Sustainable Applications of Nanofibers in Agriculture and Water Treatment: A Review
Abstract
:1. Introduction
2. Natural Fibers and Nanofibers
3. Producing Nanofibers from Agro-Wastes
4. Applications of Nanofibers in Agriculture
5. Nanofibers for Water/Wastewater Treatment
6. Nanofibers for Food Packaging
7. Nanofibers for Biomedical Fields
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thyavihalli Girijappa, Y.G.; Mavinkere Rangappa, S.; Parameswaranpillai, J.; Siengchin, S. Natural fibers as sustainable and renewable resource for development of eco-friendly composites: A comprehensive review. Front. Mater. 2019, 6, 226. [Google Scholar] [CrossRef]
- Zindani, D.; Kumar, S.; Maity, S.R.; Bhowmik, S. Mechanical characterization of bio-epoxy green composites derived from sodium bicarbonate treated punica granatum short fiber agro-waste. J. Polym. Environ. 2021, 29, 143–155. [Google Scholar] [CrossRef]
- Mahmud, S.; Hasan, K.M.F.; Jahid, M.A.; Mohiuddin, K.; Zhang, R.; Zhu, J. Comprehensive review on plant fiber-reinforced polymeric biocomposites. J. Mater. Sci. 2021, 56, 7231–7264. [Google Scholar] [CrossRef]
- Gundloori, R.V.; Singam, A.; Killi, N. Nanobased intravenous and transdermal drug delivery systems,” in applications of targeted nano drugs and delivery systems. Appl. Target. Nano Drugs Deliv. Syst. 2019, 551–594. [Google Scholar] [CrossRef]
- Dikshit, V.; Goh, G.D.; Nagalingam, A.P.; Goh, G.L.; Yeong, W.Y. Recent progress in 3D printing of fiber-reinforced composite and nanocomposites. Fiber-Reinf. Nanocomp. Fundam. Appls 2020, 371–394. [Google Scholar] [CrossRef]
- Behera, A.; Mallick, P. Application of nanofibers in aerospace industry. Fiber-Reinf. Nanocomp. Fundam. Appl. 2020, 449–457. [Google Scholar] [CrossRef]
- Hasan, K.M.F.; Horváth, P.G.; Alpár, T. Potential natural fiber polymeric nanobiocomposites: A review. Polymers 2020, 12, 1072. [Google Scholar] [CrossRef]
- Barua, P.; Mahato, A.; Datta, P.; Sen, R.; Nandi, S.K.; Kundu, B. Fiber nanobiocompositions for cranioplasty and other orthopedic applications. Fiber-Reinf. Nanocomp. Fundam. Appl. 2020, 525–558. [Google Scholar] [CrossRef]
- Patil, A.Y.; Banapurmath, N.R.; Kotturshettar, B.B.; Lekha, K.; Roseline, M. Limpet teeth-based polymer nanocomposite: A novel alternative biomaterial for denture base application. Fiber-Reinf. Nanocomp. Fundam. Appl. 2020, 477–523. [Google Scholar] [CrossRef]
- Hallad, S.A.; Banapurmath, N.R.; Hunashyal, A.M.; Shravansa, S.S.; Shettar, A.S. Nanofiber-reinforced nanocomposites for structural applications. Fiber-Reinf. Nanocomp. Fundam. Appl. 2020, 559–567. [Google Scholar] [CrossRef]
- Sathishkumar, T.P.; Ramakrishnan, S. Mechanical properties of nanococonut shell filler mixed jute mat-reinforced epoxy composites for structure application. Fiber-Reinf. Nanocomp. Fundam. Appl. 2020, 459–476. [Google Scholar] [CrossRef]
- Nishino, T. Cellulose fiber/nanofiber from natural sources including waste-based sources. Green Comp. 2017, 19–38. [Google Scholar] [CrossRef]
- De Jorge, B.C.; Gross, J. Smart nanotextiles for application in sustainable agriculture. Nanosens. Nanodev. Smart Multifunct. Text. 2021, 203–227. [Google Scholar] [CrossRef]
- Kumar, S.K.S.; Prakash, C. Characterization of electrospun polyurethane/polyacrylonitrile nanofiber for protective textiles. Iran. Polym. J. 2021, 30, 1263–1271. [Google Scholar] [CrossRef]
- Mallakpour, S.; Radfar, Z.; Hussain, C.M. Current advances on polymer-layered double hydroxides/metal oxides nanocomposites and bionanocomposites: Fabrications and applications in the textile industry and nanofibers. Appl. Clay Sci. 2021, 206, 106054. [Google Scholar] [CrossRef]
- Morais, F.P.; Carta, A.M.M.S.; Amaral, M.E.; Curto, J.M.R. Micro/nano-fibrillated cellulose (MFC/NFC) fibers as an additive to maximize eucalyptus fibers on tissue paper production. Cellulose 2021, 28, 6587–6605. [Google Scholar] [CrossRef]
- Sethi, J.; Oksman, K.; Illikainen, M.; Sirviö, J.A. Sonication-assisted surface modification method to expedite the water removal from cellulose nanofibers for use in nanopapers and paper making. Carbohydr. Polym. 2018, 197, 92–99. [Google Scholar] [CrossRef]
- Solikhin, A.; Pranata, A.W.; Muchtar, T.; Suzuki, S.; Kojima, Y.; Kobori, H. Research mapping of Indonesia nano-lignocellulose fiber studies and its potential for industrial application. SN Appl. Sci. 2020, 2, 564. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Wang, Y.; Gong, C.; Liu, B.; Wei, G. Production, structural design, functional control, and broad applications of carbon nanofiber-based nanomaterials: A comprehensive review. Chem. Eng. J. 2020, 402, 126189. [Google Scholar] [CrossRef]
- Kumar, K.A.; Zare, E.N.; Torres-Mendieta, R.; Wacławek, S.; Makvandi, P.; Černík, M.; Padil, V.V.; Varma, R.S. Electrospun fibers based on botanical, seaweed, microbial, and animal sourced biomacromolecules and their multidimensional applications. Int. J. Biol. Macromol. 2021, 171, 130–149. [Google Scholar] [CrossRef]
- Campano, C.; Merayo, N.; Negro, C.; Blanco, Á. Low-fibrillated bacterial cellulose nanofibers as a sustainable additive to enhance recycled paper quality. Int. J. Biol. Macromol. 2018, 114, 1077–1083. [Google Scholar] [CrossRef]
- Zamel, D.; Khan, A.U. Bacterial immobilization on cellulose acetate based nanofibers for methylene blue removal from wastewater: Mini-review. Inorg. Chem. Commun. 2021, 131, 108766. [Google Scholar] [CrossRef]
- Shao, Y.; Fan, Z.; Zhong, M.; Xu, W.; He, C.; Zhang, Z. Polypyrrole/bacterial cellulose nanofiber composites for hexavalent chromium removal. Cellulose 2021, 28, 2229–2240. [Google Scholar] [CrossRef]
- Wang, B.; Ji, P.; Ma, Y.; Song, J.; You, Z.; Chen, S. Bacterial cellulose nanofiber reinforced poly(glycerolsebacate) biomimetic matrix for 3D cell culture. Cellulose 2021, 28, 8483–8492. [Google Scholar] [CrossRef]
- Huang, Q.; Zhao, C.; Li, X. Enhanced electrolyte retention capability of separator for lithium-ion battery constructed by decorating ZIF-67 on bacterial cellulose nanofiber. Cellulose 2021, 28, 3097–3112. [Google Scholar] [CrossRef]
- Partheniadis, I.; Nikolakakis, I.; Laidmäe, I.; Heinämäki, J. A mini-review: Needleless electrospinning of nanofibers for pharmaceutical and biomedical applications. Processes 2020, 8, 673. [Google Scholar] [CrossRef]
- Badgar, K.; Prokisch, J.; El-Ramady, H. Nanofibers for sustainable agriculture: A short communication. Egypt. J. Soil. Sci. 2021, 61, 3. [Google Scholar] [CrossRef]
- Bensalah, H.; Raji, M.; Abdellaoui, H.; Essabir, H.; Bouhfid, R.; el kacem Qaiss, A. Thermo-mechanical properties of low-cost ‘green’ phenolic resin composites reinforced with surface modified coir fiber. Int. J. Adv. Manuf. Technol. 2021, 112, 1917–1930. [Google Scholar] [CrossRef]
- Kuram, E. Advances in development of green composites based on natural fibers: A review. Emergent Mater. 2021. [Google Scholar] [CrossRef]
- Barhoum, A.; Rasouli, R.; Yousefzadeh, M.; Rahier, H.; Bechelany, M. Nanofiber technologies: History and development. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A.S.H., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 3–43. [Google Scholar]
- Mahendra, I.P.; Putra, A.E.; Ghifari, M.A.; Yanti, D.D.; Ariwahjoedi, B.; Ngoc, H.M.; Mendez, J.A. Lignocellulose nanofiber incorporated N-TiO2 for wound dressing. Cellulose 2021, 28, 10477–10483. [Google Scholar] [CrossRef]
- Sakovich, A.G.V.; Skiba, E.A.; Gladysheva, E.K.; Golubev, D.S.; Budaev, V.V. Miscanthus as a feedstock for the production of bacterial nanocellulose. Dokl. Chem. 2020, 495, 205–208. [Google Scholar] [CrossRef]
