Biochar-Assisted Phytostabilization for Potentially Toxic Element Immobilization
Abstract
:1. Introduction
2. Materials and Methods
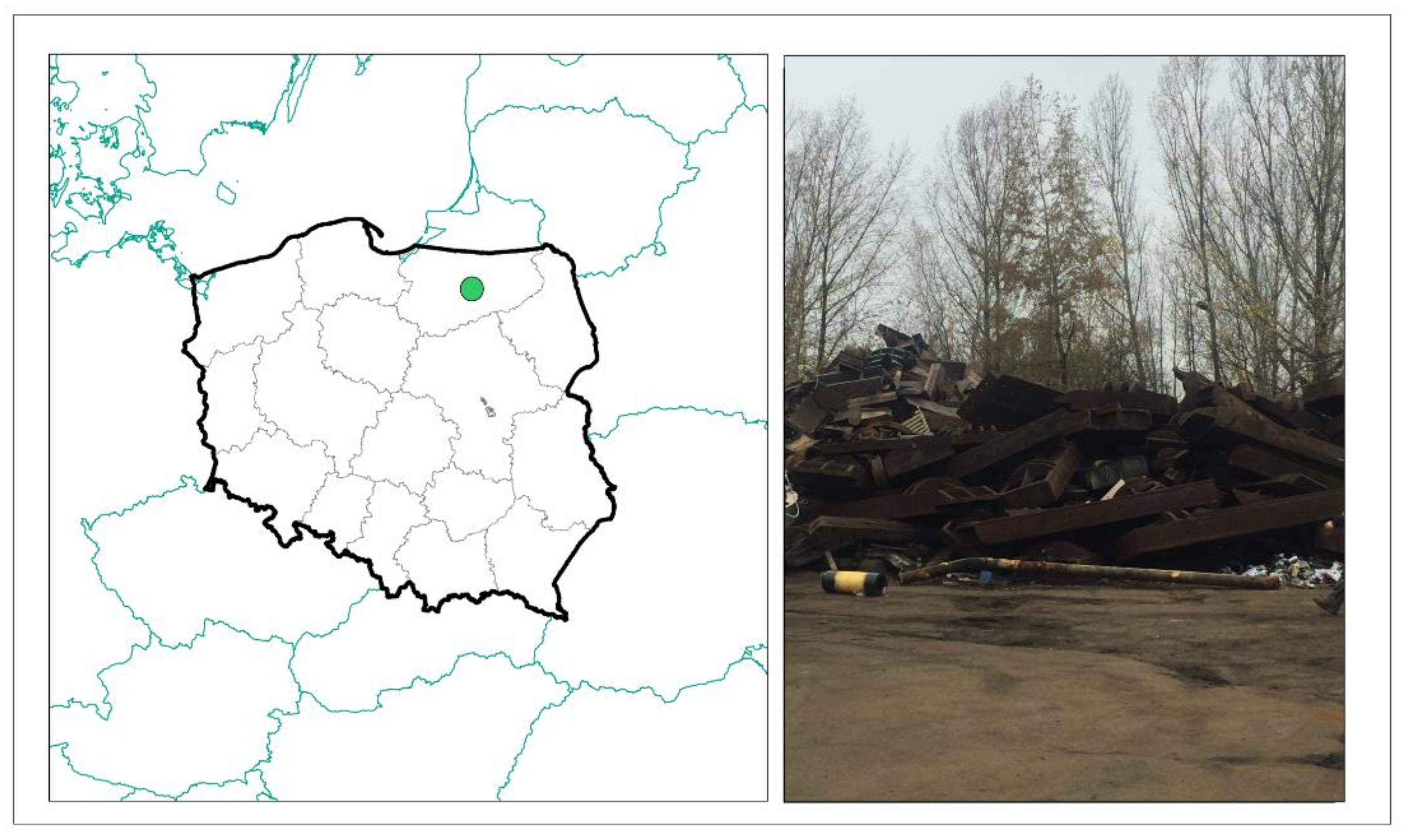
2.1. Study Area, Soil Sampling and Sample Preparation
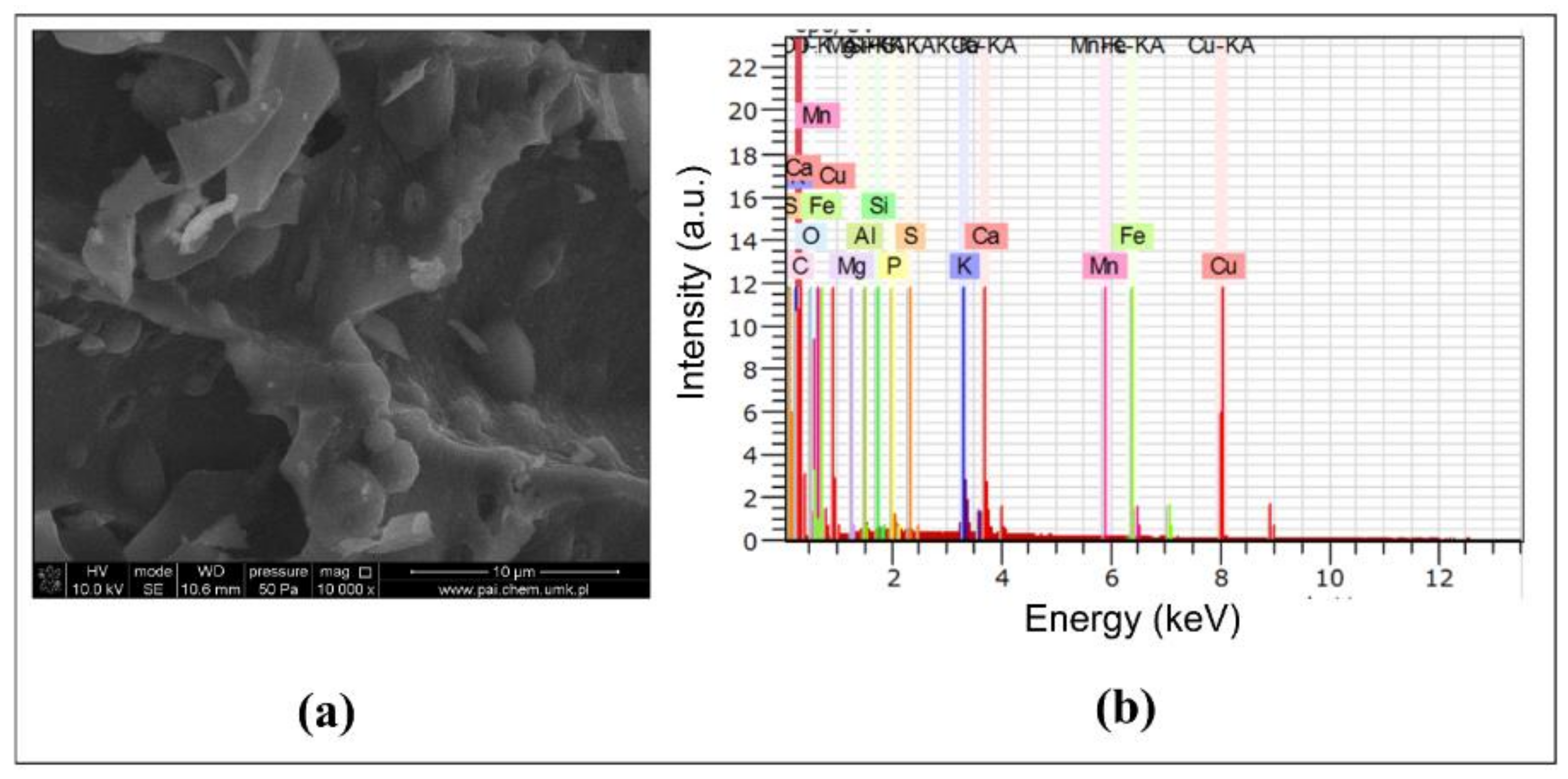
2.2. Biochar Characterization
2.3. Biochar-Assisted Phytostabilization Experiment
2.4. Soil and Plant Analyses
2.5. Statistical Analysis
3. Results and Discussion
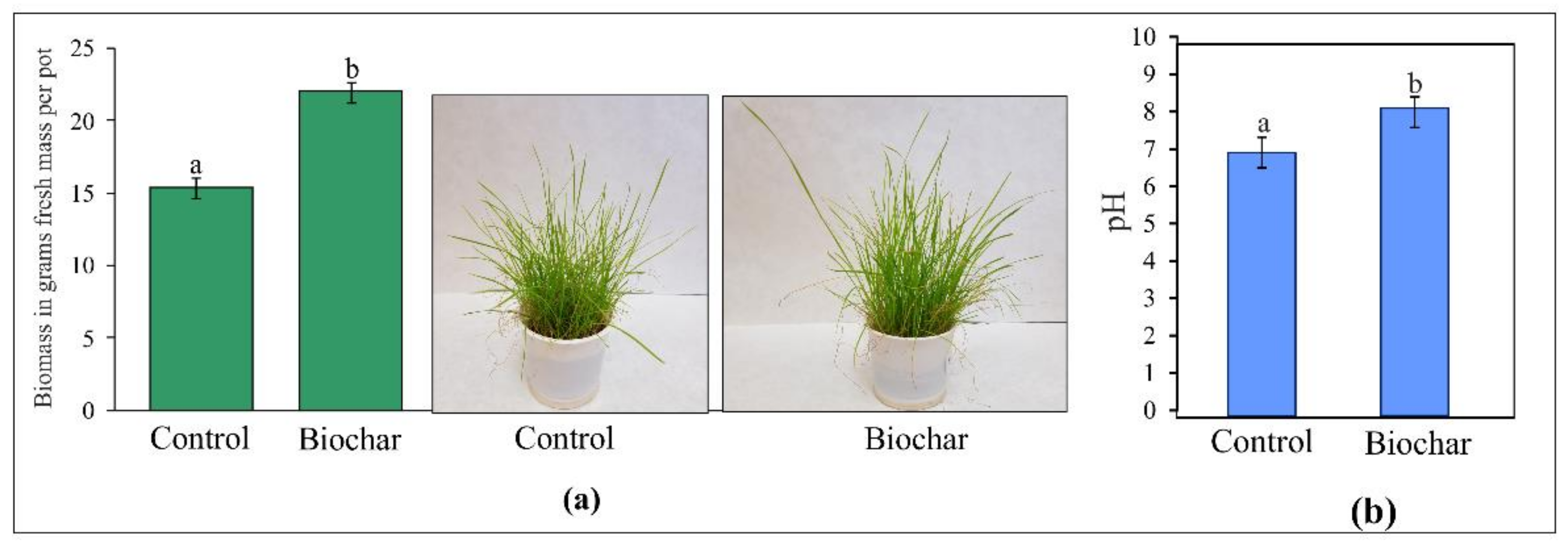
3.1. Dactylis Glomerata L. Biomass after Biochar Application
3.2. Soil pH after Biochar Application
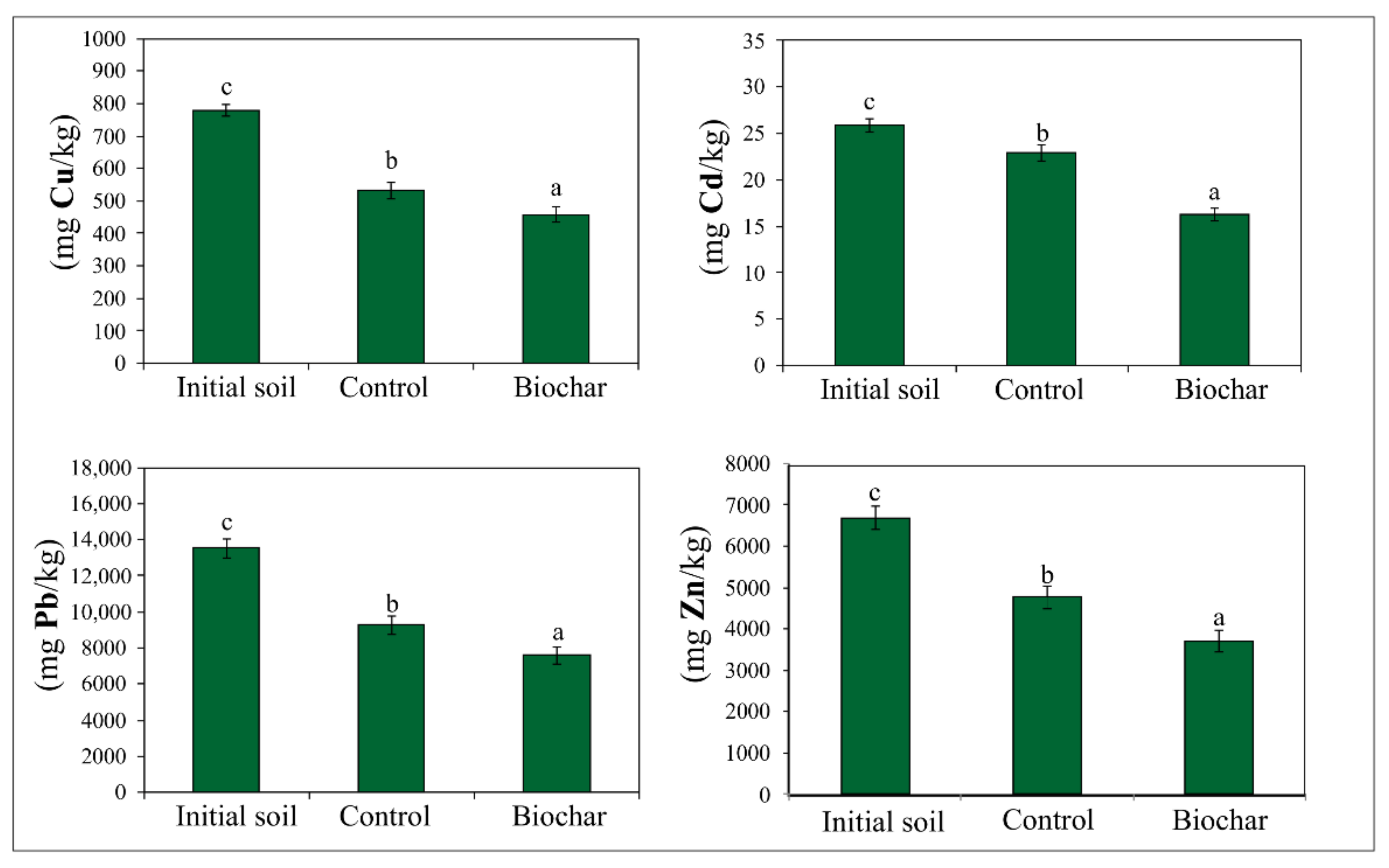
3.3. The Total Content of Cu, Cd, Pb and Zn in Soil after Biochar-Assisted Phytostabilization
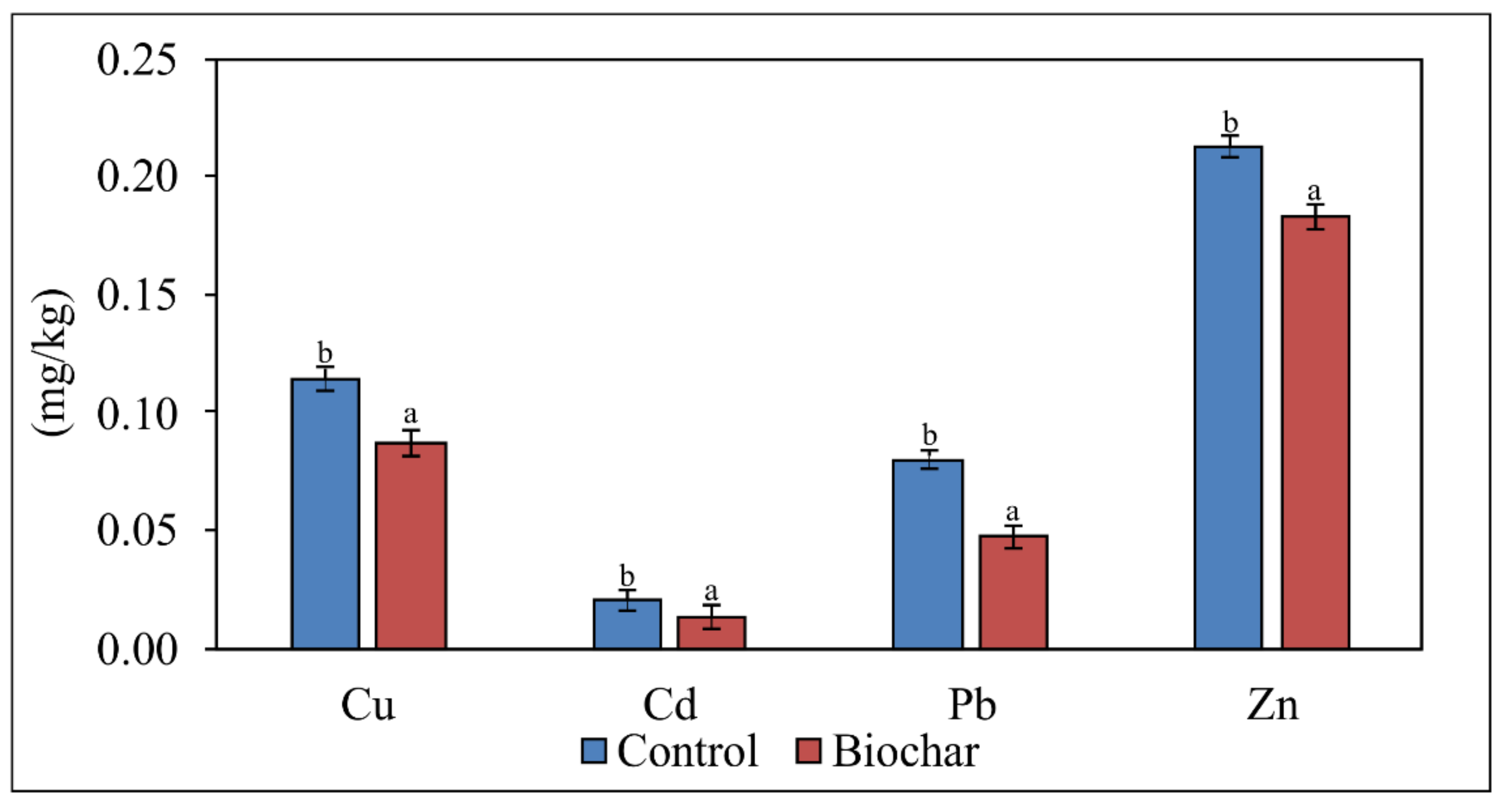
3.4. The CaCl2-Extractable Content of Cu, Cd, Pb and Zn in Soil after Biochar-Assisted Phytostabilization
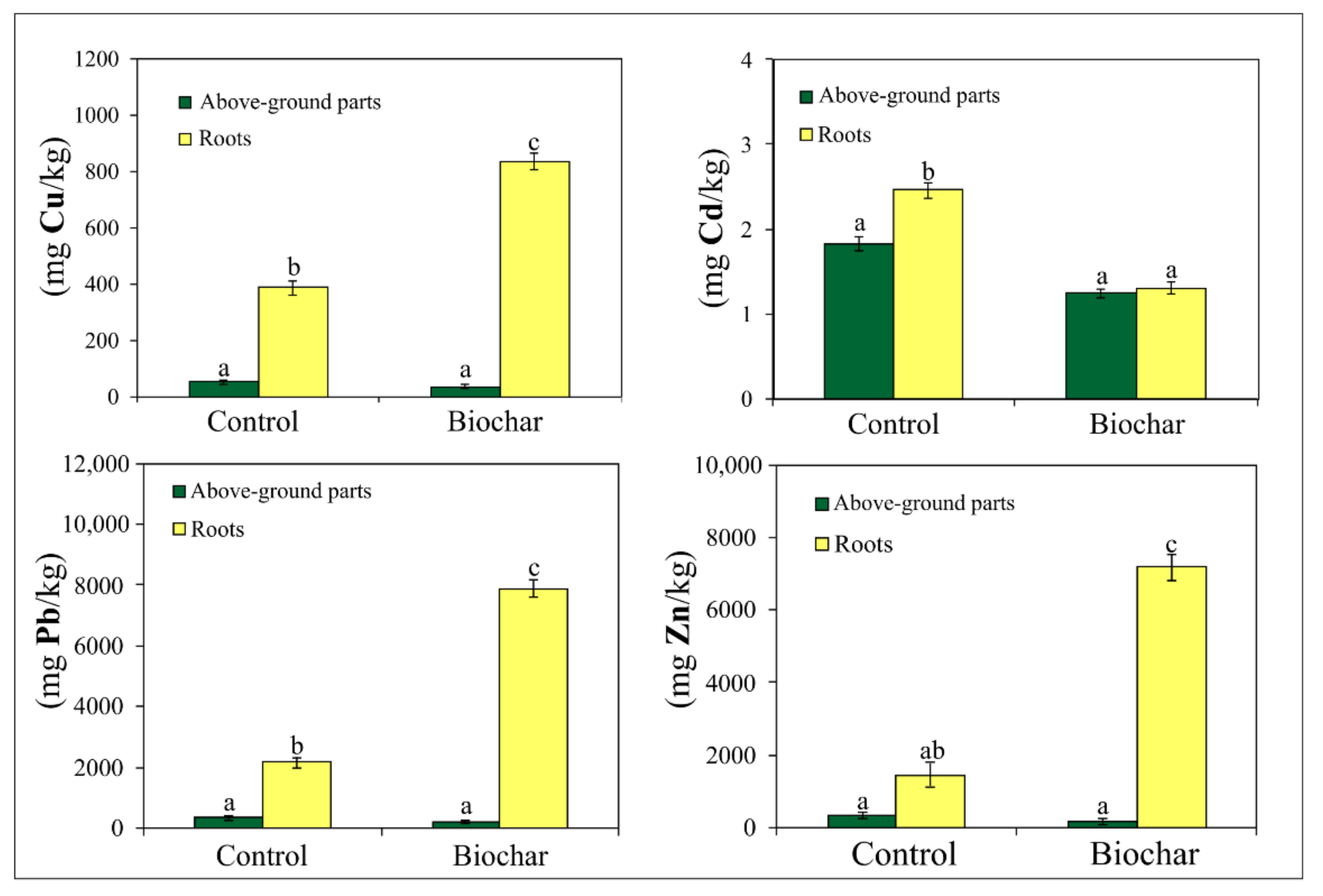
3.5. Content of Cu, Cd, Pb and Zn in Roots and Above-Ground Parts of Dactylis glomerata L.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, C.; Jin, H.; Zhong, C.; Wang, J.; Sun, M.; Xie, M. Estimating the contribution of atmosphere on heavy metals accumulation in the aboveground wheat tissues induced by anthropogenic forcing. Environ. Res. 2020, 189, 109955. [Google Scholar] [CrossRef]
