Waste Clay Bricks as a Geopolymer Binder for Pavement Construction
Abstract
:1. Introduction
2. Review Methodology
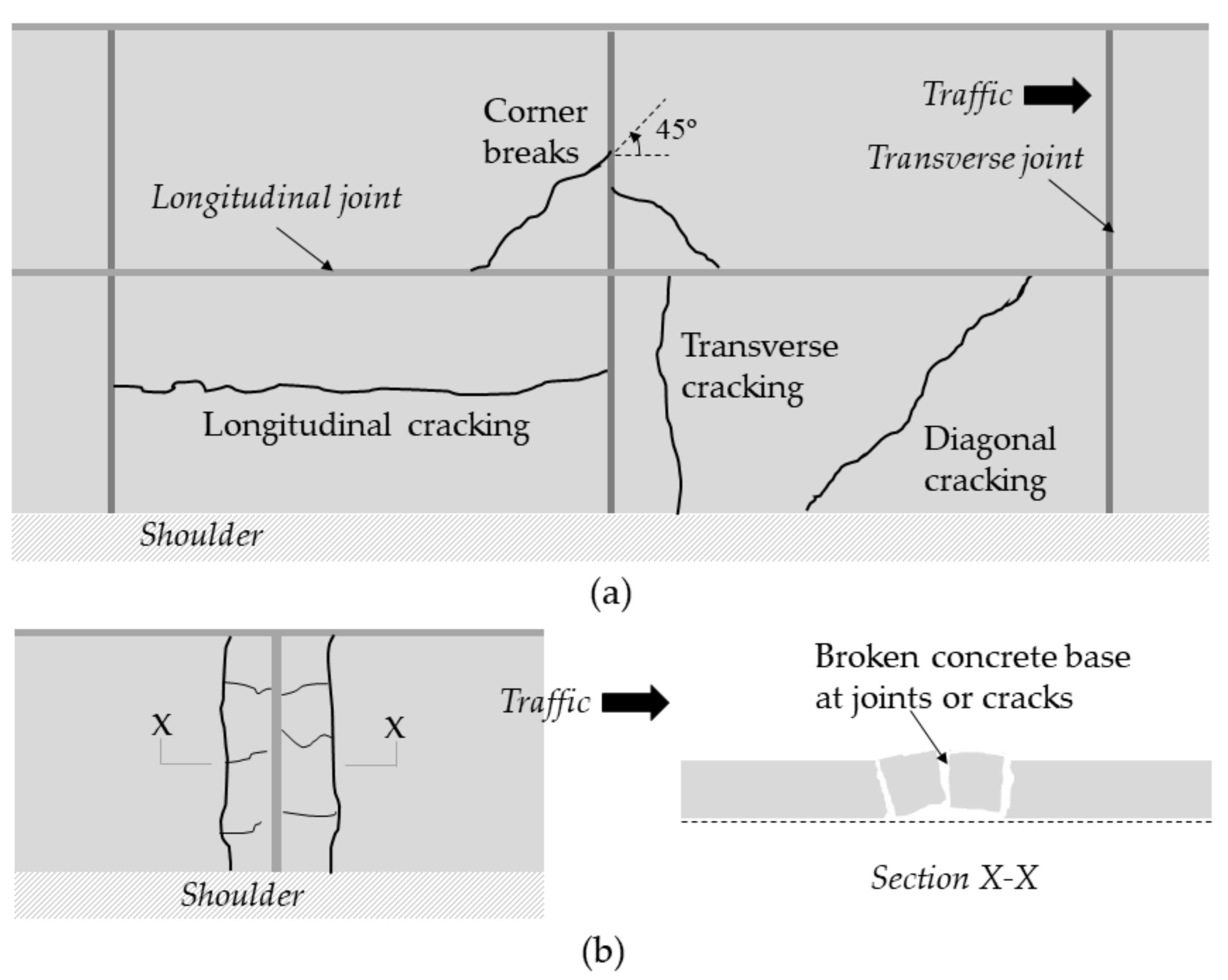
3. Design of Concrete Pavements
4. Properties of WCB as a Binder
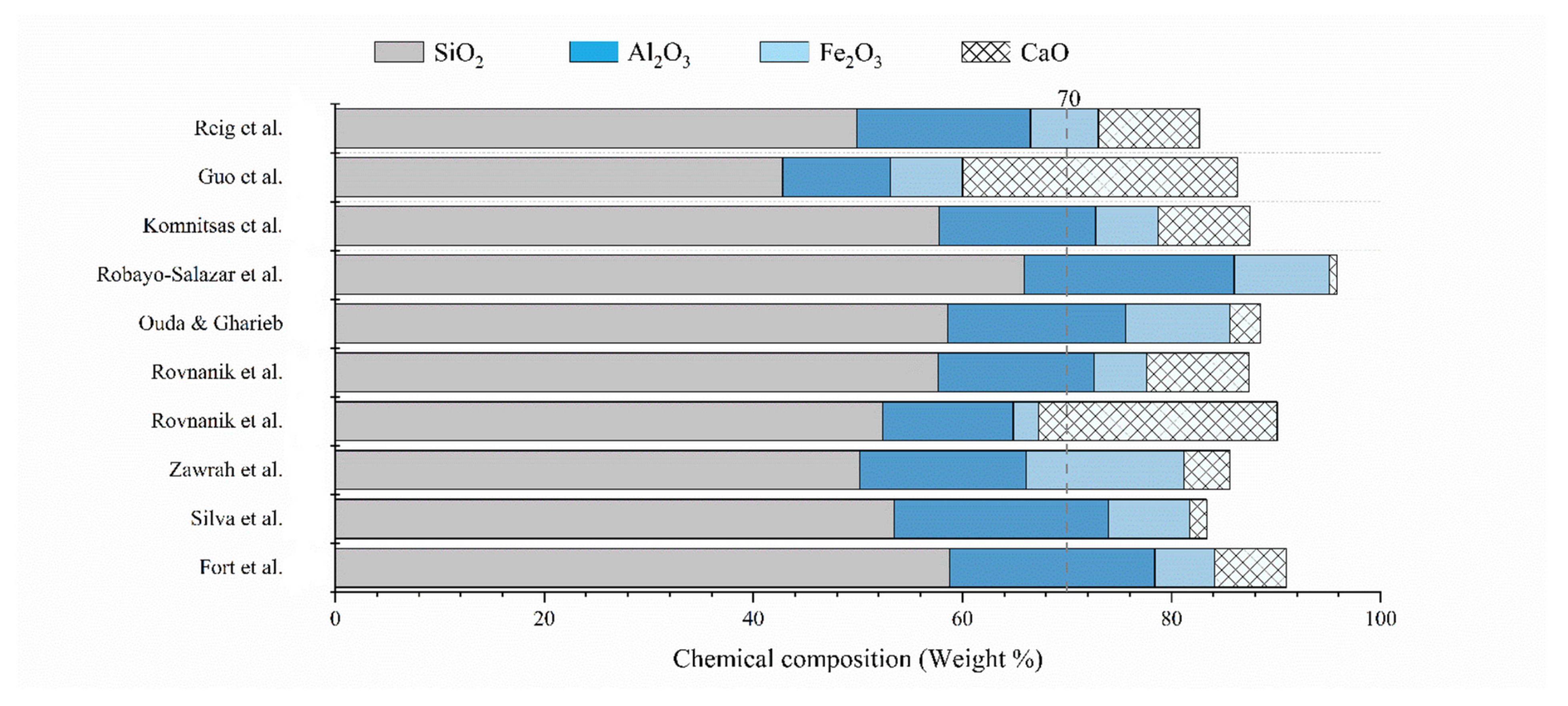
4.1. Chemical and Mineralogical Properties
4.2. Particle Size Distribution
5. Effect of the Types and Concentrations of Activators in WCB-Based Geopolymers
5.1. Methods of Mixing Activators
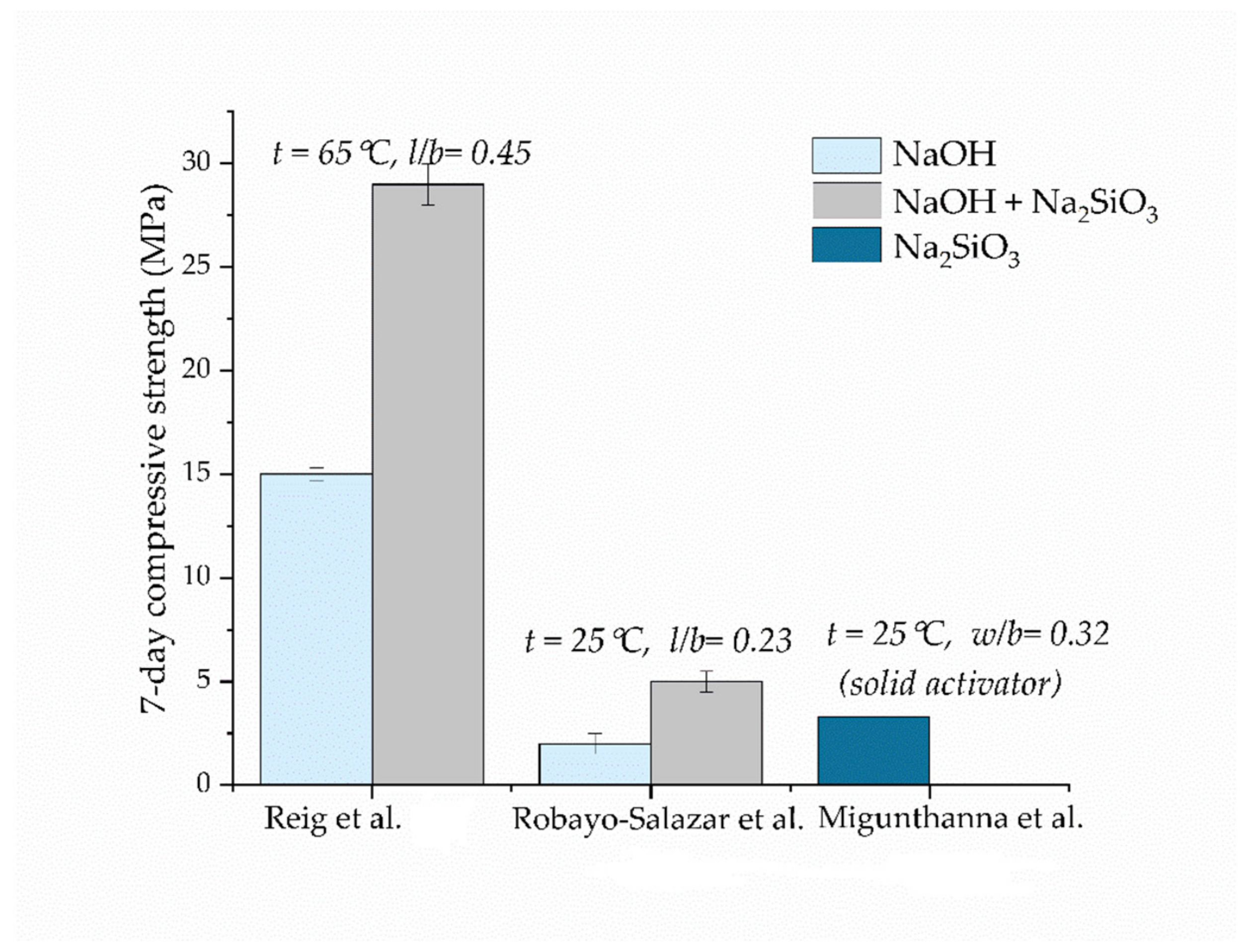
5.2. Types of Activators
5.3. Concentration of Activators
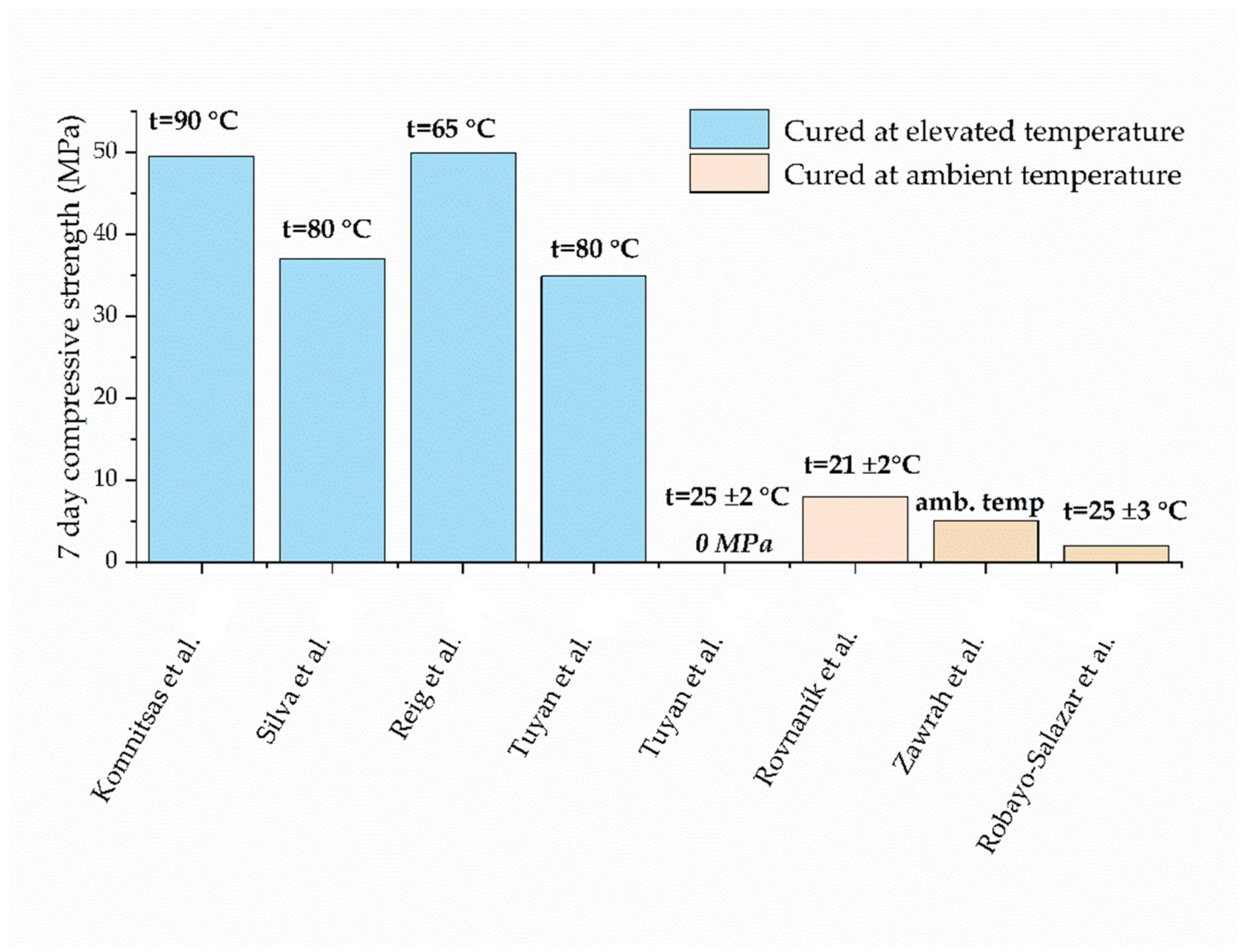
6. Effect of Curing Conditions
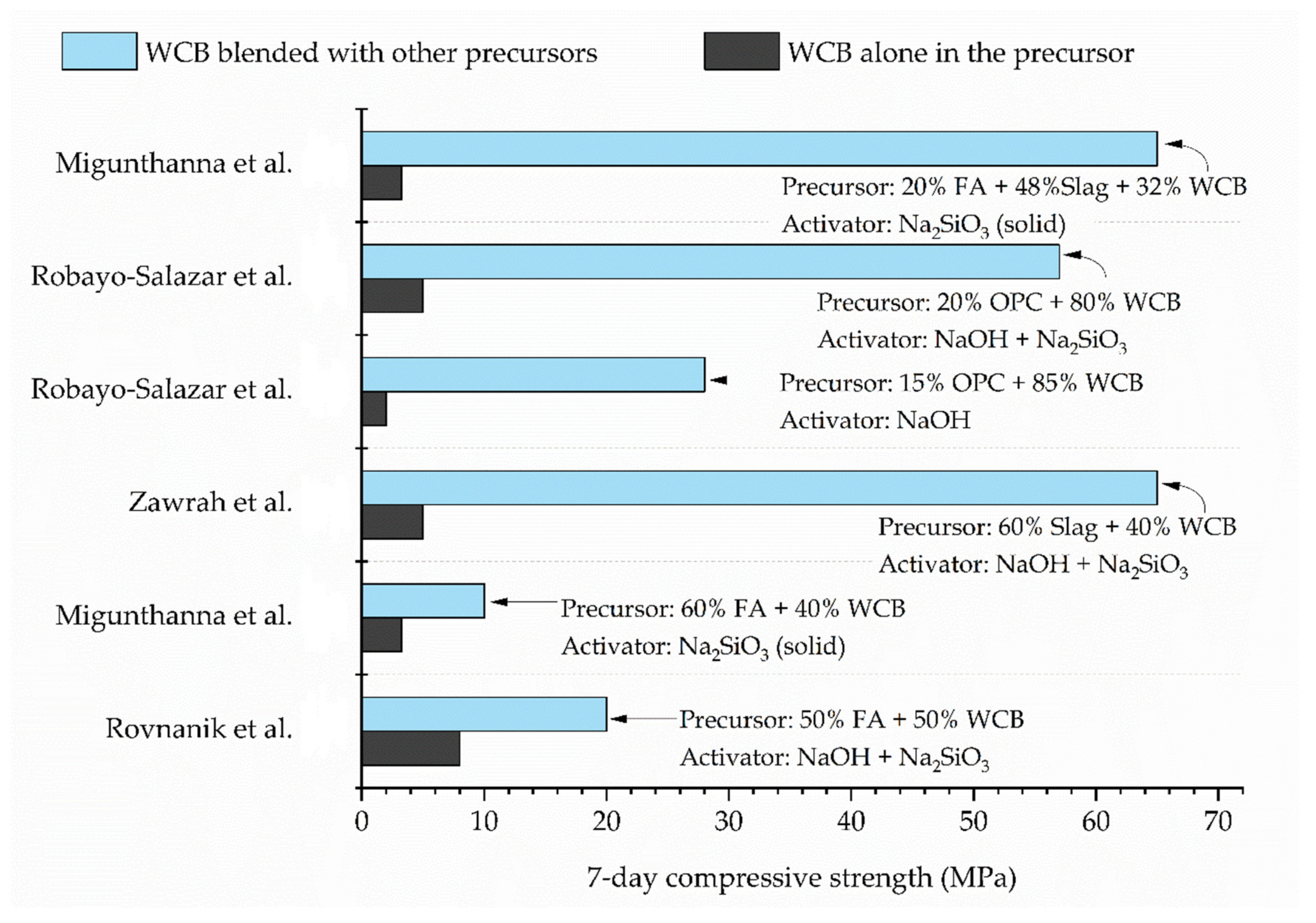
7. Binary and Ternary Geopolymer Systems with WCB and Other Aluminosilicate Materials
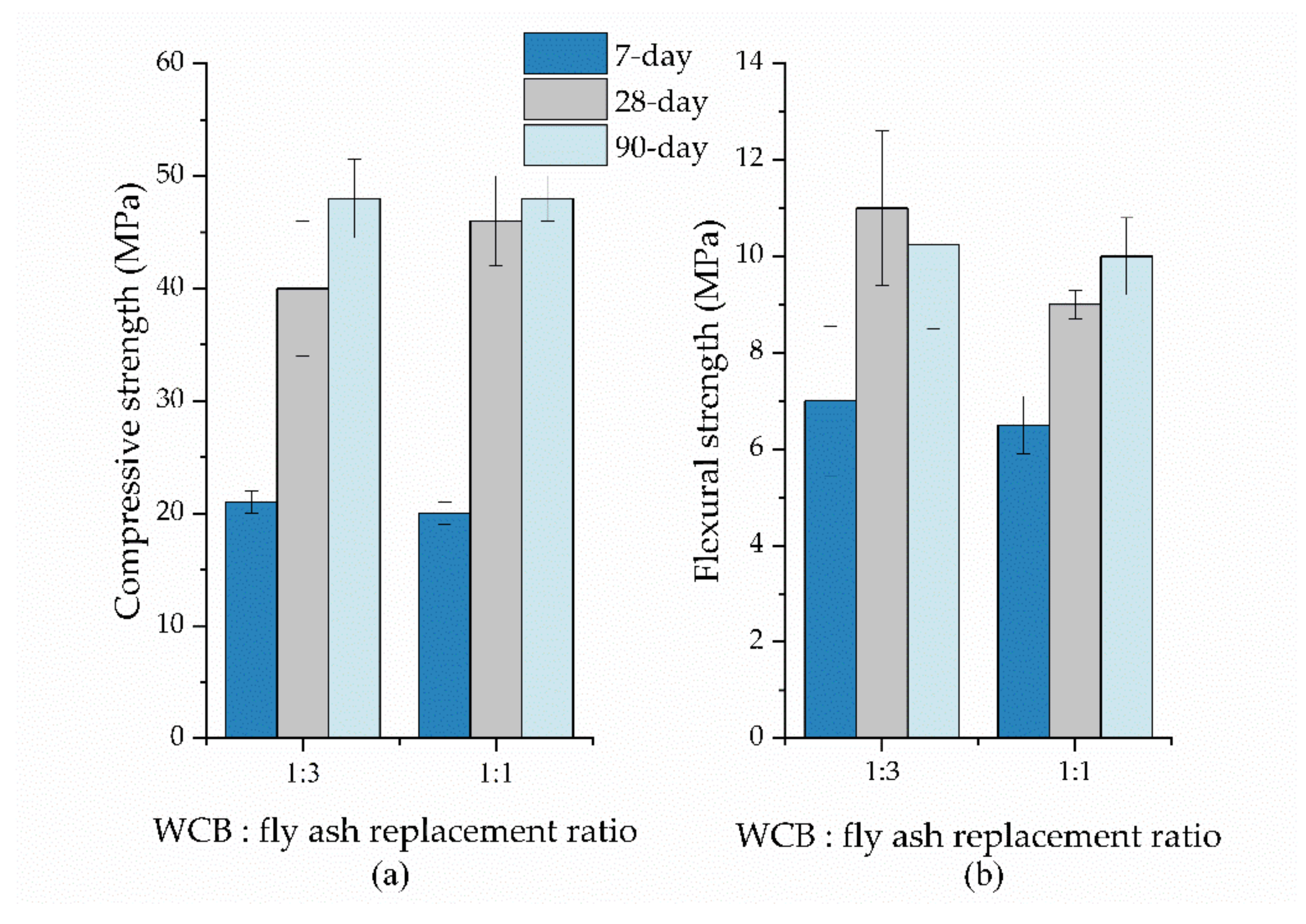
8. Effect of the Liquid-to-Binder Ratio
9. Mineralogical and Microstructural Properties of WCB Geopolymers
