Table 5.
Summary of some hybrid techniques with RO for brine treatment with low salinity (brine from low-salinity water: TDS in water 500–30,000 mg/L).
Table 5.
Summary of some hybrid techniques with RO for brine treatment with low salinity (brine from low-salinity water: TDS in water 500–30,000 mg/L).
| Combined Techniques with RO/Feed Water | Operation Conditions | Advantages | Challenges | Research Highlights | Ref. |
|---|---|---|---|---|---|
| (CP)/Colorado River Water | CP/RO/Filtration -Solid contact reactor facilitate efficient separation of the precipitates Chemicals used for scale precipitation: Sodium hydroxide and sodium bicarbonate | High water recovery | Costs Large footprint Biofouling of membranes | 70%: rate of removed scale ions 95%: water recovery | [81] |
| CP/Brackish groundwater | CP/adsorption/enmeshment/RO Chemical added: Lime (Ca(OH)2) and soda ash/NaOH | High removal rate of scale forming ions High water recovery | Cost study optimization of the hybrid process via pilot scale study | Removed species: Mg2+, Ca2+, Sr2+, Ba2+, and SiO2 97%: water recovery | [82] |
| SP | Seed slurry: Calcium carbonate/ Magnesium hydroxide -Column: Open channel | Less chemical Pretreatment High removal rate of scale forming ions High water recovery | Cost study | Hardness removal (90%) Water recovery (95%) Membrane: open-channel spiral wound modules could be an alternative to tubular RO system | [83] |
| IEX/water from oil field | -Hybrid filtration/cation exchange/RO system/ | Effective toxicity control Effective scale control | Post-treatment of the treated water to control boron removal. Cost analysis Ion exchange regeneration | Pilot-scale results: -TDS reduction (96%) -conductivity reduction (98%) -Reduction in different water quality parameters (80–100%) | [84] |
| IEX/low salinity river water desalination | -A hybrid anion exchanger with doped ferric oxide nanoparticles and a shallow shell weak acid cation exchanger -Use CO2 as sole regenerant for two column ion exchange | Effective scale control multifunctional pretreatment: TDS and scale forming ions are both reduced | Organic fouling of the membrane. Design and optimization of the hybrid ion exchange/RO Cost optimization | -More than 80% removal of calcium, sulfate, and phosphate, -water recovery (98%) -TDS reduction (50%) | [85] |

Table 6.
Summary of some hybrid techniques with RO for brine treatment with high salinity (brine from high-salinity water: TDS in water 30,000–50,000 mg/L).
Table 6.
Summary of some hybrid techniques with RO for brine treatment with high salinity (brine from high-salinity water: TDS in water 30,000–50,000 mg/L).
| Combined Techniques with RO/Feed Water | Operation Conditions | Advantages | Challenges | Research Highlights | Ref. |
|---|---|---|---|---|---|
| ED/high salinity brine | Counter-flow ED units hybridized with RO | Production of Highly concentrated brine (It could be utilized for salt production) | Modelling and optimization of the process to avoid membrane resistances (both ohmic and free energy losses) | High water recoveries are limited when applied to treatment brine with TDS 120,000 ppm | [86] |
| EDR/saline basal aquifer water | Pilot scale: pretreatment steps, included sedimentation, microfiltration and ultrafiltration, have been used before EDR/RO | Dual function of EDR: high efficiency in scaling mitigation & production of highly concentrated brine (125,000 mg/L). No chemical addition | Electrical energy consumption should be reduced | 77% water recoveries | [87] |
| ED/sea water | Nanofiltration (NF)/RO/ED | Production of highly concentrated brine, close to saturation | Pilot-scale study | Water recovery (69%) Energy consumption (6.9 kWh/m3) | [88] |
4.1.4.1. Combined Techniques with Reverse Osmosis (RO)-Brine from Low-Salinity Water
- (a)
- Chemical Precipitation-RO:
Chemical precipitation is a possible method to be used in conjunction with the RO process to remove specified sparingly soluble salts to maximize water recovery and reduce the risk of membrane scaling/fouling. In this approach, mineral-scale ions are removed as solid precipitates from the brine of a primary RO step, allowing subsequent product-water recovery in a secondary RO (SRO) phase of the pretreated brine. Steps such as adding chemicals, pH adjustment, and solids separation are included in the chemical precipitation process [3]. Many authors have evaluated different chemicals for the precipitation process. For example, sodium hydroxide (NaOH), lime (Ca(OH)2), soda ash (Na2CO3), and sodium bicarbonate (NaHCO3) were used to enhance precipitation of some substances such as calcium and silica, helping to reduce the hardness of the second RO feed water [81,89,90]. Generally, the choice to add chemicals is determined by the nature of the concentrate to be treated, as well as chemical prices.
Rioyo et al. [82] evaluated a high-pH process for removal of scale-forming precursors including magnesium, calcium, strontium, barium, and SiO2 from primary RO concentrate of brackish groundwater by precipitation and adsorption/enmeshment. The proposed design for RO brine minimization is shown in Figure 4. The authors found that lime and soda ash softening treatments outperformed the sodium hydroxide treatment. The integration of an intermediate ‘high-pH precipitation treatment’ with lime and soda ash, pH re-adjustment, and antiscalant addition between consecutive RO stages might allow the overall water recovery to be increased from 80 to 97%. The simulation achievement, on the other hand, should be followed by a pilot-scale cost study and further optimization of the hybrid process. Similarly, the experimental results found by Lu et al. [91] showed that the lime–soda ash softening removed more than 98.5% of Ca2+/Ba2+ and more than 80% of Mg2+/Sr2+/Si from RO concentrate (produced from a coal chemical industry).
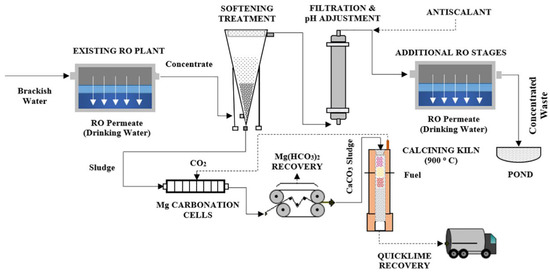
Figure 4.
Proposed plant design for RO by Rioyo et al., 2018. Reprinted with permission [82].
For brackish water (low-salinity) desalination, a high-recovery desalination system (95–98%), combining membrane RO desalting with accelerated precipitation softening (APS), was explored. Sodium hydroxide and calcite seeding were employed to alkalinize the PRO concentrate, followed by a microfiltration and acid dosing to lower the pH and avoid calcite scaling in the RO desalting process [79]. Gabelich et al. [81] examined the efficacy of combining an intermediate chemical demineralization (ICD) stage with a solid contact reactor (SCR) to improve water recovery during the RO desalination procedure at a desalination plant located near the Colorado River. Chemical precipitation was aided by the addition of NaOH to the SCR influent, while calcium removal was aided by the addition of NaHCO3. To enhance flocculation, ferric sulfate (Fe2(SO4)3) was also added. Finally, prior to SRO, a filtration (microfiltration or dual-media filtration) step was performed. The results revealed that calcium removal was closely linked with the removal of both barium and strontium.
In a pilot-scale demonstration [89], an overall water recovery of 97% was achieved by operating the primary RO at around 85% recovery, followed by the lime softening of the concentrate to reduce silica concentration, and then a second RO for a further high water recovery (from desert wells). However, in another studies, the same authors have found that the lime chemical precipitation is insufficient for the treatment of the primary RO concentrate from brackish water. A fouling of the SRO could occur because of colloidal particles generated by the precipitative treatment. The authors suggested that silica polymerization control by acidified cation of the primary RO concentrate as an additional treatment is an alternative to the lime-softening approach. Scaling has been completely controlled using a combination of antiscalants and pH control. As a result, a super-concentrate depleted of bicarbonates could be produced, due to silica control, at the highest possible pump pressures by using a seawater RO system in the second stage [90]. Nonetheless, more research is required for the evaluation and optimization of energy consumption.
As the chemical precipitation has the benefit of being easily coupled with the RO process and having the high removal rates of scale-forming ions, the integration of precipitation softening by chemical addition with an SRO for the treatment of brine has been evaluated in different desalination processes with high water recoveries. While chemical precipitation softening is generally promising for achieving high recovery, there are several difficulties in controlling pH and chemical addition that can be affected by temporal changes of the feed, high chemical demand, and sludge production [6,78].
- (b)
- Seeded Precipitation-RO:
To improve softening before RO treatment, seeded precipitation can be used as an alternative approach. Generally, lime (Ca(OH)2) or caustic soda (NaOH) are used to adjust the pH of the solution, and then crystal seeds such as gypsum, dolomite marble powder, barium sulphate, or calcite are added. Brine composition and seed type are critical factors in determining the effectiveness of hardness removal. Throughout the last two decades, a significant effort has been invested in developing seeding techniques to increase water recovery [83,92,93]. Membrane-scale formation is prevented because scale ions prefer to grow on seed crystals. A tubular membrane configuration is the most used for seeded precipitation because of the need for seed slurry circulation within the membrane modules [6].
In a feasibility study of chemically enhanced seeded precipitation (CESP) of primary RO (PRO) concentrate of brackish water with high salinity [92], the treated PRO concentrate was filtered to remove solids and then further desalted in an SRO stage to improve the overall water recovery (Figure 5). Desupersaturation with calcium sulphate salt was achieved by seeded gypsum precipitation in the absence of residual antiscalants from the PRO concentrate. It was demonstrated that the partial lime treatment stage of CESP is necessary for eliminating the residual antiscalants. This hybrid system can reach overall water recovery of about 93%. However, antiscalant makeup in the SRO and the recycling of the concentrate to the CESP process would be required.
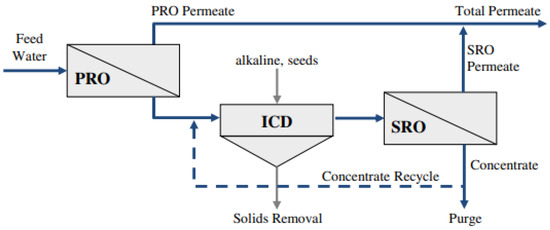
Figure 5.
Schematic of a hybrid CESP-RO process by McCool et al., 2013. Reprinted with permission [92].
In another study, scaling saturation during the second stage of the RO process has been prevented by employing seeded slurry precipitation through a patented process called SPARRO [94]. In this process, a seed crystal slurry (gypsum crystals) was added to precipitate calcium sulphate and silicon dioxide on seed crystals. Slurry-based seed crystals in SPARRO acted as nucleation sites for the precipitates, and were further attracted to the slurry. A cyclone separator was used to separate the seed crystals from the treated brine fed to the second RO process and, as a result, more than 90% of freshwater has been recovered from low-salinity mine water. Nevertheless, the damage of the membrane by seed crystals and tubular membrane channel blockage may limit the use of this process, mainly for the treatment of the concentrate from high-salinity feed [78].
Although the reviewed approaches are appealing to control scaling from brine, their industrial application is quite limited because of their high operational costs. This arises because of the necessity of proper maintenance of the tubular membrane for high precipitate recovery, leaving no footprints on it. Therefore, a novel technique using ‘open-channel’ spiral wound membrane modules has been developed and patented to simplify pretreatment and increase recovery while reducing flow resistance [83]. This membrane with new features obtained by upgrading the conventional spiral wound modules to improve membrane channel geometry and avoid formation of ‘dead areas’ showed hydrodynamical properties similar to that of a tubular membrane. The developed technique could provide an efficient solution to reduce calcium carbonate from RO concentrate by seed crystallization and reach 95% or more overall water recovery. It should be noted that a caustic solution was required for seed-crystal production.
To enhance the calcium carbonate supersaturation ratio, air stripping can be used for treating the concentrate to increase the pH value without using chemicals. Recently, seeded aeration softening in real brackish water desalination concentrates was explored [93]. CaCO3 precipitation was shown to be chemical-free because of the successful in demineralization of concentrate by aeration, making water recovery easier. The percentage of calcium removed was around 73% (92% calcite, i.e., 1.05 g-CaCO3 L−1 h−1) and this could further reduce the operational costs. The kinetic parameters found in this study can be used to design a continuous seeded aeration softening system allowing the recovery of CaCO3. However, carbon dioxide (CO2) gas emission into the atmosphere is the major drawback of this method (Equation (1)) as CO2 is a major contributor to global warming. Bubbling the emission gas in the softened concentrate to induce commercial algae cultivation may solve this problem. In the future works, this suggestion should be taken into consideration in designing a hybrid process combining seeded aeration softening of the RO brine and SRO.
Another recent study found that combining seeded precipitation through calcite seeding with precipitative step by CaO addition and MgSO4 dosing for silica removal improved the RO recovery from 89.2% up to 96.3% for zero liquid discharge in oil refineries [95]. It is worth noting that the BaSO4 DE supersaturation step upstream from the primary RO operation has also been applied. The authors explained this improvement at the membranes by the added process steps to remove scaling. The life-cycle analysis and the eco-efficiency evaluation have shown that the combined treatment proposed could enhance all eco-efficiency measures. However, challenges and future prospects of this integrated system are required for the idea to be practiced on a large scale because the technical and process requirements were not considered in these analyses.
- (c)
- Ion Exchange-RO:
Ion exchange resin is an insoluble material made up of a complicated cross-linked polymer matrix that contains groups that can be exchanged with ions in an electrolyte solution [96]. These hybrid IX-RO processes have the potential for decreasing membrane scaling and fouling to give high water recovery and lower the amount of concentrate generated.
The properties of the selected resin have an impact on ion-exchange efficiency. Weak base anion (WBA), weak acid cation (WAC), strong base anion (SBA), and strong acid cation (SAC) are generally the four most common forms of commercial resins [6]. Cation exchange resins are used to remove polyvalent metals including calcium, magnesium, and copper, whereas anion exchange resins are used to remove silica and SO4−2 from water. In addition, natural organic matter and organic micropollutants may be reduced by ion exchange resin through both ion exchange and adsorption. Uncharged groups can be adsorbed onto the resin surface via Van der Waal’s force or hydrophobic interaction, while charged groups can be transferred onto the IX functional groups via electrostatic force [97].
By lowering both TDS and scale-forming ions, ion exchange might possibly accomplish multifunctional pretreatment [85]. Generally, resins can withstand hardness and TDS levels of 500–2000 and 5000–30,000 mg/L, respectively [6]. Combining anion and cation exchange in RO pretreatment has been proven to increase the flux in subsequent RO operations [98]. Murray-Gulde et al. [84] employed a hybrid filtration/cation exchange/RO system for treatment and reuse of waters that have interacted with an oil field (Figure 6). The hybrid pilot-scale RO unit reduced the TDS and conductivity by 96% and 98%, respectively. Hence, the treated water was suitable for discharge to surface water or for irrigation. Nevertheless, a minimal additional treatment may be needed to control boron removal.
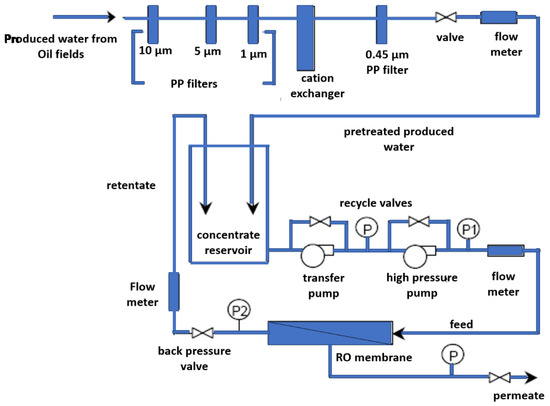
Figure 6.
Schematic diagram of the hybrid pilot-scale RO unit by Murray-Gulde et al., 2003. P, P1 and P2 stands for pressure pumps. Reproduced with permission [84].
A hybrid system specifically developed for treating waters containing high levels of dissolved silica is the patented high-efficiency reverse osmosis (HERO) process [99]. This process consists first of lime softening of the feed for hardness reduction. In the second step, divalent ions are essentially removed by weak acid cation exchange (from the primary RO concentrate), enabling the SRO to be operated at high pH to reduce scaling restraints. The third step is degasification to remove the dissolved CO2 (decarbonation) to prevent the formation of carbonate scale on the RO membrane. It is worth mentioning that the pH of the pretreated concentrate (after the second and third step) should be raised by adding sodium hydroxide to convert silica into anionic form, resulting in preventing RO membrane from silica scaling, as well as biological and organic fouling. As a result of this multistage treatment, more than 90% water recovery could be obtained for different brackish waters and the hybrid process was able to operate with silica levels in the concentrate stream as high as 1500 mg/L without membrane fouling. Although the HERO system is beneficial for brine management, RO waste is still a limiting factor because the process produces high-pH concentrate streams containing a high level of dissolved silica, which necessitates further treatment. It has been found that conventional brine treatment technologies had difficulties with HERO brine [100]. In addition, CO2 (greenhouse gas) emission from the feed water to the atmosphere during the degasification step should be quantified and considered to avoid unintended environmental impacts.