- Kumar, A.; Biswal, M.; Mohanty, S.; Nayak, S.K. Recent developments of lignocellulosic natural fiber reinforced hybrid thermosetting composites for high-end structural applications: A review. J. Polym. Res. 2021, 28, 459. [Google Scholar] [CrossRef]
- Shafik, E.S. Natural rubber biocomposites based on nanocrystalline and modified nanocrystalline cellulose: Curing, mechanical, thermal and electrical properties. J. Polym. Res. 2021, 28, 390. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Separat. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Gugulothu, D.; Barhoum, A.; Nerella, R.; Ajmer, R.; Bechelany, M. Fabrication of nanofibers: Electrospinning and non-electrospinning techniques. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A.S.H., Eds.; Springer Nature: Cahm, Switzerland, 2019; pp. 45–77. [Google Scholar] [CrossRef]
- Malik, S.; Sundarrajan, S.; Hussain, T.; Nazir, A.; Ayyoob, M.; Berto, F.; Ramakrishna, S. Sustainable nanofibers in tissue engineering and biomedical applications. Mat. Des. Process. Comms. 2021, 3, e202. [Google Scholar] [CrossRef]
- Naidu, K.C.B.; Kumar, N.S.; Banerjee, P.; Reddy, B.V.S. A review on the origin of nanofibers/nanorods structures and applications. J. Mater. Sci. Mater. Med. 2021, 32, 68. [Google Scholar] [CrossRef] [PubMed]
- Muralidhar, N.; Vadivuchezhian, K.; Arumugam, V.; Srinivasula Reddy, I. Flexural modulus of epoxy composite reinforced with Arecanut husk fibre (AHF): A mechanics’ approach. Mater. Today Proc. 2020, 27, 2265–2268. [Google Scholar] [CrossRef]
- Singh, T. Optimum design based on fabricated natural fiber reinforced automotive brake friction composites using hybrid CRITIC-MEW approach. J. Mater. Res. Technol. 2021, 14, 81–92. [Google Scholar] [CrossRef]
- Wei, W.; Luo, Q.; Liu, Y.; Qu, R.; Sun, D.; Gao, F.; Li, B.; Wu, M. Feasibility of preparing nanofiber reinforcer of gelatin hydrogel from waste peach branches. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Falade, A.O. Valorization of agricultural wastes for production of biocatalysts of environmental significance: Towards a sustainable environment. Environ. Sustain. 2021, 4, 317–328. [Google Scholar] [CrossRef]
- Nang An, V.; Chi Nhan, H.T.; Tap, T.D.; Van, T.T.T.; Van Viet, P.; Van Hieu, L. Extraction of high crystalline nanocellulose from biorenewable sources of vietnamese agricultural wastes. J. Polym. Environ. 2020, 25, 1465–1474. [Google Scholar] [CrossRef]
- Pennells, J.; Godwin, I.D.; Amiralian, N.; Martin, D.J. Trends in the production of cellulose nanofibers from non-wood sources. Cellulose 2020, 27, 575–593. [Google Scholar] [CrossRef]
- Shih, Y.-F.; Kotharangannagari, V.K.; Tsou, T.-C. Development of eco-friendly modified cellulose nanofiber reinforced polystyrene nanocomposites: Thermal, mechanical, and optical properties. J. Polym. Res. 2020, 27, 181. [Google Scholar] [CrossRef]
- Ali, M.A.S.S.; Jimat, D.N.; Nawawi, W.M.F.W.; Sulaiman, S. Antibacterial, mechanical and thermal properties of PVA/Starch composite film reinforced with cellulose nanofiber of sugarcane bagasse. Arab. J. Sci. Eng. 2021. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Kano, F.S.; Rosa, D.S. Lignocellulosic nanofiber from eucalyptus waste by a green process and their influence in bionanocomposites. Waste Biomass Valor. 2020, 11, 3761–3774. [Google Scholar] [CrossRef]
- Mousavi Kalajahi, S.E.; Alizadeh, A.; Hamishehkar, H.; Almasi, H.; Asefi, N. Orange juice processing waste as a biopolymer base for biodegradable film formation reinforced with cellulose nanofiber and activated with nettle essential oil. J. Polym. Environ. 2021. [Google Scholar] [CrossRef]
- Naeimi, A.; Khoshkam, S.; Eslaminejad, T. Natural cellulose fibers from Quinoa wastes reinforced carbon nanotube/ZnO bio-nanocomposite as a novel recyclable catalyst for oxidation reaction. Polym. Bull. 2021. [Google Scholar] [CrossRef]
- Narita, C.; Okahisa, Y.; Yamada, K. A novel approach of adhesive property of cellulose nanofibers obtained from the discarded wooden part of Kozo plant. Appl. Nanosc. 2021, 11, 2717–2726. [Google Scholar] [CrossRef]
- Saadat, S.; Emam-Djomeh, Z.; Askari, G. Antibacterial and antioxidant gelatin nanofiber scaffold containing ethanol extract of pomegranate peel: Design, characterization and in vitro assay. Food Bioprocess. Technol. 2021, 14, 935–944. [Google Scholar] [CrossRef]
- Asada, C.; Katsura, K.; Suzuki, A.; Nakamura, Y. Extraction, separation, and utilization of components contained in waste bamboo by pressurized microwave-assisted ethanol solvent treatment. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Laosirisathian, N.; Saenjum, C.; Sirithunyalug, J.; Eitssayeam, S.; Chaiyana, W.; Sirithunyalug, B. PVA/PVP K90 nanofibers containing punica granatum peel extract for cosmeceutical purposes. Fibers Polym. 2021, 22, 36–48. [Google Scholar] [CrossRef]
- De Souza, A.G.R.; Barbosa, F.S.; Rosa, D.S. Nanocellulose from industrial and agricultural waste for further use in PLA composites. J. Polym. Environ. 2020, 28, 1851–1868. [Google Scholar] [CrossRef]
- Asmatulu, R.; Khan, W.S. Electrospun nanofibers for agriculture and food industries, in Synthesis and Applications of Electrospun Nanofibers. Synth. Appl. Electrospun Nanofibers 2019, 89–109. [Google Scholar] [CrossRef]
- Meraz-Dávila, S.; Pérez-García, C.E.; Feregrino-Perez, A.A. Challenges and advantages of electrospun nanofibers in agriculture: A review. Mater. Res. Express 2021, 8, 042001. [Google Scholar] [CrossRef]
- Raja, K.; Prabhu, C.; Subramanian, K.S.; Govindaraju, K. Electrospun polyvinyl alcohol (PVA) nanofibers as carriers for hormones (IAA and GA3) delivery in seed invigoration for enhancing germination and seedling vigor of agricultural crops (groundnut and black gram). Polym. Bull. 2021, 78, 6429–6440. [Google Scholar] [CrossRef]
- Saito, H.; Yamashita, Y.; Sakata, N.; Ishiga, T.; Shiraishi, N.; Usuki, G.; Nguyen, V.T.; Yamamura, E.; Ishiga, Y. Covering soybean leaves with cellulose nanofiber changes leaf surface hydrophobicity and confers resistance against Phakopsora pachyrhizi. Front. Plant Sci. 2021, 12, 726565. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, L.M.; Genro, C.; Roggia, I.; Bender, S.D.S.; Bender, R.; Pereira, C. Innovative rice seed coating (Oryza sativa) with polymer nanofibres and microparticles using the electrospinning method. J. Res. Updates Polym. Sci. 2014, 3, 33–39. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Elumalai, G.; Rajiv, S. Environment friendly synthesis of polyvinylpyrrolidone nanofibers and their potential use as seed coats. New J. Chem. 2016, 40, 3268–3276. [Google Scholar] [CrossRef]
- Farias, B.V.; Pirzada, T.; Mathew, R.; Sit, T.L.; Opperman, C.; Khan, S.A. Electrospun polymer nanofibers as seed coatings for crop protection. ACS Sustain. Chem. Eng. 2019, 7, 19848–19856. [Google Scholar] [CrossRef]
- Sivalingam, S.; Kunhilintakath, A.; Nagamony, P.; Paspulathi Parthasarathy, V. Fabrication, toxicity and biocompatibility of Sesamum indicum infused graphene oxide nanofiber—A novel green composite method. Appl. Nanosci. 2021, 11, 679–686. [Google Scholar] [CrossRef]
- Urbina, L.; Corcuera, M.Á.; Gabilondo, N.; Eceiza, A.; Retegi, A. A review of bacterial cellulose: Sustainable production from agricultural waste and applications in various fields. Cellulose 2021, 28, 8229–8253. [Google Scholar] [CrossRef]
- Bose, P. Agricultural Applications of Nanofibers. AZoNano. 2021. Available online: https://www.azonano.com/article.aspx?ArticleID=5834 (accessed on 10 November 2021).