- Sabouhi, M.; Ali-Taleshi, M.S.; Bourliva, A.; Nejadkoorki, F.; Squizzato, S. Insights into the anthropogenic load and occupational health risk of heavy metals in floor dust of selected workplaces in an industrial city of Iran. Sci. Tot. Environ. 2020, 744, 140762. [Google Scholar] [CrossRef] [PubMed]
- Chotpantarat, S.; Thamrongsrisakul, J. Natural and anthropogenic factors influencing hydrochemical characteristics and heavy metals in groundwater surrounding a gold mine, Thailand. J. Asian Earth Sci. 2021, 211, 104692. [Google Scholar] [CrossRef]
- Pourret, O.; Bollinger, J.C.; Hursthouse, A. Heavy metal: A misused term? Acta Geochim. 2021, 40, 466. [Google Scholar] [CrossRef]
- Pourret, O.; Hursthouse, A. It’s Time to Replace the Term “Heavy Metals” with “Potentially Toxic Elements” When Reporting Environmental Research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourret, O.; Bollinger, J.C. “Heavy metal”—What to do now: To use or not to use? Sci. Total Environ. 2018, 610, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Pourret, O. On the necessity of banning the term “heavy metal” from the scientific literature. Sustainability 2018, 10, 2879. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, P.G.; Aragão, O.O.S.; Martins, G.C.; Rodrigues, M.; Souza, J.N.P.; Moreira, F.M.S.; Li, Y.C.; Guilherme, L.R.G. Hydrothermally-altered feldspar reduces metal toxicity and promotes plant growth in highly metal-contaminated soils. Chemosphere 2022, 286, 131768. [Google Scholar] [CrossRef]
- Khan, I.; Awan, S.A.; Rizwan, M.; Ali, S.; Hassan, M.J.; Brestic, M.; Zhang, X.; Huang, L. Effects of silicon on heavy metal uptake at the soil-plant interphase: A review. Ecotox. Environ. Saf. 2021, 222, 112510. [Google Scholar] [CrossRef]
- Huang, S.; Xiao, L.; Zhang, Y.; Wang, L.; Tang, L. Interactive effects of natural and anthropogenic factors on heterogenetic accumulations of heavy metals in surface soils through geodetector analysis. Sci. Tot. Environ. 2021, 789, 147937. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Liu, X.; Liu, X.; Li, X.; Ren, Y.; Wang, J.; Dong, L. Partitioning and geochemical fractions of heavy metals from geogenic and anthropogenic sources in various soil particle size fractions. Geoderma 2018, 312, 104–113. [Google Scholar] [CrossRef]
- Radziemska, M.; Gusiatin, Z.M.; Cydzik-Kwiatkowska, A.; Cerdà, A.; Pecina, V.; Bęś, A.; Datta, R.; Majewski, G.; Mazur, Z.; Dzięcioł, J.; et al. Insight into metal immobilization and microbial community structure in soil from a steel disposal dump phytostabilized with composted, pyrolyzed or gasified wastes. Chemosphere 2021, 272, 129576. [Google Scholar] [CrossRef]
