Development and Optimization of a Topical Formulation with Castanea sativa Shells Extract Based on the Concept “Quality by Design”
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extract Preparation
2.2.2. Preparation of the Cream Formulations
2.2.3. Quality by Design
Definition of QTPP and CQAs
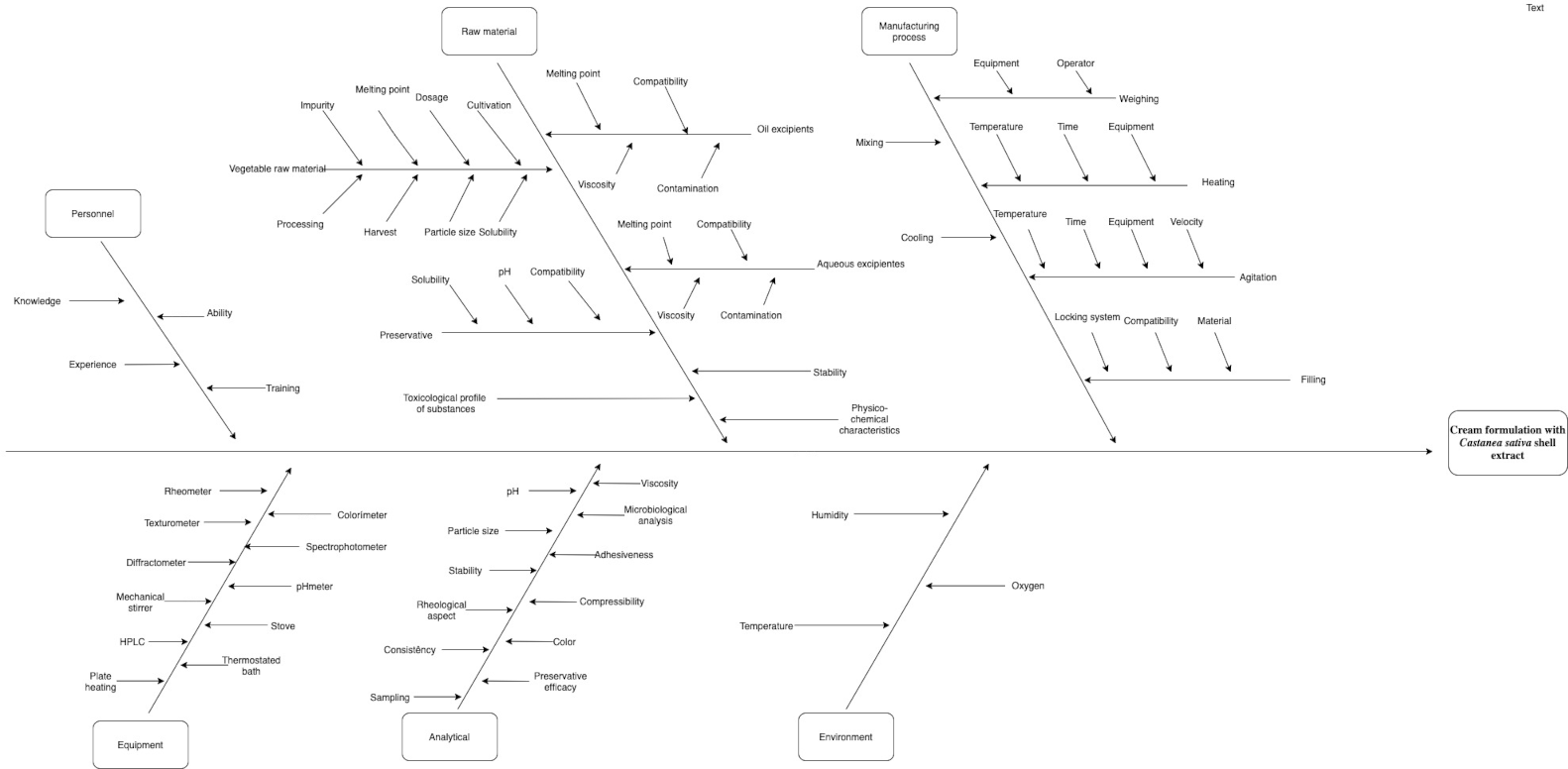
Risk Assessment
Design of Experiments
2.2.4. Characterization of the Formulations
Appearance
Determination of pH
Rheological Tests
Texture Analysis
Size of the Internal Phase
2.3. Statistical Analysis
3. Results
3.1. Preliminary Study
3.2. Definition of QTPP, CQAs, CMAs and CPPs
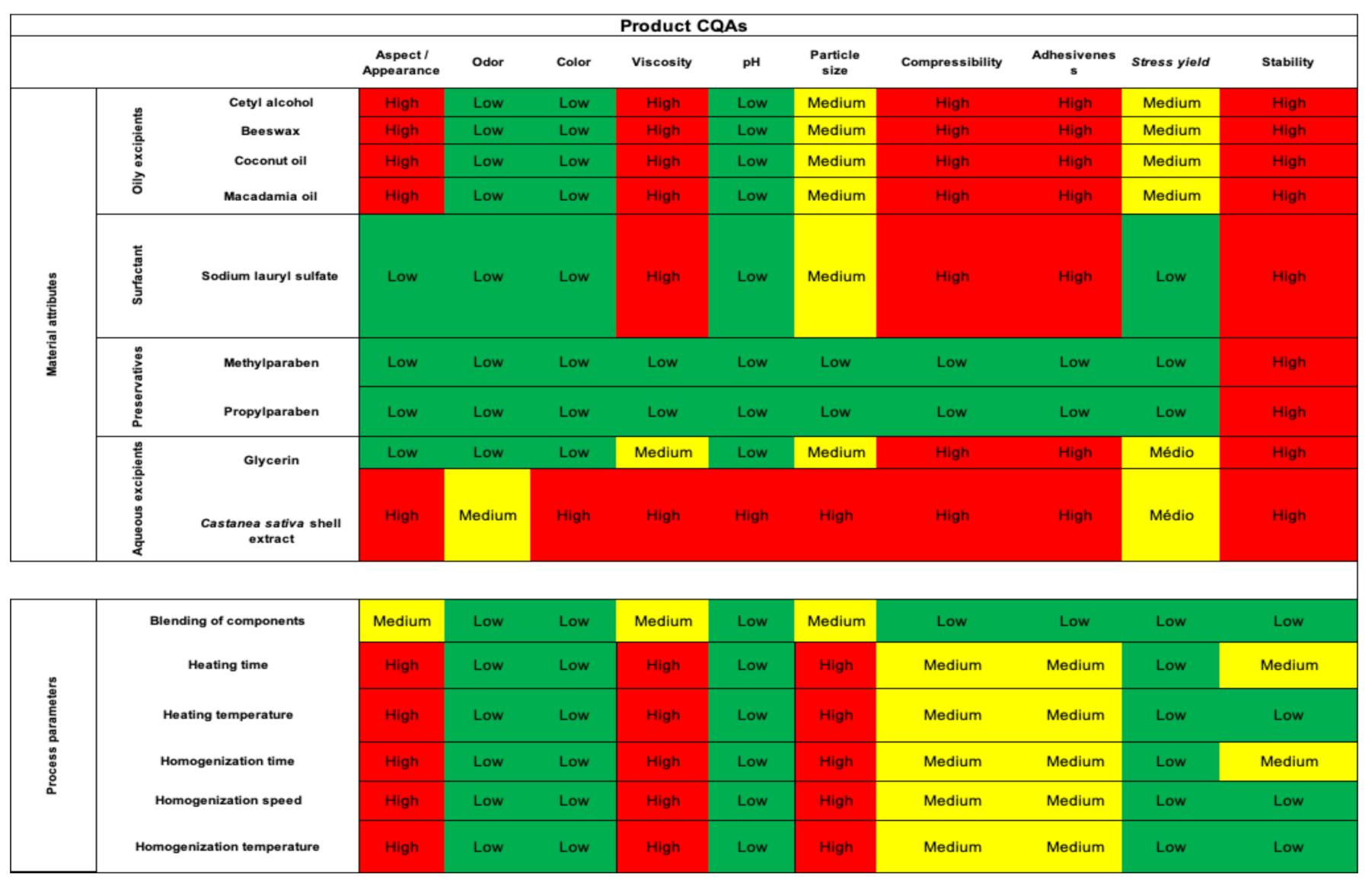
3.3. Initial Risk Assessement
3.4. Design of Experiments
3.4.1. Effect of Independent Variables on Dependent Variables
Effects on pH
Effects on Viscosity
Effects on Adhesiveness
Optimization and Validation by Box–Behnken Response Surface Methodology
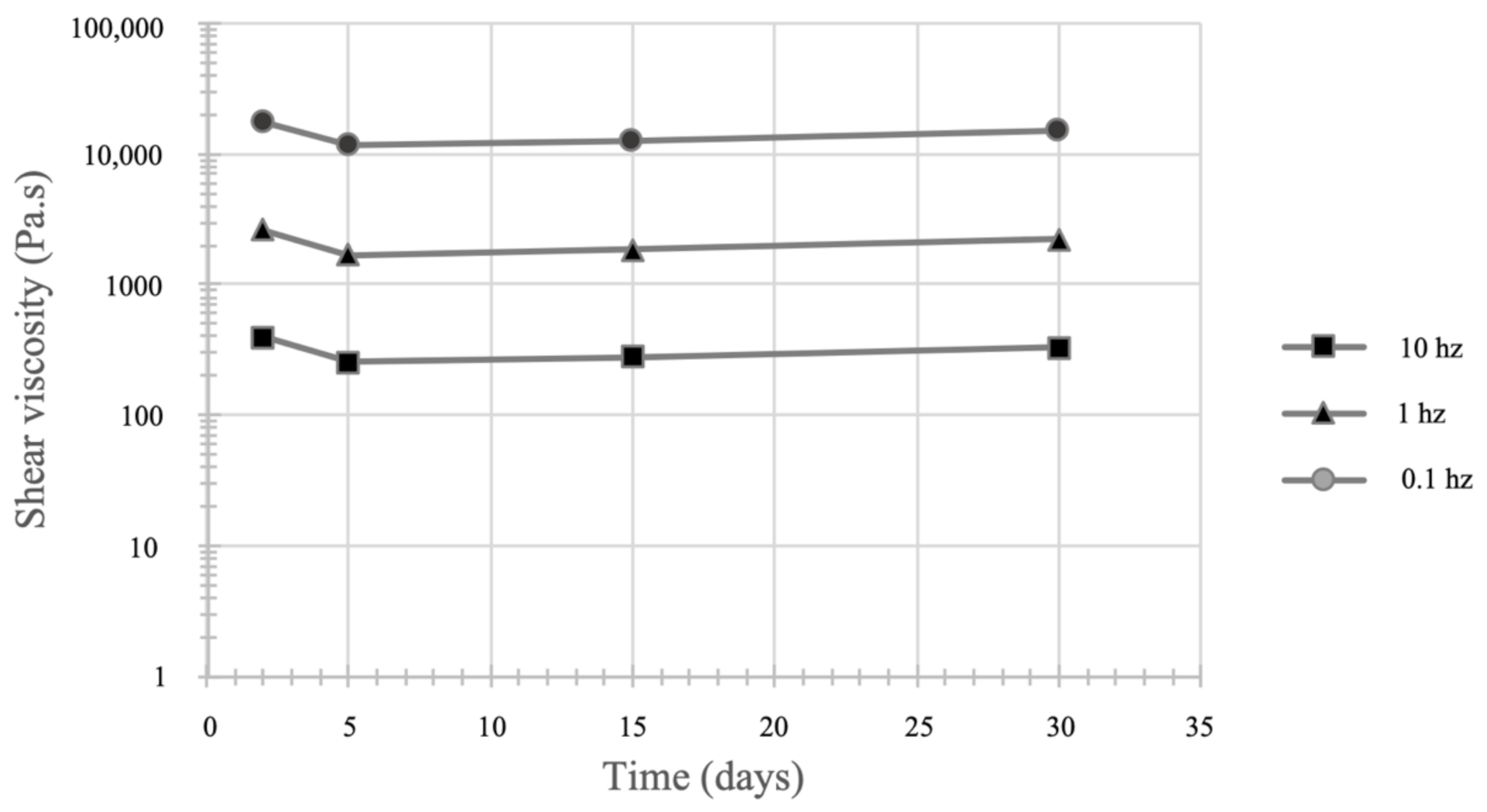
3.5. Characterization of the Optimized Formulation over Time
3.5.1. Appearance
3.5.2. Determination of pH
3.5.3. Rheological Tests
3.5.4. Texture Analysis