A hybrid IEX-RO process, using a feed with TDS 941 mg/L, has been designed and simulated by Venkatesan et al. [101] (Figure 7). Retentate from PRO is first softened by ion exchange (IEX) and then delivered to SRO. A tertiary RO (TRO) could be an option in this process (after pH adjustment and heating). When it comes to calcium, magnesium, and barium ions that contribute to scale formation, the authors found that IEX was effective in removing them, while pH modification and heating helped to increase the silica scaling issue. The use of brine from the last RO for IEX regeneration eliminates the need for chemical regeneration to make the process self-sustaining, which lowers the IEX operating cost and brine disposal cost. In future, more precise economic calculations are required to establish whether heating is beneficial in a certain situation since the economic analysis is dependent on energy expenses.
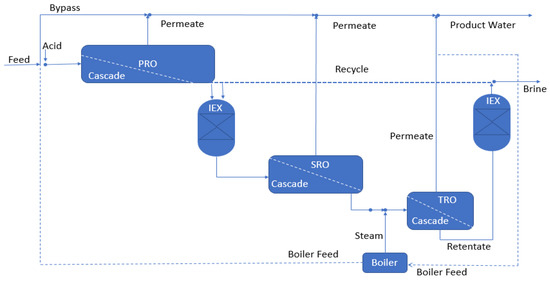
Figure 7.
Schematic diagram of a hybrid IEX-RO process by Venkatesan et al., 2012. Reproduced with permission [101].
In the same context, Vanoppen et al. [102] have shown that pretreatment with cationic ion exchange resin would alleviate membrane scaling and increase RO recovery. Additionally, they have demonstrated that RO concentrate could be recycled for regeneration of the ion exchange resin. The results showed that without the use of chemicals, RO recovery could be improved. However, this was only possible when the input stream included high monovalent/multivalent cation-ratios. In fact, the effectiveness of multivalent cation removal is mostly determined by the ratio of monovalent to multivalent cations present in the input stream, which has an impact on ion-exchange efficiency and might be regarded as a disadvantage of the proposed hybrid ion-exchange/RO process.
The development and modification of the resin structure has gained importance in recent years to reduce resin brine production. The patented Shallow Shell™ Technology (SST®) was an alternative cation ion exchange to the ordinary ion-exchange resin used in water softening. According to the findings, the SST® regeneration necessitates 15% less salt and 50% less water for rinse and dilution because the traditional ion-exchange sites that are more difficult to reach and regenerate are no longer present in this new resin structure [97]. Based on these results, a hybrid ion-exchange desalination process (HIX-Desal) has been recently proposed, as multifunctional RO pretreatment of impaired water sources containing 150–1250 mg/L TDS, to facilitate RO recovery enhancement and the upcycling of industrial carbon emissions [85]. The HIX-Desal process can simultaneously desalt the feed water and remove multiple scale-forming ions such as calcium, sulfate, and phosphate. The originality in this process is the use of carbon dioxide as the sole regenerant for a two-column train containing a hybrid anion exchanger with doped ferric oxide nanoparticles and a shallow-shell weak acid cation exchanger. The HIX-Desal process was able to reduce the risk of RO membrane scaling: TDS reduction of more than 50% and removal of more than 80% of calcium, concurrently with sulphate and phosphate, has been recorded. It should be noted that the focus of this study was to reduce scale-forming ions from RO concentrate. The referenced study did not evaluate the hybrid process HIX-Desal/RO to further enhance the feed-water recovery after ion-exchange treatment. Thus, further design optimization of the combined process (ion exchange/RO), with a focus on the potential of using HIX-Desal to prevent organic fouling, as well as cost optimization, is required.
Brine treatment via a combination of ion-exchange resin and RO is a promising approach for the prevention of RO membrane scaling and, hence, the minimizing of brine volume and recovering water as well. An overall water recovery of greater than 90% could be achieved in certain situations. However, progress is required in the study of the hybrid system for treatment of brine from high salinity. Indeed, pretreatment of highly concentrated brine by ion exchange has not been properly investigated because of the high operation cost related to either an increase in the amount of required resin or the cost of resin regeneration. Further research is also needed to optimize such an integrated process by considering the resin regeneration challenges. Reducing or eliminating the resin waste and decreasing the resin regeneration cost are important factors that influence the effectiveness of the process. The possible regeneration of resin by RO concentrate offers an advantage compared to chemical regeneration methods as it is a chemical-free regeneration method. As a result, waste is reduced and a major regeneration cost is eliminated. Despite the potential of RO brine reuse for resin waste management, more studies are needed to optimize the concentration range of brine without the additional supplement of purchased salt and to avoid resin waste [103]. The advantages and disadvantages of the reviewed pretreatment processes in combination with the RO process for brine management, as well as the estimated cost of the pretreatment technologies, are listed in Table 7.

Table 7.
Advantages and disadvantages and cost evaluation of the pretreatment process combined with RO for brine treatment with low salinity [104].
Table 7.
Advantages and disadvantages and cost evaluation of the pretreatment process combined with RO for brine treatment with low salinity [104].
| Pretreatment Method | Advantages | Disadvantages | Estimated Cost | |
|---|---|---|---|---|
| Capital Cost | Operating Cost | |||
| Chemical precipitation | High efficiency in scaling mitigation Retrofit easily into an existing desalination plant | Sensitivity to temporal changes of brine characteristics High chemical demand Sludge production Process performance can be affected by the residual antiscalant in brine High operating cost Difficulties in managing organic compounds Difficulties in controlling pH | 0.275 USD/m3 | 0.554 USD/m3 |
| Seeded precipitation | Less chemical intensive Seeded precipitation could enhance the precipitation kinetics and the efficiency of separation | Sludge production High cost to recover precipitated salts Large foot print due to the tubular RO membrane The residual antiscalant could inhibit crystal growth | 10.8 USD/m3 (SPARRO) | 0.06 USD/m3 (SPARRO) |
| Ion exchange | Effective scale control with high ion selectivity Organic compounds from brine could be reduced No sludge production | Removal depends on type of ion exchange resin Waste for resin regeneration Resin saturation High operating cost: cost of resin regeneration & increase in the amount of required resin | - | 0.08–0.21 USD/m3 |
4.1.4.2. Combined Techniques with Reverse Osmosis (RO) Brine from High-Salinity Water
- (a)
- Electrodialysis technologies (ED)/RO:
As mentioned above, RO is one of the most energy-efficient techniques but it requires a significant pretreatment, especially for brine volume minimization while improving the water recovery. The pretreatment technologies, prior to SRO brine treatment, discussed above are generally restricted to feed from low-salinity sources including brine from brackish water, waste waters, and moderately saline inland water [21]. However, electrodialysis technologies, i.e., ED or electrodialysis reversal (EDR), seem to be suitable for high efficiency when used as a pretreatment for brine from both low- and high-salinity sources. In fact, combining ED and RO allows high-salinity feeds to be treated and the use of RO eliminates ED operation with low conductivity inputs [86,105]. In addition, the hybridization of seawater RO (SWRO) with ED technologies, such as EDR, leads in a significant reduction in energy usage as EDR is suitable for energy generation with moderately concentrated solutions. Research was initially focused on the designing and simulating of this integrated membrane system and then extended to large-scale systems to reduce the energy consumption for the treatment of hypersaline feed [106].
Hybrid EDR/RO systems have been studied and modeled by McGovern et al. [86,105] for brackish water desalination, as well as for high-salinity brine treatment. The study of the hybrid system for brine treatment from brackish water has been conducted through two hybrid configurations: the first is a simple hybrid configuration, in which the RO brine is delivered into the ED unit and the permeates from the two units are mixed, and the second is a recirculated hybrid configuration, in which the RO brine is delivered into the ED unit and ED product is recirculated back to the RO unit. The comparative study showed that the simple hybridization was the most cost effective. These authors demonstrated that the simple hybrid ED/RO system is recommended when the cost of water from a standalone RO system working at 50% recovery is roughly 60–70% of the cost of water from a single ED system. Such a hybrid system can offer, on one hand, the benefits of significant reduction in the total membrane area as high rates of salt removal are attainable at high salinities because of current densities. On the other hand, relaxing the ED out-put requirement as the final product is a blend of RO and ED permeates. For the study of water desalination at high salinity, the same research team proposed a new combined system comprised of counter-flow ED units hybridized with RO as shown in Figure 8 [86]. The objective of this setup is to use ED for its ability to attain high osmotic pressures and RO for its ability to reach low salinities when ohmic resistances are high in ED. The highly concentrated brine from the concentrate stream could be further utilized for salt production [106]. However, when such a hybrid system is operated to treat brine with high salinity (brine at 120,000 ppm), the authors suggested that further research is needed in the modelling and optimization of the process. Indeed, the performance at high recoveries in the ED system was limited because of the difference in concentration between streams, therefore increasing water transport via osmosis, decreasing energy efficiency, and increasing the levelized cost of water (LCW). The current density that corresponds to the lowest water cost (to minimize LCW) was significantly greater than the density required for maximum efficiency.
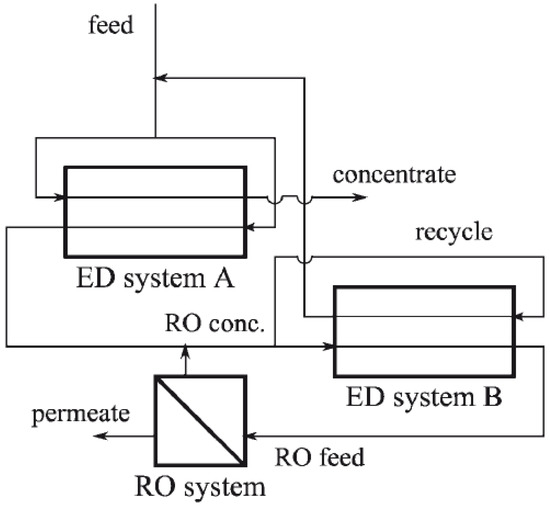
Figure 8.
Schematic diagram of hybrid ED-RO system for high-saline brines by McGovern et al., 2014. Reprinted with permission [86].
The theoretical base for establishing an EDR/RO hybrid system has been studied by modeling several configurations of the hybrid processes in terms of energy consumption [107]. The efficiencies of two basic modes (EDR (pretreatment)/RO and RO/EDR (in the second configuration, EDR is a post-treatment unit for RO process)) and two complex modes (EDR/RO/EDR and EDR/RO, in this last configuration a portion of RO brine is recycled and used in the EDR unit) were found to assess their specific energy consumption and the concentration of the discharged brine. Based on the modeling results, it has been shown that the four configurations of the EDR/RO hybrid processes could significantly reduce the specific energy and give greater control over the brine concentration compared to a standalone seawater RO process. The authors [105] suggested that the total energy consumption could be reduced more by using mechanical energy-recovery devices. However, such hybrid processes should be tested experimentally for industrial application purposes. The limitations of EDR systems could be solved by improving electrodes and membranes, and feed-solution optimization [106]. From this viewpoint, biologically treated secondary effluents (which have higher concentrations than river water) might be used in the EDR process as a low-salinity solution for increasing the efficiency [107].
Recent research toward achieving a full-scale system for industrial application has been carried out by operating a salt-splitting ED/RO pilot process using coal plant wastewater (Figure 9) [108]. The novelty in this hybrid system is the use of a salt-splitting ED unit to eliminate scaling restrictions by changing divalent-scaling low solubility ion pairs into non-scaling high solubility ion pairs. As a result, non-scaling Na2SO4 and CaCl2 aqueous solutions are produced and transported into individual brine partitions. The comparative study with a soda-ash-softened RO system showed that the overall water recovery reached 90% and the brine volume was reduced by two-thirds using the innovative hybrid membrane system (ED/RO), eliminating the need for expensive soda ash softening. The low volume and concentrated brines could be treated with a low temperature crystallizer for additional freshwater and solids production. Because there is 67% less brine, the required evaporator system could be reduced by 67% compared to the conventional RO evaporator system, and thereby reduce the cost of the process. Besides its efficiency in scaling control and in producing low volumes of concentrated brines, another advantage of the salt-splitting ED is that it can be hybridized with any RO unit. Although this hybrid system seems promising, progress is required in studying the recovery of the valuable products, as well as in using different types of feeds.
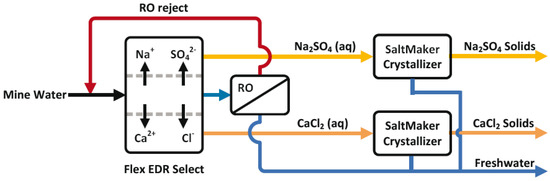
Figure 9.
Salt-splitting ED-RO-salt maker process by Man et al., 2018. Reprinted with permission [108].
Loganathan et al. [87] demonstrated a pilot EDR/RO system combined with a low-temperature crystallizer for near-ZLD discharge to treat saline basal aquifer water. In this hybrid system, EDR has a dual function of softening water prior to feeding the RO unit and concentrating the RO brine reject. High efficiency in scaling mitigation has been achieved by EDR. As a result, the EDR/RO hybrid system achieved about 77% recovery without chemical addition and concentrated the brine up to two times more than that of RO alone; the EDR brine concentration could reach 125,000 mg/L and could be further concentrated by the evaporator-crystallizer to approach ZLD. In addition, TDS concentrations in the RO unit input was around 20,000 mg/L, allowing a reduction of the RO pressure in a large-scale plant. Nevertheless, the overall energy of an EDR-RO plant, 17 kWh/m3 influent, was relatively high and should be reduced in future. In another study, to increase water recovery and decrease energy consumption, four different configurations for seawater desalination plants have been designed and compared: single-stage SWRO, SWRO-ED, NF-SWRO, and NF-SWRO-ED [88]. The results of the comparative study showed that the NF-SWRO-ED hybrid system (Figure 10) should be considered as the best choice with a water recovery of 69% and energy consumption of 6.9 kWh/m3. In addition, a highly concentrated brine, close to saturation, was produced and could be used in evaporated salt production or in the chloralkali industry. These findings should be confirmed and optimized at pilot-scale level. It has been recently published [109] that a pilot plant is now being tested for the proposed process in the “Debiensko” plant located in Poland (Nanos project, 2010). However, no results have been published so far. To decrease the desalination cost of the proposed hybrid process, as well as the methane emission from coal mines, captured methane using membrane technologies could be used as a source of energy [109]. To the best of our knowledge, this low-cost energy source has not been tested in the NF-SWRO-ED hybrid system.
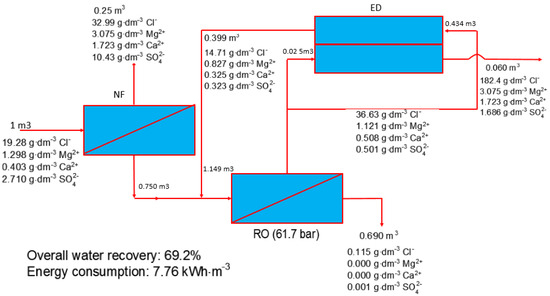
Figure 10.
A scheme of hybrid NF-SWRO-ED. Reproduced with permission [86].
In an innovative hybrid process, MD has been implemented between RO and reverse electrodialysis (RE) [110], as shown in Figure 11, for simultaneous extraction of freshwater and electrical energy production. It has been demonstrated that with more fresh water extracted from MD, the RO brine could be concentrated to a level of 5.4 M, resulting in a volume reduction factor of 83.6%. The RE efficiency, when fed with MD brine (in high concentration compartment—HCC) and seawater (low concentration compartment—LCC) has been improved through an enhancement in the overall water recovery and the power density. In another study [111], the energy performance has been examined to evaluate the economic feasibility of the integrated system. Based on the lab-scale results, the calculations presented by the authors demonstrated that the specific energy consumption in an RO-MD-RE system decreased by 16% and 6%, respectively, compared to the standalone RO system as the electrochemical and thermal potential of hot membrane distillation hypersaline brine could be recovered and converted into energy via reverse electrodialysis. In perspective, individual process optimization with a pilot-scale study might be a big step toward the implementation of the ZLD approach. A brief details of advantages, disadvantages, and energy consumption of the electrodialysis technologies combined with RO for brine treatment with high salinity is presented in Table 8.
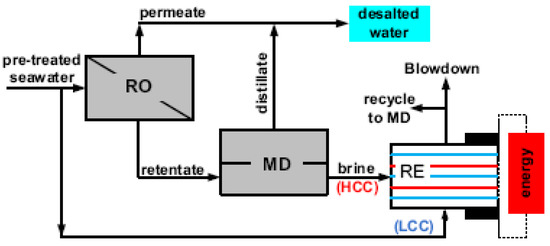
Figure 11.
Integrated membrane system for simultaneous production of water and renewable energy by Tufa et al., 2015. Reprinted with permission [110].

Table 8.
Advantages, disadvantages, and energy consumption of the electrodialysis technologies combined with RO for brine treatment with high salinity.