- Radziemska, M.; Bęś, A.; Gusiatin, Z.M.; Majewski, G.; Mazur, Z.; Bilgin, A.; Jaskulska, I.; Brtnický, M. Immobilization of potentially toxic elements (PTE) by mineral-based amendments: Remediation of contaminated soils in post-industrial sites. Minerals 2020, 10, 87. [Google Scholar] [CrossRef] [Green Version]
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Liu, L.; Yu, H. Application of co-pyrolysis biochar for the adsorption and immobilization of heavy metals in contaminated environmental substrates. J. Hazard. Mat. 2021, 420, 126655. [Google Scholar] [CrossRef]
- Trippe, K.M.; Manning, V.A.; Reardon, C.L.; Klein, A.M.; Weidman, C.; Ducey, T.F.; Novak, J.M.; Watts, D.W.; Rushmiller, H.; Spokas, K.A.; et al. Phytostabilization of acidic mine tailings with biochar, biosolids, lime, and locally-sourced microbial inoculum: Do amendment mixtures influence plant growth, tailing chemistry, and microbial composition? Appl. Soil Ecol. 2021, 165, 103962. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Souki, K.S.S.; Waterlot, C.; Douay, F.; Pelfrêne, A.; Ridošková, A.; Louvel, B.; Pourrut, B. Potentials of Miscanthus x giganteus for phytostabilization of trace element-contaminated soils: Ex situ experiment. Ecotoxicol. Environ. Saf. 2021, 214, 112125. [Google Scholar] [CrossRef]