3.5.5. Size of the Internal Phase
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell-Goldman, E.; Murphy, G.F. The pathobiology of skin aging: New insights into an old dilemma. Am. J. Clin. Pathol. 2020, 190, 1356–1369. [Google Scholar] [CrossRef]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell aging and cellular senescence in skin aging—Recent advances in fibroblast and keratinocyte biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- Sunder, S. Relevant Topical Skin Care Products for Prevention and Treatment of Aging Skin. Facial Plast. Surg. Clin. 2019, 27, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Birch-Machin, M.; Bowman, A. Oxidative stress and ageing. Br. J. Dermatol. 2016, 175, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.; Fontenla, E.; Santos, J.; Freire, M.; González-Álvarez, J.; Antorrena, G. Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind. Crops Prod. 2008, 28, 279–285. [Google Scholar] [CrossRef]
- Almeida, I.F.; Pinto, A.; Monteiro, C.; Monteiro, H.; Belo, L.; Fernandes, J.; Bento, A.; Duarte, T.; Garrido, J.; Bahia, M.F. Protective effect of C. sativa leaf extract against UV mediated-DNA damage in a human keratinocyte cell line. J. Photochem. Photobiol. B Biol. 2015, 144, 28–34. [Google Scholar] [CrossRef]
- Rodrigues, F.; Santos, J.; Pimentel, F.B.; Braga, N.; Palmeira-de-Oliveira, A.; Oliveira, M.B.P. Promising new applications of Castanea sativa shell: Nutritional composition, antioxidant activity, amino acids and vitamin E profile. Food Funct. 2015, 6, 2854–2860. [Google Scholar] [CrossRef]
- Pinto, D.; Braga, N.; Silva, A.M.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Chestnut. In Valorization of Fruit Processing By-Products; Galanakis, C.M., Ed.; Elsevier: London, UK, 2020; pp. 127–144. [Google Scholar]
- De Vasconcelos, M.D.C.B.M.; Bennett, R.N.; Rosa, E.A.; Cardoso, J.V.F. Primary and secondary metabolite composition of kernels from three cultivars of Portuguese chestnut (Castanea sativa Mill.) at different stages of industrial transformation. J. Agric. Food Chem. 2007, 55, 3508–3516. [Google Scholar] [CrossRef]
- Vázquez, G.; González-Alvarez, J.; Santos, J.; Freire, M.; Antorrena, G. Evaluation of potential applications for chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind. Crops Prod. 2009, 29, 364–370. [Google Scholar] [CrossRef]
- Gonçalves, B.; Borges, O.; Costa, H.S.; Bennett, R.; Santos, M.; Silva, A.P. Metabolite composition of chestnut (Castanea sativa Mill.) upon cooking: Proximate analysis, fibre, organic acids and phenolics. Food Chem. 2010, 122, 154–160. [Google Scholar] [CrossRef]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; De Lucia, A.; Colucci, G.; Cara, F.L.; Morana, A. Chestnut (Castanea sativa Mill.) industrial wastes as a valued bioresource for the production of active ingredients. Process Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Pharmaceutical Development; Q8 (R2); ICH: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Lawrence, X.Y.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar]
- Lolas, A.G.; Rathore, A.S. Regulatory Challenges in the QbD Paradigm. BioPharm Int. 2012, 25, 44. Available online: https://www.biopharminternational.com/view/regulatory-challenges-qbd-paradigm (accessed on 25 October 2021).