4.2. Mineral’s Recovery Technologies
A major route by which we can make the brine treatment economical is by the recovery of profitable minerals. FO, membrane-based technologies, or other advanced brine treatment techniques include sophisticated amenities, which on the other hand can only be balanced by the profit envisaged by mining brine for commercially relevant products. Mineral recovery ideas have gained momentum based on this very fundamental aspect [112]. From the economic point of view, metal recovery from seawater desalination or geothermal brine leaves less environment footprint when compared to traditional mining and purification processes. However, its extraction is a matter of controversy. Only limited mineral recovery technologies are so far commercialized and implemented in industrial scale for real-time applications. Despite finding it to be highly dependable with potential for yielding high throughput, even the most modern technologies are far from the ideal necessities of global market. However, before heading to the recovery, it is important to know the global demand. For example, rubidium (Rb) is known for being a high-priced element but stands for its low requirements, whereas Li has high market demand and therefore is more viable for extraction. Other economically viable metals are magnesium (Mg), cesium (Cs), uranium (U), etc. [112,113,114]. Moreover, while opting a suitable recovery technology, other factors such as fast reaction kinetics, ability to withstand quite high temperature (60–200 °C), and pressure 15–25 bar must also be considered. This is because most brine-management plants are working on high flow rates and temperatures. Similar to water-recovery technologies, electrodialysis and membrane distillation have also been adopted for metal recovery from brine solution (see Table 9). The other commonly used methodologies for mineral recovery are adsorption, crystallization, precipitation, and hybrid processes. The proceeding sections discuss major effective practices reported recently for the recovery of industrially relevant metals/minerals from the high-concentrate brine. Table 9 summarizes the recent studies on metal/minerals recovery from various concentrated brines.

Table 9.
Summary of metals/minerals recovered using several technologies based on recent reports.
Table 9.
Summary of metals/minerals recovered using several technologies based on recent reports.
| Minerals/Components Recovered | Brine Source | Mineral Recovery Method | Ref. |
|---|---|---|---|
| Lithium (Li) | Bolivian salt-lake brine | Adsorption by column packed with iron-doped lithium manganese oxides, Li1.33FexMn1.67−xO4 (x = 0.15, 0.30, and 0.40) | [115] |
| Li | - | Adsorption using dihydrate lithium acetate (C2H3LiO2·2H2O) | [116] |
| Li | Salt-lake brine | Electrochemical adsorption or capacitive deionization using oxygen vacancy-rich CoP/Co3O4-graphene aerogel (GA/CoP/Co3O4) as bifunctional anode and cathode | [117] |
| Li | Salt-lake brine | Adsorption by column packed with layered lithium-aluminum hydroxides | [118] |
| Li | Salt-lake brine | Adsorption by Mn-based cylindrical granular adsorbent EP/HMO (Epoxyresin/H4Mn5O12) | [119] |
| Li | Seawater brine | Adsorption by lithium-ion sieves (LIS) embedded in a cross-linked hydroxyethyl cellulose (HEC) | [120] |
| Li | East Taigener Salt-lake brine | granulated lithium adsorbents made of puckered layer double hydroxide NH4Al3(SO4)2(OH)6 | [121] |
| Li | Seawater brine | Adsorption by spinel-type manganese oxide (Li1.33Mn1.67O4) ion sieve | [122] |
| Li | Salt-lake brine | Adsorption on porous titanium-based Lithium-ion sieves (LIS) nanofibers | [117] |
| Li | Salt brine eluate | solar evaporation crystallization | [123] |
| Li | Salt-lake brine | Constant-current electrodialysis (ED) | [124] |
| Li | Salt-lake brine | Selective-electrodialysis (S-ED) for recovering Li from Mg2+/Li+ ratio brines | [125] |
| Li | Lake brines | Sandwiched liquid-membrane electrodialysis used by combining liquid-membrane extraction and electrodialysis | [126] |
| Lithium hydroxide (LiOH) | Lithium-rich salt-lake brine | mass transfer based on three-chamber bipolar membrane electrodialysis based | [127] |
| Li | Salt-lake brine | GO-composite-based pervaporation membrane and crystallizer | [128] |
| Magnesium (Mg) | Brackish water mimicked RO brine | Electrochemical nitrate removal with simultaneous magnesium recovery | [129] |
| Rubidium (Rb) | SWRO brine | integrated submerged MD-adsorption by granular KCuFC | [130,131] |
| Salt-lake brine | Adsorption by biomass-derived adsorbents (BCA@STS, CBCA@STS) modified with sodium titanium silicate (STS) | [132] | |
| Cesium (Cs) | Salt-lake brine | Adsorption by titanosilicate modified BCA and CBCA (carbonized biomass carbonaceous aerogel) | [132] |
| Calcium (Ca) | RO brine, seawater and stored urine | Eutectic freeze crystallization (EFC) | [133] |
| Boron (B) | Salt-lake brine | Electrochemical adsorption or capacitive deionization using oxygen vacancy-rich CoP/Co3O4-graphene aerogel (GA/CoP/Co3O4) as bifunctional anode and cathode | [117] |
| Neodymium (Nd), Gadolinium (Gd), Holmium (Ho) | Acid mine drainage or geothermal fluid | Adsorption using ligand-functionalized silica particles | [134] |
| Sodium chloride (NaCl) | Seawater brine | quartz glass fibrous filter membrane enabled solar crystallizer coupled with a salt crystallization inhibitor | [135] |
| Magnesium sulphate (MgSO4) | Seawater brine | Precipitation using Slaked dolomite | [136] |
| Magnesium hydroxide Mg(OH)2 | RO brine | Precipitation using NaOH | [137] |
| Lithium phosphate (Li3PO4) | Salt-lake brine | Precipitation using facet engineered Li3PO4 seed | [138] |
| MgSO4 | Seawater brine | Precipitation using paper sludge ash, sulfuric acid, and ethanol | [139] |
4.2.1. Adsorption
Adsorption is a broadly recognized strategy with great flexibility for customization on account of efficient removal or recovery of metals/minerals from brine. In this practice, a solid material called adsorbent is introduced into the feed brine to sorb the targeted mineral/metal ion selectively. It is anticipated that almost complete recovery could be achieved by adsorption [2]. It is worthwhile to consider certain prerequisites for an adsorbent to meet the demands of energy economics of a resource recovery system. They are the fast adsorption–desorption rate, high physicochemical durability, selectivity, less sensitivity toward the variations in pH, temperature and concentration of the medium, etc. Typically, adsorbents are in the form of fine powder or microcrystals [140]. Nonetheless, their difficulty to extract from the treatment site and poor cyclic reusability limits their reliability. Thus, the challenge is to develop a promising adsorbent in a self-sustained form. For instance, packing them in fixed-bed columns can certainly restrain the easy and trouble-free filtration of feed [118,130]. A multitude of alternate routes has also been adopted for the hassle-free recovery and the most important of them are granulation, foaming, membrane formation, integration into perforated fibers, etc. In many of these strategies, adsorbents are integrated with polymer/binding agents. Apparently, affinity and effective surface and structural integrity of the adsorbent must be preserved throughout the brine treatment process without compromising the adsorptivity/desorptivity. The major adsorbent types are discussed below:
- (i)
- Granules: The most straightforward method is to make the adsorbent powders into granules by combining with polymeric adhesive/binding/crosslinking agents such as (polyvinyl alcohol) (PVA) [141], poly(vinyl chloride) (PVC)/poly methyl methacrylate (PMMA) [142], epoxy resin-dicyanamide [119] alginates [121], etc. Both cylindrical [119] and spherical granular adsorbents were reported [142]. Naidu et al., attempted to fabricate granular KCuFC adsorbent for rubedium (Rb) by encapsulating in the polyacrylonitrile (PAN) [131].
- (ii)
- Foam: Adsorbents can be made as in the form of ion-permeable network structures with interconnected pores and therefore can attain an augmented surface area available for adsorption. A widely adopted strategy is to make the pore walls activated toward specific ions (e.g., Li) [116]. Such porous structures with ion selectivity are often termed as ion sieves. Quite a large number of works have concentrated on providing meso- and macro-porosity by incorporating onto a perforated/network structure [117,120]. Maintaining adequate structural and mechanical integrity without losing their performance over continuous adsorption–desorption cycles are the bottleneck for the commercialization of foam adsorbents.
- (iii)
- Membranes: In the resource recovery section, we have already discussed principles of a membrane-enabled water treatment process, which relies on the size-exclusion principle. Having said that, the adsorption by membranes is working on selective adsorption in conjunction with a size-exclusion mechanism. It has the major advantage of simultaneous recovery of both pure water and the targeted mineral. The membranes made of suitable components could either be used to embed adsorbent or be functionalized appropriately to make it selective toward a particular ion. Thus, a membrane is conveniently modified to captivate the targeted ion. There are ion imprinted membranes (IIM) and ion sieve membranes (ISM) through which the recovery of valuable minerals could be achieved in a selective manner. The key difference between these adsorbents lies in their adsorbing mechanism. IIM is formed by a functionalizing membrane surface with supramolecular cationic receptors such as calyxarenes or crown ethers [143]. An ISM, on the other hand, could be made better at selection by virtue of intercalation of ions between the interstices. However, precautions must be taken for not blocking the pores by depositing other precipitating agents present in the medium. The membrane further required regeneration by desorbing the ions by chemical treatment [143].
- (iv)
- Nanofibers: Being a relatively novel method compared to the above discussed strategies, only limited numbers of reports have come in this area [119,144]. Here perforated nanofibrous structures are fabricated by incorporating the powder adsorbent into suitable polymeric binding agents. For such a system, electrospinning is a highly viable tool to achieve fibrous morphology. For instance Lai et al. fabricated a highly porous HMn2O4 nanofiber, exploiting electrospinning and subsequent calcination by using polyvinylpyrrolidone (PVP) as a binding agent [119].
4.2.2. Crystallization
A crystallizer is considered as the bottleneck in ZLD technology. Because progressive removal of fluid content always leaves high-concentrated brine to be treated at later stages of brine treatment, most of the studies in brine management are devoted to brine concentration with lower to medium saturation level. Thus, medium-saturated brine could be managed well owing to the advancements made with FO, MD, and electrodialysis techniques. However, relatively less progress has so far been made for the treatment of saturated or near-saturated brine because of the high tendency for scale formation and fouling [135]. In this respect, crystallization is the paramount technique that could be used to retrieve less soluble metal salts from a saturated or near-saturated brine. Besides, this is considered as the modest method for extracting certain minerals from a challenging complex medium like seawater RO brine. A fundamental of crystallization involves controlling the saturation level of a solute in such a way that the ionic product (IP) of the targeted ionic salt exceeds the solubility product (SP) in the medium, prompting crystallization. This is typically achieved by: (1) removing solvents from the brine, (2) controlling temperature, and (3) the converting into salt of the ion with lower-solubility products [63]. These methods are discussed after this section.
Thermal evaporation and the subsequent concentration of brine is the most conventional type among crystallization strategies. Here, solvent has been removed from the brine by thermal management, helping the solution to attain super saturation. Negative-temperature-induced crystallization is apt for certain compounds, whereas some others crystallize at higher temperature. Yet, a few others show no significant changes at all over varying temperatures. This is because temperature is a crucial parameter that determines the solubility of a compound. Figure 12 shows the effect of temperature variation with the extent of solubility [63]. No matter, high-energy input is a fundamental requirement for the bulk heating of brine, making it difficult for industrial-scale water management. Recently, Zhang et al. [135] have come up with a quartz glass fibrous filter membrane-enabled solar-driven crystallizer for the trouble-free separation/recovery of NaCl from the concentrated brine. In their strategically designed 3D model (Figure 13), the thermal evaporation interface has been separated by a high-conducting aluminum sheet. A high evaporation rate (2.42 kg m−2 h−1) was attained for a brine concentration of 21.6 wt% SWRO brine when coupled with a salt crystallization inhibitor, nitrilotriacetic acid (NTA). Unlike the existing solar-evaporation-based technologies, this technique particularly enables enhanced evaporation performance in addition with the crystal-out of salts with self-exfoliating/self-separating benefit. However, the authors self-proclaimed that highly complex brine (e.g., sea brine), wherein many scale-forming precursors are present, can adversely affect the evaporation performance of the as-introduced model. Therefore, its application is largely limited to recovery from pure brine.
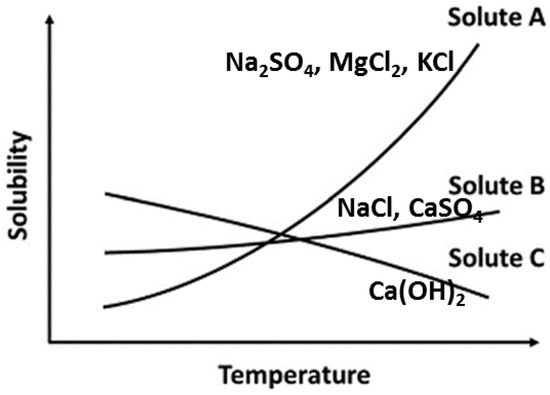
Figure 12.
Different types of solubility curves of solutes. Reprinted with permission [63].
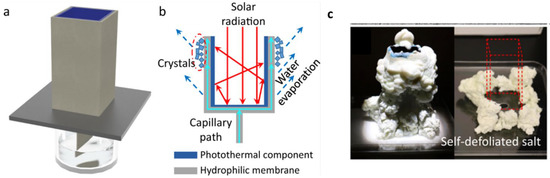
Figure 13.
(a) 3D model and (b) cross-section of quartz glass fibrous filter membrane-enabled solar-driven crystallizer. (c) Photographs showing self-exfoliation of crystallized salt. Reprinted with permission [135].
Freeze crystallization is an alternate method with relatively less energy requirement. This is because the latent heat of freezing (HLF) is significantly less (for instance, seven times less) compared to the latent heat of evaporation (HLV). In a multicomponent system such as SWRO brine, the least soluble salts will get crystallized out with ice (at their eutectic temperature) when a sufficiently lower temperature is attained. This technique is termed as EFC [145]. Randal et al. have reported a useful method to evaluate the thermodynamic feasibility and possible profitability of various brine sources for the crystallization of salts at their EFC condition [133].
4.2.3. Precipitation
Precipitation/softening is a basic requirement of a brine treatment system. As it selectively separates the targeted ion by virtue of a precipitating agent, this technique also has implications in mineral/metal ion recovery. Many variants of precipitation have so far been employed to retrieve useful ions from brine as their profitable salt. While crystallization helps the separation of a compound from its saturated solution, precipitation involves a forced crystallization/precipitation step, where a highly soluble compound of an ion is converted into a different compound of the same ion with less solubility. Nevertheless, it must be noted that the ions here are recovered as a different but profitable salt and not as an original compound. Important salts that are economically profitable such as gypsum (CaSO4. H2O), CaCO3, Mg(OH)2, Na2SO4, CaCl2, and NaCl can be recovered from saline water through selective extraction using precipitation using the SAL-PROC™ process, a proprietary integrated commercial brine treatment system involving sequential recovery of metals/minerals as their valuable products [146].
Being valuable, salt ions Ca and Mg ions could be recovered from RO brine by precipitation using suitable precipitants. A most widely employed method is to use dolomite, lime, or dolomite–lime for precipitating Mg [136,147]. Many other precipitating agents being used so far are listed in Table 9. For example, Dong et al. have presented an initial set of data for the recovery of Mg as Mg(OH)2 from brine, employing NaOH as a precipitant [137]. However, lower grade salts result because of the presence of NaCl as impurities (the grade is governed by the nature of brine [139]), but it is still considered for applications such as fertilizers, pH controlling, etc. Besides, scale-forming ions (such as SO42− and HCO3−) can affect the precipitation, which calls for removal of scale-forming ions by some means, normally by ED [148]. Be it said that there are only limited reports available for the extraction of Li as carbonate by precipitation from carbonate-rich brine. This is due to the higher pH of brine, which is unfavorable for precipitation. Precipitation of lithium as phosphate is an alternative for such brine but it demands higher thermal energy (T > 60 °C) to initiate nucleation (by virtue of a high nucleation energy barrier). Liu et al. have introduced novel, highly active facet-engineered Li3PO4 crystals as seeds for the energy-efficient recovery of Li from carbonate-rich brine by precipitation at ≤30 °C [138].
4.2.4. Miscellaneous
The recovery of a targeted metal/mineral has to face great hurdles because of the unfavorable concentration ratio and presence of competing ions in some complex brines. Thus, to get a cleaner and efficient recovery of resources, alternative options are often suggested. Many technologies have been brought to market by the combination of two or more methods discussed above and are in their early stages of commercialization. For instance, a recently emerged scheme is capacitive deionization (CDI), wherein electro adsorption of charged ions is impelled on the surface of customized porous electrodes (most commonly carbon-based aerogels) [149]. Jin et al. put forward a novel green CDI strategy for the simultaneous recovery of boron (B) and lithium (Li). They customized a highly hydrophilic graphene aerogel with bifunctional oxygen vacancy-rich CoP/Co3O4 with augmented selectivity and adsorption–desorption kinetics. The so-reported design claims the adaptability for high-Mg/Li-ratio salt-lake brines (Figure 14) [117]. However, it should be noted that the high concentration of TDS in the brine causes the scaling of electrodes. Therefore, fouling control methods are the major challenges in CDI processes [149]. The integration of pretreatment techniques would alleviate inorganic scaling and organic fouling of the electrodes in CDI.