10. Structural Integrity and Durability of WCB-Based Geopolymers
11. Summary and Conclusions
- In terms of the chemical and mineralogical composition, WCB has the potential to serve as an aluminosilicate precursor to synthesize geopolymer binders.
- An optimal Ms in the range of 0.6–2.0 and a Na2O content of 7%–10% of the binder weight are ideal for a WCB geopolymer.
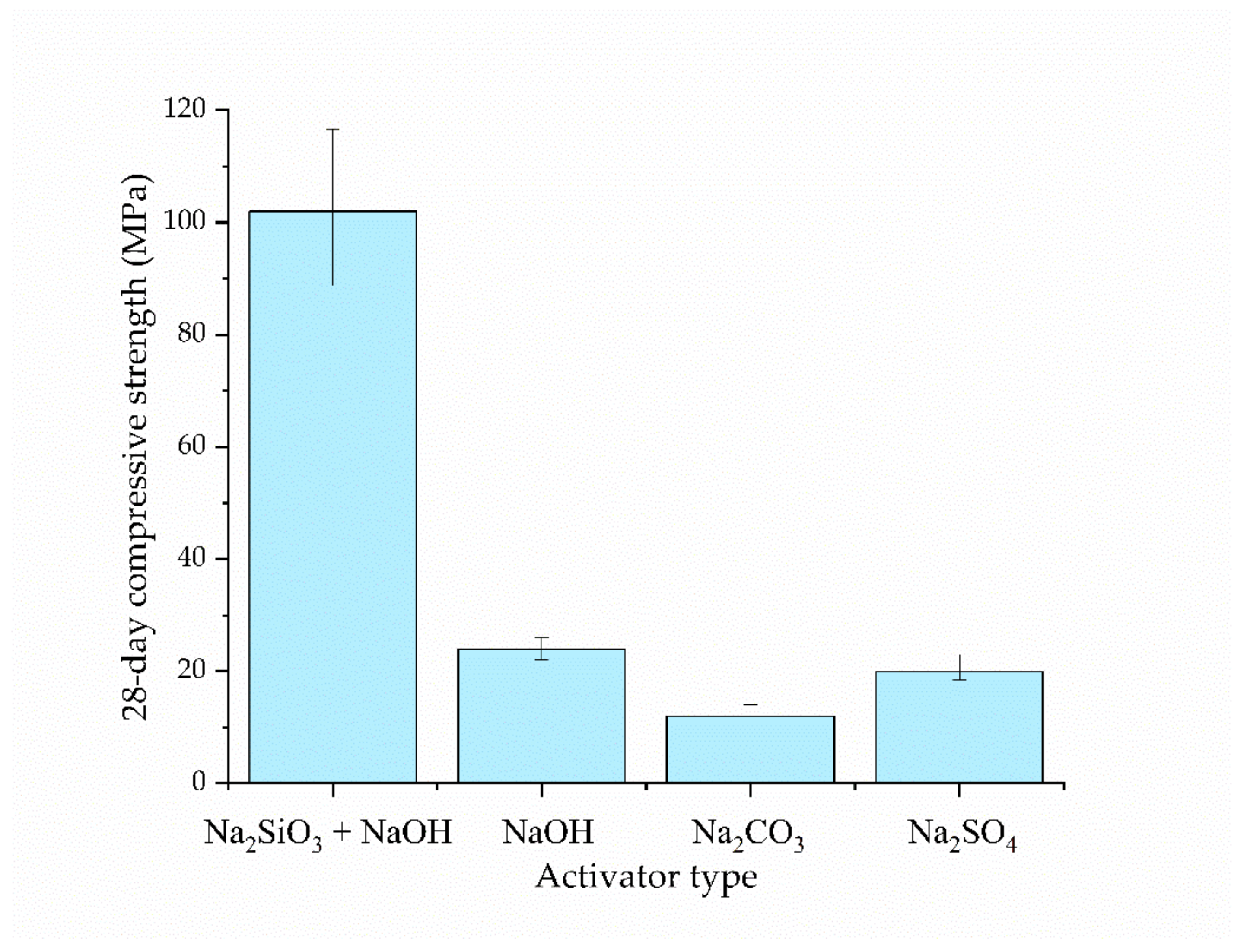
- The incorporation of Na2SiO3 in the activation is favorable in terms of improving both the strength and durability, compared to NaOH alone, Na2SO4, and Na2CO3.
- A curing temperature of 80 °C is identified as being optimal for producing the 100% WCB geopolymer.
- With ambient curing, WCB alone cannot achieve satisfactory strengths. Strength can be notably improved by using binary and ternary blends of WCB with other precursors, such as fly ash, metakaolin, slag, and OPC.
- Considering the high early strength gain, better long-term strength development, and a low-porous and compacted microstructure, the use of slag or OPC with WCB is recommended for developing pavement concretes. Incorporating fly ash is beneficial in situations where reducing shrinkage is critical.
- Conducting more comprehensive research on replacing hazardous activators, such as NaOH, and developing WCB binders using Na2SiO3 as the sole activator.
- More investigations on producing WCB-based one-part geopolymers, to find a more OPC-like alternative for the construction industry.
- Developing WCB-based pavement-grade geopolymer concrete (GPC) and optimizing the mix designs for use in pavement base and subbase constructions.
- Investigating the WCB binder and WCB-based GPC properties, such as strength (i.e., compressive and flexural) and durability (i.e., shrinkage, creep behavior, alkali–aggregate reaction, sulfate attack, permeability, and abrasion resistance).