- Marto, J.; Gouveia, L.; Gonçalves, L.; Gaspar, D.; Pinto, P.; Carvalho, F.; Oliveira, E.; Ribeiro, H.; Almeida, A. A quality by design (QbD) approach on starch-based nanocapsules: A promising platform for topical drug delivery. Colloids Surf. B. 2016, 143, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Yang, L.; Wang, Y.; Yu, S.; Guo, Y.; Deng, J.; Zhao, Q.; Jin, X. Formulation, development, and optimization of a novel octyldodecanol-based nanoemulsion for transdermal delivery of ceramide IIIB. Int. J. Nanomed. 2017, 12, 5203. [Google Scholar] [CrossRef]
- Lauterbach, A.; Ekelund, K. Rheological temperature sweeping in a quality by design approach for formulation development and optimization. Int. J. Pharm. 2019, 568, 118533. [Google Scholar] [CrossRef]
- Simões, A.; Veiga, F.; Vitorino, C. Developing cream formulations: Renewed interest in an old problem. J. Pharm. Sci. 2019, 108, 3240–3251. [Google Scholar] [CrossRef] [PubMed]
- Thakur, K.; Mahajan, A.; Sharma, G.; Singh, B.; Raza, K.; Chhibber, S.; Katare, O.P. Implementation of Quality by Design (QbD) approach in development of silver sulphadiazine loaded egg oil organogel: An improved dermatokinetic profile and therapeutic efficacy in burn wounds. Int. J. Pharm. 2020, 576, 118977. [Google Scholar] [CrossRef]
- Mitchell, M. Determining criticality-process parameters and quality attributes part II; design of experiments and data-driver criticality. BioPharm 2014, 1, 9. [Google Scholar]
- Lourenço, F.R.; Francisco, F.L.; Ferreira, M.R.S.; de Jesus Andreoli, T.; Löbenberg, R.; Bou-Chacra, N. Design space approach for preservative system optimization of an anti-aging eye fluid emulsion. J. Pharm. Pharm. Sci. 2015, 18, 551–561. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saviano, A.M.; Francisco, F.L.; Ostronoff, C.S.; Lourenço, F.R. Development, optimization, and validation of a microplate bioassay for relative potency determination of linezolid using a design space concept, and its measurement uncertainty. J. AOAC Int. 2015, 98, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Freitas, V.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Optimizing the extraction of phenolic antioxidants from chestnut shells by subcritical water extraction using response surface methodology. Food Chem. 2021, 334, 127521. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Quality Risk Management; Q9. Current Step 4; ICH: Amsterdam, The Netherlands, 2005; Volume 4, p. 408. [Google Scholar]
- Aslan, N.; Cebeci, Y. Application of Box–Behnken design and response surface methodology for modeling of some Turkish coals. Fuel 2007, 86, 90–97. [Google Scholar] [CrossRef]