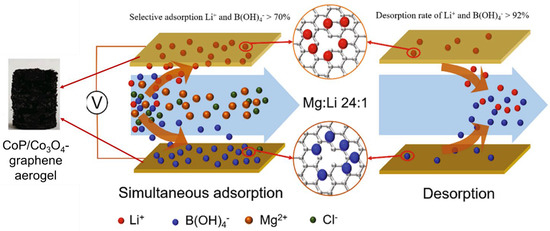
Figure 14.
Schematic illustration of electro-adsorption/desorption process for the simultaneous recovery of Li and B using strategically designed graphene aerogel as electrode materials. Reprinted with permission [117].
4.2.5. Hybrid Processes
As mentioned in the preceding sections, desalination brine has the potential to be a rich resource for the production of commercial products such as metals, mineral salts, and valuable chemicals. Although the economic impact of desalination could be mitigated by recovering materials using the standalone techniques, the high capital cost is still a limiting issue and prevents the extracted product to be commercialized. The hybrid technologies would be required to achieve optimal transformation of the brine into valuable material and reduce the overall desalination cost. The most effective hybrid techniques are those that integrate diverse processes to recover water while producing commercial products. Some of those integrated with RO, already discussed in the freshwater recovery sub-section, could be used in a near or zero liquid discharge approach to reduce the environmental impact by recovering as much material as feasible. This part focuses on the above-mentioned integrated hybrid technologies used for materials recovery, as well as water desalination, to achieve a zero-liquid discharge. The sustainability approach of some integrated processes is discussed as well.
4.2.5.1. Combined Techniques with RO for Resource Recovery Using ZLD Approach
The integration of pressure-driven membrane technology, RO, with the pretreatment methods (chemical precipitation, seeded precipitation, and ion exchange) and/or electrodialysis technologies seems promising for water and valuable products recovery while achieving ZLD. Table 10 presents how the integrated RO process could be used to desalinate brine and collect products.

Table 10.
Hybrid techniques used for valuable product recovery using the ZLD approach.
Table 10.
Hybrid techniques used for valuable product recovery using the ZLD approach.
| Recovering Component | Hybrid Method Other Details | Brine Source | Advantages | Challenges | Ref. |
|---|---|---|---|---|---|
| Calcium carbonate (CaCO3)/calcium oxide (CaO) | CP/RO Precipitant: Lime and soda ash CaCO3 and CaO recovery from the precipitated sludge through CO2 gas injection to selectively dissolve magnesium | Simulated RO brine from Brackish ground water | Economic return from mineral precipitation | Cost study Study of CO2 emission to the atmosphere | [82] |
| CaCO3 | Chemical precipitation/FO/RO Lime and soda ash softening | Real RO brine from a coal chemical industry | Production of High purity product (92.2% purity): −5.6 kg/m3 ROC | FO membrane scaling and cleaning strategies Study of the integrated FO/RO system | [91] |
| Seeded aeration softening/RO | Real brine from brackish water | Production of, chemical free, 1.05 g-CaCO3 L−1 h−1. High purity product: 92% | Reduction of CO2 emission | [93] | |
| -Divalent salt calcium sulphate (CaSO4) -Super concentrate for sodium and potassium salts | CP/RO Acidification + degasification antiscalant before SRO | Real (RO) brine/Brackish water | Divalent salts could be used for soil and dust control Production of super concentrate depleted of bicarbonates and with less scaling for thermal evaporation | Cost evaluation Additional treatment of the super concentrate is may needed Study of the integrated system CP/RO with thermal treatment | [90] |
| Production of highly concentrated brine | ED/RO | Simulated highly saline brines from both brackish and sea waters | High salinities, beyond the range of RO alone, could be reached (Brine from brackish water) | Limitations to treat brine with TDS 120,000 ppm (Brine from sea water) | [86] |
| Sodium sulfate and CaCl2 | Salt-splitting ED/RO pilot unit combined with crystallizer | Coal plant wastewater | Production of low volumes of concentrated brines Smaller evaporator system ED is easily combined with any RO unit | Separation of products Cost study | [108] |
| Highly concentrated brine close to the saturation limit of the water chemistry (TDS of 239,000 mg/L) | EDR/RO system combined with a low-temperature crystallizer | Real Brine from saline basal aquifer water | Production of highly concentrated brine up to two times more than that of RO alone | Lowering the Energy consumption Solid separation | [87] |
| Saturated brine: >180 g dm−3 as Cl−: | NF-RO-ED | Simulated Coal mine water | Production of a raw material for evaporated salt production | Membrane development to reduce the cost | [109] |
| Na2SO4 | Fractional submerged MD-Crystallization (Other hybrid technique) | Simulated sea water RO brine | The concentration/temperature gradient enhances water and salt recoveries | Quantitative analysis of ammonia in MD permeate Study with real sea water brine | [150] |
Recovery of salt from desalination brine can been achieved by using combined RO systems, as shown in Table 10. The salts could be employed for various application [151]. Magnesium and calcium salts such as CaCO3 are the major products that could be recovered by integrating the chemical and seeded precipitation techniques with the RO system. The use of lime and soda ash seems to be promising for the valorization of the calcium and magnesium by-product from RO brine through chemical precipitation [82,91]. Other chemicals such as sodium phosphate and sodium carbonate could be used as chemical precipitants to extract salts from RO concentrate [21]. In another study, sodium carbonate and sodium hydroxide were used as precipitant to collect calcium and magnesium salt. However, the authors demonstrated that the recovery of calcium and magnesium salt were masked by antiscalants and ions that are present in the RO concentrate. Consequently, they used ED to decrease the quantity of antiscalants. The recovered salts could be employed for pH control and the recovery of phosphate fertilizers from wastewater treatment [152]. The combined process ED/RO could be integrated with crystallizer to increase water and extract a variety of salts from RO brines [151]. In this combined system, ED technologies were employed as a pretreatment step for concentrating brine followed by RO and crystallization. A recent modeling study has been conducted by Mitko et al. [109] to predict the highest chloride concentration that may be attained in an industrial-scale electrodialyzer. It was shown that the ED could produce saturated brine, greater than 180 g dm−3 as Cl−, from coal mine water as long as an NF-RO system was used to pre-concentrate it. In fact, the use of cation- and anion-exchange membranes positioned between electrodes allows and facilitates the separation of salts from brine. The effect of operational conditions on performance efficiency, such as membrane properties, current density, and initial brine concentration, was examined [21]. It has been demonstrated that the treatment of RO brine with ED or EDR in combination with pressure-driven membrane processes and other technologies might be sufficient to achieve ZLD. Although the costs of studying these hybrid membrane-based systems for producing salts are relatively high [87,151], innovative designs have been established with the goal of lowering capital costs. For example, in pilot studies combining RO/ED for the treatment of brackish water to reach ZLD approach, Oren et al. [153] demonstrated that the innovative hybrid system achieved the dual function of expanding the salinity limit of RO while lowering energy consumption. Prior to a side-loop crystallizer and wind-aided enhanced evaporation, EDR had the benefit of concentrating the RO brine to a salinity of 100,000–200,000 mg/L. Several studies combining other technologies have also been conducted to increase the performance. For example, Choi et al. [150] demonstrated that 223.73 g Na2SO4 crystals were successfully extracted from simulated SWRO brine while achieving high water recovery using fractional submerged MD-crystallization. This hybrid system could reduce energy consumption and avoid crystallization on the membrane surfaces membrane. Such hybrid processes will be further discussed in future work.
4.2.5.2. Hybrid Process for Simultaneous Water-Mineral Recovery and Carbon Capture
A hybrid process treating two or more pollutants in a single reaction could be an efficient solution to improve the efficiency of the desalination process by using cost-effective and environmentally friendly strategies and ensure the sustainability of brine management approaches. Several recent studies have focused on simultaneously treating desalination brine and carbon dioxide (CO2), which is a major contributor to global warming and climate change and can be released from different industrial sectors such as the power generation that is required for desalination operations. Combining the utilization of the concentrated brine and CO2 offers the benefit of tackling two environmental problems and shifting to a green production of useful products such as sodium bicarbonate (NaHCO3), sodium carbonate (Na2CO3), nesquehonite (MgCO3·3H2O), and lansfordite (MgCO3·5H2O) while obtaining a treated brine that might be suitable for irrigation or human consumption [154,155]. Different studies have demonstrated the feasibility of recovering salts and minerals from concentrated brine as a way of CO2 capture and utilization.
In general, there are two methods for using CO2 with brine whether direct precipitation of earth alkaline cations (Ca2+ and Mg2+) by CO2 gas to form CaCO3 and MgCO3 or indirect precipitation of Na+ to form sodium bicarbonate [156]. The modified Solvay processes, using the second method, have the potential to manage these two wastes in a combined process while recovering useful resources. Therefore, it might be an effective solution and a business opportunity to improve the desalination process and make it more environmentally friendly and sustainable. Generally, the Solvay process is employed to produce sodium carbonate (soda ash) through the reaction between CO2 and saturated sodium chloride solution (the brine), in the presence of ammonia (NH3), to form soluble ammonium chloride and a precipitate of sodium bicarbonate (NaHCO3), which could be converted to soda ash by calcination [155]. Soda ash is a common raw chemical that can be used in different industrial sectors [156]. Mustafa et al. have recently reviewed the effect of different parameters on the overall combined process efficiency, including both carbon capture and metal removal efficiencies, as well as product yield [157].
Several studies reported that the optimum reaction temperature of CO2 with ammoniated brine was between 20 and 25 °C for both CO2 capture and sodium bicarbonate formation. In fact, increasing the temperature causes the evaporation of the highly volatile ammonia, decreases the solubility of CO2 in the liquid phase, and increases the solubility of products. Therefore, a low temperature is needed to improve CO2 capture efficiency and recovered product yield [157]. The type of solvent is also one of the important parameters that might have a significant impact on the combined process efficiency. NH3 is typically used as a solvent in the process. Although the ammonia is not involved in the overall reaction of the modified Solvay process, it has a significant effect on the intermediate reactions. Its role is to maintain a basic pH in the solution and increase sodium bicarbonate precipitation. El Naas et al. [155] found that the optimum reaction temperature was about 20 °C and the optimum NH3/NaCl ratio was 2 for synthetic brine solutions and 3 for actual brine to achieve 40% of sodium removal and more than 90% of CO2 removal from a CO2–CH4 gas mixture. Generally, in the conventional Solvay process, the resulting soluble ammonium chloride can be reacted with calcium oxide (CaO) obtained from limestone burning to recover and recycle the ammonia. Nevertheless, the utilization of lime as a source of CaO causes an increase in CO2 emission. Therefore, different alkaline solid waste with high CaO content have been suggested as a lime alternative [156]. In this context, Pinto et al. [158] demonstrated that a partial replacement of lime by steel slag milk in the ammonia recovery step is possible. The use of steel slag, a low-cost and abundant waste material, as a source of CaO seems promising as it can decrease the CO2 emissions and reduce the costs of products because of the low cost of solid waste and the reduction in energy consumption. The authors found that 40% of the total ammonia could be recovered thanks to the high CaO content (40.1% CaO) in the steel slag. However, it is worth remembering that the high volatility of ammonia and its health hazard effects are among the major drawbacks of ammonia. The utilization of solvents alternative to ammonia could open gateways to overcome the drawbacks of the conventional Solvay process. Therefore, different alternative solvents have been studied [154,155]. Other amines, such as methyl aminoethanol and 2–amino, and 2-methyl propanol, were used as alternatives to ammonia and good results were achieved. However, the main drawback of using amines is the formation of hazardous byproducts in the brine during the carbonation step. Therefore, researchers have opted recently for other alternative solvents. For example, El Naas et al., 2017, proposed a modified Solvay process where ammonia is replaced by calcium hydroxide [155]. The experimental results illustrated that the modified Solvay process using calcium hydroxide is superior in terms of CO2 capture efficiency, sodium removal, and energy consumption. In addition, the calcium-based Solvay process was less costly than the conventional process because of the lower costs of calcium hydroxide relative to the cost of ammonia and the elimination of the energy-intensive ammonia regeneration. However, further research is required in this study as there is a lack of information about the purity of sodium bicarbonate, as well as the calcium chloride salt. Additionally, coupling mitigation techniques with the modified Solvay process is needed to eliminate fouling of equipment caused by CaCO3 scale [157]. Dind et al. have suggested the use of mixed-metal oxides derived from the calcination of Mg-Al-layered double hydroxide (LDH) and demonstrated the efficacity of the modified process to produce a pure sodium bicarbonate better than other modified Solvay processes [154]. The mixed-metal oxide showed high potential to remove chloride from brine during the first step before being separated from the mixture in the second step. The removal of chloride results in raising the OH ions in the brine derived from the used mixed-metal oxide. Consequently, the resulting brine with high alkalinity could be then employed for production of sodium bicarbonate and CO2 absorption. The novelty in this modified process is its ability to remove both sodium and chloride ions from brine and the production of a valuable high-purity product. However, the efficiencies of sodium removal and CO2 capture were relatively low. Moreover, the use of mixed-metal oxides in the combined reaction needs to be explored further.
The types and concentrations of salts are also important factors that affect the type of recovered product and the yield of reaction [157]. It has been demonstrated that the solubility of NaHCO3 decreases with increasing the concentration of NaCl in the solution [155]. Reaction time, CO2 concentration, and catalysts, as well, can considerably improve the CO2 capture and metal removal and thereby can reduce the cost of the reactor.
This subsection discussed briefly the most up-to-date modified Solvay process to produce soda ash and baking soda in the context of brine management and CO2 utilization. The combined process using multi-stage treatment could offer a great opportunity for shifting to a green production of soda ash mainly by using not only exhausted CO2 and brine but also alkaline solid wastes such as steel slag and fly ash [159]. Thus, three environmental problems could be tackled at the same time, while reducing the capital cost. However, there are challenges that require further research and technological development initiatives for the idea to be industrially implemented, such as improving the reactor design, purification of products, and techno-economic analysis of the process.
4.3. Energy Recovery Technologies
In recent times, along with the recovery of minerals and water, energy is also recovered during brine management by utilizing the salinity gradient power (SGP), which is obtained from the release of Gibbs’ free energy during the mixing of solutions with different concentrations. As the salinity gradient power is an unexploited area of renewable energy resources, countless research studies going on nowadays are based on harvesting salinity gradient power. SGP plays a significant role for balancing the carbon footprints and the greenhouse emissions by the desalination plants [16]. Several techniques are utilizing the salinity gradient power and, among those, three important techniques are pressure-retarded osmosis (PRO), reverse electrodialysis (RED), and capacitive mixing (CapMix). The following segments discuss these three various SGP techniques for the recovery of energy.
4.3.1. Pressure Retarded Osmosis
Pressure-retarded osmosis is an excellent technology that can be explained by means of the utilization of Gibbs free energy of mixing freshwater with brine to generate power. It is hydraulic power generation using the osmotic pressure difference between high-osmotic-pressure solution such as seawater and low-osmotic-pressure solution such as freshwater. It utilizes semipermeable membranes (that only allow water molecules to pass through) for the effective recovery of energy by harvesting Gibbs free energy of mixing by allowing water to transport from a low-concentration solution (feed solution (freshwater)) to a high-concentration solution (draw solution (seawater)). While the high concentration solution (draw solution) is diluted by the arrival of the low-concentration solution, the Gibbs free energy will be converted to hydraulic pressure, which is used as the propelling force for the pressure exchanger to develop the mechanical energy to operate the turbine for the production of electrical energy.