- Espinoza Márquez, E.; Soto Zarazúa, G.M.; de Pérez Bueno, J.J. Prospects for the use of electrooxidation and electrocoagulation techniques for membrane filtration of irrigation water. Environ. Process. 2020, 7, 391–420. [Google Scholar] [CrossRef]
- Latha, M.; Raja, K.; Subramanian, K.; Karthikeyan, M.; Lakshmanan, A. Fabrication and characterization of tebuconazole loaded PVA nanofiber. Int. J. Agric. Sci. 2019, 10, 8514–8517. [Google Scholar]
- Osanloo, M.; Arish, J.; Sereshti, H. Developed methods for the preparation of electrospun nanofibers containing plant-derived oil or essential oil: A systematic review. Polym. Bull. 2020, 77, 6085–6104. [Google Scholar] [CrossRef]
- Mehrani, Z.; Ebrahimzadeh, H.; Moradi, E. Use of aloin-based and rosin-based electrospun nanofibers as natural nanosorbents for the extraction of polycyclic aromatic hydrocarbons and phenoxyacetic acid herbicides by microextraction in packed syringe method prior to GC-FID detection. Microchim. Acta 2020, 187, 401. [Google Scholar] [CrossRef]
- Ma, J.; Yu, Z.; Liu, S.; Chen, Y.; Lv, Y.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y.; Liu, M.; et al. Efficient extraction of trace organochlorine pesticides from environmental samples by a polyacrylonitrile electrospun nanofiber membrane modified with covalent organic framework. J. Hazard. Mater. 2022, 424, 127455. [Google Scholar] [CrossRef]
- Dilfi, A.K.F.; Che, Z.; Xian, G. Grafting of nano-silica onto ramie fiber for enhanced mechanical and interfacial properties of ramie/epoxy composite. J. Zhejiang Univ. Sci. A 2019, 20, 660–674. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Q.; Sun, X.; Yue, Y.; Tubana, B.; Yang, R.; Cheng, HN. Novel alginate-cellulose nanofiber-poly(vinyl alcohol) hydrogels for carrying and delivering nitrogen, phosphorus and potassium chemicals. Int. J. Biol. Macromol. 2021, 172, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Mirheidari, F.; Hatami, M.; Ghorbanpour, M. Effect of different concentrations of IAA, GA3 and chitosan nano-fiber on physio-morphological characteristics and metabolite contents in roselle (Hibiscus sabdariffa L.). S. Afr. J. Bot 2021, S0254629921002805. [Google Scholar] [CrossRef]
- Natarelli, C.V.L.; Lopes, C.M.S.; Carneiro, J.S.S.; Melo, L.C.A.; Oliveira, J.E.; Medeiros, E.S. Zinc slow-release systems for maize using biodegradable PBAT nanofibers obtained by solution blow spinning. J. Mater. Sci. 2021, 56, 4896–4908. [Google Scholar] [CrossRef]
- Tamilarasan, C.; Raja, K.; Subramanian, K.; Selvaraju, P. Synthesis and development of nano formulation for hastening seed quality in groundnut. Res. J. Agric. Sci. 2019, 10, 50–57. [Google Scholar]
- Nooeaid, P.; Chuysinuan, P.; Pitakdantham, W.; Aryuwananon, D.; Techasakul, S.; Dechtrirat, D. Eco-friendly polyvinyl alcohol/polylactic acid core/shell structured fibers as controlled-release fertilizers for sustainable agriculture. J. Polym. Environ. 2021, 29, 552–564. [Google Scholar] [CrossRef]
- Itroutwar, P.D.; Govindaraju, K.; Tamilselvan, S.; Kannan, M.; Raja, K.; Subramanian, K.S. Seaweed-based biogenic zno nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.). J. Plant Growth Regul. 2020, 39, 717–728. [Google Scholar] [CrossRef]
- Sundaran, S.P.; Reshmi, C.R.; Sagitha, P.; Manaf, O.; Sujith, A. Multifunctional graphene oxide loaded nanofibrous membrane for removal of dyes and coliform from water. J. Environ. Manag. 2019, 240, 494–503. [Google Scholar] [CrossRef]
- Raza, Z.A.; Munim, S.A.; Ayub, A. Recent developments in polysaccharide-based electrospun nanofibers for environmental applications. Carbohydr. Res. 2021, 510, 108443. [Google Scholar] [CrossRef] [PubMed]
- Naragund, V.S.; Panda, P.K. Electrospun polyacrylonitrile nanofiber membranes for air filtration application. Int. J. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Sakib, M.N.; Mallik, A.K.; Rahman, M.M. Update on chitosan-based electrospun nanofibers for wastewater treatment: A review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100064. [Google Scholar] [CrossRef]
- Yavari Maroufi, L.; Ghorbani, M.; Mohammadi, M.; Pezeshki, A. Improvement of the physico-mechanical properties of antibacterial electrospun poly lactic acid nanofibers by incorporation of guar gum and thyme essential oil. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126659. [Google Scholar] [CrossRef]
- Sonwane, N.D.; Kondawar, S.B. Enhanced room temperature ammonia sensing of electrospun nickel cobaltite/polyaniline composite nanofibers. Mater. Lett. 2021, 303, 130566. [Google Scholar] [CrossRef]
- Xia, L.; Feng, H.; Zhang, Q.; Luo, X.; Fei, P.; Li, F. Centrifugal spinning of lignin amine/cellulose acetate nanofiber for heavy metal ion adsorption. Fibers Polym. 2021. [Google Scholar] [CrossRef]
- Yadav, P.; Farnood, R.; Kumar, V. HMO-incorporated electrospun nanofiber recyclable membranes: Characterization and adsorptive performance for Pb(II) and As(V). J. Environ. Chem. Eng. 2021, 9, 106507. [Google Scholar] [CrossRef]
- Zhang, W.; He, Z.; Han, Y.; Jiang, Q.; Zhan, C.; Zhang, K.; Li, Z.; Zhang, R. Structural design and environmental applications of electrospun nanofibers. Compos. Part A Appl. Sci. Manuf. 2020, 137, 106009. [Google Scholar] [CrossRef]
- Li, J.; Bendi, R.; Malla, R.; Shah, K.J.; Parida, K.; You, Z. Cellulose nanofibers-based green nanocomposites for water environmental sustainability: A review. Emergent Mater. 2021, 4, 1259–1273. [Google Scholar] [CrossRef]