- Conducting field studies with WCB-based GPC.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Austroads. Guide to pavement technology part 2. In Pavement Structural Design; Austroads: Sydney, Australia, 2017. [Google Scholar]
- Ovidiu, G. Methods for determining the optimal solution for the rehabilitation of cement concrete road pavements. Fiabil. Durabilitate 2012, 1, 271–275. [Google Scholar]
- Ioana, P.; Eleftherios, A. Development of low-cost concrete for road pavements. Rev. Romana Mater. 2012, 42, 11. [Google Scholar]
- El-Hassan, H.; Kianmehr, P. Pervious concrete pavement incorporating GGBS to alleviate pavement runoff and improve urban sustainability. Road Mater. Pavement Des. 2018, 19, 167–181. [Google Scholar] [CrossRef]
- ASCP. Concrete Pavement Value: Economic, Operational and Community Benefits; Australian Society for Concrete Pavements (ASCP): Sydney, Australia, 2020. [Google Scholar]
- CCANZ. Benefits of Building Concrete Roads in New Zealand; Cement Concrete Association Of New Zealand (CCANZ): Wellington, New Zealand, 2013. [Google Scholar]
- CCAA. Concrete Roads—Better Value Across the Life of a Project; Cement Concrete Aggregates Australia (CCAA): Sydney, Australia, 2018. [Google Scholar]
- Miller, T.D.; Bahia, H.U. Sustainable Asphalt Pavements: Technologies, Knowledge Gaps and Opportunities; Modified Asphalt Research Center (MARC): Madison, WI, USA, 2009. [Google Scholar]
- Mallick, R.B.; El-Korchi, T. Pavement Engineering: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Mehta, A.; Siddique, R. Sustainable geopolymer concrete using ground granulated blast furnace slag and rice husk ash: Strength and permeability properties. J. Clean. Prod. 2018, 205, 49–57. [Google Scholar] [CrossRef]
- Nasier, S.; Amin, M. Experimental evaluation of eco-friendly no-fines geopolymer concrete for sustainable pavement applications. Indian J. Sci. Technol. 2018, 11, 1–10. [Google Scholar]
- Alhawat, M.; Ashour, A.; Yildirim, G.; Aldemir, A.; Sahmaran, M. Properties of geopolymers sourced from construction and demolition waste: A review. J. Build. Eng. 2022, 50, 104104. [Google Scholar] [CrossRef]
- Ozer, H.; Yang, R.; Al-Qadi, I.L. Quantifying sustainable strategies for the construction of highway pavements in Illinois. Transp. Res. Part D Transp. Environ. 2017, 51, 1–13. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Sanjayan, J.; Rajeev, P.; Cheema, D.S. Durability of low-calcium fly ash based geopolymer concrete culvert in a saline environment. Cem. Concr. Res. 2017, 100, 297–310. [Google Scholar] [CrossRef]
- Tho-in, T.; Sata, V.; Chindaprasirt, P.; Jaturapitakkul, C. Pervious high-calcium fly ash geopolymer concrete. Constr. Build. Mater. 2012, 30, 366–371. [Google Scholar] [CrossRef]
- Nazari, A.; Sanjayan, J.G. Handbook of Low Carbon Concrete; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar]
- Nematollahi, B.; Sanjayan, J.; Shaikh, F.U.A. Synthesis of heat and ambient cured one-part geopolymer mixes with different grades of sodium silicate. Ceram. Int. 2015, 41, 5696–5704. [Google Scholar] [CrossRef]
- Tuyan, M.; Andiç-Çakir, Ö.; Ramyar, K. Effect of alkali activator concentration and curing condition on strength and microstructure of waste clay brick powder-based geopolymer. Compos. Part B Eng. 2018, 135, 242–252. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Labrincha, J.; Leonelli, C.; Palomo, A.; Chindaprasit, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Ulugöl, H.; Kul, A.; Yıldırım, G.; Şahmaran, M.; Aldemir, A.; Figueira, D.; Ashour, A. Mechanical and microstructural characterization of geopolymers from assorted construction and demolition waste-based masonry and glass. J. Clean. Prod. 2021, 280, 124358. [Google Scholar] [CrossRef]
- Vasić, M.V.; Terzić, A.; Radovanović, Ž.; Radojević, Z.; Warr, L.N. Alkali-activated geopolymerization of a low illitic raw clay and waste brick mixture. An alternative to traditional ceramics. Appl. Clay Sci. 2022, 218, 106410. [Google Scholar] [CrossRef]
- Silva, G.; Castañeda, D.; Kim, S.; Castañeda, A.; Bertolotti, B.; Ortega-San-Martin, L.; Nakamatsu, J.; Aguilar, R. Analysis of the production conditions of geopolymer matrices from natural pozzolana and fired clay brick wastes. Constr. Build. Mater. 2019, 215, 633–643. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Use of reservoir clay sediments as raw materials for geopolymer binders. Adv. Appl. Ceram. 2013, 112, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Molino, B.; de Vincenzo, A.; Ferone, C.; Messina, F.; Colangelo, F.; Cioffi, R. Recycling of clay sediments for geopolymer binder production. A new perspective for reservoir management in the framework of italian legislation: The occhito reservoir case study. Materials 2014, 7, 5603–5616. [Google Scholar] [CrossRef]
- Peyne, J.; Joussein, E.; Gautron, J.; Doudeau, J.; Rossignol, S. Feasibility of producing geopolymer binder based on a brick clay mixture. Ceram. Int. 2017, 43, 9860–9871. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Mohd Mustafa, A.B.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Abadel, A.A.; Albidah, A.S.; Altheeb, A.H.; Alrshoudi, F.A.; Abbas, H.; Al-Salloum, Y.A. Effect of molar ratios on strength, microstructure & embodied energy of metakaolin geopolymer. Adv. Concr. Constr. 2021, 11, 127–140. [Google Scholar]
- Fořt, J.; Vejmelková, E.; Koňáková, D.; Alblová, N.; Čáchová, M.; Keppert, M.; Rovnaníková, P.; Černý, R. Application of waste brick powder in alkali activated aluminosilicates: Functional and environmental aspects. J. Clean. Prod. 2018, 194, 714–725. [Google Scholar] [CrossRef]
- Reig, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.; Cheeseman, C.R.; Payá, J. Properties and microstructure of alkali-activated red clay brick waste. Constr. Build. Mater. 2013, 43, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Zawrah, M.F.; Gado, R.A.; Feltin, N.; Ducourtieux, S.; Devoille, L. Recycling and utilization assessment of waste fired clay bricks (Grog) with granulated blast-furnace slag for geopolymer production. Process Saf. Environ. Prot. 2016, 103, 237–251. [Google Scholar] [CrossRef]
- Baronio, G.; Binda, L. Study of the pozzolanicity of some bricks and clays. Constr. Build. Mater. 1997, 11, 41–46. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Vlachou, A.; Bartzas, G.; Galetakis, M. Effect of synthesis parameters on the quality of construction and demolition wastes (CDW) geopolymers. Adv. Powder Technol. 2015, 26, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Robayo-Salazar, R.A.; Rivera, J.F.; de Gutiérrez, R.M. Alkali-activated building materials made with recycled construction and demolition wastes. Constr. Build. Mater. 2017, 149, 130–138. [Google Scholar] [CrossRef]