Pressure-retarded osmosis is focused on a method that could regenerate energy from the salinity difference between the concentrated brine and pure water and, at the same time, as an efficient system to replace the SWRO system, thereby solving the environmental problem caused by the harmful SWRO brine released back into the sea. In 2010, Statkraft AS, a Norwegian energy company, had put forward the idea of the first prototype plant. They used semipermeable membranes in a flat sheet spiral-wound configuration, where seawater was used as the draw solution and river water used as the feed solution [160]. Keiichiro, et al. analyzed the toughest challenges, such as concentration polarizations and membrane fouling, which were the main challenges faced by prototype plants. Using hollow-fiber modules, they have generated salinity power by a pressure-retarded osmosis system between pure water and concentrated brine [161]. Concentration polarization effects can be reduced by the modifications in the number of open ports in the modules by increasing them from three to four. The prototype plant reported the maximum output power density of 7.7 W/m2. Figure 15 shows the schematic view of the prototype plant. In addition, membrane fouling was analyzed and found to be reduced by treatments with low-pressure RO membrane and coagulation–sedimentation method with ozonation and, also, good results were achieved through these methods. To examine the potential of the system, Waqas et al. developed a mathematical model of a pressure-retarded osmosis system as an energy recovery process for the desalination plants [162]. Disposed brine of an MSF desalination plant was used as the draw solution and seawater was used as the feed solution. The power generated at a hydraulic pressure of 1010 kPa was 30.8 kW, which was found to be less than the power required by pumps of an MSF desalination plant. The plant’s performance also depends upon the properties provided by the membranes. Membrane properties such as the water permeability coefficient and the mass transfer coefficient have significant roles. Several attempts were made by many research groups for improving power density from the system. Kim, Yu Chang, and Menachem Elimelech together proposed the scenario of osmotic pressure generation by pressure-retarded osmosis using a variety of salinity gradient resources. Brine solution from an RO desalination plant was used as the high-salinity draw solution and the low-salinity feed solution used was the municipal wastewater effluent. A water flux of 13.9 Lm−2 h−1 and power density of 4.7 W/m2 at a hydraulic pressure difference of 12.5 bar was obtained when a 2 M draw/0.5 M feed at 30 °C was used. The team demonstrated a hybrid process of FO desalination, as well as pressure-retarded osmosis osmotic power generation, and conveyed the potential viability of the osmotic power generation using seawater as the feed solution [163]. Straub et al. investigated the power density that can be obtained in pressure-retarded osmosis from highly concentrated solutions where greater energy of mixing can be harnessed from such high concentration solutions [164]. Thin film composite (TFC) membranes with woven mesh supported by tricot fabric feed spacers were designed specifically to improvise the operating pressure of the system to 48 bar for greater than 10 h. The power density was reached up to 60 W/m2, thereby exploring the high-power density potential of the concentrated draw solution by providing a proper membrane, spacer, flow channel, etc. for the pressure-retarded osmosis operation at increased hydraulic pressure. A high-pressure system has a greater significance in harnessing the renewable energy stored in salinity gradients and thereby can be considered as a commercially feasible method. In 2014, another approach was introduced by Andrea et al. in the pilot-scale production of a combination system of RO-pressure-retarded osmosis, which is a next-generation system for low-energy desalination [165]. A pilot-scale system was designed accordingly, where RO energy reduction was evaluated using pressure-retarded osmosis. This combination system utilizes energy from a volume of water transferred from atmospheric pressure to elevated pressure across the semipermeable membrane to pre-pressurize RO subsystems and it is claimed to be the first system to utilize energy like this. The average power densities, which were closer to the values required for economically feasible systems, was obtained (1.1 to 2.3 W/m2). Integrated projects always gained proper attention and, among those, SWRO and pressure-retarded osmosis integrated systems focused on not only less energy consumption, but also engrossed on the low-cost, high-power density, low fouling, etc. Nevertheless, being an efficient process, SWRO is still having issues of consuming large amounts of energy to pressurize and pump water. Environmental problems are severe because of the disposal of concentrated brine from the SWRO plant and thereby develop a negative impact on society [166,167,168].
Integrating the pressure-retarded osmosis with SWRO provides advantages such as less fouling because of the pretreatment of seawater brine conducted in the SWRO system. A maximum power density of 13.3 W/m2 was obtained with a hydraulic pressure difference of 27 bar for the system put forward by the Megaton Water Project from Japan where SWRO brine was used as the draw solution and freshwater as the feed solution [161]. However, the process needs detailed optimization, since most of the process designs are missing in reported literature. Wan, Chun Feng, and Tai-Shung Chung proposed detailed configurations of two integrated processes of novel SWRO–pressure-retarded osmosis explained in terms of the positions and functions of each pressure exchanger (PX) and high-pressure pump (HP). The system was optimized by the determination of the dilution factor and corresponding operating pressure. While operating SWRO at 25% and 50% recovery, with the brines diluted to the seawater level, the specific energy consumption to produce 1 m3 of desalinated water can reduced from 1.08 to 1.14 KWh, respectively [169]. A python-based model called Propmod was introduced by Benjamin et al. that utilized both internal and user-defined inputs to simulate how a full-scale system works economically [170]. It could efficiently save 9% of the energy consumed per m3 of permeate. It was designed in such a way as to simulate any saltwater-based pressure-retarded osmosis process and was tested with salinities ranging from DI to RO concentrations.
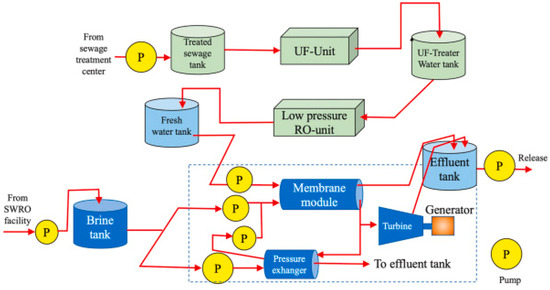
Figure 15.
Schematic drawing of pressure-retarded osmosis prototype plant. Reprinted with permission [161,171].
4.3.2. Electrodialysis Reversal (EDR)
Another SGP technology having an exceptional possibility for commercialization is electrodialysis reversal (EDR). EDR also grabs the Gibbs energy from the mixing of two solutions such as PRO; instead, ion-exchange membranes are used to capture this renewable energy. It consists of two kinds of membranes, the anion exchange membranes (AEM) and (CEM) cation exchange membranes, which are stacked together in an alternate fashion, while the flow of cations and anions may create an ionic flux that can be converted at the electrodes to generate power. Two important factors for electricity generation using EDR are power density and energy efficiency. Power density means the power per membrane area (W/m2) and energy efficiency may be termed as the fraction of the potentially available energy from the salinity difference that is converted into electrical energy (%). Most of the research is based on the enhancement of power densities and is mainly focused on the use of seawater and river water as feeds because of the availability of large volumes of these water types. Vermaas et al. for the first time presented an overview of experimentally determined power density and energy efficiency values over a wide spectrum of feed-water concentrations that can be used in EDR [172]. The research was focused on varying the concentration of feed solution concerning power density, energy efficiency, permselectivity of the membrane, and electrical resistance. It was understandable that the permselectivity was dependent on the concentration of the feed solution and it is selective for a low-concentration solution more than the higher. The highest energy efficiencies were obtained for feed water with low salt concentrations, especially with low salinity gradients. Power density increases gradually with higher salinity gradients. The highest power density obtained at the uttermost concentrations such as 0.01 M and 5 M NaCl are approximately peaking at 3.8 W/m2 and the power density was found to increase to 6.7 W/m2 while increasing the temperature up to 60 °C at 5 M brine solution concentration. However, this effect of temperature was not found in the case of permselectivity and energy efficiency. Kwon et al. [173] proposed the modified model where river water and seawater were considered as the diluted solutions and the power generation was improved using the brines that came out of two different membrane-based desalination processes, i.e., RO and FO. According to the intermembrane distance and the inlet flow rate, they have characterized the maximum power density and net power density of RED. They also looked over the combined effects of integrating RED with the desalination processes. A reduction was found in specific energy consumption (SEC) on account of the chemical energy recovery using RED. Power density was increased 1.5-fold for RO (1.48 W/m2) and 2-fold for FO (1.86 W/m2). The computed energy costs showed that the energy consumption could be lowered to approximately 7.8% from the normal value of RO and 13.5% for FO. Coming to the pilot-plant RED studies, the first pilot plant on RED belongs to southern Italy, which represents the final accomplishment of the REAPower Project. It was consisting of 125 cell pairs of IEMs and both artificial and natural feed solutions were tested. An overall power density up to 40 W where each cell pair contributes 1.6 W/m2 was reached using natural solutions as the feed. While using artificial NaCl solutions, an increase of 60% was observed, the power output reaching up to 65 W, which means 2.7 W/m2 of cell pair. Tedesco et al. [174] expanded a process simulator for the RED plant using high-saline solutions. The scale-up was planned accordingly with an expectation of 1 kW power output. A simplified schematic of the plant is given in Figure 16. Concerning the real-time application of the RED process using natural seawater and river water, the performance may depend upon the temperature of the climate as well. Mehdizadeh et al. [175] analyzed the temperature of feed solutions using two types of pilot-scale RED stacks consisting of 200 cell pairs having a total effective membrane area of 40 m2 with different intermediate distances. Increasing temperature showed linear relationships to the factors such as pump energy, gross power output, membrane resistance, open circuit voltage (OCV), which was evaluated in the study. Hulme et al. [176] aimed to investigate the scalability of RED for energy generation from high concentration salinity gradients by three process scales: a standard laboratory-scale stack (10 × 10 cm), a 10 × 20 cm stack, and a commercially available (10 × 40 cm) stack. For rectangular stacks, fixing the velocity doubles residence time when the length scale was increased two-fold to sustain the same residence time; crossflow velocity must be doubled when length scale is increased two-fold.
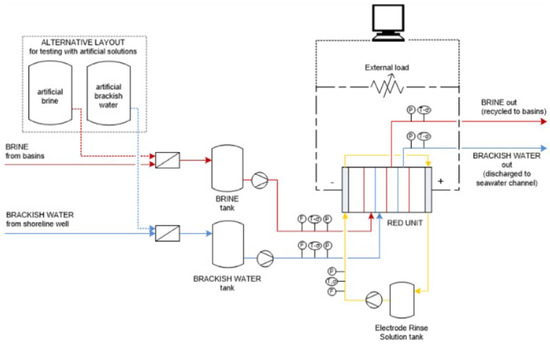
Figure 16.
Schematic of the pilot-plant layout used for the first reverse electrodialysis pilot plant. Reprinted with permission [174].
4.3.3. Capacitive Mixing (CapMix)
Capacitive mixing (CapMix) is an additional interesting SGP technology, where energy generation is possible from brine through the mixing of solutions having different saline concentrations. The cell contains two electrodes that are plunged into an ionic solution, thereby forming a supercapacitor. The CapMix energy extraction process is said to be the reverse of the brackish water desalination technique termed capacitive deionization (CDI) [177]. Based on the ability to accept and release ions into electrodes, CapMix can have three different methodologies: (1) capacitive double-layer expansion (CDLE), (2) capacitive Donnan potential (CDP), and (3) mixing entropy battery (MEB). The fixed porous carbon electrodes are always intermittent in their nature for the power production and, hence, the ion capturing/releasing will be limited for such a CapMix system. However, this limitation is somehow solved by the flow electrodes used in a CapMix system [178]. The system is called flow electrode capacitive mixing (F-CapMix). Flow electrodes are used in the system, which consists of a suspension of capacitive particles in the electrolyte that triggers the process of the energy production using CapMix [179]. High surface-area materials such as activated carbon are used for flow electrodes and they are utilized as high-concentration slurries in the electrolyte solution. About CDLE, the selectivity of ions is accomplished by charging the electrodes by using an external input power. CDP corresponds considerably to the CDLE methodology; instead, for the selective transport of ions, it uses a permselective membrane. Like in EDR technology, CDP also used the AEMs, CEMs, and a spacer.
Jiho, et al. [180] put forward a hybrid CapMix for harvesting energy. They achieved a superior energy harvesting performance with better power output by the hybrid system and to capture/release ions they used sodium manganese oxide (NMO) and porous activated carbon (AC) with an anion-exchange membrane (AEM). They analyzed the practicability in multi-ion solutions also. It was concluded that the hybrid system could be a practically viable one and can be an alternative for the recovery of the energy.
5. Conclusions and Future Recommendation for Brine Management
Disposal of brine or brine management is very crucial. As mentioned earlier, its discharge can cause severe impacts to the environment and ecosystem since it contains microbial disinfection byproducts such as scavengers and biocides, and coagulants like ferric chloride, alum, and heavy metals (Fe, Cu, Cr, Ni, Mo, etc.). Conventional discharge methods such as discharge into surface water, seawater, deep well injection, land application, and evaporation ponds can inversely affect marine ecosystems and soil quality owing to the presence of the above-mentioned constituents that can induce eutrophication, salinity variations, pH fluctuations, temperature changes, etc. The development of zero liquid discharge technologies have produced great responses considering the ability for resource recovery. A detailed techno-economic report of various ZLD processes adopted so far are presented in Table 11.

Table 11.
Techno-economic analysis of commonly adopted ZLD processes.
Table 11.
Techno-economic analysis of commonly adopted ZLD processes.
| Method | Brine TDS Level (g/L) | Recovery Rates | Energy | Cost | Industrial Scale Piloting | Ref. |
|---|---|---|---|---|---|---|
| FO | 200 | Up to 98% | 0.8–13 kWh/m3 | 0.63 USD/m3 | 15 kLD | [41] |
| ED | 150 | More than 90% | 20–40 kWh/m3 | 4–8 USD/m3 | - | [181] |
| MD | 350 | 90% | 39–67 kWh/m3 | 1.17 USD/m3 | - | [8] |
| CP/RO | More than 90% | - | 0.6 USD/m3 | 4546 kLD | [94] | |
| PRO-IEX-SRO-Heat-TRO | 941 | 96.4% | - | 0.739 USD/m3 | 96 Mgpd (363,399.6 kLD) | [101] |
| SP/RO | RO brine from low-salinity mine water | More than 90% | - | 10.8 USD/m3 | - | [104] |
| ED/RO | 60 | 69% | 6.9 kWh/m3 | - | - | [88] |
| RO/ED/ crystallizer | 6–120 | - | 191 kWhe/tonne-salt | 111 $/tonne-salt. | 100,000 tonnes/year salt | [182] |
The state of the art of these technologies also discovered that hybrid processes could be more beneficial, considering individual technology, as clearly seen from Table 11, because of their cost and energy effectiveness. It is reported that hybrid process can significantly reduce the energy consumption and cost of individual ZLD processes by up to 50–70% [5,24,40,46,95,183,184,185]. Hence, the major concern of treating RO concentrate with individual ZLD processes could be addressed by coupling either with ED, FO, or MD processes. ED technologies could achieve the softening of primary brines before the secondary stage of the RO in either simple or complex designs. It is worth mentioning that MD could also be integrated with ED technologies and RO to handle brine streams with higher salinity and develop a reliable technological approach toward an efficient implementation of ZLD. Integrating EDR with RO provides not only the benefits listed above but EDR also offers the advantage of decreasing the capital cost of the produced water as the high salinity of the brine could improve the power generation from salinity gradients. Despite the significant progress of the hybrid ED/RO system achieved, additional studies are still required for further reduction of the energy cost of the combined system. At present, the research should focus on improving the design for high-salinity water treatment, optimization of the performance of the hybrid system in a full-scale level, and using technologies to recover energy and reach the self-sufficient desalination systems.
Metal recovery technologies are truly beneficial for their ability to recover minerals of high economic value such as Li, Ru, and Cs. However, at present, very limited recovery technologies are implemented commercially from the industrial point of view. Adsorption, crystallization, and precipitation can be considered as an effective method in recovering the minerals in a cost-effective way. Still, there is always competition between several ions in the system reducing the separation efficacy, purity considerations, and secondary byproducts. As mentioned, membrane-based systems such as electrodialysis and membrane distillation always suffered from obstacles such as energy requirement, fouling, and scaling. A combination of membrane-based technologies with low-cost thermal technologies, i.e., hybrid processes, using chemicals as waste for softening and resource recovering, involving the salinity gradient power as a source of energy and integration of renewable energy systems, are needed to benefit from the strengths of the combined technologies and evaluate the feasibility of this hybrid approach in the desalination industry. PRO, EDR, and CapMix are highly relevant methods in view of energy harvesting from brine; few plant implementations have been demonstrated so far. However, these still require more development to improve the economic effectiveness and sturdiness. Hence, there is need to focus more on the development of hybrid processes to develop productive methodologies for large-scale industrial implementations with technical and economical points.
A hybrid process treating two or more pollutants in a single reaction could be an efficient solution to improve the efficiency of the desalination process by using cost-effective and environmentally friendly strategies and ensure sustainability of brine management approaches. In this context, a framework of an effective hybrid management could be formulated and developed to meet the near-ZLD approach in many countries in the world, particularly the gulf countries that are suffering from water scarcity as shown in Figure 17. The economic, environmental, and social performances are taken into consideration in the developed framework. Consideration of waste reduction in the hybrid process design is an indispensable key to the attainment of a sustainable development strategy. The focus of future research should be on the development of a combined hybrid process using multiple-stage brine treatment that involves a minimum of two or more pollutants while producing more commercial products and a better quality of treated water than previous works. The pretreatment of the brine with high levels of scale ions and organics could be performed by seeded and chemical precipitation. For example, magnesium and calcium and other scale ions could be precipitated with alkaline solutions such as lime and soda ash to recover additional commercial products. A careful consideration of the purity of the sealable products in this step would contribute toward cost saving by generating revenue. The cost of purchasing chemicals for brine pretreatment could be saved through the use of chemicals as waste, as mentioned above. For further purification of brine from heavy metals, and organic and inorganic pollutants, ultrafiltration could be applied to concentrate the softened brine. Next, the modified Solvay process could be integrated in the hybrid process to ensure the sustainability approach. Therefore, the highly concentrated pretreated brine along with carbon dioxide as a waste would be the raw materials to produce additional saleable solid carbonate such as soda ash. The amount and the quality of the produced solid carbonates, with a reduction of the treatment cost as well as waste, should be taken into consideration to address the aforementioned gaps that have been previously encountered during the study of the modified Solvay process. The integration of ultrafiltration with chemical softening, as well as the reduction of sodium, via the modified Solvay process would alleviate membrane scaling and additional RO brine treatment and/or electrodialysis, with lower cost, will be possible in a post-treatment step to obtain water suitable for human consumption and meet the near-ZLD approach.