- Wetle, R.; Bensko-Tarsitano, B.; Johnson, K.; Sweat, K.G.; Cahill, T. Uptake of uranium into desert plants in an abandoned uranium mine and its implications for phytostabilization strategies. J. Environ. Radioact. 2020, 220–221, 106293. [Google Scholar] [CrossRef]
- Radziemska, M.; Gusiatin, Z.M.; Bęś, A.; Czajkowska, J.; Mazur, Z.; Hammerschmiedt, T.; Sikorski, Ł.; Kobzova, E.; Klik, B.K.; Sas, W.; et al. Can the application of municipal sewage sludge compost in the aided phytostabilisation technique provide an effective waste management method? Energies 2021, 14, 1984. [Google Scholar] [CrossRef]
- Scattolin, M.; Peuble, S.; Pereira, F.; Paran, F.; Moutte, J.; Menad, N.; Faure, O. Aided-phytostabilization of steel slag dumps: The key-role of pH adjustment in decreasing chromium toxicity and improving manganese, phosphorus and zinc phytoavailability. J. Hazard. Mat. 2021, 405, 124225. [Google Scholar] [CrossRef]
- Xu, Y.; Qi, F.; Bai, T.; Yan, Y.; Wu, C.; An, Z.; Luo, S.; Huang, Z.; Xie, P. A further inquiry into co-pyrolysis of straws with manures for heavy metal immobilization in manure-derived biochars. J. Hazard. Mat. 2019, 380, 120870. [Google Scholar] [CrossRef]