- Llatas, C. A model for quantifying construction waste in projects according to the European waste list. Waste Manag. 2011, 31, 1261–1276. [Google Scholar] [CrossRef]
- Ouda, A.S.; Gharieb, M. Development the properties of brick geopolymer pastes using concrete waste incorporating dolomite aggregate. J. Build. Eng. 2020, 27, 100919. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Ding, S.; Zhang, H.; Xia, F.; Wang, J.; Zhou, M. Utilization of red mud in geopolymer-based pervious concrete with function of adsorption of heavy metal ions. J. Clean. Prod. 2019, 207, 789–800. [Google Scholar] [CrossRef]
- Villoria Saez, P.; del Rio Merino, M.; Porras-Amores, C. Estimation of construction and demolition waste volume generation in new residential buildings in Spain. Waste Manag. Res. 2012, 30, 137–146. [Google Scholar] [CrossRef]
- Yuan, H.; Shen, L. Trend of the research on construction and demolition waste management. Waste Manag. 2011, 31, 670–679. [Google Scholar] [CrossRef]
- ECRC. Never Waste a Crisis: The Waste and Recycling Industry in Australia; Environment and Communications References Committee (ECRC): Canberra, Australia, 2018. [Google Scholar]
- Pickin, J.; Randell, P.; Trinh, J.; Grant, B. National Waste Report 2018; Blue Environment Pty Ltd.: Docklands, Australia, 2018. [Google Scholar]
- Rovnaník, P.; Řezník, B.; Rovnaníková, P. Blended Alkali-activated Fly Ash/Brick Powder Materials. Procedia Eng. 2016, 151, 108–113. [Google Scholar] [CrossRef] [Green Version]
- Migunthanna, J.; Rajeev, P.; Sanjayan, J. Investigation of waste clay brick as partial replacement of geopolymer binders for rigid pavement application. Constr. Build. Mater. 2021, 305, 124787. [Google Scholar] [CrossRef]
- Migunthanna, J.; Rajeev, P.; Sanjayan, J. Waste Clay Brick Binders for Rigid Pavement Subbase and Base Concretes; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar]
- Debieb, F.; Kenai, S. The use of coarse and fine crushed bricks as aggregate in concrete. Constr. Build. Mater. 2008, 22, 886–893. [Google Scholar] [CrossRef]
- Cachim, P.B. Mechanical properties of brick aggregate concrete. Constr. Build. Mater. 2009, 23, 1292–1297. [Google Scholar] [CrossRef]
- Adamson, M.; Razmjoo, A.; Poursaee, A. Durability of concrete incorporating crushed brick as coarse aggregate. Constr. Build. Mater. 2015, 94, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Nepomuceno, M.C.S.; Isidoro, R.A.S.; Catarino, J.P.G. Mechanical performance evaluation of concrete made with recycled ceramic coarse aggregates from industrial brick waste. Constr. Build. Mater. 2018, 165, 284–294. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.; Chen, F.; Liu, C.; Chen, X. Utilization of waste clay bricks as coarse and fine aggregates for the preparation of lightweight aggregate concrete. J. Clean. Prod. 2018, 201, 706–715. [Google Scholar] [CrossRef]
- Zheng, C.; Lou, C.; Du, G.; Li, X.; Liu, Z.; Li, L. Mechanical properties of recycled concrete with demolished waste concrete aggregate and clay brick aggregate. Results Phys. 2018, 9, 1317–1322. [Google Scholar] [CrossRef]
- Ossa, A.; García, J.L.; Botero, E. Use of recycled construction and demolition waste (CDW) aggregates: A sustainable alternative for the pavement construction industry. J. Clean. Prod. 2016, 135, 379–386. [Google Scholar] [CrossRef]
- Aboutalebi Esfahani, M. Evaluating the feasibility, usability, and strength of recycled construction and demolition waste in base and subbase courses. Road Mater. Pavement Des. 2018, 21, 156–178. [Google Scholar] [CrossRef]
- Delongui, L.; Matuella, M.; Núñez, W.P.; Fedrigo, W.; Filho, L.C.P.d.S.; Ceratti, J.A.P. Construction and demolition waste parameters for rational pavement design. Constr. Build. Mater. 2018, 168, 105–112. [Google Scholar] [CrossRef]
- Perera, S.; Arulrajah, A.; Wong, Y.C.; Horpibulsuk, S.; Maghool, F. Utilizing recycled PET blends with demolition wastes as construction materials. Constr. Build. Mater. 2019, 221, 200–209. [Google Scholar] [CrossRef]
- Perera, S.; Arulrajah, A.; Wong, Y.; Maghool, F.; Horpibulsuk, S. Evaluation of shear strength properties of unbound PET plastic in blends with demolition wastes. Constr. Build. Mater. 2020, 262, 120545. [Google Scholar] [CrossRef]
- Bassani, M.; Tefa, L.; Coppola, B.; Palmero, P. Alkali-activation of aggregate fines from construction and demolition waste: Valorisation in view of road pavement subbase applications. J. Clean. Prod. 2019, 234, 71–84. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, F.; Zhang, Y. Use of building-related construction and demolition wastes in highway embankment: Laboratory and field evaluations. J. Clean. Prod. 2019, 230, 1051–1060. [Google Scholar] [CrossRef]
- Fořt, J.; Novotný, R.; Vejmelková, E.; Trník, A.; Rovnaníková, P.; Keppert, M.; Pommer, V.; Černý, R. Characterization of geopolymers prepared using powdered brick. J. Mater. Res. Technol. 2019, 8, 6253–6261. [Google Scholar] [CrossRef]
- Sassoni, E.; Pahlavan, P.; Franzoni, E.; Bignozzi, M.C. Valorization of brick waste by alkali-activation: A study on the possible use for masonry repointing. Ceram. Int. 2016, 42, 14685–14694. [Google Scholar] [CrossRef]
- Rovnaník, P.; Rovnaníková, P.; Vyšvařil, M.; Grzeszczyk, S.; Janowska-Renkas, E. Rheological properties and microstructure of binary waste red brick powder/metakaolin geopolymer. Constr. Build. Mater. 2018, 188, 924–933. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Mejía-Arcila, J.M.; de Gutiérrez, R.M. Eco-efficient alkali-activated cement based on red clay brick wastes suitable for the manufacturing of building materials. J. Clean. Prod. 2017, 166, 242–252. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Wei, X. Pore properties, inner chemical environment, and microstructure of nano-modified CFA-WBP (class C fly ash-waste brick powder) based geopolymers. Cem. Concr. Compos. 2017, 79, 53–61. [Google Scholar] [CrossRef]
- Vafaei, M.; Allahverdi, A. Strength development and acid resistance of geopolymer based on waste clay brick powder and phosphorous slag. Struct. Concr. J. FIB 2019, 20, 1596–1606. [Google Scholar] [CrossRef]
- Mahmoodi, O.; Siad, H.; Lachemi, M.; Dadsetan, S.; Sahmaran, M. Optimization of brick waste-based geopolymer binders at ambient temperature and pre-targeted chemical parameters. J. Clean. Prod. 2020, 268, 122285. [Google Scholar] [CrossRef]