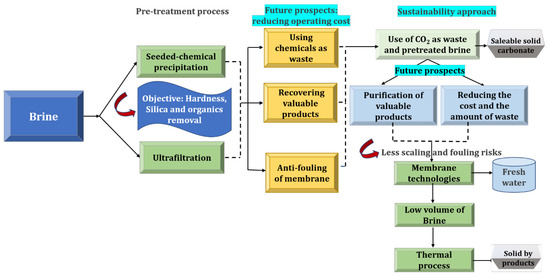
Figure 17.
A suggested framework representation of effective hybrid management to meet the futuristic near-ZLD approach for the world, majorly focusing on the gulf countries.
Author Contributions
Conceptualization, S.N.B. and I.B.; methodology, S.N.B., I.B. and N.K.; validation, M.A.A., M.S.T. and T.L.; investigation, S.N.B., I.B., N.K., R.T.T. and G.P.; writing—original draft preparation, S.N.B., I.B., N.K., R.T.T. and G.P.; writing—review and editing, M.A.A., M.S.T. and T.L.; supervision, M.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Institute of Sciences & Engineering, University of Sharjah for providing the funds for this research. The APC was funded by Water Desalination Research Group Operational Grant, University of Sharjah. Kallayi, N.; would like to acknowledge the DBT-RA fellowship, Government of India, for the financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing.
References
- Bello, A.S.; Zouari, N.; Da’ana, D.A.; Hahladakis, J.N.; Al-Ghouti, M.A. An Overview of Brine Management: Emerging Desalination Technologies, Life Cycle Assessment, and Metal Recovery Methodologies. J. Environ. Manag. 2021, 288, 112358. [Google Scholar] [CrossRef]
- Al-Absi, R.S.; Abu-Dieyeh, M.; Al-Ghouti, M.A. Brine Management Strategies, Technologies, and Recovery Using Adsorption Processes. Environ. Technol. Innov. 2021, 22, 101541. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; Chabib, C.M.; Mustafa, I.; Al Ghaferi, A.; AlMarzooqi, F. Brine Management in Desalination Industry: From Waste to Resources Generation. Desalination 2019, 472, 114187. [Google Scholar] [CrossRef]
- Soliman, M.N.; Guen, F.Z.; Ahmed, S.A.; Saleem, H.; Khalil, M.J.; Zaidi, S.J. Energy Consumption and Environmental Impact Assessment of Desalination Plants and Brine Disposal Strategies. Process Saf. Environ. Prot. 2021, 147, 589–608. [Google Scholar] [CrossRef]
- Kress, N.; Gertner, Y.; Shoham-Frider, E. Seawater Quality at the Brine Discharge Site from Two Mega Size Seawater Reverse Osmosis Desalination Plants in Israel (Eastern Mediterranean). Water Res. 2020, 171, 115402. [Google Scholar] [CrossRef]
- Ogunbiyi, O.; Saththasivam, J.; Al-Masri, D.; Manawi, Y.; Lawler, J.; Zhang, X.; Liu, Z. Sustainable Brine Management from the Perspectives of Water, Energy and Mineral Recovery: A Comprehensive Review. Desalination 2021, 513, 115055. [Google Scholar] [CrossRef]
- Sola, I.; Zarzo, D.; Carratalá, A.; Fernández-Torquemada, Y.; de-la-Ossa-Carretero, J.A.; Del-Pilar-Ruso, Y.; Sánchez-Lizaso, J.L. Review of the Management of Brine Discharges in Spain. Ocean Coast. Manag. 2020, 196, 105301. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.J.; Loizidou, M. Desalination Brine Disposal Methods and Treatment Technologies—A Review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Levitt, J. Water, Radiation, Salt, and Other Stresses; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Mannan, M.; Alhaj, M.; Mabrouk, A.; Desalination, S.A.-G. Examining the Life-Cycle Environmental Impacts of Desalination: A Case Study in the State of Qatar. Desalination 2019, 452, 238–246. [Google Scholar] [CrossRef]
- Abushaban, M. Assessing Bacterial Growth Potential in Seawater Reverse Osmosis Pretreatment: Method Development and Applications; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Stein, S.; Michael, H.A.; Dugan, B. Injection of Desalination Brine into the Saline Part of the Coastal Aquifer; Environmental and Hydrological Implications. Water Res. 2021, 207, 117820. [Google Scholar] [CrossRef]
- Klas, S.; Peretz, Y. Fish Survival in Groundwater-Desalination Concentrate. Aquac. Eng. 2020, 88, 102037. [Google Scholar] [CrossRef]
- Sadhwani Alonso, J.J.; Melián-Martel, N. Environmental Regulations—Inland and Coastal Desalination Case Studies. In Sustainable Desalination Handbook; Butterworth-Heinemann: Oxford, UK, 2018; pp. 403–435. [Google Scholar] [CrossRef]
- Yang Teh, C.; Mori Budiman, P.; Pui Yee Shak, K.; Yeong Wu, T. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Shu, L.; Jegatheesan, V. A Review of the Management and Treatment of Brine Solutions. Environ. Sci. Water Res. Technol. 2017, 3, 625–658. [Google Scholar] [CrossRef]
- Mohamed, F.M.; Alfalous, K.A. The Effectiveness of Activated Silica Derived from Rice Husk in Coagulation Process Compared with Inorganic Coagulants for Wastewater Treatment. Egypt. J. Aquat. Res. 2020, 46, 131–136. [Google Scholar] [CrossRef]
- Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M. Recent Achievements in Polymer Bio-Based Flocculants for Water Treatment. Materials 2020, 13, 3951. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A Review on Industrial Wastewater Treatment via Electrocoagulation Processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Milne, N.A.; O’Reilly, T.; Sanciolo, P.; Ostarcevic, E.; Beighton, M.; Taylor, K.; Mullett, M.; Tarquin, A.J.; Gray, S.R. Chemistry of Silica Scale Mitigation for RO Desalination with Particular Reference to Remote Operations. Water Res. 2014, 65, 107–133. [Google Scholar] [CrossRef]
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine Management Methods: Recent Innovations and Current Status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Boo, C.; Qi, H.; Billinge, I.H.; Shah, K.M.; Fan, H.; Yip, N.Y. Thermomorphic Hydrophilicity Base-Induced Precipitation for Effective Descaling of Hypersaline Brines. ACS ES T Eng. 2021, 1, 1351–1359. [Google Scholar] [CrossRef]
- Xiong, R.; Chen, Q.; Liu, J.; Wei, C. Experimental Study on Seeded Precipitation Assisted Reverse Osmosis for Industrial Wastewater Reuse. J. Water Process Eng. 2017, 20, 78–83. [Google Scholar] [CrossRef]
- Van Houwelingen, G.; Bond, R.; Seacord, T.; Fessler, E. Experiences with Pellet Reactor Softening as Pretreatment for Inland Desalination in the USA. Desalin. Water Treat. 2010, 13, 259–266. [Google Scholar] [CrossRef] [Green Version]
- Azerrad, S.P.; Isaacs, M.; Dosoretz, C.G. Integrated Treatment of Reverse Osmosis Brines Coupling Electrocoagulation with Advanced Oxidation Processes. Chem. Eng. J. 2019, 356, 771–780. [Google Scholar] [CrossRef]
- Justo, A.; González, O.; Aceña, J.; Pérez, S.; Barceló, D.; Sans, C.; Esplugas, S. Pharmaceuticals and Organic Pollution Mitigation in Reclamation Osmosis Brines by UV/H2O2 and Ozone. J. Hazard. Mater. 2013, 263, 268–274. [Google Scholar] [CrossRef]
- Westerhoff, P.; Moon, H.; Minakata, D.; Crittenden, J. Oxidation of Organics in Retentates from Reverse Osmosis Wastewater Reuse Facilities. Water Res. 2009, 43, 3992–3998. [Google Scholar] [CrossRef]
- Pérez, G.; Fernández-Alba, A.R.; Urtiaga, A.M.; Ortiz, I. Electro-Oxidation of Reverse Osmosis Concentrates Generated in Tertiary Water Treatment. Water Res. 2010, 44, 2763–2772. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I. Degradation of Pharmaceutical Compounds in Water by Non-Thermal Plasma Treatment. Water Res. 2015, 81, 124–136. [Google Scholar] [CrossRef]
- Magureanu, M.; Bilea, F.; Bradu, C.; Hong, D. A Review on Non-Thermal Plasma Treatment of Water Contaminated with Antibiotics. J. Hazard. Mater. 2021, 417, 125481. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Qin, L.; Tian, X.; Wang, A.; Zhou, Y.; Meng, L.; Chen, Y. Removal of Refractory Organics in Nanofiltration Concentrates of Municipal Solid Waste Leachate Treatment Plants by Combined Fenton Oxidative-Coagulation with Photo—Fenton Processes. Chemosphere 2016, 146, 442–449. [Google Scholar] [CrossRef]
- Justo, A.; González, Ó.; Aceña, J.; Mita, L.; Casado, M.; Pérez, S.; Piña, B.; Sans, C.; Barceló, D.; Esplugas, S. Application of Bioassay Panel for Assessing the Impact of Advanced Oxidation Processes on the Treatment of Reverse Osmosis Brine. J. Chem. Technol. Biotechnol. 2014, 89, 1168–1174. [Google Scholar] [CrossRef]
- Rodríguez, D.C.; Ramírez, O.; Mesa, G.P. Behavior of Nitrifying and Denitrifying Bacteria in a Sequencing Batch Reactor for the Removal of Ammoniacal Nitrogen and Organic Matter. Desalination 2011, 273, 447–452. [Google Scholar] [CrossRef]
- Ashoori, N.; Teixido, M.; Spahr, S.; LeFevre, G.H.; Sedlak, D.L.; Luthy, R.G. Evaluation of Pilot-Scale Biochar-Amended Woodchip Bioreactors to Remove Nitrate, Metals, and Trace Organic Contaminants from Urban Stormwater Runoff. Water Res. 2019, 154, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Barkle, G.; Stenger, R.; Moorhead, B.; Clague, J. Nitrate Removal and Secondary Effects of a Woodchip Bioreactor for the Treatment of Subsurface Drainage with Dynamic Flows under Pastoral Agriculture. Ecol. Eng. 2020, 148, 105786. [Google Scholar] [CrossRef]
- Zhao, J.; He, Q.; Chen, N.; Peng, T.; Feng, C. Denitrification Behavior in a Woodchip-Packed Bioreactor with Gradient Filling for Nitrate-Contaminated Water Treatment. Biochem. Eng. J. 2020, 154, 107454. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P.C.; Pulkkinen, J.T.; Kiuru, T.; Koskela, J.; Vielma, J. Efficient Water Treatment Achieved in Recirculating Aquaculture System Using Woodchip Denitrification and Slow Sand Filtration. Environ. Sci. Pollut. Res. 2021, 28, 65333–65348. [Google Scholar] [CrossRef]
- Christianson, L.E.; Lepine, C.; Sibrell, P.L.; Penn, C.; Summerfelt, S.T. Denitrifying Woodchip Bioreactor and Phosphorus Filter Pairing to Minimize Pollution Swapping. Water Res. 2017, 121, 129–139. [Google Scholar] [CrossRef]
- Díaz-García, C.; Martínez-Sánchez, J.J.; Maxwell, B.M.; Franco, J.A.; Álvarez-Rogel, J. Woodchip Bioreactors Provide Sustained Denitrification of Brine from Groundwater Desalination Plants. J. Environ. Manag. 2021, 289, 112521. [Google Scholar] [CrossRef]
- Tong, T.; Elimelech, M. The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef]
- Suwaileh, W.; Pathak, N.; Shon, H.; Hilal, N. Forward Osmosis Membranes and Processes: A Comprehensive Review of Research Trends and Future Outlook. Desalination 2020, 485, 114455. [Google Scholar] [CrossRef]
- Kim, Y.; Woo, Y.C.; Phuntsho, S.; Nghiem, L.D.; Shon, H.K.; Hong, S. Evaluation of Fertilizer-Drawn Forward Osmosis for Coal Seam Gas Reverse Osmosis Brine Treatment and Sustainable Agricultural Reuse. J. Memb. Sci. 2017, 537, 22–31. [Google Scholar] [CrossRef] [Green Version]
- McGinnis, R.L.; Hancock, N.T.; Nowosielski-Slepowron, M.S.; McGurgan, G.D. Pilot Demonstration of the NH3/CO2 Forward Osmosis Desalination Process on High Salinity Brines. Desalination 2013, 312, 67–74. [Google Scholar] [CrossRef]
- Martinetti, C.R.; Childress, A.E.; Cath, T.Y. High Recovery of Concentrated RO Brines Using Forward Osmosis and Membrane Distillation. J. Memb. Sci. 2009, 331, 31–39. [Google Scholar] [CrossRef]
- El Zayat, H.; Nasr, P.; Sewilam, H. Investigating Sustainable Management of Desalination Brine through Concentration Using Forward Osmosis. Environ. Sci. Pollut. Res. 2021, 28, 39938–39951. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, Y.; Kim, A.S.; Hong, S. Evaluation of Membrane-Based Desalting Processes for RO Brine Treatment. Desalin. Water Treat. 2016, 57, 7432–7439. [Google Scholar] [CrossRef]
- Tang, W.; Ng, H.Y. Concentration of Brine by Forward Osmosis: Performance and Influence of Membrane Structure. Desalination 2008, 224, 143–153. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Song, X.; Yu, J. Forward Osmosis for Multi-Effect Distillation Brine Treatment: Performance and Concentration Polarization Evaluation. Can. J. Chem. Eng. 2021, 99, S114–S125. [Google Scholar] [CrossRef]
- Khazaie, F.; Shokrollahzadeh, S.; Bide, Y.; Sheshmani, S.; Shahvelayati, A.S. High-Flux Sodium Alginate Sulfate Draw Solution for Water Recovery from Saline Waters and Wastewaters via Forward Osmosis. Chem. Eng. J. 2021, 417, 129250. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X. Volume Reduction and Water Reclamation of Reverse Osmosis Concentrate from Coal Chemical Industry by Forward Osmosis with an Osmotic Backwash Strategy. Water Sci. Technol. 2020, 81, 2674–2684. [Google Scholar] [CrossRef]
- Abdul Wahid, R.; Ang, W.L.; Mohammad, A.W.; Johnson, D.J.; Hilal, N. Evaluating Fertilizer-Drawn Forward Osmosis Performance in Treating Anaerobic Palm Oil Mill Effluent. Membranes 2021, 11, 566. [Google Scholar] [CrossRef]
- Iskander, S.M.; Zou, S.; Brazil, B.; Novak, J.T.; He, Z. Energy Consumption by Forward Osmosis Treatment of Landfill Leachate for Water Recovery. Waste Manag. 2017, 63, 284–291. [Google Scholar] [CrossRef]
- Choi, Y.; Cho, H.; Shin, Y.; Jang, Y.; Lee, S. Economic Evaluation of a Hybrid Desalination System Combining Forward and Reverse Osmosis. Membranes 2016, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.; He, Z. Enhancing Wastewater Reuse by Forward Osmosis with Self-Diluted Commercial Fertilizers as Draw Solutes. Water Res. 2016, 99, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Gulied, M.; Al Momani, F.; Khraisheh, M.; Bhosale, R.; AlNouss, A. Influence of Draw Solution Type and Properties on the Performance of Forward Osmosis Process: Energy Consumption and Sustainable Water Reuse. Chemosphere 2019, 233, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, Y.; Zhang, Z.; Xu, T. Electrodialysis of Concentrated Brine from RO Plant to Produce Coarse Salt and Freshwater. J. Memb. Sci. 2014, 450, 323–330. [Google Scholar] [CrossRef]
- Korngold, E.; Aronov, L.; Daltrophe, N. Electrodialysis of Brine Solutions Discharged from an RO Plant. Desalination 2009, 242, 215–227. [Google Scholar] [CrossRef]
- Al-Anzi, B.S.; Al-Rashidi, A.; Abraham, L.; Fernandes, J.; Al-Sheikh, A.; Alhazza, A. Brine Management from Desalination Plants for Salt Production Utilizing High Current Density Electrodialysis-Evaporator Hybrid System: A Case Study in Kuwait. Desalination 2021, 498, 114760. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghyselbrecht, K.; Meesschaert, B.; Pinoy, L.; Van der Bruggen, B. Electrodialysis on RO Concentrate to Improve Water Recovery in Wastewater Reclamation. J. Memb. Sci. 2011, 378, 101–110. [Google Scholar] [CrossRef]
- Zhao, D.; Lee, L.Y.; Ong, S.L.; Chowdhury, P.; Siah, K.B.; Ng, H.Y. Electrodialysis Reversal for Industrial Reverse Osmosis Brine Treatment. Sep. Purif. Technol. 2019, 213, 339–347. [Google Scholar] [CrossRef]