- Penido, E.S.; Martins, G.C.; Mendes, T.B.M.; Melo, L.C.A.; Guimarães, I.R.; Guilherme, L.R.G. Combining biochar and sewage sludge for immobilization of heavy metals in mining soils. Ecotox. Environ. Saf. 2019, 172, 326–333. [Google Scholar] [CrossRef]
- Morales, V.L.; Pérez-Reche, F.J.; Hapca, S.M.; Hanley, K.L.; Lehmann, J.; Zhang, W. Reverse Engineering of Biochar. Biores. Tech. 2015, 183, 163. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, Y.A.; Li, D.; Tang, B.C.; Man, S.S.L.; Jia, Y.F.; Xu, H. Vermicompost and biochar as bio-conditioners to immobilize heavy metal and improve soil fertility on cadmium contaminated soil under acid rain stress. Sci. Tot. Environ. 2018, 621, 1057–1065. [Google Scholar] [CrossRef]
- Bis, Z.; Nowak, W. Method and Appliance for Auto-Thermal Valorization of Waste Solid Fuels as Well as the Biomass used for Pure Generation of Electric Power and Heat. PL204294 (B1), 31 December 2009. Available online: https://pl.espacenet.com/publicationDetails/description?locale=pl_PL&CC=PL&date=20091231&NR=204294B1&ND=5&KC=B1&rnd=1640378047723&FT=D&DB=; https://worldwide.espacenet.com/patent/search/family/035396229/publication/PL204294B1?q=PL204294 (accessed on 24 December 2021).