- Salehi, M.; Sharafoddinzadeh, D.; Mokhtari, F.; Esfandarani, M.S.; Karami, S. Electrospun nanofibers for efficient adsorption of heavy metals from water and wastewater. CTR 2021, 1, 1–33. [Google Scholar] [CrossRef]
- Assaifan, A.K.; Aijaz, M.O.; Luqman, M.; Drmosh, Q.A.; Karim, M.R.; Alharbi, H.F. Removal of cadmium ions from water using coaxially electrospun PAN/ZnO-encapsulated PVDF nanofiber membranes. Polym. Bull. 2021. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, L. Preparation of flexible electrospun AOPAN/PVDF membranes for removing Pb2+ from water. Appl. Water Sci. 2021, 11, 51. [Google Scholar] [CrossRef]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Chi, K.; Fung, E.; Aubrecht, K.; Keroletswe, N.; Chigome, S.; Hsiao, B.S. Nitro-oxidized carboxycellulose nanofibers from moringa plant: Effective bioadsorbent for mercury removal. Cellulose 2021, 28, 8611–8628. [Google Scholar] [CrossRef]
- Cárdenas Bates, I.I.; Loranger, É.; Mathew, A.P.; Chabot, B. Cellulose reinforced electrospun chitosan nanofibers bio-based composite sorbent for water treatment applications. Cellulose 2021, 28, 4865–4885. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, B.; Fan, L.; Chen, F.; Fu, Q. Adsorbability of Modified PBS nanofiber membrane to heavy metal ions and dyes. J. Polym. Environ. 2021, 29, 3029–3039. [Google Scholar] [CrossRef]
- Fiol, N.; Tarres, Q.; Vasquez, M.G.; Pereira, M.A.; Mendonca, R.T.; Mutje, P.; Delgado-Aguilar, M. Comparative assessment of cellulose nanofibers and calcium alginate beads for continuous Cu(II) adsorption in packed columns: The influence of water and surface hydrophobicity. Cellulose 2021, 28, 4327–4344. [Google Scholar] [CrossRef]
- He, Y.; Tian, H.; Xiang, A.; Wang, H.; Li, J.; Luo, X.; Rajulu, AV. Fabrication of PVA nanofibers grafted with octaamino-poss and their application in heavy metal adsorption. J. Polym. Environ. 2021, 29, 1566–1575. [Google Scholar] [CrossRef]
- Heidarzadeh-Samani, M.; Behzad, T.; Mehrabani-Zeinabad, A. Development of a continuous fixed-bed column to eliminate cadmium(II) ions by starch-g-poly(acrylic acid)/cellulose nanofiber bio-nanocomposite hydrogel. Environ. Sci. Pollut. Res. Int. 2021, 28, 57902–57917. [Google Scholar] [CrossRef]
- Juntadech, T.; Nantasenamat, C.; Chitpong, N. Oxidized regenerated cellulose nanofiber membranes for capturing heavy metals in aqueous solutions. Cellulose 2021, 28, 11465–11482. [Google Scholar] [CrossRef]
- Mohammed, Y.A.Y.A.; Ma, F.; Liu, L.; Zhang, C.; Dong, H.; Wang, Q.; Xu, X.; Al Wahbi, A.A. Preparation of electrospun polyvinylidene fluoride/amidoximized polyacrylonitrile nanofibers for trace metal ions removal from contaminated water. J. Porous Mater. 2021, 28, 383–392. [Google Scholar] [CrossRef]
- Rajabi, S.; Shaki, H. Efficient removal of lead and copper from aqueous solutions by using modified polyacrylonitrile nanofiber membranes. Fibers Polym. 2021, 22, 694–702. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, C.; Qin, X. A visually observable copper ion adsorption membrane by electrospinning combined with copper ion probe. Fibers Polym. 2021, 22, 1844–1852. [Google Scholar] [CrossRef]
- Mahmoud, R.K.; Kotp, A.A.; El-Deen, A.G.; Farghali, A.A.; Abo El-Ela, F.I. Novel and effective Zn-Al-GA LDH anchored on nanofibers for high-performance heavy metal removal and organic decontamination: Bioremediation approach. Water Air Soil Pollut. 2020, 231, 363. [Google Scholar] [CrossRef]
- Xue, L.; Ren, J.; Wang, S.; Qu, D.; Wei, Z.; Yang, Q.; Li, Y. Preparation of nanofiber aerogels by electrospinning and studying of its adsorption properties for heavy-metal and dyes. J. Porous Mater. 2020, 27, 1589–1599. [Google Scholar] [CrossRef]
- Agrawal, S.; Ranjan, R.; Lal, B.; Rahman, A.; Singh, S.P.; Selvaratnam, T.; Nawaz, T. Synthesis and Water Treatment Applications of Nanofibers by Electrospinning. Processes 2021, 9, 1779. [Google Scholar] [CrossRef]
- Uddin, Z.; Ahmad, F.; Ullan, T.; Nawab, Y.; Ahmad, S.; Azam, F.; Rasheed, A.; Zafar, M.S. Recent trends in water purification using electrospun nanofibrous membranes. Int. J. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Chen, H.; Huang, M.; Liu, Y.; Meng, L.; Ma, M. Functionalized electrospun nanofiber membranes for water treatment: A review. Sci. Total Environ. 2020, 739, 139944. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Sazali, N.; Salleh, W.N.W.; Ismail, A.F. Nanocellulose-based materials and recent application for heavy metal removal. Water Air Soil Pollut. 2021, 232, 305. [Google Scholar] [CrossRef]
- Jahan, I.; Zhang, L. Natural polymer-based electrospun nanofibrous membranes for wastewater treatment: A review. J. Polym. Environ. 2021. [Google Scholar] [CrossRef]
- Marinho, B.A.; de Souza, S.M.A.G.U.; de Souza, A.A.U.; Hotza, D. Electrospun TiO2 nanofibers for water and wastewater treatment: A review. J. Mater. Sci. 2021, 56, 5428–5448. [Google Scholar] [CrossRef]
- Sjahro, N.; Yunus, R.; Abdullah, L.C.; Rashid, S.A.; Asis, A.J.; Akhlisah, Z.N. Recent advances in the application of cellulose derivatives for removal of contaminants from aquatic environments. Cellulose 2021, 28, 7521–7557. [Google Scholar] [CrossRef]
- El-Aswar, E.I.; Ramadan, H.; Elkik, H.; Taha, A.G. A comprehensive review on preparation, functionalization and recent applications of nanofiber membranes in wastewater treatment. J. Environ. Manag. 2022, 301, 113908. [Google Scholar] [CrossRef] [PubMed]