- Wong, C.L.; Mo, K.H.; Alengaram, U.J.; Yap, S.P. Mechanical strength and permeation properties of high calcium fly ash-based geopolymer containing recycled brick powder. J. Build. Eng. 2020, 32, 101655. [Google Scholar] [CrossRef]
- Cong, P.; Cheng, Y.; Ge, W.; Zhang, A. Mechanical, microstructure and reaction process of calcium carbide slag-waste red brick powder based alkali-activated materials (CWAAMs). J. Clean. Prod. 2022, 331, 129845. [Google Scholar] [CrossRef]
- American Association of State Highway and Transportation Officials. AASHTO Guide for Design of Pavement Structures; AASHTO: Washington, DC, USA, 1993. [Google Scholar]
- FDOT. Rigid Pavement Design Manual; Office of Design, Pavement Management Section: Tallahassee, FL, USA, 2019. [Google Scholar]
- Austroads. Specification of Geopolymer Concrete: General Guide; Austroads: Sydney, Australia, 2016. [Google Scholar]
- Yoder, E.J.; Witczak, M.W. Principles of Pavement Design; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Adlinge, S.S.; Gupta, A. Pavement Deterioration and its Causes. Int. J. Innov. Res. Dev. 2013, 2, 437–450. [Google Scholar]
- Pereira-de-Oliveira, L.A.; Castro-Gomes, J.P.; Santos, P.M.S. The potential pozzolanic activity of glass and red-clay ceramic waste as cement mortars components. Constr. Build. Mater. 2012, 31, 197–203. [Google Scholar] [CrossRef]
- ASTM C618-19; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2017.
- Abdullah, M.; Ming, L.Y.; Yong, H.C.; Tahir, M. Clay-based materials in geopolymer technology. In Cement Based Materials; InTech: London, UK, 2018; p. 239. [Google Scholar]
- Bayuaji, R.; Yasin, A.K.; Susanto, T.E.; Darmawan, M.S. A review in geopolymer binder with dry mixing method (geopolymer cement). In Proceedings of the AIP Conference Proceedings Vol 1887, Melang, Indonesia, 8–9 August 2017; AIP Publishing LLC: New York, NY, USA, 2017. [Google Scholar]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; van Deventer, J.S.J. Characterisation of one-part geopolymer binders made from fly ash. Waste Biomass Valorization 2016, 8, 225–233. [Google Scholar] [CrossRef]
- Nematollahi, B.; Sanjayan, J. Effect of different superplasticizers and activator combinations on workability and strength of fly ash based geopolymer. Mater. Des. 2014, 57, 667–672. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef] [Green Version]
- Allahverdi, A.; Kani, E.N. Use of Construction and Demolition Waste (CDW) for Alkali-Activated or Geopolymer Cements; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 439–475. [Google Scholar]
- Austroads. Specification and Use of Geopolymer Concrete in the Manufacture of Structural and Non-Structural Components: Review of Literature; Austroads: Sydney, Australia, 2016. [Google Scholar]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Provis, J.L.; Harrex, R.M.; Bernal, S.A.; Duxson, P.; van Deventer, J.S.J. Dilatometry of geopolymers as a means of selecting desirable fly ash sources. J. Non-Cryst. Solids 2012, 358, 1930–1937. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, V.F.; MacKenzie, K.J.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Puertas, F.; Torres-Carrasco, M.; Alonso, M.M. Reuse of urban and industrial waste glass as a novel activator for alkali-activated slag cement pastes: A case study. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing: Sawston, UK, 2015; pp. 75–109. [Google Scholar]
- Alonso, S.; Palomo, A. Alkaline activation of metakaolin and calcium hydroxide mixtures: Influence of temperature, activator concentration and solids ratio. Mater. Lett. 2001, 47, 55–62. [Google Scholar] [CrossRef]
- Lampris, C.; Lupo, R.; Cheeseman, C.R. Geopolymerisation of silt generated from construction and demolition waste washing plants. Waste Manag. 2009, 29, 368–373. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, Z.; Chen, W.; Zhang, J.; Kang, Z.; Pu, S.; Wan, Y. Effect of synthesis parameters on the development of unconfined compressive strength of recycled waste concrete powder-based geopolymers. Constr. Build. Mater. 2021, 292, 123264. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Yan, F. Synthesis and mechanical properties of metakaolinite-based geopolymer. Colloids Surf. A Physicochem. Eng. Asp. 2005, 268, 1–6. [Google Scholar] [CrossRef]
- Singh, B.; Rahman, M.R.; Paswan, R.; Bhattacharyya, S.K. Effect of activator concentration on the strength, ITZ and drying shrinkage of fly ash/slag geopolymer concrete. Constr. Build. Mater. 2016, 118, 171–179. [Google Scholar] [CrossRef]
- Escalante-García, J.; Gorokhovsky, A.; Mendoza, G.; Fuentes, A. Effect of geothermal waste on strength and microstructure of alkali-activated slag cement mortars. Cem. Concr. Res. 2003, 33, 1567–1574. [Google Scholar] [CrossRef]
- Görhan, G.; Aslaner, R.; Şinik, O. The effect of curing on the properties of metakaolin and fly ash-based geopolymer paste. Compos. Part B Eng. 2016, 97, 329–335. [Google Scholar] [CrossRef]
- Noushini, A.; Castel, A. The effect of heat-curing on transport properties of low-calcium fly ash-based geopolymer concrete. Constr. Build. Mater. 2016, 112, 464–477. [Google Scholar] [CrossRef]
- Austroads. Guide to Pavement Technology Part 4E. In Recycled Materials; Austroads: Sydney, Australia, 2009. [Google Scholar]
- Austroads. Guide to pavement technology part 4C. In Materials for Concrete Road Pavements; Austroads: Sydney, Australia, 2017. [Google Scholar]
- Nath, P.; Sarker, P.K. Use of OPC to improve setting and early strength properties of low calcium fly ash geopolymer concrete cured at room temperature. Cem. Concr. Compos. 2015, 55, 205–214. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; Provis, J.L.; van Deventer, J.S.J. Effect of calcium silicate sources on geopolymerisation. Cem. Concr. Res. 2008, 38, 554–564. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Van Deventer, J.S.; Provis, J.L.; Duxson, P.; Lukey, G.C. Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J. Hazard. Mater. 2007, 139, 506–513. [Google Scholar] [CrossRef]
- Nath, P.; Sarker, P.K.; Rangan, V.B. Early Age Properties of Low-calcium Fly Ash Geopolymer Concrete Suitable for Ambient Curing. Procedia Eng. 2015, 125, 601–607. [Google Scholar] [CrossRef] [Green Version]
- Rovnaník, P. Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr. Build. Mater. 2010, 24, 1176–1183. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Fernandez-Jimenez, A.; Palomo, A. Hydration kinetics in hybrid binders: Early reaction stages. Cem. Concr. Compos. 2013, 39, 82–92. [Google Scholar] [CrossRef]