- Candioti, L.V.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental design and multiple response optimization. Using the desirability function in analytical methods development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, E.S.; Noor, A.M.; Sakeena, M.H.; Abdullah, G.Z.; Abdulkarim, M.F.; Sattar, M.A. Formulation and in vitro release evaluation of newly synthesized palm kernel oil esters-based nanoemulsion delivery system for 30% ethanolic dried extract derived from local Phyllanthus urinaria for skin antiaging. Int. J. Nanomed. 2011, 6, 2499. [Google Scholar] [CrossRef]
- Maia Filho, D.C.; Ramalho, J.B.; Spinelli, L.S.; Lucas, E.F. Aging of water-in-crude oil emulsions: Effect on water content, droplet size distribution, dynamic viscosity and stability. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 208–212. [Google Scholar] [CrossRef]


| Independent Variables (Factors) | Levels | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| X1: Sodium lauryl sulfate (%) | 0.5 | 1 | 1.5 |
| X2: Beeswax (%) | 2 | 3.5 | 5 |
| X3: Macadamia oil (%) | 6 | 8 | 10 |
| QTPP | Target | CQAs | Justification |
|---|---|---|---|
| Product type | Cosmetic | - | - |
| Dosage form | Cream | - | Multiphase preparations consisting of a lipophilic phase and an aqueous phase. |
| Route of administration | Topic | - | Product administration location. |
| Dosage | NA | - | - |
| Aspect/Appearance | Homogeneous smooth cream with Castanea sativa Mill. Shell extract | Yes | Changes in the aspect/appearance of the product may affect quality, safety and effectiveness. |
| Changes in aspect/appearance are related to possible changes in the physical-chemical and/or microbiological stability of the product. | |||
| The aspect/appearance has an influence on the acceptance and adherence. | |||
| Odor | Characteristic | Yes | Changes in the odor of the product may indicate physical, chemical and/or microbiological changes in the product. |
| Odor has an influence on consumer acceptance and adherence. | |||
| Color | Light brown | Yes | The color of the product indicates the presence of C. sativa shells which have a brown color, in addition to indicating product stability. |
| Color has an influence on consumer acceptance and adherence. | |||
| Viscosity | 700–2500 Pa.s | Yes | The determination of viscosity is eventually used to assess the quality of a product. |
| Changes in the product’s viscosity demonstrate a possible change in the physical structure of the product, which may interfere with the time the product will remain in the application site. | |||
| Viscosity is a critical factor in assessing product stability. | |||
| Identification | Eur. Ph/USP | - | It is a critical factor for product safety and effectiveness. |
| pH | 4.0–8.0 | Yes | The pH has an influence on the physical-chemical stability of the product. |
| The pH of the product must be compatible with the pH of the skin in order to avoid irritation and damage to the integrity of the skin after application of the product. | |||
| Crystallization | Eur. Ph/USP | Yes | Crystallization has an impact on the uniformity and stability of the formulation. |