- Ajalloueian, F.; Guerra, P.R.; Bahl, M.I.; Torp, A.M.; Hwu, E.T.; Licht, T.R.; Boisen, A. Multi-layer PLGA-pullulan-PLGA electrospun nanofibers for probiotic delivery. Food Hydrocoll. 2022, 123, 107112. [Google Scholar] [CrossRef]
- Shi, Y.; Li, D.; Kong, Y.; Zhang, R.; Gua, Q.; Hu, M.; Tian, S.; Jin, W. Enhanced antibacterial efficacy and mechanism of octyl gallate/beta-cyclodextrins against Pseudomonas fluorescens and Vibrio parahaemolyticus and incorporated electrospun nanofibers for Chinese giant salamander fillets preservation. Int. J. Food Microbiol. 2022, 361, 109460. [Google Scholar] [CrossRef] [PubMed]
- Maria Leena, M.; Vimala Bharathi, S.K.; Moses, J.A.; Anandharamakrishnan, C. Potential applications of nanofibers in beverage. Nanoeng. Beverage Ind. 2020, 20, 333–368. [Google Scholar] [CrossRef]
- Forghani, S.; Almasi, H.; Moradi, M. Electrospun nanofibers as food freshness and time-temperature indicators: A new approach in food intelligent packaging. Innov. Food Sci. Emerg. Technol. 2021, 73, 102804. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, SA.; Razavi, R.; Rasouli, Y.; Ghorbani, M.; Divsalar, E.; Tajik, H.; Guimarães, JT.; Ibrahim, S.A. Review of microbiological methods for testing protein and carbohydrate-based antimicrobial food packaging. Trends Food Sci. Technol. 2021, 111, 595–609. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res. Int. 2021, 141, 110113. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Pandey, G.; Rawtani, D. Functionalized nanomaterials driven antimicrobial food packaging: A technological advancement in food science. Food Control 2022, 131, 108469. [Google Scholar] [CrossRef]
- Lakshmi Balasubramaniam, S.; Patel, A.S.; Nayak, B.; Howell, C.; Skonberg, D. Antioxidant and antimicrobial modified cellulose nanofibers for food applications. Food Biosci. 2021, 44, 101421. [Google Scholar] [CrossRef]
- Aman Mohammadi, M.; Ramezani, S.; Hosseini, H.; Mortazavian, A.M.; Hosseini, S.M.; Ghorbani, M. Electrospun antibacterial and antioxidant zein/polylactic acid/hydroxypropyl methylcellulose nanofibers as an active food packaging system. Food Bioprocess Technol. 2021, 14, 1529–1541. [Google Scholar] [CrossRef]
- Duan, M.; Yu, S.; Sun, J.; Jiang, H.; Zhao, J.; Tong, C.; Hu, Y.; Pang, J.; Wu, C. Development and characterization of electrospun nanofibers based on pullulan/chitin nanofibers containing curcumin and anthocyanins for active-intelligent food packaging. Int. J. Biol. Macromol. 2021, 187, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Heidari, H.; Khomeiri, M.; Yousefi, H.; Rafieian, M.; Kashiri, M. Chitin nanofiber-based nanocomposites containing biodegradable polymers for food packaging applications. J. Consum. Prot. Food Saf. 2021, 16, 237–246. [Google Scholar] [CrossRef]
- Bodbodak, S.; Shahabi, N.; Mohammadi, M.; Ghorbani, M.; Pezeshki, A. Development of a novel antimicrobial electrospun nanofiber based on polylactic acid/hydroxypropyl methylcellulose containing pomegranate peel extract for active food packaging. Food Bioprocess Technol. 2021, 14, 2260–2272. [Google Scholar] [CrossRef]
- Asim, N.; Badiei, M.; Mohammad, M. Recent advances in cellulose-based hydrophobic food packaging. Emergent Mater. 2021. [Google Scholar] [CrossRef]
- Civan, S.; Aydin, S.; Aladag Tanik, N.; Aykut, Y. Cellulose monoacetate/tetraethyl orthosilicate hybrid nanofibers for electrochemical DNA biosensors. Fibers Polym. 2021, 22, 981–988. [Google Scholar] [CrossRef]
- Winzenburg, G.; Schmidt, C.; Fuchs, S.; Kissel, T. Biodegradable polymers and their potential use in parenteral veterinary drug delivery systems. Adv. Drug Deliv. Rev. 2004, 56, 1453–1466. [Google Scholar] [CrossRef]
- Souza, M.A.; Sakamoto, K.Y.; Mattoso, L.H.C. Release of the diclofenac sodium by nanofibers of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) obtained from electrospinning and solution blow spinning. J. Nanomater. 2014, 2014, e129035. [Google Scholar] [CrossRef] [Green Version]
- Karuppannan, C.; Sivaraj, M.; Kumar, J.K.; Seerangan, R.; Balasubramanian, S.; Gopal, D.R. Fabrication of progesterone-loaded nanofibers for the drug delivery applications in bovine. Nanoscale Res. Lett. 2017, 12, 116. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, A.P.A.; Conte-Junior, C.A. Food-derived biopolymer kefiran composites, nanocomposites and nanofibers: Emerging alternatives to food packaging and potentials in nanomedicine. Trends Food Sci. Technol. 2021, 116, 370–386. [Google Scholar] [CrossRef]
- Ghajarieh, A.; Habibi, S.; Talebian, A. Biomedical applications of nanofibers. Russ. J. Appl. Chem. 2021, 94, 847–872. [Google Scholar] [CrossRef]
- Karthega, M.; Pranesh, M.; Poongothai, C.; Srinivasan, N. Poly caprolactone/titanium dioxide nanofiber coating on AM50 alloy for biomedical application. J. Magnes. Alloys 2021, 9, 532–547. [Google Scholar] [CrossRef]
- Rivelli, G.G.; Perez, A.C.; Silva, P.H.R.; de Gomes, E.C.L.; de Moreira, C.P.S.; Tamashiro, E.; Valera, F.C.P.; Anselmo-Lima, W.T.; Pianetti, G.A.; Silva-Cunha, A. Biodegradable electrospun nanofibers: A new approach for rhinosinusitis treatment. Eur. J. Pharm. Sci. 2021, 163, 105852. [Google Scholar] [CrossRef]
- Zhao, X.-H.; Niu, Y.-N.; Mi, C.-H.; Gong, H.-L.; Yang, X.-Y.; Cheng, S.-Y.; Zhou, Z.-Q.; Liu, J.-L.; Peng, X.-L.; Wei, D.-X. Electrospinning nanofibers of microbial polyhydroxyalkanoates for applications in medical tissue engineering. J. Polym. Sci. 2021, 59, 1994–2013. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, J.; Wu, J.; Du, J. Electrospinning nanofibers to 1D, 2D, and 3D scaffolds and their biomedical applications. Nano Res. 2022, 15, 787–804. [Google Scholar] [CrossRef]