- Suwan, T.; Fan, M. Influence of OPC replacement and manufacturing procedures on the properties of self-cured geopolymer. Constr. Build. Mater. 2014, 73, 551–561. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Nicolas, R.S.; Brice, D.G.; Kilcullen, A.R.; Hamdan, S.; van Deventer, J.S.J. Influence of fly ash on the water and chloride permeability of alkali-activated slag mortars and concretes. Constr. Build. Mater. 2013, 48, 1187–1201. [Google Scholar] [CrossRef]
- Alahverdi, A.; Mehrpour, K.; Najafikani, E. Taftan pozzolan-based geopolymer cement. Int. J. Eng. Sci. 2008, 19, 1–5. [Google Scholar]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Geopolymerisation of low calcium ferronickel slags. J. Mater. Sci. 2006, 42, 3073–3082. [Google Scholar] [CrossRef]
- Nkinamubanzi, P.C.; Mantellato, S.; Flatt, R.J. Superplasticizers in practice. In Science and Technology of Concrete Admixtures; Elsevier: Amsterdam, The Netherlands, 2016; pp. 353–377. [Google Scholar]
- Vafaei, M.; Allahverdi, A.; Dong, P.; Bassim, N.; Mahinroosta, M. Resistance of red clay brick waste/phosphorus slag-based geopolymer mortar to acid solutions of mild concentration. J. Build. Eng. 2021, 34, 102066. [Google Scholar] [CrossRef]
- Bernal, S.A.; de Gutierrez, R.M.; Provis, J.L.; Rose, V. Effect of silicate modulus and metakaolin incorporation on the carbonation of alkali silicate-activated slags. Cem. Concr. Res. 2010, 40, 898–907. [Google Scholar] [CrossRef]
- Tashima, M.M.; Akasaki, J.L.; Castaldelli, V.N.; Soriano, L.; Monzó, J.; Payá, J.; Borrachero, M.V. New geopolymeric binder based on fluid catalytic cracking catalyst residue (FCC). Mater. Lett. 2012, 80, 50–52. [Google Scholar] [CrossRef]
- Lemougna, P.N.; MacKenzie, K.J.D.; Melo, U.F.C. Synthesis and thermal properties of inorganic polymers (geopolymers) for structural and refractory applications from volcanic ash. Ceram. Int. 2011, 37, 3011–3018. [Google Scholar] [CrossRef]
- CCAA. Guide to Industrial Floors and Pavements—Design, Construction and Specification; Cement Concrete Aggregates Australia (CCAA): Sydney, Australia, 2009. [Google Scholar]
- Austroads. Specification and Use of Geopolymer Concrete; Austroads: Sydney, Australia, 2017. [Google Scholar]
- Provis, J.L.; van Deventer, J.S.J. (Eds.) Alkali Activated Materials State-of-the-Art Report, RILEM TC 224-AAM, 1st ed.; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]

| Reference | Characterization Techniques | Mechanical Properties | Durability Properties | Type of Study | Other Assessment Criteria | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| XRF | XRD | SEM | FTIR | Compressive Strength | Flexural Strength | Laboratory | Field | |||
| Komnitsas et al. [32] | ✓ | ✓ | ✓ | ✓ | 7, 28 days | - | Freeze-thaw response, Water immersion | ✓ | - | - |
| Silva et al. [22] | ✓ | ✓ | ✓ | ✓ | 7-day | - | - | ✓ | - | - |
| Reig et al. [29] | ✓ | ✓ | ✓ | ✓ | 3, 7 days | - | Porosity, Thermogravimetric analysis | ✓ | - | - |
| Fort et al. [28] | ✓ | ✓ | - | - | 28-day | 28-day | - | ✓ | - | Environmental impacts (greenhouse gas emissions and energy consumption) |
| Fort et al. [57] | ✓ | ✓ | ✓ | - | - | - | Porosity, Thermogravimetric analysis | ✓ | - | Reaction heat evaluation |
| Sassoni et al. [58] | ✓ | ✓ | ✓ | - | - | - | Porosity, Water absorption, Efflorescence formation, Water vapor permeability | ✓ | - | - |
| Tuyan et al. [18] | ✓ | ✓ | ✓ | ✓ | 1, 3, 5, 7, 28, 90 days | - | Porosity, Thermogravimetric analysis | ✓ | - | - |
| Rovnaník et al. [41] | ✓ | ✓ | ✓ | - | 7, 28, 90 days | 7, 28, 90 days | - | ✓ | - | - |
| Rovnaník et al. [59] | ✓ | ✓ | ✓ | ✓ | 7, 14 days | 7, 14 days | - | ✓ | - | Rheological properties |
| Zawrah et al. [30] | ✓ | ✓ | ✓ | ✓ | 3, 7, 28, 90 days | - | - | ✓ | - | Elemental mapping |
| Ouda and Gharieb [35] | ✓ | - | ✓ | ✓ | 1, 7, 14, 28 days | - | Thermogravimetric analysis | ✓ | - | - |
| Robayo-Salazar et al. [60] | ✓ | ✓ | ✓ | - | 7, 28 days | - | - | ✓ | - | Life cycle analysis |
| Robayo-Salazar et al. [33] | ✓ | ✓ | ✓ | - | 28-day | - | - | ✓ | - | Potential applications (bricks, pavers, roof tiles, tiles) |
| Guo et al. [61] | ✓ | ✓ | ✓ | ✓ | - | - | Pore properties | ✓ | - | - |
| Vafaei and Allahverdi [62] | ✓ | ✓ | ✓ | ✓ | 28-day | - | Acid resistance | ✓ | - | |
| Vafaei et al. [62] | ✓ | ✓ | ✓ | ✓ | At different durations over 24 months after exposure to acid | - | Acid resistance at mild exposures | ✓ | - | - |
| Mahmoodi et al. [63] | ✓ | ✓ | ✓ | ✓ | 7, 28 days | - | - | ✓ | - | Fresh properties (setting time and flowability tests) Environmental impacts (greenhouse gas emissions and energy consumption) |
| Ulugöl et al. [20] | ✓ | ✓ | ✓ | - | 24, 48, 72 h | - | - | ✓ | - | - |
| Wong et al. [64] | ✓ | ✓ | ✓ | - | 1, 28 days | - | - | ✓ | - | - |
| Migunthanna et al. [42] | ✓ | ✓ | ✓ | - | 1, 3, 5, 7, 14, 28, 60, 90 days | - | - | ✓ | - | Environmental impacts (CO2 emissions and energy consumption) |
| Migunthanna et al. [43] | ✓ | ✓ | ✓ | ✓ | 3, 7, 28 days | - | - | ✓ | - | Development of concrete targeting pavement applications |
| Cong et al. [65] | ✓ | ✓ | ✓ | ✓ | 7, 28 days | - | Thermogravimetric analysis | ✓ | - | Use of calcium carbide slag to act as both a precursor and activator |
| Reference | Compressive Strength (MPa) | Days | Curing Temperature (°C) | Relative Humidity (%) | Activator Type | Activator Content (by wt% of Binder) | Liquid/ Binder Ratio | Particle Size | Source of Brick Waste |
|---|---|---|---|---|---|---|---|---|---|
| Komnitsas et al. [32] | 49.5 | 7 | 90 | - | NaOH + Na2SiO3 | NaOH: 8M, 5% Na2SiO3: Ms 3.5, 6% | 0.38 | davg * = 140 µm d50 * = 6.6 µm | C&D waste |
| Silva et al. [22] | 37 ± 3 | 7 | 80 | - | NaOH + Na2SiO3 (act Ms = 0.6) | act Na2O = 8% | 0.27 | davg = n.a. * d50 = 19.66 µm | C&D waste |
| Reig et al. [29] | 14.75 ± 0.25 | 7 | 65 | - | NaOH | 5 M | 0.45 | davg = n.a. d50 = 20.9 µm | C&D waste |
| 29 ± 1 | 7 | 65 | - | NaOH + Na2SiO3 (act Ms = 1.6) | act Na2O = 7% | 0.45 | |||
| 50 ± 2.5 | 7 | 65 | - | NaOH + Na2SiO3 (act Ms = 2.0) | act Na2O = 7% | 0.30 | |||
| Fořt et al. [28] | 41.9 | 28 | 20 | - | NaOH + Na2SiO3 (act Ms = 1.4) | NaOH: 1.3% Na2SiO3: 35% | 0.57 | davg = 125 µm d50 = n.a. | Waste from brick grinding |
| Sassoni et al. [58] | Not assessed | - | - | - | NaOH + Na2SiO3+NaAlO2 | - | n.a | davg = n.a. d50 = 10 µm | Bricks with physical and geometric defects |
| Fořt et al. [57] | Not assessed | - | 60, 80 | 50 | NaOH + Na2SiO3 (act Ms = 0.8) | act Na2O = 7% | - | davg = 125 µm d50 = 50 µm | Waste from brick grinding |
| Tuyan et al. [18] | 36.2 | 5 | 90 | 40 | NaOH + Na2SiO3 (act Ms = 1.6) | act Na2O = 10% | 0.46 | davg = 100 µm d50 = n.a. | Bricks damaged during production |
| 34.9 | 7 | 80 | |||||||