| Particle size | 100 nm–100 μm | Yes | Particle size has an impact on effectiveness and stability. |
| Compressibility | 20.0–60.0 N.mm | Yes | The compressibility is related to product consistency. Changes in the consistency of the product may modify the characteristics of the product, impacting its spreadability and adherence. |
| Compressibility has an influence on acceptance and adherence. | |||
| Adhesiveness | 20.0–60.0 N.mm | Yes | Adhesiveness is related to cream retention on the skin. It has an impact on the preservation of cream in situ. |
| Preservative testing | Eur. Ph/USP | Yes | The testing of preservatives present in the product will ensure the safety and stability of the formulation. |
| Microbial limits | Eur. Ph/USP | Yes | Change in microbiological limits may impact product safety. |
| Stability | ICH Q1A/Eur. Ph/USP | Yes | Changes in product stability may impact the quality of the product during the storage period. |
| It is a quality requirement. | |||
| Container closure system | Appropriate for the dosage form | - | The packing material must be appropriate to contain the physical form of the product. |
| Material integrity | No failure | - | The integrity of the material must be maintained in order to guarantee the quality, effectiveness and safety. |
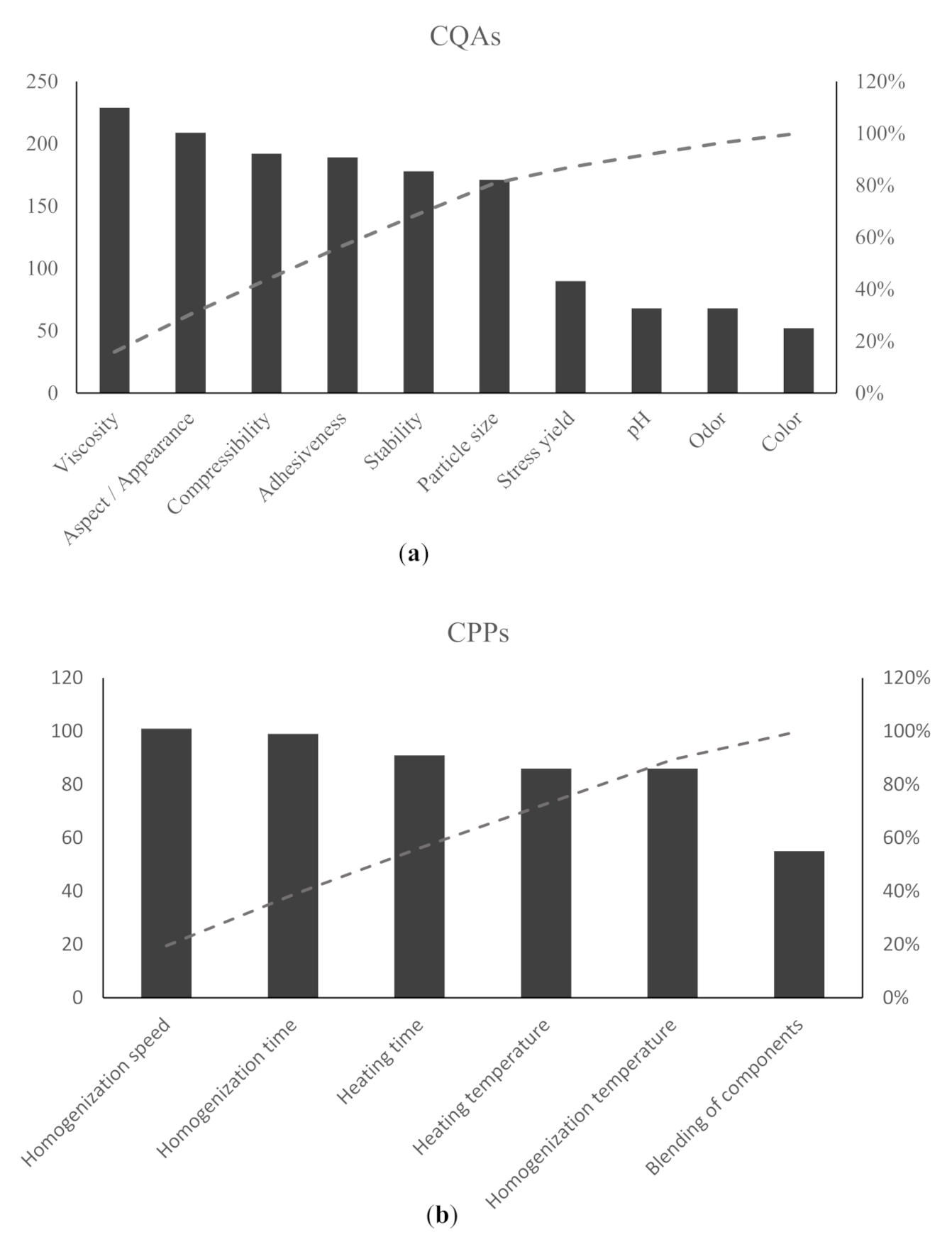
| Independent Variables | Dependent Variables | |||||
|---|---|---|---|---|---|---|
| Run | X1: Sodium Lauryl Sulfate | X2: Beeswax | X3:Macadamia Oil | Y1: pH | Y2: Viscosity | Y3: Adhesiveness |
| (%) | (%) | (%) | (Pa s) | (N.mm) | ||
| C1 | 1.5 | 5 | 8 | 7.11 ± 0.09 | 1520.6 ± 90.45 | 40.39 ± 5.91 |
| C2 | 1.5 | 3.5 | 10 | 7.30 ± 0.13 | 931.3 ± 187.31 | 30.15 ± 1.73 |
| C3 | 1 | 3.5 | 8 | 7.43 ± 0.13 | 1820.8 ± 276.21 | 35.83 ± 6.58 |
| C4 | 1 | 3.5 | 8 | 7.33 ± 0.06 | 2086.2 ± 229.12 | 41.30 ± 3.62 |
| C5 | 0.5 | 5 | 8 | 7.14 ± 0.03 | 2831.9 ± 1951.98 | 26.09 ± 14.41 |
| C6 | 1.5 | 3.5 | 6 | 7.43 ± 0.09 | 1029.0 ± 813.49 | 49.31 ± 8.54 |
| C7 | 0.5 | 3.5 | 6 | 7.32 ± 0.04 | 1415.9 ± 1074.74 | 22.86 ± 4.71 |
| C8 | 0.5 | 3.5 | 10 | 7.22 ± 0.03 | 1353.5 ± 1171.70 | 28.85 ± 9.91 |
| C9 | 1.5 | 2 | 8 | 7.33 ± 0.01 | 700.3 ± 585.02 | 46.56 ± 9.63 |
| C10 | 1 | 3.5 | 8 | 7.30 ± 0.06 | 1517.1 ± 1341.24 | 44.62 ± 0.33 |
| C11 | 1 | 5 | 10 | 7.50 ± 0.01 | 1667.5 ± 1465.21 | 58.82 ± 18.07 |
| C12 | 1 | 5 | 6 | 7.43 ± 0.03 | 1428.0 ± 1205.44 | 57.80 ± 3.67 |
| C13 | 1 | 2 | 6 | 7.86 ± 0.03 | 1015.6 ± 896.53 | 47.35 ± 1.52 |
| C14 | 1 | 3.5 | 8 | 7.56 ± 0.07 | 1670.7 ± 1344.08 | 52.13 ± 4.33 |
| C15 | 0.5 | 2 | 8 | 7.50 ± 0.03 | 1804.7 ± 1433.45 | 23.18 ± 3.38 |
| C16 | 1 | 3.5 | 8 | 7.66 ± 0.05 | 1288.0 ± 1117.73 | 50.98 ± 3.38 |
| C17 | 1 | 2 | 10 | 7.60 ± 0.01 | 877.7 ± 709.41 | 44.97 ± 7.80 |
| Sum of Squares | Mean of Squares | F-Value | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y1 | Y2 | Y3 | Y1 | Y2 | Y3 | Y1 | Y2 | Y3 | Y1 | Y2 | Y3 | |