- Imani, R.; Yousefzadeh, M.; Nour, S. Functional nanofiber for drug delivery applications. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A.S.H., Eds.; Springer Nature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Balusamy, B.; Celebioglu, A.; Senthamizhan, A.; Uyar, T. Progress in the design and development of ‘fast-dissolving’ electrospun nanofibers based drug delivery systems —A systematic review. J. Control. Release 2020, 326, 482–509. [Google Scholar] [CrossRef] [PubMed]
- Dodero, A.; Schlatter, G.; Hébraud, A.; Vicini, S.; Castellano, M. Polymer-free cyclodextrin and natural polymer-cyclodextrin electrospun nanofibers: A comprehensive review on current applications and future perspectives. Carbohydr. Polym. 2021, 264, 118042. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Verma, S.; Joshi, K.; Utreja, P.; Sharma, S. Nanofiber as a novel vehicle for transdermal delivery of therapeutic agents: Challenges and opportunities. Future J. Pharm. Sci. 2021, 7, 175. [Google Scholar] [CrossRef]
- Haidar, M.K.; Timur, S.S.; Demirbolat, G.M.; Nemutlu, E.; Gürsoy, R.N.; Ulubayram, K.; Öner, L.; Eroğlu, H. Electrospun nanofibers for dual and local delivery of neuroprotective drugs. Fibers Polym. 2021, 22, 334–344. [Google Scholar] [CrossRef]
- Pandey, A. Pharmaceutical and biomedical applications of cellulose nanofibers: A review. Environ. Chem. Lett. 2021, 19, 2043–2055. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Wang, L.L.; Cai, H.X.; Qi, Y.Y.; Zou, X.H.; Ouyang, H.W. Electrospun nanofibrous matrix improves the regeneration of dense cortical bone. J. Biomed. Mater. Res. 2010, 95, 49–57. [Google Scholar] [CrossRef]
- Schneider, O.D.; Mohn, D.; Fuhrer, R.; Klein, K.; Kämpf, K.; Nuss, K.M.R.; Sidler, M.; Zlinszky, K.; von Rechenberg, B.; Stark, W.J. Biocompatibility and bone formation of flexible, cotton wool-like PLGA/calcium phosphate nanocomposites in sheep. Open Orthop. J. 2011, 5, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Mousa, H.M.; Hussein, K.H.; Sayed, M.M.; Abd El-Rahman, M.K.; Woo, H.-M. Development and Characterization of Cellulose/Iron Acetate Nanofibers for Bone Tissue Engineering Applications. Polymers 2021, 13, 1339. [Google Scholar] [CrossRef]
- Sadat-Hosseini, S.M.A.; Moslemi, H.R.; Nourbakhsh, M.S.; Ghaffari-Khaligh, S. Evaluating the effect of electrospun polyvinyl alcohol nanofiber containing eucalyptus globules extract on the healing of experimental achilles tendon injury in rat. Iran. J. Vet. Surg. 2021. [Google Scholar] [CrossRef]
- Man, S.; Gao, W.; Zhang, Y.; Huang, L.; Liu, C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia 2010, 81, 703–714. [Google Scholar] [CrossRef]
- Shinohara, S.; Kihara, T.; Sakai, S.; Matsusaki, M.; Akashi, M.; Taya, M.; Miyake, J. Fabrication of in vitro three-dimensional multilayered blood vessel model using human endothelial and smooth muscle cells and high-strength PEG hydrogel. J. Biosci. Bioeng. 2013, 116, 231–234. [Google Scholar] [CrossRef]
- Stoppato, M.; Stevens, H.Y.; Carletti, E.; Migliaresi, C.; Motta, A.; Guldberg, R.E. Effects of silk fibroin fiber incorporation on mechanical properties, endothelial cell colonization and vascularization of PDLLA scaffolds. Biomaterials 2013, 34, 4573–4581. [Google Scholar] [CrossRef] [Green Version]
- Pennel, T.; Fercana, G.; Bezuidenhout, D.; Simionescu, A.; Chuang, T.-H.; Zilla, P.; Simionescu, D. The performance of cross-linked acellular arterial scaffolds as vascular grafts; pre-clinical testing in direct and isolation loop circulatory models. Biomaterials 2014, 35, 6311–6322. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Cui, Y.; Wang, J.; Yang, X.; Wu, Y.; Wang, K.; Gao, X.; Li, D.; Li, Y.; Zheng, X.-L.; et al. The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 2014, 35, 5700–5710. [Google Scholar] [CrossRef]
- Chen, R.; Huang, C.; Ke, Q.; He, C.; Wang, H.; Mo, X. Preparation and characterization of coaxial electrospun thermoplastic polyurethane/collagen compound nanofibers for tissue engineering applications. Colloids Surf. B Biointerfaces 2010, 79, 315–325. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, J.; Cui, Y.; Xu, R.; Wang, Z.; Zhang, J.; Wang, K.; Li, Y.; Zhao, Q.; Kong, D. Effect of sustained heparin release from PCL/chitosan hybrid small-diameter vascular grafts on anti-thrombogenic property and endothelialization. Acta Biomater. 2014, 10, 2739–2749. [Google Scholar] [CrossRef]
- Li, W.-J.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials 2005, 26, 599–609. [Google Scholar] [CrossRef]
- Wu, T.; Jiang, B.; Wang, Y.; Yin, A.; Huang, C.; Wang, S.; Mo, X. Electrospun poly(L-lactide-co-caprolactone)–collagen–chitosan vascular graft in a canine femoral artery model. J. Mater. Chem. B 2015, 3, 5760–5768. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liao, X.; Liu, C.; Li, M.; Chen, Y.; Shao, W.; Weng, K.; Li, F.; Ou, K.; He, J. Poly(l-lactide-co-caprolactone)/tussah silk fibroin nanofiber vascular scaffolds with small diameter fabricated by core-spun electrospinning technology. J. Mater. Sci. 2020, 55, 7106–7119. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoo, J.J.; Lim, G.J.; Atala, A.; Stitzel, J. In vitro evaluation of electrospun nanofiber scaffolds for vascular graft application. J. Biomed. Mater. Res. Part A 2007, 83, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- MacKintosh, S.B.; Serino, L.P.; Iddon, P.D.; Brown, R.; Conlan, R.S.; Wright, C.J.; Maffeis, T.G.G.; Raxworthy, M.J.; Sheldon, I.M. A three-dimensional model of primary bovine endometrium using an electrospun scaffold. Biofabrication 2015, 7, 025010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Cao, D.; Liu, W.; Zhou, G.; Zhang, W.J.; Cao, Y. In vivo engineering of a functional tendon sheath in a hen model. Biomaterials 2010, 31, 3894–3902. [Google Scholar] [CrossRef] [PubMed]