| 0 | 3 | 25 ± 2 | |||||||
| 0 | 7 | ||||||||
| 5.3 | 28 | ||||||||
| 18.7 | 90 | ||||||||
| Rovnaník et al. [41] | 8 ± 0.5 | 7 | 21 ± 2 | 50 ± 2 | NaOH + Na2SiO3 (act Ms = 1.0) | 25% | 0.38 | davg = n.a. d50 = n.a. | Waste from brick skiving |
| 21 ± 1 | 28 | ||||||||
| 41 ± 5 | 90 | ||||||||
| Zawrah et al. [30] | ~3 | 3 | Ambient temperature | - | NaOH + Na2SiO3 (Vol. Na2SiO3 /NaOH =2.5) | 30% | 0.30 | davg = n.a. d50 = 1.1 µm | Waste produced during brick manufacturing |
| ~5 | 7 | ||||||||
| ~10 | 28 | ||||||||
| 15 | 90 | ||||||||
| Rovnaník et al. [59] | 7 ± 0.75 | 7 | 40 °C for 20h, after, at ambient temperature | - | NaOH + Na2SiO3 (act Ms = 1.4) | - | - | davg = n.a. d50 = 8.5 µm | Waste from brick grinding |
| Ouda and Gharieb [35] | ~15 | 7 | 80 °C for 24 h, after, at 40 °C | >95% | NaOH | 12% | 0.275 | davg = 90 µm d50 = n.a. | C&D waste |
| Robayo-Salazar et al. [33] | 7.49 | 28 | 25 °C | 90% | NaOH | Na2O = 10% | 0.23 | davg = n.a. d50 = 24.25 µm | C&D waste |
| 11.24 | 28 | 70 °C for 24 h, after, at 25 °C | NaOH | Na2O = 8% | |||||
| 54.38 | 28 | 25 °C | 90% | NaOH + Na2SiO3 (act Ms = 1.9) | Na2O = 10% | ||||
| 66.56 | 28 | 70 °C for 24 h, after, at 25 °C | NaOH + Na2SiO3 (act Ms = 1.9) | Na2O = 10% | |||||
| Robayo-Salazar et al. [60] | 2 ± 0.5 | 7 | 25 ± 3 | 80% | NaOH | 10% | 0.23 | davg = n.a. d50 = 24.25 µm | Rejected samples from the brick industry |
| 7 ± 0.5 | 28 | ||||||||
| 5 ± 0.5 | 7 | 25 ± 3 | 80% | NaOH + Na2SiO3 | 44% | 0.23 | |||
| 60 ± 2 | 28 | ||||||||
| Migunthanna et al. [42] | 5.4 | 28 | 25 ± 2 | - | Na2SiO3 (act Ms = 1, solid form) | 10% | 0.32 | davg = n.a. d50 = n.a. dmax = 150 µm | C&D waste |
| 10.5 | 90 | ||||||||
| ~2 | 28 | 25 ± 2 | - | Na2SiO3 (act Ms = 1, solid form) | 20% | 0.32 | |||
| ~5.5 | 90 | ||||||||
| Ulugöl et al. [20] | ~42 | 2 | 95 | - | NaOH | 10% | 0.35 | d50 = 7.6 µm d90 = 46.9 µm | C&D waste |
| ~45 | 3 | 105 | - | NaOH | 12% | ||||
| ~43 | 2 | 115 | - | NaOH | 15% |
| Reference | Maximum Compressive Strength (MPa) | Days | Curing Temperature (°C) | Relative Humidity (%) | Activator | Activator Content (by % Weight of Binder) | Liquid /Binder Ratio | Composition of the Precursor |
|---|---|---|---|---|---|---|---|---|
| Rovnaník et al. [41] | 21 ± 1 | 7 | 21 ± 2 | 50 ± 2 | NaOH + Na2SiO3 (activator Ms = 1.0) | 25% | 0.36 | 75% fly ash + 25% WCB |
| 20 ± 1.5 | 7 | 21 ± 2 | 50 ± 2 | 0.38 | 50% fly ash + 50% WCB | |||
| Guo et al. [61] | Not assessed | - | - | - | - | - | - | 70% fly ash + 30% WCB |
| Wong et al. [64] | 44.2 | 28 | amb.t | - | NaOH + Na2SiO3 (Wt. Na2SiO3/NaOH = 2.5) | 10M NaOH | 0.25 | 90% fly ash + 10% WCB |
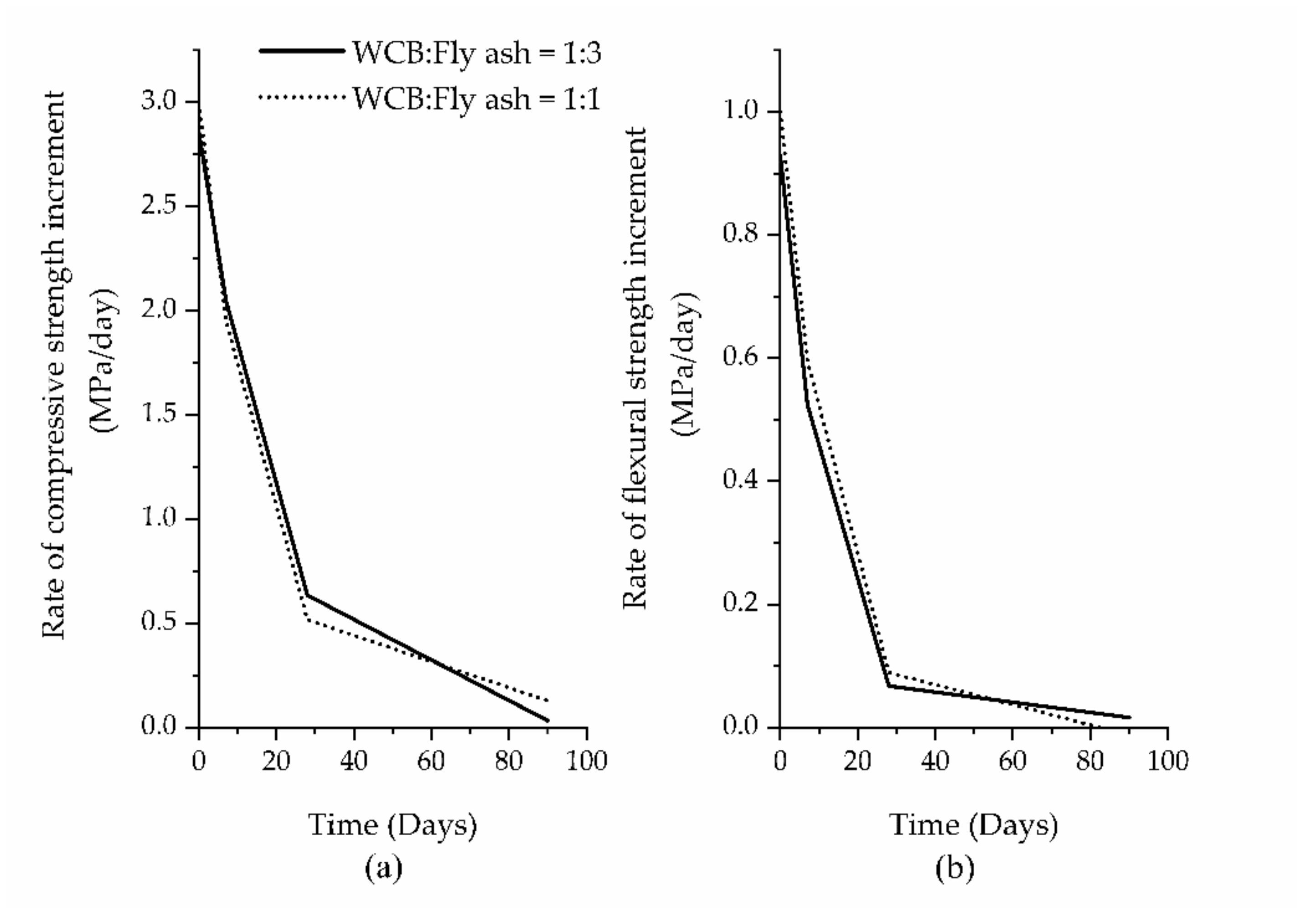
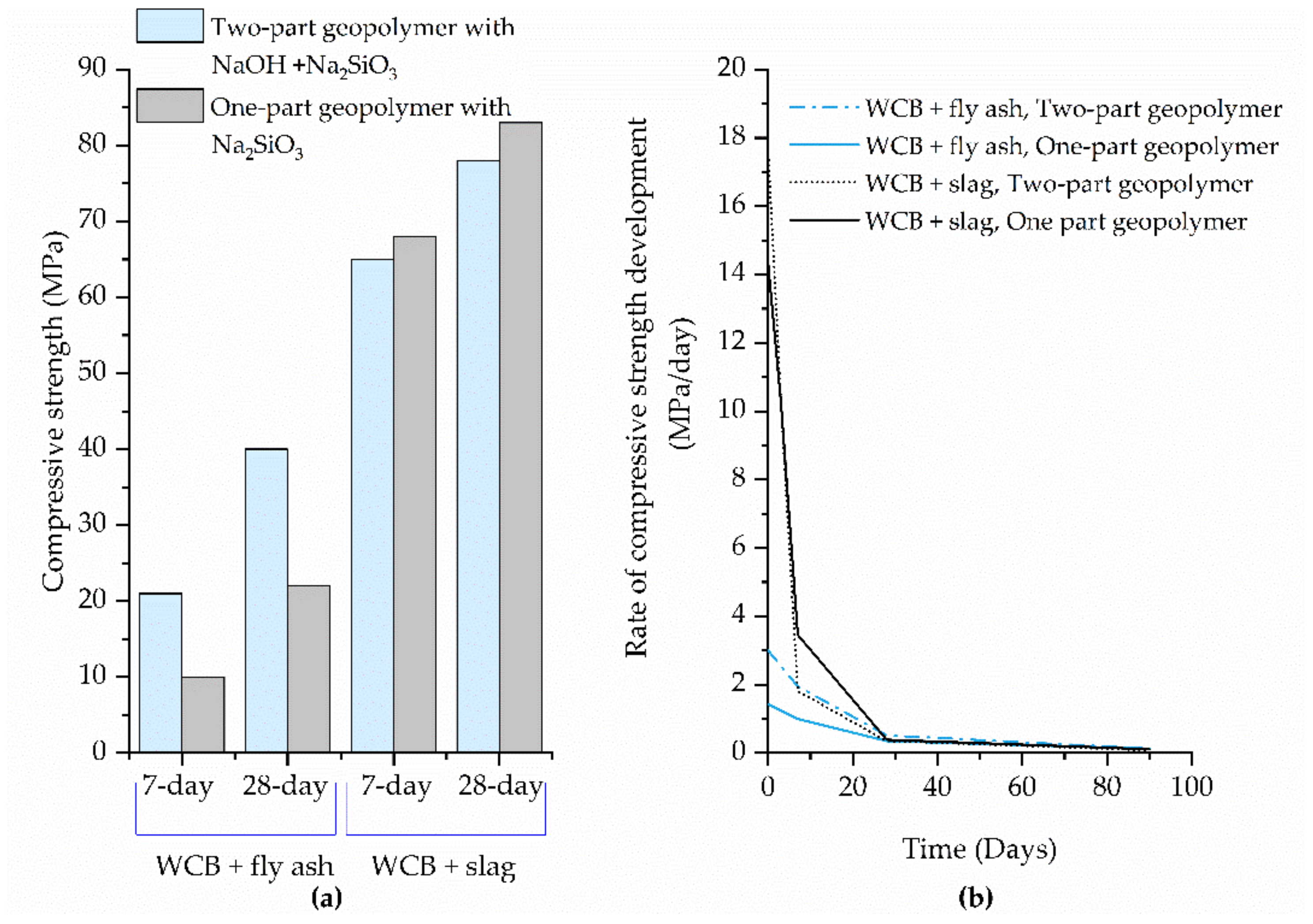
| Migunthanna et al. [42] | ~10 | 7 | 25 ± 2 | - | Na2SiO3 (solid) | 10% | 0.32 | 60% fly ash + 40% WCB |
| 22.4 | 28 | |||||||
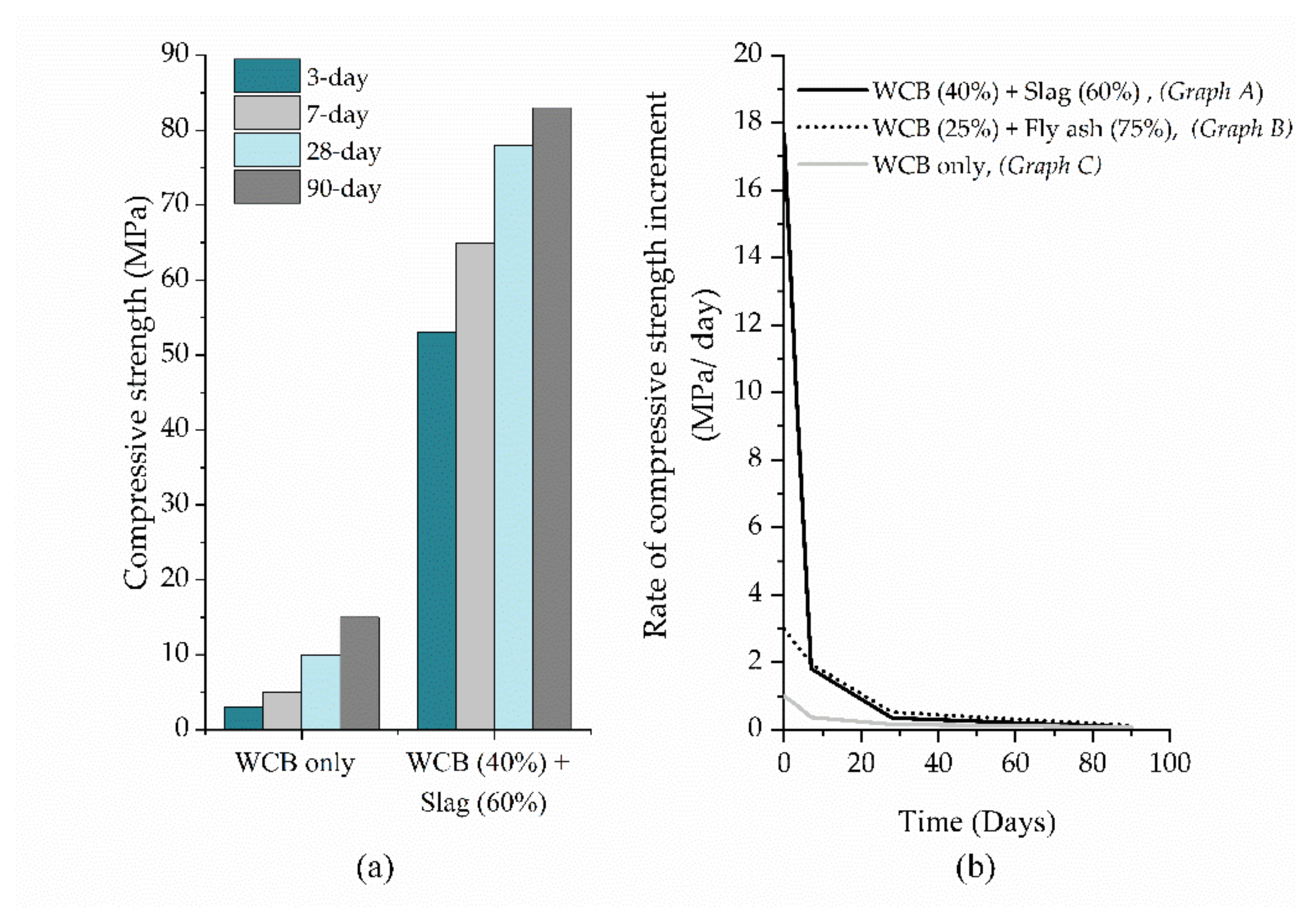
| Zawrah et al. [30] | ~65 | 7 | amb.t | - | NaOH + Na2SiO3 (Vol. Na2SiO3 /NaOH = 2.5) | 30% | 0.30 | 60% slag + 40% WCB |
| ~78 | 28 | |||||||
| Migunthanna et al. [42] | 68.3 | 7 | 25 ± 2 | - | Na2SiO3 (solid) | 10% | 0.32 | 60% slag + 40% WCB |
| 81.8 | 28 | |||||||
| Rovnaník et al. [59] | 23 ± 4 | 7 | 40 °C for 20 h, after, at amb. temp | - | NaOH + Na2SiO3 (act Ms = 1.4) | - | - | 75% metakaolin + 25% WCB |
| Ouda and Gharieb [35] | ~39 | 7 | 80 °C for 24 h, after, at 40 °C | >95 | NaOH | 12 M | 0.243 | 20% waste concrete powder + 80% WCB |
| ~34 | 7 | 0.263 | 10% calcined waste concrete powder + 90% WCB | |||||
| ~50 | 28 | 0.256 | 5% calcined waste concrete powder + 95% WCB | |||||
| Robayo-Salazar et al. [33] | 41.39 | 28 | 25 | 90 | NaOH | Na2O = 8% | 0.23 | 10% OPC + 90% WCB |
| 102.59 | 28 | 25 | 90 | NaOH + Na2SiO3 (act Ms = 1.3) | ~Na2O = 8.7% | 0.23 | 20% OPC + 80% WCB | |
| Robayo-Salazar et al. [60] | 26 ± 2 | 7 | 25 ± 3 | 80 | Na2SO4 | 6% | 0.23 | 30% OPC + 70% WCB |
| 34 ± 0.5 | 28 | |||||||
| 14 ± 1 | 7 | 25 ± 3 | 80 | Na2CO3 | 6% | 0.23 | 30% OPC + 70% WCB | |
| 22 ± 2 | 28 | |||||||
| 22 ± 1 | 7 | 25 ± 3 | 80 | NaOH | 9.3% | 0.23 | 10% OPC + 90% WCB | |
| 42 ± 5 | 28 | |||||||
| 28 ± 2 | 7 | 25 ± 3 | 80 | NaOH | 8.8% | 0.23 | 15% OPC + 85% WCB | |
| 35 ± 1 | 28 | |||||||
| 57 ± 3 | 7 | 25 ± 3 | 80 | NaOH + Na2SiO3 | 36% | 0.23 | 20% OPC + 80% WCB | |
| 102 ± 14 | 28 | |||||||
| Migunthanna et al. [43] | ~54 | 7 | 25 ± 2 | - | Na2SiO3 (solid) | 10% | 0.32 | 10% fly ash + 36% slag + 54% WCB |
| ~75 | 28 | |||||||
| ~65 | 7 | 25 ± 2 | - | Na2SiO3 (solid) | 10% | 0.32 | 20% fly ash + 48% slag + 32% WCB | |
| ~89 | 28 | |||||||
| ~66 | 7 | 25 ± 2 | - | Na2SiO3 (solid) | 10% | 0.32 | 30% fly ash + 56% slag + 14% WCB | |
| ~92 | 28 |
| Binder Composition (kg/m3) | W/B Ratio | Aggregate Composition (AC, kg/m3) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| WCB | Slag | Fly Ash | Activator | Fine Aggregates | Coarse Aggregates | ||||
| 14 mm | 7 mm | ||||||||
| Binder 1 | 144 | 216 | 0 | 40 | 0.49 | ||||
| Binder 2 | 129.6 | 194.4 | 36 | 40 | 0.47 | AC1 | 567.0 | 793.8 | 529.2 |
| Binder 3 | 115.2 | 172.8 | 72 | 40 | 0.45 | AC2 | 661.5 | 737.1 | 491.4 |
| Binder 4 | 50.4 | 201.6 | 108 | 40 | 0.45 | AC3 | 756.0 | 680.4 | 453.6 |
| Reference | Acid | Mass Loss | Compressive Strength Loss | ||
|---|---|---|---|---|---|
| WCB Mortar | OPC Mortar | WCB Mortar | OPC Mortar | ||
| Vafei et al. [108] | HCL, pH = 3 | 4.5% after 720 days | 16% after 720 days | 2.6% after 720 days | 68.2% after 720 days |
| H2SO4, pH = 3 | 43.4% after 720 days | 56.6% after 720 days | 83.1% after 720 days | 100% after 450 days | |
| Vafaei and Allahverdi [62] | HCL, pH = 1 | 36% after 180 days | 48% after 180 days | 100% after 150 days | 100% after 120 days |
| H2SO4, pH = 1 | 34% after 180 days | 58% after 180 days | 78% after 90 days | 100% after 90 days | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migunthanna, J.; Rajeev, P.; Sanjayan, J. Waste Clay Bricks as a Geopolymer Binder for Pavement Construction. Sustainability 2022, 14, 6456. https://doi.org/10.3390/su14116456
Migunthanna J, Rajeev P, Sanjayan J. Waste Clay Bricks as a Geopolymer Binder for Pavement Construction. Sustainability. 2022; 14(11):6456. https://doi.org/10.3390/su14116456
Chicago/Turabian StyleMigunthanna, Janitha, Pathmanathan Rajeev, and Jay Sanjayan. 2022. "Waste Clay Bricks as a Geopolymer Binder for Pavement Construction" Sustainability 14, no. 11: 6456. https://doi.org/10.3390/su14116456
APA StyleMigunthanna, J., Rajeev, P., & Sanjayan, J. (2022). Waste Clay Bricks as a Geopolymer Binder for Pavement Construction. Sustainability, 14(11), 6456. https://doi.org/10.3390/su14116456