- Cui, H.; Li, H.; Zhang, S.; Yi, Q.; Zhou, J.; Fang, G.; Zhou, J. Bioavailability and mobility of copper and cadmium in polluted soil after phytostabilization using different plants aided by limestone. Chemosphere 2020, 242, 125252. [Google Scholar] [CrossRef]
- Mazur, Z.; Radziemska, M.; Fronczyk, J.; Jeznach, J. Heavy metal accumulation in bioindicators of pollution in urban areas of northeastern Poland. Fresenius Environ. Bull. 2015, 24, 216–223. [Google Scholar]
- Visconti, D.; Álvarez-Robles, M.J.; Fiorentino, N.; Fagnano, M.; Clemente, R. Use of Brassica juncea and Dactylisglomerata for the phytostabilization of mine soils amended with compost or biochar. Chemosphere 2020, 260, 127661. [Google Scholar] [CrossRef]
- Glina, B.; Kowalska, J.B.; Łuczak, K.; Mazurek, R.; Spychalski, W.; Mendyk, Ł. Potentially toxic elements in fen peatland soils located near lignite-fired power plants in Central Poland. Geoderma Reg. 2021, 25, e00370. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Radziemska, M. Influence of chromium (III) and (VI) on the concentration of mineral elements in oat (Avena sativa L.). Fres. Environ. Bull. 2013, 22, 979–986. [Google Scholar]
- Shah, V.; Daverey, A. Effects of sophorolipids augmentation on the plant growth and phytoremediation of heavy metal contaminated soil. J. Clean. Prod. 2021, 280, 124406. [Google Scholar] [CrossRef]
- Ke, T.; Guo, G.; Liu, J.; Zhang, C.; Tao, Y.; Wang, P.; Xu, Y.; Chen, L. Improvement of the Cu and Cd phytostabilization efficiency of perennial ryegrass through the inoculation of three metal-resistant PGPR strains. Environ. Poll. 2021, 271, 16314. [Google Scholar] [CrossRef] [PubMed]
- Zine, H.; Midhat, L.; Hakkou, R.; Adnani, M.E.; Ouhammou, A. Guidelines for a phytomanagement plan by the phytostabilization of mining wastes. Sci. Afr. 2020, 10, e00654. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Nandillon, R.; Léger, J.C.; Hattab-Hambli, N.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Assisted phytostabilization of a multi contaminated mine technosol using biochar amendment: Early stage evaluation of biochar feedstock and particle size effects on As and Pb accumulation of two Salicaceae species (Salix viminalis and Populus euramericana). Chemosphere 2018, 194, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Khare, P.; Mishra, D.; Shanker, K.; Singh, P.; Singh, R.P.; Das, P.; Yadav, R.; Saikia, B.K.; Baruah, B.P. Biochar aided aromatic grass [Cymbopogon martini (Roxb.) Wats.] vegetation: A sustainable method for stabilization of highly acidic mine waste. J. Hazard. Mat. 2020, 390, 121799. [Google Scholar] [CrossRef]
- Gonzaga, M.I.S.; Silva, P.S.O.; Santos, J.C.J.; Junior, L.F.G.O. Biochar increases plant water use efficiency and biomass production while reducing Cu concentration in Brassica juncea L. in a Cu-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 183, 109557. [Google Scholar] [CrossRef]
- Terzano, R.; Rascio, I.; Allegretta, I.; Porfido, C.; Spagnuolo, M.; Khanghahi, M.Y.; Crecchio, C.; Sakellariadou, F.; Gattullo, C.E. Fire effects on the distribution and bioavailability of potentially toxic elements (PTEs) in agricultural soils. Chemosphere 2021, 281, 130752. [Google Scholar] [CrossRef]
- Matin, N.H.; Jalali, M.; Buss, W. Synergistic immobilization of potentially toxic elements (PTEs) by biochar and nanoparticles in alkaline soil. Chemosphere 2020, 241, 124932. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2012, 5, 202–214. [Google Scholar] [CrossRef]
- Mosharrof, M.; Uddin, M.K.; Sulaiman, M.F.; Mia, S.; Shamsuzzaman, S.M.; Haque, A.N.A. Combined Application of Rice Husk Biochar and Lime Increases Phosphorus Availability and Maize Yield in an Acidic Soil. Agriculture 2021, 11, 793. [Google Scholar] [CrossRef]
- Cruz-Méndez, A.S.; Ortega-Ramírez, E.; Lucho-Constantino, C.A.; Arce-Cervantes, O.; Vázquez-Rodríguez, G.A.; Coronel-Olivares, C.; Beltrán-Hernández, R.I. Bamboo biochar and a nopal-based biofertilizer as improvers of alkaline soils with low buffer capacity. Appl. Sci. 2021, 11, 6502. [Google Scholar] [CrossRef]
- Karim, M.R.; Halim, M.A.; Gale, N.V.; Thomas, S.C. Biochar Effects on Soil Physiochemical Properties in Degraded Managed Ecosystems in Northeastern Bangladesh. Soil Syst. 2020, 4, 69. [Google Scholar] [CrossRef]
- Campos, P.; Knicker, H.; López, R.; De la Rosa, J.M. Application of Biochar Produced from Crop Residues on Trace Elements Contaminated Soils: Effects on Soil Properties, Enzymatic Activities and Brassica rapa Growth. Agronomy 2021, 11, 1394. [Google Scholar] [CrossRef]
- Novak, J.; Busscher, W.; Laird, D.; Ahmedna, M.; Watts, D.; Niandou, M. Impact of Biochar Amendment on Fertility of a Southeastern Coastal Plain Soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Wang, X.; Cheng, H.; Tao, S. Effect of aging on stabilization of Cd and Ni by biochars and enzyme activities in a historically contaminated alkaline agricultural soil simulated with wet–dry and freeze–thaw cycling. Environ. Poll. 2021, 268, 115846. [Google Scholar] [CrossRef]
- Nworie, O.E.; Lin, C. Seasonal Variation in Tissue-borne Heavy Metal(loid)s in Herbaceous Plants Growing in Contaminated Soils Developed from Industrial Wastes of Industrial Revolution Age. Environ. Adv. 2021, 5, 100113. [Google Scholar] [CrossRef]
- Wei, J.; Li, H.; Liu, J. Heavy metal pollution in the soil around municipal solid waste incinerators and its health risks in China. Environ. Res. 2022, 203, 111871. [Google Scholar] [CrossRef]
- No 165, 1359. Regulation of the Minister of the Environment on September 2016 on the standards of the soil quality and ground quality 1.09.2016. Dziennik Ustaw: Warsaw, Poland 2016. Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar]
- Jiang, J.; Xu, R.K.; Jiang, T.Y.; Li, Z. Immobilization of Cu (II), Pb (II) and Cd (II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. J. Hazard. Mat. 2012, 229, 145–150. [Google Scholar] [CrossRef]
- Hamon, R.E.; Holm, P.E.; Lorenz, S.E.; McGrath, S.P.; Christensen, T.H. Metal uptake by plants from sludge-amended soils: Caution is required in the plateau interpretation. Plant. Soil 1999, 216, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.J.; Shen, X.-J.; Domene, X.; Alcañiz, J.-M.; Liao, X.; Palet, C. Comparison of biochars derived from diferent types of feedstock and their potential for heavy metal removal in multiple-metal solutions. Sci. Rep. 2019, 9, 9869. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.S.; Pei, J.; Huang, H. Using biochar for remediation of soils contaminated with heavy metals and organic pollutants. Environ. Sci. Poll. Res. 2013, 20, 8472. [Google Scholar] [CrossRef]
- Available online: https://www.wiredchemist.com/chemistry/data/metallic-radii (accessed on 24 December 2021).