- Du, C.; Runhong Du, J.; Zhao, X.; Cheng, F.; Ali, M.E.; Feng, X. Treatment of Brackish Water RO Brine via Bipolar Membrane Electrodialysis. Ind. Eng. Chem. Res. 2021, 60, 3115–3129. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Song, X.; Yu, J. Separation of mono- and di-valent ions from seawater reverse osmosis brine using selective electrodialysis. Environ. Sci. Pollut. Res. 2021, 28, 18754–18767. [Google Scholar] [CrossRef]
- Choi, Y.; Naidu, G.; Nghiem, L.D.; Lee, S.; Vigneswaran, S. Membrane distillation crystallization for brine mining and zero liquid discharge: Opportunities, challenges, and recent progress. Environ. Sci. Water Res. Technol. 2019, 5, 1202–1221. [Google Scholar] [CrossRef]
- Francis, L.; Ahmed, F.E.; Hilal, N. Electrospun Membranes for Membrane Distillation: The State of Play and Recent Advances. Desalination 2022, 526, 115511. [Google Scholar] [CrossRef]
- Abdallah, H.; El-Gendi, A.; Khedr, M.; El-Zanati, E. Hydrophobic Polyethersulfone Porous Membranes for Membrane Distillation. Front. Chem. Sci. Eng. 2015, 9, 84–93. [Google Scholar] [CrossRef]
- Gryta, M. The Application of Polypropylene Membranes for Production of Fresh Water from Brines by Membrane Distillation. Chem. Pap. 2017, 71, 775–784. [Google Scholar] [CrossRef]
- Mansour, S.; Giwa, A.; Hasan, S.W. Novel graphene nanoplatelets-coated polyethylene membrane for the treatment of reject brine by pilot-scale direct contact membrane distillation: An optimization study. Desalination 2018, 441, 9–20. [Google Scholar] [CrossRef]
- Gryta, M. The Study of Performance of Polyethylene Chlorinetrifluoroethylene Membranes Used for Brine Desalination by Membrane Distillation. Desalination 2016, 398, 52–63. [Google Scholar] [CrossRef]
- Mohd Ramli, M.R.; Ahmad, A.L.; Leo, C.P. Surface modification of polytetrafluoroethylene hollow fiber membrane for direct contact membrane distillation through low-density polyethylene solution coating. ACS Omega 2021, 6, 4609–4618. [Google Scholar] [CrossRef]
- Li, J.; Guan, Y.; Cheng, F.; Liu, Y. Treatment of high salinity brines by direct contact membrane distillation: Effect of membrane characteristics and salinity. Chemosphere 2015, 140, 143–149. [Google Scholar] [CrossRef]
- Sanmartino, J.A.; Khayet, M.; García-Payo, M.C.; El-Bakouri, H.; Riaza, A. Treatment of Reverse Osmosis Brine by Direct Contact Membrane Distillation: Chemical Pretreatment Approach. Desalination 2017, 420, 79–90. [Google Scholar] [CrossRef]
- Woo, Y.C.; Kim, Y.; Shim, W.G.; Tijing, L.D.; Yao, M.; Nghiem, L.D.; Choi, J.S.; Kim, S.H.; Shon, H.K. Graphene/PVDF Flat-Sheet Membrane for the Treatment of RO Brine from Coal Seam Gas Produced Water by Air Gap Membrane Distillation. J. Memb. Sci. 2016, 513, 74–84. [Google Scholar] [CrossRef]
- Zheng, G.; Yao, L.; You, X.; Liao, Y.; Wang, R.; Huang, J.J. Effects of Different Secondary Nano-Scaled Roughness on the Properties of Omniphobic Membranes for Brine Treatment Using Membrane Distillation. J. Memb. Sci. 2021, 620, 118918. [Google Scholar] [CrossRef]
- Kharraz, J.A.; Bilad, M.R.; Arafat, H.A. Flux Stabilization in Membrane Distillation Desalination of Seawater and Brine Using Corrugated PVDF Membranes. J. Memb. Sci. 2015, 495, 404–414. [Google Scholar] [CrossRef]
- Yao, M.; Tijing, L.D.; Naidu, G.; Kim, S.H.; Matsuyama, H.; Fane, A.G.; Shon, H.K. A Review of Membrane Wettability for the Treatment of Saline Water Deploying Membrane Distillation. Desalination 2020, 479, 114312. [Google Scholar] [CrossRef]
- Geng, H.; Wang, J.; Zhang, C.; Li, P.; Chang, H. High Water Recovery of RO Brine Using Multi-Stage Air Gap Membrane Distillation. Desalination 2015, 355, 178–185. [Google Scholar] [CrossRef]
- Cerda, A.; Quilaqueo, M.; Barros, L.; Seriche, G.; Gim-Krumm, M.; Santoro, S.; Avci, A.H.; Romero, J.; Curcio, E.; Estay, H. Recovering water from lithium-rich brines by a fractionation process based on membrane distillation-crystallization. J. Water Process Eng. 2021, 41, 102063. [Google Scholar] [CrossRef]
- Subramani, A.; Jacangelo, J.G. Treatment technologies for reverse osmosis concentrate volume minimization: A review. Sep. Purif. Technol. 2014, 122, 472–489. [Google Scholar] [CrossRef]
- Rahardianto, A.; Gao, J.; Gabelich, C.J.; Williams, M.D.; Cohen, Y. High Recovery Membrane Desalting of Low-Salinity Brackish Water: Integration of Accelerated Precipitation Softening with Membrane RO. J. Memb. Sci. 2007, 289, 123–137. [Google Scholar] [CrossRef]
- Subramani, A.; Schlicher, R.; Long, J.; Yu, J.; Lehman, S.; Jacangelo, J.G. Recovery optimization of membrane processes for treatment of produced water with high silica content. Desalin. Water Treat. 2011, 36, 297–309. [Google Scholar] [CrossRef]
- Gabelich, C.J.; Rahardianto, A.; Northrup, C.R.; Yun, T.I.; Cohen, Y. Process evaluation of intermediate chemical demineralization for water recovery enhancement in production-scale brackish water desalting. Desalination 2011, 272, 36–45. [Google Scholar] [CrossRef]
- Rioyo, J.; Aravinthan, V.; Bundschuh, J.; Lynch, M. Research on ‘High-PH Precipitation Treatment’ for RO concentrate minimization and salt recovery in a municipal groundwater desalination facility. Desalination 2018, 439, 168–178. [Google Scholar] [CrossRef]
- Pervov, A.G. Precipitation of calcium carbonate in reverse osmosis retentate flow by means of seeded techniques—A tool to increase recovery. Desalination 2015, 368, 140–151. [Google Scholar] [CrossRef]
- Murray-Gulde, C.; Heatley, J.E.; Karanfil, T.; Rodgers, J.H.; Myers, J.E. Performance of a hybrid reverse osmosis-constructed wetland treatment system for brackish oil field produced water. Water Res. 2003, 37, 705–713. [Google Scholar] [CrossRef]
- Dong, H.; German, M.; Tian, L.; SenGupta, A.K. Multifunctional Ion Exchange Pretreatment Driven by Carbon Dioxide for Enhancing Reverse Osmosis Recovery during Impaired Water Reuse. Desalination 2020, 485, 114459. [Google Scholar] [CrossRef]
- McGovern, R.K.; Zubair, S.M.; Lienhard, V.J.H. Hybrid Electrodialysis Reverse Osmosis System Design and Its Optimization for Treatment of Highly Saline Brines. IDA J. Desalin. Water Reuse 2014, 6, 15–23. [Google Scholar] [CrossRef]
- Loganathan, K.; Chelme-Ayala, P.; Gamal El-Din, M. Treatment of Basal Water Using a Hybrid Electrodialysis Reversal–Reverse Osmosis System Combined with a Low-Temperature Crystallizer for near-Zero Liquid Discharge. Desalination 2015, 363, 92–98. [Google Scholar] [CrossRef]
- Turek, M.; Mitko, K.; Laskowska, E.; Chorążewska, M.; Piotrowski, K.; Jakóbik-Kolon, A.; Dydo, P. Energy consumption and gypsum scaling assessment in a hybrid nanofiltration-reverse osmosis-electrodialysis system. Chem. Eng. Technol. 2018, 41, 392–400. [Google Scholar] [CrossRef]
- Ning, R.Y.; Tarquin, A.; Trzcinski, M.; Patwardhan, G. Recovery Optimization of RO Concentrate from Desert Wells. Desalination 2006, 201, 315–322. [Google Scholar] [CrossRef]
- Ning, R.Y.; Tarquin, A.J. Crystallization of Salts from Super-Concentrate Produced by Tandem RO Process. Desalin. Water Treat. 2010, 16, 238–242. [Google Scholar] [CrossRef]
- Lu, J.; You, S.; Wang, X. Forward osmosis coupled with lime-soda ash softening for volume minimization of reverse osmosis concentrate and CaCO3 recovery: A case study on the coal chemical industry. Front. Environ. Sci. Eng. 2020, 15, 9. [Google Scholar] [CrossRef]
- McCool, B.C.; Rahardianto, A.; Faria, J.I.; Cohen, Y. Evaluation of chemically-enhanced seeded precipitation of RO concentrate for high recovery desalting of high salinity brackish water. Desalination 2013, 317, 116–126. [Google Scholar] [CrossRef]
- Rom, I.; Klas, S. Kinetics of CaCO3 precipitation in seeded aeration softening of brackish water desalination concentrate. Chemosphere 2020, 260, 127527. [Google Scholar] [CrossRef]
- Juby, G.; Eastern Municipal Water District. Evaluation and Selection of Available Processes for a Zero-Liquid Discharge System for the Perris, California, Ground Water Basin; Desalination and Water Purification Research and Development Program Report; U.S. Department of the Interior, Bureau of Reclamation, Technical Service Center, Water and Environmental Services Division, Water Treatment Engineering Research Team: Perris, CA, USA, 2008. [Google Scholar]
- Ronquim, F.M.; Sakamoto, H.M.; Mierzwa, J.C.; Kulay, L.; Seckler, M.M. Eco-Efficiency Analysis of Desalination by Precipitation Integrated with Reverse Osmosis for Zero Liquid Discharge in Oil Refineries. J. Clean. Prod. 2020, 250, 119547. [Google Scholar] [CrossRef]
- Al Abdulgader, H.; Kochkodan, V.; Hilal, N. Hybrid Ion Exchange—Pressure Driven Membrane Processes in Water Treatment: A Review. Sep. Purif. Technol. 2013, 116, 253–264. [Google Scholar] [CrossRef]
- Liu, Z.; Haddad, M.; Sauvé, S.; Barbeau, B. Alleviating the Burden of Ion Exchange Brine in Water Treatment: From Operational Strategies to Brine Management. Water Res. 2021, 205, 117728. [Google Scholar] [CrossRef] [PubMed]
- Indarawis, K.A.; Boyer, T.H. Superposition of Anion and Cation Exchange for Removal of Natural Water Ions. Sep. Purif. Technol. 2013, 118, 112–119. [Google Scholar] [CrossRef]
- Jun, L.; McClain, B.; Seah, A.; Mukhopadhyay, D. Semiconductors: High-Purity Water System Upgrade in Singapore Using High-Efficiency RO. Ultrapure Water 2004, 21, 27–32. [Google Scholar]
- Chen, Y.; Baygents, J.C.; Farrell, J. Evaluating Electrocoagulation and Chemical Coagulation for Removing Dissolved Silica from High Efficiency Reverse Osmosis (HERO) Concentrate Solutions. J. Water Process Eng. 2017, 16, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, A.; Wankat, P.C. Desalination of the colorado river water: A hybrid approach. Desalination 2012, 286, 176–186. [Google Scholar] [CrossRef]
- Vanoppen, M.; Stoffels, G.; Demuytere, C.; Bleyaert, W.; Verliefde, A.R.D. Increasing RO efficiency by chemical-free ion-exchange and donnan dialysis: Principles and practical implications. Water Res. 2015, 80, 59–70. [Google Scholar] [CrossRef]
- Vermeulen, T.; Tleimat, B.W.; Klein, G. Ion-exchange pretreatment for scale prevention in desalting systems. Desalination 1983, 47, 149–159. [Google Scholar] [CrossRef]
- Semblante, G.U.; Lee, J.Z.; Lee, L.Y.; Ong, S.L.; Ng, H.Y. Brine pre-treatment technologies for zero liquid discharge systems. Desalination 2018, 441, 96–111. [Google Scholar] [CrossRef]
- McGovern, R.K.; Zubair, S.M.; Lienhard, V.J.H. The benefits of hybridising electrodialysis with reverse osmosis. J. Memb. Sci. 2014, 469, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Hybrid technologies: The future of energy efficient desalination—A review. Desalination 2020, 495, 114659. [Google Scholar] [CrossRef]
- Li, W.; Krantz, W.B.; Cornelissen, E.R.; Post, J.W.; Verliefde, A.R.D.; Tang, C.Y. A novel hybrid process of reverse electrodialysis and reverse osmosis for low energy seawater desalination and brine management. Appl. Energy 2013, 104, 592–602. [Google Scholar] [CrossRef]
- Man, M.; Sparrow, B.; Low, M. A Cradle to Grave Treatment Solution for Mine Waters. In Proceedings of the 11th ICARD|IMWA|MWD Conference–“Risk to Opportunity”, Pretoria, South Africa, 10–14 September 2018. [Google Scholar]
- Mitko, K.; Noszczyk, A.; Dydo, P.; Turek, M. Electrodialysis of Coal Mine Water. Water Resour. Ind. 2021, 25, 100143. [Google Scholar] [CrossRef]
- Tufa, R.A.; Curcio, E.; Brauns, E.; van Baak, W.; Fontananova, E.; Di Profio, G. Membrane distillation and reverse electrodialysis for near-zero liquid discharge and low energy seawater desalination. J. Memb. Sci. 2015, 496, 325–333. [Google Scholar] [CrossRef]
- Tufa, R.A.; Noviello, Y.; Di Profio, G.; Macedonio, F.; Ali, A.; Drioli, E.; Fontananova, E.; Bouzek, K.; Curcio, E. Integrated membrane distillation-reverse electrodialysis system for energy-efficient seawater desalination. Appl. Energy 2019, 253, 113551. [Google Scholar] [CrossRef]
- Kumar, A.; Naidu, G.; Fukuda, H.; Du, F.; Vigneswaran, S.; Drioli, E.; Lienhard, J.H. Metals recovery from seawater desalination brines: Technologies, opportunities, and challenges. ACS Sustain. Chem. Eng. 2021, 9, 7704–7712. [Google Scholar] [CrossRef]
- Diallo, M.S.; Rao Kotte, M.; Cho, M. Mining critical metals and elements from seawater: Opportunities and challenges. Environ. Sci. Technol. 2015, 49, 9390–9399. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Zhang, Y.; Jegatheesan, V. A review of resource recovery from seawater desalination brine. Rev. Environ. Sci. Bio/Technol. 2021, 20, 333–361. [Google Scholar] [CrossRef]
- Chitrakar, R.; Makita, Y.; Ooi, K.; Sonoda, A. Synthesis of iron-doped manganese oxides with an ion-sieve property: Lithium adsorption from bolivian brine. Ind. Eng. Chem. Res. 2014, 53, 3682–3688. [Google Scholar] [CrossRef]
- Gu, D.; Sun, W.; Han, G.; Cui, Q.; Wang, H. Lithium Ion Sieve Synthesized via an Improved Solid State Method and Adsorption Performance for West Taijinar Salt Lake Brine. Chem. Eng. J. 2018, 350, 474–483. [Google Scholar] [CrossRef]
- Jin, W.; Hu, M.; Sun, Z.; Huang, C.H.; Zhao, H. Simultaneous and Precise Recovery of Lithium and Boron from Salt Lake Brine by Capacitive Deionization with Oxygen Vacancy-Rich CoP/Co3O4-Graphene Aerogel. Chem. Eng. J. 2021, 420, 127661. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Y.; Yu, J. Application of Concentration-Dependent HSDM to the Lithium Adsorption from Brine in Fixed Bed Columns. Sep. Purif. Technol. 2020, 241, 116682. [Google Scholar] [CrossRef]
- Lai, X.; Yuan, Y.; Chen, Z.; Peng, J.; Sun, H.; Zhong, H. Adsorption–desorption properties of granular EP/HMO composite and its application in lithium recovery from brine. Ind. Eng. Chem. Res. 2020, 59, 7913–7925. [Google Scholar] [CrossRef]
- Liu, C.; Tao, B.; Wang, Z.; Wang, D.; Guo, R.; Chen, L. Preparation and characterization of lithium ion sieves embedded in a hydroxyethyl cellulose cryogel for the continuous recovery of lithium from brine and seawater. Chem. Eng. Sci. 2021, 229, 115984. [Google Scholar] [CrossRef]
- Luo, Q.; Dong, M.; Nie, G.; Liu, Z.; Wu, Z.; Li, J. Extraction of Lithium from Salt Lake Brines by Granulated Adsorbents. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 628, 127256. [Google Scholar] [CrossRef]