- Hajiali, H.; Shahgasempour, S.; Naimi-Jamal, M.R.; Peirovi, H. Electrospun PGA/gelatin nanofibrous scaffolds and their potential application in vascular tissue engineering. Int. J. Nanomed. 2011, 6, 2133–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essa, W.K.; Yasin, S.A.; Saeed, I.A.; Ali, G.A.M. Nanofiber-Based Face Masks and Respirators as COVID-19 Protection: A Review. Membranes 2021, 11, 250. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.; Progri, H.; Arasu, D.T.; Nikfarjam, S.; Shamim, N. Use of Polycaprolactone Electrospun Nanofiber Mesh in a Face Mask. Materials 2021, 14, 4272. [Google Scholar] [CrossRef]
- Tebyetekerwa, M.; Xu, Z.; Yang, S.; Ramakrishna, S. Electrospun Nanofibers-Based Face Masks. Adv. Fiber Mater. 2020, 2, 2,161–166. [Google Scholar] [CrossRef]
- Zhang, Z.; Ji, D.; He, H.; Ramakrishna, S. Electrospun ultrafine fibers for advanced face masks. Mater. Sci. Eng. R Rep. 2021, 143, 100594. [Google Scholar] [CrossRef]
- Blosi, M.; Costa, A.L.; Ortelli, S.; Belosi, F.; Ravegnani, F.; Varesano, A.; Tonetti, C.; Zanoni, I.; Vineis, C. Polyvinyl alcohol/silver electrospun nanofibers: Biocidalfilter media capturing virus-size particles. J. Appl. Polym. Sci. 2021, 138, e51380. [Google Scholar] [CrossRef]
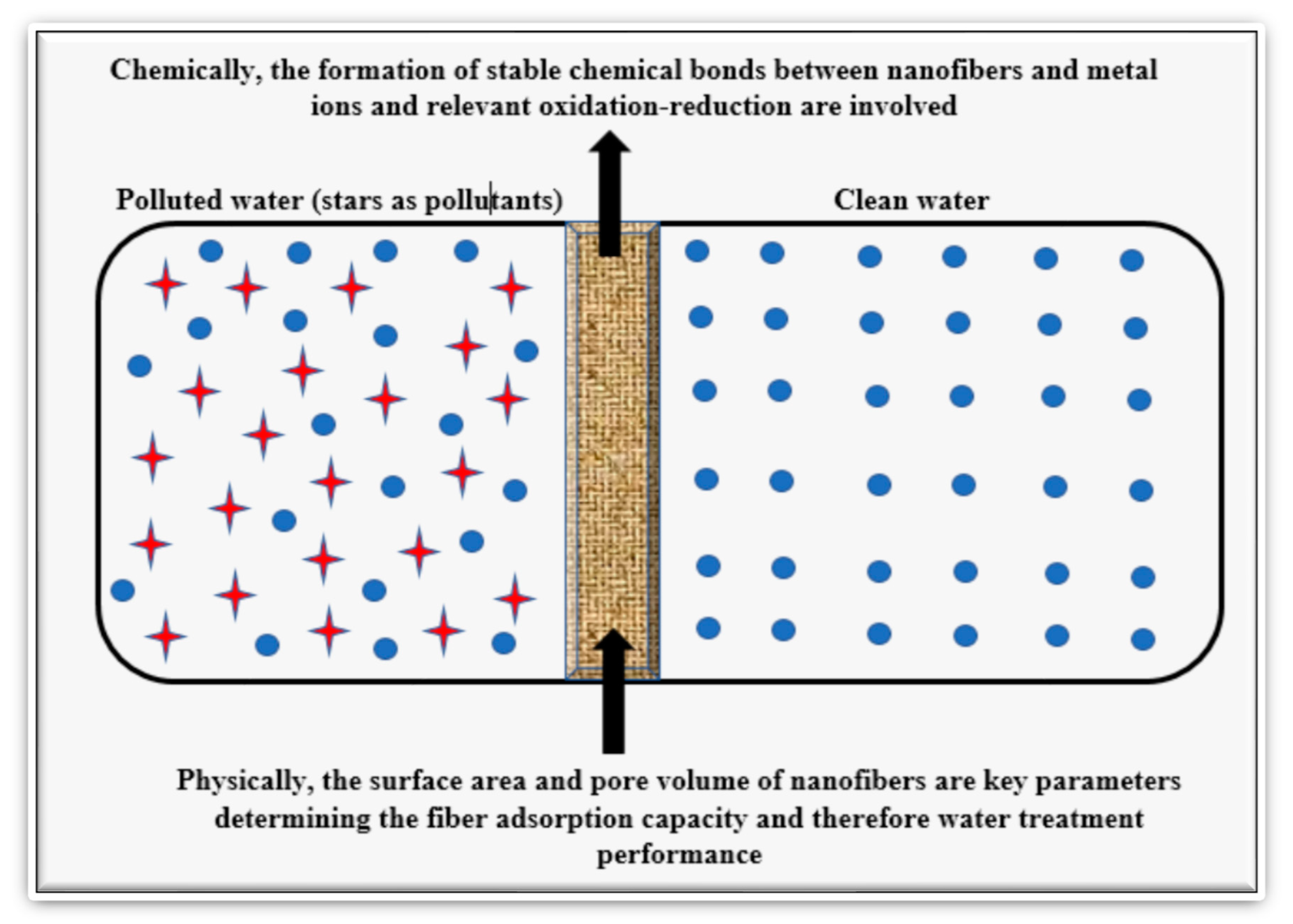
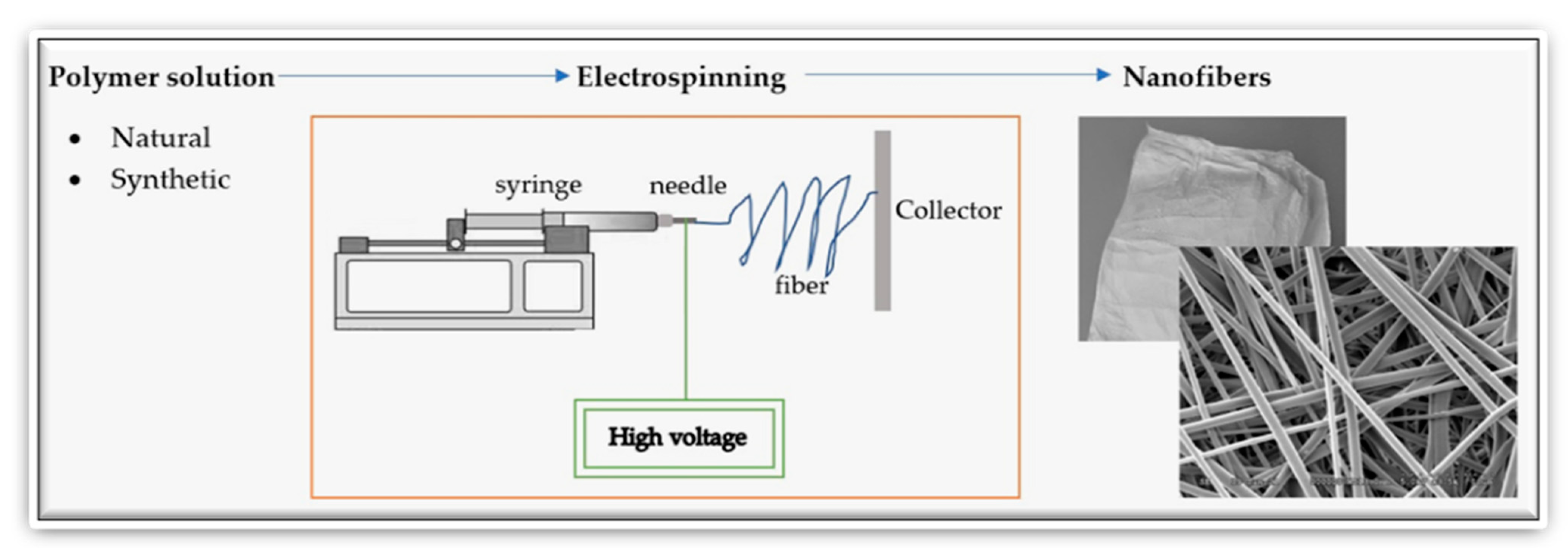
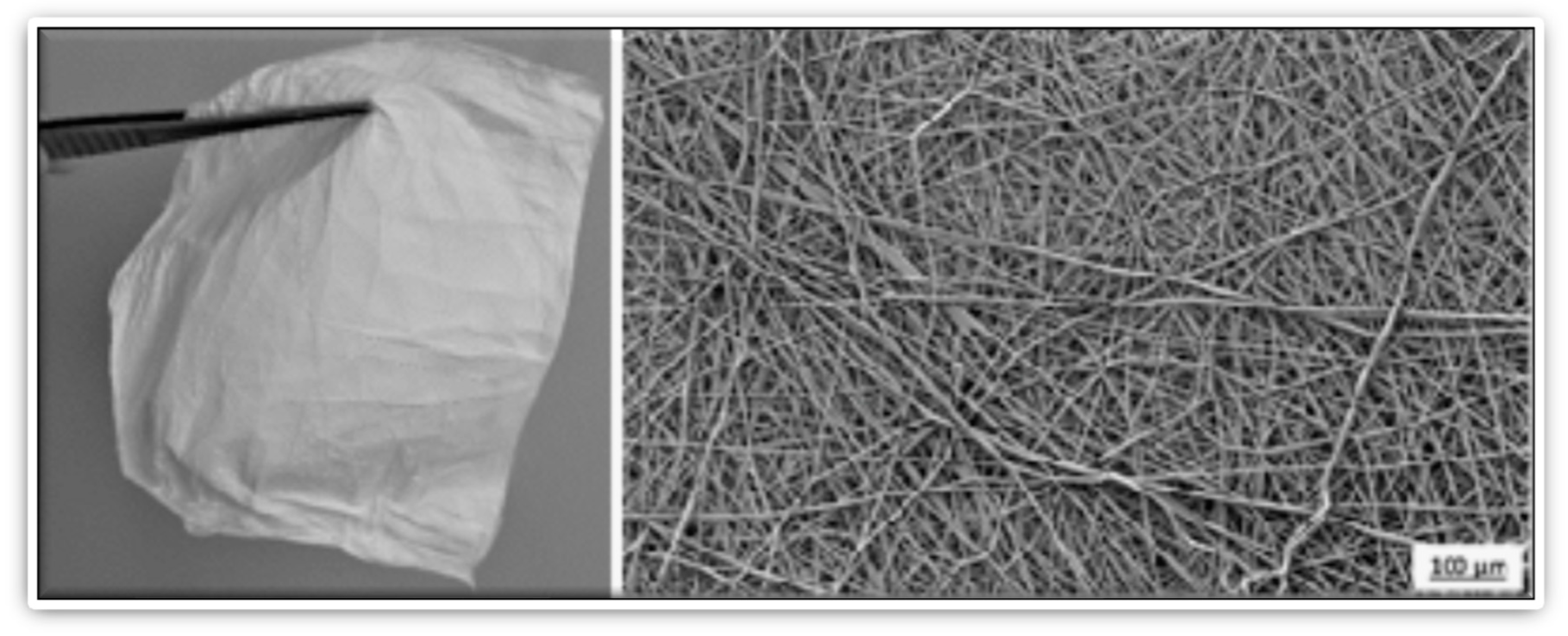
| Natural Fibers | Nanofibers |
|---|---|
| Definition | |
| The fiber is defined as a substrate of natural origin, which its length/diameter ratio is more than 1:200 [26] | “Nanofibers could be defined as the fibers which have their diameters in nanometric range” [27] |
| The main sources | |
| Green composites based on natural fibers compared to petroleum-based fiber composites [28,29] | Nanofibers are generally classified based on their composition into metal oxides, polymers, metals, carbon, ceramics, and hybrid [30] |
| Main categories of natural fiber | Main types of nano-lignocellulose fibers |
| 1—Mineral fibers (asbestos, basalt, and brucite) | 1—Lignocellulose nanofiber [31] |
| 2—Animal fibers (hair, silk, and wool) | 2—Bacterial nanocellulose [32] |
| 3—Plant fibers (lignocellulose) [33] | 3—Nanocrystalline cellulose [34] |
| 4—Nano-fibrillated cellulose [16] | |
| Main treatments for natural fibers | Main fabrication techniques of nanofibers |
| Chemical (acetylation, alkaline, benzoylation, peroxide, potassium permanganate, silane, and stearic acid) and surface treatments [1] | Non-electrospinning techniques (i.e., phase separation, drawing, template synthesis, and self-assembly), electrospinning, and hydrothermal techniques [35,36,37,38] |
| The main applications of natural fibers | The main applications of nanofibers |
| Automobile, construction, aerospace, and marine structural industries [39,40] | Aerospace, 3D printing industry, orthopedic and structural applications, polyurethane matrix, paper and textile industry [13,14,15] |
| Nanofibers Obtained from Agro-Wastes and Used Method | Comment on Nanofibers | References |
|---|---|---|
| Polyvinyl alcohol/starch nanocomposite film reinforced with cellulose nanofiber of sugarcane bagasse was produced using alkaline acid treatment under ultrasonication | Nanocomposite film reinforced with cellulose nanofiber | [46] |
| Using wastes of bamboo (Phyllostachys pubescens) as lignocellulosic biomass using microwave-assisted ethanol solvent treatment to produce cellulose nanofiber | Cellulose nanofibers | [52] |
| Lignocellulosic nanofiber can be produced by washing the Eucalyptus sawdust with an aqueous surfactant solution | Bio-nanocomposite films | [47] |
| Wastes obtained from orange juice processing can be used to obtain biodegradable film of reinforced cellulose nanofiber | Nano-biocomposite films | [48] |
| Using pomegranate (Punica granatum L.) peel extract beside polyvinylpyrrolidone and polyvinyl alcohol | Nanofibers for cosmeceutical purposes | [53] |
| Quinoa wastes incorporated with multi-walled C-nano tubes-ZnO can be used to obtain natural cellulose fibers | Bio-nanocomposite | [49] |
| Producing cellulose nanofibers obtained from the discarded wooden bark of Kozo plant by acidified sodium chlorite and acetic acid | Cellulose nanofibers | [50] |
| In vitro assay of nanofibers obtained from ethanolic extract of pomegranate peel used electrospinning method | Gelatin nanofiber | [51] |
| Peach branches used under high-pressure homogeneous to produce peach branches–cellulose nanofiber | Nanofiber reinforcer of gelatin hydrogel | [41] |
| Crystalline nanocellulose was generated using coconut husk, and rice husk by hydrolysis disintegration | Mechanically reinforced polymer composites | [43] |
| Nanocellulose incorporated in poly-lactic acid matrix obtained from cotton wastes by acid hydrolysis | Production of nanocellulose | [54] |
| Producing cellulose nanofiber from pineapple leaf wastes, which reinforced into a polystyrene substrate | Cellulose nanofiber reinforced polystyrene nanocomposites | [45] |