- McBride, M.B. Environmental Chemistry of Soils; Oxford University Press: New York, NY, USA, 1994. [Google Scholar]
- Faheem; Bao, J.; Zheng, H.; Tufail, H.; Irshada, S.; Du, J. Adsorption-assisted decontamination of Hg (II) from aqueous solution by multi-functionalized corncob-derived biochar. RSC Adv. 2018, 8, 38425. [Google Scholar] [CrossRef] [Green Version]
- Lou, H.; Cao, X.; Yan, X.; Wang, L.; Chen, Z. Adsorption performance of Cd(II), Cr(III), Cu(II), Ni(II), Pb(II) and Zn(II) by aminated solution-blown polyacrylonitrile micro/nanofibers. Water Sci. Technol. 2018, 2017, 378. [Google Scholar] [CrossRef]
- Gomes, P.C.; Fontes, M.P.F.; da Silva, A.G.; Mendonça, E.D.S.; Netto, A.R. Selectivity Sequence and Competitive Adsorption of Heavy Metals by Brazilian Soils. Soil Sci. Soc. Am. J. 2001, 65, 1115. [Google Scholar] [CrossRef]
- Karami, N.; Clemente, R.; Moreno-Jiménez, E.; Lepp, N.W.; Beesley, L. Efficiency of green waste compost and biochar soil amendments for reducing lead and copper mobility and uptake to ryegrass. J. Hazard. Mat. 2011, 191, 41–48. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Radziemska, M. The effect of chromium content in soil on the concentration of some mineral elements in plants. Fresenius Environ. Bull. 2009, 18, 1039–1045. [Google Scholar]
- Moon, D.H.; Park, J.W.; Chang, Y.Y.; Ok, Y.S.; Lee, S.S.; Ahmad, M.; Koutsospyros, A.; Park, J.H.; Baek, K. Immobilization of lead in contaminated firing range soil using biochar. Environ. Sci. Pollut. Res. 2013, 20, 8464. [Google Scholar] [CrossRef] [Green Version]
- Ok, Y.S.; Kim, S.C.; Kim, D.K.; Skousen, J.G.; Lee, J.S.; Cheong, Y.W.; Kim, S.J.; Yang, J.E. Ameliorants to immobilize Cd in rice paddy soils contaminated by abandoned metal mines in Korea. Environ. Geochem. Health 2011, 33, 23. [Google Scholar] [CrossRef]
- Dai, S.; Chen, Q.; Jiang, N.; Wang, B.; Xie, Z.; Yu, N.; Zhou, Y.; Li, S.; Wang, L.; Hua, Y.; et al. Colonized extremophile Deinococcusradiodurans alleviates toxicity of cadmium and lead by suppressing heavy metal accumulation and improving antioxidant system in rice. Environ. Poll. 2021, 284, 117127. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, H.; Xi, H.; Zhu, Y. Co-remediation of PTEs contaminated soil in mining area by heat modified sawdust and herb. Chemosphere 2021, 281, 130908. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radziemska, M.; Gusiatin, Z.M.; Mazur, Z.; Hammerschmiedt, T.; Bęś, A.; Kintl, A.; Galiova, M.V.; Holatko, J.; Blazejczyk, A.; Kumar, V.; et al. Biochar-Assisted Phytostabilization for Potentially Toxic Element Immobilization. Sustainability 2022, 14, 445. https://doi.org/10.3390/su14010445
Radziemska M, Gusiatin ZM, Mazur Z, Hammerschmiedt T, Bęś A, Kintl A, Galiova MV, Holatko J, Blazejczyk A, Kumar V, et al. Biochar-Assisted Phytostabilization for Potentially Toxic Element Immobilization. Sustainability. 2022; 14(1):445. https://doi.org/10.3390/su14010445
Chicago/Turabian StyleRadziemska, Maja, Zygmunt Mariusz Gusiatin, Zbigniew Mazur, Tereza Hammerschmiedt, Agnieszka Bęś, Antonin Kintl, Michaela Vasinova Galiova, Jiri Holatko, Aurelia Blazejczyk, Vinod Kumar, and et al. 2022. "Biochar-Assisted Phytostabilization for Potentially Toxic Element Immobilization" Sustainability 14, no. 1: 445. https://doi.org/10.3390/su14010445
APA StyleRadziemska, M., Gusiatin, Z. M., Mazur, Z., Hammerschmiedt, T., Bęś, A., Kintl, A., Galiova, M. V., Holatko, J., Blazejczyk, A., Kumar, V., & Brtnicky, M. (2022). Biochar-Assisted Phytostabilization for Potentially Toxic Element Immobilization. Sustainability, 14(1), 445. https://doi.org/10.3390/su14010445