- Ohashi, F.; Tai, Y. Lithium Adsorption from natural brine using surface-modified manganese oxide adsorbents. Mater. Lett. 2019, 251, 214–217. [Google Scholar] [CrossRef]
- Ooi, K.; Sonoda, A.; Makita, Y.; Chitrakar, R.; Tasaki-Handa, Y.; Nakazato, T. Recovery of lithium from salt-brine eluates by direct crystallization as lithium sulfate. Hydrometallurgy 2017, 174, 123–130. [Google Scholar] [CrossRef]
- Nie, X.Y.; Sun, S.Y.; Song, X.; Yu, J.G. Further Investigation into Lithium Recovery from Salt Lake Brines with Different Feed Characteristics by Electrodialysis. J. Memb. Sci. 2017, 530, 185–191. [Google Scholar] [CrossRef]
- Ji, P.Y.; Ji, Z.Y.; Chen, Q.B.; Liu, J.; Zhao, Y.Y.; Wang, S.Z.; Li, F.; Yuan, J.S. Effect of coexisting ions on recovering lithium from high Mg2+/Li+ Ratio Brines by Selective-Electrodialysis. Sep. Purif. Technol. 2018, 207, 1–11. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, G.; Jia, H.; He, L. Sandwiched Liquid-Membrane Electrodialysis: Lithium Selective Recovery from Salt Lake Brines with High Mg/Li Ratio. J. Memb. Sci. 2020, 596, 117685. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiang, X.; Wang, M.; Wang, H.; Li, Y.; Li, J.; Yang, H. Preparation of LiOH through BMED Process from Lithium-Containing Solutions: Effects of Coexisting Ions and Competition between Na+ and Li+. Desalination 2021, 512, 115126. [Google Scholar] [CrossRef]
- Cha-umpong, W.; Li, Q.; Razmjou, A.; Chen, V. Concentrating Brine for Lithium Recovery Using GO Composite Pervaporation Membranes. Desalination 2021, 500, 114894. [Google Scholar] [CrossRef]
- Ma, X.; Li, M.; Feng, C.; He, Z. Electrochemical Nitrate Removal with Simultaneous Magnesium Recovery from a Mimicked RO Brine Assisted by in Situ Chloride Ions. J. Hazard. Mater. 2020, 388, 122085. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Ryu, S.; Naidu, G.; Lee, S.; Vigneswaran, S. Integrated submerged membrane distillation-adsorption system for rubidium recovery. Sep. Purif. Technol. 2019, 218, 146–155. [Google Scholar] [CrossRef]
- Naidu, G.; Loganathan, P.; Jeong, S.; Johir, M.A.H.; To, V.H.P.; Kandasamy, J.; Vigneswaran, S. Rubidium Extraction Using an Organic Polymer Encapsulated Potassium Copper Hexacyanoferrate Sorbent. Chem. Eng. J. 2016, 306, 31–42. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Fang, D.; Ye, X.; Hu, Y.; Tan, X.; Liu, H.; Wu, Z. Novel Biomass-Derived Adsorbents Grafted Sodium Titanium Silicate with High Adsorption Capacity for Rb+ and Cs+ in the Brine. ChemistrySelect 2019, 4, 13630–13637. [Google Scholar] [CrossRef]
- Randall, D.G.; Nathoo, J. Resource Recovery by Freezing: A Thermodynamic Comparison between a Reverse Osmosis Brine, Seawater and Stored Urine. J. Water Process Eng. 2018, 26, 242–249. [Google Scholar] [CrossRef]
- Callura, J.C.; Perkins, K.M.; Noack, C.W.; Washburn, N.R.; Dzombak, D.A.; Karamalidis, A.K. Selective Adsorption of Rare Earth Elements onto Functionalized Silica Particles. Green Chem. 2018, 20, 1515–1526. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, Y.; Shi, L.; Li, H.; Li, R.; Hong, S.; Zhuo, S.; Zhang, T.; Wang, P. Designing a next generation solar crystallizer for real seawater brine treatment with zero liquid discharge. Nat. Commun. 2021, 12, 998. [Google Scholar] [CrossRef]
- Carson, R.C.; Simandl, J. Kinetics of magnesium hydroxide precipitation from seawater using slaked dolomite. Miner. Eng. 1994, 7, 511–517. [Google Scholar] [CrossRef]
- Dong, H.; Unluer, C.; Yang, E.H.; Al-Tabbaa, A. Recovery of Reactive MgO from Reject Brine via the Addition of NaOH. Desalination 2018, 429, 88–95. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z.; He, L.; Zhao, Z. Facet Engineered Li3PO4 for Lithium Recovery from Brines. Desalination 2021, 514, 115186. [Google Scholar] [CrossRef]
- Na, H.; Kim, M. Determination of Optimal Conditions for Magnesium Recovery Process from Seawater Desalination Brine Using Paper Sludge Ash, Sulfuric Acid, and Ethanol. Desalin. WATER Treat. 2019, 157, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Naidu, G.; Jeong, S.; Choi, Y.; Song, M.H.; Oyunchuluun, U.; Vigneswaran, S. Valuable Rubidium Extraction from Potassium Reduced Seawater Brine. J. Clean. Prod. 2018, 174, 1079–1088. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Zhang, Y.; Dou, X.; Wu, X.; Yang, M. Granulation of Fe–Al–Ce Trimetal Hydroxide as a Fluoride Adsorbent Using the Extrusion Method. Chem. Eng. J. 2012, 185–186, 211–218. [Google Scholar] [CrossRef]
- Zhong, J.; Lin, S.; Yu, J. Lithium Recovery from Ultrahigh Mg2+/Li+ Ratio Brine Using a Novel Granulated Li/Al-LDHs Adsorbent. Sep. Purif. Technol. 2021, 256, 117780. [Google Scholar] [CrossRef]
- Zavahir, S.; Elmakki, T.; Gulied, M.; Ahmad, Z.; Al-Sulaiti, L.; Shon, H.K.; Chen, Y.; Park, H.; Batchelor, B.; Han, D.S. A review on lithium recovery using electrochemical capturing systems. Desalination 2021, 500, 114883. [Google Scholar] [CrossRef]
- Wei, S.; Wei, Y.; Chen, T.; Liu, C.; Tang, Y. Porous lithium ion sieves nanofibers: General synthesis strategy and highly selective recovery of lithium from brine water. Chem. Eng. J. 2020, 379, 122407. [Google Scholar] [CrossRef]
- Hasan, M.; Rotich, N.; John, M.; Louhi-Kultanen, M. Salt recovery from wastewater by air-cooled eutectic freeze crystallization. Chem. Eng. J. 2017, 326, 192–200. [Google Scholar] [CrossRef]
- Ahmed, M.; Arakel, A.; Hoey, D.; Thumarukudy, M.R.; Goosen, M.F.A.; Al-Haddabi, M.; Al-Belushi, A. Feasibility of Salt Production from Inland RO Desalination Plant Reject Brine: A Case Study. Desalination 2003, 158, 109–117. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.; Shin, S.; Kim, G. Production of High-Purity MgSO4 from Seawater Desalination Brine. Desalination 2021, 518, 115288. [Google Scholar] [CrossRef]
- Landsman, M.R.; Lawler, D.F.; Katz, L.E. Application of electrodialysis pretreatment to enhance boron removal and reduce fouling during desalination by nanofiltration/reverse osmosis. Desalination 2020, 491, 114563. [Google Scholar] [CrossRef]
- Liu, E.; Lee, L.Y.; Ong, S.L.; Ng, H.Y. Treatment of Industrial Brine Using Capacitive Deionization (CDI) towards Zero Liquid Discharge—Challenges and Optimization. Water Res. 2020, 183, 116059. [Google Scholar] [CrossRef]
- Choi, Y.; Naidu, G.; Lee, S.; Vigneswaran, S. Recovery of Sodium Sulfate from Seawater Brine Using Fractional Submerged Membrane Distillation Crystallizer. Chemosphere 2020, 238, 124641. [Google Scholar] [CrossRef]
- Kim, D.H. A review of desalting process techniques and economic analysis of the recovery of salts from retentates. Desalination 2011, 270, 1–8. [Google Scholar] [CrossRef]
- Casas, S.; Aladjem, C.; Larrotcha, E.; Gibert, O.; Valderrama, C.; Cortina, J.L. Valorisation of Ca and Mg By-Products from Mining and Seawater Desalination Brines for Water Treatment Applications. J. Chem. Technol. Biotechnol. 2014, 89, 872–883. [Google Scholar] [CrossRef]
- Oren, Y.; Korngold, E.; Daltrophe, N.; Messalem, R.; Volkman, Y.; Aronov, L.; Weismann, M.; Bouriakov, N.; Glueckstern, P.; Gilron, J. Pilot Studies on High Recovery BWRO-EDR for near Zero Liquid Discharge Approach. Desalination 2010, 261, 321–330. [Google Scholar] [CrossRef]
- Dindi, A.; Quang, D.V.; AlNashef, I.; Abu-Zahra, M.R.M. A Process for Combined CO2 Utilization and Treatment of Desalination Reject Brine. Desalination 2018, 442, 62–74. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Mohammad, A.F.; Suleiman, M.I.; Al Musharfy, M.; Al-Marzouqi, A.H. A New Process for the Capture of CO2 and Reduction of Water Salinity. Desalination 2017, 411, 69–75. [Google Scholar] [CrossRef]
- Viet Quang, D.; Dindi, A.; Zahra, M. The Utilization of CO2, Alkaline Solid Waste, and Desalination Reject Brine in Soda Ash Production. In CO2 Separation, Purification and Conversion to Chemicals and Fuels; Springer: Singapore, 2019; pp. 153–184. ISBN 978-981-13-3295-1. [Google Scholar]
- Mustafa, J.; Mourad, A.A.H.I.; Al-Marzouqi, A.H.; El-Naas, M.H. Simultaneous Treatment of Reject Brine and Capture of Carbon Dioxide: A Comprehensive Review. Desalination 2020, 483, 114386. [Google Scholar] [CrossRef]
- De Carvalho Pinto, P.C.; de Oliveira Carvalho, M.M.; Linhares, F.M.; da Silva, T.R.; de Lima, G.M. A Cleaner Production of Sodium Hydrogen Carbonate: Partial Replacement of Lime by Steel Slag Milk in the Ammonia Recovery Step of the Solvay Process. Clean Technol. Environ. Policy 2015, 17, 2311–2321. [Google Scholar] [CrossRef]
- He, L.; Yu, D.; Lv, W.; Wu, J.; Xu, M. A Novel Method for CO2 Sequestration via Indirect Carbonation of Coal Fly Ash. Ind. Eng. Chem. Res. 2013, 52, 15138–15145. [Google Scholar] [CrossRef]
- Skilhagen, S.E. Osmotic Power—A New, Renewable Energy Source. Desalin. Water Treat. 2010, 15, 271–278. [Google Scholar] [CrossRef]
- Saito, K.; Irie, M.; Zaitsu, S.; Sakai, H.; Hayashi, H.; Tanioka, A. Power Generation with Salinity Gradient by Pressure Retarded Osmosis Using Concentrated Brine from SWRO System and Treated Sewage as Pure Water. Desalin. Water Treat. 2012, 41, 114–121. [Google Scholar] [CrossRef]
- Akram, W.; Sharqawy, M.H.; Lienhard, J.H. Energy Utilization of Brine from An Msf Desalination Plant by Pressure Retarded Osmosis. In Proceedings of the International Desalination Association World Congress on Desalination and Water Reuse, Tianjin, China, 20–25 October 2013. [Google Scholar]
- Kim, Y.C.; Elimelech, M. Potential of Osmotic Power Generation by Pressure Retarded Osmosis Using Seawater as Feed Solution: Analysis and Experiments. J. Memb. Sci. 2013, 429, 330–337. [Google Scholar] [CrossRef]
- Straub, A.P.; Yip, N.Y.; Elimelech, M. Raising the Bar: Increased hydraulic pressure allows unprecedented high power densities in pressure-retarded osmosis. Environ. Sci. Technol. Lett. 2014, 1, 55–59. [Google Scholar] [CrossRef]
- Achilli, A.; Prante, J.L.; Hancock, N.T.; Maxwell, E.B.; Childress, A.E. Experimental Results from RO-PRO: A Next Generation System for Low-Energy Desalination. Environ. Sci. Technol. 2014, 48, 6437–6443. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse osmosis desalination: Water sources, technology, and today’s challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Liyanaarachchi, S.; Shu, L.; Muthukumaran, S.; Jegatheesan, V.; Baskaran, K. Problems in Seawater Industrial Desalination Processes and Potential Sustainable Solutions: A Review. Rev. Environ. Sci. Bio/Technol. 2014, 13, 203–214. [Google Scholar] [CrossRef]
- Peñate, B.; García-Rodríguez, L. Current Trends and Future Prospects in the Design of Seawater Reverse Osmosis Desalination Technology. Desalination 2012, 284, 1–8. [Google Scholar] [CrossRef]
- Wan, C.F.; Chung, T.S. Energy Recovery by Pressure Retarded Osmosis (PRO) in SWRO–PRO Integrated Processes. Appl. Energy 2016, 162, 687–698. [Google Scholar] [CrossRef]
- Benjamin, J.; Arias, M.E.; Zhang, Q. A Techno-Economic Process Model for Pressure Retarded Osmosis Based Energy Recovery in Desalination Plants. Desalination 2020, 476, 114218. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Al-Othman, A.; Abdelwahab, N.; Alami, A.H.; Olabi, A.G. Recent Developments in Pressure Retarded Osmosis for Desalination and Power Generation. Renew. Sustain. Energy Rev. 2021, 138, 110492. [Google Scholar] [CrossRef]
- Daniilidis, A.; Vermaas, D.A.; Herber, R.; Nijmeijer, K. Experimentally Obtainable Energy from Mixing River Water, Seawater or Brines with Reverse Electrodialysis. Renew. Energy 2014, 64, 123–131. [Google Scholar] [CrossRef]
- Kwon, K.; Han, J.; Park, B.H.; Shin, Y.; Kim, D. Brine Recovery Using Reverse Electrodialysis in Membrane-Based Desalination Processes. Desalination 2015, 362, 1–10. [Google Scholar] [CrossRef]
- Tedesco, M.; Scalici, C.; Vaccari, D.; Cipollina, A.; Tamburini, A.; Micale, G. Performance of the First Reverse Electrodialysis Pilot Plant for Power Production from Saline Waters and Concentrated Brines. J. Memb. Sci. 2016, 500, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Mehdizadeh, S.; Yasukawa, M.; Abo, T.; Kuno, M.; Noguchi, Y.; Higa, M. The Effect of Feed Solution Temperature on the Power Output Performance of a Pilot-Scale Reverse Electrodialysis (RED) System with Different Intermediate Distance. Membranes 2019, 9, 73. [Google Scholar] [CrossRef] [Green Version]
- Hulme, A.M.; Davey, C.J.; Tyrrel, S.; Pidou, M.; McAdam, E.J. Scale-up of Reverse Electrodialysis for Energy Generation from High Concentration Salinity Gradients. J. Memb. Sci. 2021, 627, 119245. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; Van Der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the Science and Technology of Water Desalination by Capacitive Deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.; Park, H.; Yeo, J.; Yang, S.; Cho, C.H.; Han, M.H.; Kim, D.K. Desalination via a New Membrane Capacitive Deionization Process Utilizing Flow-Electrodes. Energy Environ. Sci. 2013, 6, 1471–1475. [Google Scholar] [CrossRef]
- Porada, S.; Weingarth, D.; Hamelers, H.V.M.; Bryjak, M.; Presser, V.; Biesheuvel, P.M. Carbon Flow Electrodes for Continuous Operation of Capacitive Deionization and Capacitive Mixing Energy Generation. J. Mater. Chem. A 2014, 2, 9313–9321. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Yoon, H.; Lee, J.; Kim, T.; Yoon, J. Extraction of Salinity-Gradient Energy by a Hybrid Capacitive-Mixing System. ChemSusChem 2017, 10, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Nayar, K.G.; Fernandes, J.; McGovern, R.K.; Dominguez, K.P.; McCance, A.; Al-Anzi, B.S.; Lienhard, J.H. Cost and Energy Requirements of Hybrid RO and ED Brine Concentration Systems for Salt Production. Desalination 2019, 456, 97–120. [Google Scholar] [CrossRef]
- Afrasiabi, N.; Shahbazali, E. RO Brine Treatment and Disposal Methods. Desalin. Water Treat. 2011, 35, 39–53. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, H.; Qu, F.; Yu, H.; Liang, H.; Li, G.; Ma, J. Reverse Osmosis Brine Treatment Using Direct Contact Membrane Distillation: Effects of Feed Temperature and Velocity. Desalination 2017, 423, 149–156. [Google Scholar] [CrossRef]
- Khan, M.; Al-Ghouti, M.A. DPSIR Framework and Sustainable Approaches of Brine Management from Seawater Desalination Plants in Qatar. J. Clean. Prod. 2021, 319, 128485. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).