| Main Applications of Nanofibers in Agricultural Sectors | References |
|---|---|
| 1—Nanofibers for good germination by coating seeds | [59,60,61,62] |
| 2—Agro-wastes for production nanofibers | [63,64] |
| 3—Nanofibers-based filters for irrigation systems | [65] |
| 4—Nanofibers for plant protection | [56] |
| 4.1 Encapsulation of fungicides | [66,67] |
| 4.2 Encapsulation of herbicides | [68] |
| 4.3 Detecting trace pesticides in water | [69] |
| 5—Nano-silica grafted fiber | [70] |
| 6—Smart nanotextiles for sustainable agriculture | [13] |
| 7—Nanofibers for encapsulation of agrochemicals | [71,72] |
| 7.1 Fertilizer application | [73] |
| 7.2 Plant hormones (e.g., indole acetic acid) | [57,74] |
| Nanofibers and Their Average Diameter | Max. Adsorption Capacity | Pollutant | References |
|---|---|---|---|
| Polyvinylidene fluoride–polyacrylonitrile-ZnO nanofiber membranes (200 nm) | 350 mg g−1 | Cd | [88] |
| Amidoxylated polyacrylonitrile/Poly-vinylidene fluoride (AOPAN/PVDF) (235–314 nm) | 89.29 mg g−1 | Pb (II) | [89] |
| Nitro-oxidized carboxy-cellulose nanofibers obtained from moringa plants (0.22 µm) | 257.07 mg g−1 | Hg | [90] |
| Electrospun chitosan–polyethylene oxide-oxidized cellulose biobased composite (159.3 nm and 21.7 µm, resp.) | 15.72 mg g−1 | Cu | [91] |
| Modified poly butylene succinate nanofibers (10 µm) | 91.2 and 122 mg g−1, respectively | Ag (I) and Hg (II) | [92] |
| TEMPO-oxidized cellulose nanofibers (diameter 6.15 nm) | 56.50 mg g−1 | Cu (II) | [93] |
| Polyvinyl alcohol (PVP)-octa-amino-POSS nanofibers (21 µm) | 37.4 and 120 mg g−1, respectively | Cu (II), Pb (II) | [94] |
| Starch-g-poly(acrylic acid)-cellulose nanofiber bio-nanocomposite hydrogel (10 µm) | 40.65 mg g−1 | Cd (II) | [95] |
| Oxidized regenerated cellulose nanofiber membrane (10 µm) | 20.78 and 206.1 mg g−1, respectively | Cu (II), Pb (II) | [96] |
| polyvinylidene fluoride–amidoximized polyacrylonitrile nanofibers (20.7 µm) | 30.1, 25.8, and 72.5 mg g−1, respectively | Cu (II), Ni (II), Pb (II) | [97] |
| Modified prepared polyacrylonitrile nanofibers (320 nm) | 22.95 and 12.36 mmol g−1, respectively | Cu and Pb | [98] |
| Centrifugal spinning of lignin amine/cellulose acetate nanofiber (756 nm) | 50.08 and 31.17 mg g−1, respectively | Cu (II), Co (II) | [83] |
| Visualized chitosan–polyacrylonitrile nanofiber membrane | 164.3 mg g−1 | Cu (II) | [99] |
| Zn/Al/gallate layered double hydroxide–polystyrene nanofibers (2–5 µm) | 190 mg g−1 | Cu (II) | [100] |
| Polyacrylonitrile–polyetherimide nanofibers (0.84 mm) | 242.7, 214.1, 258.3 mg g−1, respectively | Cu (II), Cr (VI), As (V) | [101] |
| Main Applications According to Different Food Processes and Industry | References |
|---|---|
| 1—Nanofibers for the field of food industry | [118] |
| 2—Nanofibers for beverage industry | [112] |
| 3—Nanofibers for encapsulation of food materials | [110] |
| 4—Nanofibers for food preservation | [111] |
| 5—Nanofibers for food packaging industry | |
| 5.1 Nanofibers for food intelligent packaging | [113] |
| 5.2 Nanofibers as an active food packaging system | [119] |
| 5.3 Nanofibers for active–intelligent food packaging | [120] |
| 5.4 Nanofibers containing biodegradable polymers | [121] |
| 5.5 Nanofiber for active food packaging | [122] |
| 5.6 Nanofibers for food freshness indicators | [113] |
| 5.7 Cellulose-based hydrophobic materials for food packaging | [123] |
| 5.8 Functionalized nanomaterials driven antimicrobial food packaging | [117] |
| Applications of Nanofibers in Medicine | Applications of Nanofibers in <break/>Pharmacology |
|---|---|
| Adhesion prevention materials | Anticancer drug delivery |
| Artificial blood vessels, cornea, and skin | Antimicrobial drug delivery |
| Drug release capsule | Antibiotic drug delivery |
| Drug release artificial skin | Anti-inflammatory drugs |
| Dialysis membrane | Cell delivery and tissue engineering |
| Facemask, skin and vascular tissue engineering | Growth factor and protein delivery |
| Nerve or organ patch | Neuroprotective drugs |
| Rhinosinusitis treatment | Nucleic acid delivery |
| Surgical adhesive sheet | Miscellaneous drug delivery |
| Transdermal absorbent | Controlled release of gentamicin |
| Wound covering and protective agent | Localized chemotherapy |
| Filling agent for artificial bone | Smart active drug release systems |
| Wound dressing and healing systems | Transdermal drug delivery |
| Wound and therapeutic applications | Double-layered planar nanofibrous scaffolds abdominal adhesion prevention |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badgar, K.; Abdalla, N.; El-Ramady, H.; Prokisch, J. Sustainable Applications of Nanofibers in Agriculture and Water Treatment: A Review. Sustainability 2022, 14, 464. https://doi.org/10.3390/su14010464
Badgar K, Abdalla N, El-Ramady H, Prokisch J. Sustainable Applications of Nanofibers in Agriculture and Water Treatment: A Review. Sustainability. 2022; 14(1):464. https://doi.org/10.3390/su14010464
Chicago/Turabian StyleBadgar, Khandsuren, Neama Abdalla, Hassan El-Ramady, and József Prokisch. 2022. "Sustainable Applications of Nanofibers in Agriculture and Water Treatment: A Review" Sustainability 14, no. 1: 464. https://doi.org/10.3390/su14010464
APA StyleBadgar, K., Abdalla, N., El-Ramady, H., & Prokisch, J. (2022). Sustainable Applications of Nanofibers in Agriculture and Water Treatment: A Review. Sustainability, 14(1), 464. https://doi.org/10.3390/su14010464