| X1: Sodium lauryl sulfate | 0.0 | 1.2999 × 106 | 535.136 | 0.0 | 1.2999 × 106 | 535.136 | 0.0 | 14.24 | 11.60 | 1.0000 | 0.0195 | 0.0271 |
| X2:Beeswax | 0.180 | 1.1626 × 106 | 55.3352 | 0.18 | 1.1626 × 106 | 55.3352 | 7.83 | 12.74 | 1.20 | 0.0489 | 0.0234 | 0.3349 |
| X3: Macadamia oil | 0.02 | 427.781 | 26.3901 | 0.02 | 427.781 | 26.3901 | 0.87 | 0.00 | 0.57 | 0.4039 | 0.9487 | 0.4915 |
| X1.X1 | 0.252737 | 765.096 | 970.21 | 0.252737 | 765.096 | 970.21 | 10.99 | 0.01 | 21.03 | 0.0295 | 0.9314 | 0.0101 |
| X1.X2 | 0.01 | 10,701.9 | 20.6116 | 0.01 | 10,701.9 | 20.6116 | 0.43 | 0.12 | 0.45 | 0.5457 | 0.7493 | 0.5404 |
| X1.X3 | 0.0 | 311.522 | 158.131 | 0.0 | 311.522 | 158.131 | 0.000 | 0.00 | 3.43 | 1.0000 | 0.9562 | 0.1378 |
| X2.X2 | 0.0127368 | 11,078.6 | 76.5096 | 0.0127368 | 11,078.6 | 76.5096 | 0.55 | 0.12 | 1.66 | 0.4981 | 0.7451 | 0.2672 |
| X2.X3 | 0.04 | 35,607.7 | 2.89 | 0.04 | 35,607.7 | 2.89 | 1.74 | 0.39 | 0.06 | 0.2577 | 0.5661 | 0.8147 |
| X3.X3 | 0.0464211 | 972,755.0 | 37.9011 | 0.0464211 | 972,755.0 | 37.9011 | 2.02 | 10.66 | 0.82 | 0.2284 | 0.0309 | 0.416 |
| Lack of adjustment | 0.02 | 383,258.0 | 124.704 | 0.00666667 | 127,753.0 | 41.5681 | 0.29 | 1.40 | 0.90 | 0.8316 | 0.3653 | 0.5147 |
| Pure error | 0.092 | 365,051.0 | 184.517 | 0.023 | 91,262.7 | 46.1292 | ||||||
| Total | 0.66 | 4.23937 × 106 | 2152.85 | |||||||||
| Y1 | Y2 | Y3 | |
|---|---|---|---|
| Experimental value | 7.43 ± 0.09 | 2214.44 ± 211.74 | 45.19 ± 2.42 |
| Intended value | 7.49 | 1779.08 | 53.66 |
| p-value | 0.61 | 0.27 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, N.; Cádiz-Gurrea, M.d.l.L.; Silva, A.M.; Macedo, C.; Rodrigues, F.; Costa, P. Development and Optimization of a Topical Formulation with Castanea sativa Shells Extract Based on the Concept “Quality by Design”. Sustainability 2022, 14, 129. https://doi.org/10.3390/su14010129
Oliveira N, Cádiz-Gurrea MdlL, Silva AM, Macedo C, Rodrigues F, Costa P. Development and Optimization of a Topical Formulation with Castanea sativa Shells Extract Based on the Concept “Quality by Design”. Sustainability. 2022; 14(1):129. https://doi.org/10.3390/su14010129
Chicago/Turabian StyleOliveira, Nágilla, Maria de la Luz Cádiz-Gurrea, Ana Margarida Silva, Catarina Macedo, Francisca Rodrigues, and Paulo Costa. 2022. "Development and Optimization of a Topical Formulation with Castanea sativa Shells Extract Based on the Concept “Quality by Design”" Sustainability 14, no. 1: 129. https://doi.org/10.3390/su14010129
APA StyleOliveira, N., Cádiz-Gurrea, M. d. l. L., Silva, A. M., Macedo, C., Rodrigues, F., & Costa, P. (2022). Development and Optimization of a Topical Formulation with Castanea sativa Shells Extract Based on the Concept “Quality by Design”. Sustainability, 14(1), 129. https://doi.org/10.3390/su14010129









